Abstract
Aim
To compare the effects of semaglutide 1.0 mg versus dulaglutide 3.0 and 4.5 mg on HbA1c and body weight in patients with type 2 diabetes.
Materials and Methods
A Bucher indirect comparison was conducted to compare efficacy outcomes of semaglutide 1.0 mg versus dulaglutide 3.0 and 4.5 mg using published results from the SUSTAIN 7 and AWARD‐11 trials. Sensitivity analyses using individual patient data from SUSTAIN 7 and aggregate data from AWARD‐11 were conducted to explore the impact of adjustment for cross‐trial imbalances in baseline characteristics.
Results
Semaglutide 1.0 mg significantly reduced HbA1c versus dulaglutide 3.0 mg, with an estimated treatment difference (ETD) of −0.24%‐points (95% confidence interval [CI] −0.43, −0.05), with comparable reductions in HbA1c versus dulaglutide 4.5 mg with an ETD of −0.07%‐points (95% CI −0.26, 0.12). Semaglutide 1.0 mg significantly reduced body weight versus dulaglutide 3.0 and 4.5 mg with an ETD of −2.65 kg (95% CI −3.57, −1.73) and −1.95 kg (95% CI −2.87, −1.03), respectively. Sensitivity analyses supported the primary analysis findings.
Conclusions
This indirect comparison showed significantly greater reductions in HbA1c with semaglutide 1.0 mg versus dulaglutide 3.0 mg and comparable HbA1c reductions versus dulaglutide 4.5 mg. Semaglutide 1.0 mg significantly reduced body weight versus both dulaglutide 3.0 and 4.5 mg. With several glucagon‐like peptide‐1 receptor agonists available, information regarding their comparative efficacy can be valuable to clinicians.
Keywords: dulaglutide, GLP‐1 receptor agonist, indirect comparison, semaglutide, type 2 diabetes
1. INTRODUCTION
Glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) are an established treatment option for people with type 2 diabetes (T2D), offering glycaemic control alongside beneficial effects on body weight and cardiovascular disease. 1 Semaglutide and dulaglutide are both long‐acting GLP‐1 RAs with once‐weekly (QW) dosing regimens and are recommended as treatment options for patients not adequately controlled on metformin alone, as well as those with established cardiovascular disease regardless of HbA1c. 2 , 3 However, semaglutide differs from dulaglutide in molecular structure and is smaller in size, which may lead to differences in metabolic effects. 3 , 4 , 5 , 6
Semaglutide was approved for use at maintenance doses of 0.5 and 1.0 mg by the Food and Drug Administration (FDA) in 2017 7 and subsequently by the European Medicines Agency (EMA) in 2018. 8 Dulaglutide was initially approved at doses of 0.75 and 1.5 mg by the FDA and EMA in 2014. 5 , 9 Semaglutide and dulaglutide have been extensively investigated as part of the SUSTAIN and AWARD clinical trial programmes, respectively. SUSTAIN 7 compared the efficacy and safety of semaglutide 0.5 mg versus dulaglutide 0.75 mg and semaglutide 1.0 mg versus dulaglutide 1.5 mg in patients with T2D inadequately controlled with metformin. 6 The superiority of semaglutide compared with dulaglutide was shown with regard to reducing HbA1c and body weight at week 40. 6 Semaglutide and dulaglutide had similar safety profiles, with comparable proportions of patients experiencing gastrointestinal (GI) adverse events and discontinuing treatment as a result of adverse events. 6
Because of the progressive nature of T2D, treatment intensification is often required to achieve and maintain good glycaemic control and to minimize complications. 10 SUSTAIN 7 and other trials illustrate that higher doses of GLP‐1 RAs are associated with greater reductions in HbA1c, although there is a need to balance treatment benefits with adverse events, particularly GI adverse events. 11 This has prompted the study of higher QW doses of both semaglutide and dulaglutide. The efficacy and safety of semaglutide 2.0 mg for the treatment of T2D is currently being assessed in the SUSTAIN FORTE trial. 12 Higher doses of dulaglutide (3.0 and 4.5 mg) were investigated in AWARD‐11 and subsequently approved in 2020 and 2021 by the FDA and EMA, respectively. 5 , 13 In AWARD‐11, treatment with dulaglutide 3.0 or 4.5 versus 1.5 mg at week 36 showed dose‐related reductions in HbA1c and body weight, with increased weight loss observed with higher doses. Dulaglutide 3.0 and 4.5 mg showed similar safety profiles to dulaglutide 1.5 mg and GI tolerability generally consistent with that previously established for dulaglutide. 14
There are currently no head‐to‐head data comparing semaglutide 1.0 mg with doses of dulaglutide of more than 1.5 mg/week. Following FDA and EMA approval of dulaglutide at more than 1.5 mg/week, 5 , 13 information on the relative efficacy of semaglutide 1.0 mg and dulaglutide at more than 1.5 mg/week would be useful for clinicians, to help guide selection of the most suitable GLP‐1 RA for patients with T2D uncontrolled on metformin. Therefore, the objective of this analysis was to compare the efficacy of semaglutide 1.0 mg with dulaglutide 3.0 or 4.5 mg, using an indirect comparison based on results from SUSTAIN 7 6 and AWARD‐11. 14
2. METHODS
An indirect comparison using the Bucher method 15 was conducted to compare efficacy outcomes of dulaglutide 3.0 and 4.5 mg with semaglutide 1.0 mg using available published results from the SUSTAIN 7 and AWARD‐11 randomized controlled trials (RCTs). The Bucher method is an established approach for performing indirect treatment comparisons (ITCs), and has been used previously for the comparison of glucose‐lowering treatments. 16 The method accounts for cross‐trial differences by measuring treatment effects relative to a common comparator arm and requires only the availability of summary‐level data for each trial. The Bucher method is appropriate if the relative treatment effect can be assumed to be the same across the two trial populations. In the current setting, this translates to assuming that the comparative efficacy results of SUSTAIN 7 would have been the same had SUSTAIN 7 been performed in an AWARD‐11 population. This is considered an appropriate assumption based on previous analyses of the impact of patient characteristics on clinical outcomes in SUSTAIN 7. 17 Moreover, both SUSTAIN 7 and AWARD‐11 RCTs were conducted in patients inadequately controlled on metformin monotherapy. Bucher indirect comparisons were calculated in SAS version 9.4. Further details of the Bucher methodology are provided in the supporting information. Sensitivity analyses are described in detail in section 2.4.
2.1. Data sources
Published aggregate data were available from both SUSTAIN 7 and AWARD‐11, and individual patient data (IPD) were also available from SUSTAIN 7. Both SUSTAIN 7 and AWARD‐11 enrolled patient populations with T2D inadequately controlled on metformin; SUSTAIN 7 reported a comparison between semaglutide 1.0 mg and dulaglutide 1.5 mg and AWARD‐11 compared dulaglutide 3.0 and 4.5 mg with dulaglutide 1.5 mg. 14 , 18 Hence, both SUSTAIN 7 and AWARD‐11 included dulaglutide 1.5 mg as a comparator in similar populations, forming the network of evidence shown in Figure 1, in which anchored ITCs of semaglutide 1.0 mg versus dulaglutide 3.0 and 4.5 mg are possible.
FIGURE 1.
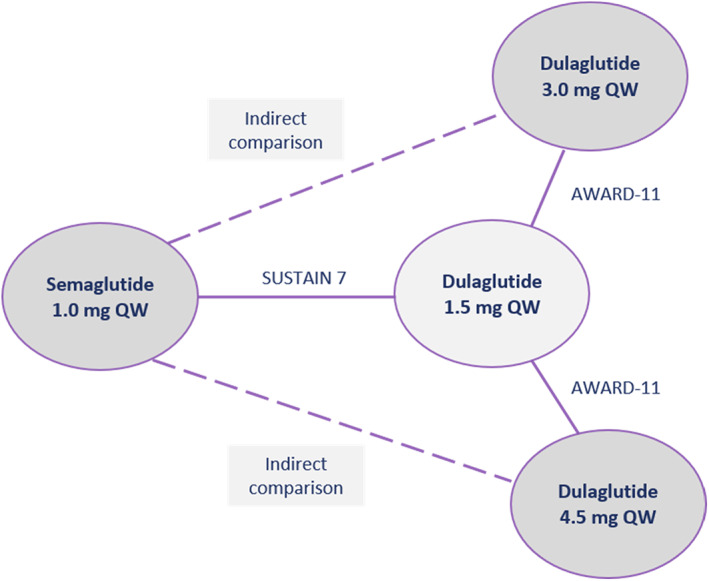
Network of evidence for semaglutide 1.0 mg versus dulaglutide 3.0 and 4.5 g. QW, once‐weekly
2.2. Outcomes assessed
Outcomes assessed in the ITCs were change from baseline in HbA1c and change from baseline in body weight, corresponding to the primary and secondary confirmatory outcomes in both SUSTAIN 7 and AWARD‐11. These outcomes were assessed at 40 and 36 weeks in SUSTAIN 7 and AWARD‐11, respectively. For these continuous outcomes, mean treatment differences with associated 95% confidence intervals (CIs) were calculated. The proportions of patients achieving HbA1c less than 7.0% were also assessed and, for this dichotomous outcome, treatment odds ratios (ORs) with associated 95% CIs were calculated.
2.3. Study and patient characteristics
Inclusion criteria of the SUSTAIN 7 and AWARD‐11 trials used in the analysis are shown in Table 1. SUSTAIN 7 was an open‐label, multinational, Phase IIIb trial and AWARD‐11 was a double‐blind, multinational, Phase III trial. Although there were some differences in terms of trial design and inclusion criteria, baseline characteristics were generally similar between the two trials, with both SUSTAIN 7 and AWARD‐11 RCTs conducted in patients inadequately controlled on metformin monotherapy, with slightly higher mean baseline HbA1c, body weight and T2D duration in AWARD‐11 compared with SUSTAIN 7 (Table 2). However, the indirect comparisons of semaglutide 1.0 mg versus dulaglutide 3.0 and 4.5 mg were anchored to dulaglutide 1.5 mg, making it possible to conduct a robust analysis without further adjustment for differences in prognostic factors across trials. In addition, based on a post hoc analysis investigating the impact of clinically relevant characteristics on relative treatment effects of semaglutide versus dulaglutide, there is no evidence to suggest that differences in baseline characteristics (age, sex, diabetes duration, glycaemic control and body mass index [BMI]) result in effect modification for semaglutide compared with dulaglutide. 17 Direct comparisons between treatments for change from baseline in HbA1c and change from baseline in body weight as reported in AWARD‐11 and SUSTAIN 7 are shown in Table S2.
TABLE 1.
Overview of study characteristics and inclusion criteria of included trials
| Study name NCT number | Study design | Inclusion criteria | Duration of follow‐up | Randomized treatment | Number of patients randomized | |||
|---|---|---|---|---|---|---|---|---|
| HbA1c | BMI | T2D duration | Background medication | |||||
| SUSTAIN 7 a NCT02648204 | Phase IIIb, open‐label, RCT multinational | 7.0%‐10.5% | No restriction | Not specified | Metformin ≥1500 mg/d (or a maximal tolerated dose) ≥90 d | 40 wk | Semaglutide 1.0 mg | 300 |
| Dulaglutide 1.5 mg | 299 | |||||||
| AWARD‐11 NCT03495102 | Phase III, double‐blind, RCT multinational | 7.5%‐11.0% | ≥25 kg/m2 | At least 6 mo | Metformin ≥1500 mg/d for ≥3 mo | 36 wk | Dulaglutide 3.0 mg | 616 |
| Dulaglutide 4.5 mg | 614 | |||||||
| Dulaglutide 1.5 mg | 612 | |||||||
Abbreviations: BMI, body mass index; RCT, randomized controlled trial; T2D, type 2 diabetes.
Note SUSTAIN 7 also included semaglutide 0.5 mg and dulaglutide 0.75 mg; however, these were not treatments of interest for the current analysis.
TABLE 2.
Characteristics of enrolled patients reported in the SUSTAIN 7 and AWARD‐11 trials
| Trial name | SUSTAIN 7 | AWARD‐11 | |||||
|---|---|---|---|---|---|---|---|
| Treatment | Semaglutide 1.0 mg (n = 300) | Dulaglutide 1.5 mg (n = 299) | Pooled treatment arms (semaglutide 1.0 mg and dulaglutide 1.5 mg) (n = 599) | Dulaglutide 3.0 mg (n = 616) | Dulaglutide 4.5 mg (n = 614) | Dulaglutide 1.5 mg (n = 612) | Pooled treatment arms (dulaglutide 3.0 mg, 4.5 mg and 1.5 mg) (n = 1842) |
| Mean age (SD), y | 55 (10.6) | 56 (10.6) | 55.6 (10.6) | 56.9 (10.2) | 56.6 (10.2) | 57.8 (9.7) | 57.1 (10.0) |
| Mean baseline HbA1c (SD), % | 8.2 (0.9) | 8.2 (0.9) | 8.2 (0.9) | 8.6 (1.0) | 8.6 (0.9) | 8.6 (0.9) | 8.6 (1.0) |
| Fasting glucose a (SD), mg/dL | 177.1 (46.5) | 172.5 (41.2) | 174.8 (44.0) | 184 (54.4) | 183.4 (48.0) | 185 (52.0) | 184.1 (51.5) |
| Mean body weight (SD), kg | 95.5 (20.9) | 93.4 (21.8) | 94.5 (21.4) | 96.3 (20.1) | 95.4 (20.6) | 95.5 (20.2) | 95.7 (20.3) |
| Mean BMI (SD), kg/m2 | 33.6 (6.5) | 33.1 (6.6) | 33.3 (6.5) | 34.3 (6.2) | 34.0 (6.2) | 34.4 (6.4) | 34.2 (6.3) |
| Mean T2D duration (SD), y | 7.3 (5.7) | 7.6 (5.6) | 7.5 (5.7) | 7.6 (5.5) | 7.7 (5.8) | 7.6 (5.8) | 7.6 (5.7) |
| Female, n (%) | 138 (46.0) | 128 (43.0) | 268 (44.4) | 288 (46.8) | 296 (48.2) | 314 (51.3) | 898 (48.8) |
| Race, n (%) | |||||||
| White | 243 (81.0) | 220 (74.0) | 463 (77.3) | 521 (84.6) | 530 (86.3) | 529 (86.4) | 1580 (85.8) |
| Black or African American | 18 (6.0) | 18 (6.0) | 36 (6.0) | 31 (5.0) | 23 (3.7) | 28 (4.6) | 82 (4.5) |
| Asian | 38 (13.0) | 55 (18.0) | 93 (15.5) | 18 (2.9) | 14 (2.3) | 13 (2.1) | 45 (2.4) |
| Other/multiple | 1 (<1) | 6 (2.0) | 7 (1.17) | 46 (7.5) | 47 (7.7) | 42 (6.9) | 135 (7.3) |
| Mean eGFR (SD), mL/min/1.73m2 | 97 (17.2) | 95 (18.0) | 96 (17.6) | 93.3 (17.8) | 93.7 (18.3) | 93.4 (18.2) | 93.5 (18.1) |
| Mean SBP (SD), mmHg | 133 (14.5) | 132 (13.6) | 132.6 (14.1) | 131.1 (14.1) | 132.1 (14.0) | 132.1 (14.2) | 131.8 (14.1) |
| Mean DBP (SD), mmHg | 82 (9.1) | 80 (8.7) | 80.8 (8.9) | 78.4 (8.7) | 79 (9.0) | 78.8 (9.3) | 78.7 (9.0) |
| Mean HR (SD), bpm | 76 (10.6) | 75 (10.5) | 75.5 (10.6) | 75.3 (9.5) | 75.5 (10.3) | 75.6 (10.1) | 75.5 (10.0) |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; FSG, fasting serum glucose; HR, heart rate; SBP, systolic blood pressure; SD, standard deviation; T2D, type 2 diabetes.
FPG recorded in SUSTAIN 7 and FSG recorded in AWARD‐11.
For the primary analysis, indirect comparisons were based on results for the trial product estimand (referred to as efficacy estimand in AWARD‐11), which was defined in both trials as including patients on treatment without rescue therapy and was the primary analysis population in SUSTAIN 7. The trial product estimand targets the treatment effect if all patients had continued to use the trial product for the planned duration of the trial without rescue medication, thus reflecting effect when treatment is used as intended. 19
2.4. Sensitivity analyses
The Bucher indirect comparison used for the primary analysis assumes that the relative treatment effect is the same in the SUSTAIN 7 population as in the AWARD‐11 population, that is, there are no differences in the trial populations that could materially impact the effect of semaglutide versus dulaglutide. While this assumption is well supported in the literature, 17 four different sensitivity analyses were conducted to assess the findings of the main analysis (Table 3).
TABLE 3.
Sensitivity analysis methods, populations and time points
| Analysis | Estimand | Aggregate data/IPD | Time point | |||||
|---|---|---|---|---|---|---|---|---|
| Bucher (unweighted) | MAIC (weighted) | Trial product | Treatment policy | Aggregate | IPD | 40 wk | 36 wk | |
| Primary analysis | X | X | X | SUSTAIN 7 | AWARD‐11 | |||
| SA1 | X | X | X (AWARD‐11) | X (subgroup of patients meeting inclusion criteria in AWARD‐11) | SUSTAIN 7 | AWARD‐11 | ||
| SA2 | X | X | X (AWARD‐11) | X (SUSTAIN 7) | SUSTAIN 7 | AWARD‐11 | ||
| SA3 | X | X | X (AWARD‐11) | X (SUSTAIN 7) | X | |||
| SA4 | X | X | X | SUSTAIN 7 | AWARD‐11 | |||
Abbreviations: IPD, individual patient data; MAIC, matching‐adjusted indirect comparison; SA, sensitivity analysis.
For the first sensitivity analysis (sensitivity analysis 1), Bucher analysis was used to account for a subgroup of subjects in SUSTAIN 7 who adhered to the inclusion criteria in AWARD‐11 (n = 414). In another sensitivity analysis (sensitivity analysis 2), a matching‐adjusted indirect comparison (MAIC) approach was used to adjust for potential effect modification attributable to baseline HbA1c, BMI and diabetes duration. A further sensitivity analysis (sensitivity analysis 3) used a MAIC approach utilizing SUSTAIN 7 results corresponding to week 36 (obtained from linear interpolation between the week 28 and week 40 visits) to assess the impact of the difference in time points at which outcomes were reported in the trials. The final sensitivity analysis (sensitivity analysis 4) used Bucher analysis of the results from the treatment policy estimand (referred to as treatment‐regimen estimand in AWARD‐11), which included efficacy data for patients regardless of treatment discontinuation or rescue medication and served to account for the potential effects of treatment discontinuation in the ITCs. Further details of the MAIC methodology are provided in the supporting information.
3. RESULTS
In total, 1842 patients were randomly assigned to a dulaglutide dose in the AWARD‐11 trial (3.0 mg, n = 616; 4.5 mg, n = 614; 1.5 mg, n = 612), with 599 out of 1199 patients randomly assigned to the highest available doses of semaglutide or dulaglutide in the SUSTAIN 7 trial (semaglutide 1.0 mg, n = 300; dulaglutide 1.5 mg, n = 299).
3.1. Change in HbA1c
In the primary analysis, semaglutide 1.0 mg was significantly more effective at reducing HbA1c from baseline compared with dulaglutide 3.0 mg with an estimated treatment difference (ETD) of −0.24%‐points (95% CI −0.43, −0.05). Semaglutide 1.0 mg offered comparable reductions in HbA1c from baseline versus dulaglutide 4.5 mg with an ETD of −0.07%‐points (95% CI −0.26, 0.12) (Figure 2A). In the primary analysis, the proportion of patients achieving HbA1c less than 7.0% was comparable for semaglutide 1.0 mg versus dulaglutide 3.0 mg with an OR of 1.31 (95% CI 0.79, 2.19) and, compared with 4.5 mg, an OR of 0.88 (95% CI 0.52, 1.48) (Figure S1).
FIGURE 2.
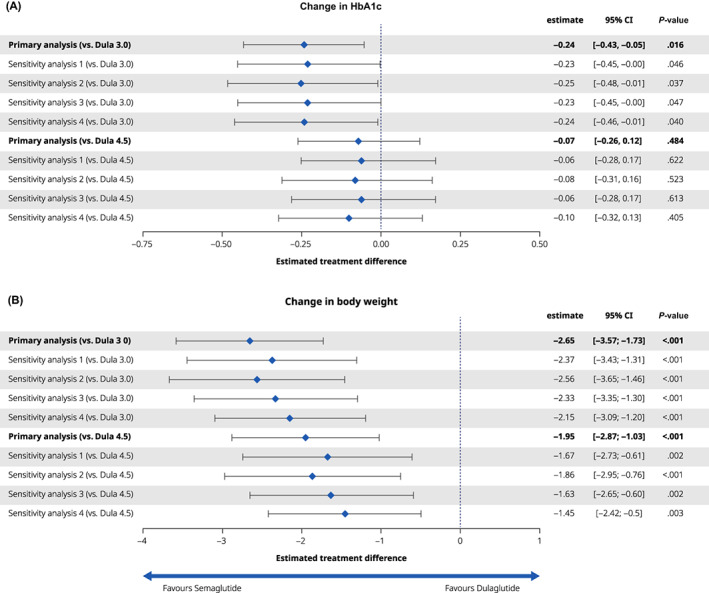
Efficacy outcomes of semaglutide (Sema) 1.0 mg versus dulaglutide (Dula) 3.0 and 4.5 mg. (A) Change in HbA1c from baseline; (B) change in bodyweight (kg) from baseline. Primary Bucher analysis, sensitivity analyses 1‐4
Sensitivity analyses adjusting for inclusion criteria (sensitivity analysis 1), baseline characteristics (sensitivity analysis 2), follow‐up length (sensitivity analysis 3) and treatment discontinuation (sensitivity analysis 4) supported the findings of the primary analysis (Figure 2A and Figure S1).
3.2. Change in body weight
In the primary analysis, semaglutide 1.0 mg was significantly more effective at reducing body weight from baseline compared with dulaglutide 3.0 and 4.5 mg with ETDs of −2.65 and −1.95 kg, respectively (95% CI −3.57, −1.73 and −2.87, −1.03, respectively) (Figure 2B).
Sensitivity analyses adjusting for inclusion criteria (sensitivity analysis 1), baseline characteristics (sensitivity analysis 2), follow‐up length (sensitivity analysis 3) and treatment discontinuation (sensitivity analysis 4) supported the findings of the primary analysis (Figure 2B).
4. DISCUSSION
This study indirectly compared the effect of two GLP‐1 RAs (semaglutide 1.0 mg vs. dulaglutide 3.0 and 4.5 mg) on reducing HbA1c and body weight from baseline in patients with T2D inadequately controlled on metformin therapy.
The primary analysis showed that semaglutide 1.0 mg was significantly more effective than dulaglutide 3.0 mg and comparable with dulaglutide 4.5 mg in reducing HbA1c from baseline. Semaglutide 1.0 mg was also significantly more effective at reducing body weight versus dulaglutide 3.0 and 4.5 mg. No significant difference was shown in the proportion of patients achieving HbA1c less than 7.0% for any comparison. All sensitivity analyses supported the findings of the primary analysis.
This is the first comparison of semaglutide 1.0 mg with dulaglutide 3.0 and 4.5 mg in patients with T2D inadequately controlled on metformin. With multiple products in the GLP‐1 RA class now available, information regarding their comparative effectiveness has become clinically relevant. Higher HbA1c levels are associated with a greater risk of complications from T2D, with only 26.4% of patients with T2D achieving the target HbA1c of less than 6.5%. 20 Approximately 90% of people with T2D are overweight or have obesity. 20 Diabetes and obesity together increase the risk of a range of chronic health conditions including cardiovascular disease, kidney disease and risk of mortality. Weight loss in patients with T2D has been shown to reduce HbA1c levels and improve cardiovascular risk factors. 21 Weight loss is therefore an important consideration in the treatment of T2D. Hence, there is a need for treatment strategies for reducing body weight, in addition to improving glycaemic control, in patients with T2D. 22 The significantly greater reductions in body weight shown with semaglutide 1.0 mg versus dulaglutide 3.0 and 4.5 mg in this indirect comparison may therefore be a factor to consider for clinicians determining the most appropriate GLP‐1 RA for individual patients. A higher dose of semaglutide (2.0 mg) for the treatment of T2D is currently being investigated in SUSTAIN FORTE. 12
The strengths of this study include the comparison method used, with findings of the primary analysis supported by sensitivity analyses. Based on the available trial data and the method including anchoring with dulaglutide 1.5 mg, a conventional Bucher ITC approach was considered the most appropriate primary analysis in this study. Specifically, the Bucher approach assumed that the effect of semaglutide versus dulaglutide would be the same in the SUSTAIN 7 population as in the AWARD‐11 population. Improvements in HbA1c and body weight with semaglutide versus dulaglutide have been reported in the literature regardless of patient characteristics, supporting the use of the Bucher method in this current study. 17 To further explore this assumption, IPD from SUSTAIN 7 were used to perform sensitivity analyses adjusting for differences between trial populations and follow‐up length. These analyses supported the findings of the primary analysis.
This ITC utilized two statistical methods to strengthen the conclusions; however, these findings can only be validated in a head‐to‐head trial. The current study focused on the indirect comparison of efficacy and did not evaluate safety outcomes. A quantitative, indirect comparison of safety outcomes is intrinsically more difficult, because of cross‐trial differences in the assessment of adverse events. Both SUSTAIN 7 and AWARD‐11 reported a similar safety profile across treatment arms; semaglutide 1.0 mg resulted in comparable adverse events versus dulaglutide 1.5 mg, and dulaglutide 1.5 mg resulted in comparable adverse events versus dulaglutide 3.0 and 4.5 mg. A limitation of this study is comparison of estimands across trials, as there are subtle differences in the handling of intercurrent events, with different criteria for initiation of rescue medication. It should also be noted that baseline HbA1c levels differed between the two studies. However, potential confounding resulting from this difference was mitigated through anchoring of analyses via dulaglutide 1.5 mg. As a result of this approach, the comparison made was between the relative treatment effects shown in each of the trials (dulaglutide 1.5 mg ‐ semaglutide 1.0 mg compared with dulaglutide 1.5 mg ‐ dulaglutide 3.0/4.5 mg). Further, when baseline HbA1c was adjusted for in sensitivity analysis 2, the impact on the results was minimal. Another limitation of this study is the comparison of data from trials with different follow‐up length. An MAIC was conducted as a sensitivity analysis specifically to account for differences in follow‐up between SUSTAIN 7 and AWARD‐11, and supported the findings of the main analysis.
Despite these limitations, this study allows for a robust indirect comparison of reduction of HbA1c and body weight with semaglutide 1.0 versus 3.0 mg and 4.5 mg dulaglutide in patients with T2D inadequately controlled on metformin.
In conclusion, this indirect comparison showed significantly greater reductions from baseline in HbA1c with semaglutide 1.0 mg versus dulaglutide 3.0 mg and a comparable HbA1c reduction with semaglutide 1.0 mg versus dulaglutide 4.5 mg. Treatment with semaglutide 1.0 mg also showed significantly greater reductions in body weight compared with both dulaglutide 3.0 and 4.5 mg. The findings of this study may help to guide clinician decisions on the most suitable product within the GLP‐1 RA treatment class for the treatment of individual patients with T2D.
CONFLICT OF INTEREST
RP reports consulting fees from AstraZeneca; consulting fees from Glytec, LLC; grants from Hanmi Pharmaceutical Co.; grants and consulting fees from Janssen; consulting fees from Merck; grants from Metavention; consulting fees from Mundipharma; grants, speaker fees and consulting fees from Novo Nordisk; consulting fees from Pfizer; grants from Poxel SA; grants and consulting fees from Sanofi; consulting fees from Scohia Pharma Inc.; consulting fees from Sun Pharmaceutical Industries; and personal consulting fees from Sanofi US Services, Inc. Except for consulting fees in February 2018 and June 2018 from Sanofi US Services, Inc., RP's services were paid for directly to AdventHealth, a non‐profit organization. AMC is an employee and shareholder of Novo Nordisk A/S. IL has received research funding, advisory/consulting fees and/or other support from Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca, Boehringer Ingelheim, Janssen, Intercept, Intarcia, TARGETPharma, Merck, Pfizer, Novartis, GI Dynamics, Mylan, Mannkind, Valeritas, Bayer and Zealand Pharma. AV has conducted research studies funded by, served as advisor for, and received lecture honoraria from Amgen, AstraZeneca, Boehringer, Daiichi, Eli Lilly, Napp, Novartis, Novo Nordisk, MannKind, Pfizer, Regeneron, Sanofi, Takeda and Tosoh. AP received consultancy fees to develop a statistical analysis plan for the analyses presented in this manuscript; she has also received personal fees from Novo Nordisk, Sanofi and Ultragenyx. JL is an employee of Novo Nordisk A/S. BC is an employee and a shareholder of Novo Nordisk A/S. AGR is an employee and minor stakeholder in Novo Nordisk A/S. NM is an employee of Novo Nordisk A/S.
AUTHOR CONTRIBUTIONS
All the authors contributed to the conception, drafting and critical editing of the manuscript. AP contributed to the planning of analyses and AGR conducted the statistical analyses.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14497.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGEMENTS
The authors would like to thank Danielle Vaughan, PhD, from DRG Abacus (part of Clarivate), for providing medical writing support, which was funded by Novo Nordisk A/S.
Pratley RE, Catarig A‐M, Lingvay I, et al. An indirect treatment comparison of the efficacy of semaglutide 1.0 mg versus dulaglutide 3.0 and 4.5 mg. Diabetes Obes Metab. 2021;23(11):2513-2520. 10.1111/dom.14497
Funding information Novo Nordisk A/S
DATA AVAILABILITY STATEMENT
Data will be shared with researchers who submit a research proposal approved by an independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion and approval of the product and product use in the EU and the USA. Individual participant data will be shared in datasets in a de‐identified and anonymised format. There will not be any limitations on how these data can be used.
REFERENCES
- 1. Leiter LA, Nauck MA. Efficacy and safety of GLP‐1 receptor agonists across the spectrum of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2017;125(7):419‐435. [DOI] [PubMed] [Google Scholar]
- 2. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lau J, Bloch P, Schäffer L, et al. Discovery of the once‐weekly glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide. J Med Chem. 2015;58(18):7370‐7380. [DOI] [PubMed] [Google Scholar]
- 4. Dalsgaard NB, Vilsbøll T, Knop FK. Effects of glucagon‐like peptide‐1 receptor agonists on cardiovascular risk factors: a narrative review of head‐to‐head comparisons. Diabetes Obes Metab. 2018;20(3):508‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. EMA . Trulicity (dulaglutide). Summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/trulicity-epar-product-information_en.pdf. Accessed February 2021.
- 6. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275‐286. [DOI] [PubMed] [Google Scholar]
- 7. FDA . Ozempic (semaglutide). Highlights of prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf. Accessed February 2021.
- 8. EMA . Ozempic (semaglutide). Summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/ozempic-epar-product-information_en.pdf. Accessed February 2021.
- 9. FDA . Trulicity (dulaglutide). Summary of product characteristics. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125469Orig1s000Lbl.pdf. Accessed February 2021.
- 10. Fonseca VA. Defining and characterising the progression of type 2 diabetes. Br J Diabetes Vasc Dis. 2008;8:S3‐S9. [Google Scholar]
- 11. Petri KCC, Ingwersen SH, Flint A, Zacho J, Overgaard RV. Exposure‐response analysis for evaluation of semaglutide dose levels in type 2 diabetes. Diabetes Obes Metab. 2018;20(9):2238‐2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ClinicalTrials.gov. A research study to compare two doses of semaglutide taken once weekly in people with type 2 diabetes (SUSTAIN FORTE). https://clinicaltrials.gov/ct2/show/NCT03989232.
- 13. FDA . Trulicity (dulaglutide). Summary of product characteristics. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125469s036lbl.pdf. Accessed February 2021.
- 14. Frias JP, Bonora E, Nevarez Ruiz L, et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin‐treated patients with type 2 diabetes in a randomized controlled trial (AWARD‐11). Diabetes Care. 2021;44:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683‐691. [DOI] [PubMed] [Google Scholar]
- 16. Evans M, Billings L, Håkan‐Bloch J, et al. An indirect treatment comparison of the efficacy of insulin degludec/liraglutide (IDegLira) and insulin glargine/lixisenatide (iGlarLixi) in patients with type 2 diabetes uncontrolled on basal insulin. J Med Econ. 2018;21(4):340‐347. [DOI] [PubMed] [Google Scholar]
- 17. Pratley RE, Aroda VR, Catarig A‐M, et al. Impact of patient characteristics on efficacy and safety of once‐weekly semaglutide versus dulaglutide: SUSTAIN 7 posthoc analyses. BMJ Open. 2020;10(11):e037883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. ClinicalTrials.gov. A study of the efficacy and safety of dulaglutide (LY2189265) in participants with type 2 diabetes (AWARD‐11). https://clinicaltrials.gov/ct2/show/NCT03495102.
- 19. Aroda VR, Saugstrup T, Buse JB, Donsmark M, Zacho J, Davies MJ. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab. 2019;21(2210):2203‐2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diabetes UK . Diabetes risk factors. https://www.diabetes.org.uk/preventing-type-2-diabetes/diabetes-risk-factors. Accessed March 2021.
- 21. Wilding JP. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract. 2014;68(6):682‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leitner DR, Frühbeck G, Yumuk V, et al. Obesity and type 2 diabetes: two diseases with a need for combined treatment strategies ‐ EASO can lead the way. Obes Facts. 2017;10(5):483‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
Data will be shared with researchers who submit a research proposal approved by an independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion and approval of the product and product use in the EU and the USA. Individual participant data will be shared in datasets in a de‐identified and anonymised format. There will not be any limitations on how these data can be used.


