Abstract
Repeated Plasmodium falciparum infections drive the development of clinical immunity to malaria in humans, however, the immunological mechanisms that underpin this response are only partially understood. Here, we investigated the impact of repeated P. falciparum infections on human γδ T cells in the context of natural infection in Malian children and adults, as well as serial controlled human malaria infection (CHMI) of U.S. adults, some of whom became clinically immune to malaria. In contrast to the predominant Vδ2+ γδ T cell population in malaria-naive Australian individuals, clonally expanded cytotoxic-Vδ1effector T cells were enriched in the γδ T cell compartment of Malian subjects. Malaria-naïve U.S. adults exposed to four sequential CHMIs defined the precise impact of P. falciparum on the γδ T cell repertoire. Specifically, innate-like Vδ2+ γδ T cells exhibited an initial robust polyclonal response to P. falciparum infection that was not sustained with repeated infections, whereas Vδ1+ γδ T cell frequencies increased in frequency with repeated infections. Moreover, repeated P. falciparum infection drove waves of clonal selection in the Vδ1+ TCR repertoire that coincided with the differentiation of Vδ1naive cells into cytotoxic-Vδ1effector cells. Finally, Vδ1+ T cells of malaria-exposed Malian and U.S. individuals were now licensed for reactivity to P. falciparum parasites in vitro. Together, our study indicates that repeated P. falciparum infection drives the clonal expansion of an adaptive γδ T cell repertoire and establishes a role for Vδ1+ T cells in the human immune response to malaria.
One Sentence Summary:
Malaria drives the adaptive differentiation of the human γδ T cell repertoire.
Introduction
In malaria-endemic regions, non-sterilizing clinical immunity to blood-stage Plasmodium falciparum parasites can be acquired, but this typically only occurs after many years of repeated infections (1). However, the mechanisms underlying this protection are only partially understood (2, 3). Recent observational studies in malaria-endemic areas, as well as clinical trials of attenuated P. falciparum sporozoite vaccine PfSPZ, have suggested that γδ T cells may contribute to protection from malaria (4–7).
Human γδ T cells are an unconventional T cell population that are thought to play an important role in immunity to microbial pathogens and cancer (8). Unlike conventional αβ T cells, γδ T cells are not restricted by classical MHC or MHC-I-like molecules to recognize antigens (9–11), but instead respond directly to non-peptidic metabolite antigens and other diverse ligands (12, 13). γδ T cells were present in the first jawed vertebrates and co-evolved with pathogenic organisms for millions of years (10, 14). In humans, the major peripheral blood population of γδ T cells (5–10% of total T cells) express a restricted TCR that consists of paired Vδ2 and Vγ9 chains (15). The Vγ9/Vδ2+ T cell population directly responds to a prenyl-pyrophosphate metabolite (PAg) (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) produced by the microbial non-mevalonate pathway (16). The Vγ9/Vδ2+ TCR repertoire is generated early in gestation and is shaped soon after birth, with a high frequency of public Vγ9 clonotypes (17–19). Innate-like Vγ9/Vδ2+ T cells can expand and comprise up to 40% of T cells during blood-stage malaria infection, a response thought to be driven by recognition of P. falciparum-derived HMB-PP (20–22) and they may participate in limiting parasite replication by targeting P. falciparum blood-stage parasites through granulysin-dependent cytotoxicity (23) and phagocytosis of antibody-coated iRBCs (24).
In contrast to innate-like Vγ9/Vδ2+ γδ T cells, a diverse biology has been established for γδ T cells that predominantly express the Vδ1+ TCR chain and circulate in blood at low frequency but are a major population in peripheral tissues (25). Firstly, Vδ1+ T cells that form tissue-associated populations in the intraepithelial lymphocyte (IEL) compartment of gut and breast tissue are thought to provide innate-like immune surveillance through host-encoded Natural Killer-receptors (NKRs) (26) and BTN-like (BTNL) 3 proteins (27). Secondly, peripheral blood and liver-resident Vδ1+ T cells possess hallmarks of adaptive T cells and comprise naïve-like (Vδ1naïve) and effector (Vδ1effector) populations with diverse or highly focused TCR repertoires, respectively (19, 25, 28). Acute cytomegalovirus (CMV) infection has been associated with the selection of a limited set of Vδ2neg γδTCR clonotypes (19, 29). Interestingly, expanded populations of Vδ1+ T cells have been observed in both children and adults with symptomatic P. falciparum infection (30–32) and in individuals residing in regions of malaria transmission (33, 34). Despite evidence that γδ T cells contribute to immunity to microbial pathogens, it remains unclear whether P. falciparum infection per se or factors associated with malaria transmission in endemic areas are responsible for the expansion of Vδ1+ T cells. Moreover, it is also unclear the impact of repeated P. falciparum infection on the phenotype, function and clonality of the γδ T cell compartment.
In this study, we investigated the γδ T cell immune repertoire response to P. falciparum malaria in a cohort of children and adults residing in a malaria-endemic region of Mali, and in malaria-naive U.S. adults serially infected with P. falciparum via mosquito bite in a controlled setting. We found that repeated P. falciparum infections drove the clonal selection and expansion of circulating cytotoxic Vδ1effector T cells that reacted to P. falciparum blood-stage parasites.
Results
Heterogeneity in the γδ T cell compartment exists across diverse geographic locations
In general, immune profiles are known to differ between children of high- and low-income countries where the latter typically suffer a disproportionately high burden of infectious disease (35). Here, we compared the circulating γδ T cell repertoire of Malian children (aged 4 – 17 years) who are exposed to intense seasonal malaria transmission (36), with that of age and gender matched children from Melbourne, Australia (aged 1 – 17 years) (Table S1). We first analyzed Mali samples collected from uninfected subjects at the end of the dry season when malaria transmission is negligible to assess γδ T cell repertoires in a relatively unperturbed state. We found that γδ T cell and Vδ1+ T cell frequencies were significantly higher in Malian children (Fig. 1A and S1A), whereas the frequency of Vγ9/Vδ2+ T cells were similar between both groups (Fig. 1A and B). We then analyzed γδ T cells in Malian adults (aged 21 – 26 years) and adults residing in an area of low malaria transmission in Kenya (aged 26 – 49 years) as well as Australian adults with no history of malaria exposure (aged 20 – 71 years). Vδ1+ T cell frequencies were lower in Kenyan and Australian adults compared to Malian children (Fig. S1B). Next, we assessed γδ T cell effector subsets in Malian children. From birth, Vγ9/Vδ2+ T cells typically form a stable innate-like T cell population composed of a CD27+ CD28+ Granzyme (Gzm) A+ GzmB+ Perforin+ compartment (18, 19, 37). In Malian individuals we found that Vγ9/Vδ2+ T cells had reduced expression of CD27+ CD28+ (Fig. 1C) and lytic Perforin (Fig. 1D), while GzmA was increased (Fig. 1E and F). In contrast, cord blood Vδ1+ T cell population is typically composed of naïve-like CD27hi CX3CR1neg GzmA/Bneg Perforinneg cells (Vδ1naive) (28). However, the Vδ1+ compartment in Malian subjects was predominantly composed of CD27lo CX3CR1+ GzmA/B+ Perforin+ effector-like cells (Vδ1eff) (Fig. 1C–F). Interestingly, a CD16+ Vγ9/Vδ2+ T cell compartment has recently been implicated in antibody-mediated phagocytosis of iRBCs (24). We found that Malian children, when compared to Australian children, tended to have increased frequencies of CD16+ Vδ1+ T cells rather than CD16+ Vγ9/Vδ2+ T cells (Fig. S1C). Together, these data suggest that the composition of the γδ T cell compartment varies significantly across geographic locations. However, it remained unclear whether high malaria transmission and/or factors associated with malaria transmission drive the proportional expansion of Vδ1+ T cells and skewing towards a Vδ1effector phenotype in the Mali cohort.
Figure 1. Increased Vδ1+ γδ T cells frequencies in Malian subjects exposed to P. falciparum infection.
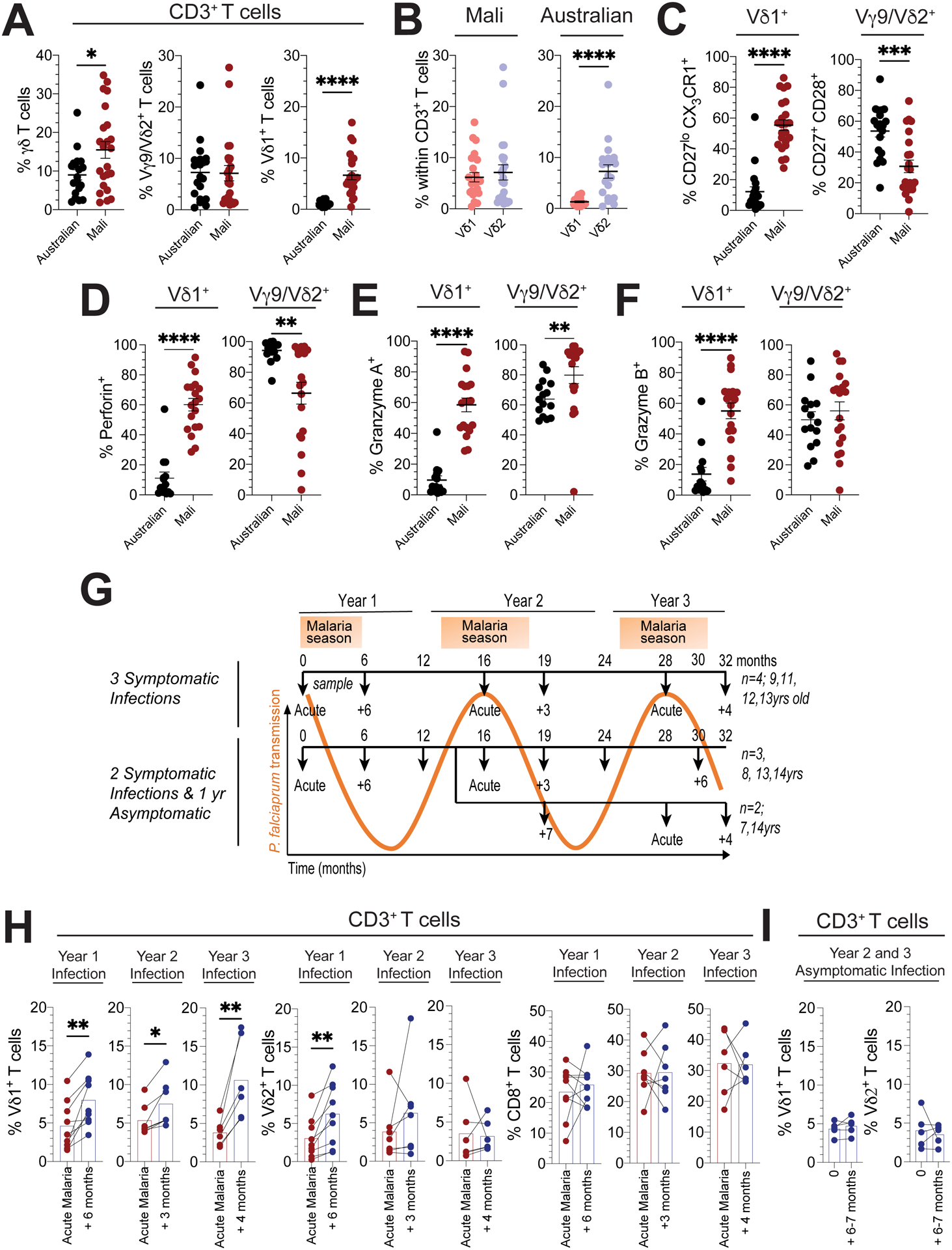
In age and gender matched Malian (n=23) or Australian subjects (n=20): A. Frequencies of total γδ, Vγ9/Vδ2+ and Vδ1+ T cells within CD3+ T cells, B. Frequencies of Vγ9/Vδ2+ and Vδ1+ T cells in total CD3+ T cells, C. Frequencies of CD27lo CX3CR1+ cells within Vδ1+ or CD27+ CD28+ cells within Vγ9/Vδ2+ T cells, D. Frequencies of perforin+, E. Gzm A +, F. Gzm B+ cells within Vδ1+ or Vγ9/Vδ2+ T cells (D, E and F: Malian n=19 and Australian n=15). G. Schematic of samples and malaria exposure for Malian subjects included in the longitudinal arm of our study. Subjects are stratified based on presentation with a confirmed febrile malaria episode in all three years (n=4) or two febrile episodes (n=5), with either one episode in year 2 (n=3) or 3 (n=2). H. Frequencies of Vδ1+, Vγ9/Vδ2+ and CD8+ T cells in total CD3+ T cells during febrile malaria and 3–6 months following treatment over the 3-year seasonal transmission periods. Year 1 (n=9), Year 2 (n=7) and Year 3 (n=6). I. Frequencies of Vδ1+ and Vγ9/Vδ2+ T cells within CD3+ T cells over a 6-month period without a febrile malaria episode (n=5; 12 – 18 months, n=2, or 24 – 30 months, n=3). Bars show the mean and error bars indicate means ± SEM. Normality was tested using the Shapiro-Wilk test.; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; p-values were determined by Mann-Whitney test (a - f) and Wilcoxon matched-pairs signed rank test (h, i).
Episodes of febrile malaria associate with fluctuations in Vδ1+ γδ T cell frequencies
To more directly investigate the potential impact of natural malaria infection on the γδ T cell compartment, we conducted a longitudinal analysis of nine Malian children (aged 8 – 14 years) over three malaria seasons (Fig. 1G). These individuals from Mali were exposed to an annual six-month malaria season in which P. falciparum transmission is intense and predictable (36). Consistent with this, P. falciparum parasite density increased during each malaria season in a subset of five children whose blood smears were examined longitudinally (Fig. S1CD). Moreover, each subject was selected because they experienced two to three febrile malaria episodes over multiple years, as detected by both passive and active clinical surveillance, allowing for longitudinal analyses of γδ T cell dynamics in response to acute symptomatic malaria followed by sustained periods without febrile malaria (Fig. 1G). We then investigated γδ T cell and CD8+ αβ T cell frequencies across consecutive episodes of febrile malaria over three seasons, these analyses pooled T cell frequencies from children who had experienced two or three episodes of febrile malaria (Fig. 1H). γδ T cell and CD8+ αβ T cell frequencies were assessed in blood samples collected on the day febrile malaria was diagnosed and again within 3 – 6 months of diagnosis. We found that Vγ9/Vδ2+ T cell frequencies increased after febrile malaria in year 1 but did not consistently change after febrile malaria in years 2 and 3 (Fig. 1H), although these observations could be due to the different sampling times in each year. CD8+ T cell frequencies were unchanged after each febrile episode (Fig. 1H). In contrast, Vδ1+ T cell frequencies were consistently decreased upon presentation with febrile malaria and increased after each febrile malaria episode across all three years (Fig. 1H). Moreover, across a sub-set of eight subjects in year 1, we also observed equivalent CD3+ lymphocyte and αβ T cell counts, and all γδ T cell populations expanded in number after febrile malaria (Fig. S1E). We then assessed a subset of children at timepoints without infection before a documented period of asymptomatic P. falciparum infection but no febrile malaria episodes (Fig. 1I; pooled from data between month 12 – 19 or 24 – 30). Vγ9/Vδ2+ and Vδ1+ T cell frequencies did not change significantly across this six to seven-month period. We noted previously that Vδ1+ T cells in Malian children were predominantly composed of Vδ1effector cells (Fig. 1C and D), however, yearly episodes of febrile malaria had little impact on Vδ1effector frequencies and CD27+ CD28+ Vγ9/Vδ2+ T cell frequencies were reduced in year 3 (Fig. S1F). Together, these data indicate that exposure to seasonal episodes of febrile malaria transiently impacts circulating frequencies of Vδ1+ γδ T cells.
Malian γδTCR repertoires are clonally skewed and change after febrile malaria
We next explored the underlying γδTCR repertoires in Malian children and whether febrile malaria could impact individual clonotypes over time. Initially, we conducted a cross-sectional analysis of blood samples collected subjects during periods of no malaria transmission (subjects 066, 521, 766) and from one subject with febrile malaria (subject 269) and compared these repertoires to those of Australian children (Fig. 2A and S3A). We analyzed both Vγ9/Vδ2+ (Vδ2+) and non-Vγ9/Vδ2 (Vδ2neg) γδ T cell populations, effectively encompassing the total γδ T cell repertoire (Fig. S2). Phenotypically, in both Malian and Australian subjects Vδ2+ γδ T cell populations were composed of effector-like populations of CD27+ CD28+ cells and Vδ2neg γδ T cells were composed CD27lo CX3CR1+ effector cells in Malian subjects and CD27hi CX3CR1neg naïve cells in Australian subjects (Fig. S3B). The Vγ9/Vδ2+ T cell subset displayed γδ TCR repertoires consistent with those seen in children and adults from Europe (18, 19, 37) (Fig. 2A and Fig. S3A), which are almost exclusively composed of Vδ2–Jδ1 (Fig. S3C) paired to Vγ9–JγP (Fig. S3D), with diverse clonotype composition and common CDR3γ9-JγP sequences shared between individuals (Fig. 2A and Fig. S3C). Vδ2neg γδ TCR repertoires were predominantly composed of Vδ1–Jδ1 sequences (Fig. S3D) that were paired to various Vγ–Jγ1/2 regions (Fig. S3D). These Vδ2neg γδTCR repertoires from Malian children exhibited expanded clonotypes, indicated by an increase in the accumulated frequency of the top 10 clonotypes in comparison to Vδ2neg γδ TCR repertoires in Australian children (Fig. 2B). In support of the skew towards expanded Vδ1 clonotypes, Malian Vδ2neg γδ TCR repertoires also showed a reduced diversity of clonotype composition (Fig. 2C) and a reduced frequency of shared sequences compared to Vδ2neg γδTCR repertoires of Australian individuals (Fig. 2D). These data suggest that the Vγ9/Vδ2+ T cell repertoires in Malian subjects are highly similar to those of Australian individuals. In contrast, Vδ2neg γδTCR repertoires of Malian individuals showed evidence of reduced clonotype sharing and diversity as a result of expanded private clonotypes.
Figure 2. γδTCR repertoires in Malian subjects evolve over time.
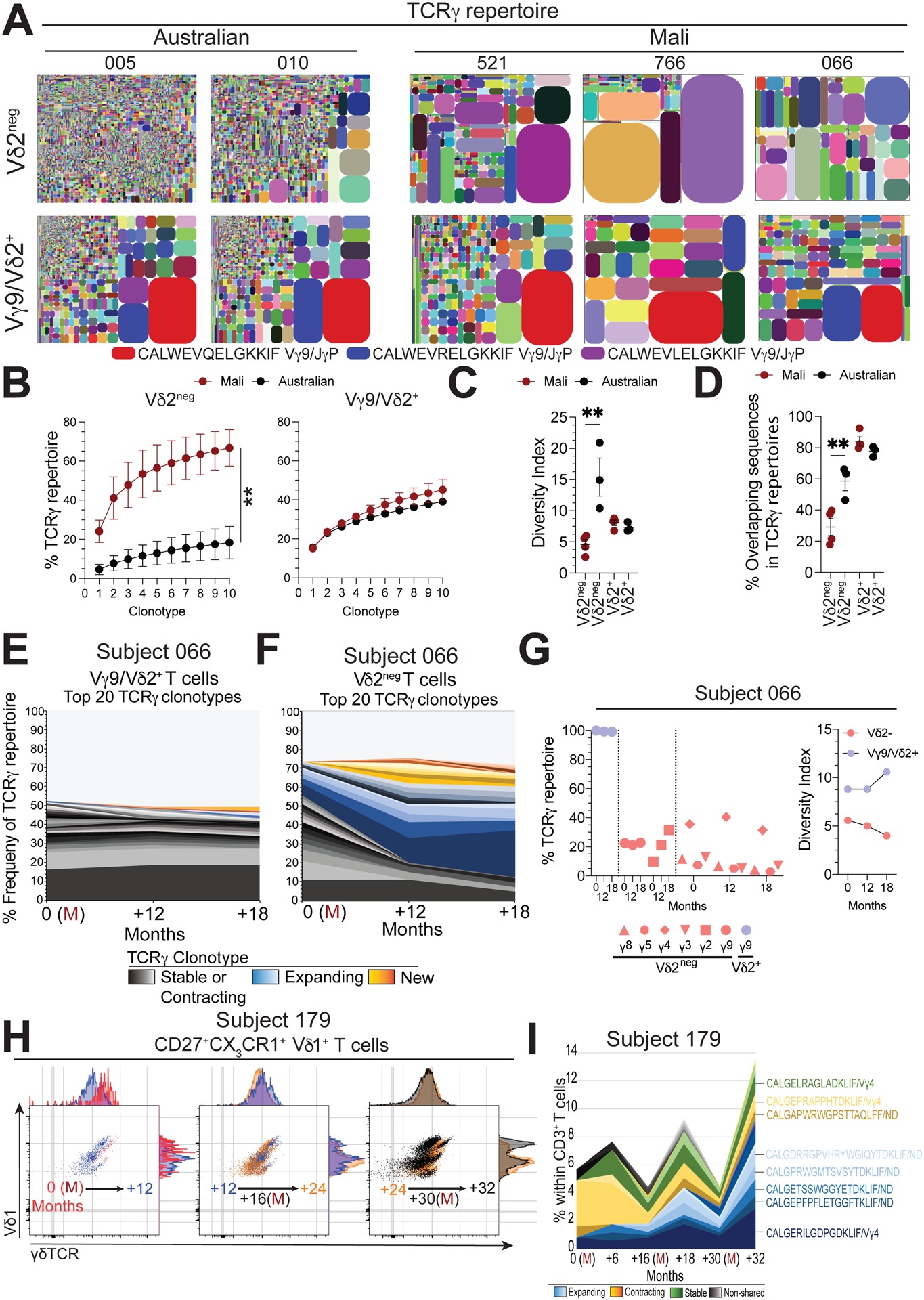
A. TCRγ clonotype tree plot analysis of Vδ2neg and Vγ9/Vδ2+ T cell populations from Australian children or Malian children during stable periods without malaria transmission. Tree plots show unique clonotypes (coloured segments) and their proportion within the total repertoire (size), in general coloured clonotypes do not match between plots unless indicated. B. Pooled accumulated frequency curves of the top 10 most prevalent clonotypes in Vδ2neg or Vγ9/Vδ2+ TCR repertoires (Australian, n=3; Mali, n=4). C. Diversity index of Vδ2neg and Vδ2+ γδ T cell repertoires in Malian (n=4) or Australian (n=3) subjects. D. Frequency of shared CDR3γ (a.a.) sequences in Vδ2neg and Vγ9/Vδ2+ T cell repertoires (Australian, n=3; Mali, n=4). E. Longitudinal tracking of the 20 most abundant TCRγ clonotypes in Vγ9/Vδ2+ and F. Vδ2neg T cell repertoires over time in subject 066. (M) indicates acute febrile malaria. G. Longitudinal analysis of Vγ chain usage and diversity index for Vδ2neg (red) and Vγ9/Vδ2+ (blue) T cell repertoires from subject 066. H. γδTCR expression patterns within (CD27lo CX3CR1+) Vδ1+effector populations in donor 179. Each flow cytometry plot has two time points overlaid, indicated by an arrow, together covering three febrile P. falciparum infections (months 0, 17 and 30). I. TCRδ clonotypes sequencing relative to total CD3+ T cells from subject 179. Error bars indicate means ± SEM. Normality was tested using the Shapiro-Wilk test.; *P < 0.05; **P < 0.01; ***P < 0.001; p-values were determined by two-way ANOVA with Sidaks post hoc testing (b) and one-way ANOVA with Holm-Sidaks post hoc testing (c, d).
Next, in a longitudinal analysis we assessed the impact of episodes of acute febrile malaria on γδTCR clonotype composition within the Vγ9/Vδ2+ and Vδ2neg γδ T cell populations. Vγ9/Vδ2+ clonotypes remained remarkably stable during and after acute febrile malaria (Fig. 2E, Fig. S3E and F). We and others have previously reported on the stability of Vδ2neg and Vδ1+ γδTCR clonotypes over several years (18, 19, 28, 29). Here, Vδ2neg γδTCR repertoires displayed changes after acute febrile malaria, characterized by contraction and expansion of existing clonotypes or emergence of new prevalent sequences (Fig. 2F, Fig. S3E and G). These changes impacted the frequency of Vδ1 sequence usage (Fig. S3H), Vγ2 usage (Fig. 2G), the overall repertoire diversity (Fig. 2G) and nucleotide length dynamics (Fig. S3I). To explore the impact of febrile malaria on clonotype composition within Vδ1effector cells, we sorted single cells from the CD27lo CX3CR1+ Vδ1effector cell compartment from samples collected over 32 months and three separate acute febrile malaria episodes from subject 179 (Fig. 2H). Interestingly, we noted by flow cytometry that Vδ1/γδTCR antibody staining intensity changed over time, with distinct Vδ1/γδTCR antibody populations emerging after each episode of febrile malaria (Fig. 2H and Fig. S3J). Underpinning these observations, single cell γδTCR sequencing revealed changes in the frequency and identity of individual Vδ1effector clonotypes over time (Fig. 2I and Fig. S3K). Together, these data suggest that Malian individuals have highly stable Vγ9/Vδ2+ T cell repertoires that are retained across episodes of febrile malaria and are shared between individuals. In contrast, γδTCR clonotypes in the Vδ1effector compartment were composed of clonotypes that varied in frequency and identity over time.
Repeated human controlled malaria infection can establish clinical immunity that correlates with increased Vδ1+ γδ T cell frequencies
To understand the precise impact of P. falciparum infection on the trajectory of γδ T cell development and selection, we assessed γδ T cell subset dynamics in PBMCs collected from five malaria-naïve adults voluntarily exposed to repeated controlled human malaria infection (CHMI). Each volunteer was exposed to the bites of five Anopheles stephensi mosquitos infected with P. falciparum (strain: NF54) on four separate occasions over 644 days (Fig. 3A). Symptomatic malaria occurs during the blood stage of the P. falciparum parasite life cycle, which typically develops after an incubation period of nine to fourteen days (36). Here, we analyzed samples at baseline (malaria naïve), immediately prior to P. falciparum infection (day 1; at CHMI1 and 3), and 21 days after infection for all CHMIs (Fig 3A). We did not observe any noticeable leukopenia measured by white blood cell counts (at day 1 or day 28; Fig. S4A) or by clinical tests prior to apheresis (day 21) at the timepoints sampled in this study. Over the course of the four CHMIs, peak parasitemia measured by blood smear did not significantly change (Fig. S4B). We then assessed the instances of febrile malaria and symptomatic disease (ranging from headaches to vomiting; Table S2). Fever was observed at CHMI1 or 2 in all but one individual and the number of symptoms observed in each individual decreased after repeated CHMIs (Fig. 3B). Three individuals had asymptomatic P. falciparum infections following CHMI4, while two volunteers remained symptomatic (Fig. 3B). Next, we analyzed γδ and αβ T cell frequencies across all CHMIs. Total αβ T cell frequencies within CD3+ T cells showed a non-significant decline with repeated CHMI (Fig. 3C). CD8+ αβ T cells increased in frequency and peaked prior to CHMI3 (Fig. 3C), coinciding with an increase in CD8+ Tnaive cells and CD8+ TCM on day 21 after CHMI2–4 (Fig. S4C). In contrast, γδ T cells frequencies increased across all CHMIs, and this was largely driven by an increase in Vγ9/Vδ2+ T cells (Fig. 3D). We also found an increase in Vδ1+ T cell frequencies across repeated CHMIs (Fig. 3D). We then analysed the relationship between γδ T cell frequencies and the risk of developing symptomatic malaria. Overall, αβ T cell and CD8+ T cell frequencies were similar in asymptomatic and symptomatic individuals (Fig. S4D). However, volunteers that progressed to asymptomatic malaria with serial CHMIs displayed robust profiles of increasing Vδ1+ and Vγ9/Vδ2+ T cells frequencies across CHMIs, while symptomatic volunteers retained frequencies of Vδ1+ and Vγ9/Vδ2+ T cells that were similar to their baseline samples (Fig. 3E). Vγ9/Vδ2+ T cells frequencies decreased between CHMIs and were not durably maintained at CHMI4 (Fig. 3E). Repeated measures correlations found a significant inverse association between the number of malaria symptoms and Vδ1+ T cell frequencies (P=0.007) (Fig. 3F), but not with αβ+ (P=0.431), γδ+ (P=0.109), CD8+ (P=0.391) or Vγ9/Vδ2+ T cell frequencies (P=0.572) (Fig. S4E). Together, these data from a highly controlled human malaria challenge model confirm that repeated in vivo P. falciparum infections drive changes in both Vδ2+ and Vδ1+ T cell frequencies. Increased Vδ1+ T cell frequencies correlated with the development of asymptomatic malaria after CHMI4, while Vγ9/Vδ2+ T cell frequencies decreased between infections and were not durably maintained after CHMI4 in asymptomatic subjects, suggesting that regulation of Vγ9/Vδ2+ T cells may contribute to symptom reduction, a hypothesis that is consistent with previous reports in the context of natural infection (38–40).
Figure 3. Repeated controlled P. falciparum infection drive clinical immunity to malaria and increased frequencies of γδ T cells.
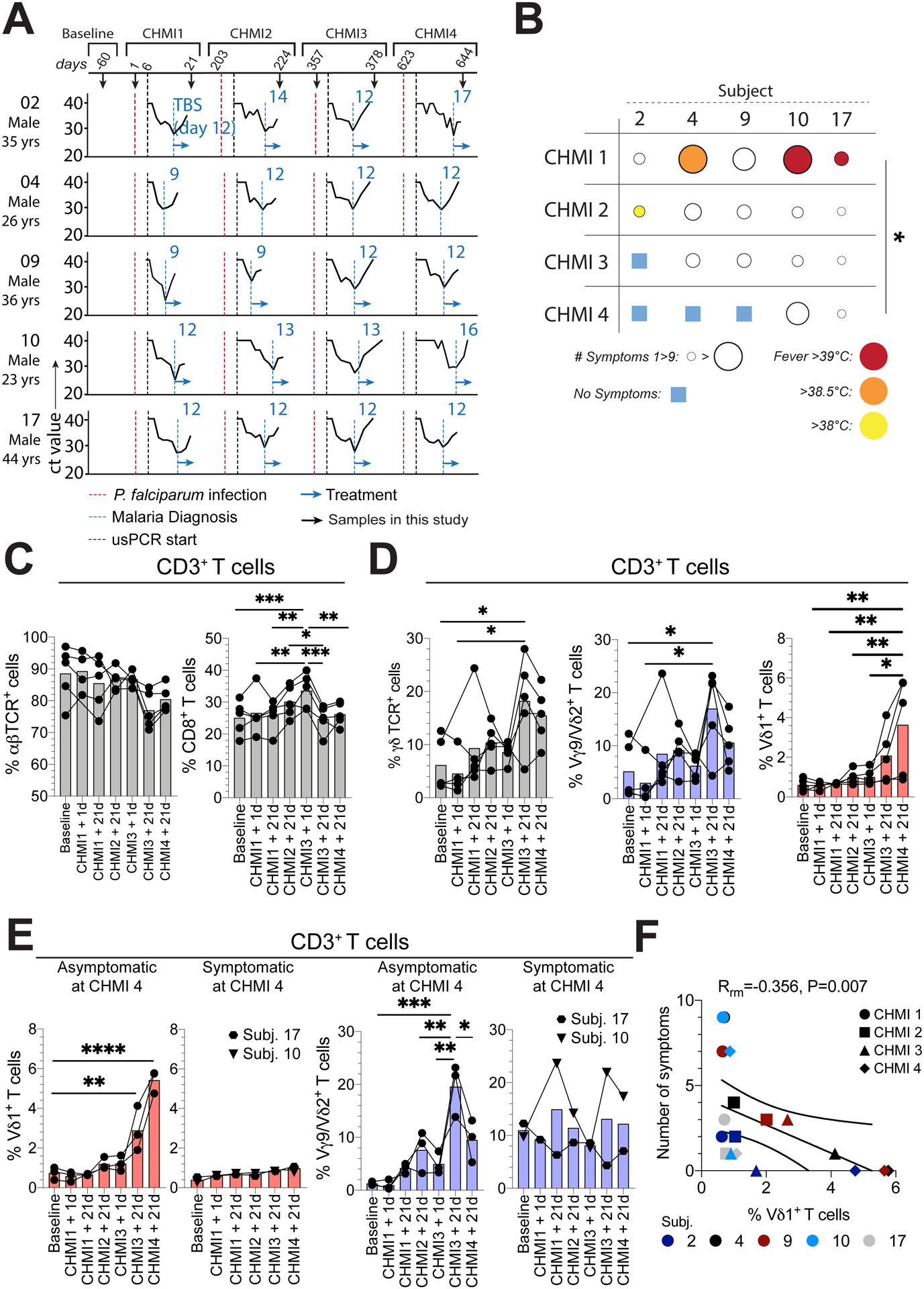
A. Controlled human malaria infection (CHMI) study subjects, samples, parasite ultra-sensitive PCR (usPCR) detection curves and diagnosis by blood smear. B. Symptomology and fever analysis of each subject during each CHMI. C-E. Within total CD3+ T cells: C. Total αβ+ and CD8+ T cell frequencies. D. Total γδ+, Vγ9/Vδ2+ (blue), and Vδ1+ (red) γδ T cells frequencies and E. Vδ1+ and Vγ9/Vδ2+ frequencies in individuals that were asymptomatic or symptomatic at CHMI4. F. Repeated measure correlation between Vδ1+ frequencies within total CD3+ T cells and the number of symptoms each individual suffered at each CHMI. Bars show the mean. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; p-values were determined by Kruskall-Wallis test (b), linear mixed effects modelling with Bonferroni’s correction (c, d, e) and repeated measures correlation (f).
Repeated P. falciparum infection initiates Vδ1naive to Vδ1effector T cell differentiation
Next, we investigated the impact of repeated P. falciparum infections on the differentiation of γδ T cell subsets. Although CD27hi CD28+ Vδ1naive T cells were the main population of Vδ1+ γδ T cells in subjects prior to CHMI (malaria naïve), this cell population decreased after repeated P. falciparum infections (Fig. 4A). Conversely, CD27lo CX3CR1+ Vδ1effector cells became the dominant population within total Vδ1+ T cells (Fig. 4B). The increase in the CD27lo CX3CR1+ Vδ1effector T cell population also correlated with a reduction in malaria symptoms (Fig. S4F). In response to a combination of inflammatory cytokines and HMB-PP stimulation, it has been proposed that Vγ9/Vδ2+ T cells switch phenotype from CD27+ CD28+ to CD27− CD28− (41); however, we found no significant changes in these populations across repeated P. falciparum infections (Fig. 4C). As noted earlier, Vγ9/Vδ2+ T cells can control parasite replication through CD16-mediated antibody-dependent cytotoxicity (24), we found that CD16 expression was upregulated on Vδ1+ T cells, but not Vγ9/Vδ2+ T cells after repeated P. falciparum infections (Fig. 4D). Interestingly, subject 17 displayed a major CD27− CD28− CD16+ Vγ9/Vδ2+ T cell population that persisted over time (Fig. 4C and D). Vδ1+ T cells consistently expressed the T cell activation marker CD38 after each P. falciparum infection, while Vγ9/Vδ2+ T cells only significantly upregulated CD38 after CHMI1 and 2 (Fig. 4E). We previously showed that Vδ1effector cells possess significant cytotoxic potential (19, 28). Here, we found that repeated P. falciparum infection drove Vδ1+ T cells to express Gzm A, B, perforin, but not Gzm K (Fig. 4F), whereas CD8+ T cells had no significant increase in Gzm A, B, perforin or Gzm K (Fig. S4G). In keeping with their pre-formed cytotoxic potential, Vγ9/Vδ2+ T cells retained robust levels of Gzm A, B, K and perforin after repeated P. falciparum infections (Fig. 4F). Our data indicate that in vivo P. falciparum infection in humans drives the differentiation of human Vδ1effector γδ T cells.
Figure 4. Repeated P. falciparum infection drives the differentiation of cytotoxic Vδ1effector T cells.
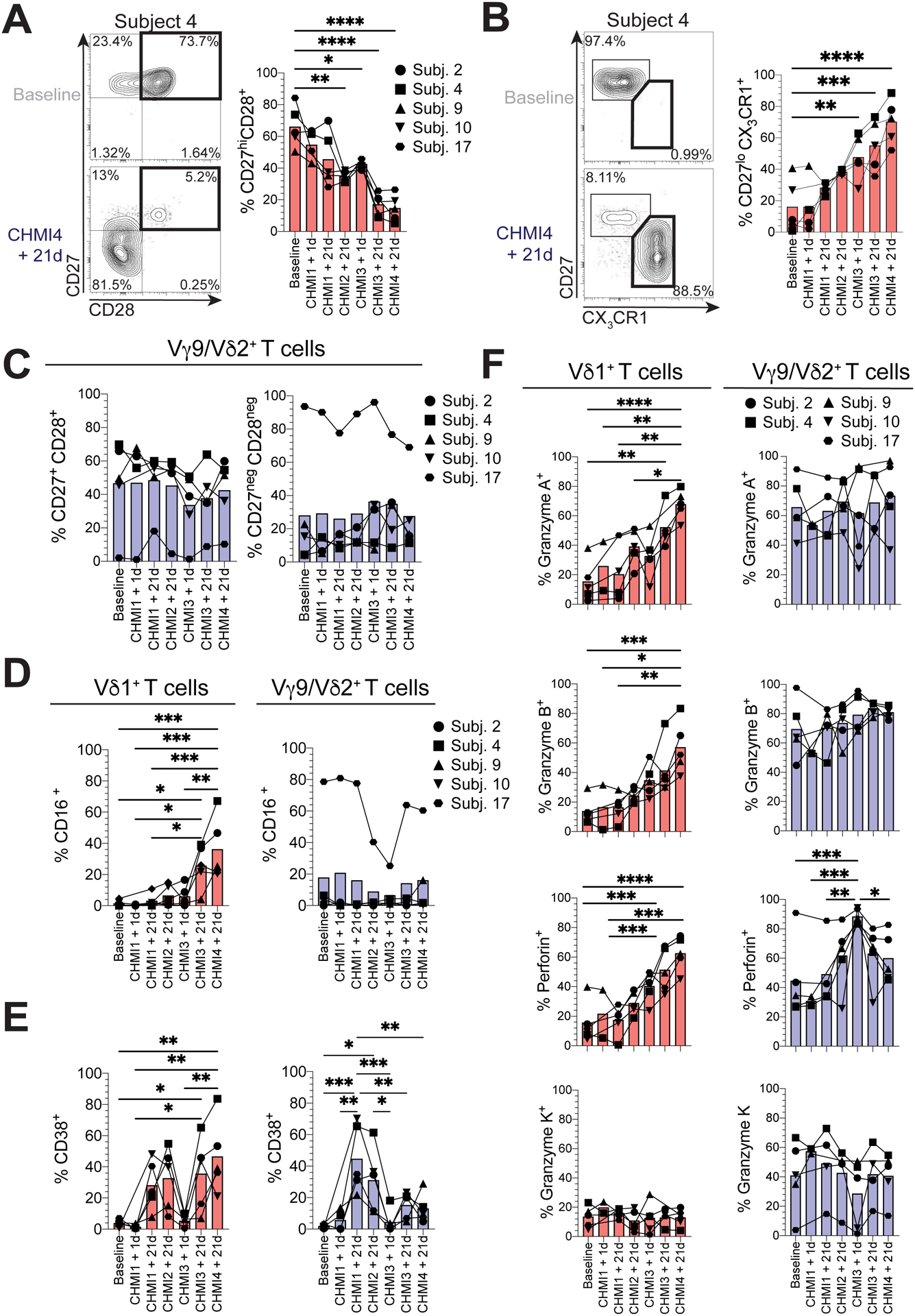
A. Representative flow cytometry plot and graph showing the frequencies of CD27+ CD28+ cells in Vδ1+ T cells after repeated CHMIs (n=5). B. Representative flow cytometry plot and graph showing the differentiation of CD27lo CX3CR1+ Vδ1+effector cells after repeated CHMIs (n=5). C. Frequencies of CD27+ CD28+ and CD27neg CD28neg cells within Vγ9/Vδ2+ T cells. D-F. Within Vδ1+ (red) and Vγ9/Vδ2+ (blue) T cells, the frequencies of: D. CD16+, E. CD38+, F. Gzm A+, B+, K+ and perforin+ cells. Bars show the mean. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; p-values were determined by linear mixed effects modelling with Bonferroni’s correction.
Repeated P. falciparum infections drive diverse waves of γδTCR selection
Next, we sought to understand whether repeated CHMIs impacted γδTCR repertoires. We used the approach described above (Fig. 2) and sorted Vδ2+ and Vδ2neg γδ T cell populations from longitudinal timepoints from all five CHMI subjects. We then analyzed the relationship between CD27lo CX3CR1+ Vδ2neg effector cells and Vδ2neg TCR repertoires prior to CHMI1 and at CHMI4 + 21d in subject 2 (Fig. 5A). At baseline, Vδ2neg γδ T cells were predominantly CD27hi CX3CR1neg and displayed a reasonably diverse γδTCR repertoire (Fig. 5A), but then after repeated CHMIs we observed a shift toward a CD27lo CX3CR1+ effector phenotype (Fig. 5A). Alongside these phenotypic changes, clonotypes found prior to CHMI1 remained stable or contracted over time, and new Vδ1+ clonotypes expanded, suggesting the potential recruitment of specific TCR sequences into the γδ T cell immune repertoire after repeated CHMIs (Fig. 5B). Analysis of the Vδ2neg γδ T cell repertoires indicated Vγ9 and Vδ1 chain usage to be the most prevalent (Fig. 5C). Overall, diversity within Vδ2neg or Vδ2+ γδ T cell repertoires did not show any significant change (Fig. S5A). Next, we assessed if CDR3 clonotype changes were occurring in Vδ2+ TCR repertoires. We found that Vδ2+ clonotypes remained stable over time, despite significant changes in the frequencies of the total population (Fig. 5D). Interestingly, in subject 17, the Vδ2+ TCR repertoire was already dominated by hyperexpanded CDR3γ and δ sequences at baseline (Fig. S5B), in contrast to all other Vδ2+ TCR repertoires in this study. Given the stability of Vδ2+ TCR clonotype repertoires at each CHMI, we then assessed the potential for dynamic changes in Vδ2neg γδ T cell repertoires at each CHMI and over time. Analysis of the γδTCR repertoire of subject 4 from baseline and over subsequent CHMI’s 1, 3 and 4 indicated dynamic changes in the TCR repertoire, with an increase in low frequency clonotypes at CHMI1 and establishment of a broader immune repertoire over time (Fig. 5E). We then analyzed the 20 most prevalent clonotypes at baseline (subject 4, 10, 17) or at CHMI1 (subject 9; Fig. S5C). We found that prevalent baseline clonotypes declined with each CHMI and we observed waves of new clonotypes that expanded into the most abundant 20 clonotypes after each CHMI (Fig. 5F). In many cases these clonotypes were found at low frequency in the preceding timepoint, suggesting that each CHMI drove rounds of γδTCR selection (Fig. 5F). Vδ2neg γδ T cell repertoire clonotypes possessed few overlapping clonotypes between individuals, while there were many inter-individual overlapping TCRγ sequences in Vδ2+ TCR repertoires (Fig. S5D). Although subject 10 and 17 were symptomatic at CHMI4 and did not have a significant increase in Vδ1+ T cell frequencies, the repertoire of their Vδ2neg γδTCR repertoire also displayed waves of clonotype selection (Fig. 5F). Together, the Vγ9/Vδ2+ T cell response to P. falciparum infection displays a highly stable polyclonal immune repertoire over time and infection. In contrast, Vδ2neg γδ T cell repertoires underwent dramatic remodeling of the γδTCR repertoire and displayed waves of clonal selection after each P. falciparum infection.
Figure 5. Repeated P. falciparum infection drives waves of Vδ1 TCR clonotype selection.
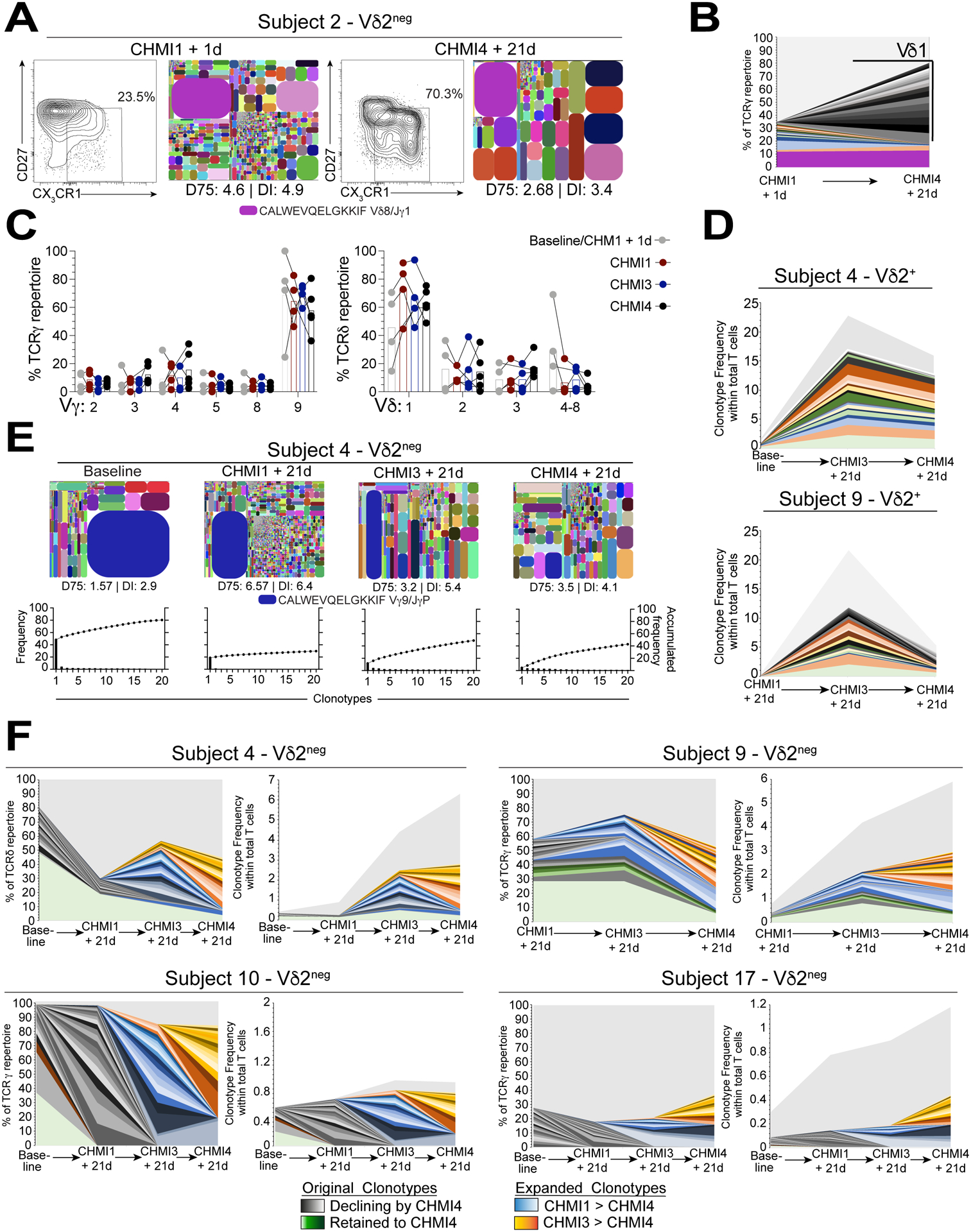
A. Flow cytometry plots showing frequencies of CD27lo CX3CR1+ Vδ2neg γδ T cells after repeated CHMI challenge in subject 2. TCRδ tree plots of the corresponding total Vδ2neg γδ T cells and DI are given for each tree plot. B. Increase in new Vδ1 sequences between baseline and CHMI4 within the top 20 clonotypes in TCRγ from subject 2. C. Vγ and Vδ usage in Vδ2neg T cell repertoires from baseline to CHMI 4 (n=4–5). D. Longitudinal tracking of the top 20 CDR3γ clonotypes in Vδ2+ T cell repertoires as a frequency of total CD3+ T cell populations. E. TCRγ tree plots showing Vδ2neg TCR repertoires at baseline and after repeated CHMIs in subject 4. The D75 and DI metrics are indicated. The graphs show the accumulated frequency of the top 20 clonotypes for each repertoire. F. Longitudinal tracking of the top 20 CDR3γ clonotypes in Vδ2neg TCR repertoires from subject 4, 9, 10, 17; displayed as a proportion of the total TCRγ repertoire (left) or within the total CD3+ T cell population (right).
Previous P. falciparum exposure licenses Vδ1+ T cells reactivity to blood-stage parasites
Finally, we explored the reactivity of γδ T cell subsets towards P. falciparum blood-stage parasites. PBMCs from Australian adults with no history of malaria exposure were co-cultured with P. falciparum infected red blood cells (PfRBC) or trophozoite/schizont extracts (PfTSE) or or intact uninfected RBCs (uRBC) or extracts (uRBCE) as controls. Vδ1+ T cells from Australian adults were unresponsive to PfRBCs or PfTSE, whereas Vγ9/Vδ2+ T cell populations were responsive (Fig. 6A), corroborating prior studies (24, 33, 42). Our in vivo results (Fig. 3, 4 and 5) prompted us to re-challenge PBMCs of Australian subjects twice over the 5-day culture period. Upon re-challenge we found that Vδ1+ T cells showed varying levels of proliferation after the second re-stimulation (Fig. 6B) but only in response to PfTSE and not PfRBCs. Previous studies have reported that Vδ1+ T cells from individuals living in malaria endemic regions of Gambia or Tanzania were unresponsive to PfRBC in vitro (33, 42, 43). Using PBMCs from two Malian subjects and a malaria-naïve subject after 2 CHMIs (subject 10 at CHMI3+1d), we found that Vδ1+ T cells proliferated in response to PfTSE but not PfRBC after a single stimulation (Fig. 6C and D). This differential responsiveness to PfRBC or PfTSE was not consistently seen in paired Vγ9/Vδ2+ T cell populations or in malaria unexposed Australian subjects (Fig. 6D). These data indicate that prior P. falciparum infection primes Vδ1+ T cells for proliferate upon re-challenge with P. falciparum parasites.
Figure 6. Previous P. falciparum exposure licenses Vδ1+ T cells for parasite reactivity.
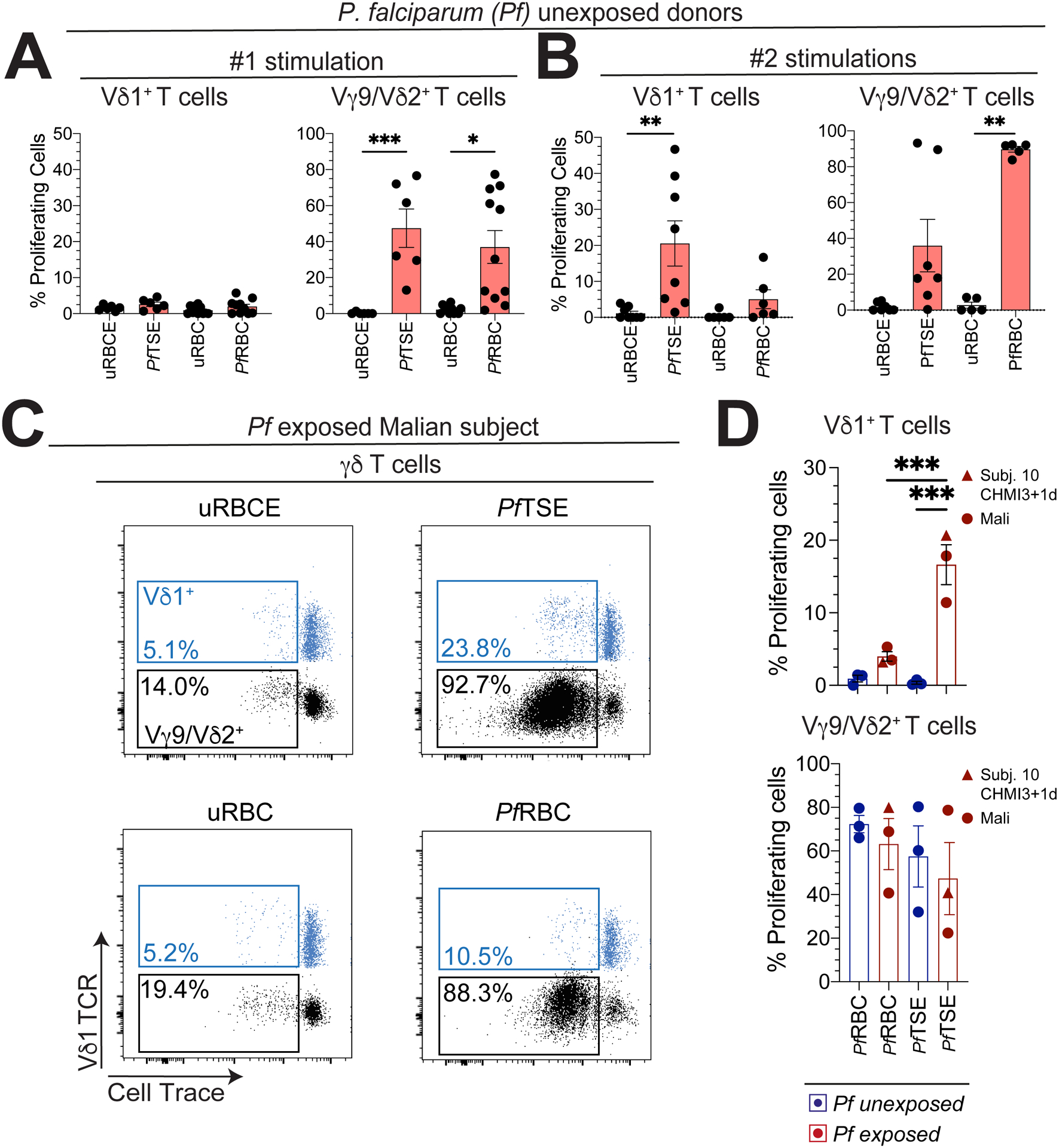
Vδ1+ and Vγ9/Vδ2+ T cells were assessed for proliferation in Australian adult donors with no history of malaria, PBMCs were labelled with cell trace and incubated for 6 days with A. One or B. two stimulations (at day 0 and 3 of culture) with P. falciparum trophozoite/early schizont extract (PfTSE) or infected red blood cells (RBCs) and uninfected RBCs (uRBC) or extract (uRBCE) (uRBCE/PfTSE: n=6; uRBC/PfRBC: n=10). C. Representative flow cytometry plots show Vδ1+ (blue) and Vδ2+ (black) T cells assessed for proliferation in the PBMCs from a Malian subject after co-culture with PfTSE. PfRBCs, uRBC or uRBCE controls. D. Graphs show the proliferation of Vδ1+ and Vγ9/Vδ2+ T cells from two Malian subjects with a history of repeated prior exposure to P. falciparum malaria, subject 10 at CHMI3 + 1d and three independent Australian donors with no history of malaria exposure. Each data point represents the proportion of proliferating cells in cultures exposed to PfRBCs or PfTSE minus the response to uRBC or uRBCE controls. Bars show the mean ± SEM. Normality was tested using the Shapiro-Wilk test.; **P < 0.01; p-values were determined by one-way ANOVA with Holm-Sidak’s post hoc testing (d).
Discussion
γδ T cells have been implicated in the immune response to pathogenic microbes, including bacteria, viruses and parasites (9). These responses in mice and humans appear to be mediated by innate-like γδ T cell populations, often utilizing semi- or invariant γδTCR repertoires that allow rapid effector responses to be mounted during the acute phases of microbial infection (9). Emerging evidence is currently re-shaping our understanding of the immunobiology of human γδ T cell populations and γδ T cells have the potential for both innate and adaptive properties (44). However, the adaptive-like features of Vδ2neg γδ T cell subsets are only partially understood (19, 28, 29, 45), and the establishment of this arm of the immune response to infectious disease has remained unclear.
Here, we show that repeated in vivo P. falciparum infection impacts populations of circulating innate-like Vδ2+ and adaptive-like Vδ1+ γδ T cells. We found that repeated P. falciparum infection triggers the differentiation of Vδ1+ T cells from a Vδ1naive phenotype into a distinct Vδ1effector subset, concomitant with dynamic clonotype selection in the γδTCR repertoire with each P. falciparum infection. Together, our data indicate that P. falciparum infection drives the selection and differentiation of the γδ T cell repertoire.
The association of human γδ T cells and malaria has been largely attributed to the remarkable responsiveness of innate-like Vγ9/Vδ2+ T cells to P. falciparum infection (43, 46, 47). In line with this, we found that Vγ9/Vδ2+ T cells were retained after natural infection in Malian subjects and increased in frequency upon exposure to repeated CHMI, an observations that is likely due to encounter with blood stage P. falciparum merozoite-derived HMB-PP (15, 24, 48), and possibly also be in response to liver stage infection (4, 7, 49). However, notwithstanding hypotheses that Vγ9/Vδ2+ T cells mount oligoclonal responses to microbial encounters (50, 51), we found that public Vγ9/Vδ2+ TCR repertoires remained highly stable over time despite significant changes in cellular frequency. The composition of these repertoires were very similar to those seen in gestation (52), cord blood, and after birth (17–19). Moreover, the cellular phenotype of Vγ9/Vδ2+ T cells after repeated P. falciparum infection was highly stable. Thus, the γδTCR repertoire of innate-like Vγ9/Vδ2+ T cells appears to allow sustained responsiveness upon P. falciparum infection.
In contrast to Vγ9/Vδ2+ T cells, the exact nature of human Vδ2− γδ T cells, and in particular Vδ1+ T cells, in the immune response to microbial pathogens is poorly defined, with recent studies identifying both innate- (26) and adaptive-like potential for these cells (28). Moreover, how Vδ1+ T cells participate in the complex immune response to P. falciparum is largely unknown (30, 33, 34, 53, 54). Current paradigms for conventional memory αβ T cells indicate that T effectors arise from T naïve cells driven by antigen-specific challenge to provide a rapid memory-response upon re-exposure to the same pathogen (55). Whether a similar paradigm applies to human γδ T cells is unclear (56). Here, we demonstrate that Vδ1effector T cells are a major population in Malian children, and that Vδ1naive cells differentiate into Vδ1effectors after repeated P. falciparum infections in malaria-naïve adults. Given that Vδ1effector γδ T cells may infiltrate peripheral tissues (25), we speculate that P. falciparum-reactive Vδ1+ γδ T cells will subsequently infiltrate the liver (25), and spleen (57). Therefore, P. falciparum-reactive Vδ1+ γδ T cells may exert cytotoxic and/or immunoregulatory functions in peripheral tissues during malaria infection and may be possible to explore under certain clinical circumstances (58). Moreover, we also found that γδTCR repertoires undergo dynamic clonotype selection after each P. falciparum infection. While only a handful of the antigenic targets are known for Vδ2− γδTCRs, nearly all identified ligands to date are endogenous host proteins (13, 59). In the case of malaria, it has been proposed that the Vδ1+ T cell response during P. falciparum infection is also driven by unknown endogenous host factors, based on the observation that Vδ1+ T cells from malaria-exposed individuals do not respond to P. falciparum antigens in vitro (33, 42). Our findings, that Vδ1+ T cells from malaria-exposed individuals react to P. falciparum lysate in vitro, suggests that Vδ1+ T cells may also have the potential to recognize parasite-derived antigens.
In malaria endemic regions, non-sterilizing immunity to symptomatic malaria is gradually acquired with repeated P. falciparum infections (60). It is hypothesized that the acquisition of immunity to malaria in humans involves resistance to severe disease followed by resistance to uncomplicated disease (3). Our study provides a window into the dynamic evolution of innate-and adaptive-like γδ T cells in the context of natural P. falciparum infection and indicates that these cells may represent an important component of the cellular immune response that contributes to immunity to malaria (4–6). However, we cannot conclude from the current study that there is an association between Vδ1effector T cell expansion and protection from febrile malaria in the context of natural infection, as Malian children who still experience febrile malaria show evidence of Vδ1effector T cell expansion. Our previous analysis of the same cohort in Mali shows that the risk of febrile malaria gradually decreases with age over years of repeated malaria exposures (1). Subjects in the age range (7–14 years) included in the longitudinal portion of the current study are at lower risk of febrile malaria than younger children in the same cohort, but generally, even 7–14-year-olds have yet to acquire immunity that fully protects against febrile malaria from year to year, leaving open the possibility that Vδ1effector T cell expansion with repeated infections may contribute to the gradual acquisition of immunity to malaria in endemic areas. Studies with larger sample sizes that encompass a broader age range and include more frequent assessments of γδ T cells relative to incident P. falciparum infections (both symptomatic and asymptomatic), will be required to assess the relationship between Vδ1effector T cells and the risk of febrile malaria in the context of natural infection.
The findings from our CHMI study suggest that initial Vγ9/Vδ2+ T cell activation may contribute to the early priming and activation of naïve Vδ1+ γδ T cells, potentially involving the capacity of Vγ9/Vδ2+ T cells to phagocytose and present parasite antigens (22, 24). Moreover, we noted a reduction in Vγ9/Vδ2+ T cells at the fourth CHMI, consistent with prior studies showing that loss and dysfunction of Vδ2+ T cells is associated with clinical immunity to malaria (40). How the emerging population of Vδ1+ γδ T cells may contribute to protection from symptomatic malaria is unclear. While the regulatory functions of γδ T cells in response to infectious diseases remains poorly understood, there is mounting evidence that these cells may play a role in regulating inflammation in the context of cancer (61, 62). Therefore, it seems plausible that repeated febrile malaria episodes could drive the expansion of a regulatory population of Vδ1+ γδ T cells that dampen inflammation through IL-10 (31), TGF-β1 (63) or other mechanisms (64).
There are several limitations of this study. First, the Mali cohort was conducted in a small rural village where the population is predominantly of a single ethnic group, limiting the generalizability of our findings. Nonetheless, we observed lower frequencies of CD16+ Vγ9/Vδ2+ T cells in the Mali cohort relative to studies in Uganda and Brazil (24, 65). Thus, it will be of interest to further investigate the impact of genetics and/or environmental factors underlying regional differences. Secondly, the number of subjects included in the CHMI study was relatively small, precluding a rigorous analysis of the factors that underlie the inter-individual variability we observed in γδ T cell responses.
In summary, our study shows that both innate and adaptive-like properties of the human γδ T cell repertoire are driven by P. falciparum infection in vivo. Vδ2+ γδ T cells mount a rapid innate-like polyclonal immune response to acute P. falciparum infection. Alongside these innate-like Vδ2+ γδ T cell responses, repeated P. falciparum infection established clonally selected populations of adaptive-like Vδ1effector γδ T cells. Together, our study suggests the importance of future studies exploring the role of the γδ T cell repertoire in contributing to the establishment of clinical immunity to malaria.
Supplementary Material
Materials and Methods
Figure S1. T cells frequencies in Malian subjects exposed to P. falciparum infection.
Figure S2. The gating strategy used to sort γδ T cells.
Figure S3. Longitudinal γδ TCR analysis in Malian subjects.
Figure S4. T cells frequencies in Malian subjects exposed to P. falciparum infection.
Figure S5. Longitudinal γδ TCR analysis in CHMI subjects.
Table S1. Study cohort.
Table S2. Symptoms recorded for each CHMI.
Table S3. Flow cytometry antibodies.
Table S4. Number of cells sorted for TRD and TRG for each sample.
Data File S1.
Acknowledgments:
We thank the study volunteers in Kenya and Mali, as well as the U.S. CHMI study volunteers for their contribution and commitment to malaria research. We thank Dr. Gregory Deye, of the National Institutes of Allergy and Infectious Diseases at the National Institutes of Health for service as program medical officer of the repetitive challenge study at the University of Maryland, Baltimore (UMB), Faith Pa’ahana-Brown, RN, Lisa Chrisley, RN, Alyson Kwon, Brenda Dorsey, Ana Raquel Da Costa, Jeffrey Crum, Kathleen Strauss, and Biraj Shrestha for their roles in the repetitive challenge study at UMB. We also thank Sanaria, Inc. for providing mosquitoes for human malaria infections. We thank FlowCore (Monash University) for assistance with cell sorting, and the Medical Genomics Facility (Hudson Institute) for their services. We also thank Dr. Eldho Paul from Monash Biostatistics Platform for statistical analyses. We also thank Prof. Benjamin E. Willcox, Dr Carrie R. Willcox, Dr Robert Seder, Prof. Matthias Eberl and Prof. Adrian Hayday for helpful discussions.
Funding:
M.S.D. is supported by an Australian Research Council (ARC) Discovery Early Career Researcher Award (DE200100292) and this work was funded by a Rebecca L. Cooper Medical Research Foundation Project Grant (PG2020668) and ARC Discovery Project (DP210103327). C.L.D., P.O., and J.D.E. are supported by grants from the U.S. National Institutes of Health (U19 AI111211 and R01 AI111948). K.C.W. and K.E.L are supported by a National Institutes of Health (NIH), Division of Allergy and Infectious Diseases (NIAID) U01 (AI-110852), distributed by the Henry M. Jackson Foundation (#1701447C). K.E.L. is further supported by additional funding from the NIAID (U01-HD092308, R01-AE141900, AI110820-06), The Geneva Foundation (V-12VAXHRFS-03), Medical Technology Enterprise Consortium (MTEC-17-01) and Pfizer Inc (C4591001, site 1002). The Mali study was funded by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. J.R. is supported by an ARC Laureate Fellowship.
Footnotes
Publisher's Disclaimer: Disclaimers: The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Competing interests: The authors declare no competing interests.
Data and materials availability:
All data are available in Data File S1. The T cell receptor (TCR) sequence data that support the findings of this study have been deposited in the Open Science Framework (OSF) and is accessible from https://osf.io/7rdm9/ and https://osf.io/3qvmh/.
References:
- 1.Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, Bathily A, Sangala J, Kone Y, Traore A, Niangaly M, Dara C, Kayentao K, Ongoiba A, Doumbo OK, Traore B, Crompton PD, An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis 57, 40–47 (2013); published online EpubJul ( 10.1093/cid/cit174). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Good MF, Doolan DL, Immune effector mechanisms in malaria. Curr Opin Immunol 11, 412–419 (1999); published online EpubAug ( 10.1016/S0952-7915(99)80069-7). [DOI] [PubMed] [Google Scholar]
- 3.Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas-Mury C, Pierce SK, Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol 32, 157–187 (2014) 10.1146/annurev-immunol-032713-120220). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, Enama ME, Gordon IJ, Chang LJ, Sarwar UN, Zephir KL, Holman LA, James ER, Billingsley PF, Gunasekera A, Chakravarty S, Manoj A, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, N. K C, Murshedkar T, DeCederfelt H, Plummer SH, Hendel CS, Novik L, Costner PJ, Saunders JG, Laurens MB, Plowe CV, Flynn B, Whalen WR, Todd JP, Noor J, Rao S, Sierra-Davidson K, Lynn GM, Epstein JE, Kemp MA, Fahle GA, Mikolajczak SA, Fishbaugher M, Sack BK, Kappe SH, Davidson SA, Garver LS, Bjorkstrom NK, Nason MC, Graham BS, Roederer M, Sim BK, Hoffman SL, Ledgerwood JE, Seder RA, Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 22, 614–623 (2016); published online EpubJun ( 10.1038/nm.4110). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaidi I, Diallo H, Conteh S, Robbins Y, Kolasny J, Orr-Gonzalez S, Carter D, Butler B, Lambert L, Brickley E, Morrison R, Sissoko M, Healy SA, Sim BKL, Doumbo OK, Hoffman SL, Duffy PE, gammadelta T Cells Are Required for the Induction of Sterile Immunity during Irradiated Sporozoite Vaccinations. J Immunol 199, 3781–3788 (2017); published online EpubDec 1 ( 10.4049/jimmunol.1700314). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong SE, van Unen V, Manurung MD, Stam KA, Goeman JJ, Jochems SP, Hollt T, Pezzotti N, Mouwenda YD, Betouke Ongwe ME, Lorenz FR, Kruize YCM, Azimi S, Konig MH, Vilanova A, Eisemann E, Lelieveldt BPF, Roestenberg M, Sim BKL, Reinders MJT, Fendel R, Hoffman SL, Kremsner PG, Koning F, Mordmuller B, Lell B, Yazdanbakhsh M, Systems analysis and controlled malaria infection in Europeans and Africans elucidate naturally acquired immunity. Nat Immunol 22, 654–665 (2021); published online EpubMay ( 10.1038/s41590-021-00911-7). [DOI] [PubMed] [Google Scholar]
- 7.Mwakingwe-Omari A, Healy SA, Lane J, Cook DM, Kalhori S, Wyatt C, Kolluri A, Marte-Salcedo O, Imeru A, Nason M, Ding LK, Decederfelt H, Duan J, Neal J, Raiten J, Lee G, Hume JCC, Jeon JE, Ikpeama I, Kc N, Chakravarty S, Murshedkar T, Church LWP, Manoj A, Gunasekera A, Anderson C, Murphy SC, March S, Bhatia SN, James ER, Billingsley PF, Sim BKL, Richie TL, Zaidi I, Hoffman SL, Duffy PE, Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Nature 595, 289–294 (2021); published online EpubJul ( 10.1038/s41586-021-03684-z). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonneville M, O’Brien RL, Born WK, Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 10, 467–478 (2010); published online EpubJul ( 10.1038/nri2781). [DOI] [PubMed] [Google Scholar]
- 9.Chien YH, Meyer C, Bonneville M, gammadelta T cells: first line of defense and beyond. Annu Rev Immunol 32, 121–155 (2014) 10.1146/annurev-immunol-032713-120216). [DOI] [PubMed] [Google Scholar]
- 10.Hayday AC, [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 18, 975–1026 (2000) 10.1146/annurev.immunol.18.1.975). [DOI] [PubMed] [Google Scholar]
- 11.Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J, T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol 33, 169–200 (2015) 10.1146/annurevimmunol-032414-112334). [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, Brenner MB, Bloom BR, Morita CT, Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci U S A 91, 8175–8179 (1994); published online EpubAug 16 ( 10.1073/pnas.91.17.8175). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willcox BE, Willcox CR, gammadelta TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol 20, 121–128 (2019); published online EpubFeb ( 10.1038/s41590-018-0304-y). [DOI] [PubMed] [Google Scholar]
- 14.Hirano M, Guo P, McCurley N, Schorpp M, Das S, Boehm T, Cooper MD, Evolutionary implications of a third lymphocyte lineage in lampreys. Nature 501, 435–438 (2013); published online EpubSep 19 ( 10.1038/nature12467). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morita CT, Jin C, Sarikonda G, Wang H, Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 215, 59–76 (2007); published online EpubFeb ( 10.1111/j.1600-065X.2006.00479.x). [DOI] [PubMed] [Google Scholar]
- 16.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H, Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett 509, 317–322 (2001); published online EpubDec 7 ( 10.1016/s0014-5793(01)03191-x). [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulou M, Dimova T, Shey M, Briel L, Veldtsman H, Khomba N, Africa H, Steyn M, Hanekom WA, Scriba TJ, Nemes E, Vermijlen D, Fetal public Vgamma9Vdelta2 T cells expand and gain potent cytotoxic functions early after birth. Proceedings of the National Academy of Sciences of the United States of America, (2020); published online EpubJul 14 ( 10.1073/pnas.1922595117). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravens S, Fichtner AS, Willers M, Torkornoo D, Pirr S, Schoning J, Deseke M, Sandrock I, Bubke A, Wilharm A, Dodoo D, Egyir B, Flanagan KL, Steinbruck L, Dickinson P, Ghazal P, Adu B, Viemann D, Prinz I, Microbial exposure drives polyclonal expansion of innate gammadelta T cells immediately after birth. Proc Natl Acad Sci U S A 117, 18649–18660 (2020); published online EpubAug 4 ( 10.1073/pnas.1922588117). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davey MS, Willcox CR, Hunter S, Kasatskaya SA, Remmerswaal EBM, Salim M, Mohammed F, Bemelman FJ, Chudakov DM, Oo YH, Willcox BE, The human Vdelta2(+) T-cell compartment comprises distinct innate-like Vgamma9(+) and adaptive Vgamma9(−) subsets. Nat Commun 9, 1760 (2018); published online EpubMay 2 ( 10.1038/s41467-018-04076-0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roussilhon C, Agrapart M, Guglielmi P, Bensussan A, Brasseur P, Ballet JJ, Human TcR gamma delta+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin Exp Immunol 95, 91–97 (1994); published online EpubJan ( 10.1111/j.1365-2249.1994.tb06020.x). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riganti C, Massaia M, Davey MS, Eberl M, Human gammadelta T-cell responses in infection and immunotherapy: common mechanisms, common mediators? Eur J Immunol 42, 1668–1676 (2012); published online EpubJul ( 10.1002/eji.201242492). [DOI] [PubMed] [Google Scholar]
- 22.Howard J, Loizon S, Tyler CJ, Duluc D, Moser B, Mechain M, Duvignaud A, Malvy D, Troye-Blomberg M, Moreau JF, Eberl M, Mercereau-Puijalon O, Dechanet-Merville J, Behr C, Mamani-Matsuda M, The Antigen-Presenting Potential of Vgamma9Vdelta2 T Cells During Plasmodium falciparum Blood-Stage Infection. J Infect Dis 215, 1569–1579 (2017); published online EpubMay 15 ( 10.1093/infdis/jix149). [DOI] [PubMed] [Google Scholar]
- 23.Costa G, Loizon S, Guenot M, Mocan I, Halary F, de Saint-Basile G, Pitard V, Dechanet-Merville J, Moreau JF, Troye-Blomberg M, Mercereau-Puijalon O, Behr C, Control of Plasmodium falciparum erythrocytic cycle: gammadelta T cells target the red blood cell-invasive merozoites. Blood 118, 6952–6962 (2011); published online EpubDec 22 ( 10.1182/blood-2011-08-376111). [DOI] [PubMed] [Google Scholar]
- 24.Junqueira C, Polidoro RB, Castro G, Absalon S, Liang Z, Sen Santara S, Crespo A, Pereira DB, Gazzinelli RT, Dvorin JD, Lieberman J, gammadelta T cells suppress Plasmodium falciparum blood-stage infection by direct killing and phagocytosis. Nat Immunol 22, 347–357 (2021); published online EpubMar ( 10.1038/s41590-020-00847-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter S, Willcox CR, Davey MS, Kasatskaya SA, Jeffery HC, Chudakov DM, Oo YH, Willcox BE, Human liver infiltrating gammadelta T cells are composed of clonally expanded circulating and tissue-resident populations. J Hepatol, (2018); published online EpubMay 18 ( 10.1016/j.jhep.2018.05.007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Kyle-Cezar F, Woolf RT, Naceur-Lombardelli C, Owen J, Biswas D, Lorenc A, Vantourout P, Gazinska P, Grigoriadis A, Tutt A, Hayday A, An innate-like Vdelta1(+) gammadelta T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci Transl Med 11, (2019); published online EpubOct 9 ( 10.1126/scitranslmed.aax9364). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, Deban L, Cipolat S, Hart R, Iannitto ML, Laing A, Spencer-Dene B, East P, Gibbons D, Irving PM, Pereira P, Steinhoff U, Hayday A, Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific gammadelta T Cell Compartments. Cell 167, 203–218 e217 (2016); published online EpubSep 22 ( 10.1016/j.cell.2016.08.030). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davey MS, Willcox CR, Joyce SP, Ladell K, Kasatskaya SA, McLaren JE, Hunter S, Salim M, Mohammed F, Price DA, Chudakov DM, Willcox BE, Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nat Commun 8, 14760 (2017); published online EpubMar 1 ( 10.1038/ncomms14760). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdorfer L, Reinhardt A, Ravens I, Beck M, Geffers R, von Kaisenberg C, Heuser M, Thol F, Ganser A, Forster R, Koenecke C, Prinz I, Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat Immunol 18, 393–401 (2017); published online EpubApr ( 10.1038/ni.3686). [DOI] [PubMed] [Google Scholar]
- 30.Hviid L, Kurtzhals JA, Adabayeri V, Loizon S, Kemp K, Goka BQ, Lim A, Mercereau-Puijalon O, Akanmori BD, Behr C, Perturbation and proinflammatory type activation of V delta 1(+) gamma delta T cells in African children with Plasmodium falciparum malaria. Infect Immun 69, 3190–3196 (2001); published online EpubMay ( 10.1128/IAI.69.5.3190-3196.2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taniguchi T, Md Mannoor K, Nonaka D, Toma H, Li C, Narita M, Vanisaveth V, Kano S, Takahashi M, Watanabe H, A Unique Subset of gammadelta T Cells Expands and Produces IL-10 in Patients with Naturally Acquired Immunity against Falciparum Malaria. Front Microbiol 8, 1288 (2017) 10.3389/fmicb.2017.01288). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worku S, Bjorkman A, Troye-Blomberg M, Jemaneh L, Farnert A, Christensson B, Lymphocyte activation and subset redistribution in the peripheral blood in acute malaria illness: distinct gammadelta+ T cell patterns in Plasmodium falciparum and P. vivax infections. Clin Exp Immunol 108, 34–41 (1997); published online EpubApr ( 10.1046/j.1365-2249.1997.d01-981.x). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodier M, Krause-Jauer M, Sanni A, Massougbodji A, Sadeler BC, Mitchell GH, Modolell M, Eichmann K, Langhorne J, Gamma delta T cells in the peripheral blood of individuals from an area of holoendemic Plasmodium falciparum transmission. Trans R Soc Trop Med Hyg 87, 692–696 (1993); published online EpubNov-Dec ( 10.1016/0035-9203(93)90299-6). [DOI] [PubMed] [Google Scholar]
- 34.Hviid L, Smith-Togobo C, Willcox BE, Human Vdelta1(+) T Cells in the Immune Response to Plasmodium falciparum Infection. Front Immunol 10, 259 (2019) 10.3389/fimmu.2019.00259). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill DL, Carr EJ, Rutishauser T, Moncunill G, Campo JJ, Innocentin S, Mpina M, Nhabomba A, Tumbo A, Jairoce C, Moll HA, van Zelm MC, Dobano C, Daubenberger C, Linterman MA, Immune system development varies according to age, location, and anemia in African children. Sci Transl Med 12, (2020); published online EpubFeb 5 ( 10.1126/scitranslmed.aaw9522). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portugal S, Tran TM, Ongoiba A, Bathily A, Li S, Doumbo S, Skinner J, Doumtabe D, Kone Y, Sangala J, Jain A, Davies DH, Hung C, Liang L, Ricklefs S, Homann MV, Felgner PL, Porcella SF, Farnert A, Doumbo OK, Kayentao K, Greenwood BM, Traore B, Crompton PD, Treatment of Chronic Asymptomatic Plasmodium falciparum Infection Does Not Increase the Risk of Clinical Malaria Upon Reinfection. Clin Infect Dis 64, 645–653 (2017); published online EpubMar 1 ( 10.1093/cid/ciw849). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papadopoulou M, Dimova T, Shey M, Briel L, Veldtsman H, Khomba N, Africa H, Steyn M, Hanekom WA, Scriba TJ, Nemes E, Vermijlen D, Fetal public Vgamma9Vdelta2 T cells expand and gain potent cytotoxic functions early after birth. Proc Natl Acad Sci U S A 117, 18638–18648 (2020); published online EpubAug 4 ( 10.1073/pnas.1922595117). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jagannathan P, Lutwama F, Boyle MJ, Nankya F, Farrington LA, McIntyre TI, Bowen K, Naluwu K, Nalubega M, Musinguzi K, Sikyomu E, Budker R, Katureebe A, Rek J, Greenhouse B, Dorsey G, Kamya MR, Feeney ME, Vdelta2+ T cell response to malaria correlates with protection from infection but is attenuated with repeated exposure. Sci Rep 7, 11487 (2017); published online EpubSep 13 ( 10.1038/s41598-017-10624-3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farrington LA, Jagannathan P, McIntyre TI, Vance HM, Bowen K, Boyle MJ, Nankya F, Wamala S, Auma A, Nalubega M, Sikyomu E, Naluwu K, Bigira V, Kapisi J, Dorsey G, Kamya MR, Feeney ME, Frequent Malaria Drives Progressive Vdelta2 T-Cell Loss, Dysfunction, and CD16 Up-regulation During Early Childhood. J Infect Dis 213, 1483–1490 (2016); published online EpubMay 1 ( 10.1093/infdis/jiv600). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagannathan P, Kim CC, Greenhouse B, Nankya F, Bowen K, Eccles-James I, Muhindo MK, Arinaitwe E, Tappero JW, Kamya MR, Dorsey G, Feeney ME, Loss and dysfunction of Vdelta2(+) gammadelta T cells are associated with clinical tolerance to malaria. Sci Transl Med 6, 251ra117 (2014); published online EpubAug 27 ( 10.1126/scitranslmed.3009793). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wragg KM, Tan HX, Kristensen AB, Nguyen-Robertson CV, Kelleher AD, Parsons MS, Wheatley AK, Berzins SP, Pellicci DG, Kent SJ, Juno JA, High CD26 and Low CD94 Expression Identifies an IL-23 Responsive Vdelta2(+) T Cell Subset with a MAIT Cell-like Transcriptional Profile. Cell Rep 31, 107773 (2020); published online EpubJun 16 ( 10.1016/j.celrep.2020.107773). [DOI] [PubMed] [Google Scholar]
- 42.Rutishauser T, Lepore M, Di Blasi D, Dangy JP, Abdulla S, Jongo S, Ramadhani K, Sim BKL, Hoffman SL, Tanner M, Daubenberger C, De Libero G, Activation of TCR Vdelta1(+) and Vdelta1(−)Vdelta2(−) gammadelta T Cells upon Controlled Infection with Plasmodium falciparum in Tanzanian Volunteers. J Immunol 204, 180–191 (2020); published online EpubJan 1 ( 10.4049/jimmunol.1900669). [DOI] [PubMed] [Google Scholar]
- 43.Goodier M, Fey P, Eichmann K, Langhorne J, Human peripheral blood gamma delta T cells respond to antigens of Plasmodium falciparum. Int Immunol 4, 33–41 (1992); published online EpubJan ( 10.1093/intimm/4.1.33). [DOI] [PubMed] [Google Scholar]
- 44.Hayday AC, gammadelta T Cell Update: Adaptate Orchestrators of Immune Surveillance. J Immunol 203, 311–320 (2019); published online EpubJul 15 ( 10.4049/jimmunol.1800934). [DOI] [PubMed] [Google Scholar]
- 45.Kaminski H, Menard C, El Hayani B, Adjibabi AN, Marseres G, Courant M, Zouine A, Pitard V, Garrigue I, Burrel S, Moreau JF, Couzi L, Visentin J, Merville P, Dechanet-Merville J, Characterization of a unique gammadelta T cell subset as a specific marker of CMV infection severity. J Infect Dis, (2020); published online EpubJul 5 ( 10.1093/infdis/jiaa400). [DOI] [PubMed] [Google Scholar]
- 46.Schmaler M, Orlova-Fink N, Rutishauser T, Abdulla S, Daubenberger C, Human unconventional T cells in Plasmodium falciparum infection. Semin Immunopathol 42, 265–277 (2020); published online EpubJun ( 10.1007/s00281-020-00791-3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behr C, Dubois P, Preferential expansion of V gamma 9 V delta 2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum. Int Immunol 4, 361–366 (1992); published online EpubMar ( 10.1093/intimm/4.3.361). [DOI] [PubMed] [Google Scholar]
- 48.Hernandez-Castaneda MA, Happ K, Cattalani F, Wallimann A, Blanchard M, Fellay I, Scolari B, Lannes N, Mbagwu S, Fellay B, Filgueira L, Mantel PY, Walch M, gammadelta T Cells Kill Plasmodium falciparum in a Granzyme- and Granulysin-Dependent Mechanism during the Late Blood Stage. J Immunol 204, 1798–1809 (2020); published online EpubApr 1 ( 10.4049/jimmunol.1900725). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL, Team VRCS, Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365 (2013); published online EpubSep 20 ( 10.1126/science.1241800). [DOI] [PubMed] [Google Scholar]
- 50.Willcox CR, Davey MS, Willcox BE, Development and Selection of the Human Vgamma9Vdelta2(+) T-Cell Repertoire. Front Immunol 9, 1501 (2018) 10.3389/fimmu.2018.01501). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pauza CD, Cairo C, Evolution and function of the TCR Vgamma9 chain repertoire: It’s good to be public. Cell Immunol 296, 22–30 (2015); published online EpubJul ( 10.1016/j.cellimm.2015.02.010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimova T, Brouwer M, Gosselin F, Tassignon J, Leo O, Donner C, Marchant A, Vermijlen D, Effector Vgamma9Vdelta2 T cells dominate the human fetal gammadelta T-cell repertoire. Proc Natl Acad Sci U S A 112, E556–565 (2015); published online EpubFeb 10 ( 10.1073/pnas.1412058112). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hviid L, Akanmori BD, Loizon S, Kurtzhals JA, Ricke CH, Lim A, Koram KA, Nkrumah FK, Mercereau-Puijalon O, Behr C, High frequency of circulating gamma delta T cells with dominance of the v(delta)1 subset in a healthy population. Int Immunol 12, 797–805 (2000); published online EpubJun ( 10.1093/intimm/12.6.797). [DOI] [PubMed] [Google Scholar]
- 54.Deroost K, Langhorne J, Gamma/Delta T Cells and Their Role in Protection Against Malaria. Front Immunol 9, 2973 (2018) 10.3389/fimmu.2018.02973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omilusik KD, Goldrath AW, The origins of memory T cells. Nature 552, 337–339 (2017); published online EpubDec 21 ( 10.1038/d41586-017-08280-8). [DOI] [PubMed] [Google Scholar]
- 56.Davey MS, Willcox CR, Baker AT, Hunter S, Willcox BE, Recasting Human Vdelta1 Lymphocytes in an Adaptive Role. Trends Immunol 39, 446–459 (2018); published online EpubJun ( 10.1016/j.it.2018.03.003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Falini B, Flenghi L, Pileri S, Pelicci P, Fagioli M, Martelli MF, Moretta L, Ciccone E, Distribution of T cells bearing different forms of the T cell receptor gamma/delta in normal and pathological human tissues. J Immunol 143, 2480–2488 (1989); published online EpubOct 15 ( [PubMed] [Google Scholar]
- 58.Kho S, Qotrunnada L, Leonardo L, Andries B, Wardani PAI, Fricot A, Henry B, Hardy D, Margyaningsih NI, Apriyanti D, Puspitasari AM, Prayoga P, Trianty L, Kenangalem E, Chretien F, Safeukui I, Del Portillo HA, Fernandez-Becerra C, Meibalan E, Marti M, Price RN, Woodberry T, Ndour PA, Russell BM, Yeo TW, Minigo G, Noviyanti R, Poespoprodjo JR, Siregar NC, Buffet PA, Anstey NM, Hidden Biomass of Intact Malaria Parasites in the Human Spleen. N Engl J Med 384, 2067–2069 (2021); published online EpubMay 27 ( 10.1056/NEJMc2023884). [DOI] [PubMed] [Google Scholar]
- 59.Willcox BE, Mohammed F, Willcox CR, gammadelta TCR Recognition of MR1: Adapting to Life on the Flip Side. Trends Biochem Sci 45, 551–553 (2020); published online EpubJul ( 10.1016/j.tibs.2020.03.012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marsh K, Kinyanjui S, Immune effector mechanisms in malaria. Parasite Immunol 28, 51–60 (2006); published online EpubJan-Feb ( 10.1111/j.1365-3024.2006.00808.x). [DOI] [PubMed] [Google Scholar]
- 61.Silva-Santos B, Serre K, Norell H, gammadelta T cells in cancer. Nat Rev Immunol 15, 683–691 (2015); published online EpubNov ( 10.1038/nri3904). [DOI] [PubMed] [Google Scholar]
- 62.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF, Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 27, 334–348 (2007); published online EpubAug ( 10.1016/j.immuni.2007.05.020). [DOI] [PubMed] [Google Scholar]
- 63.Bhagat G, Naiyer AJ, Shah JG, Harper J, Jabri B, Wang TC, Green PH, Manavalan JS, Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Invest 118, 281–293 (2008); published online EpubJan ( 10.1172/JCI30989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siegers GM, Lamb LS Jr., Cytotoxic and regulatory properties of circulating Vdelta1+ gammadelta T cells: a new player on the cell therapy field? Mol Ther 22, 1416–1422 (2014); published online EpubAug ( 10.1038/mt.2014.104). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farrington LA, Callaway PC, Vance HM, Baskevitch K, Lutz E, Warrier L, McIntyre TI, Budker R, Jagannathan P, Nankya F, Musinguzi K, Nalubega M, Sikyomu E, Naluwu K, Arinaitwe E, Dorsey G, Kamya MR, Feeney ME, Opsonized antigen activates Vdelta2+ T cells via CD16/FCgammaRIIIa in individuals with chronic malaria exposure. PLoS Pathog 16, e1008997 (2020); published online EpubOct ( 10.1371/journal.ppat.1008997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Figure S1. T cells frequencies in Malian subjects exposed to P. falciparum infection.
Figure S2. The gating strategy used to sort γδ T cells.
Figure S3. Longitudinal γδ TCR analysis in Malian subjects.
Figure S4. T cells frequencies in Malian subjects exposed to P. falciparum infection.
Figure S5. Longitudinal γδ TCR analysis in CHMI subjects.
Table S1. Study cohort.
Table S2. Symptoms recorded for each CHMI.
Table S3. Flow cytometry antibodies.
Table S4. Number of cells sorted for TRD and TRG for each sample.
Data File S1.
Data Availability Statement
All data are available in Data File S1. The T cell receptor (TCR) sequence data that support the findings of this study have been deposited in the Open Science Framework (OSF) and is accessible from https://osf.io/7rdm9/ and https://osf.io/3qvmh/.


