Abstract
Background
In‐depth insight into haemodynamic changes during normotensive pregnancy may help identify women at risk for gestational hypertensive complications.
Objectives
To determine the magnitude of changes in cardiac output and its determinants stroke volume and heart rate, and total peripheral vascular resistance during singleton normotensive and hypertensive pregnancies.
Search strategy
PubMed (NCBI) and Embase (Ovid) databases were searched from their inception up to November 2019.
Selection criteria
Studies reporting original measurements of haemodynamic parameters during pregnancy together with a non‐pregnant reference measurement. Studies including women using antihypertensive medication were excluded.
Data collection and analysis
Pooled mean differences between pregnant and non‐pregnant women, and absolute values of haemodynamic parameters were calculated for predefined gestational intervals using a random‐effects model in normotensive and hypertensive pregnancy. Meta‐regression analysis was used to analyse group differences in adjustments and absolute values during pregnancy.
Main results
In normotensive pregnancies, cardiac output increased from the first weeks on, reaching its highest level early in the third trimester (mean difference, 1.41 l·min1; 95% CI 1.18–1.63 l·min). In parallel, vascular resistance decreased progressively until its nadir in the early third trimester (mean difference, −331 dyn·sec–1·cm–5; 95% CI −384 to −277 dyn·sec–1·cm–5) and then increased slightly at term. In hypertensive pregnancies, the initial cardiac output increase was higher and vascular resistance did not change throughout gestation compared with reference values.
Conclusions
Hemodynamic changes in women who eventually develop hypertensive complications are substantially different. Serial monitoring and plotting against developed normograms can identify women at risk and may allow timely intervention.
Tweetable abstract
Monitoring haemodynamic changes in pregnancy helps identify women at risk for hypertensive complications.
Keywords: Cardiovascular disease, gestational hypertension, pre‐eclampsia, pregnancy
Tweetable abstract
Monitoring haemodynamic changes in pregnancy helps identify women at risk for hypertensive complications.
Introduction
Healthy pregnancy is accompanied by major haemodynamic changes that benefit the uteroplacental circulation. A drop in systemic vascular resistance during the first trimester triggers several compensatory mechanisms to maintain blood pressure, such as an increase in plasma volume and cardiac output (CO). 1 , 2
Pregnancies complicated by gestational hypertension and/or pre‐eclampsia may show deviant haemodynamic changes long before clinical presentation. An exaggerated rise in CO is associated with gestational hypertension and late‐onset pre‐eclampsia, whereas a shallow rise in CO with no drop in vascular resistance predisposes to the much less common early‐onset pre‐eclampsia along with impaired fetal growth. 3 , 4 , 5 , 6 Recognition of haemodynamic changes during normal pregnancy may identify early deviant haemodynamic changes in women destined to develop severe maternal hypertensive complications, enhancing possibilities for early prevention and intervention.
This study aimed to assess adjustments in CO and its determinants stroke volume (SV) and heart rate (HR), and total peripheral vascular resistance (TPVR) during singleton normotensive and hypertensive pregnancies. We performed a systematic review and meta‐analysis of existing data to provide detailed insight into the magnitude and moment of haemodynamic changes during normotensive and hypertensive pregnancies, and to construct reference curves for normotensive gestation.
Methods
No patients or public were involved in the design and conduct of this research. There was no specific funding for this research, and no core outcome set was used.
Data sources and searches
This article follows the previously designed series of meta‐analyses on physiologic adjustments of maternal cardiovascular and cardiometabolic parameters during pregnancy, and follows the Preferred Reporting Guidelines for Systematic Reviews and Meta‐Analyses (PRISMA). 1 , 7 , 8 We conducted an extensive literature search of studies evaluating CO, TPVR, SV and HR during normotensive and hypertensive pregnancies in the MEDLINE (PubMed) and Embase (Ovid) electronic databases. We included articles published from inception (1946 in PubMed and 1974 in Embase) to November 2019. Part one of the search string consisted of pregnancy and hypertensive complications of pregnancy and was similar to the search string used in previously published systematic reviews. Part two of the search string focused on haemodynamic parameters, CO, TPVR, SV, HR and blood pressure (Appendix S1). Filters were used to limit the search to studies that included human participants and journal articles as publication type, and the studies had to be published in English or Dutch to be able to assess quality.
Study selection
The identified studies were assessed for eligibility in two phases. First, every study was screened independently for eligibility by two investigators based on the title and abstract. Several investigators participated in this process (EM, SdH, ZM, NS, FAH, FA). Secondly, full‐text articles were obtained and screened for eligibility based on the inclusion and exclusion criteria by two of four investigators (EM, SdH, ZM, NS). Discrepancies were resolved by mutual agreement or by consulting a third investigator. Studies were included if they were original studies measuring haemodynamic parameters during human singleton pregnancies. To be included, studies had to report a reference measurement, either measured before conception, ≥6 weeks postpartum in the same study group, or in a non‐pregnant control group. Moreover, haemodynamic parameters had to be reported as numerical values (mean with standard deviation [SD], standard error [SE] or 95% confidence interval [CI]). Requests were made to retrieve additional information from authors if data were graphically presented, if haemodynamic indices were indexed for anthropometric measures or if data were incomplete. We excluded case reports, and studies with unknown pregnancy outcome. We also excluded studies that included a proportion of women with a history of cardiovascular complications, including chronic hypertension, diabetes mellitus, gestational diabetes and thyroid disease. Studies of complicated pregnancies were excluded if women could have used antihypertensive medication.
Data extraction and quality assessment
Data from included articles were extracted and entered in predesigned data collection forms. We registered study characteristics (study design, sample size, measurement method and maternal position during measurement), participant characteristics (age, non‐pregnant body mass index [BMI] and parity), whether women had a normotensive or hypertensive pregnancy and how the latter was defined, and outcome measures. We chose to analyse crude values of haemodynamic parameters, unadjusted for body surface area or other anthropometric measurements. Haemodynamic parameters of interest were CO (l·min–1), TPVR (dyne·sec–1·min–1·cm–5), SV (ml) and HR (bpm). Detailed data on systolic and diastolic blood pressure, and mean arterial pressure have been handled in a separate manuscript. When studies had focused on position‐dependent or exercise‐dependent changes of haemodynamic parameters during pregnancy, only baseline values measured at rest were extracted. If the exact gestational age when the measurements were collected was not mentioned, they were included as 7 weeks for the first trimester, 21 weeks for the second trimester, and 34 weeks for the third trimester. If both non‐pregnant controls and a postpartum reference measurement were given, the latter was selected to reduce between‐subject variance due to unpaired measurements. When a study provided multiple postpartum values, the last measurement after delivery was used as the reference measurement.
Quality of the included studies was assessed using a modified list of items described in the Quality In Prognosis Studies tool, made suitable for the purpose of this review. 9 Studies were scored with a plus or minus in six domains including study participation, study attrition, variable measurement, data reporting and study design. Items that were not mentioned or not applicable received a negative score. Cross‐sectional studies were scored negative for study attrition because loss‐to‐follow‐up reporting is not applicable for this study design. Studies with a score >60% were defined as high quality, between 30 and 60% as moderate quality, and <30% as low quality.
Data synthesis and analysis
The haemodynamic parameters were categorised into five different predefined intervals of gestational age: first trimester (up to 14 weeks), early second trimester (15–21 weeks), late second trimester (22–28 weeks), early third trimester (29–35 weeks) and late third trimester (36–41 weeks). The primary outcome was the weighted mean difference of the haemodynamic parameters, and the relative change presented as a percentage with a 95% CI between pregnant women and a non‐pregnant reference group. In these, measurements from pre‐pregnant and postpartum women and non‐pregnant controls were combined in the non‐pregnant group. Pooled estimates of the absolute values with a 95% CI for the haemodynamic parameters were also analysed. SE or 95% CI values were converted to SD according to the Cochrane Handbook for Systematic Reviews of Interventions. 10 If data were reported as median with interquartile range, we inquired whether the data were normally distributed. If so, studies were included and the SD was calculated by dividing the difference of the 25th and the 75th percentile by 1.35. 10 If a study reported multiple measurements in the same women at one gestational interval, the mean and SD were pooled into one combined measurement according to the Cochrane Handbook. Pooled mean differences in CO, TPVR, SV and HR were calculated separately for these intervals using a random‐effects meta‐analysis as described by DerSimonian and Laird. 11 This random‐effects model allows inter‐study variation and was chosen, as observational data on study and characteristics from different populations were used and heterogeneity was anticipated between studies. Publication bias was evaluated for each gestational interval in normotensive pregnancies by Egger’s regression test for funnel plot asymmetry. 12 If present, the pooled effect sizes were corrected for publication bias using the trim‐and‐fill method described by Duval and Tweedie. 13 The ratio between total heterogeneity and total variability (I 2 statistic) is presented as a measure of heterogeneity. I 2 can distinguish true heterogeneity from sampling variance and is expressed as a percentage. 14 Sources of heterogeneity (type of reference group, quality of study, measurement method, position during measurement, parity and differences between normotensive and hypertensive pregnancies) were analysed by meta‐regression analyses using a mixed‐effects model. Reference curves were constructed assuming that changes were normally distributed over the course of the pregnancy. To establish reference curves, we computed the 5th, 50th and 95th percentiles weighted by study sample size, using a restricted cubic splines model. All analyses were performed in R version 4.0.2. 15 The meta‐analyses and meta‐regression analyses were performed using the meta package. 16
Results
Study selection
The search strategy in PubMed and Embase yielded 32 604 unique articles (Figure 1), 3934 of which were excluded based on language restrictions. After screening of title and abstracts, we excluded 27 558 articles that concerned other topics. This left 1112 articles for full‐text evaluation, 17 of which could not be retrieved despite attempts to obtain them from other libraries in the Netherlands or by contacting the authors via Research Gate. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 Of the 1095 manuscripts with full text available, 981 were excluded. Studies were excluded because a reference group was absent (n = 526), the study design was unsuitable (n = 166), the data were unusable (n = 152), the data were not original (n = 44), only blood pressure was reported (n = 43) and for other reasons (n = 51). Often, contacted authors could not provide the requested data, such as unindexed parameters, because the data were stored on storage media incompatible with modern computers.
Figure 1.
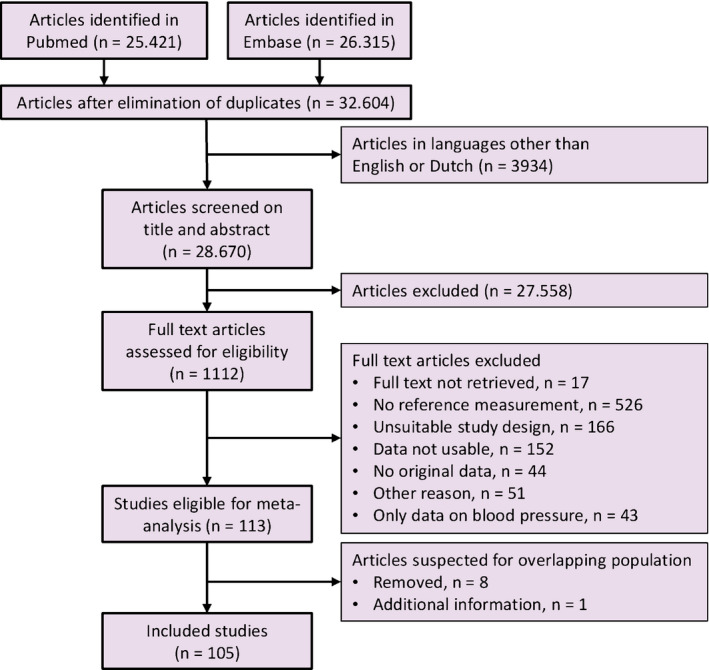
Flowchart summarising selection of studies in the meta‐analysis.
Of the 113 eligible articles, nine were suspected to report data from previously published samples because the authors, method and/or study period were similar. 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 Eight of these studies were excluded; one was included because supplemental data on hypertensive pregnancies were provided. 40 In total, 105 studies were included, and the full references are presented in Appendix S2. Eighteen studies reported data on pregnancies complicated by hypertension. Thirteen studies reported on pregnancies complicated by pre‐eclampsia, and five studies included women with pregnancy‐induced hypertension or combined complications. The criteria used to define hypertension and pre‐eclampsia are reported in Table S2. One study did not report the non‐pregnant SD for HR, so the ratio between non‐pregnant and pregnant SD in the complete dataset was used to estimate the SD. 43 Three studies compared different measurement methods, and Doppler echocardiography measurements were extracted, as most other studies used this method. 44 , 45 , 46
Summary of studies
A summary of included studies and their population is presented in Table S1. More than half of the studies reported gravidity or parity of women. Most studies included a mixed parous group, whereas 14 studies included only nulliparous women.
Quality assessment of included studies
The quality assessment is summarised in Table S3. Of the 105 included studies, 13 (12%) were scored as low quality, 70 (67%) as moderate quality, and 22 (21%) as high quality. The items that scored the lowest among all studies were: (1) reporting baseline characteristics of participants who were lost to follow‐up (4%) and (2) the use of a pre‐conception measurement as the reference value (13%). Items that scored the highest were: (1) methods and setting were the same for all participants and throughout follow‐up (88%) and (2) clear reporting of gestational age at measurement (88%).
Synthesis of results
Normotensive pregnancies
During normotensive pregnancies, CO started to increase from the first trimester, reaching a maximum mean difference of 1.41 l·min–1 between 29 and 35 weeks of pregnancy (Table 1, Figure 2). In parallel, TPVR decreased in the first 14 weeks by 188 dyne·sec–1·cm–5 and continued to decrease, reaching its minimum between 22 and 28 weeks. SV started to increase in the first trimester and peaked between 15 and 21 weeks of pregnancy. In the second half of the pregnancy, the increase in SV diminished and between 36 and 41 weeks of pregnancy, SV did not differ between pregnant and non‐pregnant women. HR started to increase in the first trimester and peaked in the early third trimester with a mean difference of 14.6 bpm, after which the increase plateaued. Forest plots of weighted mean differences and absolute values per gestational age interval of all haemodynamic parameters in normotensive pregnancies are presented in Figures S1–S8. During early normotensive pregnancy, both systolic and diastolic blood pressure significantly decreased, reaching their nadir in the second trimester (−4 mmHg, 95% CI −6 to −2 mmHg and −4 mmHg, 95% CI −5 to −3 mmHg respectively). Thereafter, blood pressure gradually increased towards non‐pregnant values at term (S. de Haas et al., unpubl. obs.).
Table 1.
Mean differences and absolute values of haemodynamic parameters during normotensive pregnancies
| Haemodynamic parameter | Non‐pregnant | Gestational age interval | |||||
|---|---|---|---|---|---|---|---|
| <14 weeks | 15–21 weeks | 22–28 weeks | 29–35 weeks | 36–41 weeks | |||
| Cardiac output (l·min–1) | MD | – | 0.68 (0.52–0.84) | 1.27 (0.98–1.55) | 1.23 (1.12–1.35) | 1.41 (1.18–1.64) | 1.16 (0.95–1.37) |
| cMD | – | – | 0.82 (0.54–1.10) | 1.41 (1.29–1.53) | – | 1.56 (1.33–1.78) | |
| % | – | 14 (11–18) | 26 (20–32) | 27 (24–29) | 29 (24–34) | 24 (20–28) | |
| Abs | 4.82 (4.68–4.97) | 5.48 (5.11–5.85) | 6.19 (5.73–6.66) | 5.95 (5.67–6.24) | 6.28 (5.91–6.65) | 6.03 (5.74–6.32) | |
| Total peripheral vascular resistance (dyne·sec–1·cm–5) | MD | – | −188 (−249 to −127) | −293 (−348 to −238) | −331 (−383 to −279) | −312 (−367 to −258) | −226 (−340 to −111) |
| cMD | – | −424 (−476 to −373) | −405 (−516 to −295) | ||||
| % | – | −14 (−19 to −10) | −23 (−28 to −19) | −25 (−28 to −21) | −24 (−29 to −20) | −17 (−26 to −9) | |
| Abs | 1358 (1299–1417) | 1178 (1070–1286) | 988 (884–1093) | 1022 (979–1064) | 972 (921–1024) | 1080 (985–1174) | |
| Stroke volume (ml) | MD | – | 5.1 (2.7–7.4) | 9.7 (6.2–13.2) | 8.5 (6.7–10.3) | 6.6 (3.4–9.9) | 2.6 (−1.7 to 6.8) |
| cMD | – | 4.7 (1.3–8.0) | |||||
| % | – | 7 (4–11) | 14 (9–19) | 13 (10–15) | 10 (5–14) | 4 (−2 to 9) | |
| Abs | 69.5 (66.9–72.2) | 73.5 (67.4–79.7) | 79.8 (73.7–85.8) | 76.8 (72.5–81.2) | 75.6 (71.5–79.6) | 74.1 (69.4–78.7) | |
| Heart rate (bpm) | MD | – | 5.9 (4.7–7.0) | 9.4 (8.2–10.6) | 10.7 (9.2–12.2) | 14.6 (13.4–15.8) | 13.5 (12.4–14.6) |
| cMD | – | 8.0 (6.8–9.3) | – | – | – | – | |
| % | – | 9 (7–10) | 14 (12–15) | 16 (13–18) | 21 (19–23) | 19 (18–21) | |
| Abs | 69.4 (68.5–70.3) | 75.0 (73.2–76.7) | 79.3 (77.4–81.3) | 80.1 (78.9–81.2) | 84.4 (82.4–86.4) | 83.6 (82.3–84.8) | |
Values are reported as mean differences (MD) and relative change (%) compared with reference and as absolute values (Abs), with 95% confidence interval. MDs corrected for publication bias (cMD) are presented for gestational age intervals with statistically significant funnel plot asymmetry.
Figure 2.
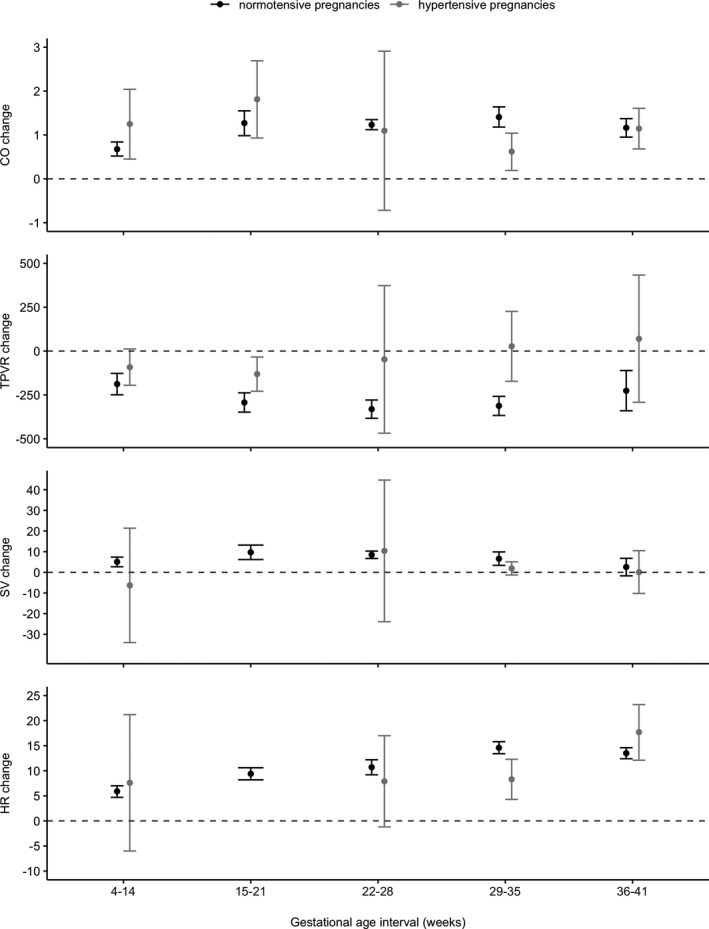
Mean differences and 95% CI as error bar of haemodynamic parameters compared with non‐pregnant reference values (pre‐conceptional, postpartum or non‐pregnant control group) in normotensive and hypertensive pregnancies per gestational interval.
In case of significant funnel plot asymmetry, which indicates publication bias, corrected mean differences were calculated (Table 1). Meta‐regression analysis showed a significant contribution of the measurement method to the heterogeneity of CO, TPVR and SV measurements. In addition, the type of reference group contributed significantly to heterogeneity in CO measurements. Study quality contributed significantly to heterogeneity in HR measurements, and maternal position contributed significantly to heterogeneity in SV measurements. Parity did not contribute to heterogeneity in any of the parameters.
Hypertensive pregnancies
The CO increase observed during the first trimester of hypertensive pregnancies was higher than the increase observed in normotensive pregnancies, on top of a higher absolute CO value in the non‐pregnant state (Table 2). TPVR and SV values in hypertensive pregnancies were not different from non‐pregnant reference values. In the last trimester, HR values in hypertensive pregnancies were substantially higher than non‐pregnant reference values. Meta‐regression analysis showed that changes in CO, TPVR and HR during complicated pregnancies were significantly different from changes during normotensive pregnancies. Forest plots of weighted mean differences and absolute values per gestational interval of haemodynamic parameters in hypertensive pregnancies are presented in Figures S9–S16. Pregnancies eventually complicated by hypertension and/or pre‐eclampsia lacked an early decrease in systolic blood pressure, even though diastolic blood pressure dropped to a greater extent compared with normotensive pregnancies. In the second half of pregnancy, systolic and diastolic blood pressure increased tremendously (detailed data presented in second manuscript; S. de Haas et al., unpubl. obs.).
Table 2.
Mean differences and absolute values of haemodynamic parameters during hypertensive pregnancies
| Hemodynamic parameter | Non‐pregnant | Gestational age interval | |||||
|---|---|---|---|---|---|---|---|
| <14 weeks | 15–21 weeks | 22–28 weeks | 29–35 weeks | 36–41 weeks | |||
| Cardiac output (l/minute) | MD* | 1.25 (0.45–2.04) | 1.81 (0.93–2.69) | 1.10 (−0.72 to 2.91) | 0.62 (0.19–1.04) | 1.15 (0.68–1.61) | |
| Abs | 5.29 (4.86–5.72) | 6.66 (4.66–8.65) | 7.84 (6.82–8.85) | 6.63 (4.51–8.75) | 5.97 (5.49–6.45) | 5.68 (3.86–7.51) | |
| Total peripheral vascular resistance (dyne·sec–1·cm–5) | MD* | – | −92 (−195 to 12) | −131 (−229 to −34) | −47 (−468 to 373) | 27 (−172 to 226) | 70 (−292 to 433) |
| Abs | 1251 (1040–1461) | 727 (564–889) | 674 (569–779) | 1222 (711–1732) | 1283 (670–1896) | 1665 (1071–2259) | |
| Stroke volume (ml) | MD | – | −6.3 (−34.0 to 21.4) | – | 10.4 (−23.9 to 44.7) | 1.9 (−1.3 to 5.1) | 0.1 (−10.2 to 10.5) |
| Abs | 71.7 (65.0–78.3) | 59.4 (36.7–82.1) | – | 81.4 (41.2–121.6) | 72.6 (64.6–80.5) | 67.9 (48.6–87.2) | |
| Heart rate (bpm) | MD* | – | 7.6 (−6.0 to 21.2) | 7.9 (−1.2 to 17.0) | 8.3 (4.3–12.3) | 17.7 (12.1–23.2) | |
| Abs | 70.4 (66.5–74.2) | 72.6 (67.5–77.6) | 82.3 (74.4–90.2) | 80.4 (76.8–84.1) | 83.1 (79.7–86.5) | ||
Values are reported as mean differences (MD) compared with non‐pregnant reference and as absolute values (Abs) with 95% confidence interval.
* Meta‐regression showed significantly different changes in hypertensive pregnancies compared with normotensive pregnancies.
Reference curves
Reference curves for absolute CO, TPVR, SV and HR values during pregnancy and in the postpartum period were constructed with data from normotensive pregnancies (Figures 3, S17–S19).
Figure 3.
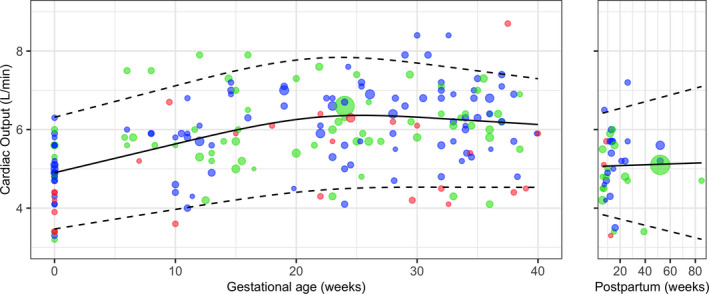
Reference curve of absolute CO values during pregnancy and in the postpartum period, with mean (solid line), and 5th and 95th percentiles (dashed lines) weighted by study sample size. Size of individual plots indicates sample size of point estimate and their colour indicates study quality: red, low quality; green, moderate quality; blue, high quality. Studies with multiple measurements during pregnancy are plotted per measurement.
Discussion
Main findings
This study comprehensively describes the magnitude and time‐point of changes in CO, its determinants SV and HR, and TPVR during normotensive and hypertensive pregnancies. During hypertensive pregnancies, the initial increase in CO is augmented, established merely by an elevation in HR, and this increase is not sustained over the course of pregnancy. In contrast to normotensive pregnancies, TPVR does not decrease much in hypertensive pregnancies.
Strength and limitations
The strength and novelty of this work are that we included only studies with a non‐pregnant reference measurement. Therefore, we provide general mean differences as our primary outcome, which makes usability method‐ and device‐independent.
There are some limitations to our study. First, the postpartum reference group may have attenuated the mean differences in our outcome parameters due to the residual time‐effect of pregnancy and breast‐feeding on haemodynamic parameters. 2 , 47 , 48 , 49 , 50 However, we performed a meta‐regression analysis to identify parameters that affected the observed changes and found no effect of the kind of reference group on heterogeneity. Secondly, we were not able to analyse nulliparous and multiparous women separately, although the magnitude of haemodynamic changes is thought to be more pronounced in multiparous women. 49 , 51 , 52 Meta‐regression analysis did not show an effect of parity on the tested parameters, although this could be due to the inclusion of nulliparous women in the mixed parous group. Thirdly, we could not assess the effect of age and BMI on haemodynamic parameters during pregnancy, as these factors were inconsistently reported. Fourthly, our references curves are constructed based on aggregated data per study and are weighted by sample size. We made assumptions on the normality of data distribution. This limitation can only be overcome with an individual patient data meta‐analysis. Finally, for funnel plot asymmetry, corrected mean differences were calculated. These are based on the assumption that publication bias is present and therefore should be interpreted with some caution.
It is well accepted that the increase of CO in normotensive pregnancy results from an increase in both SV and HR. 2 , 46 , 53 , 54 , 55 Our study confirmed that HR increased over the course of a normotensive pregnancy. This is most likely explained by an increased sympathetic activity and involvement of the Bainbridge reflex, which is an increase in HR to prevent damming of blood in response to increased venous filling. In contrast, the SV increase lessens in the second half of pregnancy, which may be the result of several factors. First, preload might be hampered because the enlarged uterus compresses the inferior vena cava at the end of pregnancy. 56 , 57 Secondly, a higher HR decreases the diastolic period, which may impair filling of the left ventricle. 7 Thirdly, cardiac remodelling during pregnancy may reduce compliance of the extensively stretched sarcomeres, which may reduce the SV. 48
In a healthy pregnancy, CO increase and TPVR decrease are thought to be caused by several early adaptations following conception. These include increased arterial compliance, decreased vascular tone, decreased vascular responsiveness to vasoconstrictors, increased release of and sensitivity to vasodilatory substances (including relaxin, nitric oxide, progesterone and oestrogen), and the opening of maternal protective regulatory micro‐circulatory and placental arterio‐venous shunts. 58 , 59 , 60 , 61 The subsequent relative circulatory underfill is counterbalanced by activation of the renin‐angiotensin‐aldosterone system, which stimulates renal volume retention and expands plasma volume, supporting a rise in SV and, with it, increased CO. 62 , 63 Plasma volume expansion leads to dilutional anaemia, which may reduce TPVR by reducing blood viscosity. 64 , 65 Marked sympathetic activation in favour of elevated inotropy and chronotropy may be a compensatory mechanism to maintain blood pressure at pre‐pregnancy levels, although resetting of baroreceptor sensitivity and reduction of neurovascular transduction in pregnancy blunts the effect of sympathetic regulation on arterial blood pressure. 37 , 66 , 67 , 68 The mechanisms responsible for healthy vascular adjustments might be compromised in gestational hypertensive diseases.
Hypertensive pregnancies
Pregnancies ending in hypertensive complications show an altered vascular response to conception. In addition to a higher CO at baseline, the initial increase in CO is more pronounced. The rise in CO predominantly originates from increased HR rather than SV, suggesting sympathetic over‐activity to counterbalance haemodynamic changes in the first trimester. Because sympathetic activation is usually accompanied by a raised SV, the absence of an SV increase in women destined to develop hypertension may originate from other factors, such as diminished circulating plasma volume resulting in shallow preload, reduced left ventricle compliance, increased afterload due to increased vascular resistance or reduced arterial compliance. 1 , 69 Concurrently, TPVR does not decrease, suggesting blunted vascular adjustments, and no change or even rise in blood pressure at any time during pregnancy before the clinical onset of hypertensive complications. Previous longitudinal studies have shown similar deviant haemodynamic changes preceding hypertensive complications. Easterling et al. 3 showed that pre‐eclamptic women had a higher CO over the course of pregnancy than normotensive women did. Bosio et al. 4 showed that evolving of gestational hypertension to pre‐eclampsia was accompanied by a crossover from a high‐output, low‐resistance circulation to a low‐output, high‐resistance circulation. Other studies have shown that severe, early‐onset pre‐eclampsia and associated fetal growth restriction may result from an initial and consistent low‐output, high‐resistance vasoconstricted circulation. 5 , 70 Because the systemic–biological pathways to complications are so divergent, combining measurements of women at different pathophysiological time points results in the wide confidence intervals found in hypertensive pregnancies. Unfortunately, we were not able to perform subgroup analyses on gestational hypertension, early‐onset pre‐eclampsia or late‐onset pre‐eclampsia because of complications and inconsistent definitions among the included studies.
Interpretation
Haemodynamic differences between normotensive and hypertensive pregnancies can be detected early in pregnancy, even when blood pressure is still within the normotensive range. Recognising abnormal changes in haemodynamic parameters during pregnancy has great potential to improve prevention and intervention opportunities. 71 Preventive measures may include daily low‐dose aspirin and calcium supplementation in women at higher risk for hypertensive complications. Even more sophisticated, tailored pharmaceutical correction of deviant haemodynamic changes towards the expected normal values could be a key modality in preventing hypertensive complications. In this line of reasoning, timely initiation of beta‐blockade in women with exaggerated high CO seems to reduce hypertensive complications during pregnancy. 72 Additional vasodilatory therapy in women with an unfavourably low output and high resistance circulation could improve flow and with it maternal and fetal outcomes. 73 Devices that are easy to use have been developed to assess CO, so it is now feasible to measure CO in the outpatient setting along with blood pressure to determine gestational haemodynamic changes in clinical practice.
Conclusion
In normotensive pregnancy, resting CO increases from the first trimester to the early third trimester by almost 1.5 l·min above non‐pregnant values, and both SV and HR contribute to this increase. In parallel, TPVR decreases, reaching its nadir in the late second trimester. Monitoring these haemodynamic changes during pregnancy can help identify those women at risk of developing hypertensive complications, as in these women the change in CO, TPVR and HR are deviate from that observed in normotensive pregnancy. Serial measurements are necessary to detect aberrant changes that precede hypertension during pregnancy.
Disclosure of interests
None declared. Completed disclosure of interests forms are available to view online as supporting information.
Contribution to authorship
EM, SdH, JvD, MS and CG were involved in designing and initiation of the study. EM, SdH, ZM, NS, FAH and FA were involved in study screening and selection. EM and SdH had full access to the data in the study and take responsibility for the integrity of the data. SvK provided statistical expertise. All authors interpreted the data. EM and SdH wrote the first draft of this protocol, and all authors critically reviewed and contributed to adjustments and approved the final version of this manuscript.
Funding
This systematic review and meta‐analysis did not receive any specific funding.
Supporting information
Figure S1. Forest plot of mean difference of CO in normotensive pregnancy.
Figure S2. Forest plot of absolute values of CO in normotensive pregnancy.
Figure S3. Forest plot of mean difference of TPVR in normotensive pregnancy.
Figure S4. Forest plot of absolute values of TPVR in normotensive pregnancy.
Figure S5. Forest plot of mean difference of SV in normotensive pregnancy.
Figure S6. Forest plot of absolute values of SV in normotensive pregnancy.
Figure S7. Forest plot of mean difference of HR in normotensive pregnancy.
Figure S8. Forest plot of absolute values of HR in normotensive pregnancy.
Figure S9. Forest plot of mean difference of CO in hypertensive pregnancy.
Figure S10. Forest plot of absolute values of CO in hypertensive pregnancy.
Figure S11. Forest plot of mean difference of TPVR in hypertensive pregnancy.
Figure S12. Forest plot of absolute values of TPVR in hypertensive pregnancy.
Figure S13. Forest plot of mean difference of SV in hypertensive pregnancy.
Figure S14. Forest plot of absolute values of SV in hypertensive pregnancy.
Figure S15. Forest plot of mean difference of HR in hypertensive pregnancy.
Figure S16. Forest plot of absolute values of HR in hypertensive pregnancy.
Figure S17. Reference curve of TPVR during pregnancy and in the postpartum period.
Figure S18. Reference curve of SV during pregnancy and in the postpartum period.
Figure S19. Reference curve of HR during pregnancy and in the postpartum period.
Table S1. Summary of included studies.
Table S2. Definitions of hypertensive pregnancies.
Table S3. Quality assessment overview.
Appendix S1. Literature search.
Appendix S2. Reference list of included studies.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Mulder EG, de Haas S, Mohseni Z, Schartmann N, Abo Hasson F, Alsadah F, van Kuijk SMJ, van Drongelen J, Spaanderman MEA, Ghossein‐Doha C. Cardiac output and peripheral vascular resistance during normotensive and hypertensive pregnancy – a systematic review and meta‐analysis. BJOG 2022; 10.1111/1471-0528.16678.129:696–707.
Data availability
Data sharing is not applicable to this article as no new data were created or analysed in this study.
References
- 1. de Haas S, Ghossein‐Doha C, van Kuijk SM, van Drongelen J, Spaanderman ME. Physiologic adaptation of plasma volume during pregnancy: a systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2017;49:177–87. [DOI] [PubMed] [Google Scholar]
- 2. Meah VL, Cockcroft JR, Backx K, Shave R, Stohr EJ. Cardiac output and related haemodynamics during pregnancy: a series of meta‐analyses. Heart 2016;102:518–26. [DOI] [PubMed] [Google Scholar]
- 3. Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol 1990;76:1061–9. [PubMed] [Google Scholar]
- 4. Bosio PM, McKenna PJ, Conroy R, O’Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol 1999;94:978–84. [DOI] [PubMed] [Google Scholar]
- 5. Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension 2008;52:873–80. [DOI] [PubMed] [Google Scholar]
- 6. Stott D, Nzelu O, Nicolaides KH, Kametas NA. Maternal hemodynamics in normal pregnancy and in pregnancy affected by pre‐eclampsia. Ultrasound Obstet Gynecol 2018;52:359–64. [DOI] [PubMed] [Google Scholar]
- 7. De Haas S, Ghossein‐Doha C, Geerts L, van Kuijk SMJ, van Drongelen J, Spaanderman MEA. Cardiac remodeling in normotensive pregnancy and in pregnancy complicated by hypertension: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2017;50:683–696. [DOI] [PubMed] [Google Scholar]
- 8. Lopes van Balen VA, van Gansewinkel TAG, de Haas S, van Kuijk SMJ, van Drongelen J, Ghossein‐Doha C, Spaanderman MEA. Physiological adaptation of endothelial function to pregnancy: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2017;50:697–708. [DOI] [PubMed] [Google Scholar]
- 9. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Available from http://www.training.cochrane.org/handbook (accessed 14 January 2016). [Google Scholar]
- 11. DerSimonian R, Laird N. Meta‐analysis in clinical trials revisited. Contemp Clin Trials 2015;45(Pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Team RC . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 16. Schwarzer G. Meta: an R package for meta‐analysis. R News 2007;7:40–5. [Google Scholar]
- 17. Siamopoulos KC, Papanikolaou S, Elisaf M, Theodorou J, Pappas H, Papanikolaou N. Ambulatory blood pressure monitoring in normotensive pregnant women. J Hum Hypertens 1996;10(Suppl 3):S51–4. [PubMed] [Google Scholar]
- 18. Welt SI, Nagey DA, Mull CG, Pupkin VX, Crenshaw C Jr. Postpartum blood pressure patterns in patients with hypertensive complications of pregnancy. Md Med J 1986;35:483–6. [PubMed] [Google Scholar]
- 19. Ihrman K. A clinical and physiological study of pregnancy in a material from northern Sweden. VI. The arterial blood pressures at rest and in orthostatic test during and after pregnancy. Acta Soc Med Ups 1960;65:314–25. [PubMed] [Google Scholar]
- 20. Salvatore CA. Capillary resistance during pregnancy. Obstet Gynecol 1961;18:96–102. [PubMed] [Google Scholar]
- 21. Walters WA, MacGregor WG, Hills M. Cardiac output at rest during pregnancy and the puerperium. Clin Sci 1966;30:1–11. [PubMed] [Google Scholar]
- 22. Rovinsky JJ, Jaffin H. Cardiovascular hemodynamics in pregnancy. I. Blood and plasma volumes in multiple pregnancy. Am J Obstet Gynecol 1965;93:1–15. [DOI] [PubMed] [Google Scholar]
- 23. Nisell H, Hjemdahl P, Linde B, Lunell NO. Cardiovascular responses to isometric handgrip exercise: an invasive study in pregnancy‐induced hypertension. Obstet Gynecol 1987;70(3 Pt 1):339–43. [PubMed] [Google Scholar]
- 24. Kuzniar J, Piela A, Skret A, Szmigiel Z, Zaczek T. Hemodynamic determinants of fetal outcome in preeclampsia. Ginekol Pol 1985;56:268–74. [PubMed] [Google Scholar]
- 25. Meher S, Neilson J. Hypertension in pregnancy. Practitioner 2004;248:720, 2, 4 passim. [PubMed] [Google Scholar]
- 26. Suonio S, Olkkonen H, Lahtinen T. Maternal circulatory response to a single dose of ritodrine hydrochloride during orthostasis in normal and hypertensive late pregnancy. Am J Obstet Gynecol 1978;130:745–7. [DOI] [PubMed] [Google Scholar]
- 27. Burgess HA. Parameters of normotensive women and women with pregnancy induced hypertension (PIH) in Lusaka. East Afr Med J 1991;68:727–34. [PubMed] [Google Scholar]
- 28. Masson GM. Plasma oestriol in pre‐eclampsia. J Obstet Gynaecol Br Commonw 1973;80:206–9. [DOI] [PubMed] [Google Scholar]
- 29. Easterling TR, Benedetti TJ. Preeclampsia: a hyperdynamic disease model. Am J Obstet Gynecol 1989;160:1447–53. [DOI] [PubMed] [Google Scholar]
- 30. Assali NS, Holm LW, Parker HR. Systemic and regional hemodynamic alterations in toxemia. Circulation 1964;30 (Suppl 2):53–62. [DOI] [PubMed] [Google Scholar]
- 31. Ritmiller LF, Messmore IL, Nicodemus RE. The significance of hypertension in pregnancy. Pennsylvania Med J 1948;51:771–5. [PubMed] [Google Scholar]
- 32. Krieger VI, Weiden S. The value of the cold pressor test in the prediction of hypertension and toxaemia in pregnancy. Med J Aust 1947;1:417–23. [DOI] [PubMed] [Google Scholar]
- 33. Fernandes RM, Franchini E, Elliott‐Sale KJ, Takito MY. Does pregnancy affect the metabolic equivalent at rest and during low intensity exercise? Curr Women’s Health Rev 2017;13:38–43. [Google Scholar]
- 34. Rovinsky JJ, Jaffin H. Cardiovascular hemodynamics in pregnancy. II. Cardiac output and left ventricular work in multiple pregnancy. Am J Obstet Gynecol 1966;95:781–6. [PubMed] [Google Scholar]
- 35. Pandey AK, Banerjee AK, Das A, Bhawani G, Kumar A, Majumadar B, et al. Evaluation of maternal myocardial performance during normal pregnancy and post partum. Indian Heart J 2010;62:64–7. [PubMed] [Google Scholar]
- 36. Mahendru AA, Everett TR, Wilkinson IB, Lees CC, McEniery CM. Maternal cardiovascular changes from pre‐pregnancy to very early pregnancy. J Hypertens 2012;30:2168–72. [DOI] [PubMed] [Google Scholar]
- 37. Usselman CW, Wakefield PK, Skow RJ, Stickland MK, Chari RS, Julian CG, et al. Regulation of sympathetic nerve activity during the cold pressor test in normotensive pregnant and nonpregnant women. Hypertension 2015;66:858–64. [DOI] [PubMed] [Google Scholar]
- 38. Estensen ME, Grindheim G, Remme EW, Swillens A, Smiseth OA, Segers P, et al. Systemic arterial response and ventriculo‐arterial interaction during normal pregnancy. Am J Hypertens 2012;25:672–7. [DOI] [PubMed] [Google Scholar]
- 39. Kametas NA, Savvidou MD, Donald AE, McAuliffe F, Nicolaides KH. Flow‐mediated dilatation of the brachial artery in pregnancy at high altitude. BJOG 2002;109:930–7. [DOI] [PubMed] [Google Scholar]
- 40. Cong J, Yang X, Zhang N, Shen J, Fan T, Zhang Z. Quantitative analysis of left atrial volume and function during normotensive and preeclamptic pregnancy: a real‐time three‐dimensional echocardiography study. Int J Cardiovasc Imaging 2015;31:805–12. [DOI] [PubMed] [Google Scholar]
- 41. Carpenter RE, Emery SJ, Uzun O, Rassi D, Lewis MJ. Influence of physical exercise on baroreceptor sensitivity during pregnancy. J Matern Fetal Neonatal Med 2017;30:514–9. [DOI] [PubMed] [Google Scholar]
- 42. Schmidt SML, Usselman CW, Martinek E, Stickland MK, Julian CG, Chari R, et al. Activity of muscle sympathetic neurons during normotensive pregnancy. Am J Physiol Regul Integr Comp Physiol 2018;314:R153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Larkin H, Gallery ED, Hunyor SN, Gyory AZ, Boyce ES. Cardiac and haemodynamic measurements in hypertensive pregnancy. Clin Sci 1980;59 (Suppl 6):357s–60s. [DOI] [PubMed] [Google Scholar]
- 44. Ducas RA, Elliott JE, Melnyk SF, Premecz S, daSilva M, Cleverley K, et al. Cardiovascular magnetic resonance in pregnancy: insights from the cardiac hemodynamic imaging and remodeling in pregnancy (CHIRP) study. J Cardiovasc Magn Reson 2014;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rang S, de Pablo LB, van Montfrans GA, Bouma BJ, Wesseling KH, Wolf H. Modelflow: a new method for noninvasive assessment of cardiac output in pregnant women. Am J Obstet Gynecol 2007;196:235.e1–8. [DOI] [PubMed] [Google Scholar]
- 46. Petersen JW, Liu J, Chi YY, Lingis M, Williams RS, Rhoton‐Vlasak A, et al. Comparison of multiple non‐invasive methods of measuring cardiac output during pregnancy reveals marked heterogeneity in the magnitude of cardiac output change between women. Physiol Rep 2017;5:e13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morris EA, Hale SA, Badger GJ, Magness RR, Bernstein IM. Pregnancy induces persistent changes in vascular compliance in primiparous women. Am J Obstet Gynecol 2015;212:633.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Melchiorre K, Sharma R, Khalil A, Thilaganathan B. Maternal cardiovascular function in normal pregnancy: evidence of maladaptation to chronic volume overload. Hypertension 2016;67:754–62. [DOI] [PubMed] [Google Scholar]
- 49. Clapp JF III, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol 1997;80:1469–73. [DOI] [PubMed] [Google Scholar]
- 50. Mezzacappa ES, Kelsey RM, Myers MM, Katkin ES. Breast‐feeding and maternal cardiovascular function. Psychophysiology 2001;38:988–97. [DOI] [PubMed] [Google Scholar]
- 51. Turan OM, De Paco C, Kametas N, Khaw A, Nicolaides KH. Effect of parity on maternal cardiac function during the first trimester of pregnancy. Ultrasound Obstet Gynecol 2008;32:849–54. [DOI] [PubMed] [Google Scholar]
- 52. Ling HZ, Guy GP, Bisquera A, Poon LC, Nicolaides KH, Kametas NA. The effect of parity on longitudinal maternal hemodynamics. Am J Obstet Gynecol 2019;221:249.e1–14. [DOI] [PubMed] [Google Scholar]
- 53. Savu O, Jurcut R, Giusca S, van Mieghem T, Gussi I, Popescu BA, et al. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging 2012;5:289–97. [DOI] [PubMed] [Google Scholar]
- 54. Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 1989;256(4 Pt 2):H1060–5. [DOI] [PubMed] [Google Scholar]
- 55. Andreas M, Kuessel L, Kastl SP, Wirth S, Gruber K, Rhomberg F, et al. Bioimpedance cardiography in pregnancy: a longitudinal cohort study on hemodynamic pattern and outcome. BMC Pregnancy Childbirth 2016;16:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Clark SL, Cotton DB, Pivarnik JM, Lee W, Hankins GD, Benedetti TJ, et al. Position change and central hemodynamic profile during normal third‐trimester pregnancy and post partum. Am J Obstet Gynecol 1991;164:883–7. [DOI] [PubMed] [Google Scholar]
- 57. Bamber JH, Dresner M. Aortocaval compression in pregnancy: the effect of changing the degree and direction of lateral tilt on maternal cardiac output. Anesth Analg 2003;97:256–8, table of contents. [DOI] [PubMed] [Google Scholar]
- 58. Spaanderman ME, Willekes C, Hoeks AP, Ekhart TH, Peeters LL. The effect of pregnancy on the compliance of large arteries and veins in healthy parous control subjects and women with a history of preeclampsia. Am J Obstet Gynecol 2000;183:1278–86. [DOI] [PubMed] [Google Scholar]
- 59. Leo CH, Jelinic M, Ng HH, Marshall SA, Novak J, Tare M, et al. Vascular actions of relaxin: nitric oxide and beyond. Br J Pharmacol 2017;174:1002–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spaanderman ME, Meertens M, van Bussel M, Ekhart TH, Peeters LL. Cardiac output increases independently of basal metabolic rate in early human pregnancy. Am J Physiol Heart Circ Physiol 2000;278:H1585–8. [DOI] [PubMed] [Google Scholar]
- 61. Jaffe R, Jauniaux E, Hustin J. Maternal circulation in the first‐trimester human placenta – myth or reality? Am J Obstet Gynecol 1997;176:695–705. [DOI] [PubMed] [Google Scholar]
- 62. Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, et al. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol 1997;273(5 Pt 2):F777–82. [DOI] [PubMed] [Google Scholar]
- 63. Duvekot JJ, Cheriex EC, Pieters FA, Menheere PP, Peeters LH. Early pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. Am J Obstet Gynecol 1993;169:1382–92. [DOI] [PubMed] [Google Scholar]
- 64. Linde T, Sandhagen B, Hägg A, Mörlin C, Wikström B, Danielson BG. Blood viscosity and peripheral vascular resistance in patients with untreated essential hypertension. J Hypertens 1993;11:731–6. [DOI] [PubMed] [Google Scholar]
- 65. Whittaker PG, Macphail S, Lind T. Serial hematologic changes and pregnancy outcome. Obstet Gynecol 1996;88:33–9. [DOI] [PubMed] [Google Scholar]
- 66. Jarvis SS, Shibata S, Bivens TB, Okada Y, Casey BM, Levine BD, et al. Sympathetic activation during early pregnancy in humans. J Physiol 2012;590:3535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Okada Y, Best SA, Jarvis SS, Shibata S, Parker RS, Casey BM, et al. Asian women have attenuated sympathetic activation but enhanced renal‐adrenal responses during pregnancy compared to Caucasian women. J Physiol 2015;593:1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Usselman CW, Skow RJ, Matenchuk BA, Chari RS, Julian CG, Stickland MK, et al. Sympathetic baroreflex gain in normotensive pregnant women. J Appl Physiol 2015;119:468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thadhani R, Ecker JL, Kettyle E, Sandler L, Frigoletto FD. Pulse pressure and risk of preeclampsia: a prospective study. Obstet Gynecol 2001;97:515–20. [DOI] [PubMed] [Google Scholar]
- 70. Foo FL, Mahendru AA, Masini G, Fraser A, Cacciatore S, MacIntyre DA, et al. Association between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertension 2018;72:442–50. [DOI] [PubMed] [Google Scholar]
- 71. McLaughlin K, Scholten RR, Kingdom JC, Floras JS, Parker JD. Should maternal hemodynamics guide antihypertensive therapy in preeclampsia? Hypertension 2018;71:550–6. [DOI] [PubMed] [Google Scholar]
- 72. Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol 1999;93(5 Pt 1):725–33. [DOI] [PubMed] [Google Scholar]
- 73. Chaffin DG, Webb DG. Outcomes of pregnancies at risk for hypertensive complications managed using impedance cardiography. Am J Perinatol 2009;26:717–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Forest plot of mean difference of CO in normotensive pregnancy.
Figure S2. Forest plot of absolute values of CO in normotensive pregnancy.
Figure S3. Forest plot of mean difference of TPVR in normotensive pregnancy.
Figure S4. Forest plot of absolute values of TPVR in normotensive pregnancy.
Figure S5. Forest plot of mean difference of SV in normotensive pregnancy.
Figure S6. Forest plot of absolute values of SV in normotensive pregnancy.
Figure S7. Forest plot of mean difference of HR in normotensive pregnancy.
Figure S8. Forest plot of absolute values of HR in normotensive pregnancy.
Figure S9. Forest plot of mean difference of CO in hypertensive pregnancy.
Figure S10. Forest plot of absolute values of CO in hypertensive pregnancy.
Figure S11. Forest plot of mean difference of TPVR in hypertensive pregnancy.
Figure S12. Forest plot of absolute values of TPVR in hypertensive pregnancy.
Figure S13. Forest plot of mean difference of SV in hypertensive pregnancy.
Figure S14. Forest plot of absolute values of SV in hypertensive pregnancy.
Figure S15. Forest plot of mean difference of HR in hypertensive pregnancy.
Figure S16. Forest plot of absolute values of HR in hypertensive pregnancy.
Figure S17. Reference curve of TPVR during pregnancy and in the postpartum period.
Figure S18. Reference curve of SV during pregnancy and in the postpartum period.
Figure S19. Reference curve of HR during pregnancy and in the postpartum period.
Table S1. Summary of included studies.
Table S2. Definitions of hypertensive pregnancies.
Table S3. Quality assessment overview.
Appendix S1. Literature search.
Appendix S2. Reference list of included studies.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


