Summary
Whether third‐generation hydroxyethyl starch solutions provoke kidney injury or haemostatic abnormalities in patients having cardiac surgery remains unclear. We tested the hypotheses that intra‐operative administration of a third‐generation starch does not worsen postoperative kidney function or haemostasis in cardiac surgical patients compared with human albumin 5%. This triple‐blind, non‐inferiority, clinical trial randomly allocated patients aged 40–85 who underwent elective aortic valve replacement, with or without coronary artery bypass grafting, to plasma volume replacement with 6% starch 130/0.4 vs. 5% human albumin. Our primary outcome was postoperative urinary neutrophil gelatinase‐associated lipocalin concentrations, a sensitive and early marker of postoperative kidney injury. Secondarily, we evaluated urinary interleukin‐18; acute kidney injury using creatinine RIFLE criteria, coagulation measures, platelet count and function. Non‐inferiority (delta 15%) was assessed with correction for multiple comparisons. We enrolled 141 patients (69 starch, 72 albumin) as planned. Results of the primary analysis demonstrated that postoperative urine neutrophil gelatinase‐associated lipocalin (median (IQR [range])) was slightly lower with hydroxyethyl starch (5 (1–68 [0–996]) ng.ml−1) vs. albumin (5 (2–74 [0–1604]) ng.ml−1), although not non‐inferior [ratio of geometric means (95%CI) 0.91 (0.57, 1.44); p = 0.15] due to higher than expected variability. Urine interleukin‐18 concentrations were reduced, but interleukin‐18 and kidney injury were again not non‐inferior. Of 11 individual coagulation measures, platelet count and function, nine were non‐inferior to albumin. Two remaining measures, thromboelastographic R value and arachidonic acid‐induced platelet aggregation, were clinically similar but with wide confidence intervals. Starch administration during cardiac surgery produced similar observed effects on postoperative kidney function, coagulation, platelet count and platelet function compared with albumin, though greater than expected variability and wide confidence intervals precluded the conclusion of non‐inferiority. Long‐term mortality and kidney function appeared similar between starch and albumin.
Keywords: cardiac surgery, hydroxyethyl starch, urinary interleukin‐18, urinary neutrophil gelatinase‐associated lipocalin
요약
3세대 하이드록시에틸 전분 용액이 심장수술을 받는 환자 에게 신장손상이나 혈액응고 장애를 유발하는지는 여전히 불 분명하다. 이에 본 연구에서는 심장수술 중 3세대 전분을 투 여해도, 수술 후 신장 기능이나 지혈이 5% 사람혈청알부민 투 여와 비교하였을 때 악화되지 않는다는 가설을 검정하였다. 이 삼중맹검 비열등성 임상 연구는 관상동맥우회술이 동반 또는 동반되지 않는 대동맥 판막 치환술을 시행받는 40‐85세 의 환자에서 혈장 보충을 의하여 5% 사람혈청알부민 혹은 6% 녹말 130/0.4%을 무작위로 사용하였다. 저자들의 일차 연 구 결과는 수술 후 신장 손상의 민감한 초기 표식인자로 알려 져 있는 소변 중성 젤라틴 효소 관련 리포칼린(urinary neu‐ trophil gelatinase‐associated lipocalin, NGAL) 농도이다. 이차 연구 결과들로는 소변 인터루킨‐18, RIFLE 기준 급성 신 손상, 혈액응고 지표, 혈소판 수 및 기능을 평가하였다. 비열 등성(델타 15%)은 다중 비교를 위해 보정하고 평가되었다. 저 자들은 141명의 환자(69 전분, 72 알부민)를 계획대로 등록하 였다. 일차 연구 결과는 수술 후 소변 NGAL (중위수 (사분위 ‐ 수범위[범위])이 알부민(5 (2–74 [0–1604]) ng.ml‐1) 대비 하이 드록시에틸 전분(5 (1–68 [0–996] ng.ml‐1)에서 약간 낮았지만 예상보다 높은 변동성으로 인해 비열등함을 보였다[기하학적 평균의 비율(95% 신뢰구간) 0.91 (0.57, 1.44); p = 0.15]. 소변 인터루킨‐18 농도는 감소하였지만 인터루킨‐18과 신장손상 또한 비열등함을 보였다. 열한 가지의 개별 응고 검사들과 혈 소판 수 및 기능 검사 중에서 9개는 알부민보다 비열등함을 보였다. 나머지 두 가지 측정치인 혈전탄성묘사도 R값과 아라 키돈산‐유도 혈소판 응집은 임상적으로 유사하였지만 신뢰구 간이 넓었다. 심장수술 중 녹말 투여는 알부민에 비해 수술 후 신장기능, 혈액응고, 혈소판 수 및 기능에 대해 유사한 효과를 나타냈지만, 측정치들의 예상보다 큰 변동성과 넓은 신뢰구간 으로 인하여 명확한 비열등성을 결론지을 수는 없었다. 장기 간 사망률과 신장 기능은 녹말과 알부민 군에서 모두 유사하 였다.
Introduction
Plasma volume replacement with hydroxyethyl starch (HES) solutions is an effective treatment for hypovolaemia during cardiac surgery. According to two studies with inconsistent results, HES appears to increase mortality and kidney injury in critically ill, including septic patients, who received HES 1, 2. In contrast, early and long‐term postoperative kidney and other multisystem complications were not increased in patients who received HES during elective abdominal surgery 3, 4, 5. However, results from non‐cardiac surgery cannot be directly extrapolated to patients undergoing cardiac surgery, because cardiopulmonary bypass induces inflammation, endothelial dysfunction and abnormal microvascular permeability 6 which may augment risk for postoperative kidney dysfunction and mortality.
The safety of modern, low‐molecular weight, low‐molar substitution HES, including 6% HES 130/0.4, on early and long‐term kidney function in adult cardiac surgical patients has not been fully evaluated. Retrospective and observational investigations report conflicting results 7, 8, 9, 10, 11, 12, 13, 14 and results of a few small and unblinded prospective investigations in cardiac surgical patients have been inconsistent 15, 16, 17. Other investigations did not include a non‐HES comparator 18 and some only enrolled children 19, 20. Several meta‐analyses combined small, heterogenous clinical trials which examined modern HES administration with low event rates, where kidney injury was not considered a primary or secondary outcome 21, 22, 23. Other reports pooled investigations of early and late generation HES products 23, 24 and did not discriminate between non‐cardiac and cardiac surgical patients 21, 22, 23. Importantly, long‐term kidney function after HES administration in cardiac surgical patients was not examined 21, 22, 23, 24.
The US Food and Drug Administration warns that patients given HES during cardiac surgery are at increased risk for coagulopathy.25 This package label warning is based on one meta‐analysis that included investigations of older generation HES characterised by higher molecular weight and molar substitution, rather than the modern, third‐generation HES products 26. These data were extrapolated to newer HES products, including 6% HES 130/0.4, even though the authors concluded that ‘insufficient data’ were available to compare the effect of modern HES solutions on peri‐operative blood loss. Indeed, modern HES solutions cause less coagulopathy than older preparations 27. As might therefore be expected, more recent meta‐analyses demonstrated conflicting results 28, 29, perhaps because small heterogenous trials were included, some of which only roughly estimated blood loss.
Our goal was to determine the safety of a third generation HES product, specifically 6% HES 130/0.4, on postoperative kidney function and haemostasis in patients having cardiac surgery. We tested the primary hypothesis that kidney function was not worse in cardiac surgery patients who received 6% HES 130/0.4 compared with patients who received human albumin 5%, as measured by a sensitive and early marker of postoperative kidney injury, urinary neutrophil gelatinase‐associated lipocalin (NGAL). Secondarily, we tested the hypothesis that urinary concentrations of interleukin‐18 (IL‐18) and kidney injury assessed by Risk Injury Failure Loss End stage renal disease (RIFLE) diagnostic criteria as well as coagulation and platelet function, were comparable in patients assigned to HES and albumin. Long‐term (1‐year) kidney outcomes and mortality were also collected.
Methods
This single‐centre, triple‐blinded (participant, investigator, outcomes assessor), parallel‐group, randomised, non‐inferiority trial was approved by Cleveland Clinic Institutional Review Board. With written informed consent, we enrolled patients scheduled for elective cardiac surgery at the Cleveland Clinic Main Campus between June 2015 and February 2018.
Inclusion criteria for eligible patients were aged 40–85 year, scheduled for elective aortic valve replacement with or without coronary artery bypass grafting with or without additional minor surgical procedures. Exclusion criteria included pre‐operative renal insufficiency (creatinine > 141.5 μmol.l−1), renal failure with oliguria or anuria not related to hypovolaemia, haemodialysis, use of hypothermic circulatory arrest, known hypersensitivity or allergy to hydroxyethyl starch or the excipients of hydroxyethyl starch, plasma volume overload, severe hypernatraemia or hyperchloraemia, intracranial bleeding, pregnant or breastfeeding women, critically ill adult patients, including patients with sepsis, severe liver disease, pre‐existing coagulation or bleeding disorders and any contra‐indications to proposed interventions.
Patients were randomly assigned (1:1) without stratification in random‐sized blocks of two to six patients to 6% HES 130/0.4 (Voluven®, Fresenius Kabi, Bad Homburg, Germany) or human albumin 5%. Allocation was concealed with a secure web randomisation site that was accessed shortly before induction of anaesthesia. Treatment assignments were generated using a reproducible algorithm in the PLAN procedure in SAS statistical software version 9.4 (SAS Institute, Carey, NC, USA).
The 6% HES 130/0.4 was suspended in 0.9% sodium chloride. Human albumin 5% was suspended in a solution that was adjusted to physiological pH with sodium bicarbonate and/or sodium hydroxide with mean (SD) total sodium content 145 (15) mEq.l−1. The Cleveland Clinic research pharmacy blinded the study solution. The assigned study solution (human albumin 5% solution or 6% HES 130/0.4) was transferred to a standard size (250 or 500 ml) glass bottle and covered with a shroud. The anaesthetic team determined when plasma volume replacement was clinically‐indicated. Anaesthesia, surgery and cardiopulmonary bypass were carried out with routine methods.
The blinded study drug (6% HES 130/0.4 or 5% human albumin) was administered in 250‐ml or 500‐ml increments per clinician preference, when hypovolaemia was indicated by any of the following conditions – cardiac output and/or cardiac index decreased ≥ 20% from baseline; heart rate increased ≥ 20% from baseline; mean or systolic blood pressure decreased ≥ 20% from baseline; vasopressor requirement increased ≥ 20% from baseline; central venous and/or pulmonary artery diastolic pressures decreased ≥ 20% from baseline or acute surgical haemorrhage. Fluid challenges were repeated until the inciting condition was rectified. The maximum dose of HES 130/0.4 was limited to 35 ml.kg−1.day−1.
Primary endpoint of kidney function was urinary concentration of NGAL, an early, predictive biomarker of acute kidney injury following bypass 30, 31. This was measured at baseline (following anaesthetic induction and before surgical incision) 1 h after arrival to Intensive care unit(ICU) and 24 h after completion of surgery.
Secondary endpoints included urinary concentrations of IL‐18 (an early and sensitive biomarker for acute kidney injury in patients having cardiac surgery 31, 32) measured at baseline, within 1 h of arrival to ICU and at 24 h following completion of surgery.
Postoperative kidney dysfunction using RIFLE diagnostic criteria was also assessed. Patients were assessed for risk for kidney dysfunction (RIFLE‐R), injury to the kidney (RIFLE‐I), failure of kidney function (RIFLE‐F) using criteria based on peak serum creatinine concentrations within the first seven postoperative days 33. Diagnostic categories including loss of kidney function (RIFLE‐L) and end‐stage kidney function (RIFLE‐E) are not based on serum creatinine concentrations and were therefore not included in this investigation. A second (post‐hoc) assessment of postoperative kidney dysfunction was also performed using RIFLE categorisation based on creatinine and urine output criteria.
Other secondary endpoints included markers of haemostatic function compared at baseline, within 1 h of arrival to ICU and at 24 h following completion of surgery. These included prothrombin time (PT), activated partial thromboplastin time (aPTT) and thromboelastography (TEG) (Haemonetics TEG 5000, Braintree, MA). We report the coagulation index, a summary variable comprised of 5 TEG parameters which provides a single number describing overall coagulation status. At our institution, a hypercoagulable state is defined as a coagulation index > 2.0 and coagulopathy is < −4.0. As the coagulation index is a calculated parameter based on TEG components 34, it was reported, but not included as an outcome measure.
We assessed platelet number and function. Platelet function was assessed by measurement of platelet aggregation in platelet‐rich plasma using turbidometric aggregometry (Chronolog Model 700 aggregometer, Havertown, PA, USA). This method measures the increase in light transmission after adding a platelet aggregating agent due to precipitation of platelet aggregates. Adenosine diphosphate (ADP), collagen and arachidonic acid were used as agonists to obtain data about the P2Y12 receptor, the GPIa/IIa pathway and cyclo‐oxygenase pathways, respectively.
All‐cause 1‐year mortality following surgery was collected from follow‐up phone calls, the patient's primary care physician or the Social Security Death Index. Long‐term kidney function measured by serum creatinine and use of renal replacement therapy between 6 months and 1 year following surgery was collected from medical records at the Cleveland Clinic and outside hospitals/physician records. Kidney function was classified into RIFLE categories based on serum creatinine.
We assessed the balance of randomised groups on baseline and procedural characteristics using absolute standardised difference (ASD), defined as the absolute difference in means, mean ranks or proportions divided by the pooled standard deviation. Baseline variables with ASD > 0.33 (i.e. 1.96 × ) were considered imbalanced and were adjusted for in primary analysis as a sensitivity analysis. Analyses were modified as intent‐to‐treat which was defined a priori as including all randomly allocated patients who received some amount of study intervention.
All analyses tested for non‐inferiority using a non‐inferiority delta of 15% of the control group mean for raw differences if the distribution of a continuous outcome measurement is approximately normal, or no more than 15% worse for ratios if the distribution of a continuous outcome is approximately log‐normal or comparing the risk for a categorical outcome. Non‐inferiority was claimed if the confidence limits for the treatment effect (estimated from analyses described below) were within the specified non‐inferiority region. P values were obtained from a 1‐tailed t‐test using a test statistic defined as , where is the estimated treatment effect, is the standard error of the treatment effect, and δ is the non‐inferiority delta.
Primarily, we assessed the effect of HES vs. albumin on NGAL at 1 h and 24 h after surgery using a repeated measures linear mixed model with an unstructured within‐subject correlation structure. We adjusted for baseline urinary NGAL to obtain a smaller standard error for the estimated treatment effect given that the baseline value is correlated with future measurements. The heterogeneity of the estimated treatment effect over time was assessed by testing the treatment‐by‐time interaction using a significance criterion of p < 0.15. A significant interaction would suggest that the treatment effect varies over time, in which case we would estimate the treatment effect individually for each time‐point measured. The significance level of the non‐inferiority test on urine NGAL was p < 0.021, which was adjusted for five interim analyses.
For secondary outcomes of kidney functions, we estimated the effect of HES vs. albumin on urinary IL‐18 at 1 h and 24 h after surgery using an analogous method as for NGAL. We originally planned to estimate the effect of HES on the ordinal RIFLE criteria as another secondary outcome. However, we observed few patients with a RIFLE classification of Risk, Injury or Failure. Therefore, we redefined this outcome to risk (or worse) vs. no risk for this analysis. The relative risk of kidney injury comparing HES to albumin was estimated using a log‐linked binomial model. The overall alpha is 0.025 for the secondary kidney function outcomes and 0.013 (0.025/2) for the non‐inferiority test on each outcome.
For secondary outcomes of coagulation and platelet function, non‐inferiority of HES to albumin was assessed for each outcome at the 0.025 level. We would claim non‐inferiority of HES to albumin if non‐inferiority was shown on all of the variables in this set. Therefore, no adjustment for multiple testing was done (i.e. intersection‐union test).
Sample size estimation was based on assessing the non‐inferiority of HES to albumin on the primary outcome of urinary NGAL. Assuming that NGAL values follow a log‐normal distribution as in previous studies 35 with a coefficient of variation of 25%, we would need 52 patients per group to have 90% power at the 0.025 significance level to be able to claim non‐inferiority of HES to albumin using a non‐inferiority delta of a ratio of geometric means of 1.15. Adjusting for the interim monitoring at each one sixth of the total, a maximum sample size of 130 patients was required. Allowing for five potential dropouts and five pilot patients (which were not included in the analyses), we planned to enrol 140 patients.
Results
A total of 149 patients met eligibility criteria and were randomly assigned. Seven patients from the HES group and one patient from the albumin group did not receive any intra‐operative study fluid and were not included for the analyses as pre‐specified in the protocol. Finally, 69 patients in the HES group and the 72 patients in albumin group were analysed (Fig. 1).
Figure 1.
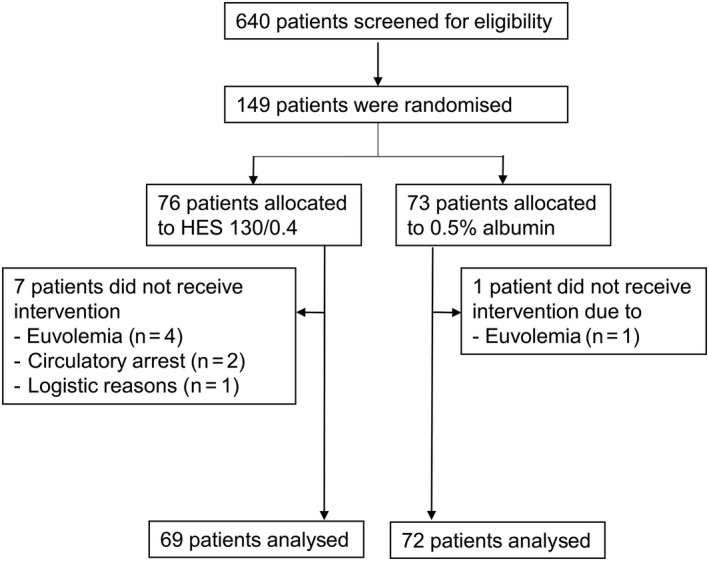
CONSORT patient flow diagram
Patients were compared on potentially confounding baseline and procedural characteristics. Most baseline variables and procedure characteristics were balanced between groups with the exception of left ventricular ejection fraction (mean (SD) was 62 (7) % HES vs. 57 (10) % albumin, ASD = 0.47), use of calcium channel blockers (number (%) was 22 (32%) vs. 11 (15%); ASD = 0.40), and Factor Xa inhibitors (number (%) was 7 (10%) vs. 1 (1%); ASD = 0.38). Additional patient characteristics, comorbidities and surgical variables are summarised in Table 1.
Table 1.
A summary of baseline, medical and procedural characteristics of the study population. Values are mean (SD), number (proportion) or median (IQR [range])
| Factor | HES 130/0.4 | Albumin 5% | ASDa | ||
|---|---|---|---|---|---|
| n = 69 | n = 72 | ||||
| Missing | Summary | Missing | Summary | ||
| Demographics | |||||
| Age; years | 0 | 71 (10) | 0 | 69 (9) | 0.19 |
| Female | 0 | 22 (32%) | 0 | 28 (39%) | 0.15 |
| Medical history | |||||
| Diabetes | 0 | 0 | 0.32 | ||
| Type 1 | 3 (4%) | 0 | |||
| Type 2 | 15 (22%) | 20 (28%) | |||
| Hypertension | 0 | 53 (77%) | 0 | 54 (75%) | 0.04 |
| Stroke | 0 | 6 (9%) | 0 | 4 (6%) | 0.12 |
| Myocardial infarction | 1 | 4 (6%) | 0 | 4 (6%) | 0.01 |
| Surgical information | |||||
| Previous cardiac surgery | 0 | 13 (19%) | 0 | 14 (19%) | 0.02 |
| Surgery type | 0 | 0 | 0.20 | ||
| Aortic valve only | 35 (51%) | 32 (44%) | |||
| Aortic valve + CABG | 12 (17%) | 16 (22%) | |||
| Aortic valve + other | 14 (20%) | 18 (25%) | |||
| Aortic valve + CABG + other | 8 (12%) | 6 (8%) | |||
| Duration of aortic cross‐clamp; min | 0 | 65 (48–91 [26–176]) | 0 | 60 (45–89 [25–375]) | 0.10 |
| Total fluid; l | 0 | 2.8 (2.3–3.2 [1.2–5.5]) | 0 | 2.8 (2.1–3.7 [0.9–0.8]) | 0.05 |
| Transfusion | 0 | 19 (28%) | 0 | 16 (22%) | 0.12 |
| RBC | 0 | 13 (19%) | 0 | 10 (14%) | 0.13 |
| Platelets | 0 | 10 (14%) | 0 | 10 (14%) | 0.02 |
| Fresh frozen plasma | 0 | 8 (12%) | 0 | 5 (7%) | 0.16 |
| Cryoprecipitate | 0 | 3 (4%) | 0 | 4 (6%) | 0.06 |
| Use of antifibrinolytic drug | 0 | 4 (6%) | 0 | 7 (10%) | 0.15 |
| Volume of study solution; ml | 0 | 500 (400–750 [200–1000]) | 0 | 750 (500–1000 [250–1000]) | 0.18 |
Absolute standardised difference (ASD), defined as the absolute difference in means, mean ranks or proportions divided by the pooled standard deviation. ASD > 0.33 were considered imbalanced.
Urinary samples were examined before surgery, and at 1 h and 24 h after surgery. There were no missing urinary NGAL levels at baseline or 1 h after surgery, but four patients in the albumin group and one patient in HES group had no available values of urinary NGAL at 24 h. Urinary NGAL concentrations at baseline, 1 h after surgery and 24 h after surgery are shown in Figure 2.
Figure 2.
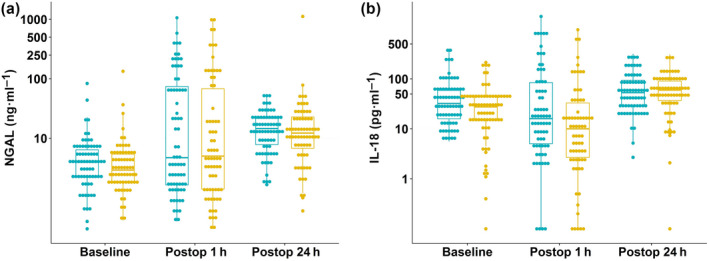
Urinary concentrations of (a) neutrophil gelatinase‐associated lipocalin (NGAL) and (b) interleukin‐18 (IL‐18) at baseline, 1 h after surgery (postop 1 h) and 24 h after surgery (postop 24 h) are shown.
We compared urine NGAL concentrations between groups according to whether specific thresholds identifying increased risk of kidney injury were reached: the frequency of patients with urinary NGAL of > 100 ng.ml−1 at 1 h after surgery was identical between groups (15 (22%) with HES and 16 (22%) with albumin), the number of patients with urine NGAL > 250 ng.ml−1 was identical between the treatment groups (7 (10%) with HES and 7 (10%) with albumin; Fig. 2). We also applied a cardiac surgery‐associated NGAL (CSA‐NGAL) score to urinary NGAL concentrations 36 Tubular damage was unlikely (urine NGAL < 50 ng.ml−1) in 50 (72%) HES patients vs. 48 (67%) patients who received albumin, tubular damage was possible (urine NGAL 50 to < 150 ng.ml−1) in three patients (4%) receiving HES vs. eight (11%) who received albumin, tubular damage occurred in 15 (22%) patients who received HES and 15 (21%) who received albumin, severe tubular damage occurred in 0 patients (0%) who received HES and 1 (1%) who received albumin.
Since the distribution of urinary NGAL was approximately log normal, summary statistics are presented for each group at two time‐points as median (IQR [range]) and the effect of HES compared with albumin as ratio of geometric means. There was no evidence that the treatment effect varied over time, with p = 0.74 for the treatment‐by‐time interaction. Therefore, we assessed the overall treatment effect collapsing over time. As shown in Fig. 3, HES was not non‐inferior to albumin, with an estimated ratio of geometric means of 0.91 (95%CI 0.57–1.44); non‐inferiority p = 0.15. A sensitivity analysis with adjustment for left ventricular ejection fraction and use of calcium channel blocker found results consistent with the primary analysis and a ratio of geometric means of 1.02 (95%CI 0.64–1.64); non‐inferiority p = 0.31.
Figure 3.
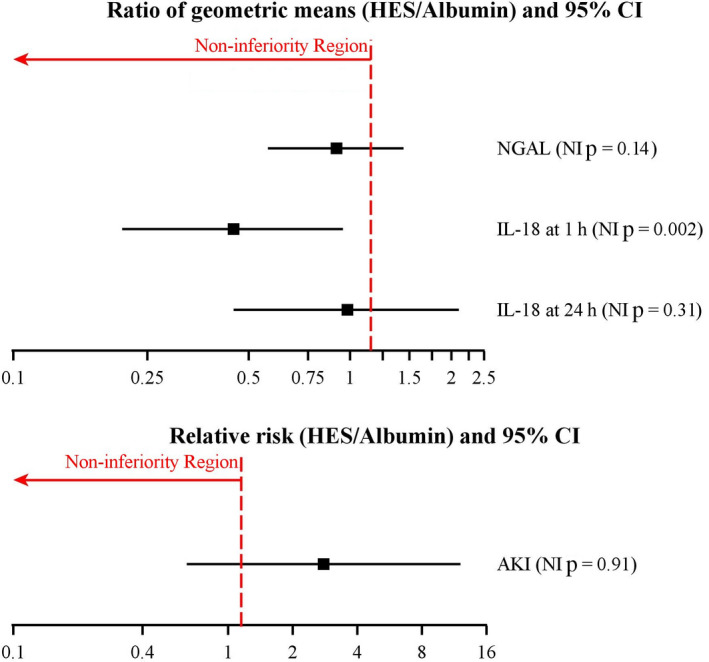
Non‐inferiority (NI) tests on the primary and secondary outcomes. Non‐inferiority was claimed if p < 0.021 for urinary NGAL, or p < 0.013 for urinary IL‐18 and AKI, defined by RIFLE categories: Risk, Injury or Failure. HES, Third‐generation hydroxyethyl starch; NGAL, urinary neutrophil gelatinase‐associated lipocalin; IL‐18, urinary interleukin‐18; AKI, acute kidney injury assessed as Risk, Injury or Failure by RIFLE classification based on creatinine values vs. No Risk.
We performed secondary analyses on kidney function. We compared urine IL‐18 concentrations between groups according to whether specific thresholds identifying risk of kidney injury were reached; the frequency of patients with urine IL‐18 of > 50 pg.ml−1 37, 38 at 1 h after surgery was lower in the HES group (13 (19%) patients receiving HES vs. 23 (32%) patients receiving albumin), the number of patients considered at risk of kidney injury with IL‐18 of > 150 pg.ml−1 32 was also lower in the HES group (8 (12%) patients given HES compared with 14 (19%) patients given albumin (Fig. 2)).
The treatment effect of HES vs. albumin was inconsistent over time (interaction p value of 0.079). Therefore, we estimated the treatment effects at 1 h and 24 h after surgery separately, adjusting for baseline IL‐18 levels. At 1 h, HES group was non‐inferior to albumin, with an estimated ratio of geometric means of 0.45 (95%CI 0.21–0.95); non‐inferiority p = 0.002. In contrast, we did not find non‐inferiority of HES compared with albumin on urinary IL‐18 at 24 h after surgery, with an estimated ratio of geometric means of 0.98 (95%CI 0.45–2.10); non‐inferiority p = 0.31 (Fig. 3).
Acute kidney injury assessed by RIFLE classification based on creatinine values was summarised as no risk, Risk, Injury or Failure. The number of patients (proportion) with any Risk/Injury/Failure vs. no risk was 8 (12%) for HES and 3 (4%) for albumin. HES was not found to be non‐inferior to albumin, with an estimated relative risk of 2.78 (95%CI 0.64–12.10); non‐inferiority p = 0.92.
As a post‐hoc analysis, we examined kidney injury using RIFLE criteria based on both creatinine levels and urine output, as recommended by Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group 33. The number of patients (proportion) with any Risk/Injury/Failure was 42 (61%) for HES and 55 (76%) for albumin; HES was non‐inferior to albumin using RIFLE criteria based on creatinine and urine output with an estimated relative risk of 0.80 (95%CI 0.61–1.03); non‐inferiority p < 0.001 (Table 2).
Table 2.
A comparison of HES vs. albumin on kidney function outcomes using RIFLE classification based on creatinine and urine output
| Outcomes | HES 130/0.4 | Albumin 5% | Relative risk of any risk/injury/failure | NI delta | NI |
|---|---|---|---|---|---|
| n = 69 | n = 72 | (95%CI) | p value2 | ||
| No risk | 27 (39%) | 16 (24%) | 0.80 (0.61, 1.03) | 1.15 | < 0.001 |
| Risk | 18 (26%) | 24 (33%) | |||
| Injury | 23 (33%) | 31 (43%) | |||
| Failure | 1 (1%) | 0 (0%) |
HES, hydroxyethyl starch; NI, non‐inferiority.
We performed secondary analyses on haemostatic function. We estimated treatment effect of HES vs. albumin and assessed non‐inferiority on each outcome except for variables INR and coagulation index, which were derived from the PT and TEG parameters. Coagulation measures (PT and aPTT), TEG parameters (K, MA, α, LY 30) and platelet function measures (ADP‐ and collagen‐induced platelet aggregation), were non‐inferior for HES vs. albumin (Fig. 4). TEG R times and arachidonic acid‐induced platelet aggregation in HES group were not non‐inferior to albumin; thus, we could not claim non‐inferiority of HES to albumin on overall coagulation and platelet function. As a post‐hoc analysis, we compared arachidonic acid induced‐platelet aggregation between HES (n = 53) and albumin (n = 58) groups in patients who either were not treated with aspirin or stopped before surgery and similar to the initial results, HES was not considered non‐inferior on arachidonic acid‐induced platelet aggregation (difference (95%CI) of −1.55 (−7.30–4.19) %max, p = 0.267).
Figure 4.
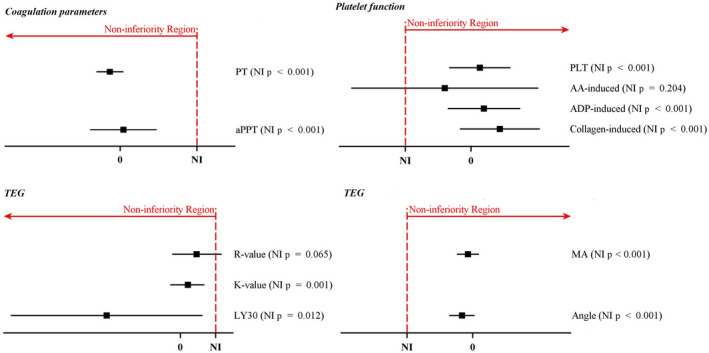
Forest plot of non‐inferiority tests on coagulation and platelet function. The estimated difference of HES compared with albumin is shown as a square and error bars indicate the 95%CI. Non‐inferiority delta is 15% of the control group mean. Non‐inferiority of each outcome was claimed if p < 0.025. NI, non‐inferiority; PT, prothrombin time; aPTT, activated partial thromboplastin time; PLT, platelet count; AA, arachidonic acid; ADP, adenosine diphosphate; TEG, thromboelastogram; R value, reaction time; K value, kinetics; LY 30, amplitude at 30 min; MA, maximum amplitude.
However, postoperative chest tube output over the first 24 h following surgery was similar between treatment groups: 590 (460–810 [110–2520]) ml in patients who received HES and 600 (410–826 [120–2170]) ml in patients who received albumin.
Patients were contacted at 1 year after surgery to assess long‐term kidney function and mortality. All patients in the HES group were alive, whereas two patients from the albumin group died within 1 year of surgery. In total, 26 (43%) patients in the HES group and 19 (30%) patients in the albumin group had creatinine measurement within 1 year. Only one patient had risk of kidney injury (HES). No patient received renal replacement therapy (Table 3).
Table 3.
Summary of 1‐year outcomes. Values are number (proportion) or median (IQR [range])
| Factor | HES 130/0.4 | Albumin 5% | ||
|---|---|---|---|---|
| n = 69 | n = 72 | |||
| Missing | Outcomes | Missing | Outcomes | |
| 1‐year mortality (%) | 2 | 0 (0) | 1 | 2 (3) |
| Creatinine (mg.dl−1) | 43 | 0.96 (0.80–1.12 [0.67–1.65]) | 54 | 0.99 (0.80–1.13 [0.51–1.44]) |
| RIFLE class (%) | 43 | 54 | ||
| No risk | 25 (96%) | 18 (100%) | ||
| Risk | 1 (4%) | 0 | ||
| Injury | 0 | 0 | ||
| Failure | 0 | 0 | ||
| Renal replacement therapy | 4 | 0 | 1 | 0 |
HES, hydroxyethyl starch.
The number of patients with missing data is shown in column titled ‘Missing’.
Discussion
Our study has demonstrated that the observed urinary NGAL and IL‐18 concentrations, which are early, predictive biomarkers of postoperative kidney dysfunction, were slightly lower in patients who received a third‐generation HES compared with another commonly used colloid, 5% human albumin. However, variability in the kidney markers was higher than expected. We thus could not claim that HES was non‐inferior. Risk for kidney injury assessed by RIFLE criteria with HES was also inconclusive. Overall, these data show that observed measures of kidney function were similar between groups and evidence of harm was lacking. Measures of coagulation and platelet function were also largely similar between HES and albumin. Nine of 11 individual measures of coagulation, platelet count and function were non‐inferior to albumin, but two exceptions, specifically, TEG R‐time and arachidonic acid‐induced platelet aggregation, precluded HES from being considered non‐inferior. The observed differences between groups on coagulation and platelet function, however, appeared minimal and clinically irrelevant.
Variability in urinary NGAL concentrations was greater than expected. Confidence intervals spanned from a 43% reduction in kidney injury with HES to a 44% increase compared with albumin. Despite high variability, the observed urinary NGAL values demonstrated minor changes from baseline and were slightly lower with HES than albumin. Use of the CSA‐NGAL score, a score designed for cardiac surgery 36, suggests that HES did not increase risk. In fact, the odds ratio for kidney injury with HES was reduced. However, broad 95% confidence intervals precluded the claim that risk was non‐inferior. A larger sample size was required to claim non‐inferiority, but our sample size was based on a report of postoperative urinary NGAL which described lower variability 35. If we had selected clinical outcomes, an even larger sample size would have been required.
Kidney injury measured by IL‐18 concentrations was non‐inferior with HES at 1 h after surgery. This result provides evidence supporting overall non‐inferiority of HES on kidney function, consistent with previous reports that urine IL‐18 early after surgery (1–2 h postoperatively) is the most predictive of kidney outcomes 31. Other clinically important cut‐off points for observed urinary IL‐18 concentrations 32, 37, 38 found that fewer patients were at risk for kidney injury with HES, though confidence intervals at the 24‐h time‐point extended past the non‐inferiority boundary.
Because only four patients (3%) experienced kidney injury or failure, we could not make any statistical inference as planned and categories of Risk, Injury and Failure defined by RIFLE were combined. Although the point estimate suggested an increased risk with HES administration, these fragile results were based on a small number of patients, low incidence of kidney injury, with wide confidence intervals. Interestingly, we assessed the effect of HES on kidney injury by RIFLE criteria post‐hoc based on serum creatinine and urine output as recommended by the Second International Consensus Conference of the Acute Dialysis Quality Initiative Group 39, which found that HES was non‐inferior to albumin.
Our investigation was designed to test for non‐inferiority with a non‐inferior delta of 15%. In other words, HES would be considered non‐inferior if the postoperative urinary NGAL concentrations and upper 95% confidence limit for the treatment effect were no more than 15% worse. The non‐inferiority margin of 15% was conservatively chosen based on clinical experience; however, diagnostic categories of kidney injury, including RIFLE, allow a 50% increase in creatinine concentration before kidney injury is diagnosed 39. Had we allowed a similar increase of 50% in urinary NGAL concentration (non‐inferiority delta of 0.5), HES would have been considered non‐inferior to albumin.
Though higher than expected, variability did not permit us to conclude that HES was non‐inferior to albumin after surgery, the observed long‐term kidney outcomes and 1‐year mortality were similar between groups. Event rates at 1 year following surgery were too low to make a definitive conclusion; however, these data suggest that intra‐operative HES administration is associated with preserved long‐term kidney function and survival. A larger sample size is needed to definitively conclude that the long‐term effect of HES and albumin are comparable on kidney function and mortality.
Peri‐operative coagulopathy and increased blood loss was a concern with older generation HES products 27. Thus, third‐generation modern HES solutions were developed to minimise interference with coagulation. Coagulation measures including PT and aPTT and nearly all TEG measures, including those that assess clot amplification, propagation, strength and lysis, were comparable in both groups. Only the Reaction time, which represents clot initiation, was slightly longer (0.4 min or 24 s) in patients who received HES. The Reaction time measures the initiation phase of enzymatic clotting factor activation, also known as the fluid phase of coagulation, which correlates with PT and aPTT. Since aPTT and PT were non‐inferior with HES, the Reaction time was slightly longer but remained within the normal reference range, and all other measures of clot formation efficiency and viscoelastic properties were non‐inferior, we conclude that there was no clinically meaningful adverse effect of HES on coagulation factors.
Platelet count, ADP‐induced and collagen‐induced platelet aggregation were comparable between groups. However, arachidonic acid‐induced platelet aggregation was not. Interestingly, arachidonic acid‐induced platelet aggregation was abnormal at baseline in both groups (normal > 70%), probably due to pre‐operative aspirin treatment in some, but not all patients. Residual aspirin may have contributed to great variability and wide confidence intervals, thus precluding the conclusion of non‐inferiority of HES on arachidonic acid‐induced platelet aggregation. Supporting the conclusion that haemostatic outcomes were clinically similar between groups, peri‐operative blood loss, blood product transfusion and postoperative chest tube output were similar between HES and albumin groups.
This study is strengthened by the inclusion of patients limited to aortic valve surgery with or without coronary artery bypass grafting, and thus reducing procedural heterogeneity and variability in peri‐operative fluid requirements and blood loss. Including only elective cardiac surgical patients with aortic stenosis, however, may limit the generalisability of our results to other cardiac pathologies, though different results are not expected in patients having coronary artery bypass grafting or other cardiac surgeries. The variability in the biomarkers of kidney function exhibited higher than expected variability and thus could not claim non‐inferiority despite minimal differences in outcomes between groups. Similar to other clinical trials examining the benefit/risk ratio of HES given during surgery 3, 4, 5, our investigation examined the effect of intra‐operative use of HES given over a few hours of administration. The amount of investigative solution in our trial was lower compared than one major clinical trial in non‐cardiac surgery 3 (0.6 l vs. 1.0 l); however, this amount of HES given during our trial comprised a significant amount (approximately 25%) of the total intravenous fluids received during surgery.
In summary, HES administration during cardiac surgery produced similar observed effects on postoperative kidney function, coagulation, platelet count and platelet function compared with albumin, though greater than expected variability and wide confidence intervals precluded the conclusion of non‐inferiority. Long‐term mortality and kidney function appeared similar between HES and albumin.
Acknowledgements
This investigation was supported by an investigator‐initiated research grant, Fresenius Kabi, Bad Homburg, Germany; Pfizer, New York City, USA; and the Departments of Cardiothoracic Anesthesia and Outcomes research, Cleveland Clinic, Cleveland, OH, USA. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication. None of the authors has a personal financial interest related to this research. This study was registered on ClinicalTrials.gov, NCT02192502. No other external funding or competing interests declared.
References
- 1. Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. New England Journal of Medicine 2012; 367: 1901–11. [DOI] [PubMed] [Google Scholar]
- 2. Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. New England Journal of Medicine 2012; 367: 124–34. [DOI] [PubMed] [Google Scholar]
- 3. Kabon B, Sessler DI, Kurz A, et al. Effect of intraoperative goal‐directed balanced crystalloid versus colloid administration on major postoperative morbidity: a randomized trial. Anesthesiology 2019; 130: 728–44. [DOI] [PubMed] [Google Scholar]
- 4. Joosten A, Delaporte A, Ickx B, et al. Crystalloid versus colloid for intraoperative goal‐directed fluid therapy using a closed‐loop system: a randomized, double‐blinded, controlled trial in major abdominal surgery. Anesthesiology 2018; 128: 55–66. [DOI] [PubMed] [Google Scholar]
- 5. Joosten A, Delaporte A, Mortier J, et al. Long‐term impact of crystalloid versus colloid solutions on renal function and disability‐free survival after major abdominal surgery. Anesthesiology 2019; 130: 227–36. [DOI] [PubMed] [Google Scholar]
- 6. Dabbagh A, Rajaei S, Bahadori Monfared A, Keramatinia AA, Omidi K. Cardiopulmonary bypass, inflammation and how to defy it: focus on pharmacological interventions. Iranian Journal of Pharmaceutical Research 2012; 11: 705–14. [PMC free article] [PubMed] [Google Scholar]
- 7. Lagny MG, Roediger L, Koch JN, et al. Hydroxyethyl starch 130/0.4 and the risk of acute kidney injury after cardiopulmonary bypass: a single‐center retrospective study. Journal of Cardiothoracic and Vascular Anesthesia 2016; 30: 869–75. [DOI] [PubMed] [Google Scholar]
- 8. Bayer O, Schwarzkopf D, Doenst T, et al. Perioperative fluid therapy with tetrastarch and gelatin in cardiac surgery–a prospective sequential analysis. Critical Care Medicine 2013; 41: 2532–42. [DOI] [PubMed] [Google Scholar]
- 9. Vives M, Callejas R, Duque P, et al. Modern hydroxyethyl starch and acute kidney injury after cardiac surgery: a prospective multicentre cohort. British Journal of Anaesthesia 2016; 117: 458–63. [DOI] [PubMed] [Google Scholar]
- 10. Ryhammer PK, Tang M, Hoffmann‐Petersen J, et al. Colloids in cardiac surgery‐friend or foe? Journal of Cardiothoracic and Vascular Anesthesia 2017; 31: 1639–48. [DOI] [PubMed] [Google Scholar]
- 11. Lee EH, Yun SC, Lim YJ, Jo JY, Choi DK, Choi IC. The effects of perioperative intravenous fluid administration strategy on renal outcomes in patients undergoing cardiovascular surgery: an observational study. Medicine 2019; 98: e14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Momeni M, Nkoy Ena L, Van Dyck M, et al. The dose of hydroxyethyl starch 6% 130/0.4 for fluid therapy and the incidence of acute kidney injury after cardiac surgery: a retrospective matched study. PLoS ONE 2017; 12: e0186403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Min JJ, Cho HS, Jeon S, Lee JH, Lee JJ, Lee YT. Effects of 6% hydroxyethyl starch 130/0.4 on postoperative blood loss and kidney injury in off‐pump coronary arterial bypass grafting: a retrospective study. Medicine 2017; 96: e6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van der Linden P, Dumoulin M, et al. Efficacy and safety of 6% hydroxyethyl starch 130/0.4 (Voluven) for perioperative volume replacement in children undergoing cardiac surgery: a propensity‐matched analysis. Critical Care 2015; 19: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Datzmann T, Hoenicka M, Reinelt H, Liebold A, Gorki H. Influence of 6% hydroxyethyl starch 130/0.4 versus crystalloid solution on structural renal damage markers after coronary artery bypass grafting: a post hoc subgroup analysis of a prospective trial. Journal of Cardiothoracic and Vascular Anesthesia 2018; 32: 205–11. [DOI] [PubMed] [Google Scholar]
- 16. Svendsen OS, Farstad M, Mongstad A, Haaverstad R, Husby P, Kvalheim VL. Is the use of hydroxyethyl starch as priming solution during cardiac surgery advisable? A randomized, single‐center trial. Perfusion 2018; 33: 483–9. [DOI] [PubMed] [Google Scholar]
- 17. Ooi JS, Ramzisham AR, Zamrin MD. Is 6% hydroxyethyl starch 130/0.4 safe in coronary artery bypass graft surgery? Asian Cardiovascular and Thoracic Annuals 2009; 17: 368–72. [DOI] [PubMed] [Google Scholar]
- 18. Joosten A, Tircoveanu R, Arend S, Wauthy P, Gottignies P, Van der Linden P. Impact of balanced tetrastarch raw material on perioperative blood loss: a randomized double blind controlled trial. British Journal of Anaesthesia 2016; 117: 442–9. [DOI] [PubMed] [Google Scholar]
- 19. Oh HW, Lee JH, Kim HC, et al. The effect of 6% hydroxyethyl starch (130/0.4) on acute kidney injury in paediatric cardiac surgery: a prospective, randomised trial. Anaesthesia 2018; 73: 205–15. [DOI] [PubMed] [Google Scholar]
- 20. Hanart C, Khalife M, De Ville A, Otte F, De Hert S, Van der Linden P. Perioperative volume replacement in children undergoing cardiac surgery: albumin versus hydroxyethyl starch 130/0.4. Critical Care Medicine 2009; 37: 696–701. [DOI] [PubMed] [Google Scholar]
- 21. Van Der Linden P, James M, Mythen M, Weiskopf RB. Safety of modern starches used during surgery. Anesthesia and Analgesia 2013; 116: 35–48. [DOI] [PubMed] [Google Scholar]
- 22. Martin C, Jacob M, Vicaut E, Guidet B, Van Aken H, Kurz A. Effect of waxy maize‐derived hydroxyethyl starch 130/0.4 on renal function in surgical patients. Anesthesiology 2013; 118: 387–94. [DOI] [PubMed] [Google Scholar]
- 23. Gillies MA, Habicher M, Jhanji S, et al. Incidence of postoperative death and acute kidney injury associated with i.v. 6% hydroxyethyl starch use: systematic review and meta‐analysis. British Journal of Anaesthesia 2014; 112: 25–34. [DOI] [PubMed] [Google Scholar]
- 24. Raiman M, Mitchell CG, Biccard BM, Rodseth RN. Comparison of hydroxyethyl starch colloids with crystalloids for surgical patients: a systematic review and meta‐analysis. European Journal of Anaesthesiology 2016; 33: 42–8. [DOI] [PubMed] [Google Scholar]
- 25. FDA . Approved blood products. 6% Hydroxyethyl Starch 130/0.4 in 0.9% Sodium Chloride Injection (Voluven 500 ml freeflex flexible plastic intravenous solution container). https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/6-hydroxyethyl-starch-13004-09-sodium-chloride-injection-voluven-500-ml-freeflex-flexible-plastic (accessed 13/06/2019).
- 26. Navickis RJ, Haynes GR, Wilkes MM. Effect of hydroxyethyl starch on bleeding after cardiopulmonary bypass: a meta‐analysis of randomized trials. Journal of Thoracic and Cardiovascular Surgery 2012; 144: 223–30. [DOI] [PubMed] [Google Scholar]
- 27. Kozek‐Langenecker SA, Jungheinrich C, Sauermann W, Van der Linden P. The effects of hydroxyethyl starch 130/0.4 (6%) on blood loss and use of blood products in major surgery: a pooled analysis of randomized clinical trials. Anesthesia and Analgesia 2008; 107: 382–90. [DOI] [PubMed] [Google Scholar]
- 28. Jacob M, Fellahi JL, Chappell D. Kurz A The impact of hydroxyethyl starches in cardiac surgery: a meta‐analysis. Critical Care 2014; 18: 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi XY, Zou Z, He XY, Xu HT, Yuan HB, Liu H. Hydroxyethyl starch for cardiovascular surgery: a systematic review of randomized controlled trials. European Journal of Clinical Pharmacology 2011; 67: 767–82. [DOI] [PubMed] [Google Scholar]
- 30. Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clinical Journal of the American Society of Nephrology 2008; 3: 665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xin C, Yulong X, Yu C, Changchun C, Feng Z, Xinwei M. Urine neutrophil gelatinase‐associated lipocalin and interleukin‐18 predict acute kidney injury after cardiac surgery. Renal Failure 2008; 30: 904–13. [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Guo W, Zhang J, et al. Urinary interleukin 18 for detection of acute kidney injury: a meta‐analysis. American Journal of Kidney Diseases 2013; 62: 1058–67. [DOI] [PubMed] [Google Scholar]
- 33. Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care 2004; 8: R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Traverso CI, Caprini JA, Arcelus JI. The normal thromboelastogram and its interpretation. Seminars in Thrombosis and Hemostasis 1995; 21(Suppl 4): 7–13. [DOI] [PubMed] [Google Scholar]
- 35. Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. Journal of the American Society of Nephrology 2012; 23: 905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Geus HR, Ronco C, Haase M, Jacob L, Lewington A, Vincent JL. The cardiac surgery‐associated neutrophil gelatinase‐associated lipocalin (CSA‐NGAL) score: a potential tool to monitor acute tubular damage. Journal of Thoracic and Cardiovascular Surgery 2016; 151: 1476–81. [DOI] [PubMed] [Google Scholar]
- 37. Parikh CR, Mishra J, Thiessen‐Philbrook H, et al. Urinary IL‐18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney International 2006; 70: 199–203. [DOI] [PubMed] [Google Scholar]
- 38. Washburn KK, Zappitelli M, Arikan AA, et al. Urinary interleukin‐18 is an acute kidney injury biomarker in critically ill children. Nephrology, Dialysis, Transplantation 2008; 23: 566–72. [DOI] [PubMed] [Google Scholar]
- 39. Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Medicine 2004; 30: 33–7. [DOI] [PubMed] [Google Scholar]


