Abstract
Objectives
Periodontal disease is a frequent diagnosis of dogs and can have severe negative impacts on welfare. It was hypothesised that breeds with skull shapes that differ most in conformation from the moderate mesocephalic skull shape have higher odds of periodontal disease.
Materials and Methods
The cohort study included a random sample of dogs under primary veterinary care in 2016 from the VetCompass Programme database. Risk factor analysis used random effects multivariable logistic regression modelling.
Results
The study included a random sample of 22,333 dogs. The 1‐year period prevalence for diagnosis with periodontal disease was 12.52% (95% CI: 12.09 to 12.97). Eighteen breeds showed increased odds compared with crossbred dogs. Breeds with the highest odds included Toy Poodle (odds ratio 3.97, 95% confidence intervals 2.21 to 7.13), King Charles Spaniel (odds ratio 2.63, 95% confidence interval 1.50 to 4.61), Greyhound (odds ratio 2.58, 95% confidence interval 1.75 to 3.80) and Cavalier King Charles Spaniel (odds ratio 2.39, 95% confidence interval 1.85 to 3.09). Four breeds showed reduced odds compared with crossbreds. Brachycephalic breeds had 1.25 times the odds (95% confidence interval 1.11 to 1.42) of periodontal disease compared with mesocephalic breeds. Spaniel types had 1.63 times the odds (95% confidence interval 1.42 to 1.87) compared with non‐spaniel types. Increasing adult bodyweight was associated with progressively decreasing odds of periodontal disease.
Clinical Significance
The high prevalence identified in this study highlights periodontal disease as a priority welfare concern for predisposed breeds. Veterinarians can use this information to promote improved dental care in predisposed dogs, especially as these dogs age.
INTRODUCTION
Periodontal disease is a syndromic diagnosis term covering the presence of at least one from a range of more specific diagnoses including gingivitis and periodontitis that exist along a continuum (Niemiec 2013, Bellows et al. 2019, Ruparell et al. 2021). Periodontal disease is one of the most frequent diagnoses made for dogs under first opinion veterinary care (Lund et al. 1999, O'Neill et al. 2014, Robinson et al. 2016), with some prospective studies suggesting prevalence values as high as 44% to 63.6% (Butković et al. 2001, Kortegaard et al. 2008). A recent study that scored the welfare impact of common disorders in dogs identified dental disease as having the highest overall welfare impact score from the common disorders of dogs (Summers et al. 2019). This finding was driven by the high prevalence (9.6%) and duration (39.9% of cases were affected for the entire study period) of periodontal disease along with a moderate severity (score of 7/21) (Summers et al. 2019). In addition to direct oral effects, dental disease is also associated with reduced overall systemic health (Pavlica 2008, Glickman et al. 2009, Bellows et al. 2019) including associations with disease in other organ systems such as kidneys, heart and liver (DeBowes et al. 1996), and even death due to starvation or secondary septicemia (de Campos Andrade et al. 2015, Janssens et al. 2016, Soe et al. 2017). Although gingivitis is considered to be clinically reversible, periodontitis is considered irreversible and leads to destruction of the periodontal ligament, cementum and alveola bone that often results in tooth loss (Wallis & Holcombe 2020).
Despite its high prevalence and welfare impact, there is limited published evidence on the epidemiology of periodontal disease in the wider dog population. This may be partially because many periodontal cases are managed completely in the primary care setting whereas much of the previous veterinary research literature emanated from the referral care or experimental research settings (Bartlett et al. 2010). Generalisability to the wider companion dog population from many of the earlier published studies on periodontal disease is limited by relatively small sample sizes of dogs that were often housed in laboratories or selected from the subset of extremely severe cases that were referred for secondary veterinary care (Butković et al. 2001, Marshall et al. 2014, Gordon et al. 2018). From the primary care studies that have been published, the breeds reported with frequent periodontal disease tended to be smaller sized dogs and include Yorkshire Terriers, Cocker Spaniels, West Highland White Terriers, Border Terriers and Poodles (Marshall et al. 2014, O'Neill et al. 2014, Wallis & Holcombe 2020). Medium‐ and large‐sized breeds, including Labrador Retrievers, Rottweilers, German Shepherd Dogs and Staffordshire Bull Terriers, tended to be reported with lower frequencies of periodontal disease (O'Neill et al. 2017a, O'Neill et al. 2017c, McGreevy et al. 2018, Pegram et al. 2020). Greyhounds are a reported exception to this trend, with a recent study identifying 39% of Greyhounds under primary veterinary care in the UK as affected during a single year (O'Neill et al. 2019c). This prevalence in Greyhounds is more than four times higher than the 9.3% prevalence previously reported across all dog breeds (O'Neill et al. 2014). Until recently, the majority of epidemiological studies focused on reporting disorder predisposition (Gough et al. 2018). However, identification of factors associated with protection from disorders could additionally be valuable to provide deeper understanding of the aetiopathogenesis and impact of individual disorders to the health profile of a breed (Pegram et al. 2020, O'Neill et al. 2020a).
Although there is substantial published evidence linking dental health to factors such as bodyweight (prevalence is reported to decrease with increasing bodyweight) (Harvey et al. 1994), diet (a soft diet is considered to increase the risk) (Hill 1998, Lund et al. 1999) and age (incidence is reported to increase with age) (Harvey et al. 1994, Kortegaard et al. 2008, Marshall et al. 2014), there is limited understanding of associations between periodontal disease and body conformation. Dogs are the most phenotypically diverse companion animal species and therefore offer unique opportunities to explore correlations between conformational morphology and health (Wayne et al. 2006, O'Neill et al. 2014). The UK Kennel Club currently recognises 218 breeds from an estimated 400 breeds that exist worldwide (The Kennel Club 2021b). Skull morphology, in particular, has attracted a great deal of attention in recent years in relation to associations with several disorders (Drake & Klingenberg 2010, O'Neill et al. 2020b, BWG 2021) but there is currently limited evidence on associations between skull conformation and periodontal disease. A recent cross‐sectional study reported no significant association between skull morphology and severity of periodontal disease but these results may have been subject to selection bias because the study dogs were all from a commercial breeding environment and the sample size was relatively small (445 dogs) (Stella et al. 2018).
Given the substantial welfare harms, such as pain and systemic infections, in combination with the high frequency of periodontal disease, greater understanding of which breeds and conformational features are either predisposed to, or protected from, periodontal disease could promote more effective and targeted health approaches to mitigate these harms (Niemiec et al. 2020). Using anonymised veterinary clinical data from the VetCompass Programme (VetCompass 2021), this study aimed to report the prevalence of diagnosis with periodontal disease in dogs overall and within both common and commonly affected breeds. The study also aimed to report on demographic risk factors for diagnosis with periodontal disease, placing special focus on associations with breed and skull conformation. The study hypothesised that dogs with brachycephalic (broad headed) and dolichocephalic (long headed) skull conformations have higher odds of diagnosis with periodontal disease than dogs with more moderate mesocephalic skull conformation. The study did not aim to report in clinical aspects of periodontal disease in dogs.
METHODS
Study design
The study population included all available dogs under primary veterinary care at clinics participating in the VetCompass Programme during 2016. Dogs under veterinary care were defined as those with either (1) at least one electronic patient record (EPR) recorded during 2016 or (2) at least one EPR recorded during both 2015 and 2017. VetCompass collates de‐identified EPR data from primary‐care veterinary practices in the UK for epidemiological research (VetCompass 2021). Data fields available to VetCompass researchers include a unique animal identifier along with species, breed, date of birth, sex, neuter status, insurance and bodyweight, and also clinical information from free‐form text clinical notes, summary diagnosis terms (The VeNom Coding Group 2021) and treatment with relevant dates.
The study used a cohort design. From the overall population of dogs under veterinary care in 2016, a random sample of dogs were selected and followed in the clinical records for a 1‐year period (2016) to identify all dogs within the sample with a recorded diagnosis of periodontal disease. The case definition for periodontal disease required evidence in the clinical records that periodontal disease existed as a clinical condition at some point during 2016. The clinical decision‐making process was at the discretion of the attending veterinary surgeons. Sample size calculations estimated that 13,621 dogs would need to be assessed to estimate prevalence for a disorder that occurred in 10.0% of dogs (O'Neill et al. 2014) with 0.5% acceptable margin of error at a 95% confidence level from a population of 905,544 dogs (Epi Info CDC 2021). Ethics approval was obtained from the Royal Veterinary College Ethics and Welfare Committee (reference SR2018‐1652).
Breed descriptive information entered by the participating practices was cleaned and mapped to a VetCompass breed list derived and extended from the VeNom Coding breed list that included both recognised purebred breeds and also designer breed terms (The VeNom Coding Group 2021). A purebred variable categorised all dogs of recognisable breeds as “purebred,” dogs with contrived names generated from two or more purebred breed terms as designers and all remaining dogs with breed information as “crossbred” (The Kennel Club 2021b). Pure breeds and designer breeds that had over 300 dogs in the overall study population or had at least 20 periodontal disease cases were included in the breed variable as individual breeds. This breed variable also included a category that grouped all remaining purebreds and a category that grouped all general crossbred dogs. This approach was taken to facilitate statistical power for the individual breed analyses (Scott et al. 2012). Breeds were further characterised by skull shape (dolichocephalic, mesocephalic, brachycephalic, non‐purebred) and spaniel (spaniel, non‐spaniel, non‐purebred) status for analysis (Appendix S1). A Kennel Club breed group variable classified breeds recognised by the UK Kennel Club into their relevant breed groups (Gundog, Hound, Pastoral, Terrier, Toy, Utility and Working) and all remaining types were classified as non‐Kennel Club recognised (The Kennel Club 2021b).
Neuter and insurance status were defined by the final available EPR value. Adult bodyweight was defined as the mean of all bodyweight (kg) values recorded for each dog after reaching 18 months old and was categorised as: less than 10.0, 10.0 to less than 15.0, 15.0 to less than 20.0, 20.0 to less than 25.0, 25.0 to less than 30.0, 30.0 to less than 40.0 and at least 40.0. Mean adult bodyweight was generated for all breed‐sex combinations with adult bodyweight available for at least 100 dogs in the overall study population and used to categorise individual dogs as “at or above the breed‐sex mean,” “below the breed‐sex mean” and “no recorded bodyweight”. Age (years) was defined at December 31, 2016, and was categorised as: up to 1.0, 1.0 to less than 2.0, 2.0 to less than 4.0, 4.0 to less than 6.0, 6.0 to less than 8.0, 8.0 to less than 10.0, 10.0 to less than 12.0 and at least 12.0.
The list of unique animal identification numbers was randomly ordered and the clinical records of a randomly selected subset of animals were reviewed manually in detail to identify all dogs that met the case definition for periodontal disease. No distinction was made between pre‐existing and incident cases of periodontal disease. Following internal validity checking and data cleaning in Excel (Microsoft Office Excel 2013, Microsoft Corp.), analyses were conducted using Stata Version 13 (Stata Corporation).
Statistical analysis
One‐year period prevalence values with 95% confidence intervals (CI) described the probability of diagnosis at least once during 2016 in dogs overall and in common breeds. The CI estimates were derived from standard errors based on approximation to the binomial distribution (Kirkwood & Sterne 2003). Risk factor analysis included dogs with periodontal disease as cases while all remaining dogs in the sample were classed as non‐cases. Binary logistic regression modelling was used to evaluate univariable associations between risk factors and periodontal disease during 2016. Because breed was a factor of primary interest for the study, variables that derived from the breed information and therefore were highly collinear with breed (skull shape, spaniel, purebred, Kennel Club recognised breed and Kennel Club breed group) were excluded from initial breed multivariable modelling. Instead, each of these variables individually replaced the breed variable in the main final breed‐focused model to evaluate their effects after taking account of the other variables. Adult bodyweight (a defining characteristic of individual breeds) replaced breed and bodyweight relative to breed‐sex mean in the final breed‐focused model. Risk factors with liberal associations in univariable modelling (P<0.2) were taken forward for multivariable evaluation. Model development used manual backwards stepwise elimination. Model building began with a full model using the relevant variables taken forward from the univariable analysis. Variables were sequentially removed and tested for their contribution using the likelihood ratio test (P<0.05) to decide on retention. All removed variables were checked in the final model to assess for confounding effect. Pair‐wise interaction effects were evaluated for the final model variables (Dohoo et al. 2009). Clinic attended was evaluated as a random effect. The area under the ROC curve and the Hosmer‐Lemeshow test were used to evaluate the quality of the model fit and discrimination (non‐random effect model) (Dohoo et al. 2009, Hosmer et al. 2013). Statistical significance was set at P<0.05. Univariable odds ratios (ORs) are reported as OR whereas multivariable ORs are reported as adjusted odds ratio (aOR).
RESULTS
Prevalence
The study included a random sample of 22,333 dogs attending 784 veterinary clinics from an overall population of 905,554 dogs under veterinary care in 2016. There were 2,797 of 22,333 dogs diagnosed with periodontal disease during 2016, yielding a 1‐year period prevalence of 12.52% (95% CI: 12.09 to 12.97). The breeds with the highest periodontal disease prevalence were Greyhound (32.21%, 95% CI 24.8 to 40.35), King Charles Spaniel (30.14%, 95% CI 19.94 to 42), Toy Poodle (25.97%, 95% CI 16.64 to 37.23), Cavalier King Charles Spaniel (25.29%, 95% CI 21.27 to 29.65), Yorkshire Terrier (22.22%, 95% CI 19.32 to 25.34) and Border Terrier (22.09%, 95% CI 17.18 to 27.66). The breeds with the lowest periodontal disease prevalence were Pug (8.96%, 95% CI 6.39 to 12.14), Labrador Retriever (7.11%, 95% CI 5.85 to 8.55), Staffordshire Bull Terrier (6.52%, 95% CI 5.3 to 8), German Shepherd Dog (3.30%, 95% CI 1.97 to 5.16) and French Bulldog (2.01%, 95% CI 0.87 to 3.92) (Fig. 1).
Fig 1.
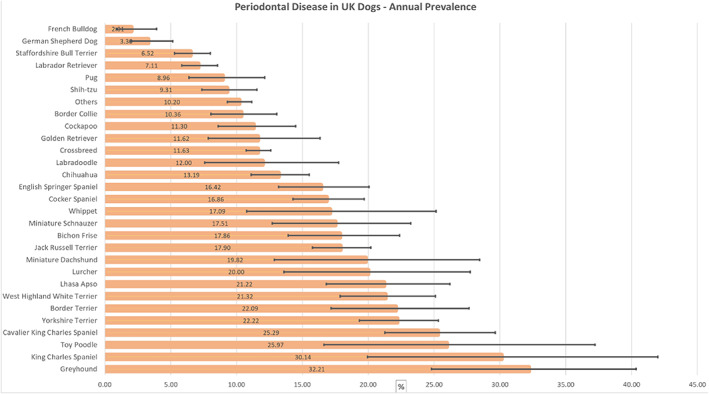
One‐year (2016) period prevalence of periodontal disease in common dog breeds under primary veterinary care in the UK. N = 22,333
Data completeness varied between the variables assessed: breed 99.68%, age 98.80%, sex 99.66%, neuter 99.66% and adult bodyweight 66.82%. The median age of dogs with periodontal disease (7.54 years, interquartile range (IQR) 5.00 to 10.50, range 0.53 to 19.27) was older than dogs without periodontal disease (3.90 years, IQR 1.65 to 7.48, range 0.01 to 20.46) (P<0.001). The median adult bodyweight of dogs with periodontal disease (10.33 kg, IQR 7.20 to 19.33, range 1.49 to 65.94) was lighter than for dogs without periodontal disease (14.90 kg, IQR 8.37 to 25.86, range 1.41 to 85.00) (P<0.001). Further demographic information is available in Tables 1 and 2.
Table 1.
Descriptive and univariable logistic regression (clinic attended included as a random effect) results for breed‐related risk factors evaluated for periodontal disease during 2016 in dogs under primary veterinary care in the VetCompass Programme in the UK
| Variable | Category | Case number (%) | Non‐case number (%) | Odds ratio | 95% CI | Category P‐value | Variable P‐value |
|---|---|---|---|---|---|---|---|
| Purebred status | Crossbred | 548 (19.61) | 4162 (21.38) | Base | 0.016 | ||
| Designer | 140 (5.01) | 1155 (5.93) | 0.90 | 0.74 to 1.10 | 0.316 | ||
| Purebred | 2107 (75.38) | 14,149 (72.69) | 1.11 | 1.01 to 1.24 | 0.038 | ||
| Breed | Crossbreed | 548 (19.59) | 4162 (21.30) | 1.00 | 0 to 0 | <0.001 | |
| Greyhound | 48 (1.72) | 101 (0.52) | 3.82 | 2.62 to 5.56 | <0.001 | ||
| King Charles Spaniel | 22 (0.79) | 51 (0.26) | 3.35 | 1.97 to 5.69 | <0.001 | ||
| Toy Poodle | 20 (0.72) | 57 (0.29) | 3.04 | 1.78 to 5.21 | <0.001 | ||
| Cavalier King Charles Spaniel | 110 (3.93) | 325 (1.66) | 2.66 | 2.08 to 3.39 | <0.001 | ||
| Yorkshire Terrier | 170 (6.08) | 595 (3.05) | 2.18 | 1.79 to 2.66 | <0.001 | ||
| Lhasa Apso | 66 (2.36) | 245 (1.25) | 2.14 | 1.59 to 2.89 | <0.001 | ||
| Border Terrier | 57 (2.04) | 201 (1.03) | 2.08 | 1.51 to 2.85 | <0.001 | ||
| West Highland White Terrier | 110 (3.93) | 406 (2.08) | 2.07 | 1.63 to 2.62 | <0.001 | ||
| Lurcher | 27 (0.97) | 108 (0.55) | 1.97 | 1.26 to 3.08 | 0.003 | ||
| Bichon Frise | 60 (2.15) | 276 (1.41) | 1.68 | 1.24 to 2.28 | 0.001 | ||
| Jack Russell Terrier | 213 (7.62) | 977 (5.00) | 1.66 | 1.39 to 1.98 | <0.001 | ||
| Miniature Schnauzer | 38 (1.36) | 179 (0.92) | 1.63 | 1.12 to 2.37 | 0.010 | ||
| Whippet | 20 (0.72) | 97 (0.50) | 1.63 | 0.98 to 2.70 | 0.058 | ||
| Miniature Dachshund | 22 (0.79) | 89 (0.46) | 1.60 | 0.97 to 2.62 | 0.064 | ||
| Cocker Spaniel | 130 (4.65) | 641 (3.28) | 1.50 | 1.21 to 1.87 | <0.001 | ||
| English Springer Spaniel | 78 (2.79) | 397 (2.03) | 1.49 | 1.14 to 1.95 | 0.003 | ||
| Chihuahua | 126 (4.50) | 829 (4.24) | 1.16 | 0.94 to 1.44 | 0.164 | ||
| Labradoodle | 21 (0.75) | 154 (0.79) | 0.98 | 0.61 to 1.59 | 0.948 | ||
| Cockapoo | 54 (1.93) | 424 (2.17) | 0.94 | 0.69 to 1.27 | 0.681 | ||
| Golden Retriever | 28 (1.00) | 213 (1.09) | 0.91 | 0.60 to 1.38 | 0.655 | ||
| Border Collie | 63 (2.25) | 545 (2.79) | 0.87 | 0.65 to 1.15 | 0.322 | ||
| Others | 440 (15.73) | 3872 (19.82) | 0.84 | 0.73 to 0.96 | 0.012 | ||
| Shih‐tzu | 74 (2.65) | 721 (3.69) | 0.82 | 0.63 to 1.07 | 0.140 | ||
| Pug | 37 (1.32) | 376 (1.92) | 0.73 | 0.51 to 1.04 | 0.080 | ||
| Labrador Retriever | 104 (3.72) | 1358 (6.95) | 0.55 | 0.44 to 0.69 | <0.001 | ||
| Staffordshire Bull Terrier | 85 (3.04) | 1219 (6.24) | 0.52 | 0.41 to 0.66 | <0.001 | ||
| German Shepherd Dog | 18 (0.64) | 528 (2.70) | 0.25 | 0.15 to 0.40 | <0.001 | ||
| French Bulldog | 8 (0.29) | 390 (2.00) | 0.15 | 0.07 to 0.30 | <0.001 | ||
| Kennel Club Breed Group | Not KC‐recognised | 728 (26.05) | 5711 (29.34) | Base | <0.001 | ||
| Toy | 587 (21.00) | 2726 (14.00) | 1.71 | 1.51 to 1.93 | <0.001 | ||
| Hound | 142 (5.08) | 656 (3.37) | 1.68 | 1.37 to 2.05 | <0.001 | ||
| Terrier | 529 (18.93) | 3119 (16.02) | 1.32 | 1.17 to 1.49 | <0.001 | ||
| Utility | 291 (10.41) | 2227 (11.44) | 1.03 | 0.89 to 1.2 | 0.649 | ||
| Gundog | 374 (13.38) | 2911 (14.95) | 0.97 | 0.84 to 1.11 | 0.633 | ||
| Pastoral | 97 (3.47) | 1228 (6.31) | 0.60 | 0.48 to 0.75 | <0.001 | ||
| Working | 47 (1.68) | 888 (4.56) | 0.41 | 0.3 to 0.55 | <0.001 | ||
| Skull conformation | Dolichocephalic | 238 (8.52) | 1506 (7.74) | Base | 0.004 | ||
| Brachycephalic | 485 (17.35) | 3684 (18.94) | 0.85 | 0.72 to 1.01 | 0.069 | ||
| Crossbred | 688 (24.62) | 5306 (27.28) | 0.83 | 0.71 to 0.98 | 0.026 | ||
| Mesocephalic | 1384 (49.52) | 8957 (46.04) | 0.97 | 0.84 to 1.13 | 0.734 | ||
| Spaniel | Non‐spaniel type | 1887 (67.47) | 13,804 (70.66) | 1.00 | <0.001 | ||
| Spaniel type | 360 (12.87) | 1500 (7.68) | 1.79 | 1.57 to 2.03 | <0.001 | ||
| Non‐purebred | 550 (19.66) | 4232 (21.66) | 0.97 | 0.87 to 1.07 | 0.526 |
Column percentages shown in brackets
CI Confidence interval
Table 2.
Descriptive and univariable logistic regression (clinic attended included as a random effect) results for non‐breed‐related risk factors evaluated for periodontal disease during 2016 in dogs under primary veterinary care in the VetCompass Programme in the UK
| Variable | Category | Case number (%) | Non‐case number (%) | Odds ratio | 95% CI | Category P value | Variable P value |
|---|---|---|---|---|---|---|---|
| Adult (>18 months) bodyweight (kg) | < 10 kg | 1114 (47.34) | 4300 (34.21) | 2.43 | 2.04 to 2.88 | <0.001 | |
| 10.0 to < 15.0 | 459 (19.51) | 2005 (15.95) | 2.13 | 1.77 to 2.58 | <0.001 | ||
| 15.0 to < 20.0 | 219 (9.31) | 1475 (11.73) | 1.35 | 1.09 to 1.68 | 0.006 | ||
| 20.0 to < 25.0 | 194 (8.24) | 1410 (11.22) | 1.24 | 1.00 to 1.55 | 0.054 | ||
| 25.0 to < 30.0 | 152 (6.46) | 1198 (9.53) | 1.15 | 0.91 to 1.46 | 0.235 | ||
| 30.0 to < 40.0 | 176 (7.48) | 1593 (12.67) | Base | <0.001 | |||
| > or = 40 | 39 (1.66) | 590 (4.69) | 0.61 | 0.42 to 0.87 | 0.007 | ||
| < 10 | 1114 (47.34) | 4300 (34.21) | 2.43 | 2.04 to 2.88 | <0.001 | ||
| Bodyweight relative to breed mean | At or above | 1027 (36.72) | 5801 (29.69) | Base | <0.001 | ||
| Below | 1322 (47.26) | 6724 (34.42) | 1.11 | 1.01 to 1.21 | 0.030 | ||
| Unrecorded | 448 (16.02) | 7011 (35.89) | 0.28 | 0.24 to 0.32 | <0.001 | ||
| Age (years) | < 1.0 | 9 (0.32) | 2499 (12.79) | 0.04 | 0.02 to 0.08 | <0.001 | |
| 1.0 to < 2.0 | 70 (2.5) | 3208 (16.42) | 0.24 | 0.18 to 0.31 | <0.001 | ||
| 2.0 to < 4.0 | 369 (13.19) | 4089 (20.93) | Base | <0.001 | |||
| 4.0 to < 6.0 | 516 (18.45) | 2937 (15.03) | 1.97 | 1.71 to 2.28 | <0.001 | ||
| 6.0 to < 8.0 | 546 (19.52) | 2252 (11.53) | 2.75 | 2.38 to 3.18 | <0.001 | ||
| 8.0 to < 10.0 | 481 (17.2) | 1767 (9.04) | 3.06 | 2.64 to 3.56 | <0.001 | ||
| 10.0 to < 12.0 | 365 (13.05) | 1208 (6.18) | 3.51 | 2.98 to 4.13 | <0.001 | ||
| > or = 12.0 | 425 (15.19) | 1325 (6.78) | 3.58 | 3.06 to 4.19 | <0.001 | ||
| Unrecorded | 16 (0.57) | 251 (1.28) | 0.74 | 0.44 to 1.24 | 0.250 | ||
| Sex/neuter | Female entire | 435 (15.55) | 5249 (26.87) | Base | <0.001 | ||
| Female neutered | 868 (31.03) | 3988 (20.41) | 2.57 | 2.27 to 2.92 | <0.001 | ||
| Male entire | 606 (21.67) | 5871 (30.05) | 1.22 | 1.07 to 1.39 | 0.003 | ||
| Male neutered | 883 (31.57) | 4358 (22.31) | 2.39 | 2.11 to 2.71 | <0.001 | ||
| Unrecorded | 5 (0.18) | 70 (0.36) | 0.87 | 0.35 to 2.2 | 0.774 | ||
| Insurance | Uninsured | 2268 (81.09) | 17,086 (87.46) | Base | <0.001 | ||
| Insured | 529 (18.91) | 2450 (12.54) | 1.42 | 1.27 to 1.60 | <0.001 |
Column percentages shown in brackets
CI Confidence interval
Risk factors
All tested variables were liberally associated with diagnosis with periodontal disease in univariable logistic regression modelling and were evaluated using multivariable logistic regression modelling as described in the methods (Tables 1 and 2). The final main breed‐focused multivariable model retained five risk factors: breed, bodyweight relative to breed‐sex mean, age, sex‐neuter and insurance (Table 3). The final model was improved by inclusion of the clinic attended as a random effect (rho: 0.01 indicating that 1% of the variability was accounted for by the clinic attended, P=0.001). The final random effects model showed acceptable model‐fit (Hosmer‐Lemeshow test statistic: P=0.231) and acceptable discrimination (area under the ROC curve: 0.767).
Table 3.
Final breed‐focused random effects multivariable logistic regression model for risk factors associated with periodontal disease in dogs under primary veterinary care in the VetCompass Programme in the UK
| Variable | Category | Odds ratio | 95% CI | Category P‐value | Variable P‐value |
|---|---|---|---|---|---|
| Breed | Crossbreed | Base | <0.001 | ||
| Toy Poodle | 3.97 | 2.21 to 7.13 | <0.001 | ||
| King Charles Spaniel | 2.63 | 1.50 to 4.61 | 0.001 | ||
| Greyhound | 2.58 | 1.75 to 3.80 | <0.001 | ||
| Cavalier King Charles Spaniel | 2.39 | 1.85 to 3.09 | <0.001 | ||
| Lhasa Apso | 2.24 | 1.63 to 3.06 | <0.001 | ||
| Yorkshire Terrier | 2.16 | 1.75 to 2.67 | <0.001 | ||
| Cockapoo | 2.11 | 1.51 to 2.94 | <0.001 | ||
| Chihuahua | 2.09 | 1.66 to 2.63 | <0.001 | ||
| Lurcher | 1.93 | 1.21 to 3.10 | 0.006 | ||
| Border Terrier | 1.85 | 1.32 to 2.58 | <0.001 | ||
| Miniature Dachshund | 1.80 | 1.06 to 3.06 | 0.029 | ||
| Whippet | 1.74 | 1.02 to 2.98 | 0.042 | ||
| Bichon Frise | 1.67 | 1.21 to 2.30 | 0.002 | ||
| Cocker Spaniel | 1.66 | 1.32 to 2.09 | <0.001 | ||
| Miniature Schnauzer | 1.66 | 1.12 to 2.46 | 0.012 | ||
| West Highland White Terrier | 1.47 | 1.15 to 1.88 | 0.002 | ||
| Jack Russell Terrier | 1.37 | 1.14 to 1.65 | 0.001 | ||
| English Springer Spaniel | 1.33 | 1.00 to 1.75 | 0.047 | ||
| Labradoodle | 1.26 | 0.77 to 2.08 | 0.363 | ||
| Pug | 1.17 | 0.81 to 1.71 | 0.405 | ||
| Others | 0.96 | 0.83 to 1.10 | 0.538 | ||
| Shih‐tzu | 0.92 | 0.70 to 1.20 | 0.523 | ||
| Golden Retriever | 0.81 | 0.53 to 1.25 | 0.346 | ||
| Border Collie | 0.77 | 0.57 to 1.03 | 0.074 | ||
| Labrador Retriever | 0.49 | 0.39 to 0.62 | <0.001 | ||
| Staffordshire Bull Terrier | 0.45 | 0.35 to 0.58 | <0.001 | ||
| French Bulldog | 0.43 | 0.21 to 0.88 | 0.022 | ||
| German Shepherd Dog | 0.25 | 0.15 to 0.40 | <0.001 | ||
| Bodyweight relative to breed mean | At or above | Base | <0.001 | ||
| Below | 1.31 | 1.19 to 1.44 | <0.001 | ||
| Unrecorded | 0.62 | 0.54 to 0.72 | <0.001 | ||
| Age (years) | < 1.0 | 0.06 | 0.03 to 0.12 | <0.001 | <0.001 |
| 1.0 to < 2.0 | 0.30 | 0.23 to 0.39 | <0.001 | ||
| 2.0 to < 4.0 | Base | ||||
| 4.0 to < 6.0 | 1.91 | 1.65 to 2.22 | <0.001 | ||
| 6.0 to < 8.0 | 2.76 | 2.37 to 3.21 | <0.001 | ||
| 8.0 to < 10.0 | 3.17 | 2.71 to 3.71 | <0.001 | ||
| 10.0 to < 12.0 | 3.76 | 3.17 to 4.46 | <0.001 | ||
| > or = 12.0 | 3.91 | 3.31 to 4.61 | <0.001 | ||
| Unrecorded | 1.34 | 0.77 to 2.32 | 0.294 | ||
| Sex/neuter | Female entire | Base | <0.001 | ||
| Female neutered | 1.41 | 1.24 to 1.62 | <0.001 | ||
| Male entire | 1.19 | 1.03 to 1.37 | 0.016 | ||
| Male neutered | 1.37 | 1.20 to 1.57 | <0.001 | ||
| Unrecorded | 1.39 | 0.51 to 3.77 | 0.522 | ||
| Insurance | Uninsured | Base | <0.001 | ||
| Insured | 1.30 | 1.14 to 1.47 | <0.001 |
Clinic attended was included as a random effect. N=22,333
CI Confidence interval
After accounting for the effects of the other variables evaluated, 18 breed types showed increased adjusted odds of periodontal disease compared with crossbred dogs. The breeds with the highest adjusted odds included Toy Poodle (aOR 3.97, 95% CI 2.21 to 7.13, P<0.001), King Charles Spaniel (aOR 2.63, 95% CI 1.50 to 4.61, P=0.001), Greyhound (aOR 2.58, 95% CI 1.75 to 3.80, P<0.001) and Cavalier King Charles Spaniel (aOR 2.39, 95% CI 1.85 to 3.09, P<0.001). Four breeds showed reduced adjusted odds of periodontal disease compared with crossbreds: German Shepherd Dog (aOR: 0.25, 95% CI 0.15 to 0.40, P<0.001), French Bulldog (aOR: 0.43, 95% CI 0.21 to 0.88, P=0.022), Staffordshire Bull Terrier (aOR: 0.45, 95% CI 0.35 to 0.58, P=0.001) and Labrador Retriever (aOR: 0.49, 95% CI 0.39 to 0.62, P=0.001).
Dogs with an adult bodyweight below their breed‐sex mean had 1.31 (95% CI 1.19 to 1.44, P<0.001) times the adjusted odds of periodontal disease compared with dogs that weighed at or above their breed‐sex mean. Increasing age was associated with progressively increasing adjusted odds of periodontal disease. Entire females had lower adjusted odds of periodontal disease compared with the other sex/neuter combinations. Insured dogs had 1.30 (95% CI 1.14 to 1.47, P<0.001) times the adjusted odds of periodontal disease compared with uninsured dogs (Table 3).
As described in the methods, some variables were used to replace the breed variable in the final breed‐focused model. Designer types had 1.63 times the adjusted odds (95% CI 1.31 to 2.02, P<0.001) of periodontal disease compared with crossbred dogs. Toy (1.89 aOR, 95% CI 1.66 to 2.15, P<0.001) and Hound (1.52 aOR, 95% CI 1.23 to 1.89, P<0.001) Kennel Club breed groups showed higher adjusted odds of periodontal disease compared with breeds that are not recognised by the Kennel Club. Spaniel types had 1.63 times the adjusted odds (95% CI 1.42 to 1.87, P<0.001) of periodontal disease compared with non‐spaniel types. Increasing adult bodyweight was associated with progressively decreasing adjusted odds of periodontal disease. Brachycephalic dog types had 1.25 times the adjusted odds (95% CI 1.11 to 1.42, P<0.001) of periodontal disease compared with mesocephalic types (Table 4).
Table 4.
Results for risk factors that directly replaced the breed variable in the final breed‐focused random effects multivariable logistic regression model (along with age, bodyweight relative to breed mean, sex/neuter and insurance status)
| Variable | Category | Odds ratio | 95% CI | Category P‐value | Variable P‐value |
|---|---|---|---|---|---|
| Purebred status | Crossbred | Base | <0.001 | ||
| Designer | 1.63 | 1.31 to 2.02 | <0.001 | ||
| Purebred | 1.13 | 1.02 to 1.26 | 0.023 | ||
| Kennel Club Breed Group | Not KC‐recognised | 1.00 | 0 to 0 | <0.001 | |
| Toy | 1.89 | 1.66 to 2.15 | <0.001 | ||
| Hound | 1.52 | 1.23 to 1.89 | <0.001 | ||
| Utility | 1.16 | 0.99 to 1.36 | 0.061 | ||
| Terrier | 0.98 | 0.86 to 1.12 | 0.775 | ||
| Gundog | 0.81 | 0.7 to 0.93 | 0.003 | ||
| Pastoral | 0.49 | 0.39 to 0.62 | <0.001 | ||
| Working | 0.39 | 0.28 to 0.53 | <0.001 | ||
| Skull conformation | Mesocephalic | Base | 0.010 | ||
| Brachycephalic | 1.25 | 1.11 to 1.42 | <0.001 | ||
| Dolichocephalic | 1.08 | 0.92 to 1.26 | 0.363 | ||
| Non‐purebred | 1.02 | 0.92 to 1.13 | 0.704 | ||
| Spaniel | Non‐spaniel type | Base | <0.001 | ||
| Spaniel type | 1.63 | 1.42 to 1.87 | <0.001 | ||
| Non‐purebred | 0.92 | 0.82 to 1.02 | 0.111 | ||
| Adult (>18 months) bodyweight (kg) | < 10.0 | 3.07 | 2.57 to 3.67 | <0.001 | <0.001 |
| 10.0 to < 15.0 | 2.48 | 2.04 to 3.02 | <0.001 | ||
| 15.0 to < 20.0 | 1.50 | 1.20 to 1.87 | <0.001 | ||
| 20.0 to < 25.0 | 1.29 | 1.03 to 1.62 | 0.027 | ||
| 25.0 to < 30.0 | 1.23 | 0.97 to 1.56 | 0.095 | ||
| 30.0 to < 40.0 | Base | ||||
| ≥ 40.0 | 0.58 | 0.40 to 0.84 | 0.004 | ||
| Unavailable | 1.07 | 0.87 to 1.31 | 0.526 |
Adult (>18 months) bodyweight (kg) replaced the breed and bodyweight relative to breed mean variables in the final breed‐focused random effects multivariable logistic regression model. These results report associations between these risk factors and periodontal disease in dogs under primary veterinary care in the VetCompass Programme in the UK. Clinic attended was included as a random effect. N=22,333
CI Confidence interval
DISCUSSION
This paper is part of a new research paradigm that goes beyond previous research tendency to focus primarily on predisposition (Gough et al. 2018). Instead, the study design has been extended to explore breeds and conformations that are either predisposed to, or protected from periodontal disease, in order to provide evidence that can support moves to select towards positive features as well as away from negative features (Pegram et al. 2020, O'Neill et al. 2020a, The Kennel Club 2021a).
The current study reports a 1‐year period prevalence of 12.52% for periodontal disease diagnosis within the general dog population under primary veterinary care. This result is in line with other retrospective studies of individual breeds based on primary care clinical data (O'Neill et al. 2017a, O'Neill et al. 2017b, O'Neill et al. 2017c, McGreevy et al. 2018, O'Neill et al. 2018, O'Neill et al. 2019a, O'Neill et al. 2019b, O'Neill et al. 2020b) but is substantially lower than reported in several prospective studies that reported prevalence as high as 63% (Butković et al. 2001, Kortegaard et al. 2008). This difference may result from a lower inclusion clinical threshold and more rigorous dental examinations within prospective studies and suggests that many true cases of periodontal disease may be missed in the primary care setting. In many dogs under first opinion care, dental health status is determined based on clinical examination alone whereas more in‐depth diagnostic methods such as full‐mouth radiography have been reported to identify additional dental disease lesions that were not noted on routine oral examination, with this difference being more noteworthy in older dogs (Kim et al. 2013). A study of 114 dogs diagnosed with active periodontal disease on full‐mouth examination under anaesthesia identified that only 82% of these dogs showed inflammation on visual examination while conscious (Queck et al. 2018). Newer methods such as the visual dental scale (Bauer et al. 2018), assessment of canine gingival margin plaque (Ruparell et al. 2021) and the thiol‐detection test (Queck et al. 2018) offer opportunities for clinicians to improve diagnostic rates for periodontal disease in the first opinion setting. It is noteworthy though that many prospective studies of periodontal disease prevalence relied on targeted selection of study animals, often in laboratory‐like environments or referral setting, which may reduce the generalisability of these findings to the wider dog population (Marshall et al. 2014, Stella et al. 2018, Wallis et al. 2018, Pereira dos Santos et al. 2019). In contrast, the current study included a random sample of dogs under primary veterinary care within the VetCompass Programme and should therefore be more representative of the general population of dogs (Bateson 2010). A study using primary care veterinary data from the USA reported higher dental disease prevalence than the current study, with 20.5% of dogs recorded with dental calculus and 19.5% recorded with gingivitis (Lund et al. 1999). None the less, the prevalence of 12.5% reported in the current study highlights periodontal disease as one of the most common disorders of dogs overall, with even more concerningly high prevalence values for many of the predisposed breeds highlighted. This high general prevalence exemplifies the significance of dental disease to general practice care and highlights the value of increasing the coverage of dental training in current veterinary undergraduate and postgraduate teaching (Anderson et al. 2017).
Periodontal disease prevalence tends to increase with age (Hamp et al. 1984, Harvey et al. 1994, Kyllar & Witter 2005, Kortegaard et al. 2008), with some authors suggesting that almost all dogs over the age of five years have some degree of periodontal disease (Hoffmann & Gaengler 1996). In line with this ageing effect, the median age of dogs in the current study with periodontal disease (7.54 years) was substantially higher than that of dogs without periodontal disease (3.90 years). The adjusted odds of periodontal disease also rose steeply with ageing by a factor of over 65 times; ranging from 0.06 times the adjusted odds in dogs aged under 1 year to 3.91 times the adjusted odds in dogs aged over 12 years, compared with dogs aged two to less than 4 years. With age being such an influential factor, veterinarians should be especially rigorous in assessing dental health during routine examinations in older dogs to promote earlier diagnosis and intervention. Greater emphasis on monitoring of dental health by veterinarians from puppyhood onwards, and discussion of at‐home dental care with owners, might help to reduce the welfare burden of periodontal disease later in life and thus improve the dog's quality of life (Roudebush et al. 2005).
Periodontal disease has long been linked to body size in dogs, with smaller breeds reportedly at greater risk than larger breeds (Harvey et al. 1994, Hoffmann & Gaengler 1996, Butković et al. 2001, Stella et al. 2018). Dental calculus has been reported in some small‐breed dogs as young as 1 year of age (Kyllar & Witter 2005). Breeds such as Yorkshire Terrier, Toy Poodle, Cocker Spaniel and Jack Russell Terrier are often cited as predisposed (Hamp et al. 1984, O'Neill et al. 2014). In the current study, the median adult bodyweight of dogs with periodontal disease (10.33 kg) was over 4 kg lighter than dogs without periodontal disease (14.90 kg). Dogs weighing under 10 kg had more than three times the adjusted odds of periodontal disease compared with dogs weighing 30 to less than 40 kg. In addition to any intrinsic genetic susceptibility, increased periodontal disease in smaller dogs may be associated with the challenges related to brushing the teeth of very small dogs, greater reluctance in smaller dogs to accept dental chews and a reputation for fussy eating habits (Mateo et al. 2020). Increased owner awareness of periodontal disease in smaller breeds should be promoted to ensure that effective preventative measures, such as tooth brushing, are put in place to reduce the likelihood of the onset of periodontal disease. It would also be valuable to explore reasons for non‐compliance by owners with current veterinary advice on routine tooth brushing in an effort to increase future compliance.
Breeds with the highest adjusted odds of periodontal disease in the current study included Toy Poodle, King Charles Spaniel, Greyhound, Cavalier King Charles Spaniel, Lhasa Apso and Yorkshire Terrier. Apart from the Greyhound, these are all small sized breeds. Conversely, many of the breeds with the lowest adjusted odds in the current study tended to be larger sized breeds, including Labrador Retriever, Staffordshire Bull Terrier and German Shepherd Dog. In an earlier study based on primary care clinical data, the least affected breeds were Border Collies (6.7% prevalence), German Shepherd Dogs (4.5%), Labrador Retrievers (3.2%) and Staffordshire Bull Terriers (2.4%) (O'Neill et al. 2014). Although the precise prevalence values in those studies differ slightly from the current study (Labrador Retriever 7.11%, German Shepherd Dog 3.30%, Staffordshire Bull Terrier 6.52%), these breeds are still seen to have a lower odds of periodontal disease diagnosis in the current study and could therefore be categorised as protected breeds. Dog skulls are generally classified into three distinct categories based on head shape profiles: dolichocephalic (“long‐headed”), mesocephalic (“middle‐headed”) and brachycephalic (“broad‐headed”), although there is ongoing debate on the precise allocation of breeds to each of the three skull categories (O'Neill et al. 2015, O'Neill et al. 2020a). Staffordshire Bull Terriers and Labrador Retrievers are almost universally categorised within the mesocephalic group, whereas German Shepherd Dogs are variously categorised as either mesocephalic or dolichocephalic. However, all three could be accepted to represent a skull shape with a more moderate conformation compared to the elongated skull of a Borzoi or the flattened brachycephalic faces of breeds such as Pugs and French Bulldogs (The Kennel Club 2021b). To date, much of the research on the effects of skull shape on the health of brachycephalic dog breeds has focused on respiratory disorders, neurological disease and ocular disease (Koch et al. 2003, O'Neill et al. 2015, Packer et al. 2015, Gordon et al. 2017, Liu et al. 2017, Knowler et al. 2019). However, there is also some evidence that brachycephalic breeds may also be predisposed to incisor overcrowding and that smaller breeds of dog are more susceptible to periodontal disease (Lund 2008, Burns 2016, Bellows et al. 2019). Dental occlusion varies considerably across skull shapes and, in extreme cases, the lower incisors can be up to 5 cm in front of the upper row (Emily & Penman 1994).
Although our results support a general predisposition for periodontal disease in brachycephalic dog types (1.25 times the adjusted odds compared with mesocephalic types), this effect was not universal across all brachycephalic breeds. French Bulldogs had a low prevalence of periodontal disease (just 2.01%) that is in line with the results from an earlier study on French Bulldogs based on UK primary‐care that did not even identify periodontal disease among the 26 most common disorders in French Bulldogs (O'Neill et al. 2018). Even after controlling for the effects of confounding from factors such as age, the current study still identified French Bulldogs as significantly protected to periodontal disease compared with crossbreds, showing just 0.43 times the adjusted odds. Evidence suggestive that this periodontal protective effect may be real in French Bulldogs comes from the results in the current study for the Pug, another small‐stature breed with extreme brachycephaly that has become very popular over the past 20 years (The Kennel Club 2021c). Although also featuring among the breeds with the lowest prevalence of periodontal disease (8.96%), the prevalence for Pugs was not nearly as low as the French Bulldog and, after controlling for confounding, there was no strong evidence of predisposition to periodontal disease in Pugs (aOR 1.17, 95% CI 0.81 to 1.71). A protective effect for periodontal disease in French Bulldogs could be an artefact influenced by diagnostic differences peculiar to this breed but such effects would also be expected to apply to other similar breeds such as the Pug. It is possible that high awareness of the presence of other health issues and concerns about anaesthetic risk in this breed reduce the primary care focus on routine dental examination. The relative youthfulness of the French Bulldog population compared with dogs overall may also lead to some residual confounding in the multivariable modelling (O'Neill et al. 2018). Equally, French Bulldogs may be truly protected to periodontal disease from effects such as breed‐related differences in food and chew stick manipulation such that French Bulldogs may benefit more from dental chews than other breeds. There is therefore scope for further research into chew efficacy in relation to breed (Mateo et al. 2020).
Although dolichocephaly was not associated with an overall increased adjusted odds of periodontal disease, certain dolichocephalic breeds such as the Greyhound (aOR 2.58) had higher adjusted odds of dental disease than other types of dog. A recent VetCompass study reported periodontal disease as the most prevalent disorder in Greyhounds (39% prevalence) (O'Neill et al. 2019c), which is slightly higher than the 32.21% prevalence reported in the current study, and supports a view that dental care should be a priority issue for Greyhounds. Predisposition to periodontal disease in Greyhounds may result in part because many of the Greyhounds seen in primary care settings are retirees from racing where maintenance of good of dental hygiene may not be deemed a priority for a working animal (EFRA 2016). Greyhound trainers often formulate their own diets composed predominantly of raw meat, which may not offer nutritional balance and could be deficient in vitamins and minerals, thus affecting dental hygiene (Hill 1998). The dolichocephalic shape of a Greyhound's skull could also put it at higher risk. With the elongation of the jaw, gaps between teeth could form, exposing more gum and allowing more food to build up between teeth. It is also possible that genetic factors are involved.
This is one of the first studies to explore periodontal disease occurrence in so‐called designer dog types, which are hybrids between differing parental purebred breeds. The results showed that designer breeds had 1.63 times the odds of diagnosis with periodontal disease compared with crossbreds and 1.44 times the odds compared with purebreds. However, the high level of periodontal disease identified in these designer types may be less to do with being designer per se and more to do with the poodle component that is common within many designer types such as the Cockapoo, Cavapoo and Labradoodle. Poodles are considered to be at high risk of periodontal disease (Hoffmann & Gaengler 1996, Dias et al. 2021), with the Toy Poodle identified as the breed with the highest odds of periodontal disease in the current study. These current results provide some evidence against a hybrid vigour effect in dogs that, if present, should have offered a protective effect against a polygenic disorder such as periodontal disease in these designer breeds (Nicholas et al. 2016).
The study had some limitations. Periodontal disease cases were defined based on a binary classification as cases or non‐cases but without further criteria such as severity, chronicity, clinical signs and diagnostics being considered in the modelling. As discussed above, it is possible that some dogs with true periodontal disease were not diagnosed clinically and may therefore have been omitted from our cases, leading to an underestimate of the true prevalence (Kim et al. 2013, Bauer et al. 2018, Queck et al. 2018). The specific type of dental disease is not always recorded in the clinical records and there are often inconsistencies in recording of the severity grading between veterinarians. Consequently, it was not always clear whether the clinical records referred to gingivitis (reversible) or periodontitis (irreversible) (Bauer et al. 2018), and therefore this distinction was not reported in the current study. The current study included clinical data gathered from a convenience sample of UK first opinion veterinary practices that participate within the VetCompass Programme. This may have selectively biased participation and therefore also the results. However, these effects should be somewhat mitigated by the large number of patients and the random sampling used for the dogs within these practices. The subset of the UK dog population that is unregistered with a veterinary practice was omitted from the study. Differential access to veterinary care could be breed‐related, whereby owners of some breeds may be more likely to seek veterinary intervention and therefore more likely to be included in studies such as the current. Such effects would reduce the generalisability of the current study to the wider UK dog population.
Periodontal disease is shown to be a common diagnosis in UK dogs, with one in eight dogs diagnosed annually. There are strong breed predispositions for periodontal disease, with Toy Poodle, Greyhound and Cavalier King Charles Spaniel at greatest risk while German Shepherd Dog, French Bulldog and Staffordshire Bull Terrier showed reduced risk. The study also highlights associations with conformation such as skull shape and body size. The risk of periodontal disease increases with ageing. Periodontal disease should be considered as a priority welfare concern for predisposed breeds and attention to good dental care is recommended for all dogs, especially as they age.
Funding
This study was supported at the RVC by an award from the Kennel Club Charitable Trust and Agria Pet Insurance. Neither the Kennel Club Charitable Trust, Agria Pet Insurance nor the Kennel Club had any input in the design of the study, the collection, analysis and interpretation of data or in writing the manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Supporting information
Appendix S1. Supporting Information.
Acknowledgements
Thanks to Noel Kennedy (RVC) for VetCompass™ software and programming development. We are grateful to the following researchers who assisted with data collection during this project: Alice Ashworth, Alison Toetz, Bethany Allen, Eleanor White, Elizabeth Ainsworth, Jasmine Broomhead, Penelope Crocker and Teresa Soares. The authors acknowledge the Medivet Veterinary Partnership, Vets4Pets/Companion Care, Goddard Veterinary Group, Independent Vet Care, Linnaeus Group, CVS Group, Beaumont Sainsbury Animal Hospital, Blue Cross, PDSA, Dogs Trust, Vets Now and the other UK practices who collaborate in VetCompass™. The authors are grateful to The Kennel Club Charitable Trust, The Kennel Club and Agria Pet Insurance for supporting VetCompass™.
References
- Anderson, J. G. , Goldstein, G. , Boudreaux, K. , et al. (2017) The state of veterinary dental education in North America, Canada, and the Caribbean: a descriptive study. Journal of Veterinary Medical Education 44, 358‐363 [DOI] [PubMed] [Google Scholar]
- Bartlett, P. C. , van Buren, J. W. , Neterer, M. , et al. (2010) Disease surveillance and referral bias in the veterinary medical database. Preventive Veterinary Medicine 94, 264‐271 [DOI] [PubMed] [Google Scholar]
- Bateson, P. (2010) Independent Inquiry into Dog Breeding. University of Cambridge, Cambridge, UK. https://dogwellnet.com/files/file/308‐independent‐inquiry‐into‐dog‐breeding‐2010‐patrick‐bateson/ Accessed July 27, 2021 [Google Scholar]
- Bauer, A. E. , Stella, J. , Lemmons, M. , et al. (2018) Evaluating the validity and reliability of a visual dental scale for detection of periodontal disease (PD) in non‐anesthetized dogs (Canis familiaris). PLoS One 13, e0203930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows, J. , Berg, M. L. , Dennis, S. , et al. (2019) 2019 AAHA dental care guidelines for dogs and cats. Journal of the American Animal Hospital Association 55, 49‐69 [DOI] [PubMed] [Google Scholar]
- Burns, K. (2016) Below the surface of anesthesia free dentistry. Journal of the American Veterinary Medical Association 248, 242‐258 [Google Scholar]
- Butković, V. , Šehič, M. , Stanin, D. , et al. (2001) Dental diseases in dogs: a retrospective study of radiological data. Acta Veterinaria Brno 70, 203‐208 [Google Scholar]
- BWG . (2021) The Brachycephalic Working Group . http://www.ukbwg.org.uk/. Accessed February 14, 2021
- de Campos Andrade, P. M. M. , Morgado, T. O. , Mallmann, P. R. , et al. (2015) Occurrence of oral diseases in neotropical wild carnivores kept in captivity at the zoo from Federal University of Mato Grosso–Cuiabá. Semina: Ciências Agrárias 36, 2633‐2639 [Google Scholar]
- DeBowes, L. J. , Mosier, D. , Logan, E. , et al. (1996) Association of periodontal disease and histologic lesions in multiple organs from 45 dogs. Journal of Veterinary Dentistry 13, 57‐60 [PubMed] [Google Scholar]
- Dias, F. G. G. , de Oliveira, A. R. , Dias, L. G. G. G. , et al. (2021) Oral disorders in animals seen in the dentistry sector of the University of Franca and prevention in the control of local and systemic impairments. Research, Society and Development 10, e25310212485 [Google Scholar]
- Dohoo, I. , Martin, W. & Stryhn, H. (2009) Veterinary Epidemiologic Research. VER Inc., Charlottetown, Canada: [Google Scholar]
- Drake, A. G. & Klingenberg, C. P. (2010) Large scale diversification of skull shape in domestic dogs: disparity and modularity. The American Naturalist 175, 289‐301 [DOI] [PubMed] [Google Scholar]
- EFRA (2016) Greyhound welfare. In: The Environment. Ed F. A. R. A. C . House of Commons, London, UK: [Google Scholar]
- Emily, P. & Penman, S. (1994) Handbook of Small Animal Dentistry. Pergamon Press Ltd, Oxford, UK: [Google Scholar]
- EPI INFO CDC (2021) Centers for Disease Control and Prevention (US): Epi Info. CDC, Atlanta, Georgia. https://www.cdc.gov/epiinfo/index.html Accessed February 21, 2021 [Google Scholar]
- Glickman, L. T. , Glickman, N. W. , Moore, G. E. , et al. (2009) Evaluation of the risk of endocarditis and other cardiovascular events on the basis of the severity of periodontal disease in dogs. Journal of the American Veterinary Medical Association 234, 486‐494 [DOI] [PubMed] [Google Scholar]
- Gordon, C. , Marioni, K. , Amengual, P. , et al. (2017) MRI‐based morphometric analysis in relation to Chiari‐like malformation in brachycephalic canine breeds. Vlaams Diergeneeskundig Tijdschrift 86, 351‐360 [Google Scholar]
- Gordon, S. M. , Camargo, G. A. , Mejia, G. C. , et al. (2018) Use of the dental electronic health record for research: assessing demographic and oral health characteristics data for clinic patients. Journal of Dental Education 82, 1249‐1257 [DOI] [PubMed] [Google Scholar]
- Gough, A. , Thomas, A. & O'Neill, D. (2018) Breed Predispositions to Disease in Dogs and Cats. Wiley‐Blackwell, Chichester, West Sussex: [Google Scholar]
- Hamp, S.‐E. , Olsson, S.‐E. , Farsø‐Madsen, K. , et al. (1984) A macroscopic and radiologic investigation of dental diseases of the dog. Veterinary Radiology 25, 86‐92 [Google Scholar]
- Harvey, C. , Shofer, F. & Laster, L. (1994) Association of age and body weight with periodontal disease in north American dogs. Journal of Veterinary Dentistry 11, 94‐105 [PubMed] [Google Scholar]
- Hill, R. C. (1998) The nutritional requirements of exercising dogs. The Journal of Nutrition 128, 2686S‐2690S [DOI] [PubMed] [Google Scholar]
- Hoffmann, T. & Gaengler, P. (1996) Epidemiology of periodontal disease in poodles. Journal of Small Animal Practice 37, 309‐316 [DOI] [PubMed] [Google Scholar]
- Hosmer, D. W. , Lemeshow, S. & Sturdivant, R. X. (2013) Applied Logistic Regression. Wiley, Hoboken, NJ, USA: [Google Scholar]
- Janssens, L. , Verhaert, L. , Berkowic, D. , et al. (2016) A standardized framework for examination of oral lesions in wolf skulls (Carnivora: Canidae: Canis lupus). Journal of Mammalogy 97, 1111‐1124 [Google Scholar]
- Kim, C.‐G. , Lee, S.‐Y. , Kim, J.‐W. , et al. (2013) Assessment of dental abnormalities by full‐mouth radiography in small breed dogs. Journal of the American Animal Hospital Association 49, 23‐30 [DOI] [PubMed] [Google Scholar]
- Kirkwood, B. R. & Sterne, J. A. C. (2003) Essential Medical Statistics. Blackwell Science, Oxford: [Google Scholar]
- Knowler, S. P. , Dumas, E. , Spiteri, M. , et al. (2019) Facial changes related to brachycephaly in Cavalier King Charles Spaniels with Chiari‐like malformation associated pain and secondary syringomyelia. Journal of Veterinary Internal Medicine 34, 237‐246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, D. A. , Arnold, S. , Hubler, M. , et al. (2003) Brachycephalic syndrome in dogs. Compendium on Continuing Education for the Practising Veterinarian ‐ North American Edition 25, 48‐55 [Google Scholar]
- Kortegaard, H. E. , Eriksen, T. & Baelum, V. (2008) Periodontal disease in research beagle dogs – an epidemiological study. Journal of Small Animal Practice 49, 610‐616 [DOI] [PubMed] [Google Scholar]
- Kyllar, M. & Witter, K. (2005) Prevalence of dental disorders in pet dogs. Veterinární Medicína 50, 496‐505 [Google Scholar]
- Liu, N.‐C. , Troconis, E. L. , Kalmar, L. , et al. (2017) Conformational risk factors of brachycephalic obstructive airway syndrome (BOAS) in pugs, French bulldogs, and bulldogs. PLoS One 12, e0181928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, E. (2008) Using data to understand periodontal disease risk. Banfield Journal, January/February, 15‐20 [Google Scholar]
- Lund, E. M. , Armstrong, P. J. , Kirk, C. A. , et al. (1999) Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. Journal of the American Veterinary Medical Association 214, 1336‐1341 [PubMed] [Google Scholar]
- Marshall, M. , Wallis, C. , Milella, L. , et al. (2014) A longitudinal assessment of periodontal disease in 52 miniature schnauzers. BMC Veterinary Research 10, 166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo, A. , Torre, C. , Crusafont, J. , et al. (2020) Evaluation of efficacy of a dental chew to reduce gingivitis, dental plaque, calculus, and halitosis in toy breed dogs. Journal of Veterinary Dentistry 37, 22‐28 [DOI] [PubMed] [Google Scholar]
- McGreevy, P. D. , Wilson, B. J. , Mansfield, C. S. , et al. (2018) Labrador retrievers under primary veterinary care in the UK: demography, mortality and disorders. Canine Genetics and Epidemiology 5, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas, F. W. , Arnott, E. R. & McGreevy, P. D. (2016) Hybrid vigour in dogs? The Veterinary Journal 214, 77‐83 [DOI] [PubMed] [Google Scholar]
- Niemiec, B. , Gawor, J. , Nemec, A. , et al. (2020) World small animal veterinary association global dental guidelines. Journal of Small Animal Practice 61, E36‐E161 [DOI] [PubMed] [Google Scholar]
- Ed Niemiec, B. A. (2013) Veterinary Periodontology. John Wiley & Sons, Ames, IA, USA: [Google Scholar]
- O'Neill, D. , Coulson, N. R. , Church, D. B. , et al. (2017a) Demography and disorders of German Shepherd Dogs under primary veterinary care in the UK. Canine Genetics and Epidemiology 4, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Ballantyne, Z. F. , Hendricks, A. , et al. (2019a) West Highland White Terriers under primary veterinary care in the UKin 2016: demography, mortality and disorders. Canine Genetics and Epidemiology 6, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Baral, L. , Church, D. B. , et al. (2018) Demography and disorders of the French Bulldog population under primary veterinary care in the UKin 2013. Canine Genetics and Epidemiology 5, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Butcher, C. , Church, D. B. , et al. (2019b) Miniature Schnauzers under primary veterinary care in the UKin 2013: demography, mortality and disorders. Canine Genetics and Epidemiology 6, 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Church, D. B. , McGreevy, P. D. , et al. (2014) Prevalence of disorders recorded in dogs attending primary‐care veterinary practices in England. PLoS One 9, 1‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Darwent, E. C. , Church, D. B. , et al. (2017b) Border terriers under primary veterinary care in England: demography and disorders. Canine Genetics and Epidemiology 4, 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Jackson, C. , Guy, J. H. , et al. (2015) Epidemiological associations between brachycephaly and upper respiratory tract disorders in dogs attending veterinary practices in England. Canine Genetics and Epidemiology 2, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Packer, R. M. A. , Lobb, M. , et al. (2020a) Demography and commonly recorded clinical conditions of Chihuahuas under primary veterinary care in the UKin 2016. BMC Veterinary Research 16, 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Pegram, C. , Crocker, P. , et al. (2020b) Unravelling the health status of brachycephalic dogs in the UKusing multivariable analysis. Scientific Reports 10, 17251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Rooney, N. J. , Brock, C. , et al. (2019c) Greyhounds under general veterinary care in the UKduring 2016: demography and common disorders. Canine Genetics and Epidemiology 6, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, D. G. , Seah, W. Y. , Church, D. B. , et al. (2017c) Rottweilers under primary veterinary care in the UK: demography, mortality and disorders. Canine Genetics and Epidemiology 4, 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer, R. M. A. , Hendricks, A. & Burn, C. C. (2015) Impact of facial conformation on canine health: corneal ulceration. PLoS One 10, 1‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlica, Z. (2008) Periodontal disease burden and pathological changes in organs of dogs. Journal of Veterinary Dentistry 25, 97‐105 [DOI] [PubMed] [Google Scholar]
- Pegram, C. , Wonham, K. , Brodbelt, D. C. , et al. (2020) Staffordshire Bull terriers in the UK: their disorder predispositions and protections. Canine Medicine and Genetics 7, 13 [Google Scholar]
- Pereira dos Santos, J. D. , Cunha, E. , Nunes, T. , et al. (2019) Relation between periodontal disease and systemic diseases in dogs. Research in Veterinary Science 125, 136‐140 [DOI] [PubMed] [Google Scholar]
- Queck, K. E. , Chapman, A. , Herzog, L. J. , et al. (2018) Oral‐fluid thiol‐detection test identifies underlying active periodontal disease not detected by the visual awake examination. Journal of the American Animal Hospital Association 54, 132‐137 [DOI] [PubMed] [Google Scholar]
- Robinson, N. J. , Brennan, M. L. , Cobb, M. , et al. (2016) Investigating preventive‐medicine consultations in first‐opinion small‐animal practice in the United Kingdom using direct observation. Preventive Veterinary Medicine 124, 69‐77 [DOI] [PubMed] [Google Scholar]
- Roudebush, P. , Logan, E. & Hale, F. A. (2005) Evidence‐based veterinary dentistry: a systematic review of homecare for prevention of periodontal disease in dogs and cats. Journal of Veterinary Dentistry 22, 6‐15 [DOI] [PubMed] [Google Scholar]
- Ruparell, A. , Wallis, C. , Haydock, R. , et al. (2021) Comparison of subgingival and gingival margin plaque microbiota from dogs with healthy gingiva and early periodontal disease. Research in Veterinary Science 136, 396‐407 [DOI] [PubMed] [Google Scholar]
- Scott, M. , Flaherty, D. & Currall, J. (2012) Statistics: how many? Journal of Small Animal Practice 53, 372‐376 [DOI] [PubMed] [Google Scholar]
- Soe, E. , Davison, J. , Süld, K. , et al. (2017) Europe‐wide biogeographical patterns in the diet of an ecologically and epidemiologically important mesopredator, the red fox Vulpes vulpes: a quantitative review. Mammal Review 47, 198‐211 [Google Scholar]
- Stella, J. L. , Bauer, A. E. & Croney, C. C. (2018) A cross‐sectional study to estimate prevalence of periodontal disease in a population of dogs (Canis familiaris) in commercial breeding facilities in Indiana and Illinois. PLoS One 13, e0191395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers, J. F. , O'Neill, D. G. , Church, D. , et al. (2019) Health‐related welfare prioritisation of canine disorders using electronic health records in primary care practice in the UK. BMC Veterinary Research 15, 163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Kennel Club . (2021a) Breed Health and Conservation Plans (BHCPs) . The Kennel Club Limited. https://www.thekennelclub.org.uk/health/breed‐health‐and‐conservation‐plans/. Accessed February 21, 2021
- The Kennel Club . (2021b) Breed Information Centre . The Kennel Club Limited. https://www.thekennelclub.org.uk/search/breeds‐a‐to‐z. Accessed February 21, 2021
- The Kennel Club . (2021c) Breed Registration Statistics . The Kennel Club Limited. https://www.thekennelclub.org.uk/media‐centre/breed‐registration‐statistics/. Accessed February 28, 2021
- The Venom Coding Group . (2021) VeNom Veterinary Nomenclature . VeNom Coding Group. http://venomcoding.org. Accessed February 21, 2021
- VetCompass (2021) VetCompass Programme. RVC Electronic Media Unit, London, UK. http://www.rvc.ac.uk/VetCOMPASS/ Accessed February 7, 2021 [Google Scholar]
- Wallis, C. & Holcombe, L. J. (2020) A review of the frequency and impact of periodontal disease in dogs. Journal of Small Animal Practice 61, 529‐540 [DOI] [PubMed] [Google Scholar]
- Wallis, C. , Patel, K. V. , Marshall, M. , et al. (2018) A longitudinal assessment of periodontal health status in 53 Labrador retrievers. Journal of Small Animal Practice 59, 560‐569 [DOI] [PubMed] [Google Scholar]
- Wayne, R. K. , Leonard, J. A. & Vila, C. (2006) Genetic analysis of dog domestication. In: Documenting Domestication: New Genetic and Archaeological Paradigms. Ed Zeder M. A.. University of California Press, Berkeley, CA, USA: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


