ABSTRACT
Myasthenia gravis (MG) is an autoimmune disease that causes neuromuscular junction transmission defect and has a predilection for the with neuromuscular junction transmission defect and predilection for extra-ocular and eyelid muscles. Most cases of ocular MG (OMG) convert later to generalised MG (GMG). Assaying acetylcholine receptor antibodies (AchRA) has been used to diagnose MG, but the reported sensitivity in OMG is lower (50%) than in GMG. We report the clinical course and the diagnostic yield of assaying AchRA in a Kuwaiti cohort of patients with OMG. We carried out a retrospective review of 47 patients diagnosed with OMG who were tested for AchRA. Ancillary tests included the ice test, single-fibre electromyography (SFMEG), and repetitive nerve stimulation electromyography (RNS). Progression to GMG occurred in 51% of OMG patients with a mean time to progression of 12.1 months (range 4 to 20 months). AchRAs were positive in 46 of 47 cases (98%), while SFEMG was positive in 31 of 34 cases (91.1%). Older age (44.25 years versus 38 years, p < .05) and higher AchRA titre (2.0 nmol/L versus 1.27 nmol/L, p < .05) were significantly associated with conversion to GMG. We have found a high rate of AchRA seropositivity in relatively younger subjects of OMG. Higher AchRA titres and older age were associated with conversion to GMG, usually within the first 2 years.
KEYWORDS: Ocular myasthenia, anti-acetyl choline receptor antibody, generalised myasthenia
Introduction
Myasthenia gravis (MG) is an autoimmune disease that leads to impaired neuromuscular transmission. Solely ocular manifestations of MG (OMG) occur in 15–50% of cases, most frequently in the form of fluctuating ptosis and diplopia.1 OMG can evolve into generalised MG (GMG), with reported rates of conversion varying between 20–60%.2,3 Because OMG can mimic various ocular motility disorders or non-myasthenia ptosis, the diagnosis can often be challenging. Therefore, ancillary testing, including assaying acetylcholine receptor antibodies (AchRA), the ice test, and electrophysiological tests, can help establish the diagnosis.4,5 The sensitivity of AchRAs in patients presenting with OMG has been reported to be much lower than in patients presenting with GMG.3,5,6 However, recent studies have shown higher AchRA sensitivity in OMG.7,8 The edrophonium (Tension) test has high rates of false positives and false negatives and can be associated with severe adverse effects, so its use has declined.9 Single-fibre electromyography (SFEMG) has been reported to be sensitive and specific in OMG, whereas repetitive nerve stimulation (RNS) is less sensitive in OMG.5,10 The objectives of this study were to determine the clinical course and the rate of conversion of OMG into GMG, the utility of AchRA testing in OMG, and to explore any independent factors that are associated with the conversion to GMG in a Kuwaiti cohort of patients.
Methods
We reviewed the medical records of patients with a diagnosis of OMG from 2008–2018. Patients with signs of GMG at the onset, such as dysphagia, dysarthria, dyspnoea, dysphonia, neck or extremity weakness, were excluded. The diagnosis of OMG was defined by the presence of ptosis or diplopia characterised by fatiguability and variability with at least one of the following: (1) positive AchRA titre; (2) significant jitter on SFEMG or abnormal RNS; (3) a clinical response to the ice test; or (4) a good response to treatment with pyridostigmine. Alternative diagnoses were excluded by appropriate laboratory investigation and neuro-imaging studies, when required. The ice test was performed in the clinic in patients with ptosis by placing surgical gloves filled with crushed ice over the ptotic eyelid in unilateral ptosis or both eyelids in bilateral ptosis for 2 minutes. An improvement of 2 mm of the ptosis had to be observed for the test to be positive. All patients underwent serum testing for AChRA using a radio-immunoassay (RIA), and values greater than 0.2 nmol/L were considered positive. The minimum follow-up duration for the inclusion in the study was 12 months, and the time to progression to GMG in patients who converted was recorded.
Ancillary tests included SFMEG and RNS according to a previously described protocol.11 SFEMG was performed in one or more of frontalis, orbicularis oculi, or masseter muscle. The normal upper limits for the mean consecutive difference (MCD) were 30 µs for individual motor endplates and 21 µs for the mean MCD per study. Abnormality was defined by abnormal values/blocking in more than 10% of the fibres studied or a mean MCD per study exceeding the upper limits of normal.
The treatment of patients consisted of pryridostigmine at a starting dosage of 60 mg three times daily, which was increased as required during follow-up. At an incremental dosing regimen starting from 10 mg/day to 40 mg/day and then tapering over two months, oral prednisolone was used as required in patients who did not respond well to pyridostigmine.
Conversion to GMG was defined as the point during follow-up at which patients developed non-ocular symptoms, such as dysarthria, dysphagia, dyspnoea, or jaw, neck, arm, or leg weakness.
The Institutional Research Review Board of Ibn Sina Hospital approved this study.
Statistical Analysis
Continuous and ordinal variables were reported as mean ± standard deviation, and categorical variables were reported as frequency and percentage. A comparison was made of variables in the patients who developed GMG and those who remained as OMG using the student’s t-test and for continuous and ordinal variables and the chi-square test for categorical variables, where appropriate. Survival analysis (Kaplan–Meier) was performed to compare the probability of conversion to GMG between different categories of patients at different time points. Multivariable regression analysis was performed to determine if there were independent variables associated with the development of GMG. Statistical analysis was done using the Statistical Product and Service Solutions statistical package (IBM Corp. IBM SPSS, Version 27.0).
Results
The clinical characteristics of the 47 study subjects with OMG are shown in Table 1. The mean follow-up duration for the whole cohort was 42.3 ± 25.2 months, ranging from 15 to 118 months. The mean follow-up duration for patients who remained as OMG and did not convert to GMG throughout the study was 31 ± 14.8 (range 15 to 71 months). Progression to GMG occurred in 51% of OMG patients with a mean time to progression of 12.1 ± 4.3 months (range from 4 to 20 months). Of the patients who progressed to GMG, 13 patients converted within the first year (54.2%), and the remaining 11 patients (45.8%) converted within the second year (Figure 1).
Table 1.
The clinical characteristics of study subjects.
| Patients (N = 47) | Mean ± standard deviation or number (%) | |||
|---|---|---|---|---|
| Age (years) |
41.2 ± 9.3 |
|||
| Follow-up duration (months) |
42.3 ± 25.2 |
|||
| Gender |
Male |
23 (49%) |
||
| Female |
25 (51%) |
|||
| Symptom duration (days) |
32 ± 6.9 |
|||
| Clinical presentation |
Ptosis |
21 (44.5%) |
||
| Diplopia |
12 (25.5%) |
|||
| Ptosis and diplopia |
14 (30%) |
|||
| Acetylcholine receptor antibody |
Positive |
46 (98%) |
||
| Negative |
1 (2%) |
|||
| Acetylcholine receptor antibody titre |
1.65 ± 1.0 |
|||
| Ice test (N = 25) |
Ptosis (N = 14) |
Positive test |
13 (92.8%) |
|
| Ptosis and diplopia (N = 12) |
10 (83.3%) |
|||
| Repetitive nerve stimulation (N = 36) |
Abnormal |
12 (33.3%) |
||
| Normal |
24 (66.6) |
|||
| Single fibre EMG (N = 34) |
Abnormal |
31 (91.1%) |
||
| Normal |
3 (8.9%) |
|||
| Progression to generalised myasthenia gravis |
24 (51%) |
|||
| Time to generalisation (months) |
12.1 ± 4.3 |
|||
| Oral steroid treatment |
11 (23.4%) |
|||
| Thymoma | 8 (17%) | |||
Symptoms duration: The duration of ocular symptoms (diplopia, ptosis) before presentation.
EMG = electromyography.
Figure 1.
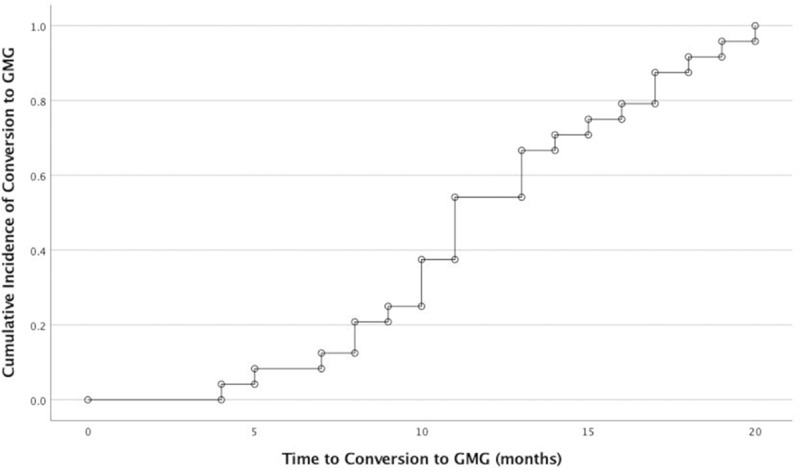
Cumulative incidence of progression to generalised myasthenia gravis in patients who generalised over time expressed in months.
GMG = generalised myasthenia gravis
The most common clinical presentation was with isolated ptosis (44.5%), followed by a combination of ptosis and diplopia (30%) and then diplopia only (25.5%). The most prevalent positive test was the AchRA assay (98%), positive in all except one case. SFEMG was performed in 34 patients and was abnormal in 31 patients (91.1%). RNS was performed in 36 patients, but it was abnormal in only 12 (33.3%). The ice test was more frequently positive in patients with isolated ptosis (92.8%) than patients with ptosis and diplopia (83.3%).
Patients who converted to GMG were older than those who remained as OMG (44.25 ± 9.0 years versus 38.08 ± 8.7 years, p < .05). Using survival analysis and various age cut-off-point intervals, we found that patients older than 45 had a significantly higher mean survival time for conversion to GMG than patients younger than 45 years (14.0 versus 10.46 months, respectively, log-rank test p-value = .029) (Figure 2a). The AchRA titre was significantly higher in patients who converted to GMG than those who did not (2.0 ± 1.1 nmol/L versus 1.27 ± 0.65 nmol/L, p < .05). Moreover, patients with titres above 1.485 nmol/L were three times more likely to convert to GMG than patients below 1.485 nmol/L (confidence intervals = 1.85–6.17, chi-square p < .001). Survival analysis, however, did not show a significant difference in the mean survival estimate of conversion to GMG between patients with AchRA titres above or below the median value of 1.485 nmol/L (11.72 versus 13.16 months, respectively, log-rank test p = .40) (Figure 2b).
Figure 2.
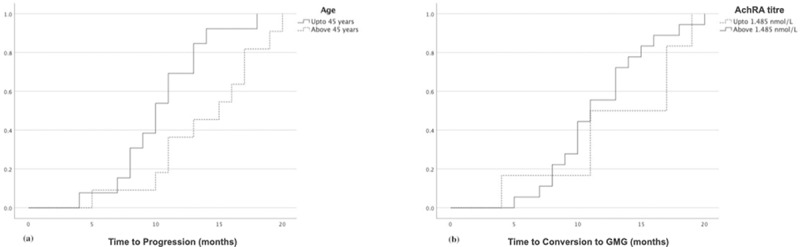
Kaplan–Meier survival curve showing the probability of conversion to generalised myasthenia gravis over time in (a) patients below and above 45 years and (b) patients with acetylcholine receptor antibody titres below and above 1.485 nmol/L.
AchRA = acetylcholine receptor antibodyGMG = generalised myasthenia gravis
There was a trend for SFEMG to be more abnormal in patients who converted to GMG (p = .09). However, there was no significant difference in the results of RNS between patients who generalised and those who did not generalise (Table 2). A total of 11 patients (23.4%) in this study used oral steroids, all of whom converted later to GMG. Of the patients who converted to GMG, 11 (45.8%) used oral steroids, and 13 (54.2%) did not use oral steroids. Multivariable regression analysis did not show an independent effect on conversion to GMG for any of the variables including gender, age, SFEMG result, and AchRA antibody titre.
Table 2.
Comparison between ocular myasthenia gravis patients who did or did not convert to generalised myasthenia gravis.
| Mean ± standard deviation or number (%) |
||||
|---|---|---|---|---|
| No conversion to GMG (N = 23) | Conversion to GMG (N = 24) |
p-value | ||
| Age (years) |
38 ± 8.7 |
44.25 ± 9.0 |
< .05 |
|
| Gender |
Male |
9 (39%) |
14 (58.3%) |
.2 |
| Female |
14 (61%) |
10 (41.7%) |
||
| Acetylcholine receptor antibody titre (nmoL/litre) |
1.27 ± 0.65 |
2.0 ± 1.1 |
< .05 |
|
| RNS |
Normal |
13 (76.5%) |
11 (58%) |
.24 |
| Abnormal |
4 (23.5%) |
8 (42%) |
||
| SFMEG | Normal |
3 (16.7%) |
0 |
.09 |
| Abnormal | 15 (83.3%) | 16 (100%) | ||
RNS = Repetitive nerve stimulation
SFEMG = Single fibre electromyography.
Discussion
In this group of OMG patients, we found that the AchRA assay was positive in 98% in cases of OMG, which is contrary to the previously reported lower AchRA rates in OMG of 45–65%.12–14 This is consistent with recent studies, which have found higher seropositive rates of AchRA in OMG.7,8 Peeler et al. also found a rate of 70.9% in a study of 223 OMG subjects.7 Chung et al. found a rate of 86.7% in patients who were eventually diagnosed with OMG.8 The discrepancy between the seropositivity rates of AchRA in this study and previous reports can be due to the variation in the clinical setting, study design, patient characteristics and selection, and referral practices. The mean age of subjects in this study (41.2 years) was slightly younger than the studies by Peeler et al. (59.2 years) and Nagia et al. (61.5 years), which may account for the higher seropositivity in our series compared with theirs (98% vs. 70–72%, respectively).7,15
Referral patterns may affect the yield of the AchRA assay testing since OMG can mimic a variety of ocular motility disorders, including strabismus and decompensated phorias, thyroid eye diseases, and cranial neuropathies. Neuro-ophthalmological assessment may improve patient selection and thus increase the positive predictive value of the AchRA assay. Chung et al. retrospectively reviewed the records of 114 patients referred to a tertiary centre for evaluation of suspected OMG. Of the patients referred, only 15 were eventually diagnosed as OMG while the rest of the patients received alternative diagnoses. Of their 15 OMG patients, 13 had positive AchRAs with a sensitivity of 80% and a positive predictive value of 92.3%.8
It is also conceivable that the new radio-immunoassays are much more sensitive and have a more standardised set reference for a positive test than older assays with no standardised reference value.
AchRA seropositivity and high AchRA titres have been associated with a greater risk of generalisation in cases of OMG.7 In this study, patients who converted to GMG were older and had higher AchRA titres than those who did not convert (Table 2). Mazzoli et al. have also shown that conversion to GMG was significantly associated with female sex, higher age of onset (> 50 years), and AchRA positivity.16 In their series, however, positive AchRA was detected in only 39.6% of patients. They also reported a much lower conversion rate to GMG of 18.4% than this study. Nagia et al., however, did not find any predictive factors for GMG conversion, including AChRA status, age, or gender, with only a trend for the presence of thymoma and AChRA titres predicting conversion to GMG.15
Eleven patients required steroids in this study, and all of them converted to GMG. Peeler et al. did not find a significant difference in the conversion rate to GMG between patients on immunosuppressive therapy and those who were not.15 Oral steroids have been found in other studies to be beneficial in alleviating OMG symptoms and reducing the risk of conversion to GMG.17,18 Because of the relatively small sample size, its retrospective nature, and the low number of patients who used oral steroids, the exact timing of starting steroids before conversion to GMG could not be determined. Therefore, our study was not designed to study the effect of immunosuppressive therapy on the rate of conversion to GMG.
The reported range of conversion to GMG varies across different studies between 20–85%.2,3,8,16,19,20 All our subjects converted to GMG in the first two years, consistent with previous reports that 80% of conversions to GMG occurred within the first year.2,3 Nagia et al. reported a median conversion time of 20 months and 69.7% converted within the first 2 years.15
SFEMG was abnormal in 31 of 34 cases (91.1%), and there was a trend (p = .09) for SFEMG to be abnormal in patients who converted to GMG (Table 2). Although SFEMG is very sensitive and specific for the diagnosis of OMG (88%–92%), the test is time-consuming and inconvenient for patients and requires technical expertise.10,21 It can also be abnormal in disorders such as Lambert-Eaton myasthenic syndrome and mitochondrial myopathies.22,23 Therefore, it is probably more helpful in the assessment of seronegative cases. Out of the 34 patients in this study who had an AchRA assay and SFEMG testing, one patient was seronegative with an abnormal SFEMG, while three patients were seropositive with a normal SFMEG. The single AchRA seronegative patient who had an abnormal SFEMG presented with ptosis and showed a good response to pyridostigmine.
We also found the ice test very useful in evaluating OMG in patients with isolated ptosis or ptosis associated with diplopia with positive tests in 92.8% and 83.3%, respectively. This is in keeping with previous reports, which found that the test to be 90% sensitive and 100% specific in OMG.24
Thymoma or thymic hyperplasia has been associated with an increased risk of generalised disease in OMG.25,26 Eight patients in this study had thymoma, and all of the patients generalised later, two of whom had thymectomy before conversion to GMG and six patients following conversion. The low prevalence of thymoma in our study (17%) is in keeping with other studies reporting lower incidence of thymoma/thymic hyperplasia in OMG compared with GMG.17
The retrospective nature and sample size are among the limitations of this study. It is possible that with longer follow-up, more cases of OMG would have converted to GMG. We have not been able to investigate the effect of more factors such as other autoimmune conditions on the development of GMG because of the low prevalence of these in this study group. Other autoantibodies, such as anti-muscle-specific kinase (MuSK) were not routinely performed.27
In summary, we have shown in a group of OMG with a slightly younger age than other reported series higher seropositivity of AchRA than reported in the literature. Almost half of the patients in this series converted to GMG in the first two years of follow-up, and this was associated with older age of onset and higher AchRA titres.
Funding Statement
The authors reported there is no funding associated with the work featured in this article.
Declaration of interest statement
No potential conflict of interest was reported by the authors.
References
- 1.Beekman R, Kuks JB, Oosterhuis HJ.. Myasthenia gravis: diagnosis and follow-up of 100 consecutive patients. J Neurol. 1997. Feb;244(2):112–118. doi: 10.1007/s004150050059. PubMed PMID: 9120493. [DOI] [PubMed] [Google Scholar]
- 2.Bever CT Jr., Aquino AV, Penn AS, et al. Prognosis of ocular myasthenia. Ann Neurol. 1983. Nov;14(5): 516–519. PubMed PMID: 6651238. doi: 10.1002/ana.410140504. [DOI] [PubMed] [Google Scholar]
- 3.Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve. 2008. Feb;37(2): 141–149. PubMed PMID: 18059039. doi: 10.1002/mus.20950. [DOI] [PubMed] [Google Scholar]
- 4.Oger J, Kaufman R, Berry K.. Acetylcholine receptor antibodies in myasthenia gravis: use of a qualitative assay for diagnostic purposes. Can J Neurol Sci. 1987. Aug;14(3):297–302. doi: 10.1017/s0317167100026652. PubMed PMID: 3664371. [DOI] [PubMed] [Google Scholar]
- 5.Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006. Jul;16(7):459–467. doi: 10.1016/j.nmd.2006.05.006. PubMed PMID: 16793269. [DOI] [PubMed] [Google Scholar]
- 6.Vincent A, Newsom Davis J. Anti-acetylcholine receptor antibodies. J Neurol Neurosurg Psychiatry. 1980. Jul;43(7):590–600. doi: 10.1136/jnnp.43.7.590. PubMed PMID: 7400823; PubMed Central PMCID: PMCPMC490626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peeler CE, Lott LB, Nagia L, et al. Clinical utility of acetylcholine receptor antibody testing in ocular myasthenia gravis. JAMA Neurol. 2015;72. doi: 10.1001/jamaneurol.2015.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung IY, Sheth SJ, Wells KK, et al. The usefulness of anti-acetylcholine receptor binding antibody testing in diagnosing ocular myasthenia gravis. J Neuroophthalmol. 2020. Aug 28. doi: 10.1097/WNO.0000000000001061. PubMed PMID: 32868574. [DOI] [PubMed] [Google Scholar]
- 9.Ing EB, Ing SY, Ing T, et al. The complication rate of edrophonium testing for suspected myasthenia gravis. Can J Ophthalmol. 2000. Apr;35(3):141–144. discussion 145. doi: 10.1016/s0008-4182(00)80007-1. PubMed PMID: 10812483. [DOI] [PubMed] [Google Scholar]
- 10.Baruca M, Leonardis L, Podnar S, et al. Single-fiber EMG as a prognostic tool in myasthenia gravis. Muscle Nerve. 2016. Dec;54(6): 1034–1040. PubMed PMID: 27144873. doi: 10.1002/mus.25174. [DOI] [PubMed] [Google Scholar]
- 11.Khuraibet AJ, Rousseff RT, Behbehani R, et al. Single-fiber electromyography of masseter muscle in myasthenia gravis. Muscle Nerve. 2008. Apr;37(4): 522–525. PubMed PMID: 17985370. doi: 10.1002/mus.20921. [DOI] [PubMed] [Google Scholar]
- 12.Limburg PC, The TH, Hummel-Tappel E, et al. Anti-acetylcholine receptor antibodies in myasthenia gravis. Part 1. Relation to clinical parameters in 250 patients. J Neurol Sci. 1983. Mar;58(3): 357–370. PubMed PMID: 6842264. doi: 10.1016/0022-510x(83)90095-3. [DOI] [PubMed] [Google Scholar]
- 13.Vincent A, Newsom-Davis J. Acetylcholine receptor antibody characteristics in myasthenia gravis. III. Patients with low anti-AChR antibody levels. Clin Exp Immunol. 1985. Jun;60(3):631–636. PubMed PMID: 2410169; PubMed Central PMCID: PMCPMC1577216. [PMC free article] [PubMed] [Google Scholar]
- 14.Padua L, Stalberg E, LoMonaco M, et al. SFEMG in ocular myasthenia gravis diagnosis. Clin Neurophysiol. 2000. Jul;111(7): 1203–1207. PubMed PMID: 10880794. doi: 10.1016/s1388-2457(00)00307-2. [DOI] [PubMed] [Google Scholar]
- 15.Nagia L, Lemos J, Abusamra K, et al. Prognosis of ocular myasthenia gravis: Retrospective multicenter analysis. Ophthalmology. 2015. Jul;122(7): 1517–1521. PubMed PMID: 25892018. doi: 10.1016/j.ophtha.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Mazzoli M, Ariatti A, Valzania F, et al. Factors affecting outcome in ocular myasthenia gravis. Int J Neurosci. 2018. Jan;128(1): 15–24. PubMed PMID: 28625092. doi: 10.1080/00207454.2017.1344237. [DOI] [PubMed] [Google Scholar]
- 17.Kupersmith MJ, Latkany R, Homel P. Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Arch Neurol. 2003. Feb;60(2):243–248. doi: 10.1001/archneur.60.2.243. PubMed PMID: 12580710. [DOI] [PubMed] [Google Scholar]
- 18.Pasnoor M, He J, Herbelin L, et al. A randomized controlled trial of methotrexate for patients with generalized myasthenia gravis. Neurology. 2016. Jul 5;87(1):57–64. doi: 10.1212/WNL.0000000000002795. PubMed PMID: 27306628; PubMed Central PMCID: PMCPMC4932232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evoli A, Tonali P, Bartoccioni E, et al. Ocular myasthenia: diagnostic and therapeutic problems. Acta Neurol Scand. 1988. Jan;77(1): 31–35. PubMed PMID: 3354309. doi: 10.1111/j.1600-0404.1988.tb06970.x. [DOI] [PubMed] [Google Scholar]
- 20.Hendricks TM, Bhatti MT, Hodge DO, et al. Incidence, epidemiology, and transformation of ocular myasthenia gravis: A population-based study. Am J Ophthalmol. 2019. Sep;205:99–105. doi: 10.1016/j.ajo.2019.04.017. PubMed PMID: 31077669; PubMed Central PMCID: PMCPMC6744973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witoonpanich R, Dejthevaporn C, Sriphrapradang A, et al. Electrophysiological and immunological study in myasthenia gravis: diagnostic sensitivity and correlation. Clin Neurophysiol. 2011. Sep;122(9): 1873–1877. PubMed PMID: 21419697. doi: 10.1016/j.clinph.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 22.Ukachoke C, Ashby P, Basinski A, et al. Usefulness of single fiber EMG for distinguishing neuromuscular from other causes of ocular muscle weakness. Can J Neurol Sci. 1994. May;21(2): 125–128. PubMed PMID: 8087737. doi: 10.1017/s0317167100049040. [DOI] [PubMed] [Google Scholar]
- 23.Oh SJ, Ohira M. Single-fiber EMG and clinical correlation in Lambert-Eaton myasthenic syndrome. Muscle Nerve. 2013. May;47(5):664–667. doi: 10.1002/mus.23638. PubMed PMID: 23505075. [DOI] [PubMed] [Google Scholar]
- 24.Kubis KC, Danesh-Meyer HV, Savino PJ, et al. The ice test versus the rest test in myasthenia gravis. Ophthalmology. 2000. Nov;107(11): 1995–1998. PubMed PMID: 11054320. doi: 10.1016/s0161-6420(00)00458-9. [DOI] [PubMed] [Google Scholar]
- 25.Farrugia ME, Cleary M, Carmichael C. A retrospective study of acetylcholine receptor antibody-positive ocular myasthenia in the West of Scotland. J Neurol Sci. 2017. Nov 15;382:84–86. doi: 10.1016/j.jns.2017.09.036. PubMed PMID: 29111026. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Hotter B, Swierzy M, et al. Generalization after ocular onset in myasthenia gravis: a case series in Germany. J Neurol. 2018. Dec;265(12): 2773–2782. PubMed PMID: 30225725. doi: 10.1007/s00415-018-9056-8. [DOI] [PubMed] [Google Scholar]
- 27.Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. 2011. Jul;44(1):36–40. doi: 10.1002/mus.22006. PubMed PMID: 21674519. [DOI] [PubMed] [Google Scholar]


