Large‐scale trials have evaluated the effects of treatments on major renal outcomes, but the definition of a renal event has varied from trial to trial. In defining a renal event, trialists included patients who needed renal replacement therapy or whose estimated glomerular filtration rate (eGFR) declined to <10–15 mL/min/1.73 m2, and they also included patients who experienced large and sustained decreases in eGFR from baseline. Different trials have designated different threshold values for a critical change in eGFR — with some trials designating a sustained ≥40% decline and other specifying a sustained ≥50% decrease. Still others have required a sustained doubling of serum creatinine, which corresponds to a ≥57% decline in eGFR.
Two large‐scale trials have evaluated the effect of sodium–glucose co‐transporter 2 (SGLT2) inhibitors in patients with heart failure and a reduced ejection fraction. 1 They reported a favourable effect of dapagliflozin and empagliflozin on heart failure hospitalizations, but the two trials prespecified different definitions of a major renal event. Both trials included patients who required renal replacement therapy or who experienced a sustained decrease in eGFR to <10–15 mL/min/1.73 m2. However, the EMPEROR‐Reduced trial also included patients with a sustained ≥40% decrease in eGFR, whereas the DAPA‐HF investigators specified a sustained ≥50% decrease in eGFR, and they also included the occurrence of renal death. 1 , 2 The definition used by the DAPA‐HF trial was used in a meta‐analysis of the two trials. 1 In DAPA‐HF, the hazard ratio for the effect of dapagliflozin using the meta‐analysis renal endpoint was 0.71 [95% confidence interval (CI) 0.44–1.16]. 1 In EMPEROR‐Reduced, the hazard ratio for the effect of empagliflozin was 0.50 (95% CI 0.32–0.77) using the EMPEROR definition, and it was 0.52 (95% CI 0.29–0.92) using the meta‐analysis definition. 1 , 2 The use of different definitions for a renal event did not influence conclusions concerning a benefit of empagliflozin on renal outcomes in heart failure and a reduced ejection fraction.
The EMPEROR‐Preserved trial was a large, international, double‐blind and placebo‐controlled trial of empagliflozin in patients with heart failure and a preserved ejection fraction. Patients with heart failure and an ejection fraction >40% were randomly assigned to placebo or empagliflozin for a median of 26 months. Empagliflozin reduced the primary endpoint of cardiovascular death or heart failure hospitalization by 21% [hazard ratio 0.79 (95% CI 0.69–0.90)] and decreased total (first and recurrent) hospitalizations for heart failure by 27% [hazard ratio 0.73 (95% CI 0.61–0.88)]. 3 When the influence of baseline ejection fraction on these results was evaluated according to prespecified subgroups of 41–49%, 50–59% and ≥60%, baseline ejection fraction did not influence the effect of empagliflozin on the primary endpoint. However, ejection fraction did influence the effect of empagliflozin on total hospitalizations for heart failure (P‐trend = 0.008), with an attenuated effect in patients with an ejection fraction ≥60%. 4
In contrast to these favourable effects of empagliflozin on heart failure outcomes in EMPEROR‐Preserved, empagliflozin did not exert a favourable effect on major renal outcomes using the EMPEROR definition, 5 which relied on a threshold of a sustained ≥40% decrease in eGFR and did not include the occurrence of renal death. The hazard ratio for the effect of empagliflozin on major renal events was 0.95 (95% CI 0.73–1.24). The neutral effect of empagliflozin on kidney outcomes was similarly observed across the prespecified ejection fraction subgroups of 41–49%, 50–59% and ≥60% (Figure 1 ). 5
Figure 1.
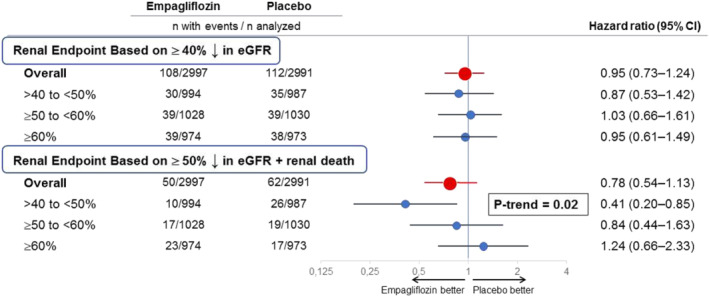
Effect of empagliflozin on major renal outcomes in EMPEROR‐Preserved, overall and in prespecified ejection fraction subgroups, using different definitions for a renal event. Shown are prespecified ejection fraction subgroups: >40% to <50%, ≥50% to <60% and ≥60%. Effect in the overall trial is shown in red, whereas effects in the ejection fraction subgroups are shown in blue. The renal endpoint based on a ≥40% sustained decline in estimated glomerular filtration rate (eGFR) was prespecified in the EMPEROR‐Reduced and EMPEROR‐Preserved trials, whereas the renal endpoint based on a ≥50% sustained decline in eGFR and including renal death was prespecified in the DAPA‐HF trial and was used in a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. 1 Both endpoints included patients who required chronic renal replacement therapy or who experienced a sustained decrease in eGFR to <10–15 mL/min/1.73 m2. There were 14 renal deaths (10 on placebo and 4 on empagliflozin). The P‐trend test refers to the linear influence of three prespecified ejection fraction subgroups on the magnitude of the effect of empagliflozin on major renal outcomes. CI, confidence interval.
Therefore, according to the analyses that had been prespecified in EMPEROR‐Preserved, we found a striking discordance between the effect of empagliflozin on heart failure outcomes and major renal events, both in the overall population and in prespecified subgroups. When considering all patients with an ejection fraction >40%, empagliflozin reduced heart failure hospitalizations with no effect on major renal outcomes; when considering prespecified subgroups, ejection fraction significantly influenced the effect of empagliflozin on heart failure admissions but not on renal events. These discordances were extraordinarily puzzling, since in prior large‐scale clinical trials, the effect of SGLT2 inhibitors on heart failure and renal outcomes had consistently tracked together. 6 , 7
To determine if the observed discordances were related to the definition that we specified for the identification of a renal event, we asked if our results would differ if we had prespecified the more conventional meta‐analysis criteria for a renal event. Accordingly, we re‐analysed the data from EMPEROR‐Preserved using the meta‐analysis definition. The hazard ratio for the effect of empagliflozin on major renal outcomes for the overall population was 0.78 (95% CI 0.54–1.13), a finding similar to that previously reported with dapagliflozin in patients with a reduced ejection fraction. 1 Additionally, using the meta‐analysis definition, ejection fraction had a significant influence on the magnitude of the effect of empagliflozin on kidney outcomes in EMPEROR‐Preserved (P‐trend = 0.02) (Figure 1 ). In patients with an ejection fraction of 41–49%, the hazard ratio was 0.41 (95% CI 0.20–0.85), an effect comparable to that which we previously reported for patients with an ejection fraction of ≤40% using the same endpoint [hazard ratio 0.52 (95% CI 0.29–0.92)] 1 — a finding consistent with the premise that patients with an ejection fraction of 41–49% should be classified as having heart failure and a reduced ejection fraction. 8 In contrast, in patients with an ejection fraction ≥60%, the hazard ratio was 1.24 (95% CI 0.66–2.33). Accordingly, the influence of ejection fraction on renal outcomes (P‐trend = 0.02) now closely paralleled the influence of ejection fraction on heart failure hospitalizations (P‐trend = 0.008), noted above. 4
Our results indicate that the definition of a major renal outcome can influence conclusions concerning the effect of a treatment on the progression of kidney disease in patients with heart failure. In the EMPEROR‐Preserved trial, we found a discordance between the effects of empagliflozin on heart failure hospitalizations and renal outcomes using the EMPEROR definition of a kidney event, but we noted a concordance between the heart failure and renal effects of SGLT2 inhibition (overall and in prespecified subgroups) when we used a more conventional definition, a finding that is closely aligned with observations of the effects of these drugs in large‐scale trials in type 2 diabetes. 6 , 7 Further exploration of these findings is warranted.
Conflict of interest: M.B., C.Z. and S.H. are employees of Boehringer Ingelheim. The other authors serve on the Executive Committee of the EMPEROR trials and receive consulting fees from Boehringer Ingelheim related to this activity.
References
- 1. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 2. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 3. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner‐La Rocca HP, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR‐Preserved Trial Investigators . Empagliflozin and cardiovascular outcomes in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 4. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Schnaidt S, Zeller C, Schnee JM, Anker SD; EMPEROR‐Preserved Trial Study Group . Effect of empagliflozin on worsening heart failure events in patients with heart failure and a preserved ejection fraction: the EMPEROR‐Preserved trial. Circulation 2021. Aug 29. 10.1161/CIRCULATIONAHA.121.056824 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Packer M, Butler J, Zannad F, Pocock SJ, Filippatos G, Ferreira JP, Brueckmann M, Jamal W, Zeller C, Wanner C, Anker SD; EMPEROR Study Group . Empagliflozin and major renal outcomes in heart failure. N Engl J Med 2021;385:1531–1533. [DOI] [PubMed] [Google Scholar]
- 6. Barbarawi M, Al‐Abdouh A, Barbarawi O, Lakshman H, Al Kasasbeh M, Chen K. SGLT2 inhibitors and cardiovascular and renal outcomes: a meta‐analysis and trial sequential analysis. Heart Fail Rev 2021. Feb 23. 10.1007/s10741-021-10083-z [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo‐Jack S, Pratley R, Greenberg M, Wang S, Huyck S, Gantz I, Terra SG, Masiukiewicz U, Cannon CP. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta‐analysis. JAMA Cardiol 2021;6:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butler J, Anker SD, Packer M. Redefining heart failure with a reduced ejection fraction. JAMA 2019;322:1761–1762. [DOI] [PubMed] [Google Scholar]


