Abstract
Objective
To assess the incidence of testicular cancer in trans women (male sex assigned at birth, female gender identity) using gender‐affirming hormonal treatment.
Patients and Methods
Data of trans women starting hormonal treatment at our gender identity clinic between 1972 and 2017 were linked to the national pathology database to obtain testicular cancer diagnoses. The standardised incidence ratio (SIR) was calculated using the number of observed testicular cancer cases in our cohort and the number of expected cases based on age‐specific Dutch incidence rates. Subgroup analyses were performed in testicular tissues sent for histopathological analysis at the time of bilateral orchidectomy, and when follow‐up exceeded 5 years.
Results
The cohort consisted of 3026 trans women with a median follow‐up time of 2.3 interquartile range (IQR) (1.6–3.7) years. Two testicular cancer cases were identified whilst 2.4 cases were expected (SIR 0.8, 95% confidence interval 0.1–2.8). In addition, one testicular cancer case was encountered in an orchidectomy specimen (0.1%). In the 523 trans women with a follow‐up time of >5 years (median [IQR] 8.9 [6.4–13.9] years), no testicular cancer was observed.
Conclusion
Testicular cancer risk in trans women is similar to the risk in cis men. The testicular cancer cases occurred within the first 5 years after commencing hormonal treatment, and the percentage of cases encountered at the time of bilateral orchidectomy was low. As no testicular cancer was observed in trans women with a long follow‐up period, long‐term hormonal treatment does not seem to increase testicular cancer risk.
Keywords: testicular cancer, transgender, gender‐affirming hormonal treatment, gender dysphoria, carcinogenesis, oestrogen, #TesticularCancer, #tscsm, #uroonc
Introduction
Testicular cancer mainly occurs in young people; the incidence in the Netherlands is 9.5 per 100 000 men, with a peak incidence of 32.4 per 100 000 men in those aged between 30 and 34 years [1]. Testicular cancers can roughly be divided into sex cord or gonadal stromal tumours and germ‐cell tumours, of which the latter most commonly occur. Germ‐cell tumours are further classified as seminoma, non‐seminoma, and mixed germ‐cell tumours. Prognosis, depending on histology, location of the primary tumour and metastases, and serum tumour marker levels, is generally better for seminoma compared to non‐seminoma [2]. Although the incidence has increased over the past 40 years in most countries, the aetiology of testicular cancer and the reasons for this rise remain unclear. Established risk factors for testicular cancer are a history of cryptorchidism, a low sperm count, presence of a contralateral testis tumour or a positive family history among first‐grade relatives for testicular cancer [2]. Some theories also suggest that a relative excess of exogenous oestrogens during pre‐ or post‐natal life (e.g. diethylstilbesterol, pesticides) may play a causal role in the development of testicular cancer [3, 4, 5]. It is hypothesised that, following endocrine disruption, some of the primordial germ cells lose track of their normal development and become premalignant cells that may develop into carcinoma in situ cells, which in their turn may develop into a complete cancer [4].
An increasing group of birth‐assigned males with long‐term exposure to exogenous oestrogens are people with gender dysphoria. Gender dysphoria refers to the distress that results from a conflict between a person’s assigned sex at birth and one’s gender identity [6]. People assigned male at birth who also identify as male are referred to as cis men, whereas birth‐assigned males who identify as female are referred to as trans women. Birth‐assigned males who neither identify as male nor female fall under the umbrella term gender queer, non‐binary, or alternative gender. Birth‐assigned males with gender dysphoria desiring to align their physical characteristics with their gender identity can choose to undergo medical treatment, consisting of gender‐affirming hormonal treatment (GAHT) and gender‐affirming surgery (GAS). The hormonal treatment protocol usually consists of antiandrogens, to suppress serum testosterone concentrations, combined with oestrogens, to achieve feminisation. For people presenting during adolescence (aged <18 years) treatment can be initiated when a person reaches puberty (Tanner Stage ≥2) and aims to suppress pubertal development by administration of GnRH agonist (GnRHa). After at least 6 months of puberty suppression and having reached the age of 16 years, treatment can be supplemented with oestrogens. GAS can involve facial feminisation, breast augmentation, and bilateral orchidectomy often combined with vaginoplasty [7]. For the sake of clarity, we will refer to birth‐assigned males seeking feminising medical treatment as trans women.
Until 2014, a sterilisation law was in place in the Netherlands, meaning that a gonadectomy was required for legal gender recognition. Therefore, almost all trans women visiting our gender identity clinic underwent this procedure until 2014. However, since this law has changed, an increasing number of people with non‐binary identities or less need to confirm to binary cis presentation choose to keep their male gonads. Consequently, in the future we might be faced with a growing population of young trans women using GAHT, who are still at risk for testicular cancer. Several studies have been conducted on the influence of androgen deprivation, oestradiol supplementation, or a combination of these two, on testicular tissue and showed incomplete spermatogenesis, a decreased diameter of seminiferous tubules, and increased peritubular hyalinisation [8, 9, 10, 11]. However, very little is known about the influence on the occurrence of testicular cancer and only a few cases of testicular cancer in trans women using GAHT have been reported [12, 13, 14, 15, 16].
The primary aim of the present study was to evaluate the incidence of testicular cancer in trans women using GAHT and, hereby, assess the safety of hormonal treatment in terms of testicular cancer risk. A secondary aim was to assess the outcome of histopathological analyses of orchidectomy specimens obtained during GAS.
Patients and Methods
Study Design and Population
For this nationwide retrospective cohort study, we identified all people who visited the gender identity clinic of the Amsterdam UMC between 1972 and September 2017. Approximately 95% of all transgender people in the Netherlands visit our centre for either psychological, endocrine, or surgical treatment. As only trans women using GAHT were eligible for inclusion, people who never used GAHT, those who underwent bilateral orchidectomy prior to the start of GAHT, or those of whom the start date of GAHT was unknown, were excluded. Other exclusion criteria involved being aged <18 years at the time of the study (2020) or having used female and male hormones alternatingly during the follow‐up period. Lastly, as data were partially obtained from the Dutch national pathology database (PALGA), which covers histopathological diagnoses nationwide since 1991, trans women were also excluded when their last visit to the gender identity clinic was before 1991 [17].
Hormonal treatment for trans women generally consists of a combination of antiandrogens and oestrogens. The most commonly prescribed antiandrogen in this cohort was cyproterone acetate (10–100 mg daily) and only sporadically spironolactone (100–200 mg daily) was used. Different administration routes for oestrogens exist, such as transdermal, oral, and intramuscular formulations. The different types of oestrogens prescribed in our centre included oestradiol patches (50–150 µg/24 h twice weekly), oestradiol gel (0.75–3.0 mg daily), oestradiol valerate (2–6 mg daily), ethinyl oestradiol (25–100 µg daily), conjugated oestrogens (0.625–1.25 mg daily), oestradiol implants (20 mg every 3–6 months), and oestradiol injections (10–100 mg every 2–4 weeks). From 2001 onward, mainly oestradiol patches, oestradiol gel, or oestradiol valerate were used. People who started hormonal treatment when they were aged <18 years, often used GnRHa, namely triptorelin, prior to the start with oestrogens and continued this medication until orchidectomy.
The Ethical Review Board of the VU University Medical Center Amsterdam, concluded that the Medical Research Involving Human Subjects Act (Wet medisch‐wetenschappelijk onderzoek met mensen [WMO]) did not apply to this study. Necessity for informed consent was waived because of the retrospective design, the large study population, and the risk of selection bias (e.g. excluding deceased trans women).
Data Collection
Data on medical history (e.g. testicular cancer, cryptorchidism), age at start of GAHT, documented hormone use, endocrine laboratory results, date of bilateral orchidectomy, date of last visit to our clinic, and data on mortality were collected from the medical files of the participants. This database was linked to PALGA to obtain data regarding testicular cancer histology (germ‐cell tumours, sex cord/stromal tumours and germ cell neoplasia in situ) and the date of testicular cancer diagnosis [17]. Data on testicular cancer diagnosis were further validated by comparing notes from the medical files with data obtained from PALGA.
Statistical Analysis
Descriptive analyses were conducted to assess the characteristics of the cohort. Normally distributed data are presented as means with standard deviations (SDs) and non‐normally distributed data as medians with interquartile ranges (IQRs). Mean oestradiol and testosterone concentrations were calculated by averaging the results from measurements performed during GAHT. In people who had started GAHT prior to their first visit to our clinic, we used the first known start date of GAHT to calculate the most accurate treatment duration. The follow‐up time was calculated as the number of years from the start of GAHT until, either the date of testicular cancer diagnosis, or the date of bilateral orchidectomy, or the date of death, or the date of the last visit to our gender identity clinic. To calculate the age‐adjusted standardised incidence ratio (SIR), we used the observed cases and the expected cases of testicular cancer in our cohort. Only testicular cancer cases that occurred after the start of GAHT were included for analysis. For our primary research aim, we only included testicular cancer cases that were discovered due to symptoms (e.g. scrotal mass or infertility), as cis men are similarly diagnosed. Expected cases were calculated based on age‐specific incidence rates obtained from the Netherlands Comprehensive Cancer Organisation [1]. Since this organisation also uses data from PALGA to generate the incidence rates of testicular cancer in the Dutch population, this allows for a reliable comparison. For the sake of clarity, we will refer to the reference population as cis men, although we were not able to verify if this was true for the whole population. The SIR with 95% CI was calculated using a mid‐exact P test. As it remains largely unknown how GAHT during puberty affects testicular architecture in terms of testicular cancer risk, a subgroup analysis was performed for trans women who initiated GAHT when aged ≥18 years. Furthermore, in order to assess the effect of long‐term hormone use more accurately, a subgroup analysis was performed for trans women with a follow‐up time of ≥5 years. Lastly, to assess how often testicular cancer was discovered in orchidectomy specimens obtained during gender‐affirming surgery, a subgroup analysis was performed for trans women whose testicular tissue was sent for histopathological analysis at the time of bilateral orchidectomy.
STATA Statistical Software, version 15.1 (Statacorp, College Station, TX, USA) and OpenEpi version 3.01 (www.OpenEpi.com) were used for statistical analyses.
Results
A total of 8015 people visited our gender identity clinic between 1972 and 2017 for either psychological, endocrine, or surgical treatment. After applying the in‐ and exclusion criteria, 3026 trans women were included in the study cohort (Fig. 1). The median (IQR) follow‐up time was 2.3 (1.6–3.7) years per person and the total follow‐up time of the entire cohort was 11 223 years (Fig. 2). The median (IQR) age at start of GAHT was 29 (22–41) years. At initiation of GAHT the median serum testosterone and oestradiol concentrations were in the normal male range. During GAHT serum oestradiol concentrations increased (median [IQR] 181 [110–296] pmol/L) and serum testosterone concentrations decreased (median [IQR] 1.0 [0.6–1.3] nmol/L). In this cohort, 1914 trans women (63%) underwent bilateral orchidectomy at a median (IQR) of 2.3 (1.7–3.4) years after commencing hormonal treatment. Last known hormone measurements before bilateral orchidectomy did not differ from the averaged results during GAHT (data not shown). Table 1 shows the characteristics of the entire study cohort.
Fig. 1.
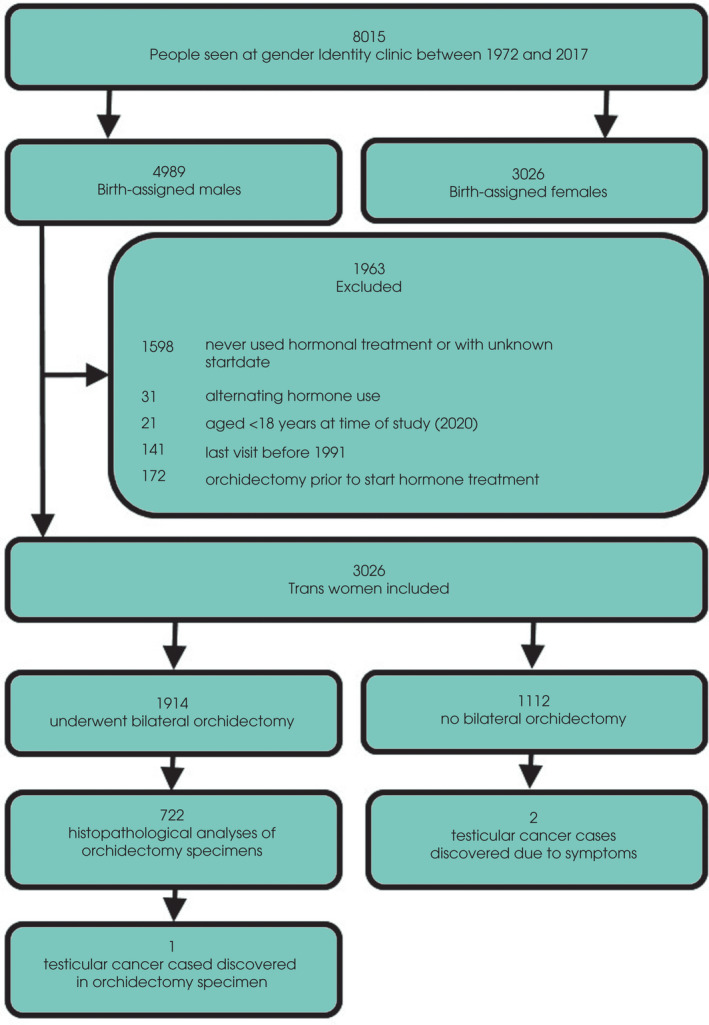
Study flow chart.
Fig. 2.

Follow‐up time of the study cohort. Dashed line indicates cohort for subgroup analysis performed for trans women with a follow‐up time of ≥5 years.
Table 1.
Characteristics of study cohort (n = 3026).
| Characteristics | N | Median (IQR) unless stated otherwise |
|---|---|---|
| Age at start of GAHT, years | 3026 | 29 (22–41) |
| History of cryptorchidism, % | 35 | 1 |
| Serum testosterone concentration at initiation of GAHT, nmol/L | 844 | 18.0 (12.0–23.0) |
| Serum oestradiol concentration at initiation of GAHT, pmol/L | 840 | 86 (68–109) |
| Serum testosterone concentration during GAHT, nmol/L | 1714 | 1.0 (0.6–1.3) |
| Serum oestradiol concentration during GAHT, pmol/L | 1756 | 181 (110–296) |
| Bilateral orchidectomy, % | 1914 | 63 |
| Follow‐up time, years | 3026 | 2.3 (1.6–3.7) |
| Total follow‐up time, years | 3026 | 11 223 |
In total, three cases of testicular cancer were observed in the study cohort. Age at the time of diagnosis of the three trans women with testicular cancer ranged from the second to the fourth decade of life. All three trans women were Caucasian, had no medical history of oncological diseases, and their family history was negative for testicular cancer. One person started treatment during adolescence, while the other two started GAHT in adulthood. Used antiandrogenic treatment involved tripterolin, cyproterone acetate, and spironolactone, resulting in serum testosterone levels of <2 nmol/L. Types of used oestrogens included oestradiol injections, oestradiol patches, and oestradiol valerate, resulting in mean serum oestradiol concentrations of between 150 and 300 pmol/L. Oestrogens were used for a duration of 1–3 years prior to diagnosis. Histology showed non‐seminoma (mature teratoma, 95%; embryonal carcinoma, <5%; and yolk sac tumour, <5%) in one case, pure seminoma grown into the rete testis in the second case, and a seminoma with syncytiotrophoblast cells in the third case. Tumour diameters ranged from 0.9 to 6.8 cm. None of the trans women had metastasis and all remained clinically stable after treatment.
Two of the previously mentioned testicular cancer cases were discovered due to symptoms of a painless scrotal mass. Based on age‐specific incidence rates in cis men, we expected 2.4 cases of testicular cancer in our cohort. This resulted in a SIR of 0.8 with a 95% CI of 0.1–2.8. Subgroup analysis of 2731 trans women who initiated GAHT when aged ≥18 years, showed two testicular cancer cases, although 2.2 cases would have been expected, resulting in a SIR of 0.9 (95% CI 0.2–3.0). In the subgroup of 523 trans women with a follow‐up of ≥5 years (median [IQR] 8.9 [6.4–13.9] years, total 5870 years), no testicular cancer cases were observed, although 1.2 cases would have been expected based on the age‐specific incidence rates in cis men.
Of the 1914 trans women who underwent bilateral orchidectomy, histopathological analysis of the resected specimens was performed in 722 trans women. Within this group, histopathological analysis showed testicular cancer in one case (0.1% of orchidectomy specimens obtained during GAS).
Discussion
The aim of the present study was to assess testicular cancer incidence in trans women using GAHT. A total of three testicular cancer cases were observed in our present cohort, of which two were discovered due to symptoms and the third was encountered during routine histopathological analysis of the bilateral orchidectomy specimen. Our observations suggest that there is no difference in testicular cancer risk between trans women using GAHT and cis men. In addition, no testicular cancer cases were observed in trans women with a follow‐up time of >5 years.
To the best of our knowledge, there is only one other epidemiological study that, in addition to other types of cancer, also assessed testicular cancer incidence in trans women, reporting an incidence ratio of 0.3 (95% CI 0.1–0.6) compared to cis men [18]. However, in contrast to our present study this proportional incidence study lacked data on GAHT and GAS. As trans women are no longer at risk for testicular cancer after bilateral orchidectomy, we feel that with our longitudinal data we were able to accurately calculate follow‐up time, and hereby, adequately assess the effect of GAHT on testicular cancer risk.
In total, five case reports have been published on testicular cancer cases in trans women. Histopathological analyses showed seminoma in three cases, non‐seminoma (mature teratoma) in one case, and mixed germ‐cell tumour (embryonal carcinoma, 75%; immature teratoma, 15%; seminoma, 9%; and yolk sac tumour, <1%) in the last case. Similar to the cases observed in our present cohort, two trans women were referred to urologists because they felt a painless scrotal mass which was, in one case, already present for several months [14, 16]. In the other three cases, testicular pathology was only discovered after extensive examination, initiated when antiandrogenic treatment failed to suppress serum testosterone concentrations [12, 13, 15]. This illustrates how diagnosis of testicular cancer in trans women may be delayed when people experience severe genital dysphoria and may ignore or be unaware of abnormalities such as a testicular mass. Furthermore, physicians might not be aware of the presence of testicles during a consultation with a phenotypical woman, which can also lead to a delayed diagnosis. Improving awareness on this topic is important to provide proper care for the increasing number of trans women who may not undergo genital GAS. In addition, it is imperative that healthcare providers are counselled on working in a trans sensitive manner [19, 20]. Furthermore, consistent with recommendations for cis men, trans women with clinical risk factors such as a family history of testicular cancer, should be encouraged to regularly perform testicular self‐examination [2, 21].
Several studies addressed the influence of GAHT on testicular tissue and mainly showed severe spermatogenic involution, reduced numbers of Leydig cells, seminiferous tubules with a decreased diameter or an absent lumen, heavy peritubular hyalinisation, and fibrosis [9]. Because of depletion of germ cells, testicular volumes have shown to generally decrease by 25% within the first year of hormonal treatment [22]. However, in previous studies, no malignant changes were observed in the orchidectomy specimens of trans women obtained during GAS [8, 23]. To the best of our knowledge this is the first reported case of testicular cancer discovered during histopathological analysis of an orchidectomy specimen, even though our centre implemented routine histopathological analysis of orchidectomy specimens obtained during GAS ˜10 years ago. For trans women who underwent bilateral orchidectomy outside the Netherlands or before routine histopathological analysis was implemented, no histology results were available. As testicular cancer was discovered in only 0.1% of the 722 analysed orchidectomy specimens, one can argue if this routine histopathological analysis is necessary when there is no suspicion of testicular pathology.
In literature, exposure to oestrogens has been implicated as a risk factor for germ‐cell tumours. Studies in rodents have shown that exposure to high oestrogen levels during either pre‐natal or adult life induces testicular tumour formation; however, it is unclear how such findings in animals apply to humans [24]. Several epidemiological studies have found a possible association between exposure to occupational and environmental oestrogenic chemicals, such as pesticides and other endocrine‐disrupting agents, and increased testicular cancer risk but require further confirmation [3, 4, 5, 25]. Also, studies have investigated the association between high maternal oestrogen levels during the first trimester of pregnancy and the development of testicular cancer in offspring but failed to produce clear evidence for this oestrogen excess hypothesis [4, 26]. However, limitations of these studies include the indirect parameters that were used to assess the effect of oestrogen exposure such as maternal age >30 years, being first‐born, and twinning. We feel that, with our present study, we are able to more directly assess the influence of oestrogens on testicular cancer risk, as the most profound difference between our cohort and cis men is the use of gender‐affirming hormones. As we found that testicular cancer risk in trans women is not increased compared to cis men, our present results do not support the hypothesis of a carcinogenic effect of post‐natal exposure to exogenous oestrogens on testicular tissue.
The major strengths of our present study include the large cohort size consisting of young people with an age range in the peak incidence of testicular cancer. Also, follow‐up time is adequately calculated by using the date that people were no longer at risk of testicular cancer or when they were last seen at our gender clinic. Furthermore, we were able to validate our data by linking our cohort to the PALGA, which has nationwide coverage [17]. Taken these factors into account, we feel that we are the first to report a reliable estimate of the testicular cancer risk in trans women.
A limitation of the present study is that, despite the large cohort size, follow‐up time is relatively short due to the fact that the majority of trans women decided to undergo bilateral orchidectomy directly after the required minimum of 12 months GAHT. Nonetheless, within the subgroup of 523 trans women using hormonal treatment for ≥5 years (median [range] 8.9 [5.0–52.1] years), no testicular cancer cases were observed, implying that a longer duration of hormonal treatment does not contribute to an increased testicular cancer risk. It might be worthwhile to repeat this study in 10 years, to establish a larger cohort size and a longer follow‐up time, and consequently draw even more reliable conclusions on testicular cancer risk in trans women. Secondly, it was not possible to compare between GAHT protocols, as, on the one hand, many trans women change often between different types of prescribed oestrogens over time and, on the other hand, they mostly use cyproterone acetate as antiandrogenic treatment. Furthermore, scrotal ultrasonography to screen for the presence of testicular cancer was not routinely performed at initiation and during GAHT, but this was also not the case for the reference population, as guidelines advice against population‐based screening [2]. Therefore, we do not expect that this affected our present results.
In conclusion, the present large nationwide cohort study in trans women using GAHT suggests that testicular cancer risk is comparable to the risk in cis men. Furthermore, results from our subgroup analysis in trans women with a long follow‐up period, suggest that longer exogenous oestrogen exposure does not increase the risk of developing testicular cancer. This is reassuring for trans women who do not wish, or do not have the option, to undergo genital GAS. However, awareness of the presence of the gonads remains important and regular testicular self‐examination is recommended.
Conflict of Interest
The authors have nothing to disclose.
Author Contributions
All authors have made a substantial contribution to the manuscript and all of them have read and approved the final version.
Abbreviations
- GAHT
gender‐affirming hormonal treatment
- GAS
gender‐affirming surgery
- GnRHa
GnRH agonist
- IQR
interquartile range
- PALGA
Dutch national pathology database
- SIR
standardised incidence ratio
References
- 1.The Netherlands Comprehensive Cancer Organization (IKNL). Dutch Cancer Figures, 2017. Available at: http://embed.nkr%2010cijfers.nl/1.1.5/#/table?embedId=557%26amp;embedTitle=Incidence,%20Testis,%202017,%20Male,%20CR. Accessed August 2020
- 2. Laguna MP, Albers P, Algaba F et al. EAU Guidelines on Testicular Cancer, 2020. European Association of Urology 2020. Available at: https://uroweb.org/guideline/testicular-cancer/. Accessed August 2021 [Google Scholar]
- 3. Fénichel P, Chevalier N. Is testicular germ cell cancer estrogen dependent? The role of endocrine disrupting chemicals. Endocrinology 2019; 160: 2981–9 [DOI] [PubMed] [Google Scholar]
- 4. Giannandrea F, Paoli D, Figà‐Talamanca I, Lombardo F, Lenzi A, Gandini L. Effect of endogenous and exogenous hormones on testicular cancer: the epidemiological evidence. Int J Dev Biol 2013; 57: 255–63 [DOI] [PubMed] [Google Scholar]
- 5. Skakkebaek NE, Rajpert‐De Meyts E, Jorgensen N et al. Germ cell cancer and disorders of spermatogenesis: an environmental connection? APMIS 1998; 106: 3–11 [DOI] [PubMed] [Google Scholar]
- 6. American Psychiatric Association (APA) . Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 7. Hembree WC, Cohen‐Kettenis PT, Gooren L et al. Endocrine treatment of gender‐dysphoric/gender‐incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2017; 102: 3869–903 [DOI] [PubMed] [Google Scholar]
- 8. Matoso A, Khandakar B, Yuan S et al. Spectrum of findings in orchiectomy specimens of persons undergoing gender confirmation surgery. Hum Pathol 2018; 76: 91–9 [DOI] [PubMed] [Google Scholar]
- 9. Schneider F, Kliesch S, Schlatt S, Neuhaus N. Andrology of male‐to‐female transsexuals: influence of cross‐sex hormone therapy on testicular function. Andrology 2017; 5: 873–80 [DOI] [PubMed] [Google Scholar]
- 10. Schulze C. Response of the human testis to long‐term estrogen treatment: morphology of Sertoli cells, Leydig cells and spermatogonial stem cells. Cell Tissue Res 1988; 251: 31–43 [DOI] [PubMed] [Google Scholar]
- 11. Huhtaniemi I, Nikula H, Parvinen M, Rannikko S. Histological and functional changes of the testis tissue during GnRH agonist treatment of prostatic cancer. Am J Clin Oncol 1988; 11(Suppl 1): S11–5 [PubMed] [Google Scholar]
- 12. Wolf‐Gould CS, Wolf‐Gould CH. A transgender woman with testicular cancer: a new twist on an old problem. LGBT Health 2016; 3: 90–5 [DOI] [PubMed] [Google Scholar]
- 13. Kvach EJ, Hyer JS, Carey JC, Bowers M. Testicular seminoma in a transgender woman: a case report. LGBT Health 2019; 6: 40–2 [DOI] [PubMed] [Google Scholar]
- 14. Kobori Y, Suzuki K, Iwahata T et al. Mature testicular teratoma with positive estrogen receptor beta expression in a transgendered individual on cross‐sex hormonal therapy: a case report. LGBT Health 2015; 2: 81–3 [DOI] [PubMed] [Google Scholar]
- 15. Elshimy G, Tran K, Harman SM, Correa R. Unmasked testicular seminoma during use of hormonal transgender woman therapy: a hidden hCG‐secreting tumor. J Endocr Soc 2020; 4: bvaa074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chandhoke G, Shayegan B, Hotte SJ. Exogenous estrogen therapy, testicular cancer, and the male to female transgender population: a case report. J Med Case Rep 2018; 12: 373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casparie MT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007; 29: 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nash R, Ward KC, Jemal A, Sandberg DE, Tangpricha V, Goodman M. Frequency and distribution of primary site among gender minority cancer patients: an analysis of U.S. national surveillance data. Cancer Epidemiol 2018; 54: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vermeir E, Jackson LA, Marshall EG. Improving healthcare providers' interactions with trans patients: recommendations to promote cultural competence. Healthc Policy 2018; 14: 11–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Polly R, Nicole J. Understanding the transsexual patient: culturally sensitive care in emergency nursing practice. Adv Emerg Nurs J 2011; 33: 55–64 [DOI] [PubMed] [Google Scholar]
- 21. Thornton CP. Best practice in teaching male adolescents and young men to perform testicular self‐examinations: a review. J Pediatr Health Care 2016; 30: 518–27 [DOI] [PubMed] [Google Scholar]
- 22. Meyer WJ 3rd, Webb A, Stuart CA, Finkelstein JW, Lawrence B, Walker PA. Physical and hormonal evaluation of transsexual patients: a longitudinal study. Arch Sex Behav 1986; 15: 121–38 [DOI] [PubMed] [Google Scholar]
- 23. Kent MA, Winoker JS, Grotas AB. Effects of feminizing hormones on sperm production and malignant changes: microscopic examination of post orchiectomy specimens in transwomen. Urology 2018; 121: 93–6 [DOI] [PubMed] [Google Scholar]
- 24. Bosland MC. Hormonal factors in carcinogenesis of the prostate and testis in humans and in animal models. Prog Clin Biol Res 1996; 394: 309–52 [PubMed] [Google Scholar]
- 25. Mester B, Behrens T, Dreger S, Hense S, Fritschi L. Occupational causes of testicular cancer in adults. Int J Occup Environ Med 2010; 1: 160–70 [PubMed] [Google Scholar]
- 26. Dieckmann KP, Pichlmeier U. Clinical epidemiology of testicular germ cell tumors. World J Urol 2004; 22: 2–14 [DOI] [PubMed] [Google Scholar]


