Abstract
Objectives
Skin rejuvenation with radiofrequency has been a widely used treatment modality for the safe and efficient remodeling of the dermis and revision of textural irregularities, achieved with minimal downtime. The efficacy of fractional radiofrequency (FRF) specifically for acne scarring has not been widely established. The objective of this clinical trial was to establish the efficacy and safety of FRF for moderate to severe acne scarring in a wide range of Fitzpatrick skin types using two different applicator tips to deliver energy to the skin (80‐pin of up to 124 mJ/pin and 160‐pin of up to 62 mJ/pin).
Methods
Enrolled subjects received a series of three FRF treatments to the full face, each 4 weeks apart. A visual analog scale was utilized to assess pain of the treatment. Subject satisfaction questionnaires were completed at follow‐up visits at 6 and 12 weeks post final treatment. Photographs were graded for change by three blinded evaluators using the Global Aesthetic Improvement Scale (GAIS).
Results
Image sets of 23 enrolled subjects were assessed by blinded evaluation, showing a statistically significant improvement (p = 0.009) from the baseline visit to the 12‐week follow‐up on the GAIS for acne scarring. Subject satisfaction was high with subjects giving an average satisfaction score of 3.27 (“satisfied”) out of 4. Pain was “mild” as treatments were rated an average of 2.15 on a 10‐point visual analog scale. The GAIS score of the 80‐pin tip improved patients' acne scars treated with that applicator by 1.06 points and 0.85 for the 160‐pin tip. Ninety‐five percent (95.5%) of subjects reported either a mild, moderate, or significant improvement to their treatment area. Ninety‐one percent of subjects reported that they would recommend the treatment to a friend.
Conclusion
FRF produced a statistically significant improvement in acne scarring when assessed by independent blinded evaluators. No serious adverse events resulted from treatment by either applicator tip. Treatment pain was low and tolerable among subjects of all Fitzpatrick skin types. Subjects had high levels of satisfaction with the results.
Keywords: acne scars, acne vulgaris, fractional radiofrequency
INTRODUCTION
Acne vulgaris is among the most common dermatological conditions worldwide. 1 It is a chronic and common inflammatory condition of the pilosebaceous unit, producing inflammatory and noninflammatory lesions chiefly on the face. 2 , 3 , 4 A frequent complication of acne vulgaris is scarring. Acne scarring can occur when a lesion experiences trauma, which leads to cutaneous inflammation that initiates an altered wound healing response, leading to an imbalance in matrix degradation and collagen biosynthesis. The end result is either an excess or diminished deposition of collagen, which produces the appearance of scarring. 5 , 6 , 7 Acne scarring may also develop de novo, the rupture and breakdown of inflammatory red papules, pustules, and deep‐seated subcutaneous abscesses in the deep dermis can lead to erosion and ulceration, which can also result in scar formation. 8 Severe scarring has been reported in approximately 30% of acne patients, whereas mild to moderate scarring has been reported in up to 95% of these patients. 9
The impact acne scarring can have on the quality of life is profound. It can be a considerable source of physical and psychological distress, sometimes increasing the incidence of depression and other mental health disorders. 10 , 11 This can often lead to a loss of self‐esteem and stigmatization of the patient, frequently resulting in a diminished quality of life. 12 , 13 , 14 One study found that people who are looking at photos of individuals with acne scars, find them less successful, less attractive, and shyer. 15 Beyond the psychological impact, scars can also be associated with disruptions in daily activities (severe itching, tenderness, pain, and sleep disturbances), imploring the necessity for more effective treatment solutions for this common aesthetic indication. 16
There are a variety of treatment modalities available to treat acne scarring, one of them being fractional radiofrequency (FRF). FRF uses an array of electrodes that produce ablative and coagulative microthermal injuries to the epidermis and dermis with interspersed areas of unaffected skin. This provokes a significant dermal wound healing response, resulting in fibroblast stimulation and subsequent collagen remodeling. This is demonstrated by the increased levels of Type I and Type III procollagen and elastin found in skin biopsy samples, post FRF treatment. 17 The added collagen volume, the improvement in elasticity, along with melanin/erythema index, contributes to the improvement of acne scars.
FRF uses multiple different electrode arrays (or pins) to heat the tissue, none of which have been studied in clinical trials. Also, the effect of different energies, related ablation, and coagulation leading to resurfacing impact have not been evaluated in treating different severity of acne scars. The objective of this clinical trial was to establish the efficacy and safety of FRF for moderate to severe acne scarring in a variety of skin types, using two different noninvasive applicator tips and related emitted energies to the skin (80‐ and 160‐pin).
MATERIALS AND METHODS
Participants
This study was a prospective, open‐label, single‐arm clinical study conducted at one clinical center between November 2018 and November 2019. The study protocol complied with the CONSORT 2010 statement for reporting randomized controlled trial (see Supporting Information) and the trial was conducted according to the Declaration of Helsinki and all its revisions. It was approved by the Institutional Review Board Advarra (IRB approval number: Pro00029469) and registered to the ClinicalTrials.gov Registry (ID number: NCT03767153). All subjects provided written informed consent to participate in the trial.
Male or female subjects who met the inclusion criteria for FRF treatment of acne scarring were studied. Subjects 22 to 71 years old and Fitzpatrick skin types (I–VI) were enrolled. Exclusion criteria included the presence of pacemaker or defibrillator, metal implants in treated anatomy, pregnancy, planning pregnancy, lactating, any past or current significant systemic illness, illness localized in the area of treatment, therapies, or medication that may have interfered with the treatment or healing process, recent surgery in the treatment area, and acute or chronic infection in the area. Women of childbearing age were required to use a reliable method of birth control at least 3 months before study enrollment.
Description of treatment
Skin in the treatment area was cleansed and dried before treatment. Treatments were performed using the Venus Viva™ (Venus Concept Inc.). To begin the treatment, subjects lay in a supine position. Topical lidocaine 23%/tetracaine 7% was applied for 30 min. The topical anesthetic was removed, and the skin cleaned with mild, lipid free cleanser. The distal section of the applicator on the device was cleaned and fitted with a new tip (160‐ or 80‐pin) per practitioner preference. The 160‐pin tip is generally used for mild disease, where the 80‐pin tip is used for more severe disease. The applicator then was held perpendicular to and in close contact with the skin surface for the duration of the treatment. Treatment consisted of a single pass over the entire face and two or three passes over severe scars in the treatment area. The full range of energies available were 215 to 271 V, pulse duration: 18–30 ms (energy 80‐pin up to 124 mJ/pin and 160‐pin up to 62 mJ/pin). The voltage used in this study ranged from 240 to 270 V, with the first treatments being lower and increasing over the course of the three treatments. The pulse duration was always at 30 ms. Treatment parameters such as voltage and pulse duration, were determined at the discretion of the primary investigator (Stephen W. Eubanks), response to treatment will vary based on skin type and severity of disease. Nothing was applied to the treatment area immediately after the treatment. Furthermore, subjects were advised to not apply anything to the treatment area, to avoid possible thermal or mechanical damage for the 24 h period after the treatment. Subjects were also instructed to use a high factor of sunscreen (SPF ≥ 30) to protect the treated area from direct sunlight beginning the next day and for the entire period of the study.
Outcome measures
Evaluations were conducted using clinical photography as well as investigator and patient assessments. At each treatment and follow‐up visit, standardized photographs were taken at 0° and 45° from both sides using the Accuview Imaging System. All photographs were taken with standardized photography equipment including stool height and anatomical alignment, illumination, and background throughout the study.
The primary outcome measure was an improvement in acne scarring at 6‐ and 12‐weeks posttreatment compared to baseline as assessed by treatment blinded evaluators by photographic assessment utilizing the five‐grade Global Aesthetic Improvement Scale (GAIS). GAIS is a 5‐point scale rating global aesthetic improvement in appearance, compared to pretreatment, as judged by the investigator. The rating categories were “worse,” “no change,” “improved,” “much improved,” and “very much improved.” GAIS was measured at baseline and during follow‐up, with results compared to the baseline pretreatment photographs. Blinded physicians were chosen at random as evaluators and were sent randomized images and asked to grade the change between the pre‐ and posttreatment images, using the GAIS measure. Secondary performance outcomes were the subjects' assessments of satisfaction with the treatment using a Subject Satisfaction Scale (SSS) at 6‐ and 12‐weeks posttreatment. Subject satisfaction was evaluated with the following 5‐point Likert scale: (4) Very satisfied, (3) satisfied, (2) no opinion, (1) unsatisfied, (0) very unsatisfied. Immediately after each treatment, subject discomfort was assessed using a 10 cm Visual Analog Scale (VAS) 18 , 19 on a scale from 0 cm (no pain) and 10 cm (pain as bad as it can be). Subjects were not permitted to view their previous VAS or SSS treatment scores. All adverse events (AEs) were recorded up to the 12‐week posttreatment visit.
Statistical analysis
Quantitative data are presented as mean, median, and/or range, whereas data are presented as percentage (%). Unless otherwise stated, standard error (SE) was shown. Two‐sided Student's paired t test was used to test for changes between two points baseline data compared to the mean of three graders at the follow‐up visits at 6 and 12 weeks after the last treatment. p < 0.05 were considered statistically significant.
RESULTS
Patient demographics
Twenty‐five subjects were enrolled and completed the study, two were lost to follow‐up, therefore, 23 subjects completed treatment. Of these, 14 (56%) were treated with the 80‐pin tip and 11 (44%) were treated with the 160‐pin. The mean age and standard deviation (SD) at study consent were 45.4 ± 11.6 years. Twenty‐one subjects (84%) were female and four (16%) were male. Four (4) subjects had Fitzpatrick's skin type II (16%), 10 had type III (40%), 7 had type IV (28%), 2 had type V (8%), and 2 had type VI (8%) (Table 1 and Figure 1). Two subjects were lost to follow‐up.
Table 1.
Demographic data of participants
| Demographic data | Results (N = 25) |
|---|---|
| Age, mean (SD) (years) | 45.4 (11.6) |
| Age, range (years) | 26–71 |
| Gender, n (%) | |
| Female | 21 (84) |
| Male | 4 (16) |
| Race, n (%) | |
| Caucasian | 19 (76) |
| Black or African descent | 3 (12) |
| American Indian/Caucasian | 1 (4) |
| Asian | 1 (4) |
| Asian/Caucasian | 1 (4) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 23 (92) |
| Hispanic or Latino | 2 (8) |
| Fitzpatrick skin type, n (%) | |
| I | 0 (0) |
| II | 4 (16) |
| III | 10 (40) |
| IV | 7 (28) |
| V | 2 (8) |
| VI | 2 (8) |
| Applicator type, n (%) | |
| 80‐pin | 14 (56) |
| 160‐pin | 11 (44) |
Figure 1.
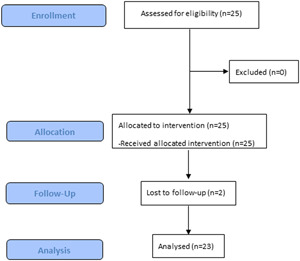
Study overview. A flow chart representing patient enrollment
Primary outcomes: GAIS
Three blinded evaluators assessed GAIS and reported a significant improvement from baseline to an average of 0.95 at 12 weeks (SE 0.11) (p = 0.009). When stratified by tips at 12 weeks, on average, the GAIS improved 1.06 (0.13) for the 80‐pin and 0.85 (0.13) for the 160‐pin tip (p = 0.12) (Figure 2). There was no correlation between any demographic subgroup and GAIS. The inter‐rater statistics between the three graders was slight to fair agreement (K values: grader 1 and 2 = 0.12, grader 2 and 3 = 0.21, and grader 1 and 3 = 0.16).
Figure 2.
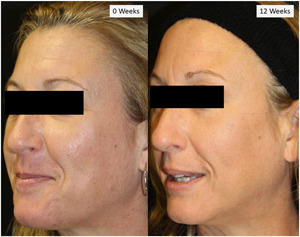
Acne scar progression at baseline (0 weeks) and after final treatment (12 weeks)
Secondary outcomes: Subject satisfaction
Subjects were consistently satisfied with their treatment, with mean scores of 3.39 (SE 0.14) at 6 weeks and 3.27 (0.15) at 12 weeks after the last treatment. At 6‐week follow‐up, 91.3% reported being “satisfied” (43.5%) or “very satisfied” (47.8%), with the remaining 8.7% reporting they had “no opinion.” No subjects reported being “unsatisfied” or “very unsatisfied.” By the 12‐week follow‐up visit, 86.4% of the subjects reported satisfaction due to their treatments, of which 40.9% reported being “very satisfied.” “No opinion” was reported by 13.6% of subjects. No subjects reported dissatisfaction during the study (Figure 3).
Figure 3.
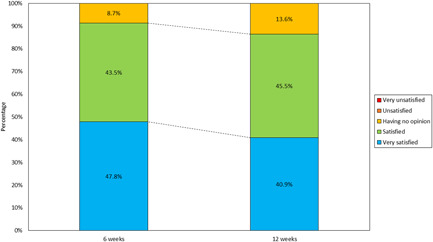
Subject Satisfaction Score distribution at 80‐ and 160‐pin after 12‐week follow‐up
Over 95% (95.5%) of subjects reported either a mild (27.3%), moderate (59.1%), or significant (9.0%) improvement to their treatment areas at 12 weeks and 91.0% of subjects would recommend this treatment to a friend (Table 2).
Table 2.
Subject evaluation of treatment effects measured by a Treatment Evaluation Questionnaire
| Question | Answer | 6 weeks, n (%) | 12 weeks, n (%) |
|---|---|---|---|
| Did the patient notice changes in skin? | Yes | 22 (95.6) | 21 (95.5) |
| No | 1 (4.4) | 1 (4.5) | |
| When did the patient notice changes in skin? | During treatment | 12 (52.2) | 7 (31.2) |
| End of treatment | 3 (13.0) | 5 (22.7) | |
| Never | 1 (4.3) | 1 (4.5) | |
| After treatment | 7 (30.4) | 0 (0.0) | |
| Level of improvement? | No change | 1 (4.3) | 1 (4.5) |
| Mild | 10 (43.5) | 6 (27.3) | |
| Moderate | 9 (39.1) | 13 (59.1) | |
| Significant | 3 (13.0) | 2 (9.0) | |
| What were the specific skin changes? | Smoother skin | 17 (73.9) | 15 (68.2) |
| Softer skin | 10 (43.5) | 7 (31.8) | |
| Firmness | 7 (30.4) | 10 (45.5) | |
| Improvement | 11 (47.8) | 10 (45.5) | |
| Specific facial areas of improvement? | Forehead | 6 (26.1) | 7 (31.8) |
| Nose | 4 (17.4) | 6 (27.3) | |
| Cheek | 18 (78.3) | 15 (68.2) | |
| Chin | 7 (30.4) | 6 (27.3) | |
| Overall skin texture improvement | 1 (4.3) | 0 (0.0) | |
| Overall lighter skin | 1 (4.3) | 0 (0.0) | |
| Would the patient recommend treatment? | Yes | 20 (87.0) | 20 (91.0) |
| No | 3 (13.0) | 2 (9.0) |
Safety outcomes: Visual Analog Scale, tolerability, and AEs
The treatments were well tolerated at all treatment sessions, and there was no difference in the discomfort or pain VAS scores at treatment three visits compared to the first treatment visit (p = 0.25) in both the 80‐ and 160‐pin. VAS for all three treatments was rated an average of 2.15 out of 10 for both the 80‐ and 160‐pin. There were no reports of AEs or unanticipated side effects during the duration of the study (Figure 4).
Figure 4.
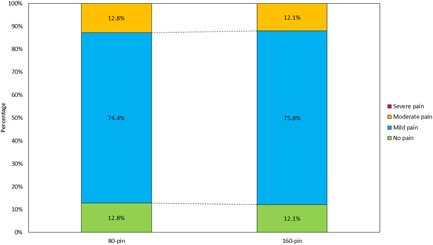
Visual Analog Scale distribution between 80‐ and 160‐pin
DISCUSSION
This was a prospective, evaluator‐blinded study of the safety and performance of FRF for the treatment and reduction of acne scarring in 25 subjects. The treated acne scars demonstrated significant improvement in their appearance as measured by the primary outcome measure, as assessed by the evaluators blinded to the treatment who reviewed the before and after treatment images. None of the subjects experienced any AEs, pain was rated low, and subjects were very satisfied with the treatment and outcomes achieved.
There are a variety of treatment methods and options available for the treatment of acne scarring, however, there are very few studies based on high‐quality evidence evaluating acne scar management. 20 The traditional method of treating acne scarring uses ablative lasers (e.g., carbon dioxide laser, erbium:yttrium‐aluminum‐garnet laser). 21 , 22 , 23 , 24 However, subjects report recovery time following this treatment tends to be lengthy (at least 1 week or more) and side effects are severe (pain, persistent erythema, edema, infection, postinflammatory hyperpigmentation, and hypopigmentation). 25 , 26 This has led to the development of technologies with a more favorable profile, such as FRF. FRF devices have been proven to decrease the propensity for postinflammatory hyperpigmentation and the aforementioned problems of ablative lasers, all while achieving a similar level of acne scar reduction. 13 , 27 , 28 The efficacy of FRF compared to other treatment modalities for acne scarring has previously been investigated in a Cochrane review. 29 Results indicated that FRF was as effective as ablative lasers in reducing acne scars (results showed no statistically significant difference between FRF and ablative lasers 30 , 31 , 32 ), but more effective than nonfractional and nonablative methods. 29 Additionally, when FRF was compared to ablative lasers, more pain with ablative lasers was noticed, as was a higher incidence of AEs. 31 , 32 Furthermore, FRF energy, unlike lasers, is not absorbed by chromophores such as melanin, making this modality potentially safer in individuals with dark skin. 33 , 34 The key advantage of the FRF modalities is their low profile of side effects (especially that of hyperpigmentation) and the low downtime associated with their use. 35 , 36
Several smaller studies have also proven the effectiveness of FRF in treating acne scarring. Sadick et al., 37 used an FRF system to treat twenty‐six subjects with facial wrinkles and acne scars, a significant reduction in the depth of acne scars was noted 4 weeks after therapy with further improvement at the 3‐month follow‐up. Another study used a multielectrode bipolar FRF device to treat mild to moderate acne scars, after three treatment sessions results showed “much improvement” in acne scarring in 60% of subjects and “improvement” in 30%. 38 Baskan et al., 39 treated nine subjects with facial acne atrophic scars with a microneedle FRF device and a clinical improvement of more than 25% was reported in almost all the subjects. Another study used a bipolar microneedle FRF device in a double‐pass technique, results showed improvement in acne scars in 70% of the subjects after 8 weeks. 40
In our analysis, the 80‐pin applicator tip reported slightly better GAIS scores when compared to the 160‐pin (1.06 vs. 0.85), suggesting the 80‐pin tip may potentially be useful in more severe cases of acne scarring. Although the difference was not statistically significant, with more participants, a significant effect may be noted. The 80‐pin tip can deliver double the amount of energy per pin compared to the 160‐pin tip (up to 124 mJ/pin for the 80‐pin tip compared to up to 62 mJ/pin for the 160‐pin tip) which results in increased depth of ablation and enhances treatment outcomes. A histology done on pig skin supports this theory, reporting that the 80‐pin applicator produced approximately 35% more ablation immediately following treatment than the 160‐pin applicator (Figure 5A,B). More severe cases of acne scarring may benefit from the increased ablation depth. Satisfaction and tolerability were consistent between groups and over the two follow‐up visits; the satisfaction scores at the 6‐week follow‐up did not differ significantly from the 12‐week follow‐up (80‐pin: p = 0.40 vs. 160‐pin: p = 0.29). Furthermore, the pain was rated low (2.15 out of 10) and did not differ between the groups (p = 0.32). Less severe cases of scarring may benefit from the 160‐pin tip, as subjects were also satisfied with the outcomes. Further research on this topic is warranted.
Figure 5.
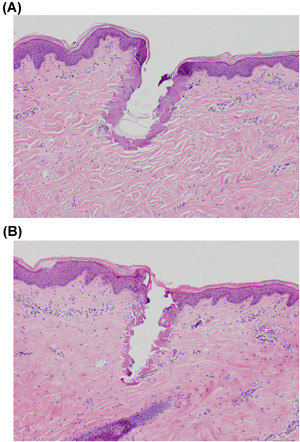
(A) Histology sample done on pig skin, showing the depth of ablation between the 80‐pin applicator, settings were 280 V and 28 ms. (B) Histology sample done on pig skin, showing the depth of ablation between the 160‐pin, settings were 280 V and 28 ms
Limitations of the study included the relatively small sample size that limited the power of the study and the ability to show a significant difference in efficacy between the 80‐ and 160‐pin tips. Additionally, a shorter time follow‐up period of GAIS could have shown results much faster than at 12 weeks, and a longer follow‐up period to fully assess the longevity of all the outcomes (e.g., 6 or 12 months after treatment) would be interesting.
CONCLUSIONS
In conclusion, our results suggest that FRF is effective in the treatment of acne scars in subjects of all skin types, without significant AEs. Furthermore, FRF treatments were safe with no AEs and subjects had limited downtime as the treatments allow for quick recovery times. The FRF device may be a viable alternative for fractional laser devices for the treatment of acne scars for subjects looking for shorter recovery times and looking to avoid the drawbacks of fractional laser treatments.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors would like to thank Rachel R. Mays for assistance in the writing of this manuscript. Venus Concept Inc. paid for the costs of the study.
Eubanks SW, Solomon JA. Safety and efficacy of fractional radiofrequency for the treatment and reduction of acne scarring: a prospective study. Lasers Surg Med. 2022;54:74–81. 10.1002/lsm.23453
REFERENCES
- 1. Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, Dessinioti C, Feldman SR, Zouboulis CC. Acne vulgaris. Nat Rev Dis Primers. 2015;1:15029. [DOI] [PubMed] [Google Scholar]
- 2. Juhl CR, Bergholdt HKM, Miller IM, Jemec GBE, Kanters JK, Ellervik C. Dairy intake and acne vulgaris: a systematic review and meta‐analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10(8):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. George RM, Sridharan R. Factors aggravating or precipitating acne in Indian adults: a hospital‐based study of 110 cases. Indian J Dermatol. 2018;63(4):328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan H‐M, Zhao H‐J, Guo D‐Y, Zhu P‐Q, Zhang C‐L, Jiang W. Gut microbiota alterations in moderate to severe acne vulgaris patients. J Dermatol. 2018;45(10):1166–71. [DOI] [PubMed] [Google Scholar]
- 5. Akaishi S, Koike S, Dohi T, Kobe K, Hyakusoku H, Ogawa R. In: Nd:YAG laser treatment of keloids and hypertrophic scars. Eplasty. 2012. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3258100/. Accessed 2 Oct 2020. [PMC free article] [PubMed]
- 6. Fabbrocini G, Annunziata MC, D'arco V, de Vita V, Lodi G, Mauriello MC, et al. Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010;2010:893080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magnani LR, Schweiger ES. Fractional CO2 lasers for the treatment of atrophic acne scars: a review of the literature. J Cosmet Laser Ther. 2014;16(2):48–56. [DOI] [PubMed] [Google Scholar]
- 8. Kurokawa I, Nakase K. Recent advances in understanding and managing acne. F1000Res. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19(4):303–8. [DOI] [PubMed] [Google Scholar]
- 10. Koo J. The psychosocial impact of acne: patients' perceptions. J Am Acad Dermatol. 1995;32(5 Pt 3):S26–30. [DOI] [PubMed] [Google Scholar]
- 11. Halvorsen JA, Lien L, Dalgard F, Bjertness E, Stern RS. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population‐based study. J Invest Dermatol. 2014;134(7):1847–54. [DOI] [PubMed] [Google Scholar]
- 12. Lanoue J, Goldenberg G. Acne scarring: a review of cosmetic therapies. Cutis. 2015;95(5):276–81. [PubMed] [Google Scholar]
- 13. Simmons BJ, Griffith RD, Falto‐Aizpurua LA, Nouri K. Use of radiofrequency in cosmetic dermatology: focus on nonablative treatment of acne scars. Clin Cosmet Investig Dermatol. 2014;7:335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan H‐H, Tan AWH, Barkham T, Yan X‐Y, Zhu M. Community‐based study of acne vulgaris in adolescents in Singapore. Br J Dermatol. 2007;157(3):547–51. [DOI] [PubMed] [Google Scholar]
- 15. Tan J, Kang S, Leyden J. Prevalence and risk factors of acne scarring among patients consulting dermatologists in the USA. J Drugs Dermatol. 2017;16(2):97–102. [PubMed] [Google Scholar]
- 16. Gordon MD. Pruritus in burns: a descriptive study. J Burn Care Rehabil. 1988;9(3):305–8. [PubMed] [Google Scholar]
- 17. Kim JE, Lee HW, Kim JK, Moon SH, Ko JY, Lee MW, et al. Objective evaluation of the clinical efficacy of fractional radiofrequency treatment for acne scars and enlarged pores in Asian skin. Dermatol Surg. 2014;40(9):988–95. [DOI] [PubMed] [Google Scholar]
- 18. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011;63(Suppl 11):S240–252. [DOI] [PubMed] [Google Scholar]
- 19. Aun C, Lam YM, Collett B. Evaluation of the use of visual analogue scale in Chinese patients. Pain. 1986;25(2):215–21. [DOI] [PubMed] [Google Scholar]
- 20. Forbat E, Al‐Niaimi F. Fractional radiofrequency treatment in acne scars: Systematic review of current evidence. J Cosmet Laser Ther. 2016;18(8):442–7. [DOI] [PubMed] [Google Scholar]
- 21. Spadoni D, Cain CL. Facial resurfacing. Using the carbon dioxide laser. AORN J. 1989;50(5):1007, 1009–13. [PubMed] [Google Scholar]
- 22. Weinstein C. Ultrapulse carbon dioxide laser removal of periocular wrinkles in association with laser blepharoplasty. J Clin Laser Med Surg. 1994;12(4):205–9. [DOI] [PubMed] [Google Scholar]
- 23. Waldorf HA, Kauvar AN, Geronemus RG. Skin resurfacing of fine to deep rhytides using a char‐free carbon dioxide laser in 47 patients. Dermatol Surg. 1995;21(11):940–6. [DOI] [PubMed] [Google Scholar]
- 24. Perez MI, Bank DE, Silvers D. Skin resurfacing of the face with the Erbium:YAG laser. Dermatol Surg. 1998;24(6):653–8. [DOI] [PubMed] [Google Scholar]
- 25. Ward PD, Baker SR. Long‐term results of carbon dioxide laser resurfacing of the face. Arch Facial Plast Surg. 2008;10(4):238–43. [DOI] [PubMed] [Google Scholar]
- 26. Alster TS, Lupton JR. Prevention and treatment of side effects and complications of cutaneous laser resurfacing. Plast Reconstr Surg. 2002;109(1):308–16. [DOI] [PubMed] [Google Scholar]
- 27. Ahn GR, Kim JM, Park SJ, Li K, Kim BJ. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52(5):396–401. [DOI] [PubMed] [Google Scholar]
- 28. Lan T, Tang L, Xia A, Hamblin MR, Jian D, Yin R. Comparison of fractional micro‐plasma radiofrequency and fractional microneedle radiofrequency for the treatment of atrophic acne scars: a pilot randomized split‐face clinical study in China. Lasers Surg Med. 2020. 10.1002/lsm.23369 [DOI] [PubMed] [Google Scholar]
- 29. Abdel Hay R, Shalaby K, Zaher H, Hafez V, Chi C‐C, Dimitri S, et al. Interventions for acne scars. Cochrane Datab Syst Rev. 2016;4:CD011946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chae WS, Seong JY, Jung HN, Kong SH, Kim MH, Suh HS, et al. Comparative study on efficacy and safety of 1550 nm Er:Glass fractional laser and fractional radiofrequency microneedle device for facial atrophic acne scar. J Cosmet Dermatol. 2015;14(2):100–6. [DOI] [PubMed] [Google Scholar]
- 31. Zhang Z, Fei Y, Chen X, Lu W, Chen J. Comparison of a fractional microplasma radio frequency technology and carbon dioxide fractional laser for the treatment of atrophic acne scars: a randomized split‐face clinical study. Dermatol Surg. 2013;39(4):559–66. [DOI] [PubMed] [Google Scholar]
- 32. Rongsaard N, Rummaneethorn P. Comparison of a fractional bipolar radiofrequency device and a fractional erbium‐doped glass 1,550‐nm device for the treatment of atrophic acne scars: a randomized split‐face clinical study. Dermatol Surg. 2014;40(1):14–21. [DOI] [PubMed] [Google Scholar]
- 33. Taub AF, Garretson CB. Treatment of acne scars of skin types II to V by sublative fractional bipolar radiofrequency and bipolar radiofrequency combined with diode laser. J Clin Aesthet Dermatol. 2011;4(10):18–27. [PMC free article] [PubMed] [Google Scholar]
- 34. Cameli N, Mariano M, Serio M, Ardigò M. Preliminary comparison of fractional laser with fractional laser plus radiofrequency for the treatment of acne scars and photoaging. Dermatol Surg. 2014;40(5):553–61. [DOI] [PubMed] [Google Scholar]
- 35. Peterson JD, Palm MD, Kiripolsky MG, Guiha IC, Goldman MP. Evaluation of the effect of fractional laser with radiofrequency and fractionated radiofrequency on the improvement of acne scars. Dermatol Surg. 2011;37(9):1260–7. [DOI] [PubMed] [Google Scholar]
- 36. Ramesh M, Gopal M, Kumar S, Talwar A. Novel technology in the treatment of acne scars: the matrix‐tunable radiofrequency technology. J Cutan Aesthet Surg. 2010;3(2):97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sadick NS, Sato M, Palmisano D, Frank I, Cohen H, Harth Y. In vivo animal histology and clinical evaluation of multisource fractional radiofrequency skin resurfacing (FSR) applicator. J Cosmet Laser Ther. 2011;13(5):204–9. [DOI] [PubMed] [Google Scholar]
- 38. Gold MH, Biron JA. Treatment of acne scars by fractional bipolar radiofrequency energy. J Cosmet Laser Ther. 2012;14(4):172–8. [DOI] [PubMed] [Google Scholar]
- 39. Baskan EB, Akin Belli A. Evaluation of the efficacy of microneedle fractional radiofrequency in Turkish patients with atrophic facial acne scars. J Cosmet Dermatol. 2018:1317–21. [DOI] [PubMed] [Google Scholar]
- 40. Cho SI, Chung BY, Choi MG, Baek JH, Cho HJ, Park CW, et al. Evaluation of the clinical efficacy of fractional radiofrequency microneedle treatment in acne scars and large facial pores. Dermatol Surg. 2012;38(7 Pt 1):1017–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


