ABSTRACT
Human induced pluripotent stem cell-derived mesenchymal stem cells (iMSCs) have been believed to be a promising alternative for the stem cell transplantation therapy. The exosomes (Exo) from iMSCs play an important role in several kinds of life activities. The role of exosomes from iMSCs in severe acute pancreatitis (SAP) induced myocardial injury (MI) has not been investigated. The Exo were isolated from iMSCs through differential centrifugation method. The SAP rat model was established with 5% sodium taurocholate injection into the distal end of the bilepancreatic duct. RT-PCR and western blotting were used to measure related gene expression. Masson trichrome and Sirius Red stainings were used to evaluate MI injury. Cardiac function was detected through cardiac ultrasound.Exo promoted cell viability through activating Akt/nuclear factor E2 related factors 2 (Nrf2)/heme oxygenase 1 (HO-1) signaling pathway in vitro. Exo improved MI induced by SAP through activating Akt/Nrf2/HO-1 signaling pathway. Exo improved cardiac function, and suppressed oxidative status in the SAP model. Exo increased the expression of von Willebrand Factor (vWF) and vascular endothelial growth factor (VEGF) through activating Nrf2/HO-1 signaling pathway. Our data indicated that the Exo from iMSCs could improve MI caused by SAP through activating Nrf2/HO-1 axis. These findings firstly unfold the potential application of Exo from iMSCs in treating MI induced by SAP.
Abbreviations: LVEF: Left ventricular ejection fraction; LVFS: left ventricular fractional shorten; LVDd: left ventricular end-diastolic diameter; LVDs: left ventricular end-systolic diameter; MI: Myocardial infarction; MSCs: Mesenchymal stem cells; iPSCs: Human-induced pluripotent stem cells; SAP: Severe acute pancreatitis; iMSCs: iPSCs derived VEGF: MSCs; vascular endothelial growth factor; Nrf2: Nuclear factor erythroid 2-related factor; RT-PCR: Real-time polymerase chain reaction; HE: Hematoxylin-eosin; MODS: Multiple organ dysfunction syndrome; PI3K: Phosphatidylinositol 3-kinase; SOD: Superoxide dismutase; FBS: Fetal bovine serum; ECL: Enhanced chemiluminescence; IHC: Immunohistochemistry.
KEYWORDS: Multiple organ dysfunction syndrome, VEGF, vWF, LY294002
1. Introduction
Mesenchymal stem cells (MSCs) have attracted extensive attention because of their multidirectional differentiation potential, low immunogenicity and good clinical therapeutic function [1,2]. However, it has been found that the number and proliferation of stem cells decreased significantly with age, which limits the clinical application of MSCs. Therefore, it is necessary to find other alternative cell sources. Human-induced pluripotent stem cells (iPSCs) are pluripotent stem cells prepared from adult stem cells in recent years [3]. They have multidirectional differentiation potential similar to embryonic stem cells and can differentiate into any type of cells, but there is no immunogenicity and ethical controversy. The iPSCs derived MSCs (iMSCs) have been widely studied, and have presented a great potential application value in several fields [3].
Exosomes are membrane vesicles secreted outside the cell after the fusion of intracellular multivesicles and cell membrane. Exosomes are cup-shaped or dish-shaped bodies with a diameter of 40–120 nm [4]. Exosomes contain a variety of components including lipids, proteins and microRNA, and exosomes play an important role in immune surveillance, nerve shaping, tissue repair, stem cell survival, and coagulation function [5,6].
Severe acute pancreatitis (SAP) is believed to be one of the most common acute abdominal inflammatory diseases, and about 20% of new SAP cases are reported every year [7]. The mortality rate of SAP in the world is 15–30%. SAP is a complex pathophysiological process involving multiple factors[8]. The pathogenesis of SAP is characterized by pancreatic self-digestion by trypsin, intestinal bacterial translocation, over activation of inflammatory cells, calcium overload, pancreatic circulatory disorder and hyperlipidemia [8]. The activation of various digestive enzymes in the pancreas could induce a large number of inflammatory cells to continuously release various cytokines. These cytokines could trigger a continuous cascade of inflammatory mediators, and a series of inflammatory reactions leading to multiple organ dysfunction syndrome (MODS) [8]. SAP can result in MODS. SAP induced myocardial injury (MI) is believed to be linked with about 10–30% mortality in SAP patients [7,9,10]. If exosomes secreted by iMSCs (Exo) could improve the MI induced by SAP has not been investigated.
Phosphatidylinositol 3-kinase (PI3K)/protein kinase B signaling pathway is one of the most critical signaling pathways regulating cell survival [11]. It plays an important role in myocardial protection, and some drug preconditioning can reduce inflammatory response and oxidative stress by activating PI3K/Akt pathway, and play a protective role in myocardial protection [12]. Nuclear factor E2 related factors 2 (Nrf2) is a redox sensitive transcription factor, and it can trigger the expression of antioxidant genes, such as superoxide dismutase (SOD) [13]. Nrf2 is an important downstream target of PI3K/Akt pathway [14]. Therefore, Akt/Nrf2 may be a valuable therapeutic target to prevent MI-induced oxidative stress and injury. If Exo could regulate MI induced by SAP through Akt/Nrf2/heme oxygenase 1 (HO-1) signaling pathway remains unknown.
In this study, we isolated Exo from iMSCs, and investigated if Exo could improve MI caused by SAP through Nrf2/HO-1 signaling pathway. This study might provide a novel idea for the prevention and treatment of MI damage caused by SAP through Nrf2/HO-1 signaling pathway
2. Methods and materials
2.1. Cell culture
DMEM high glucose medium (Gibco, US) containing 10% fetal bovine serum (FBS, Gibco, US) was used to culture cardiomyocytes in an incubator with 37°C and 5% CO2. When the cell confluence reached to 70–80%, the cells were digested with 0.25% trypsin for the experiment.
2.2. Establishment of hypoxia cell model
The condition of 2% O2, 3% CO2, and 95% N2 was used to incubate cells for 24 h to establish hypoxia cell model. After treatment with Exo (3 × 105 particles/50 µL, 50 µL) or LY294002 (Nanjingjiancehng, China) for 24 h, the cells were used for detections of migration, invasion, apoptosis, and proliferation.
2.3. Characterization of hiPSC-MSCs
The iPSCs used in this study were purchased from South China Institute for Stem Cell Biology and Regenerative Medicine Group of the Chinese Academy of Sciences. DMEM medium containing 10% FBS, 0.2 mM non-essential amino acids, 1 mM L-glutamine, 0.5% penicillin were used to culture iPSCs. The iPSCs were cultured under this condition for 2 weeks. Then, the iPSCs were harvested and cultured in 0.1% gelatin-coated dishes with MSC medium. After developing a homogeneous fibroblastic morphology, the cells were used for detection of MSCs phenotype and differentiation potentials.
The surface markers of MSCs were detected using flow cytometer. The digested cells were washed using PBS, and incubated with related antibodies (20 min) in the dark. After PBS washing, the cells were analyzed with flow BD Accuri™ C6 cytometer. Osteogenic differentiation medium, adipogenic differentiation medium, and chondrogenic differentiation medium (Gibco, US) were used to induce cells. The cells were used for IHC staining to detect the differentiation of MSCs. For the IHC staining, anti-human aggrecan antibody, anti-mouse FABP4 antibody, and anti-human osteocalcin antibody were used in this study.
2.4. Isolation and identification of Exo from hiPSC-MSCs
All isolation operations were carried out at 4°C condition. 200 ml cell supernatant was collected and centrifuged at 300 g for 10 min to remove living cells from the supernatant. 2000 g centrifugation (10 min) was performed to remove dead cells and debris. 10,000 g centrifugation (30 min) was conducted to remove large vesicles in the supernatant. After filtration, centrifugation at 100,000 g for 70 min was performed, and the precipitation was collected. The precipitation was resuspended and the exosomes filtered before use.
The prepared exosomes (20 µL) were dropped on the sample carrying copper net of electron microscope (pore size: 2 nm). After 3 min, the floating liquid was absorbed using filter paper. Three percent phosphotungstic acid solution (20 µL) was added. After the copper mesh was dried, the exosomes were observed by electron microscope (Zeiss, Germany). NanoFCM (Flow Nanoanalyzer) was used to measure the particle size and concentration of exosomes.
2.5. Establishment of SAP animal model
Clean grade Wistar rats (200–250 g) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. Rats were fed on the condition of 24 ± 2°C and 40–60% humidity with 12 hours light/12 hours darkness. They were randomly divided into sham operation group, SAP model group, group SAP+Exo and group SAP+Exo+LY294002. The animals were fasted for 12 hours with free access to water before operation. After anaesthetization with pentobarbital sodium intraperitoneally, 5% sodium taurocholate (0.1 mL/100 g body weight) was retrogradely injected into the distal end of the bilepancreatic duct. Animals in the group sham only underwent surgery without injection of sodium taurocholate. The animals in the group SAP+Exo were injected with Exo (100 µg) in PBS through tail vein 24 h before SAP induction. One dose immediately after SAP induction, and another one dose after 12 h were performed. The animals in the group SAP+Exo+LY294002 were treated as same as group SAP+Exo except for the additional treatment with LY294002 (50 µM, 100 µL). The rats in the group sham and group SAP were treated with same amount of 0.9% NaCl solution. After 24 h of SAP induction, the animals were euthanized and tissues were collected. All protocols were approved by the Ethic Committee of Fujian Medical University Union Hospital.
2.7. Real-time polymerase chain reaction (RT-PCR)
After collecting the cells of each group, the total RNA was extracted with Trizol reagent. The concentration of nucleic acid was determined, reverse-transcribed into cDNA with SuperScriptTM II Reverse Transcriptase (Thermo, US). The 25 μL PCR reacting system was used, and listed as follows: PCR Master mix (12 µL), ddH2O (11 µL), upstream and downstream primers (1 µL), cDNA template (1 µL). The primers are listed in Table 1. ΔΔCt method was applied to detect relative mRNA expression.
Table 1.
Primers used in this study.
| Genes | Forward primers | Reverse primers |
|---|---|---|
| AKT | ACTCATTCCAGACCCACGAC | AGCCCGAAGTCCGTTATCTT |
| Nrf2 | AAACCAGTGGATCTGCCAAC | ACGTAGCCGAAGAAACCTCA |
| HO-1 | TCTCCGATGGGTCCTTACACTC | GGCATAAAGCCCTACAGCAACT |
| LPL | TGGAGGTACTTTTCAGCCAGGAT | CGTGGGAGCACTTCACTAGCT |
| OCN | CCCCCTCTAGCCTAGGACC | ACCAGGTAATGCCAGTTTGC |
| Sox9 | AGCGCCCCCACTTTTGCTCT | GCTCGCCCTTGGGGAACGTG |
| β-Actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
2.8. Western blotting
The protein was extracted with lysis buffer containing phenylmethanesulfonyl fluoride (PMSF, Nanjingjiancehng, China). Protein concentration was determined by BCA method (Beyotime, China). The protein was separated by polyacrylamide gel electrophoresis and transferred to the PVDF membrane at 200 mA for 2 h. The membranes were blocked with 5% skimmed milk containing 0.1% TBST for 2 hours. After washing with TBS for 3 times (10 min/time), the membrane was incubated with primary antibody at 4°C overnight. The membranes were washed with TBS at 37°C for 3 times, incubated with secondary antibody at 37°C for 1 h. After washing with TBS for 3 times (10 min/time), the membranes were incubated with enhanced chemiluminescence (ECL) reagent at room temperature. The protein gray was analyzed using Image J software. The antibodies are listed as follows: Rabbit polyclonal to phospho-AKT (ab38449, Abcam), rabbit monoclonal to AKT (ab179463, Abcam), rabbit monoclonal to Nrf2 (ab62352, Abcam), rabbit monoclonal to HO-1 (ab52947, Abcam), rabbit polyclonal to GAPDH (ab9485, Abcam), goat anti-rabbit second antibody (ab96899, Abcam).
2.9. Transwell assay
The cell density was adjusted to 5 × 105/ml using serum-free medium. 200 μL cell suspension was added to the upper chamber, and 600 μL cell suspension containing 20% FBS was added into the lower chamber. After 24 h, PBS was used to wash 3 times, and wipe off the cells not penetrated on the membrane with a cotton swab. The cells were fixed with precooled pure methanol for 15 min. After washing with PBS for 3 times, 0.1% crystal violet was used to stain cells for 30 min. An inverted microscope was used to take photos.
2.10. Wound healing method
2 × 105 cells per well in logarithmic growth stage were inoculated into 6-well plates. After reaching about 80% confluence, 200 μL pipette tip was used to make wound healing. The scratch areas were recorded at 0 h and 48 h latter.
2.11. Flow cytometry
The cells were cultured and treated as described in part 2.1 and 2.2. The treated cells were collected and washed twice with PBS. After centrifugation at 2000 g for 15 min, the cells were suspended using PBS. Propidium iodide and Annexin V-FITC were used to incubate cells for 20 min in the dark. Finally, cell apoptosis was detected.
2.12. Hematoxylin-eosin staining
The heart tissue samples were fixed in 4% paraformaldehyde solution for 24 hours, and then dehydrated in a series of gradient ethanol (50%, 75%, 85%, 95% and 100%). The tissues were embedded in paraffin, and cut into 4-μm sections. The slides were stained with hematoxylin (Beyotime, China) for 5 minutes, differentiated with 1% hydrochloric acid ethanol for 3 s and stained with eosin for 3 minutes at room temperature, and dehydrated with ethanol (75%, 95%, 100%), respectively. The dehydrated samples were soaked in dimethyl solution I, II and III for 5 min, and mounted before observing with an inverted optical microscope.
2.13. Masson trichrome staining
The Masson trichrome staining was performed with the following steps: dewaxing, gradient ethanol hydration, running water washing, 1% eosin staining for 1 h, distilled water washing for 2 min, hematoxylin staining for 5 min, distilled water washing for 2 min, Ponceau staining for 10 min, 0.2% glacial acetic acid soaking for 3 min, 1% aluminum phosphate solution differentiation for 1 min, 2% bright green staining for 1 min, 0.2% glacial acetic acid socking for 3 min, dehydrate with gradient ethanol, clear xylene and neutral gum seal. Through optical microscope, collagen fibers were dyed blue and myocardial fibers were dyed pink. Image pro6.0 software was used to measure the central muscle fibrosis area and myocardial infarction area.
2.14. Sirius red staining
After dewaxing, the sections were stained with Lapis Lazuli blue solution for 10 min. After washing with distilled water for 3 times, the sections were stained with Sirius red saturated picric acid for 20 min. The slides were differentiated and dehydrated with anhydrous alcohol. The sections were sealed with neutral gum, and observed with an inverted microscope.
2.15. Immunohistochemistry (IHC) staining
The heart tissues were firstly de-paraffined. Endogenous peroxidase was inactivated with 3% hydrogen peroxide incubation for 10 min. The sections were incubated with HCA buffer and heated in microwave oven for 5 min for antigen repair. After PBS washing (3 times, 5 min/time), 5% skimmed milk was used to block nonspecific antigen. After washing with PBS 3 times, the membrane was incubated with primary antibody at 4°C overnight. The membranes were washed with PBS for 3 times, incubated with secondary antibody at 37°C for 2 h. After washing with PBS for 3 times, the sections were incubated with DAB (3,3’-Diaminobenzidine, Beyotime, China) regent, and observed with an inverted microscope.
2.16. Cell proliferation
Cells (1 × 105/well) were seeded into 96-well plate and cultured as described in the part 2.1 and 2.2. Cell counting kit-8 (CCK8) regent (Beyotime, China) was used to incubate the cells for 4 h, and the OD value at 450 nm was detected.
2.17. Cardiac function detection through cardiac ultrasound
Three animals in each group were randomly selected. The cardiac function parameters of animals in each group: left ventricular ejection fraction (LVEF), left ventricular fractional shorten (LVFS), left ventricular end-diastolic diameter (LVDd), and left ventricular end-systolic diameter (LVDs) were measured by cardiac ultrasound diagnostic instrument. The mean values were compared for five cardiac cycles.
2.18. Statistical analysis
All experiments were repeated at least 3 times. The experimental results were expressed as mean ± SD, and analyzed using SPSS 18. 0 software (Chicago, United States). One way ANOVA was used for the comparison of more than three groups, and t-test was used for the comparison of the two groups. P < 0.05 was believed as statistically significant.
3. Results
3.1. Identification of iMSCs and Exo
The iMSCs were derived from hiPSCs, and some MSCs markers were identified. iMSCs expressed the MSCs markers, inCcluding CD29, CD90, and CD73. However, some hematopoietic cell markers including CD45 and CD34 were not expressed in the iMSCs (Figure 1(a)). The ability of iMSCs differentiating into chondrocytes, osteocytes, and adipocytes through staining aggrecan, osteocalcin, and FABP4 after related differentiation (Figure 1(b)). OCN, Sox9, and LPL were linked with osteocytes, chondrocytes, and adipocytes differentiation ability [1,15]. They were remarkably up-regulated in iMSCs (Figure 1(c)) indicating that iMSCs had the properties and multipotency of MSCs. The iMSCs were used to isolate exosomes, which were identified using transfer electron microscopy. Homogeneous spheroidal Exo were obtained from iMSCs (Figure 1(d)). The average size of exosomes was 65.18 nm measured by size distribution (Figure 1(e)), and the exosomes concentrations were 6.09E particles/mL (Figure 1(f)).
Figure 1.
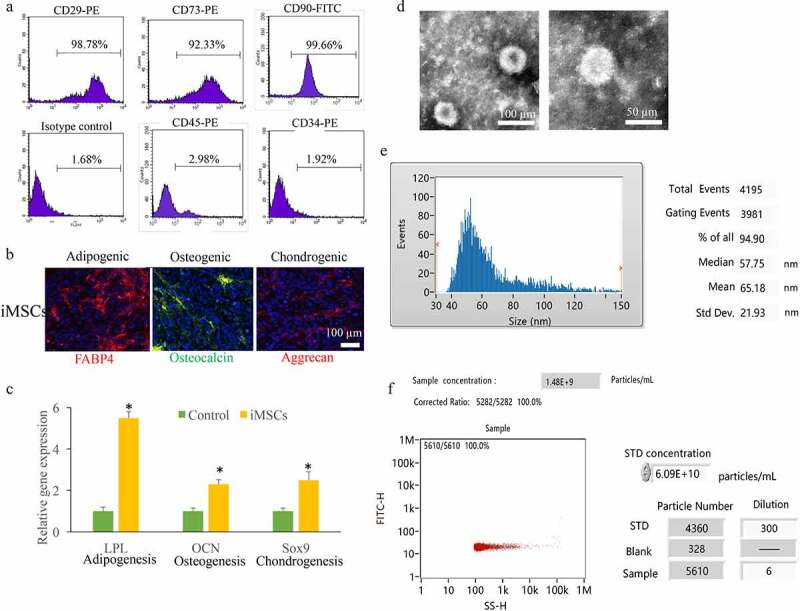
Identification iMSCs and isolated Exo. (a) The MSCs markers including CD29, CD90, CD73, CD45, and CD34 were measured. (b) The ability of iMSCs differentiating into chondrocytes, osteocytes, and adipocytes through staining aggrecan, osteocalcin, and FABP4. (c) OCN, Sox9, and LPL expression were measured. (d) The isolated exosomes were measured using transfer electron microscopy. (e) The average size of exosomes was measured by size distribution. (f) The exosomes concentrations were detected. * P < 0.05 compared with the group control. These results were obtained from at least three independent experiments. Sex-determining region Y-box 9 (Sox9); Osteocalcin (OCN); Lipoprotein lipase (LPL).
3.2. Exo promoted cell viability through activating Akt/Nrf2/HO-1 signaling pathway in vitro
The expression of Akt/Nrf2/HO-1 signaling pathway after Exo treatment was measured. We found that the levels of p-Akt, Nrf2, and HO-1 were significantly inhibited in the hypoxia cells, but Exo treatment remarkably reversed the influence of hypoxia, and promoted the expression of Akt/Nrf2/HO-1 signaling pathway (Figure 2(a-b)). In addition, the cell migration (Figure 2(c-d)), proliferation (Figure 2(e)), invasion (Figure 2(f-g)) were inhibited, but the cell apoptosis (Figure 2(i)-h) was increased by hypoxia treatment. The incubation with Exo remarkably reversed the influence of hypoxia, and promoted the cell viability (Figure 2(c-i)). In addition, the treatment with LY294002, the inhibitor of Akt/Nrf2/HO-1 signaling pathway, greatly suppressed the effects of Exo.
Figure 2.
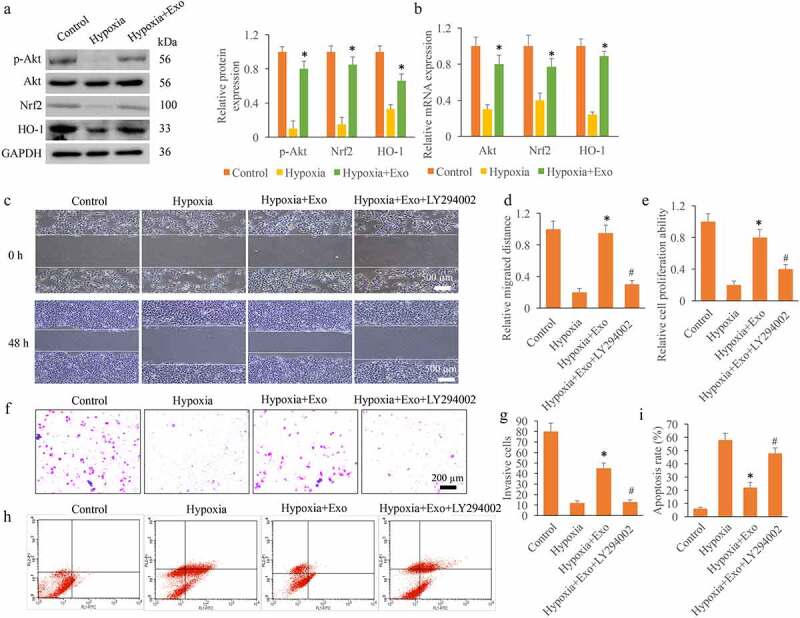
Exo promoted cell viability through activating Akt/Nrf2/HO-1 signaling pathway in vitro. (a) The protein expression levels of p-Akt, Nrf2, and HO-1 were measured. (b) The mRNA expression levels of p-Akt, Nrf2, and HO-1 were measured. (c) The cell migration was measured with wound healing assay. (d) The cell migration was analyzed. (e) The cell proliferation was detected using CCK8 method. (f) The cell invasion was measured with Transwell method. (g) The cell invasion was analyzed. (h) The cell apoptosis was detected with flow cytometry. (i) The cell apoptosis was analyzed. * P < 0.05 compared with group hypoxia. # P < 0.05 compared with the group hypoxia+Exo. These results were obtained from at least three independent experiments.
3.3. Exo improved MI induced by SAP through activating Akt/Nrf2/HO-1 signaling pathway
The SAP animal model was established, and the SAP induced MI injury was investigated. A lot of necrosis, space, and disorderly arranged muscle fibers could be observed in the group SAP measured with HE staining (Figure 3(a)), but the morphological disorder was greatly changed by Exo. However, the incubation with LY294002 reversely changed the influence of Exo, and accelerated the myocardial tissue injury (Figure 3(a)). Sirius Red and Masson staining methods were used to measure collagen deposition. Similar findings in terms of collagen deposition were obtained using these two methods. Significant increase of infarction area was found in the group SAP compared with group Sham, but Exo treatment remarkably inhibited the infarction ratio (Figure 3(b-d)). However, the supplement with LY294002 markedly reversed the inhibition effect of Exo on infarction ratio, and promoted it (Figure 3(b-d)).
Figure 3.
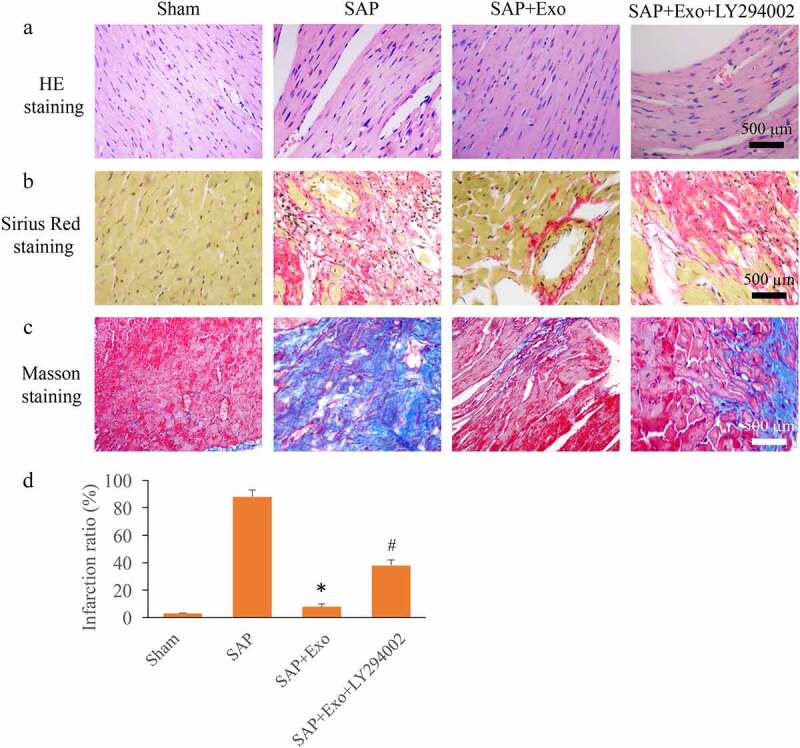
Exo improved MI induced by SAP through activating Akt/Nrf2/HO-1 signaling pathway. (a) The influence of Exo and LY294002 on morphological changes of myocardial tissues was assessed using HE staining. (b) Sirius Red staining was used to assess collagen deposition. (c) Masson staining was used to assess collagen deposition. (d) The influence of Exo and LY294002 on infarction ratio was analyzed. * P < 0.05 compared with group SAP. # P< 0.05 compared with the group SAP+Exo. 10 rats in each group were used in this study, and the infraction ratio was calculated with at least three independent experiments.
3.4. Exo improved cardiac function, and suppressed oxidative status in the SAP model
LVEF, LVFS, LVDd, and LVDs are commonly used to assess the cardiac function items. We found that the decreased LVEF and LVFS in the group SAP could be signifcantly promoted by Exo treatment. However, the supplement with LY294002 remarkably suppressed the effect of Exo (Figure 4(a)). On the contrary, the significant increased LVDs and LVDd induced by SAP were markedly decreased by Exo treatment. Meanwhile, treatment with the inhibitor of Nrf2/HO-1 greatly increased the levels of LVDs and LVDd (Figure 4(b)). In addition, the levels of cTnl, and some redox markers were measured. We found that Exo could greatly suppressed the contents of cTnl and MDA, but increased SOD and GSH-Px compared with group SAP. However, the influence of Exo was markedly reversed by LY294002 (Figure 4(c-d)).
Figure 4.
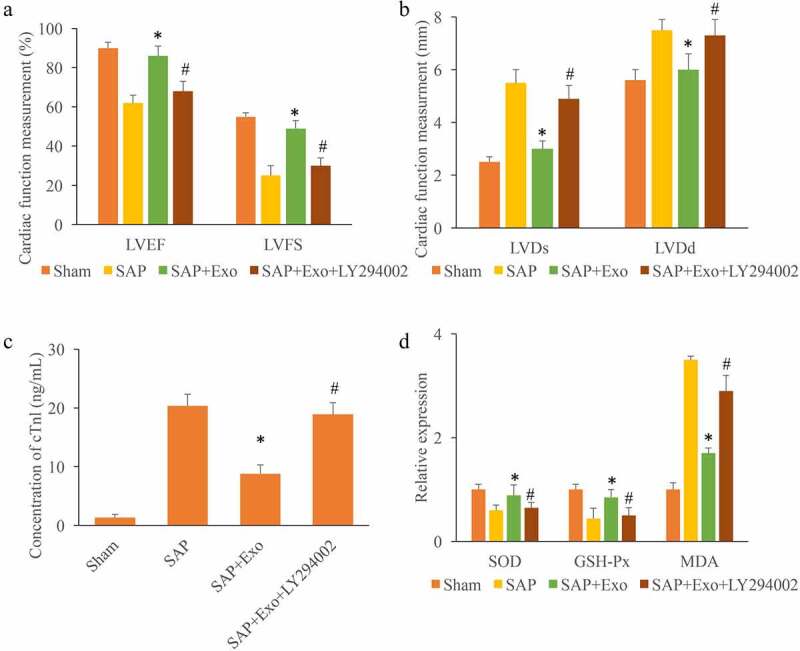
Exo improved cardiac function, and suppressed oxidative status in the SAP model. (a) The influence of Exo and LY294002 on LVEF and LVFS was measured. (b) The influence of Exo and LY294002 on LVDs and LVDd was measured. (c) The influence of Exo and LY294002 on cTnl was measured. (d) The influence of Exo and LY294002 on SOD, GSH-Px, and MDA was measured. * P < 0.05 compared with group SAP. # P < 0.05 compared with the group SAP+Exo. These results were obtained from at least three independent experiments.
3.5. Exo increased the expression of von Willebrand Factor (vWF) and vascular endothelial growth factor (VEGF) through activating Nrf2/HO-1 signaling pathway
We further investigated the role of Exo and LY294002 on the expression of Nrf2/HO-1 signaling pathway and the levels of vWF and VEGF. We found that the Akt/Nrf2/HO-1 signaling pathway was suppressed in the group SAP (Figure 5(a-c)). However, the expression of Nrf2/HO-1 signaling pathway was significantly elevated by Exo, but deceased by LY294002 (Figure 5(a-c)). The levels of vWF and VEGF were markedly suppressed in the group SAP, but increased by Exo treatment (Figure 5(d-e)). However, the treatment with LY294002 remarkably inhibited the effect of Exo on the expression of vWF and VEGF (Figure 5(d-e)). Therefore, Exo might affect the expression of vWF and VEGF through activating Nrf2/HO-1 signaling pathway.
Figure 5.
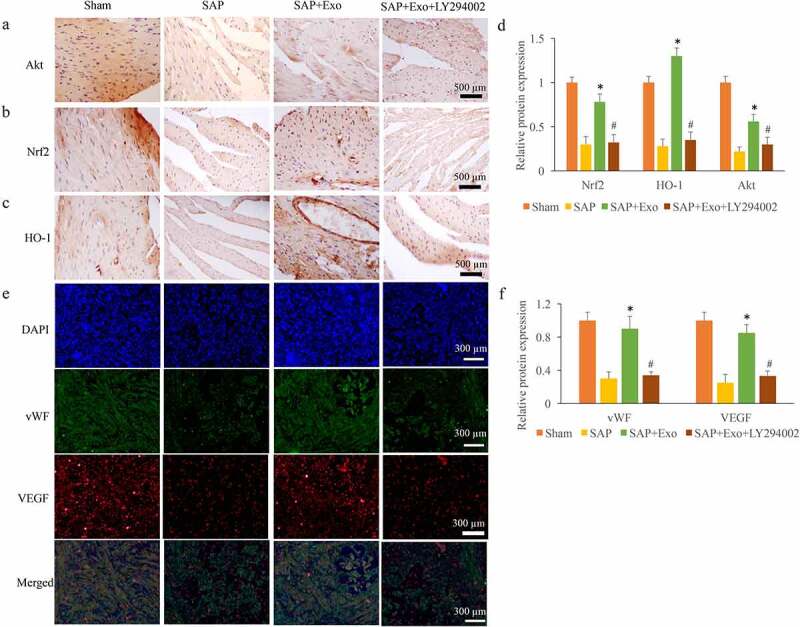
Exo increased the expression of vWF and VEGF through activating Nrf2/HO-1 signaling pathway. (a) The expression of Akt was measured through IHC staining. (b) The expression of Nrf2 was measured through IHC staining. (c) The expression of HO-1 was measured through IHC staining. (d) The protein expression of Nrf2 and HO-1 was analyzed using Image J software. (e) The expression levels of VEGF and vWF were measured through Immunofluorescence staining. (f) The expression levels of VEGF and vWF were analyzed. * P < 0.05 compared with group SAP. # P < 0.05 compared with the group SAP+Exo. These results were obtained from at least three independent experiments.
4. Discussion
hiPSCs have a wide range of sources and can be obtained in a variety of ways without immune rejection and ethical restrictions, which makes them have unique advantages in disease treatment, organ regeneration and transplantation, and drug development [16]. MSCs are important seed cells in regenerative medicine field [17]. Although adult tissues are rich in distribution, the proliferation ability of adult MSCs is generally limited, and its acquisition is also limited. iMSCs have high self-renewal and proliferation ability, which provide a potential alternative source for MSCs [18]. We successfully isolated iMSCs, and identified them using specific marker and differentiation potential experiment (Figure 1(a-c)). The morphology, size diameter, and concentration of Exo secreted from iMSCs were also measured (Figure 1(d-f)).
The application of Exo from MSCs has been widely studied. It was reported that Exo had the same ability to promote angiogenesis as MSC [19]. In the animal model of acute myocardial infarction, Exo could improve blood flow recovery and reduce infarct area, which indicated that Exo could protect heart tissue at least by promoting angiogenesis [20,21]. Meanwhile, Exo could improve cardiac fibrosis, inhibit inflammatory response and improve cardiac function in MI animal model [22]. We demonstrated that Exo significantly activated Akt/Nrf2/HO-1 signaling pathway, and promoted the cell viability of vascular endothelial cell in vitro (Figure 2). In addition, the improvement of cardiac function, decreased of infarction ratio, and suppression of oxidative level were achieved by Exo treatment and these effects of Exo were remarkably reversed by LY294002 (Figures 3–4).
The imbalance of redox balance is a sign of many pathological processes. Nrf2 is a key factor in regulating the expression of genes encoding antioxidants, detoxification and cytoprotective molecules, such as heme oxygenase 1, SOD, glutathione S-transferase and glutamic cysteine ligase [23]. Abnormal Nrf2 expression is related to the pathogenesis of MI, inflammatory diseases, cancer and aging. Some studies have shown that PI3K/Akt signaling pathway can activate Nrf2 and then induce increased expression of antioxidant enzymes, including HO-1 [24,25].
HO-1 is the rate limiting enzyme of heme degradation. It can not only protect cells and reduce the damage of oxidative stress to cells, but also affect inflammatory response and apoptosis [26]. Some animal experiments have confirmed that HO-1 can reduce tissue damage caused by inflammatory response caused by different proinflammatory factors, and play an organ protective role [27]. Nrf2/HO-1 activated by PI3K/Akt pathway can prevent ROS-induced cell injury and further reduce MI injury [28].
It was reported that Exo secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-κB signaling pathway and Nrf2/HO-1 axis [29]. Meanwhile, astrocyte-derived Exo could ameliorate oxidative stress by activating Nrf2/HO-1 signaling in the hippocampus of traumatic brain injury rats [30]. In the present study, we found that the activation of Akt/Nrf2/HO-1 signaling pathway caused by Exo could be reversed by LY294002 treatment in the MI animal model induced by SAP (Figure 6). In addition, the expression of vWF and VEGF in the myocardial tissue have been proved to be closely related with the improvement of MI [31,32]. In this study, we found that the increased expression of vWF and VEGF caused by Exo were remarkably inhibited by LY294002 (Figure 5(d-e)).
Figure 6.
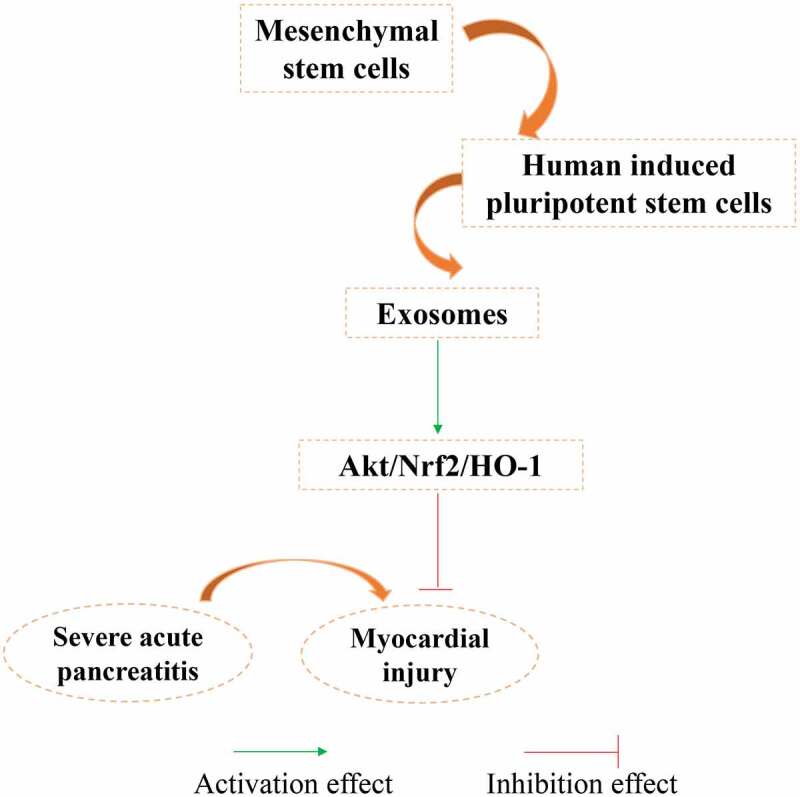
Schematic image of function mechanism of Exo.
5. Conclusion
In this study, we successfully isolated Exo from iMSCs, and identified Exo. Exo could significantly promote cardiomyocyte viability in vitro, improve cardiac function, decrease infarction ratio, inhibit oxidation and cTnl levels. However, these effects of Exo were significantly reversed by the inhibitor of Akt/Nrf2/HO-1 pathway, LY294002. Therefore, Exo might improve MI induced by SAP through promoting Akt/Nrf2/HO-1 pathway. This study expands the application of Exo in the field of SAP induced MI, and provides a novel thought for the prevention and treatment of MI using Exo through targeting Nrf2/HO-1 pathway.
Funding Statement
The authors reported there is no funding associated with the work featured in this article.
Authors’ contributions
CC conceived and designed the experiments; MC, JC, and CC performed the experiments; CC wrote the paper. All authors read and approved the final manuscript.
Consent for publication
All authors agree the publication of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
Data supporting this study has been presented in the manuscript, the data required by editor, reviewer and reader could be provided by the corresponding author.
Ethics approval and consent to participate
The study has been approved by the Ethics Committee of Fujian Medical University Union Hospital.
References
- [1].Elhussieny A, Nogami K, Sakai-Takemura F, et al. Mesenchymal stem cells derived from human induced pluripotent stem cells improve the engraftment of myogenic cells by secreting urokinase-type plasminogen activator receptor (uPAR). Stem Cell Res Ther. 2021;12:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liang X, Lin F, Ding Y, et al. Conditioned medium from induced pluripotent stem cell-derived mesenchymal stem cells accelerates cutaneous wound healing through enhanced angiogenesis. Stem Cell Res Ther. 2021;12:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhou M, Xi J, Cheng Y, et al. Reprogrammed mesenchymal stem cells derived from iPSCs promote bone repair in steroid-associated osteonecrosis of the femoral head. Stem Cell Res Ther. 2021;12:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maleki B, Alani B, Tamehri Zadeh SS, et al. MicroRNAs and exosomes: cardiac stem cells in heart diseases. Pathol Res Pract. 2021;229:153701. [DOI] [PubMed] [Google Scholar]
- [6].Balbi C, Vassalli G.. Exosomes: beyond stem cells for cardiac protection and repair. Stem Cells. 2020;38:1387–1399. [DOI] [PubMed] [Google Scholar]
- [7].Li L, Li YQ, Sun ZW, et al. Qingyi decoction protects against myocardial injuries induced by severe acute pancreatitis. World J Gastroenterol. 2020;26:1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pan L, Niu Z, Gao Y, et al. Silencing of CREB inhibits HDAC2/TLR4/NF-kappaB cascade to relieve severe acute pancreatitis-induced myocardial injury. Inflammation. 2021;44:1565–1580. [DOI] [PubMed] [Google Scholar]
- [9].Wen Y, Sun HY, Tan Z, et al. Abdominal paracentesis drainage ameliorates myocardial injury in severe experimental pancreatitis rats through suppressing oxidative stress. World J Gastroenterol. 2020;26:35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ren S, Pan L, Yang L, et al. miR-29a-3p transferred by mesenchymal stem cells-derived extracellular vesicles protects against myocardial injury after severe acute pancreatitis. Life Sci. 2021;272:119189. [DOI] [PubMed] [Google Scholar]
- [11].Wang D, Liu J, Jiang H.. Triclosan regulates the Nrf2/HO-1 pathway through the PI3K/Akt/JNK signaling cascade to induce oxidative damage in neurons. Environ Toxicol. 2021;36:1953–1964. [DOI] [PubMed] [Google Scholar]
- [12].Zhuang S, Yu R, Zhong J, et al. Rhein from Rheum rhabarbarum inhibits hydrogen-peroxide-induced oxidative stress in intestinal epithelial cells partly through PI3K/Akt-mediated Nrf2/HO-1 pathways. J Agric Food Chem. 2019;67:2519–2529. [DOI] [PubMed] [Google Scholar]
- [13].Brasil FB, Bertolini Gobbo RC, de Almeida Fj S, et al. The signaling pathway PI3K/Akt/Nrf2/HO-1 plays a role in the mitochondrial protection promoted by astaxanthin in the SH-SY5Y cells exposed to hydrogen peroxide. Neurochem Int. 2021;146:105024. [DOI] [PubMed] [Google Scholar]
- [14].Baraka SA, Tolba MF, Elsherbini DA, et al. Rosuvastatin and low-dose carvedilol combination protects against isoprenaline-induced myocardial infarction in rats: role of PI3K/Akt/Nrf2/HO-1 signalling. Clin Exp Pharmacol Physiol. 2021;48:1358–1370. [DOI] [PubMed] [Google Scholar]
- [15].Lin Y, Liu L, Li Z, et al. Pluripotency potential of human adipose-derived stem cells marked with exogenous green fluorescent protein. Mol Cell Biochem. 2006;291:1–10. [DOI] [PubMed] [Google Scholar]
- [16].Pichard L, Brondelo JM, Becker F, et al. Generation of human pluripotent stem cell lines (iPSCs) from mesenchymal stem cells (MSCs) from three elderly patients with osteoarthritis. Stem Cell Res. 2020;44:101721. [DOI] [PubMed] [Google Scholar]
- [17].Jakob M, Hambrecht M, Spiegel JL, et al. Pluripotent stem cell-derived mesenchymal stem cells show comparable functionality to their autologous origin. Cells. 2020;10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu Z, Liu W, Wang Z, et al. Mesenchymal stem cells derived from iPSCs expressing interleukin-24 inhibit the growth of melanoma in the tumor-bearing mouse model. Cancer Cell Int. 2020;20:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qi X, Zhang J, Yuan H, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12:836–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu X, Li Q, Niu X, et al. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int J Biol Sci. 2017;13:232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hu GW, Li Q, Niu X, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim S, Lee SK, Kim H, et al. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dai H, Wang P, Mao H, et al. Dynorphin activation of kappa opioid receptor protects against epilepsy and seizure-induced brain injury via PI3K/Akt/Nrf2/HO-1 pathway. Cell Cycle. 2019;18:226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang J, Chen R, Liu C, et al. Antidepressant mechanism of catalpol: involvement of the PI3K/Akt/Nrf2/HO-1 signaling pathway in rat hippocampus. Eur J Pharmacol. 2021;909:174396. [DOI] [PubMed] [Google Scholar]
- [25].Wu X, Wang J, Song L, et al. Catalpol weakens depressive-like behavior in mice with streptozotocin-induced hyperglycemia via PI3K/AKT/Nrf2/HO-1 signaling pathway. Neuroscience. 2021;473:102–118. [DOI] [PubMed] [Google Scholar]
- [26].Xu L, He S, Yin P, et al. Punicalagin induces Nrf2 translocation and HO-1 expression via PI3K/Akt, protecting rat intestinal epithelial cells from oxidative stress. Int J Hyperthermia. 2016;32:465–473. [DOI] [PubMed] [Google Scholar]
- [27].Xiao Q, Piao R, Wang H, et al. Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/AKT activation. Int J Biol Macromol. 2018;118:747–755. [DOI] [PubMed] [Google Scholar]
- [28].Lin CC, Lin WN, Cho RL, et al. Induction of HO-1 by mevastatin mediated via a Nox/ROS-dependent c-Src/PDGFRalpha/PI3K/Akt/Nrf2/ARE cascade suppresses TNF-alpha-induced lung inflammation. J Clin Med. 2020;9:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ning H, Chen H, Deng J, et al. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-kappaB signaling pathway and Nrf2/HO-1 axis. Stem Cell Res Ther. 2021;12:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang W, Hong J, Zhang H, et al. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging (Albany NY). 2021;13:21642–21658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zou Y, Li L, Li Y, et al. Restoring cardiac functions after myocardial infarction-ischemia/reperfusion via an exosome anchoring conductive hydrogel. ACS Appl Mater Interfaces. 2021;13:56892–56908. [DOI] [PubMed] [Google Scholar]
- [32].Chen Q, Huang M, Wu J, et al. Exosomes isolated from the plasma of remote ischemic conditioning rats improved cardiac function and angiogenesis after myocardial infarction through targeting Hsp70. Aging (Albany NY). 2020;12:3682–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting this study has been presented in the manuscript, the data required by editor, reviewer and reader could be provided by the corresponding author.


