Summary
Haematoma after thyroid surgery can lead to airway obstruction and death. We therefore developed guidelines to improve the safety of peri‐operative care of patients undergoing thyroid surgery. We conducted a systematic review to inform recommendations, with expert consensus used in the absence of high‐quality evidence, and a Delphi study was used to ratify recommendations. We highlight the importance of multidisciplinary team management and make recommendations in key areas including: monitoring; recognition; post‐thyroid surgery emergency box; management of suspected haematoma following thyroid surgery; cognitive aids; post‐haematoma evacuation care; day‐case thyroid surgery; training; consent and pre‐operative communication; postoperative communication; and institutional policies. The guidelines support a multidisciplinary approach to the management of suspected haematoma following thyroid surgery through oxygenation and evaluation; haematoma evacuation; and tracheal intubation. They have been produced with materials to support implementation. While these guidelines are specific to thyroid surgery, the principles may apply to other forms of neck surgery. These guidelines and recommendations provided are the first in this area and it is hoped they will support multidisciplinary team working, improving care and outcomes for patients having thyroid surgery.
Keywords: airway, emergency, haematoma, haemorrhage, thyroid
Recommendations
All staff potentially interacting with patients undergoing thyroid surgery should be trained to recognise haematoma following thyroid surgery. This includes ward staff where patients are nursed and doctors of all grades and speciality.
Minimum monitoring includes wound inspection, early warning and pain scoring, as well as awareness for more subtle signs (agitation; anxiety; difficulty in breathing; discomfort).
A post‐thyroid surgery emergency box should be available at the bed‐side of patients who have undergone thyroid surgery during the postoperative period, including during transfers.
Emergency front‐of‐neck airway equipment (scalpel; bougie; tracheal tube) must be readily available on wards caring for patients after thyroid surgery.
If concerned about potential haematoma following thyroid surgery, immediate senior surgical review (e.g. registrar or consultant) must be arranged. If senior surgical review is not immediately available, or if there are signs of airway compromise, a senior anaesthetist should be informed immediately.
If the patient shows signs of airway compromise due to haematoma, a systematic approach should be taken to open the wound at the bed‐side. For this we recommend using the SCOOP approach (skin exposure; cut sutures; open skin; open muscles (superficial and deep layers); pack wound).
When emergency haematoma evacuation has taken place, it is important for the surgical team, usually the consultant, to communicate with the patient, including after discharge. This should include offering referral for clinical psychology support or similar.
All organisations offering thyroid surgery should support members of the multidisciplinary team, including but not exclusive to anaesthetists, nursing staff, members of the cardiac arrest team and surgeons, to attain and maintain competency and skills required to manage complications.
Why were these guidelines developed?
Postoperative haemorrhage is a well‐recognised complication of thyroid surgery with an incidence between 0.45% and 4.2% [1, 2, 3, 4]. Rapid haematoma formation, even with small volumes, can result in significant airway obstruction requiring emergency intervention. The unpredictability and potential risk of death due to haematoma following thyroid surgery supports the need for training that enables early recognition and management of the airway when haematoma is suspected [5, 6]. Our aim was to produce recommendations that would improve patient safety by identifying key areas in clinical management, training and institutional preparedness. These guidelines aim to support early recognition of haematoma following thyroid surgery and describe a systematic approach to airway management in patients who have had thyroid surgery, through individual and organisational preparedness. These guidelines are specific to thyroid surgery (lobectomy and total thyroidectomy), but the principles may apply to other forms of neck surgery.
What guidelines currently exist?
There are several guidelines that describe the management of an anticipated difficult airway, and others describing the management of an unanticipated difficult airway [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20]. These are usually in the setting of pre‐operative or emergency airway management [21]. However, the management of postoperative airway complications in the setting of head and neck surgery has not been previously published. Specifically, to our knowledge, there are no guidelines available supporting the multidisciplinary management of haematoma following thyroid surgery, either in the anaesthetic or in the surgical literature.
How do these guidelines differ from existing guidelines?
At the time of writing, there were no consensus recommendations, or nationally or internationally agreed guidelines on the management of suspected haematoma with or without airway compromise following thyroid surgery.
Disclaimer
These guidelines are not intended to represent a minimum standard of practice, nor are they to be regarded as a substitute for good clinical judgement. They present key principles and suggested strategies for the management of haematoma after thyroid surgery. This document is intended to guide appropriately trained healthcare professionals.
Introduction
Patients requiring thyroid surgery are at risk of pre‐, intra‐ and postoperative airway compromise. The risk of pre‐operative and intra‐operative airway obstruction can be mitigated with numerous strategies in surgical patients with predicted or unpredicted difficult airways [7, 8]. In these settings, airway compromise usually occurs within controlled environments with immediate access to skilled staff to manage complications. However, airway obstruction following thyroid surgery may present once patients are in more remote areas, with higher patient to staff ratios, less frequent monitoring and lack of immediate access to appropriately skilled staff. This population is, therefore, potentially at greater risk of undetected or late‐presenting airway compromise and subsequent mortality. Moreover, the nature of the anatomical structures in the neck places patients at risk of rapid clinical deterioration in the event of even a small haemorrhage, especially in the context of laryngeal oedema. Prompt assessment and management is required, occasionally necessitating bed‐side haematoma evacuation with or without emergency tracheal intubation to avert significant morbidity and mortality.
There are more than 11,000 thyroid procedures performed annually in England alone and >150,000 in the USA; the scale of peri‐operative adverse events in this cohort is substantial [22, 23]. Postoperative haemorrhage is a well‐recognised complication of thyroid surgery, with an incidence between 0.45% and 4.2%, which has not been reducing over time [1, 2, 3, 4]. Evidence suggests that subsequent haematoma formation leads to acute airway compromise necessitating emergency bed‐side intervention in up to a quarter of these patients [24, 25]. Although the incidence of mortality is poorly reported, death due to haematoma following thyroid surgery continues to occur. Some reported deaths have prompted institutional practice changes in the UK [6]. While haemorrhage is the underlying mechanism, it is haematoma formation which usually requires immediate intervention due to risks of airway obstruction. For clarity, these guidelines specifically describe recognition and management of haematoma following thyroid surgery, and not haemorrhage.
Given the risk of death due to haematoma after thyroid surgery, there is a specific need to ensure safe peri‐operative care for this cohort of patients. To date, postoperative safety guidance has not been published, despite this being a period associated with high risk of morbidity and mortality [6, 26]. We therefore aimed to produce guidelines to support members of the multidisciplinary team in the management of postoperative haematoma following anterior cervical approach thyroidectomy by providing clear recommendations to enable organisational preparedness, early recognition and prompt clinical management. While the scope of these guidelines is specific, it is hoped that the principles presented may apply to other types of neck surgery, such as parathyroid, cervical spine or vascular surgery, and offer a basis to support future work in this area.
Methods
These multidisciplinary consensus guidelines were developed by an 11‐member Working Group with representation from the Difficult Airway Society (DAS), the British Association of Endocrine and Thyroid Surgeons (BAETS) and the British Association of Otorhinolaryngology, Head and Neck Surgery (ENT‐UK). A patient representative from the British Thyroid Foundation, a nursing representative, trainee representatives and consultants were also members of the Working Group. These guidelines adhered to the appraisal of guidelines for research and evaluation (AGREE) reporting checklist [27].
To inform our recommendations, we performed a systematic review in accordance with the PRISMA reporting checklist and prospectively registered this with PROSPERO. Full details of the methodology and results of the systematic review are reported in online Supporting Information Appendix S1. The results of the systematic review were then used to inform a Delphi study to formulate recommendations [28, 29]. An initial long list of 47 recommendations was proposed by the Working Group, with each reviewed and rated for content and clarity (see online Supporting Information Appendix S2). The proposed recommendations were based on a number of factors including: strength of supporting evidence; applicability of evidence in practice; multidisciplinary team involvement; and practical implications of the recommendations. Each recommendation was anonymously voted on using a structured Microsoft Excel sheet (Microsoft, Inc., Redmond, USA) by all members of the Working Group as either ‘Accept’, ‘Reject’ or ‘Unsure’, as well as whether each recommendation should be made a key recommendation. Recommendations supported by eight or more members of the Working Group were included in the manuscript. A second round of rating was undertaken with the 13 recommendations with the greatest acceptance proceeding to a third and final round of discussions, which was a virtual round‐table discussion to finally vote on and ratify recommendations.
We also performed a survey to refine the recommended box contents. In the first round of the survey, an initial long list of 31 potential content items was presented to the Working Group and all members of the three represented Executive Committees (n = 31) to review and select essential items. An essential item was defined as “an item without which you could not perform the procedure.” We prospectively determined to shortlist items of equipment receiving more than 20 votes. The items on this list were then ratified during a virtual round‐table discussion by members of the Working Group.
The strength of recommendations was graded using a modified version of the system developed by the Centre for Evidence‐based Medicine [30], as was used for previous airway guideline recommendations [7]. Recommendations were graded A to D according to the strength of the available evidence [7, 31]. During development, drafting and finalising of these guidelines and recommendations the Working Group met 11 times remotely over 10 months. A supplementary document aimed to support implementation of the guidelines and recommendations was developed concurrently (see online Supporting Information Appendix S3). A draft of the guidelines and supporting material was then circulated to all stakeholder organisations for comment, as well as external expert anaesthetists, nurses and surgeons. The comments and feedback received were used to further refine and inform the final recommendations.
Monitoring
Haemorrhage and subsequent haematoma most frequently occur within the first 24 h following thyroid surgery, with approximately half occurring within 6 h [5, 24, 25, 26, 32, 33, 34, 35]. Thus, routine patient observations should be carried out at least hourly for first 6 h postoperatively [2, 5, 24, 25, 32, 33, 34, 35, 36, 37] (Grade C). Following the initial 6‐h period, the frequency of observations may be tailored according to individual patient risk and local policies [2, 5, 24, 32, 34, 35, 36, 37] (Grade C). Staff should be aware of signs that may suggest a need for an increase in the required frequency of observations [2, 5, 24, 32, 34, 35, 36, 37] (Grade D). Although several cases of haematoma following thyroid surgery have been reported after 24 h, this is extremely rare. Patients are usually discharged 1 day following surgery [1, 32, 33, 38].
The potential for rapid deterioration and adverse patient outcomes in the event of haematoma formation underscores the necessity for optimal patient visibility to nursing staff wherever possible. Therefore, patients should be preferentially nursed in a bed where they can easily attract the attention of nursing staff. This may be in an open ward or bed located near to the nursing station [39] (Grade C). In addition to increasing direct visibility for nursing staff, open wards and multi‐bedded areas may also allow others on the ward to alert nursing staff in the event of acute deterioration. The awareness of concerning features requiring urgent clinical review and ability to manage patients appropriately is also critical, and thus patients should be nursed on a ward where staff have had training in recognition and management of haematoma following thyroid surgery [40, 41] (Grade C).
Minimum monitoring should include wound inspection, early warning score (respiratory rate; heart rate; blood pressure; temperature; arterial oxygen saturations; Glasgow Coma Scale) and pain score, as well as awareness for more subtle signs (agitation; anxiety; difficulty in breathing; discomfort) [2, 5, 24, 32, 34, 35, 36, 37] (Grade C). These may be recorded through electronic or paper‐based systems. Supplementing standardised early warning scores with additional charts may facilitate trend capture and analysis, enabling prompt detection of patients potentially at risk of clinical deterioration [42]. However, the practicalities of implementing such charts and adherence to recording may prove difficult in practice. It is important to recognise that haematoma following thyroid surgery may present acutely with minimal prior warning. New technologies such as acoustic respiratory rate monitoring may play a role in the detection and alert of acute observational changes in the future [43], but they are currently beyond the immediate access of most institutions.
Several factors have been associated with an increased risk of haematoma following thyroid surgery including increasing age and male sex, but the patterns and individual risk factors have been shown to be unpredictable [4, 26]. However, any recognised risk factors should be considered when determining appropriate postoperative monitoring and subsequent management strategies.
Recognition
Early recognition of potential airway complications secondary to haematoma following thyroid surgery is critical. The acronym DESATS has been created to aid identification, which includes: difficulty swallowing/discomfort; increase in early warning score (EWS) or national early warning score (NEWS); swelling; anxiety; tachypnoea/difficulty breathing; and stridor (DESATS). This acronym has been developed as part of the recommended post‐thyroid surgery regular review (Fig. 1) to act as a cognitive aid and support the early recognition of patients manifesting signs of potential postoperative haematoma. It is important to recognise that the order of signs within the DESATS acronym is not based on the timing of presentation in the evolution of haematoma, as any of the signs may herald the complication. All these identified signs are based on trends identified in our literature review (see online Supporting Information Appendix S1) and supported by expert opinion. They represent ‘triggers’ that should raise the suspicion of a haematoma following thyroid surgery and prompt appropriate management, with a suggested algorithm presented in Fig. 2. Staff should be aware of signs that may indicate an at‐risk patient requiring urgent clinical review (any of DESATS) [2, 5, 24, 32, 34, 35, 36, 37] (Grade D). Although some triggers may herald other postoperative complications, including delirium given the demographic of patients having thyroid surgery, a high index of suspicion for haematoma following thyroid surgery is still warranted.
Figure 1.

The Difficult Airway Society (DAS), British Association of Endocrine and Thyroid Surgeons (BAETS) and British Association of Otorhinolaryngology, Head and Neck Surgery (ENT‐UK) post‐thyroid surgery regular review. This figure forms part of the consensus guidelines for the management of haematoma after thyroid surgery and should be used in conjunction with the text. EWS, early warning score; NEWS, national early warning score. © DAS, BAETS, ENT‐UK 2021.
Figure 2.
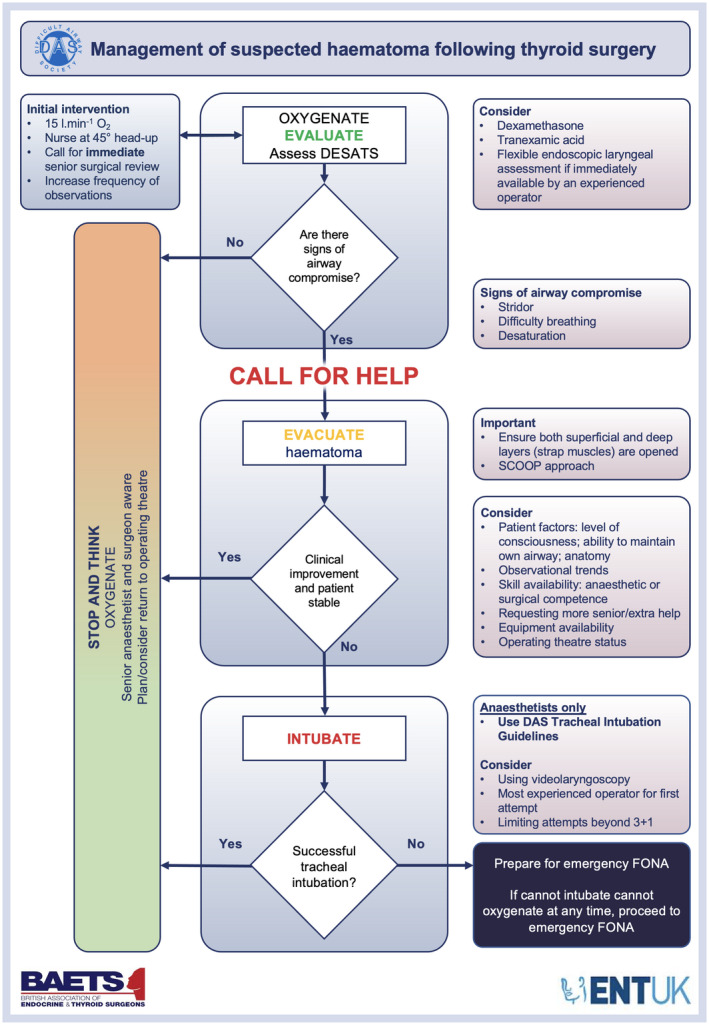
The Difficult Airway Society (DAS), British Association of Endocrine and Thyroid Surgeons (BAETS) and British Association of Otorhinolaryngology, Head and Neck Surgery (ENT‐UK) management of suspected haematoma following thyroid surgery. This figure forms part of the consensus guidelines for the management of haematoma after thyroid surgery and should be used in conjunction with the text. DESATS, difficulty swallowing/discomfort, EWS/NEWS, swelling, anxiety, tachypnoea/difficulty breathing, stridor; SCOOP, skin exposure, cut sutures, open skin, open muscles, pack wound; FONA, front‐of‐neck airway. © DAS, BAETS, ENT‐UK 2021.
There is no evidence to support the use of drains for prevention of haematoma in routine thyroid surgery [26]. When used, it is important that staff are aware clot formation may prevent free drainage and provide false reassurance as haematoma can still form in the presence of drains.
Post‐thyroid surgery emergency box
The need for easy and rapid access to essential equipment in time‐sensitive situations is well recognised in airway management. Tracheostomy guidelines promote bed‐side availability of key emergency equipment [40] and others recommend dedicated sections for emergency front‐of‐neck airway equipment on difficult airway trolleys [44]. This principle of immediate availability of essential equipment should be extended to patients following thyroid surgery. Patients who have had a thyroidectomy with an open anterior approach should have a post‐thyroid surgery emergency box at the bed‐side containing emergency equipment required for opening the neck wound in the event of haematoma [40, 44] (Grade C). Recommended content of such boxes is outlined in Table 1.
Table 1.
Recommended contents of post‐thyroid surgery emergency box.
| Should include |
| Artery clip |
| Management of suspected haematoma following thyroid surgery guideline |
| SCOOP guideline |
| Scalpel |
| Scissors |
| Sterile gauze or medium wound pack |
| Could also include |
| Gloves |
| Staple remover (if staples used) |
SCOOP; skin exposure, cut sutures, open skin, open muscles, pack wound.
Identifying and ensuring suitable equipment for airway management is available during transfers of at‐risk patients is highlighted in several national and international transfer guidelines [45, 46, 47]. Post‐thyroid surgery emergency boxes should accompany patients during transfers [40, 44, 45, 46, 47] (Grade B).
Management of suspected haematoma following thyroid surgery
In the event of a suspected haematoma following thyroid surgery, management should start with concurrent oxygenation and clinical evaluation progressing to haematoma evacuation and tracheal intubation when indicated (Fig. 2).
Oxygenate and evaluate
Concern about haemorrhage and or haematoma following thyroid surgery should prompt the immediate administration of supplemental oxygen (Grade D) and patients should be nursed in a head‐up position before further assessment [48] (Grade C). The availability of portable lighting in ward settings may be advantageous (Grade D).
The evaluation of airway patency should be concurrent with immediate management steps. The signs of airway compromise (arterial oxygen desaturation; difficulty breathing; stridor; tachypnoea) or concern about deterioration due to rapidly expanding neck swelling indicate a need to immediately call for help, which may be via local peri‐arrest protocols. Note that stridor may be a late sign and may warrant immediate management. In patients with signs of airway compromise, a senior anaesthetist should be informed immediately (Grade D), and clinicians should progress to the next stage of the algorithm and evacuate the haematoma.
If no immediate airway compromise is identified but where concerns have been raised, immediate on‐site senior surgical review (e.g. registrar or consultant) must be arranged. If senior surgical review is not immediately available, arrange on‐site senior anaesthetic review (Grade D). Out‐of‐hours considerations may include relevant cross‐covering speciality experience and adoption of a multidisciplinary team approach, which may be beneficial in clinical evaluation. Flexible endoscopic laryngeal assessment by an experienced operator [7] (Grade D). Intravenous dexamethasone and tranexamic acid should be considered [49, 50] (Grade C). Dexamethasone may improve upper airway obstruction and oedema and tranexamic acid may reduce bleeding, though neither will be immediate [49, 50]. Furthermore, the frequency of observations should be increased (Grade D). If the patient is stable but there are ongoing concerns of potential haemorrhage and/or haematoma, transfer to the operating theatre, post‐anaesthesia care unit (PACU) or ICU for close observation may be considered (Grade D).
Evacuate
If the patient shows signs of airway compromise due to suspected haematoma, a systematic approach should be taken to open the wound at the bed‐side, ensuring the superficial and deep layers (strap muscles) are opened to prevent ongoing haematoma formation. For this we recommend using the following ‘SCOOP’ approach: skin exposure; cut sutures; open skin; open muscles; pack wound (Grade D; Fig. 3) [51]. The SCOOP approach was originally developed in response to a patient incident and has been adopted by several organisations since introduction [6, 25, 51]. Local anaesthetic infiltration is not required when opening the wound (Grade D).
Figure 3.
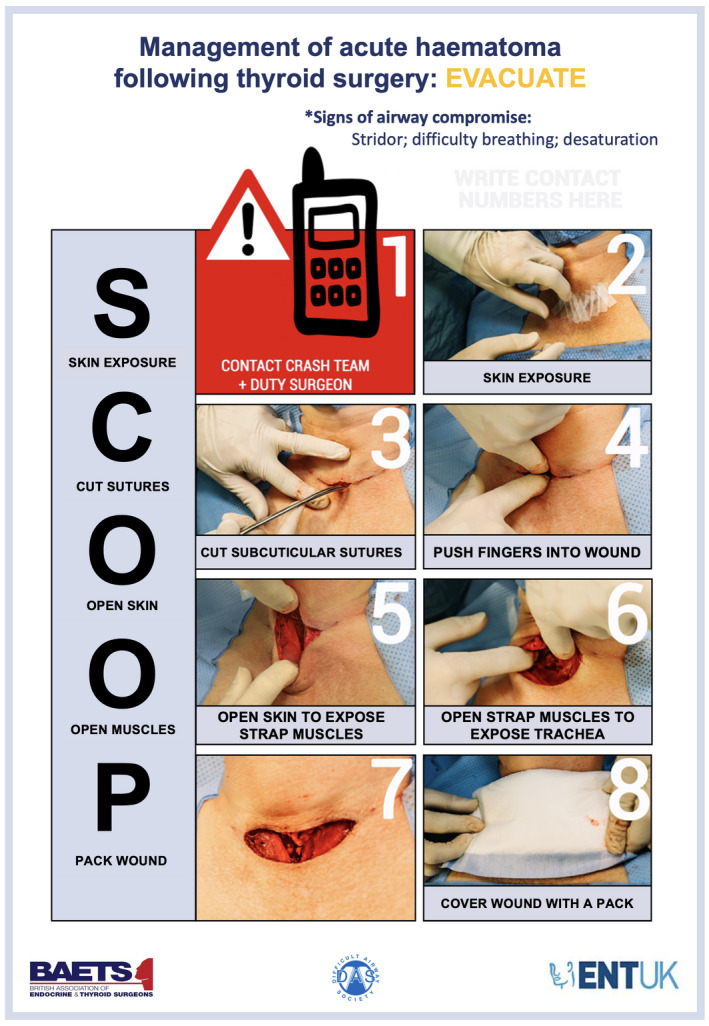
The Difficult Airway Society (DAS), British Association of Endocrine and Thyroid Surgeons (BAETS) and British Association of Otorhinolaryngology, Head and Neck Surgery (ENT‐UK) SCOOP protocol. This figure forms part of the consensus guidelines for the management of haematoma after thyroid surgery and should be used in conjunction with the text. It is presented as an edited version with permission from its original creator. © DAS, BAETS, ENT‐UK 2021.
Should haematoma evacuation fail to stabilise the patient, with no resolution of airway compromise and/or further patient deterioration, tracheal intubation is indicated. Operating theatre staff should be made aware of the patient to arrange a timely return to theatre (Grade D). Local procedures may require this to be done separately to informing the anaesthetist.
Intubate
In the event of further deterioration and no resolution of airway compromise, emergency tracheal intubation must be considered, regardless of patient location (Grade D). If indicated, trained and experienced anaesthetists should attempt tracheal intubation as per the DAS guidelines [7, 8, 52]. This includes consideration of videolaryngoscopy at the first attempt of tracheal intubation and limiting the number of attempts at tracheal intubation [52] (Grade C). If the clinical presentation is appropriate, awake tracheal intubation (ATI) should be considered in line with the DAS ATI guidelines and performed by anaesthetists with appropriate expertise [7]. In time‐critical settings where life‐threatening airway compromise is imminent, tracheal intubation should be attempted after opening of the wound and evacuation of the haematoma [8, 52] (Grade D). This should help optimise tracheal intubation conditions by preventing worsening laryngeal oedema in the setting of an already potentially difficult airway and improve the likelihood of a successful first attempt at tracheal intubation. Tracheal intubation may be more successful with the use of a smaller tracheal tube and/or the use of adjuncts such as a bougie. Success of tracheal intubation should be confirmed with capnography [53, 54] (Grade C).
Multiple attempts at tracheal intubation are likely to exacerbate laryngeal oedema resulting in further airway compromise, increasingly difficult tracheal intubation and ventilation, and delayed oxygenation. Therefore, early progression to front‐of‐neck airway should be considered (Grade D). In a cannot intubate, cannot oxygenate situation, scalpel cricothyroidotomy or emergency tracheostomy are preferred to cannula cricothyroidotomy [8, 55, 56] (Grade D). Both of these techniques reduce the likelihood of gas trapping, maximising respiratory gas exchange and subsequent oxygenation. They are also associated with fewer complications such as hypercapnia, barotrauma, subcutaneous emphysema and obstruction of the tube compared with cannula cricothyroidotomy, and allow egress of gas in the event of complete upper airway obstruction, while providing a definitive airway to support ongoing management [55, 56].
Emergency front‐of‐neck airway equipment, including a scalpel (number 10 blade), bougie and tracheal tube (cuffed 6.0 mm internal diameter) must be immediately available on wards to patients who have had thyroid surgery [40, 44, 57] (Grade C). This may be as a dedicated and readily identifiable pack or drawer on the emergency trolley or as separate items and is in keeping with the DAS guidelines for emergency front‐of‐neck airway (Plan D) [8]. Rather than making a new incision in the patient’s neck, the scalpel can be used to both open the existing neck wound as well as make incisions in the cricothyroid membrane or trachea to insert a tracheal or tracheostomy tube. Ongoing bleeding is not a contraindication to performing an emergency front‐of‐neck airway.
Cognitive aids
The use of cognitive aids can help when managing difficult clinical situations by outlining critical steps in the management process. They can improve communication by flattening hierarchies and structuring interactions [58]. During stressful critical incidents, they can reduce the cognitive load of the user and make it easier to take a structured approach [58, 59]. Therefore, cognitive aids should be available to support early recognition and management of haematoma following thyroid surgery [58, 59] (Grade D).
These guidelines include a number of cognitive aids (Fig. 1, 2, 3). They are related and designed to be used together. They cover key aspects of recognition and management relevant to the multidisciplinary team, rather than being directed at any single group of healthcare professionals. It is recognised that they will need to be used in tandem with other more specific guidelines, in particular for tracheal intubation and emergency front‐of‐neck airway [7, 8, 11, 52], and have been designed to complement other training such as Advanced Life Support [60, 61].
Post‐haematoma evacuation care
Emergency haematoma evacuation is a distressing event for both patients and staff involved. Patients will need to return to the operating theatre for definitive management and close postoperative observation. Following emergency evacuation and definitive surgical management, transfer to an area with level 2 or 3 care should be considered (Grade D). If local or national databases exist, reporting of these critical incidents is warranted.
The psychological impact of such an event on patients cannot be underestimated [62, 63]. Where emergency haematoma evacuation has taken place, it is important for the surgical team, usually the consultant, to communicate with the patient including after discharge. This should include offering referral for clinical psychology support or similar [62, 63, 64, 65, 66] (Grade B). When haematoma has occurred, especially if there has been an airway complication, a duty of candour letter should be sent to the patient describing what has happened and offering ongoing support, when appropriate [64, 65, 66] (Grade B). The impact of events on staff must also be considered. Following a postoperative haematoma event, debriefing by those involved should be encouraged [67, 68, 69, 70] (Grade B). Psychological support should be available to those involved in events should they require it [67, 68, 69] (Grade C).
Day‐case thyroid surgery
Some units offer day‐case thyroid surgery [4, 71]. Discussion on the suitability of thyroidectomy as a day‐case procedure falls outside the scope of these guidelines. However, where day‐case surgery is performed, patients should stay in hospital and be monitored for a minimum of 6 h postoperatively [24, 25, 26, 32, 33, 34] (Grade B). Patients should only be discharged if there are no concerns following review after 6 h (Grade D). The decision to undertake day‐case surgery should include discussion with the patient about the possibility of haematoma at home and the subsequent actions required [64, 72] (Grade B).
Training
Successful management of patients with haematoma following thyroid surgery after staff training has been demonstrated [73]. The multidisciplinary team should be aware of the need to recognise and manage potential haematoma following thyroid surgery in patients. Thus, staff potentially interacting with patients having thyroid surgery should be trained to recognise and manage haematoma following thyroid surgery [73, 74] (Grade B). This includes emergency department staff in centres offering day‐case surgery. Local training should reference the post‐thyroid surgery emergency box used and enable individuals to familiarise themselves with its exact contents [40] (Grade D). The frequency of local training should reflect staff changeovers, ensuring education opportunities for new members of the team (Grade D). All members of the multidisciplinary team are equally responsible in attaining and maintaining skills. Educational opportunities should be maximised wherever possible. Teaching should ideally prioritise simulation, enable familiarisation with anatomy and encourage appreciation of the multidisciplinary team dynamics [73, 75] (Grade D). Individual training should be repeated every 3 y, as a minimum [73] (Grade D). A range of supporting materials, including training resources have been made available to aid implementation of these guidelines and recommendations (see online Supporting Information Appendix S3).
All organisations offering thyroid surgery should support members of the multidisciplinary team, including but not exclusive to anaesthetists, members of the arrest team, nursing staff and surgeons to attain and maintain competencies and skills required to manage acute complications specifically related to thyroid surgery [53, 54] (Grade C).
Consent and pre‐operative communication
There are several potential surgical complications highlighted to patients when acquiring informed consent for thyroidectomy [1]. As a recognised complication of thyroid surgery, pre‐operative communication of the risk of haemorrhage and/or haematoma with patients should be in accordance with the General Medical Council principles and the Montgomery ruling [72, 76]. The risk of haemorrhage following thyroidectomy is approximately 1:100 [1, 2, 3, 4, 36]. The risk of haematoma is poorly defined, but studies suggest it leads to acute airway compromise necessitating emergency bed‐side intervention up to approximately 1:400 thyroidectomies [24, 25]. This is in the context of other recognised operative and postoperative risks. Therefore, when appropriate, surgical teams should include in the pre‐operative consent process, including in leaflets or written consent, the possibility of postoperative haematoma which may require a return to the operating theatre or emergency treatment on the ward [64, 65, 72, 76] (Grade B).
Postoperative communication
The safe transfer of patient care between healthcare teams within different areas of the hospital is a key consideration. The World Health Organization (WHO) surgical safety checklist aims to improve safety through clear communication and includes the theatre sign out [77]. Any concerns for postoperative complications, including the potential risk of haematoma, are highlighted during the sign out, and should be cascaded through explicit handovers which must take place when transferring to the PACU and thereafter the ward. This should include communication of postoperative risks and specific concerns of bleeding [78, 79] (Grade C). Direct surgical handover of any concerns to PACU and/or ward nurses can enhance staff awareness and improve patient safety. Therefore, surgical transfer and/or review of patients in recovery in addition to handover by the anaesthetist and the operating theatre nursing team to the recovery staff should be considered [78, 79, 80] (Grade B). If postoperative concerns arise in patients undergoing day‐case surgery, suitability for same day discharge should be re‐assessed.
Institutional policies
The guidelines and recommendations set out in this document aim to improve patient safety. They serve to support organisational preparedness through recognition of risk and identifying mitigating actions to enable management of that risk. Multidisciplinary team involvement, supported by a systematic review, in management of haematoma following thyroid surgery has been central to their production. The complex nature of risk means the responsibility does not lie with any single individual or group. Institutions offering thyroid surgery should have a nominated local risk lead to coordinate multidisciplinary staff training, implementation of recommendations and review of any critical incidents. This could be the local Airway Lead or another nominated senior member of the multidisciplinary team [81] (Grade D).
Discussion
These guidelines have been developed largely based on expert opinion due to the paucity of high‐quality evidence, as demonstrated by the low grade of many recommendations, warranting cautious interpretation. Thus, there is a need to further develop the evidence base in this area, and national databases may be warranted. The growing number of novel surgical techniques, including endoscopic or transoral approaches to thyroid surgery, makes the application of these recommendations to other surgical procedures uncertain. However, it is likely that the principles may apply to other types of neck surgery, for example parathyroid, cervical spine or vascular surgery, and offer a basis to support future work in this area [82, 83]. The lack of national or international guidance on management of haematoma following thyroid surgery leading to airway compromise demonstrates an area of airway management that was previously unaddressed. Ultimately, the impact that these guidelines and recommendations have on clinical practice should be reviewed to facilitate shared learning and enable improvements to future iterations.
There are other limitations to methodology and recommendations made. For example, there are areas that remain important to the care of patients having thyroidectomy that may not have been described, such as tracheal extubation, which should follow the DAS guidelines for the management of tracheal extubation [84]. We focus on the management of haematoma, but risk mitigation strategies have not been described due to their complexity and context specificity. Moreover, the data suggest that there are few modifiable risk factors, thus prevention is more challenging to make specific recommendations about within the scope of this document. Many recommendations require institutional buy‐in, thus there is a responsibility for healthcare professionals to drive local change. Further, the Working Group and majority of reviewers were from the UK, and thus generalisability to other countries and regions is unclear. Finally, while we conducted a systematic review, there is a possibility that we may have missed data that could have influenced the recommendations made, including those in the grey literature or unpublished results.
These guidelines were primarily produced to prioritise patient safety, by providing recommendations for members of the multidisciplinary team to manage patients with suspected haematoma following thyroid surgery. They aim to support current practice and complement staff training. The supporting evidence available was limited despite postoperative haematoma being a well‐recognised complication of thyroid surgery [1]. The necessity of these guidelines is underscored by previous complications leading to patient deaths [6]. Previously, the awareness of critical stages in recognition and management of haematoma following thyroid surgery may have been limited to those rotating through specialist teams. However, multidisciplinary management is necessary, which is highlighted by the diverse professional backgrounds of the Working Group. Institutional responsibility in identification and mitigation of risk requires organisations offering thyroid surgery to support the implementation of these recommendations in the interest of patient safety. The guidelines and recommendations provided are the first in this area and it is hoped they will support multidisciplinary team working, improving care and outcomes for patients having thyroid surgery.
Supporting information
Appendix S1. Methodology and results of the systematic review.
Appendix S2. Items used in the Delphi study and survey.
Appendix S3. Supporting resources to aid implementing guidance.
Acknowledgements
This systematic review was prospectively registered with PROSPERO (CRD42020214858). We thank Dr R. Bhagrath (UK), Dr A. Chakladar (UK), Ms H. Doran (UK), Dr F. Eatock (UK), Dr Y. Endlich (Australia), Miss S. Fraser (UK), Professor G. Frova (Italy), Dr A. Higgs (UK), Mr O. Hilmi (UK), Professor N. Kumar (UK), Dr B. McGuire (UK), Dr A. McNarry (UK), Mr R. Mihai (UK), Dr F. Mir (UK), Mr R. Moorthy (UK), Mr C. Murray (UK), Professor S. M. Myatra (India), Mr J. Ramsingh (UK), Professor G.W. Randolph (USA), Ms S. Roberts (UK), Dr T. Rope (UK), Mr G. Sadler (UK), Mr D. Scott‐Coombes (UK), Dr M. Sorbello (Italy), Mr A. C. Swift (UK) and Professor P. Tostevin (UK) for review and comment on these guidelines. KE is an Editor for Anaesthesia and he or his institution have previously received educational, research or travel support from Fisher and Paykel, Ambu, GE Healthcare and Edwards Lifesciences. IA has previously received honoraria as educational grants from Stortz, Ambu, Fisher and Paykel, Flexicare, Verathon Medical and BioMarin. TT has previously received honoraria for consulting for Ambu and Smiths Medical. No other external funding or competing interests declared.
Contributor Information
H. A. Iliff, @iliff_helen.
K. El‐Boghdadly, Email: elboghdadly@gmail.com, @elboghdadly.
I. Ahmad, @dr_imranahmad.
S. Khan, @ShadKhanSurgeon.
G. Rees, @grees34.
T. S. Tatla, @tarantatla2.
References
- 1. The British Association of Endocrine and Thyroid Surgeons . The British Association of Endocrine and Thyroid Surgeons Fifth National Audit Report. 2017. https://www.baets.org.uk/wp‐content/uploads/BAETS‐Audit‐National‐Report‐2017.pdf (accessed 04/07/2021).
- 2. Godballe C, Madsen AR, Pedersen HB, et al. Post‐thyroidectomy hemorrhage: a national study of patients treated at the Danish departments of ENT Head and Neck Surgery. European Archives of Oto‐Rhino‐Laryngology 2009; 266: 1945–52. [DOI] [PubMed] [Google Scholar]
- 3. Swirta J, Barczyński M. Krwotok po operacji tarczycy [Haemorrhage after thyroid surgery]. Przegląd Lekarski 2014; 71: 82–5. [PubMed] [Google Scholar]
- 4. Doran HE, Wiseman SM, Palazzo FF, Chadwick D, Aspinall S. Post‐thyroidectomy bleeding: analysis of risk factors from a national registry. British Journal of Surgery 2021; 108: 851–7. [DOI] [PubMed] [Google Scholar]
- 5. Lee HS, Lee BJ, Kim SW, et al. Patterns of post‐thyroidectomy hemorrhage. Clinical and Experimental Otorhinolaryngology 2009; 2: 72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oxford University Hospitals NHS Foundation Trust . Learning from Deaths. 2019. https://www.ouh.nhs.uk/about/trust‐board/2019/may/documents/TB2019.50‐learning‐from‐deaths.pdf (accessed 04/07/2021).
- 7. Ahmad I, El‐Boghdadly K, Bhagrath R, et al. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia 2020; 75: 509–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. British Journal of Anaesthesia 2015; 115: 827–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mushambi MC, Kinsella SM, Popat M, et al. Obstetric Anaesthetists’ Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia 2015; 70: 1286–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petrini F, Accorsi A, Adrario E, et al. Recommendations for airway control and difficult airway management. Minerva Anestesiologica 2005; 71: 617–57. [PubMed] [Google Scholar]
- 11. Australian and New Zealand College of Anaesthetists . Guidelines for the management of evolving airway obstruction: transition to the can’t intubate can’t oxygenate airway emergency. 2017. https://www.anzca.edu.au/getattachment/71f54974‐314a‐4d96‐bef2‐c03f39c8a8e9/PS61‐Guideline‐for‐the‐management‐of‐evolving‐airway‐obstruction‐transition‐to‐the‐Can’t‐Intubate‐Can’t‐Oxygenate‐airway‐emergency (accessed 20/02/2021).
- 12. Law JA, Broemling N, Cooper RM, et al. The difficult airway with recommendations for management – Part 1 – Intubation encountered in an unconscious/induced patient. Canadian Journal of Anesthesia 2013; 60: 1089–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013; 118: 251–70. [DOI] [PubMed] [Google Scholar]
- 14. Myatra SN, Shah A, Kundra P, et al. All India difficult airway association 2016 guidelines for the management of unanticipated difficult tracheal intubation in adults. Indian Journal of Anaesthesia 2016; 60: 885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramkumar V, Dinesh E, Shetty SR, et al. All India difficult airway association 2016 guidelines for the management of unanticipated difficult tracheal intubation in obstetrics. Indian Journal of Anaesthesia 2016; 60: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langeron O, Bourgain J‐L, Francon D, et al. Difficult intubation and extubation in adult anaesthesia. Anaesthesia Critical Care and Pain Medicine 2018; 37: 639–51. [DOI] [PubMed] [Google Scholar]
- 17. Rehn M, Hyldmo PK, Magnusson V, et al. Scandinavian SSAI clinical practice guideline on pre‐hospital airway management. Acta Anaesthesiologica Scandinavica 2016; 60: 852–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piepho T, Cavus E, Noppens R, et al. S1‐Leitlinie Atemwegsmanagement [S1 guidelines on airway management]. Anaesthesist 2015; 64: 859–73. [DOI] [PubMed] [Google Scholar]
- 19. Japanese Society of Anaesthesiologists . JSA airway management guideline 2014: to improve the safety of induction of anesthesia. Journal of Anesthesia 2014; 28: 482–93. [DOI] [PubMed] [Google Scholar]
- 20. Cook TM, El‐Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID‐19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia 2020; 75: 785–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edelman DA, Perkins EJ, Brewster DJ. Difficult airway management algorithms: a directed review. Anaesthesia 2019; 74: 1175–85. [DOI] [PubMed] [Google Scholar]
- 22. Gray WK, Aspinall S, Tolley N, Day J, Lansdown M. The volume and outcome relationship for thyroidectomy in England. Langenbeck’s Archives of Surgery 2021; Epub 9 June. 10.1007/s00423-021-02223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al‐Qurayshi Z, Robins R, Hauch A, Randolph GW, Kandil E. Association of surgeon volume with outcomes and cost savings following thyroidectomy. Journal of the American Medcial Association Otolaryngology‐Head & Neck Surgery 2016; 142: 32–9. [DOI] [PubMed] [Google Scholar]
- 24. Farooq MS, Nouraei R, Kaddour H, Saharay M. Patterns, timing and consequences of post‐thyroidectomy haemorrhage. Annals of the Royal College of Surgeons of England 2017; 99: 60–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Edafe O, Cochrane E, Balasubramanian SP. Reoperation for bleeding after thyroid and parathyroid surgery: incidence, risk factors, prevention, and management. World Journal of Surgery 2020; 44: 1156–62. [DOI] [PubMed] [Google Scholar]
- 26. Liu J, Sun W, Dong W, et al. Risk factors for post‐thyroidectomy haemorrhage: a meta‐analysis. European Journal of Endocrinology 2017; 176: 591–602. [DOI] [PubMed] [Google Scholar]
- 27. Brouwers MC, Kerkvliet K, Spithoff K, Consortium ANS . The AGREE reporting checklist: a tool to improve reporting of clinical practice guidelines. British Medical Journal 2016; 352: i1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. Journal of Clinical Epidemiology 2014; 67: 401–9. [DOI] [PubMed] [Google Scholar]
- 29. Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One 2011; 6: e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pandit JJ, Popat MT, Cook TM, et al. The Difficult Airway Society “ADEPT” Guidance on selecting airway devices: the basis of a strategy for equipment evaluation. Anaesthesia 2011; 66: 726–37. [DOI] [PubMed] [Google Scholar]
- 31. Du Rand IA, Blaikley J, Booton R, et al. Summary of the British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax 2013; 68: 786–7. [DOI] [PubMed] [Google Scholar]
- 32. Doran HE, England J, Palazzo F. Questionable safety of thyroid surgery with same day discharge. Annals of the Royal College of Surgeons of England 2012; 94: 543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leyre P, Desurmont T, Lacoste L, et al. Does the risk of compressive hematoma after thyroidectomy authorize 1‐day surgery? Langenbeck’s Archives of Surgery 2008; 393: 733–7. [DOI] [PubMed] [Google Scholar]
- 34. Dixon JL, Snyder SK, Lairmore TC, Jupiter D, Govednik C, Hendricks JC. A novel method for the management of post‐thyroidectomy or parathyroidectomy hematoma: a single‐institution experience after over 4,000 central neck operations. World Journal of Surgery 2014; 38: 1262–7. [DOI] [PubMed] [Google Scholar]
- 35. Pontin A. Postoperative bleeding after thyroid surgery: care instructions. Medical Bulletin of Sisli Hospital 2019; 53: 329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dehal A, Abbas A, Al‐Tememi M, Hussain F, Johna S. Impact of surgeon volume on incidence of neck hematoma after thyroid and parathyroid surgery: ten years’ analysis of nationwide in‐patient sample database. American Surgeon 2014; 80: 948–52. [PubMed] [Google Scholar]
- 37. Shandilya M, Kieran S, Walshe P, Timon C. Cervical haematoma after thyroid surgery: management and prevention. Irish Medical Journal 2006; 99: 266–8. [PubMed] [Google Scholar]
- 38. Calò PG, Erdas E, Medas F, et al. Late bleeding after total thyroidectomy: report of two cases occurring 13 days after operation. Clinical Medicine Insights: Case Reports 2013; 6: 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maben J, Griffiths P, Penfold C, et al. Southampton (UK): NIHR Journals Library. Evaluating a major innovation in hospital design: workforce implications and impact on patient and staff experiences of all single room hospital accommodation. 2015. https://www.ncbi.nlm.nih.gov/books/NBK274434/ (accessed 04/07/2021). [PubMed]
- 40. McGrath BA, Bates L, Atkinson D, Moore JA. Multidisciplinary guidelines for the management of tracheostomy and laryngectomy airway emergencies. Anaesthesia 2012; 67: 1025–41. [DOI] [PubMed] [Google Scholar]
- 41. McGrath BA, Brenner MJ, Warrillow SJ, et al. Tracheostomy in the COVID‐19 era: global and multidisciplinary guidance. Lancet Respiratory Medicine 2020; 8: 717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kramer AA, Sebat F, Lissauer M. A review of early warning systems for prompt detection of patients at risk for clinical decline. Journal of Trauma and Acute Care Surgery 2019; 87: S67–73. [DOI] [PubMed] [Google Scholar]
- 43. Ishikawa M, Namizato D, Sakamoto A. The value of acoustic respiratory rate monitoring in a patient with postoperative hemorrhage after thyroidectomy: a case report. Journal of Clinical Monitoring and Computing 2020; 34: 147–50. [DOI] [PubMed] [Google Scholar]
- 44. Chrimes N, Bradley WPL, Gatward JJ, Weatherall AD. Human factors and the ‘next generation’ airway trolley. Anaesthesia 2019; 74: 427–33. [DOI] [PubMed] [Google Scholar]
- 45. Bourn S, Wijesingha S, Nordmann G. Transfer of the critically ill adult patient. British Journal of Anaesthesia Education 2018; 18: 63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Faculty of Intensive Care Medicine . Guidance on: the transfer of the critically Ill adult. 2019. https://www.ficm.ac.uk/sites/default/files/transfer_critically_ill_adult_2019.pdf (accessed 04/07/2021).
- 47. Kulshrestha A, Singh J. Inter‐hospital and intra‐hospital patient transfer: recent concepts. Indian Journal of Anaesthesia 2016; 60: 451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee BJ, Kang JM, Kim DO. Laryngeal exposure during laryngoscopy is better in the 25° back‐up position than in the supine position. British Journal of Anaesthesia 2007; 99: 581–6. [DOI] [PubMed] [Google Scholar]
- 49. Lee CH, Peng MJ, Wu CL. Dexamethasone to prevent postextubation airway obstruction in adults: a prospective, randomized, double‐blind, placebo‐controlled study. Critical Care 2007; 11: R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hunt BJ. The current place of tranexamic acid in the management of bleeding. Anaesthesia 2015; 70: 50–3. [DOI] [PubMed] [Google Scholar]
- 51. British Association of Endocrine and Thyroid Surgeons Management of post‐operative haemorrhage in thyroid and parathyroid surgery. https://www.baets.org.uk/management‐of‐post‐operative‐haemorrhage‐in‐thyroid‐and‐parathyroid‐surgery/ (accessed 09/02/2021).
- 52. Higgs A, McGrath BA, Goddard C, et al. Guidelines for the management of tracheal intubation in critically ill adults. British Journal of Anaesthesia 2018; 120: 323–52. [DOI] [PubMed] [Google Scholar]
- 53. Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. British Journal of Anaesthesia 2011; 106: 617–31. [DOI] [PubMed] [Google Scholar]
- 54. Cook TM, Woodall N, Harper J, Benger J. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. British Journal of Anaesthesia 2011; 106: 632–42. [DOI] [PubMed] [Google Scholar]
- 55. Spackman DR, Kellow N, White SA, Seed PT, Feneck RO. High frequency jet ventilation and gas trapping. British Journal of Anaesthesia 1999; 83: 708–14. [DOI] [PubMed] [Google Scholar]
- 56. Sasano N, Tanaka A, Muramatsu AI, et al. Tidal volume and airway pressure under percutaneous transtracheal ventilation without a jet ventilator: comparison of high‐flow oxygen ventilation and manual ventilation in complete and incomplete upper airway obstruction models. Journal of Anesthesia 2014; 28: 341–6. [DOI] [PubMed] [Google Scholar]
- 57. Qazi I, Mendonca C, Sajayan A, Boulton A, Ahmad I. Emergency front of neck airway: what do trainers in the UK teach? A national survey. Journal of Anaesthesiology Clinical Pharmacology 2019; 35: 318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Agarwala AV, Spanakis SG, Nixon H. Cognitive aids: does patient safety depend on a manual? International Anesthesiology Clinics 2019; 57: 48–61. [DOI] [PubMed] [Google Scholar]
- 59. Marshall S. The use of cognitive aids during emergencies in anesthesia: a review of the literature. Anesthesia and Analgesia 2013; 117: 1162–71. [DOI] [PubMed] [Google Scholar]
- 60. Villanueva T. ALERT course (Acute Life threatening Events—Recognition and Treatment). British Medical Journal 2007; 334: s49. [Google Scholar]
- 61. Soar J, Deakin C, Lockey A, Nolan J, Perkins G. Resusitation Council UK; Guidelines: adult advanced life support. 2015. https://www.resus.org.uk/library/2021‐resuscitation‐guidelines/adult‐advanced‐life‐support‐guidelines (accessed 04/07/2021).
- 62. Qi W, Gevonden M, Shalev A. Prevention of post‐traumatic stress disorder after trauma: current evidence and future directions. Current Psychiatry Reports 2016; 18: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Karatzias T, Murphy P, Cloitre M, et al. Psychological interventions for ICD‐11 complex PTSD symptoms: systematic review and meta‐analysis. Psychological Medicine 2019; 49: 1761–75. [DOI] [PubMed] [Google Scholar]
- 64. Naik G, Ahmed H, Edwards AGK. Communicating risk to patients and the public. British Journal of General Practice 2012; 62: 213–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cikalo M, Fitzgerald A, Brown S, Edwards M & Glanville J National Institute for Health and Care Excellence. National Institute for Health and Care Excellence: overview of systematic reviews exploring complex risk communication final report; 2014. https://www.nice.org.uk/guidance/ng34/evidence/evidence‐review‐1‐overview‐of‐systematic‐reviews‐exploring‐complex‐risk‐communication‐2311295437 (accessed 19/02/2021).
- 66. Cruikshanks A, Bryden DC. What to do when complications occur. Anaesthesia 2018; 73: 95–101. [DOI] [PubMed] [Google Scholar]
- 67. Twigg S. Clinical event debriefing. Current Opinion in Pediatrics 2020; 32: 337–42. [DOI] [PubMed] [Google Scholar]
- 68. Magyar J, Theophilos T. Debriefing critical incidents in the emergency department. Emergency Medicine Australasia 2010; 22: 499–506. [DOI] [PubMed] [Google Scholar]
- 69. Webb K. Online Research at Cardiff (ORCA). Enhancing frontline health professionals’ resilience: MedTRiM‐An evaluation of the effectiveness of a training programme to assist in improving the resilience of health professionals on the frontline of healthcare delivery final report. 2018. https://orca.cardiff.ac.uk/123443/1/MedTRiM%20Report%20final.pdf (accessed 04/07/2021).
- 70. Johnson TJ, Millinchamp FJ, Kelly FE. Use of a team immediate debrief tool to improve staff well‐being after potentially traumatic events. Anaesthesia 2021; 76: 1001–2. [DOI] [PubMed] [Google Scholar]
- 71. Mirnezami R, Sahai A, Symes A, Jeddy T. Day‐case and short‐stay surgery: the future for thyroidectomy? International Journal of Clinical Practice 2007; 61: 1216–22. [DOI] [PubMed] [Google Scholar]
- 72. General Medical Council . Guidance on professional standards and ethics for doctors Decision making and consent. 2020. https://www.gmc‐uk.org/‐/media/documents/gmc‐guidance‐for‐doctors–‐decision‐making‐and‐consent‐english_pdf‐84191055.pdf?la=en&hash=BE327A1C584627D12BC51F66E790443F0E0651DA (accessed 05/07/2021).
- 73. Bélanger M‐È, Tanoubi I, Georgescu M, et al. Successful management of a neck hematoma following simulation training. Anaesthesia Critical Care and Pain Medicine 2017; 36: 237–8. [DOI] [PubMed] [Google Scholar]
- 74. The King’s Fund . Variations in health care : the good, the bad, and the inexplicable. 2001. https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/Variations‐in‐health‐care‐good‐bad‐inexplicable‐report‐The‐Kings‐Fund‐April‐2011.pdf (accessed 05/07/2021).
- 75. Coyle M, Martin D, McCutcheon K. Interprofessional simulation training in difficult airway management: a narrative review. British Journal of Nursing 2020; 29: 36–43. [DOI] [PubMed] [Google Scholar]
- 76. SC 11 [2015] 1 AC 1430 . Montgomery (Appellant) v Lanarkshire Health Board (Respondent). 2015. https://www.supremecourt.uk/cases/docs/uksc‐2013‐0136‐judgment.pdf (accessed 05/07/2021).
- 77. Papadakis M, Meiwandi A, Grzybowski A. The WHO safer surgery checklist time out procedure revisited: strategies to optimise compliance and safety. International Journal of Surgery 2019; 69: 19–22. [DOI] [PubMed] [Google Scholar]
- 78. Desmedt M, Ulenaers D, Grosemans J, Hellings J, Bergs J. Clinical handover and handoff in healthcare: a systematic review of systematic reviews. International Journal for Quality in Health Care 2021; 33: mzaa170. [DOI] [PubMed] [Google Scholar]
- 79. Bukoh MX, Siah CR. A systematic review on the structured handover interventions between nurses in improving patient safety outcomes. Journal of Nursing Management 2020; 28: 744–55. [DOI] [PubMed] [Google Scholar]
- 80. Raeisi A, Rarani MA, Soltani F. Challenges of patient handover process in healthcare services: a systematic review. Journal of Education and Health Promotion 2019; 8: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McNarry AF, M. Cook T, Baker PA, O'Sullivan EP. The Airway Lead: opportunities to improve institutional and personal preparedness for airway management. British Journal of Anaesthesia 2020; 125: e22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ahmad I, Onwochei DN, Muldoon S, Keane O, El‐Boghdadly K. Airway management research: a systematic review. Anaesthesia 2019; 74: 225–36. [DOI] [PubMed] [Google Scholar]
- 83. Ahmad I, El‐Boghdadly K. From evidence based on practice to evidence‐based practice: time for a difficult airway management research strategy. Anaesthesia 2019; 74: 135–9. [DOI] [PubMed] [Google Scholar]
- 84. Mitchell V, Dravid R, Patel A, et al. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012; 67: 318–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Methodology and results of the systematic review.
Appendix S2. Items used in the Delphi study and survey.
Appendix S3. Supporting resources to aid implementing guidance.


