ABSTRACT
Transplantation of pancreatic islets is a promising approach to controlling glucose levels in type 1 diabetes mellitus (T1DM), but islet survival is still limited. To overcome this, islet co-culture with mesenchymal stromal cells (MSCs) together with safe immunosuppressive agents like squalene-gusperimus nanoparticles (Sq-GusNPs) may be applied. This could support islet survival and engraftment. Here, we studied how Sq-GusNPs and adipose-derived stem cells (ASCs) influence islets response under pro-inflammatory conditions. Through qRT-PCR, we studied the expression of specific genes at 24 hours in human and rat islets and ASCs in co-culture under indirect contact with or without treatment with Sq-GusNPs. We characterized how the response of islets and ASCs starts at molecular level before impaired viability or function is observed and how this response differs between species. Human islets and ASCs responses showed to be principally influenced by NF-κB activation, whereas rat islet and ASCs responses showed to be principally mediated by nitrosative stress. Rat islets showed tolerance to inflammatory conditions due to IL-1Ra secretion which was also observed in rat ASCs. Human islets induced the expression of cytokines and chemokines with pro-angiogenic, tissue repair, and anti-apoptotic properties in human ASCs under basal conditions. This expression was not inhibited by Sq-GusNPs. Our results showed a clear difference in the response elicited by human and rat islets and ASCs in front of an inflammatory stimulus and Sq-GusNPs. Our data support the use of ASCs and Sq-GusNP to facilitate engraftment of islets for T1DM treatment.
KEYWORDS: Squalene-gusperimus nanoparticles, adipose-derived stromal cells (ASCs) immunosuppressant, gene expression, qRT-PCR, pancreatic islets
Graphical abstract
Introduction
Transplantation of pancreatic islets is a promising alternative for exogenous insulin in patients with type 1 diabetes mellitus (T1DM). The advantage of islet transplantation over other therapies is that it regulates glucose on a minute-to-minute level.1 This approach has reached a certain degree of success, but its broad application is hampered by the high variation in the survival rate of the islets after the grafting procedure. The high variation has been attributed to factors such as differences in islet quality but also sensitivity to hypoxia and low nutrient conditions after implantation as well as to the cellular and humoral response triggered after transplantation.1,2 To overcome this, many strategies have been proposed to increase islet survival. Among these strategies is the use of new, safe immunosuppressive agents that are released in a controlled fashion to provide an anti-inflammatory microenvironment around islets in the transplantation site as well as the use of mesenchymal stromal cells that have shown to increase islets survival rate.3–9
Mesenchymal stromal cells (MSCs) are a somatic cell population present in many perinatal and adult tissues.10 They can be isolated from several tissues such as bone marrow, adipose tissue, connective tissue, umbilical cord, menstrual blood, and nasal mucosa.11 MSCs can self-renew, are easy to culture in vitro, and can differentiate into different cell types.10,12 These cells also have a high regenerative effect on surrounding tissue due to the capacity to produce soluble trophic factors.13 This can promote survival, regeneration, and function of different cells including those of pancreatic islets through paracrine mechanisms driven by MSCs-derived growth factors, cytokines, and chemokines.9,14 The factors secreted by MSCs can also modulate the immune system by inhibition of activation and proliferation of lymphocytes T and B, and dendritic cells, as well as by induction of polarization of macrophages from M1 to M2 state.12 Therefore, co-transplantation of pancreatic islets with MSCs is considered to have a high potential to prolong functional graft survival.9
Although MSCs have been proposed as a regeneration-supporting and anti-inflammatory cell-based approach for pancreatic islet transplantation,9 so far it has not been studied whether MSCs maintain these functional traits when exposed to immunosuppressive conditions. This is relevant as currently, islet transplantation is in the vast majority of cases applied in patients that are receiving a kidney transplant because of end-stage renal failure and chronic immunosuppression.15 Also, new methodologies are currently developed to apply local immunosuppression and controlled release of drugs to manage inflammatory responses around the grafts but their effects on MSCs function co-transplanted with islets is unknown.16–19
During the isolation and transplant process islets are submitted to harsh conditions that reduce the functional mass of the graft limiting its success. Especially the immediate posttransplant period is an extremely delicate period in which more than 60% of the islets have been reported to die due to enhanced sensitivity to inflammatory stress.20 This enhanced sensitivity is caused by a combination of disruption of the interactions between the islets and the peri-insular extracellular matrix as well as interruption of direct vascular access during the isolation process leading to low nutrient supply, high oxidative and hypoxic stress, and significant loss of insulin-producing cells posttransplant.14 To lower this loss we presented in a previous study a new controlled release system to modulate immunity in the immediate period after implantation.20 This involves squalene-gusperimus nanoparticles (Sq-GusNPs), a nanoparticulate system designed to encapsulate the immunosuppressive drug gusperimus to enhance the efficacy of this powerful anti-inflammatory drug.21
Adipose-derived stem cells (ASCs) are often used as an MSCs source because they have shown superiority regarding the secretion of paracrine molecules and they can be relatively easily isolated from the patient itself by taking a fat biopsy.14 So, they can serve as an autologous treatment in islet transplantation. Additionally, Sq-GusNPs have been shown to maintain the viability and function of human pancreatic islets under inflammatory conditions without cytotoxic effects.20 Here, we co-cultured pancreatic islets with ASCs in the presence and absence of IFN-γ, IL-1β, and TNF-α. This was done to gain insight into how islets and ASCs mutually influence each other in the presence or absence of an immunosuppressive agent under inflammatory or basal conditions. We determined the gene expression of pro-inflammatory, regulatory cytokines, or cell-death associated genes in both islets and ASCs after stimulation and/or treatment in both human and rat islets and ASCs. With this, we aimed to characterize the inflammatory response in the islets and how ASCs are influenced by the inflammatory conditions at molecular level in the first 24 hours. Additionally, we studied the differences in expression profiles between rat and human cells and whether potential synergistic effects between ASCs and Sq-GusNPs occur that contribute to functional survival of pancreatic islets.
Materials and methods
Materials
The bioconjugate squalene-gusperimus (Sq-Gus) was obtained starting from squalene and through consecutive reaction steps until obtention of the squalene carbocyclic acid derivative which was further reacted with gusperimus as previously reported.21 Absolute ethanol (EtOH) and HEPES were purchased from Merck (Darmstadt, Germany). DMEM supplemented with 4.5 g/L glucose and L-Glutamine was purchased from LONZA (Walkersville, MD, USA). CMRL-1066, Glutamax, penicillin-streptomycin, L-Glutamine, alamarBlueTM reagent, TRIzolTM reagent, and SuperScript II Reverse Transcriptase were purchased from Life Technologies Europe BV (Bleiswijk, The Netherlands). Fetal calf serum (FCS), D-(+)-Glucose, FastStart Universal SYBR Green Master (Rox), Histopaque®-1119, Histopaque®-1077, DNase I Roche, Corning® Transwell® polyester membrane cell culture inserts pore size 0.4 μm, membrane diameter 12 mm, and human and rat primers associated with gene expression of different cytokines and inflammatory factors were purchased from Sigma-Aldrich Chemie N.V. (Zwijndrecht, The Netherlands). Recombinant human interferon-gamma (rh IFN-γ), recombinant human interleukin-1 beta (rh IL-1β), recombinant human tumor necrosis factor-alpha (rh TNF-α), recombinant rat interferon-gamma (rr IFN-γ), recombinant rat interleukin-1 beta (rr IL-1β), and recombinant rat tumor necrosis factor-alpha (rr TNF-α) were purchased from ImmunoTools (Friesoythe, Germany). Human insulin ELISA kit Mercodia was purchased from Bio-Connect Diagnostics B.V. (Te Huissen, The Netherlands). Ultra-sensitive Rat Insulin ELISA Kit was purchased from Crystal Chem Europe (Zaandam, The Netherlands). Collagenase NB4 Standard Grade was purchased from Nordmark Pharma GmbH (Uetersen, Germany). Collagenase NB8 was purchased from Serva Electrophoresis (Heidelberg, Germany). The Invitrogen™ Quant-iT™ PicoGreen™ dsDNA reagent was purchased from Fisher Scientific (Landsmeer, The Netherlands).
Squalene-gusperimus nanoparticles (Sq-GusNPs) preparation
Sq-GusNPs were prepared through nanoprecipitation as previously reported.21 Briefly, the Sq-Gus bioconjugate was dissolved in EtOH at a concentration of 2 mg/mL. Later, 380 μL of the solution was added drop by drop to 1 mL of deionized water under stirring (500 rpm) for 10 minutes after which EtOH was evaporated using the concentrator SpeedVac SPD2010 (Thermo Fisher Scientific, Bleiswijk, The Netherlands). This resulted in an aqueous suspension of pure NPs with a size of 189.2 ± 73.83 nm as measured by dynamic light scattering (DLS) with the particle size analyzer NICOMP 380 ZLS (Particle Sizing Systems, Inc., Santa Barbara, CA, USA).
Cell culture
Pancreatic human or rat islets were cultured in CMRL-1066 medium supplemented with 8.3 mM glucose, 20 mM HEPES, 10% FCS heat-inactivated, 2 mM Glutamax, and 50 μg/mL penicillin/streptomycin as previously reported.22 Adipose tissue-derived stem cells (ASCs) from human or rat were cultured in DMEM supplemented with 4.5 g/L glucose and L-Glutamine, 10% FCS heat-inactivated, L-glutamine (2 mM), and 50 μg/mL penicillin/streptomycin. ASCs and islets were cultured in an incubator at 37°C and 5% CO2.
Islet isolation
Human islets
Human pancreatic islets were isolated from pancreata of deceased donors as previously described.23 The islets were obtained from the Leiden University Medical Center or through the JDRF award 31–2008-416 (European Consortium for Islet Transplantation, Islet for Basic Research program, Milan, Italy). Donor characteristics are specified in Table 1. Islets were used for research when the quality and/or number were insufficient for clinical application according to national laws and when research consent was available. All the procedures were approved and carried out under the code of proper secondary use of human tissue in The Netherlands as formulated by the Dutch Federation of Medical Scientific Societies. After shipment to the University Medical Center Groningen islets were cultured for 24 hours as described in the cell culture section before starting the experiments.
Table 1.
Donor information for human islets.
|
Donor |
Age (years) |
Gender |
BMI (Kg/m2) |
Islet isolation center |
Death cause |
Purity (%) |
Viability (%) |
| 1 | 54 | Male | 35 | LUMCa | Non-cardiac | 55 | >80 |
| 2 | 59 | Male | 23 | ECIT2 | Cerebral bleeding | 80 | 95 |
| 3 | 74 | Female | 26 | LUMC | Non-cardiac | 75 | >80 |
| 4 | 57 | Female | 27 | ECIT | Cerebral bleeding | 60 | 95 |
| 5 | 53 | Female | 27.2 | ECIT | Cerebral bleeding | 75 | 90 |
aLUMC: Leiden University Medical Center;2ECIT: European Consortium for Islet Transplantation
Rat islets
The Dutch Central Committee on Animal Testing (CCD) and Animal Welfare Authority at the University of Groningen approved all described animal procedures (CCD project number: AVD1050020185726). All experiments and procedures were performed in accordance with the Institution Animal Care Committee of the University of Groningen. All animals received animal care in compliance with the Dutch law on experiment animal care. Rat pancreatic islets were isolated from different and independent groups of Sprague-Dawley rats (Envigo, Horst, The Netherlands) weighing 250–300 g as previously described.24,25 Briefly, under anesthesia, the pancreas was distended by injecting a solution of collagenase NB8/DNase I into the bile duct. After dissection, the tissue was further digested by incubating the distended pancreas for 18 min in a water bath at 37°C under shaking. Subsequently, islets were separated from the exocrine tissue by a Histopaque (1119, 1077) density gradient. Islets with a diameter of 30–150 µm were handpicked, counted, and cultured for 24 hours as described in the cell culture section before starting the experiments.
Adipose tissue-derived stem cells (ASCs) isolation from perirenal fat
Human perirenal fat was obtained from surgical waste material of healthy kidney donors. For this purpose, no informed consent is required according to guidelines approved by University Medical Center Groningen (UMCG). Rat perirenal fat was isolated from Sprague-Dawley rats. Perirenal fat tissue was transferred to a petri dish and 2 mL of Hank’s balanced salt solution buffer (HBSS) was added. After cutting the tissue into small pieces, it was transferred to a 50 mL conical tube with 10 mL of HBSS and centrifuged at 500 ×g for 5 minutes at room temperature. Subsequently, HBSS solution was removed, washed again with additional 10 mL of HBSS, centrifuged at 500 ×g for 5 minutes at room temperature, and HBSS solution removed. Then, 0.5 mg/mL collagenase NB4 solution in DMEM supplemented with 1% penicillin/streptomycin was added to the fat and incubated for 30 minutes in a water bath at 37°C under shaking. Next, the mixture was vortexed and to stop digestion we added 20 mL of DMEM supplemented with 10% FCS, 1% penicillin/streptomycin, and 1% glutamine. The tube was centrifuged at 700 ×g for 7 minutes at room temperature, and the content washed again with 20 mL DMEM supplemented with 10% FCS, 1% penicillin/streptomycin, and 1% glutamine. Finally, cells were centrifuged at 700 ×g for 7 minutes at room temperature, fat and medium were removed, and ASCs in the pellet were transferred to a culture flask and cultured as indicated in cell culture section until use. The passage number of ASCs used in the experiments was 1–6. ASCs were seeded 24 h before starting the experiments.
Cell viability with alamarBlueTM
Cell viability was determined using the alamarBlueTM reagent as previously reported.20 Briefly, the reagent was diluted in culture medium (10% v/v). After treatment, ASCs or islets were washed with PBS and incubated for four hours with 0.5 mL of the diluted reagent. Later, fluorescence was measured with the plate reader CLARIOstarPlus (BMG LABTECH, Offenburg, Germany) Ex/Em 560/590 nm. For the islets, results were normalized to total DNA content as was determined using the Quanti-iT PicoGreen dsDNA kit (Invitrogen). Fluorescence obtained from ASCs or islets without any treatment was used as reference and control. Results were expressed as percentage of the control.
Glucose-stimulated insulin secretion (GSIS) test
After treatment, 25 handpicked islets were washed 2 times with 2.75 mM glucose solution prepared in 25 mM KRH buffer (low glucose solution). After a 90 minutes preincubation period (human islets) or 30 minutes (rat islets) with low glucose solution, islets were incubated for 60 min (human islets) or 45 min (rat islets) with low glucose solution, followed by a 60 min (human islets) or 45 min (rat islets) incubation with a 16.5 mM glucose solution prepared in 25 mM KRH buffer (high glucose solution). Finally, islets were washed a single time with low glucose solution and incubated for 60 min (human islets) or 45 min (rat islets) with low glucose solution. From each incubation step, supernatants were collected, and insulin secretion was determined using the insulin ELISA kit Mercodia for human islets or Crystal Chem for rat islets. Results were normalized to total DNA content as was determined using the Quanti-iT PicoGreen dsDNA kit (Invitrogen). The stimulation index (SI) was calculated by dividing the amount of insulin secreted after incubation with the high glucose solution by the amount secreted after the first incubation with low glucose solution.
Insulin ELISA
Sandwich ELISA for human insulin (Mercodia) and rat insulin (Crystal Chem) were performed according to the manufacturer’s instructions using a microplate spectrophotometer Benchmark Plus BIO-RAD (Bio-Rad Laboratories B.V., Veenendaal, The Netherlands) at 450 nm with correction at 630 nm for the rat insulin assay.
Effect of treatment with Sq-GusNPs, ASCs, and inflammatory cytokines on viability and function of pancreatic islets
To determine how Sq-GusNPs, ASCs, and inflammatory cytokines influence viability or function of pancreatic islets, 40 handpicked islets were cultured alone or cocultured with ASCs (50.000 ASCs/mL) without direct contact in presence or absence of Sq-GusNPs at a concentration of 22.31 µg/mL or a blend of proinflammatory cytokines (IFN-γ 2000 U/mL, IL-1β 150 U/mL, and TNF-α 2000 U/mL) in 24 well Transwell plates in a total volume of 1 mL of the culture medium used for islet culture. After 24 hours incubation, viability of islets and ASCs was determined with the alamarBlueTM reagent, and islet function was tested through GSIS. ASCs and islets without treatment were used as control group.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
A qRT-PCR was performed to determine the expression of genes associated with the secretion of different cytokines and factors associated with an inflammatory response. After treatment, islets or ASCs were homogenized with TRIzolTM reagent, and total RNA was isolated following the manufacturer’s instructions. Later cDNA was synthesized using SuperScript II Reverse Transcriptase. Finally, qPCR was performed with a FastStart Universal SYBR Green Master for the genes associated with the secretion of different cytokines (primer sequences are listed in Tables 2 and 3). Reactions were carried out in 384-well PCR plates (Thermo Scientific) using a ViiA7 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). Delta Ct (ΔCt) values were calculated and normalized to the housekeeping genes GAPDH, and β-actin. The 2−(ΔΔCT) method was used for the comparative quantification of gene expression.26
Table 2.
Human primer sequences used for qRT-PCR.
| Gene | Forward sequence 5’ – 3’ | Reverse sequence 5’ – 3’ |
|---|---|---|
| GAPDH | CAAATTCCATGGCACCGTCAA | AGCATCGCCCCACTTGATTT |
| β-actin | CGCGAGAAGATGACCCAGAT | AGCACAGCCTGGATAGCAAC |
| TNF-α | GAGGCCAAGCCCTGGTATG | CGGGCCGATTGATCTCAGC |
| IL-8 | ACTCCAAACCTTTCCACCCC | TTCTCAGCCCTCTTCAAAAACTTC |
| IL-6 | ACTCACCTCTTCAGAACGAATTG | CCATCTTTGGAAGGTTCAGGTTG |
| IL-1β | AGCTACGAATCTCCGACCAC | CGTTATCCCATGTGTCGAAGAA |
| IL-10 | TCAAGGCGCATGTGAACTCC | GATGTCAAACTCACTCATGGCT |
| IL-4 | ACAGCCTCACAGAGCAGAAGACT | TGTTCTTGGAGGCAGCAAAGA |
| IL-13 | CCTCATGGCGCTTTTGTTGAC | TCTGGTTCTGGGTGATGTTGA |
| IL-1Ra | CAATGCTGACTCAAAGGAGACGA | TCCCTCCATGGATTCCCAAGA |
| NF-κB (p50) | GCAGCACTACTTCTTGACCACC | TCTGCTCCTGAGCATTGACGTC |
| iNOS | GCCACAGAAGAGCCTGAGAG | GATCTCTGTGGGCGTGTGAT |
| MCP-1 | GAAAGTCTCTGCCGCCCTT | GGTGACTGGGGCATTGATTG |
| IP-10 | CTGTACGCTGTACCTGCATCA | TGATGGCCTTCGATTCTGGA |
| IL-15 | AACAGAAGCCAACTGGGTGAATG | CTCCAAGAGAAAGCACTTCATTGC |
| RIPK1 | AGCTCCTGGGCGTCATCATA | AGGTCTGCGATCTCGGCTTT |
| RIPK3 | TAATGTGGGCAGTGCTTGCT | GTCTGTCCTTGGGCTCACTG |
| BAD | CCCAGAGTTTGAGCCGAGTG | CCCATCCCTTCGTCGTCCT |
| BID | GGCCTACCCTAGAGACATGGA | AGACATCACGGAGCAAGGAC |
| Caspase 3 | CATACTCCACAGCACCTGGTT | TCAAGCTTGTCGGCATACTGT |
| Caspase 9 | TTTGAGGACCTTCGACCAGC | CGAAACAGCATTAGCGACCC |
Table 3.
Rat primer sequences used for qRT-PCR.
| Gene | Forward sequence 5’–3’ | Reverse sequence 5’–3’ |
|---|---|---|
| GAPDH | AAGGTCGGTGTGAACGGATTT | CTTTGTCACAAGAGAAGGCAGC |
| β-actin | GAACCCTAAGGCCAACCGTGAA | TACGTACATGGCTGGGGTGT |
| TNF-α | CATCAAGAGCCCTTGCCCTA | CTGGAAGACTCCTCCCAGGTA |
| CXCL-1 | CCACACTCAAGAATGGTCGC | ACTTGGGGACACCCTTTAGC |
| IL-6 | CCTACCCCAACTTCCAATGCT | ATGGTCTTGGTCCTTAGCCAC |
| IL-1β | CAGGATGAGGACCCAAGCAC | GTCGTCATCATCCCACGAGT |
| IL-10 | GGGAGAGAAGCTGAAGACCC | ATTCATGGCCTTGTAGACACCT |
| IL-4 | TGTAGAGGTGTCAGCGGTCT | TCAGTGTTGTGAGCGTGGAC |
| IL-13 | AGTTGCAATGCCATCCACAG | CCACATCCGAGGCCTTTTGG |
| IL-1Ra | GGGACCTTACAGTCACCTAATCTC | GGTCTTTTCCCAGCAGGGTG |
| NF-κB (p65) | ATCAATGGCTACACGGGACC | AGTTCATGTGGATGAGGCCG |
| iNOS | GGAGAAAACCCCAGGTGCTATT | TCGATGGAGTCACATGCAGC |
| MCP-1 | GATCCCAATGAGTCGGCTGG | ACAGAAGTGCTTGAGGTGGTT |
| IP-10 | GAATCCGGAATCTGAGGCCA | ACGGAGCTCTTTTTGACCTTCT |
| IL-15 | TACTGCAATGAACTGCTTTCTCC | GCTGTTTGCAAGGTAGAGCAC |
| RIPK1 | GACCGAGTTCACAACCACCA | TGTTAGCGAAGACGGCTTGA |
| RIPK3 | TATGGCTCAATGGTGCGTCA | AGTCCCACTCGAGGTTCTCA |
| BAD | GAATGAGCGATGAATTTGAGGG | CCTTTCCCCAAATTTCGATCC |
| BID | TGGACTCTGAGGTCAGCAATG | TTCGGAGAAAGCCGAACACC |
| Caspase 3 | AGAGCTGGACTGCGGTATTG | TCGGCCTCCACTGGTATCTT |
| Caspase 9 | GAGGATATTCAGCGGGCAGG | GCAGGAGATGAAGCGAGGAA |
Gene expression in pancreatic islets and ASCs after co-culture or treatment with Sq-GusNPs and inflammatory cytokines
To determine how Sq-GusNPs, ASCs, and inflammatory cytokines affect gene expression in pancreatic islets, 40 handpicked islets were cultured alone or cocultured with ASCs (50.000 ASCs/mL) without contact in presence or absence of Sq-GusNPs at a concentration of 22.31 µg/mL and/or a blend of proinflammatory cytokines (IFN-γ 2000 U/mL, IL-1β 150 U/mL, and TNF-α 2000 U/mL) in 24 well Transwell plates in a total volume of 1 mL culture medium. After 24 hours incubation, the expression of different genes associated with cytokine secretion was determined by qRT-PCR. Islets and ASCs without treatment were used as control group.
Statistics
The experiments were performed 5 times and statistical analysis was carried out in GraphPad Prism, Version 8.2.0 (GraphPad Software Inc., USA). Normal distribution of data was confirmed using the D’Agostino-Pearson omnibus (K2) test. Where indicated data transformation using the function Y = Log(Y) was applied for analysis. Comparisons were done using one- or two-way ANOVA with Dunnett’s post hoc test. A p-value <0.05 was considered statistically significant. Results are expressed as mean ± standard error of the mean (SEM).
Results
Recently, we developed Sq-GusNPs, a technology for reducing inflammatory responses in the immediate period post-transplantation that involves nanoencapsulation and temporary, controlled release of the anti-inflammatory agent gusperimus in the vicinity of the graft.20,21 Here, we investigate whether immunosuppression with Sq-GusNPs in the presence or absence of ASCs can contribute to reduced release of cytokines in the islets or reduce initiation of cell-death processes.
For this study, we co-cultured islets with ASCs and/or Sq-GusNPs under IFN-γ, IL-1β, and TNF-α induced inflammatory stress. This was done with both human and rat islets to determine species specificity. It is well known that this inflammatory stress does not immediately lead to impaired viability or reduced insulin secretion in the first 24 hours of exposure. Also, here we did not find any effects on viability or the glucose-induced insulin release (Figure S1 and S2 of the supplementary information). It has been reported that this exposure to IFN-γ, IL-1β, and TNF-α does influence the islets cytokine and chemokine secretion and other factors associated with an inflammatory response.27,28 Therefore, we determined here the impact of IFN-γ, IL-1β, and TNF-α in the presence and absence of ASCs and/or Sq-GusNPs on gene expression for both islets and ASCs. We focused on the expression of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and genes associated with the activation of the NF-κB route, namely, the oxidative stress indicator inducible nitric oxide synthase (iNOS), and relevant cytokines, chemokines, and cell-death genes.
Impact of Sq-GusNPs and co-culture on NF-κB gene expression in islets and ASCs
We first studied how IFN-γ, IL-1β, and TNF-α exposure in presence or absence of Sq-GusNPs and/or co-culture influenced the expression of the NF-κB gene in human and rat islets and ASCs. Human islets and ASCs showed no upregulation of NF-κB under basal conditions but exposure to the cytokine mixture did upregulate its expression in both human islets and ASCs (Figure 1A and 1B). The cytokine mixture increased the expression of the NF-κB gene by 2.9-fold (p = .0106) in human islets compared to untreated, control islets (Figure 1A). In human ASCs the cytokine-induced enhancement of NF-κB was 4.5-fold (p = .0006) compared to untreated, control ASCs (Figure 1B). No attenuation of NF-κB expression was observed for cytokine-exposed human islets or ASCs when co-cultured with either Sq-GusNPs or ASCs or a combination thereof (Table S1 of the supplementary information).
Figure 1.
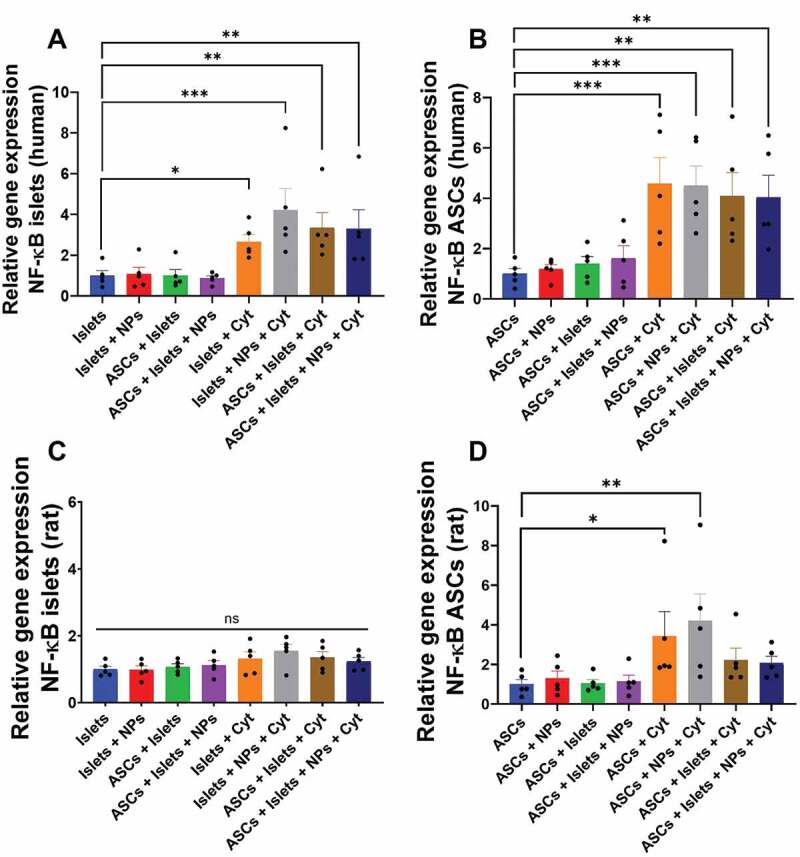
NF-κB gene expression in human and rat islets and ASCs. Human or rat pancreatic islets were co-cultured in the presence and absence of IFN-γ, IL-1β, and TNF-α with ASCs, Sq-GusNPs, or a combination of both. A) NF-κB expression in human islets. B) NF-κB expression in human ASCs. C) NF-κB expression in rat islets. D) NF-κB expression in rat ASCs. Comparisons were made using one-way ANOVA with Dunnet’s multiple comparison test after data transformation using the function Y = Log(Y). Islets (Islets without treatment); Islets + NPs (Islets treated with Sq-GusNPs); ASCs + Islets (Adipose-derived stem cells co-cultured with pancreatic islets); ASCs + Islets + NPs (Adipose-derived stem cells co-cultured with islets and treated with Sq-GusNPs); Islets + Cyt (Islets stimulated with the cytokine mixture); Islets + NPs + Cyt (Islets treated with Sq-GusNPs and stimulated with the cytokine mixture); ASCs + Islets + Cyt (Adipose-derived stem cells co-cultured with islets and stimulated with cytokine cocktail); ASCs + Islets + NPs + Cyt (Adipose-derived stem cells co-cultured with islets, treated with Sq-GusNPs, and stimulated with the cytokine mixture). Data represent mean values ± SEM of five independent experiments. p < .0009 (***); p ≤ .005 (**); p < .05 (*).
Rat islets showed no upregulation of NF-κB gene expression neither under basal nor under cytokine exposed conditions (Figure 1C). This was different in ASCs, as rat ASCs showed an increase in the expression of NF-κB of 3.2-fold (p < .05) after IFN-γ, IL-1β, and TNF-α exposure (Figure 1D). Treatment of cytokine exposed rat ASCs with Sq-GusNPs did not reduce or inhibit NF-κB. However, co-culture of ASCs with rat islets and addition of Sq-GusNPs to the stimulated co-culture avoided the upregulation of the NF-κB gene (Table S2 of the supplementary information).
Impact of Sq-GusNPs and co-culture on iNOS gene expression in islets and ASCs
As inflammatory stress induces nitric oxide (NO) production by islets,29 we studied how exposure to the cytokine mixture contributes to iNOS expression in human and rat islets and ASCs. iNOS is a gene that encodes the inducible nitric oxide synthase enzyme responsible for the production of NO from L-arginine in presence of NADPH and oxygen.30
In human islets, increase in the expression of the iNOS gene was only observed when cytokine exposed islets were co-cultured with ASCs in presence of Sq-GusNPs which enhanced iNOS expression 4.6-fold (p = .0107) (Figure 2A, Table S3 of the supplementary information). Human ASCs showed no increase in gene expression for iNOS neither unstimulated nor when exposed to cytokines (Figure 2B).
Figure 2.
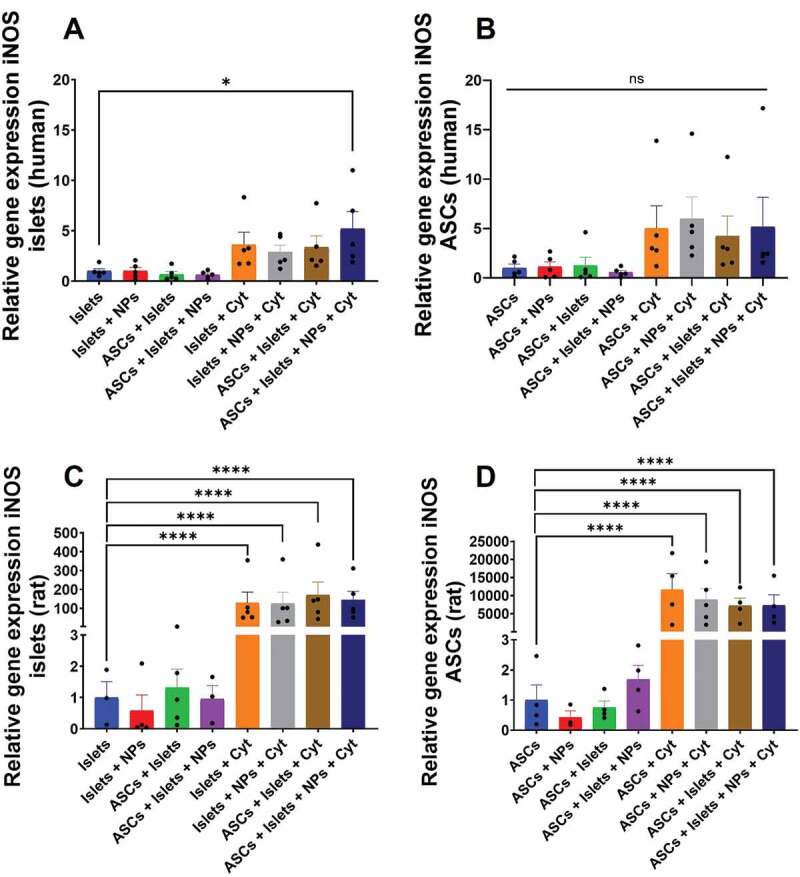
iNOS gene expression in human and rat islets and ASCs. Human or rat pancreatic islets were co-cultured in the presence and absence of IFN-γ, IL-1β, and TNF-α with ASCs, Sq-GusNPs, or a combination of both. A) iNOS expression in human islets. B) iNOS expression in human ASCs. C) iNOS expression in rat islets. D) iNOS expression in rat ASCs. Comparisons were made using one-way ANOVA with Dunnet’s multiple comparison test after data transformation using the function Y = Log(Y). Islets (Islets without treatment); Islets + NPs (Islets treated with Sq-GusNPs); ASCs + Islets (Adipose-derived stem cells co-cultured with pancreatic islets); ASCs + Islets + NPs (Adipose-derived stem cells co-cultured with islets and treated with Sq-GusNPs); Islets + Cyt (Islets stimulated with the cytokine mixture); Islets + NPs + Cyt (Islets treated with Sq-GusNPs and stimulated with the cytokine mixture); ASCs + Islets + Cyt (Adipose-derived stem cells co-cultured with islets and stimulated with cytokine cocktail); ASCs + Islets + NPs + Cyt (Adipose-derived stem cells co-cultured with islets, treated with Sq-GusNPs, and stimulated with the cytokine mixture). Data represent mean values ± SEM of five independent experiments. p < .0001 (****); p < .05 (*); ns (non-significant statistical difference).
Rat cells showed to be more sensitive for cytokine-induced iNOS expression. Rat islets showed a profound increase of 152.7-fold in iNOS expression after cytokine exposure (Figure 2C). Rat ASCs showed a rather high increase in iNOS expression compared to rat islets by 12455.1-fold (Figure 2D) after cytokine exposure. Co-culture and/or treatment with Sq-GusNPs did not reduce or increase the expression of this gene under cytokine-stimulated conditions significantly (Table S4 of the supplementary information).
Impact of Sq-GusNPs and co-culture on inflammatory cytokines/chemokines-associated gene expression in islets and ASCs
Next, we studied how IFN-γ, IL-1β, and TNF-α exposure in presence and absence of Sq-GusNPs and/or ASCs influence the expression of specific genes associated with the activation of the NF-κB route and support the expression of key cytokines and chemokines in human and rat islets and ASCs under inflammatory or basal conditions.
Inflammatory cytokines-associated gene expression
Here, we studied the expression of the genes associated with secretion of the cytokines tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), interleukin 1 beta (IL-1β), and interleukin 15 (IL-15). In absence of an inflammatory stimulus, we did not observe increase in gene expression in human nor in rat islets in presence or absence of either ASCs and/or Sq-GusNPs (Figure 3A and 3C). Cytokine exposure of human islets enhanced the expression of all the evaluated genes (Figure 3A). TNF-α was increased by 8.8-fold (p = .0011), IL-6 was increased by 26.6-fold (p = .008), IL-1β was increased by 20.4-fold (p < .0001), and IL-15 was increased by 3.7-fold (p = .0196). Treatment of the stimulated islets with Sq-GusNPs inhibited the increase of IL-15 but did not inhibit the increased expression of TNF-α, IL-6, and IL-1β. Co-incubation with ASCs or co-incubation and treatment with Sq-GusNPs did not reduce the expression of the evaluated genes after cytokine stimulation (Table S5 of the supplementary information).
Figure 3.
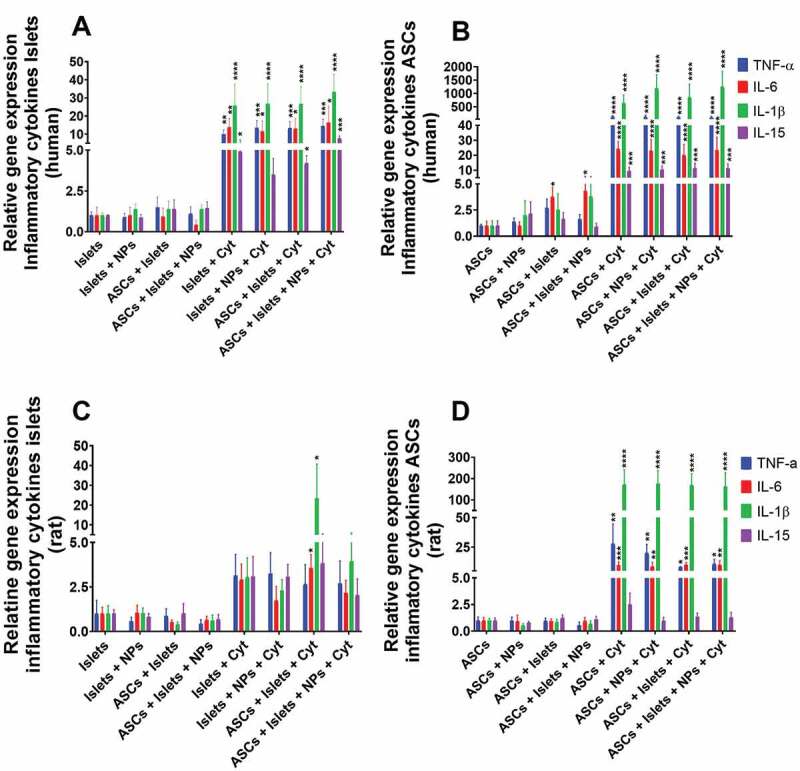
Gene expression associated with secretion of proinflammatory cytokines for human and rat islets and ASCs. Human or rat pancreatic islets were co-cultured in the presence and absence of IFN-γ, IL-1β, and TNF-α with ASCs, Sq-GusNPs, or a combination of both. A) Inflammatory gene expression in human islets. B) Inflammatory gene expression in human ASCs. C) Inflammatory gene expression in rat islets. D) Inflammatory gene expression in rat ASCs. Comparisons were made using one-way ANOVA with Dunnet’s multiple comparison test after data transformation using the function Y = Log(Y). Islets (Islets without treatment); Islets + NPs (Islets treated with Sq-GusNPs); ASCs + Islets (Adipose-derived stem cells co-cultured with pancreatic islets); ASCs + Islets + NPs (Adipose-derived stem cells co-cultured with islets and treated with Sq-GusNPs); Islets + Cyt (Islets stimulated with the cytokine mixture); Islets + NPs + Cyt (Islets treated with Sq-GusNPs and stimulated with the cytokine mixture); ASCs + Islets + Cyt (Adipose-derived stem cells co-cultured with islets and stimulated with cytokine cocktail); ASCs + Islets + NPs + Cyt (Adipose-derived stem cells co-cultured with islets, treated with Sq-GusNPs, and stimulated with the cytokine mixture). Data represent mean values ± SEM of five independent experiments. p < .0001 (****); p < .0009 (***); p < .005 (**); p < .05 (*).
We also determined the expression of these genes in ASCs and how islets might influence gene expression in ASCs by crosstalk. As shown in Figure 3B, ASCs had an enhanced expression of the IL-6 gene when exposed to human islets. Human islets enhanced expression of IL-6 by 5.2-fold (p = .0292) in ASCs in absence of the proinflammatory cytokines. The increased expression was not inhibited for the treatment with Sq-GusNPs and was maintained 5.2-fold (p = .0293) higher than in the control group (untreated ASCs). Under cytokine stimulation, ASCs increased expression of TNF-α, IL-6, IL-1β, and IL-15 by 105.2-fold (p < .0001), 36.3-fold (p < .0001), 389.0-fold (p < .0001), and 11.8-fold (p = .0007) respectively. No significative reduction or inhibition in gene expression for cytokine-stimulated ASCs was observed when these cells were co-cultured with islets and/or treated with Sq-GusNPs (Table S6 of the supplementary information).
Rat islets showed a different expression profile than human islets with fewer expression of proinflammatory cytokine genes. No increase in the expression of these genes was seen when islets were cultured with the cytokine mixture with or without Sq-GusNPs (Figure 3C). When islets were co-cultured with ASCs IL-6 and IL-1β expression was increased by 4.4- (p = .0274), and 9.9-times (p = .0261) respectively in presence of cytokines. Addition of Sq-GusNPs to the stimulated co-culture inhibited the expression of both cytokines (Table S7 of the supplementary information).
Also, with the rat experiments, we studied the impact of cytokine exposure, co-culture, and Sq-GusNPs treatment in rat ASCs gene expression (Figure 3D). TNF-α, IL-6, and IL-1β expression was increased by 17.0-fold (p = .0034), 12.5-fold (p = .0009), and 118.6-fold (p < .0001) respectively. This increased expression was not reduced significantly by Sq-GusNPs exposure, co-culture with rat islets, or a combination thereof. Noticeably, the rat ASCs were strong producers of IL-1β and unlike human ASCs, rat ASCs did not increase the expression of IL-15 after exposure to the cytokine mixture (Table S8 of the supplementary information).
Inflammatory chemokines-associated gene expression
We also studied some key genes associated with secretion of inflammatory chemokines whose production is induced by inflammatory cytokines via the NF-κB route, namely, interleukin 8 (IL-8) in humans or its equivalent cytokine in rat chemokine (C-X-C motif) ligand 1 (CXCL-1), monocyte chemoattractant protein-1 (MCP-1), and interferon-gamma inducible protein-10 (IP-10). In absence of cytokine exposure, no increase in the expression of the evaluated chemokines was observed neither in human or rat islets even when they were co-cultured with ASCs irrespective of the presence of Sq-GusNPs (Figure 4A and 4C).
Figure 4.
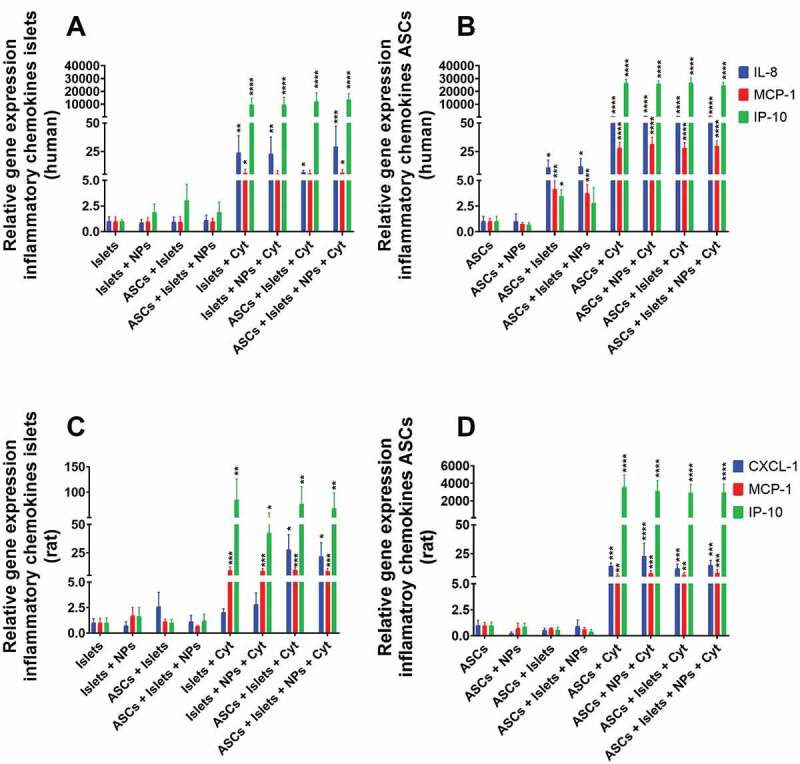
Gene expression associated with secretion of proinflammatory chemokines for human and rat islets and ASCs. Human or rat pancreatic islets were co-cultured in the presence and absence of IFN-γ, IL-1β, and TNF-α with ASCs, Sq-GusNPs, or a combination of both. A) Chemokine expression in human islets. B) Chemokine expression in human ASCs. C) Chemokine expression in rat islets. D) Chemokine expression in rat ASCs. Comparisons were made using one-way ANOVA with Dunnet’s multiple comparison test after data transformation using the function Y = Log(Y). Islets (Islets without treatment); Islets + NPs (Islets treated with Sq-GusNPs); ASCs + Islets (Adipose-derived stem cells co-cultured with pancreatic islets); ASCs + Islets + NPs (Adipose-derived stem cells co-cultured with islets and treated with Sq-GusNPs); Islets + Cyt (Islets stimulated with the cytokine mixture); Islets + NPs + Cyt (Islets treated with Sq-GusNPs and stimulated with the cytokine mixture); ASCs + Islets + Cyt (Adipose-derived stem cells co-cultured with islets and stimulated with cytokine cocktail); ASCs + Islets + NPs + Cyt (Adipose-derived stem cells co-cultured with islets, treated with Sq-GusNPs, and stimulated with the cytokine mixture). Data represent mean values ± SEM of five independent experiments. p < .0001 (****); p < .0009 (***); p < .005 (**); p < .05 (*).
When human islets were exposed to the cytokine mixture all the studied chemokine genes were upregulated (Figure 4A). The expression was increased by 19.5-fold for IL-8 (p = .0015), 5.0-fold for MCP-1 (p = .0403), and an extreme enhancement of 6456.5-fold (p < .0001) was observed for IP-10. Sq-GusNPs or co-culture with human ASCs inhibited the expression of MCP-1 and maintained an increased expression of IL-8 and IP-10 in cytokine-stimulated conditions (Table S9 of the supplementary information). For human islets co-cultured with ASCs and treated with Sq-GusNPs no inhibition in gene expression under inflammatory stimulus was observed and all the evaluated chemokines were upregulated. For IL-8, MCP-1, and IP-10 the expression was increased by 23.6-fold (p = .0007), 5.9-fold (p = .0192), and 10715.2-fold (p < .0001) respectively.
Under basal conditions human islets induced the expression of IL-8, MCP-1, and IP-10 by 8.4-fold (p = .0325), 4.6-fold (p = .0008), and 5.7-fold (p = .0184) respectively in human ASCs (Figure 4B). Addition of Sq-GusNPs to the co-culture without cytokine stimulation did not inhibit IL-8 and MCP-1 expression but did inhibit the expression of IP-10 in ASCs (Table S10 of the supplementary information). Human ASCs upregulated gene expression for all the evaluated genes when they were exposed to the cytokine mixture. IL-8 was increased by 554.6-fold (p < .0001), MCP-1 was increased by 31.9-fold (p < .0001), and as for human islets, IP-10 expression was increased to a higher extent by 46131.8-fold (p < .0001). When stimulated ASCs were treated with Sq-GusNPs, co-cultured with human islets, or co-cultured and treated with Sq-GusNPs no inhibition in gene expression was observed (Table S10 of the supplementary information).
Rat islets showed an increased expression of two of the evaluated genes with a higher increase of IP-10 when they were stimulated with the cytokine mixture (Figure 4C). MCP-1 was increased by 10.7-fold (p = .0003) and IP-10 was increased by 58.3-fold (p = 0.0011). This expression was not reduced significantly when rat islets were co-cultured with ASCs, treated with Sq-GusNPs, or co-cultured and treated with Sq-GusNPs under inflammatory stimulus (Table S11 of the supplementary information). Interestingly, when stimulated rat islets were co-cultured with ASCs an increase in the expression of CXCL-1 by 22.2-fold (p = 0.0124) was observed. Addition of Sq-GusNPs to the stimulated co-culture did not inhibit the increased expression of CXCL-1.
In rat ASCs, the expression of CXCL-1, MCP-1, and IP-10 was also increased under inflammatory conditions with a further elevated expression for IP-10 in comparison to rat islets (Figure 4D). The CXCL-1 expression was increased by 28.5-fold (p = 0.0001), MCP-1 was increased by 6.2-fold (p = 0.003), and IP-10 was increased by 2517.7-fold (p < 0.0001) when ASCs were exposed to the cytokine mixture. When rat ASCs were treated with Sq-GusNPs and/or co-cultured with islets in presence of the cytokine mixture no reduction or inhibition in the expression of any of these genes was observed (Table S12 of the supplementary information).
Impact of Sq-GusNPs and co-culture on anti-inflammatory cytokines-associated gene expression in islets and ASCs
In addition to the pro-inflammatory genes, we also studied the expression of the regulatory cytokines interleukin 10 (IL-10), interleukin 4 (IL-4), interleukin 13 (IL-13), and interleukin 1 receptor antagonist (IL-1Ra) in human and rat islets and ASCs. In human islets, we did not observe any statistically significant effect on IL-10, IL-4, IL-13, and IL-1Ra in the absence or presence of IFN-γ, IL-1β, and TNF-α (Figure 5A). Also, co-culture with ASCs and/or treatment with Sq-GusNPs did not influence expression profiles. The same applied to human ASCs (Figure 5B).
Figure 5.
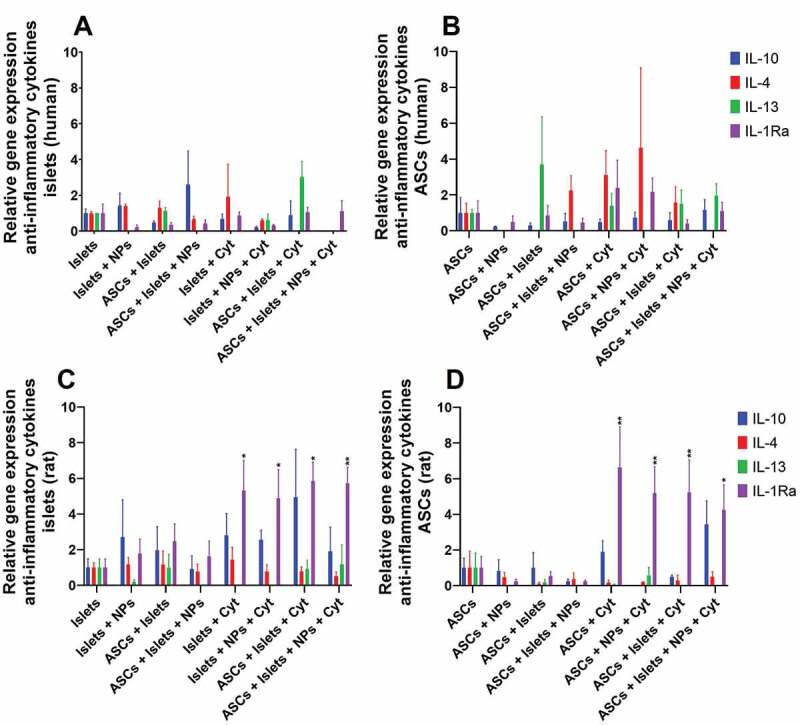
Regulatory cytokine expression in human and rat islets and ASCs. Human or rat pancreatic islets were co-cultured in the presence and absence of IFN-γ, IL-1β, and TNF-α with ASCs, Sq-GusNPs, or a combination of both. A) Regulatory cytokine expression in human islets. B) Regulatory cytokine expression in human ASCs. C) Regulatory cytokine expression in rat islets. D) Regulatory cytokine expression in rat ASCs. Comparisons were made using one-way ANOVA with Dunnet’s multiple comparison test after data transformation using the function Y = Log(Y). Islets (Islets without treatment); Islets + NPs (Islets treated with Sq-GusNPs); ASCs + Islets (Adipose-derived stem cells co-cultured with pancreatic islets); ASCs + Islets + NPs (Adipose-derived stem cells co-cultured with islets and treated with Sq-GusNPs); Islets + Cyt (Islets stimulated with the cytokine mixture); Islets + NPs + Cyt (Islets treated with Sq-GusNPs and stimulated with the cytokine mixture); ASCs + Islets + Cyt (Adipose-derived stem cells co-cultured with islets and stimulated with cytokine cocktail); ASCs + Islets + NPs + Cyt (Adipose-derived stem cells co-cultured with islets, treated with Sq-GusNPs, and stimulated with the cytokine mixture). Data represent mean values ± SEM of five independent experiments. p < .009 (**); p < .05 (*).
Results were different for the rat experiments. Expression of IL-1Ra in rat islets was increased by 5.7-fold (p = 0.0253) when stimulated with IFN-γ, IL-1β, and TNF-α (Figure 5C). Treatment of the stimulated islets with Sq-GusNPs and/or co-culture with ASCs did not inhibit the expression of IL-1Ra. The expression when stimulated islets were treated with Sq-GusNPs was increased 5.3-fold (p = 0.0354) and co-culture with ASCs or a combination of the two increased the expression by 7.6-fold (p < 0.05) compared to untreated islets.
In the rat ASCs, a similar behavior was observed. The cytokine challenge increased IL-1Ra expression 11.6-fold (p = 0.004). Treatment of the stimulated ASCs with Sq-GusNPs or co-culture with islets increased the expression to a lower extent by 9.7- (p = 0.0083), and 9.8-times (p = 0.0079) respectively. A lower increase of 7.9-times (p = 0.0187) in the expression of this gene under cytokine stimulation was observed when ASCs were co-cultured with islets and treated with Sq-GusNPs (Figure 5D).
Impact of Sq-GusNPs and co-culture on cell death-associated gene expression in islets and ASCs
Finally, we evaluated the expression of six genes associated with cell death processes, two genes associated with necroptosis, receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and receptor-interacting serine/threonine-protein kinase 3 (RIPK3), and four genes associated with cell death via apoptosis, namely, BCL2 associated agonist of cell death (BAD), BH3 interacting-domain death agonist (BID), cysteine-aspartic acid protease 3 (Caspase 3), and cysteine-aspartic acid protease 9 (Caspase 9).
In human islets, we observed no upregulation of the evaluated genes when exposed to IFN-γ, IL-1β, and TNF-α. Only when human islets were co-cultured with ASCs, treated with Sq-GusNPs, and stimulated with cytokines a significant increase of 2.9 times (p < 0.05) of caspase 3 expression was observed (Figure 6A). Human ASCs showed an increased expression of the necroptotic gene RIPK3 and the apoptotic gene BID after stimulation with the cytokine mixture by 6.3-fold (p = 0.0008) and 4.2-fold (p = 0.0004), respectively (Figure 6B). After treatment of the stimulated ASCs with Sq-GusNPs, co-culture with human islets, or co-culture and treatment with Sq-GusNPs no decrease in the upregulated genes was observed (Table S13 of the supplementary information).
Figure 6.
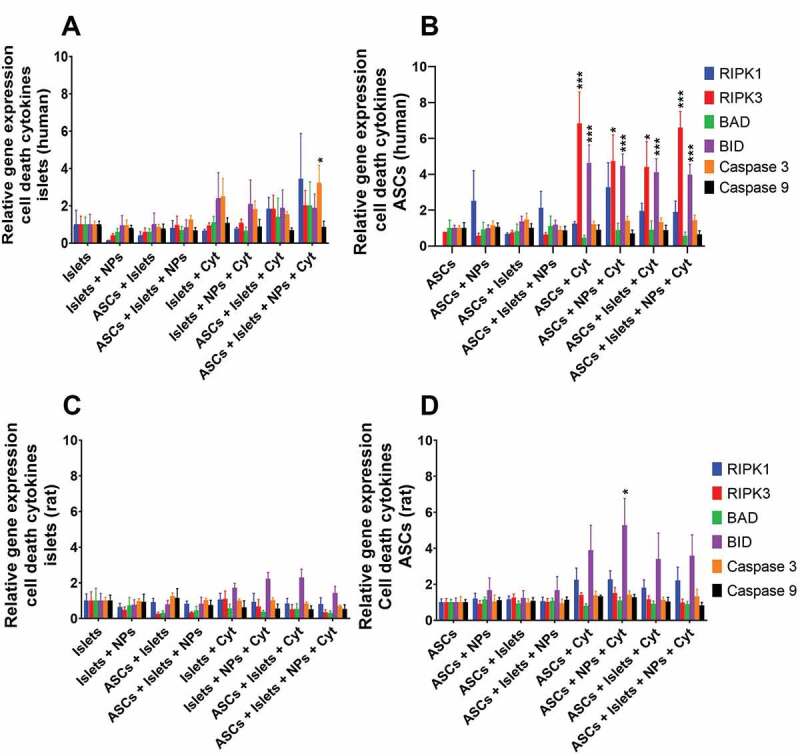
Cell-death associated gene expression in human and rat islets and ASCs. Human or rat pancreatic islets were co-cultured in the presence and absence of IFN-γ, IL-1β, and TNF-α with ASCs, Sq-GusNPs, or a combination of both. A) Gene expression in human islets. B) Gene expression in human ASCs. C) Gene expression in rat islets. D) Gene expression in rat ASCs. Comparisons were made using one-way ANOVA with Dunnet’s multiple comparison test after data transformation using the function Y = Log(Y). Islets (Islets without treatment); Islets + NPs (Islets treated with Sq-GusNPs); ASCs + Islets (Adipose-derived stem cells co-cultured with pancreatic islets); ASCs + Islets + NPs (Adipose-derived stem cells co-cultured with islets and treated with Sq-GusNPs); Islets + Cyt (Islets stimulated with the cytokine mixture); Islets + NPs + Cyt (Islets treated with Sq-GusNPs and stimulated with the cytokine mixture); ASCs + Islets + Cyt (Adipose-derived stem cells co-cultured with islets and stimulated with cytokine cocktail); ASCs + Islets + NPs + Cyt (Adipose-derived stem cells co-cultured with islets, treated with Sq-GusNPs, and stimulated with the cytokine mixture). Data represent mean values ± SEM of five independent experiments. p ≤ .0009 (***); p < .05 (*).
In rat islets, no upregulation of any of the evaluated genes was observed. Not under basal or when exposed to the cytokine mixture. The treatment with Sq-GusNPs, co-culture with ASCs, or a combination thereof did not change this (Figure 6C). Rat ASCs only showed upregulation of the pro-apoptotic gene BID when ASCs were stimulated with cytokines and treated with Sq-GusNPs. This induced 5.3 times (p = 0.0131) increase in BID expression (Figure 6D).
Discussion
Here we studied, to the best of our knowledge, for the first time how pancreatic islets and ASCs mutually influence each other in the presence or absence of an immunosuppressive agent and under inflammatory or basal conditions. To this end, we studied in islets and ASCs genes related to proinflammatory and regulatory responses as well as genes involved in different cell-death processes. We choose to focus on the first 24 hours of exposure to cytokines as this is the period in which insulin secretion is not yet impaired but cellular molecular processes are dysregulated.27,31 We demonstrated that in the first 24 hours viability or function was not impaired in human or rat islets. Also, the viability of ASCs was not affected. This allowed us to study in islets the effects of co-culture with ASCs and/or treatment with Sq-GusNPs in a period in which irreversible damage to ß-cells is not yet complete.
Our data show that in human or rat islets in absence of cytokine exposure the coculture with ASCs, treatment with Sq-GusNPs, or a combination of both did not enhance the expression of inflammatory cytokines or chemokines. This indicates that Sq-GusNPs or ASCs have no immunogenic influence on pancreatic islets. However, we found that in human ASCs the expression of IL-6, IL-8, MCP-1, and IP-10 was enhanced by human islets in absence of cytokine stimulation. The enhanced expression of these cytokines in human ASCs should not be considered as an observation that negatively impacts graft survival. We consider the expression of IL-6, IL-8, and MCP-1 induced by human islets in human ASCs as beneficial for engraftment as these chemokines have been proposed to be not only inflammatory but also to be proangiogenic,32–34 anti-apoptotic, proliferative, and having tissue repair effects through paracrine mechanisms.35–37,14 This observation suggests that co-incubation of human islets with ASCs in the pre-transplantation period might precondition human ASCs to support engraftment.
The enhanced expression of cytokines in human ASCs induced by human islets was only minorly suppressed by the immunosuppressive agent Sq-GusNPs. Only IP-10 was inhibited. This, however, can also be considered to be beneficial as IP-10 is a potent inhibitor of angiogenesis.34 As IL-6, IL-8, and MCP-1 expression was not reduced in human ASCs by Sq-GusNPs we conclude that application of this immunosuppressive regimen does not interfere with processes involved in engraftment of the transplant mediated by human ASCs.
Human cells showed higher activity of NF-κB under inflammatory stress than rat cells indicating a higher sensitivity for inflammatory stress in human cells. This enhanced sensitivity was illustrated by a stronger expression of the studied genes in both human islets as well as in human ASCs. In presence of IFN-γ, IL-1β, and TNF-α, human islets expressed all the studied cytokines and chemokines. In contrast, rat islets did not express any of the studied cytokines, but they did express MCP-1 and IP-10 after cytokine stimulation. Additionally, human ASCs showed a rather high expression of all the evaluated cytokines and chemokines under inflammatory stress than rat ASCs. The observation of higher sensitivity for cytokine stress could explain why human islets compared to rat islets perform worse in different transplantation models, which up to now is usually attributed to differences in islet-quality and lower engraftment of human islets compared to rat islets.8
The generation of NO has been identified as one of the main mediators of cytokine-induced β-cell damage.29 iNOS gene expression is an indicator of the production of NO by cells.30 NO secretion in β-cells has been associated with inhibition of insulin secretion, mitochondrial dysfunction, and DNA damage which can lead to cell death.28,38 In rat cells, in contrast to human cells, proinflammatory conditions did induce nitrosative stress as was observed in both rat islets and in rat ASCs which both expressed iNOS to a high extend. This might indicate that nitrosative stress is less involved in human islet cell death than in rat islets under inflammatory conditions.38,39
After 24 hours exposure of human islets to IFN-γ, IL-1β, and TNF-α, Sq-GusNPs inhibited IL-15 and MCP-1. IL-15 is a potent growth factor, chemoattractant, and activator for T cells and natural killer (NK) cells.34 MCP-1 induces chemotaxis of monocytes, recruits T cells, and NK cells.40 This suggests that Sq-GusNPs can avoid migration and activation of different kinds of cells from the innate and adaptative arms of the immune system and therewith contribute to the prevention of graft rejection by directly suppressing cytokines in human islets under inflammatory stress. Additionally, Sq-GusNPs does also influence immune cells in the vicinity of the graft and have been shown to downregulate the secretion in macrophages of TNF-α, IL-6, and IL-8.20 This again demonstrates that immunosuppression by Sq-GusNPs does not negatively but rather beneficially influence processes related to inflammation and engraftment of especially human islets.
Rat islets were less sensitive for the inflammatory cytokines than human islets and showed no upregulation of genes associated with inflammation. However, in contrast to human islets, co-culture of cytokine-exposed rat islets with rat ASCs increased expression of IL-6 and IL-1β in rat islets. Here, Sq-GusNPs exerted an inhibitory effect on the expression of IL-6 and IL-1β in rat islets even under the influence of IL-1β highly expressed by the rat ASCs in co-culture. Again, this illustrates a major difference between human and rat islet-responses to ASCs and Sq-GusNPs. It also shows the protective effect that Sq-GusNPs can have on rat islets in co-culture conditions with ASCs.
Rat islets and ASCs in contrast to human islets and ASCs expressed the regulatory cytokine IL-1Ra. This could be one of the reasons why rat islets were less susceptible to cytokine exposure. IL-1Ra has been shown to neutralize pro-inflammatory cytokines and chemokines such as IL-1β, IL-6, TNF-α, KC, MCP-1, and MIP-1α.30,41 This finding suggests that rat islets are more tolerant to inflammatory conditions than human islets thanks to autoregulation driven by IL-1Ra secretion. By their part and according to our results, the observation of an increased IL-1Ra expression in rat ASCs suggests that rat ASCs may reinforce anti-inflammatory effects on rat islets through a paracrine effect as has been previously described.12
Finally, human and rat islets showed a low tendency or no tendency to express genes related to cell death at 24 hours. This can explain why islet-cell death is not observed at an early stage after inflammatory stimulation. Even human islets, despite their enhanced sensitivity to inflammatory conditions, only showed an increased expression of caspase 3 in co-culture with human ASCs after cytokine exposure and treatment with Sq-GusNPs. This indicates that in principle a combination of immunosuppression with Sq-GusNPs and ASCs may induce cell death by apoptosis in human islets in a caspase 3 dependent pathway under pro-inflammatory conditions. Notably, before apoptosis occurs not only caspase 3 should be activated but also e.g. cytochrome c release and caspase 9 should be upregulated to eventually induce cell death.42 It remains to be determined whether the combination of co-culture of human islets with human ASCs and treatment with Sq-GusNPs can induce apoptosis in human islets under pro-inflammatory conditions.
Surprisingly, human ASCs showed to be more prone to cell death processes under inflammatory conditions than rat ASCs. Human ASCs increased the expression of the necroptotic gen RIPK3 and the apoptotic gene BID without increasing the expression of the RIPK1 gene. This suggests that inflammatory conditions may induce necroptotic death in human ASCs in a RIPK1 independent mechanism.43 This consideration is corroborated by the increased expression of the BID gene in human ASCs, since, even though BID is considered a pro-apoptotic gene,44 its expression together with RIPK3 has shown to be involved in complement-dependent cytotoxicity leading to necroptosis in a pathway that depends on both RIPK3 and MLKL.45,46 For cytokine-stimulated rat ASCs, only the treatment with Sq-GusNPs produced an increased expression of the pro-apoptotic gene BID.47 This suggests that Sq-GusNPs may induce cell death via apoptosis in rat ASCs under inflammatory conditions in a BID-dependent pathway.
Conclusion
The beneficial impact of ASCs on islet cells is not influenced by immunosuppression induced by Sq-GusNPs. Sq-GusNPs treatment did only minorly reduce cytokines and chemokines in human ASCs that are associated with anti-apoptotic, proangiogenic, proliferative, and tissue repair capacity effects. However, there are major differences between human and rat islets. The species-dependent effects were observed after exposure to IFN-γ, IL-1β, and TNF-α as well as when exposed to ASCs and/or treatment with Sq-GusNPs. Exposure to the cytokines had a strong impact on human islets by NF-κB dependent activation but not via nitrosative stress whereas in rat islets the impact was mainly on nitrosative responses and less on NF-κB activation. Rat islets showed higher tolerance to inflammatory conditions which seem to be driven by IL-1Ra secretion by rat islets and rat ASCs. Our data support the use of ASCs and Sq-GusNP to manage inflammatory responses and to support engraftment of human islets pre- and post-transplantation for T1DM treatment. This study is the basis for further studies in which later times, protein expression measurements, and inclusion of additional genes such as cytochrome C and MLKL will be included to corroborate synergistic effects of Sq-GusNPs and ASCs in the functional survival of pancreatic islets.
Supplementary Material
Acknowledgments
This work was supported by the Abel Tasman Talent Program of the University of Groningen and the COLCIENCIAS project “Preparation and characterization of Gusperimus nanocarriers with potential application in the process of implantation of cellular islets for the treatment of type 1 diabetes mellitus” contract 747-2018, N° 111580763077.
Funding Statement
This work was supported by the Departamento Administrativo de Ciencia, Tecnología e Innovación (COLCIENCIAS); Rijksuniversiteit Groningen.
Data availability
Data supporting the findings of this study are available within the article, in the supplementary file, and from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19382014.2022.2100191
References
- 1.Hu S, Kuwabara R, Navarro Chica CE, Smink AM, Koster T, Medina JD, de Haan BJ, Beukema M, Lakey JRT, García AJ, et al. Toll-like receptor 2-modulating pectin-polymers in alginate-based microcapsules attenuate immune responses and support islet-xenograft survival. Biomaterials. 2021;266:120460. [DOI] [PubMed] [Google Scholar]
- 2.De Vos P, Van Hoogmoed CG, De Haan BJ, Busscher HJ.. Tissue responses against immunoisolating alginate-PLL capsules in the immediate posttransplant period. J Biomed Mater Res. 2002;62:430–437. doi: 10.1002/jbm.10345. [DOI] [PubMed] [Google Scholar]
- 3.Bünger CM, Tiefenbach B, Jahnke A, Gerlach C, Freier T, Schmitz KP, Hopt UT, Schareck W, Klar E, De Vos P. Deletion of the tissue response against alginate-pll capsules by temporary release of co-encapsulated steroids. Biomaterials. 2005;26(15):2353–2360. doi: 10.1016/j.biomaterials.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Bocca N, Pileggi A, Molano RD, Marzorati S, Wu W, Bodor N, Ricordi C, Buchwald P. Soft corticosteroids for local immunosuppression: exploring the possibility for the use of loteprednol etabonate for islet transplantation. Pharmazie. 2008;63:226–232. [PubMed] [Google Scholar]
- 5.Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. [DOI] [PubMed] [Google Scholar]
- 6.Ryan AJ, O’Neill HS, Duffy GP, O’Brien FJ. Advances in polymeric islet cell encapsulation technologies to limit the foreign body response and provide immunoisolation. Curr Opin Pharmacol. 2017;36:66–71. doi: 10.1016/j.coph.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Inoue R, Nishiyama K, Li J, Miyashita D, Ono M, Terauchi Y, Shirakawa J. The feasibility and applicability of stem cell therapy for the cure of type 1 diabetes. Cells. 2021;10:1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubber EL, Rackham CL, Jones PM. Protecting islet functional viability using mesenchymal stromal cells. Stem Cells Transl Med. 2021;10:674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu Z, Lou Q, Cooper DKC, Pu Z, Lu Y, Chen J, Ni Y, Zhan Y, Chen J, Li Z, et al. Potential roles of mesenchymal stromal cells in islet allo- and xenotransplantation for type 1 diabetes mellitus. Xenotransplantation. 2021;28:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Docherty FM, Sussel L, Bagnato VS, Romero MP. Islet regeneration: endogenous and exogenous approaches. Int J Mol Sci. 2021;23(1):22. doi: 10.3390/ijms23010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackel A, Aksamit A, Bruderek K, Lang S, Brandau S. TNF-α and IL-1β sensitize human MSC for IFN-γ signaling and enhance neutrophil recruitment. Eur J Immunol. 2021;51(2):319–330. doi: 10.1002/eji.201948336. [DOI] [PubMed] [Google Scholar]
- 12.Gao G, Fan C, Li W, Liang R, Wei C, Chen X, Yang Y, Zhong Y, Shao Y, Kong Y, Li Z. Mesenchymal stem cells: ideal seeds for treating diseases. Hum Cell. 2021;1:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasse GF, Van Os L, De Jager M, Jonker MR, Borghuis T, Van Den Toorn LT, Jellema P, White ES, Van Rijn P, Harmsen MC, et al. Adipose stromal cell-secretome counteracts profibrotic signals from ipf lung matrices. Front Pharmacol. 2021;12:1–15. doi: 10.3389/fphar.2021.669037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza BM, Rodrigues M, de Oliveira FS, da Silva LPA, Bouças AP, Portinho CP, Dos Santos BP, Camassola M, Rocha D, Lysakowski S, et al. Improvement of human pancreatic islet quality after co-culture with human adipose-derived stem cells. Mol Cell Endocrinol. 2020;505:110729. doi: 10.1016/j.mce.2020.110729. [DOI] [PubMed] [Google Scholar]
- 15.Niclauss N, Meier R, Bédat B, Berishvili E, Berney T. Beta-cell replacement: pancreas and islet cell transplantation. Endocr Dev. 2016;31:146–162. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JT, Haller CA, Qu Z, Cui W, Urlam MK, Chaikof EL. Biomolecular surface engineering of pancreatic islets with Thrombomodulin. Acta Biomater. 2010;6:1895–1903. doi: 10.1016/j.actbio.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozlovskaya V, Zavgorodnya O, Chen Y, Ellis K, Tse HM, Cui W, Thompson JA, Kharlampieva E. Ultrathin polymeric coatings based on hydrogen-bonded polyphenol for protection of pancreatic islet cells. Adv Funct Mater. 2012;22:3389–3398. doi: 10.1002/adfm.201200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong JH, Yook S, Hwang JW, Jung MJ, Moon HT, Lee DY, Byun Y. Synergistic effect of surface modification with poly(ethylene glycol) and immunosuppressants on repetitive pancreatic islet transplantation into antecedently sensitized rat. Transplant Proc. 2013;45:585–590. doi: 10.1016/j.transproceed.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Ricci M, Blasi P, Giovagnoli S, Rossi C, Macchiarulo G, Luca G, Basta G, Calafiore R. Ketoprofen controlled release from composite microcapsules for cell encapsulation: effect on post-transplant acute inflammation. J Control Release. 2005;107:395–407. doi: 10.1016/j.jconrel.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Navarro Chica CE, Qin T, de Haan BJ, Faas MM, Smink AM, Sierra L, López BL, de Vos P. In vitro studies of squalene‐gusperimus nanoparticles in islet‐containing alginate microcapsules to regulate the immune response in the immediate posttransplant period. Adv NanoBiomed Res. 2021;1(11):2100055. doi: 10.1002/anbr.202100055. [DOI] [Google Scholar]
- 21.Navarro Chica CE, de Haan BJ, Faas MM, Smink AM, Sierra L, de Vos P, López BL. Design and characterization of Squalene-Gusperimus nanoparticles for modulation of innate immunity. Int J Pharm. 2020;590:119893. [DOI] [PubMed] [Google Scholar]
- 22.Smink AM, De Haan BJ, Paredes-Juarez GA, Wolters AHG, Kuipers J, Giepmans BNG, Schwab L, Engelse MA, Van Apeldoorn AA, De Koning E, et al. Selection of polymers for application in scaffolds applicable for human pancreatic islet transplantation. Biomed Mater. 2016;11:35006. doi: 10.1088/1748-6041/11/3/035006. [DOI] [PubMed] [Google Scholar]
- 23.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 24.De Haan BJ, Faas MM, Spijker H, Van Willigen JW, De Haan A, De Vos P. Factors influencing isolation of functional pancreatic rat islets. Pancreas. 2004;29(1):15–22. doi: 10.1097/00006676-200407000-00063. [DOI] [PubMed] [Google Scholar]
- 25.Smink AM, Skrzypek K, Liefers-Visser JAL, Kuwabara R, De Haan BJ, De Vos P, Stamatialis D. In vivo vascularization and islet function in a microwell device for pancreatic islet transplantation. Biomed Mater. 2021;16(3):035036. doi: 10.1088/1748-605X/abf5ec. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Llacua LA, de Haan BJ, de Vos P. Laminin and collagen IV inclusion in immunoisolating microcapsules reduces cytokine-mediated cell death in human pancreatic islets. J Tissue Eng Regen Med. 2018;12(2):460–467. doi: 10.1002/term.2472. [DOI] [PubMed] [Google Scholar]
- 28.Cardozo AK, Heimberg H, Heremans Y, Leeman R, Kutlu B, Kruhøffer M, Ørntoft T, Eizirik DL. A comprehensive analysis of cytokine-induced and nuclear factor-κb-dependent genes in primary rat pancreatic β-cells. J Biol Chem. 2001;276:48879–48886. [DOI] [PubMed] [Google Scholar]
- 29.Cetkovic-Cvrlje M, Eizirik DL. TNF-α and IFN-γ potentiate the deleterious effects of IL-1β on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine. 1994;6(4):399–406. doi: 10.1016/1043-4666(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 30.Cieślak M, Wojtczak A, Cieślak M. Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment. Acta Biochim Pol. 2015;62(1):15–21. doi: 10.18388/abp.2014_853. [DOI] [PubMed] [Google Scholar]
- 31.Hostens K, Pavlovic D, Zambre Y, Ling Z, Van Schravendijk C, Eizirik DL, Pipeleers DG. Exposure of human islets to cytokines can result in disproportionately elevated proinsulin release. J Clin Invest. 1999;104(1):67–72. doi: 10.1172/JCI6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeForge LE, Remick DG. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem Biophys Res Commun. 1991;174(1):18–24. doi: 10.1016/0006-291X(91)90478-P. [DOI] [PubMed] [Google Scholar]
- 33.Zhou D, Yu T, Chen G, Brown SA, Yu Z, Mattson MP, Thompson JS. Effects of NF-κB1 (p50) targeted gene disruption on ionizing radiation-induced NF-κB activation and TNFα, IL-1α, IL-1β and IL-6 mRNA expression in vivo. Int J Radiat Biol. 2001;77(7):67–72. doi: 10.1080/09553000110050047. [DOI] [PubMed] [Google Scholar]
- 34.Cardozo AK, Proost P, Gysemans C, Chen M-C, Mathieu C, Eizirik DL. IL-1β and IFN-γ induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia. 2003;46(2):255–266. doi: 10.1007/s00125-002-1017-0. [DOI] [PubMed] [Google Scholar]
- 35.Kwon YW, Heo SC, Jeong GO, Yoon JW, Mo WM, Lee MJ, Jang IH, Kwon SM, Lee JS, Kim JH. Tumor necrosis factor-α-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim Biophys Acta - Mol Basis Dis. 2013;1832:2136–2144. doi: 10.1016/j.bbadis.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Xiang C, Xie QP. Protection of mouse pancreatic islet function by co-culture with hypoxia pre-treated mesenchymal stromal cells. Mol Med Rep. 2018;18:2589–2598. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi H, Sakata N, Yoshimatsu G, Hasegawa S, Kodama S. Regenerative and transplantation medicine: cellular therapy using adipose tissue-derived mesenchymal stromal cells for type 1 diabetes mellitus. J Clin Med. 2019;8:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delaney CA, Pavlovic D, Hoorens A, Pipeleers DG, Eizirik DL. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology. 1997;138:2610–2614. [DOI] [PubMed] [Google Scholar]
- 39.Eizirik DL, Sandler S, Welsh N, Cetkovic-Cvrlje M, Nieman A, Geller DA, Pipeleers DG, Bendtzen K, Hellerström C. Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest. 1994;93:1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melzi R, Mercalli A, Sordi V, Cantarelli E, Nano R, Maffi P, Sitia G, Guidotti LG, Secchi A, Bonifacio E, et al. Role of CCL2/MCP-1 in islet transplantation. Cell Transplant. 2010;19:1031–1046. [DOI] [PubMed] [Google Scholar]
- 41.Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, Kergoat M, Portha B, Homo-Delarche F, et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci U S A. 2009;106:13998–14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brentnall M, Rodriguez-Menocal L, Ladron R, Guevara D, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis; 2013. [DOI] [PMC free article] [PubMed]
- 43.Song G, Ma Z, Liu D, Qian D, Zhou J, Meng H, Zhou B, Song Z. Bone marrow-derived mesenchymal stem cells attenuate severe acute pancreatitis via regulation of microRNA-9 to inhibit necroptosis in rats. Life Sci. 2019;223:9–21. doi: 10.1016/j.lfs.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 44.BID . BH3 interacting domain death agonist [Homo sapiens (human)] - Gene – NCBI; 2021. Accessed October 6, 2021. https://www.ncbi.nlm.nih.gov/gene/637
- 45.Ziporen L, Donin N, Shmushkovich T, Gross A, Fishelson Z. Programmed necrotic cell death induced by complement involves a bid-dependent pathway. J Immunol. 2009;182:515–521. [DOI] [PubMed] [Google Scholar]
- 46.Lusthaus M, Mazkereth N, Donin N, Fishelson Z. Receptor-interacting protein kinases 1 and 3, and mixed lineage kinase domain-like protein are activated by sublytic complement and participate in complement-dependent cytotoxicity. Front Immunol. 2018;9:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renshaw SA, Dempsey CE, Barnes FA, Bagstaff SM, Dower SK, Bingle CD, Whyte MKB. Three novel bid proteins generated by alternative splicing of the human bid gene. J Biol Chem. 2004;279:2846–2855. doi: 10.1074/jbc.M309769200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available within the article, in the supplementary file, and from the corresponding author upon reasonable request.


