Abstract
OBJECTIVE
Waxes are used as structuring agents in lipsticks. There are a variety of waxes combined in a single lipstick to provide good stability, pleasant texture and good pay‐off. Due to a significant growth for natural, green and sustainable products, there is a constant search for alternatives to animal‐derived and petroleum‐derived ingredients. In this study, a green, non‐animalderived wax, namely long‐chain ketones (referred to as alkenones), sourced from marine microalgae was formulated into lipsticks and evaluated as a structuring agent.
METHODS
Alkenones were used as a substitute for microcrystalline wax, ozokerite and candelilla wax, typical structuring agents. In total, 384 lipsticks were formulated: L1 (control, no alkenones), L2 (alkenones as a substitute for ozokerite), L3 (alkenones as a substitute for microcrystalline wax) and L4 (alkenones as a substitute for candelilla wax). Products were tested for hardness (bending force), stiffness, firmness (needle penetration), pay‐off (using a texture analyser and a consumer panel), friction, melting point and stability for 12 weeks at 25 and 45°C.
RESULTS
Alkenones influenced each characteristic evaluated. In general, lipsticks with alkenones (L2‐L4) became softer and easier to bend compared to the control (L1). In terms of firmness, lipsticks were similar to the control, except for L4, which was significantly (P < 0.05) firmer. The effect on pay‐off was not consistent. L2 and L3 had higher pay‐off to skin and fabric than L1. In addition, L4 had the lowest amount transferred, but it still had the highest colour intensity on skin. Alkenones influenced friction (glide) positively; the average friction decreased for L2‐L4. The lowest friction (i.e. best glide) was shown in L4. Melting point of the lipsticks was lower when alkenones were present. Overall, L4, containing 7% of 4 alkenones in combination with microcrystalline wax, ozokerite and carnauba wax, was found to have the most desirable attributes, including ease of bending, high level of firmness, low pay‐off in terms of amount, high colour intensity on skin and low friction (i.e. better glide). Consumers preferred L4 the most overall.
CONCLUSION
Results of this study indicate that alkenones offer a sustainable, non‐animal and non‐petroleum‐derived choice as a structuring agent for lipsticks.
Keywords: colour cosmetics, formulation/stability, statistics, alkenones, lipstick
In this study, a green, non‐animal derived wax, namely alkenones, sourced from marine microalgae was formulated into lipsticks and evaluated as a structuring agent and compared to microcrystalline wax, ozokerite and candelilla wax. Overall, the lipstick with 7% of alkenones (L4) in combination with microcrystalline wax, ozokerite and carnauba wax was found to have the most desirable attributes, including ease of bending, high level of firmness, low pay‐off in terms of amount, high colour intensity on skin and low friction (i.e. better glide). Consumers also preferred L4 the most overall in the consumer study.

Résumé
OBJECTIF
Les cires sont utilisées comme agents de structuration dans les rouges à lèvres. Un rouge à lèvres contient plusieurs cires, afin d’obtenir une bonne stabilité, une texture agréable et un bon transfert de matière. En raison d’une croissance significative de la demande en produits naturels, écologiques et durables, les chercheurs s’efforcent constamment de trouver des alternatives aux ingrédients d’origine animale et dérivés du pétrole. Dans cette étude, les cétones à longue chaîne (appelés alkénones), une cire verte qui n’est pas d’origine animale, mais provenant de microalgues marines, a été formulée pour les rouges à lèvres et évaluée comme agent de structuration.
MÉTHODES
Les alkénones ont été utilisés comme substitut pour la cire microcristalline, l’ozokérite et la cire de candelilla, des agents de structuration courants. Au total, 384 rouges à lèvres ont été formulés : L1 (contrôle, sans alkénone), L2 (alkénones comme substitut de l’ozokérite), L3 (alkénones comme substitut de la cire microcristalline) et L4 (alkénones comme substitut de la cire de candelilla). Des tests ont été réalisés sur les produits pour évaluer la dureté (force de flexion), la rigidité, la fermeté (pénétration de l’aiguille), le transfert de matière (à l’aide d’un analyseur de texture et d’un panel de consommateurs), la friction, le point de fusion et la stabilité pendant 12 semaines à 25 et 45 °C.
RÉSULTATS
Les alkénones ont eu une influence sur chacune des caractéristiques évaluées. En général, les rouges à lèvres contenant des alkénones (L2 à L4) sont devenus plus mous et ont présenté une flexion plus facile que dans le cas du contrôle (L1). En termes de fermeté, les rouges à lèvres étaient similaires au contrôle, à l’exception de L4, qui était significativement (P < 0,05) plus ferme. L’effet sur le transfert de matière a été variable. L2 et L3 ont présenté un transfert de matière sur la peau et le tissu supérieur à celui de L1. En outre, dans le cas de L4, la quantité transférée était la plus faible, mais l’intensité de la couleur sur la peau était toujours la plus élevée. Les alkénones ont eu un effet positif sur la friction (glissement) ; la friction moyenne a diminué pour L2 à L4. La friction la plus basse (c.‐à‐d. le meilleur glissement) a été observée dans le cas de L4. Le point de fusion des rouges à lèvres était plus bas lorsque des alkénones étaient présents. Dans l’ensemble, L4, contenant 7 % d’alkénones en combinaison avec de la cire microcristalline, de l’ozokérite et de la cire de carnauba, s’est révélée avoir les caractéristiques les plus souhaitables, notamment une facilité de flexion, une fermeté élevée, un faible transfert de matière en termes de quantité, une intensité de couleur élevée sur la peau et une faible friction (c.‐à‐d. un meilleur glissement). En général, les consommateurs ont préféré L4.
CONCLUSION
Les résultats de cette étude indiquent que les alkénones offrent un choix durable, non issu de l’animal et non dérivé du pétrole comme agent de structuration pour les rouges à lèvres.
Introduction
Lipstick has been one of the most popular colour cosmetic products worldwide for decades across generations 1, 2, 3, 4. They serve the purpose of enhancing the appearance of the lips by adding colour and/or shine. Waxes are key components in lipsticks because they provide rigidity and appropriate hardness to the stick. Typically, multiple waxes are combined in a single lipstick to achieve the desired hardness, stability, texture and pay‐off 5, 6. A variety of waxes are available on the market today for lipsticks; they mainly differ in hardness, melting point and source. Commonly used waxes include microcrystalline wax (a synthetic ingredient derived from petroleum), ozokerite (a mineral wax derived from shale), beeswax (animal‐derived) and candelilla wax and carnauba wax (plant‐derived ingredients). Candelilla wax and carnauba wax are only available in certain parts of the world. Candelilla wax is obtained from the leaves of a small shrub native to northern Mexico and the southwestern United States, Euphorbia cerifera and Euphorbia antisyphilitica 7. Carnauba wax is obtained from the leaves of Brazilian Carnauba palm tree (Copernicia cerifera) that grows along riverbanks, valleys and lagoons in the northern and northeastern part of Brazil 8. Availability of these waxes could be potentially affected by environmental factors, which has been seen in the case of candelilla wax. In the last two decades, there has been a shortage of candelilla wax on the market due to climatic factors. According to forecasts 9, candelilla wax supply and price will continue to fluctuate. As a result, there is a high demand for suitable structuring agent alternatives in lipsticks and lip balms 10, 11.
Cosmetic consumers are becoming more conscious and increasingly concerned with ingredients used in their products 12. As trends, such as green, sustainable, natural and vegan, are gaining popularity 13, synthetic and animal‐derived ingredients are less favoured among certain consumer groups. Therefore, companies are continuously searching for new natural and sustainable ingredients they could use in their products.
Alkenones are an off‐white waxy solid at room temperature (Figure 1) with a melting point range of 71.1–77.4°C 14. Alkenones are a family of unique lipids biosynthesized by certain haptophyte microalgae 15, including the industrially grown Isochrysis sp. (Chromista, Haptophyta) 16. Hence, they are a marine‐based vegan ingredient. They are renewable, and therefore, green which fits well into the popular natural/sustainable and vegan trend. Alkenones can be grown in many locations; therefore, their availability is not as limited as that of some other waxes. Marine‐based ingredients, including microalgae‐sourced ingredients, are becoming popular in cosmetics 17 due to their natural origin and richness in vitamins 18, minerals 19, proteins and essential fatty acids 20. Given their waxy nature and reasonably high melting point, we argue that alkenones represent an attractive and as‐yet‐undeveloped class of natural ingredients that may find useful applications in a variety of cosmetic and personal care applications. Alkenones used in this study have been previously described and characterized extensively 21, 22, 23, and their potential application in personal care products, specifically in sunscreens, has been studied by our research group 14, 24.
Figure 1.

(a) Structure of a common alkenone, that is 37:2 methyl alkenone, isolated from Isochrysis microalgae. Alkenones contain trans double bonds that are not methylene‐interrupted, and a methyl or ethyl ketone. (b) Alkenones wax.
In a previous study, we found alkenones to be a viable alternative to microcrystalline wax and ozokerite in lipsticks and lip balms 14 based on visual observations during a 10‐week period at two different temperatures. The goal of this study was to compare alkenones to waxes commonly used in lipsticks and instrumentally evaluate the effect of alkenones on the hardness, stiffness, firmness and pay‐off of lipsticks. Additionally, we evaluated consumers’ preference for lipsticks made in this study.
Materials and methods
Materials
The marine microalgae Isochrysis was purchased from Necton S.A. (Olhão, Portugal). Alkenones were isolated and purified from the Isochrysis biomass as previously described 25. Castor oil (INCI: Ricinus Communis (Castor) Seed Oil), triglyceride (INCI: Caprylic/Capric Triglyceride), isoeicosane, meadowfoam seed oil (INCI: Limnanthes Alba (Meadowfoam) Seed Oil), microcrystalline wax, ozokerite, candelilla wax (INCI: Euphorbia Cerifera (Candelilla) Wax), carnauba wax (INCI: Copernicia Cerifera (Carnauba) Wax), Red 7 Lake dispersion (INCI: Red 7 (and) Ricinus Communis (Castor) Seed Oil), mica, Vitamin E (INCI: tocopherol) and a preservative blend (Propylene glycol (and) diazolidinyl urea (and) methylparaben (and) propylparaben) were purchased from Making Cosmetics (Snoqualmie, WA, USA). All ingredients were cosmetic grade. Two commercial lipsticks were tested in this study as controls, including Wet and Wild Silk Finish Lipstick in Dark Wine colour (hereinafter referred to as ‘C1’) purchased at a local store (Dollar General, Toledo, OH) and L’Oréal Colour Riche Comfortable Creamy Matte Lipstick in Matte‐ly in Love colour (hereinafter referred to as ‘C2’) purchased online (Amazon.com).
In our preliminary study 14, we selected microcrystalline wax and ozokerite to compare to the alkenones. Our reasoning was the that these waxes and alkenones have similar melting points (microcrystalline wax 64–72°C 26, ozokerite 73–76°C 27, and alkenones 71.1–77.4°C 14). The melting point of candelilla wax (68.5–72.5°C 28) is also close to that of the alkenones; therefore, we included candelilla wax in this study as well.
In this study, we formulated four types of lipsticks (Table 1). Lipstick 1 (L1) contained a combination of four waxes, not including alkenones; therefore, L1 was the control formula. Lipsticks 2–4 contained alkenones as a replacement for either microcrystalline wax (L2), or ozokerite (L3), or candelilla wax (L4), respectively.
Table 1.
Composition of lipsticks in this study.
| Ingredient INCI names |
L1 % (w/w) |
L2 % (w/w) |
L3 % (w/w) |
L4 % (w/w) |
|---|---|---|---|---|
| Phase A | ||||
| Ricinus Communis (Castor) Seed Oil | 30.8 | 30.8 | 30.8 | 30.8 |
| Caprylic/Capric Triglyceride | 16.0 | 16.0 | 16.0 | 16.0 |
| Isoeicosane | 17.0 | 17.0 | 17.0 | 17.0 |
| Limnanthes Alba (Meadowfoam) Seed Oil | 5.0 | 5.0 | 5.0 | 5.0 |
| Microcrystalline wax | 3.5 | 3.5 | ‐ | 3.5 |
| Ozokerite | 3.5 | ‐ | 3.5 | 3.5 |
| Alkenones | ‐ | 3.5 | 3.5 | 7.0 |
| Euphorbia Cerifera (Candelilla) Wax | 7.0 | 7.0 | 7.0 | ‐ |
| Copernicia Cerifera (Carnauba) Wax | 3.0 | 3.0 | 3.0 | 3.0 |
| Phase B | ||||
| Red 7 (and) Ricinus Communis (Castor) Seed Oil | 2.0 | 2.0 | 2.0 | 2.0 |
| Mica | 11.0 | 11.0 | 11.0 | 11.0 |
| Phase C | ||||
| Tocopherol | 0.2 | 0.2 | 0.2 | 0.2 |
| Propylene glycol (and) diazolidinyl urea (and) methylparaben (and) propylparaben | 1.0 | 1.0 | 1.0 | 1.0 |
Methods
Formulation of lipsticks
Phase A was heated to 80°C to melt the waxes. Phase B components were added to melted phase A, and then, the mixture was removed from heat. Phase C was added to phase A/B, and the mixture was poured into a metal lipstick mould whereas still hot. When settled, the sticks were topped off and the mould was placed in the freezer (−18°C) for 15 min. The lipsticks were then removed from the mould and inserted into plastic lipstick cases.
Bending force and stiffness
Samples were kept in stability cabinets (at 25 and 45°C) for 24 h before testing. Bending force of each lipstick was determined using a TA.XTPlus texture analyser (Texture Technologies Corp., Hamilton, MA) with a TTC bending force fixture (Figure 2a). To determine the bending force and stiffness, each lipstick was raised to maximum length and clamped horizontally. The upper bending fixture stroke of the sample was approximately 3 mm away from the tip. Test mode was set to ‘measure compression’, and target mode was set to ‘distance’. Trigger force was 20.0 g. The pre‐test, test and post‐test speeds were set to 1.0, 1.0 and 10.0 mm s−1, respectively. Five lipsticks of each type were tested. Exponent Stable Micro Systems software (version 6.1.10.0) was used to generate hardness curves.
Figure 2.
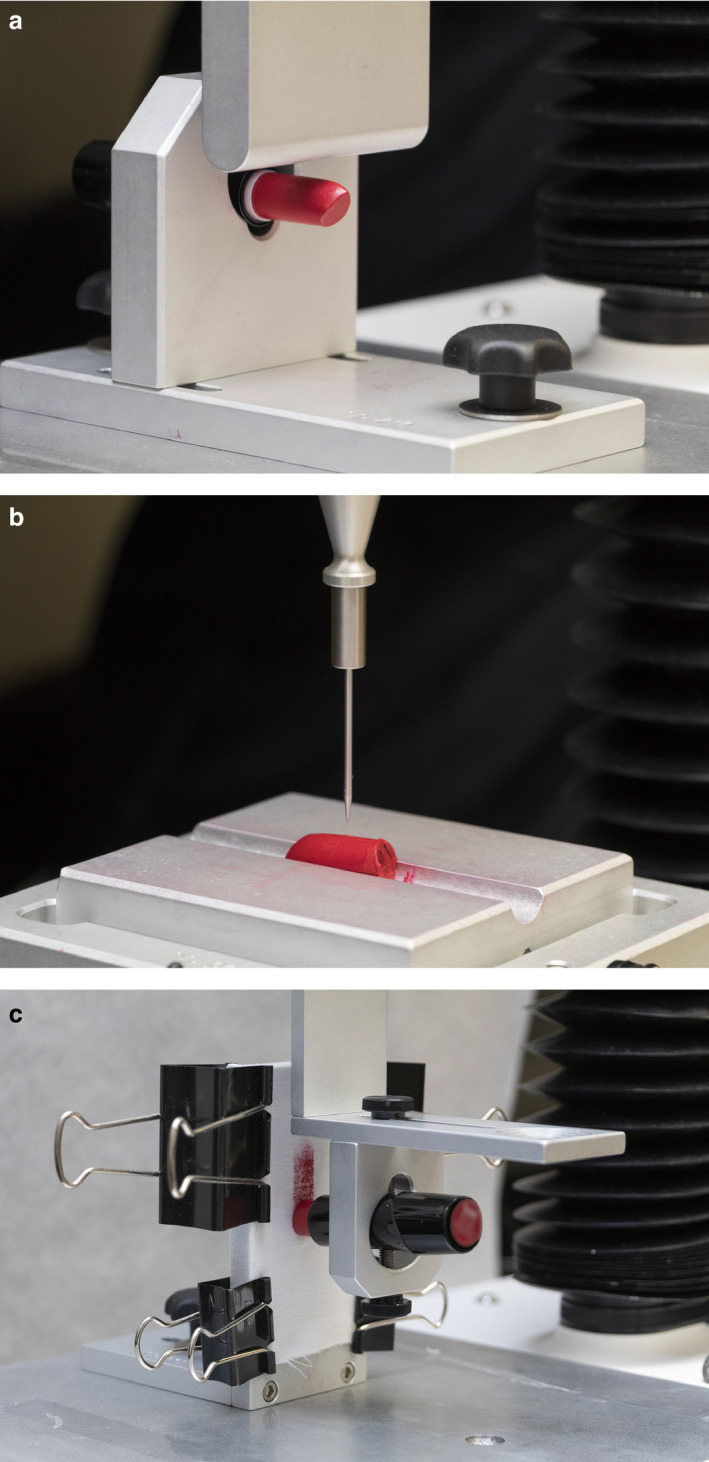
(a) Bending force test. (b) Needle penetration testing. (c) Pay‐off testing to fabric.
Once the trigger force was attained, the probe moved down 7 mm and the sample was bent until it broke away from the main body. This was shown as the maximum force value, which indicated the hardness of the sample. The gradient of the slope during the bending action referred to ‘stiffness’ (or resilience) of the sample 29.
Needle penetration test
Samples were kept in stability cabinets for 24 h before testing. Firmness of each lipstick was determined using a TA.XTPlus texture analyser (Texture Technologies Corp., Hamilton, MA) with a TTC needle penetration fixture (Figure 2b). Hardness was tested at room temperature (25°C) and elevated temperature (45°C). To determine hardness, each lipstick was placed on the side onto a plate placed centrally under the needle probe. Test mode was set to ‘measure compression’, and target mode was set to ‘distance’. The needle penetration distance was 7 mm, and trigger force was 5.0 g. The pre‐test, test and post‐test speeds were set to 1.0, 2.0 and 10.0 mm s−1, respectively. Five lipsticks of each type were tested. Exponent Stable Micro Systems software (version 6.1.10.0) was used to generate hardness curves.
During the test, the needle moved down to penetrate the sample. The maximum force value was measured as the force required to penetrate the sample to the specified distance (i.e. 7 mm).
The needle penetration test can also indicate the presence of unwanted air bubbles or grainy texture, which would be seen as fluctuations in the force values during penetration, as a result of either incomplete colorant dispersion or the working and cooling processes during manufacture. This ‘grainy’ texture would be perceived by the consumer as undesirable.
Pay‐off
Pay‐off to fabric of each lipstick was determined using a TA.XTPlus texture analyser (Texture Technologies Corp., Hamilton, MA) with a TTC vertical friction rig and a stationary vertical plate (Figure 2c). The vertical friction rig was secured onto the machine arm and placed vertically, parallel to the stationary vertical plate, with a 5 mm distance between the rig and plate. The fabric was cut into 15 × 13 cm pieces (Mainstays 200 thread count fitted sheet, Walmart, Bentonville, AR, USA) and ironed. To determine pay‐off, each lipstick was prepared by cutting a clean flat surface with a sharp blade and attached to the vertical friction rig. Each piece of fabric was weighed before the study, then mounted on the stationary vertical plate using four binder clips to create a flat, smooth surface. Test setting was ‘Cycle Until Count in Tension’ for three cycles for a distance of 55 mm at a speed of 5 mm s−1. Each piece of fabric was weighed after the test, and weight difference was calculated. Kinetic friction, that is resistance to maintain the movement at a specific constant speed, was also measured using the same setup. Two lipsticks from each batch were used for the pay‐off and friction test, and each lipstick was tested three times.
Pay‐off to skin (i.e. forearm) was also tested using volunteers. Each lipstick was applied to the forearm in three layers (moving the lipstick up‐down‐up) onto a 5‐cm long area of the inner forearm. Each lipstick was weighed on a balance before and after the test in order to calculate the amount of lipstick transferred to skin. At the end of the pay‐off test, a picture was taken of each volunteer’s forearm and colour intensity was evaluated visually.
Consumer study
Fourteen consumers, of ages ranging between 18 and 29 years, were recruited for the panel. Consumers from both genders and any ethnicity were invited to participate. A main inclusion criterium was that participants must had prior experience with lipstick. The majority of participants were female (93%). Ethnicities included Caucasian/White/European (68%), Asian/Pacific Islander (18%), African American/African/Black/Caribbean (7%) and other (7%).
Panellists were asked not to wear any lipstick or lip balm prior to arriving to the consumer test. They were instructed to first look at the lipsticks in the lipstick case, then apply each lipstick one by one to the lips, applying as much lipstick as they typically would in real‐world conditions, using the same number of strokes for each lipstick. In addition, panellists were asked to complete a paper‐based survey before, during, and after applying the lipsticks. Testing was administered in a research laboratory under artificial daylight type of illumination at room temperature (between 22°C and 25°C). The consumer panel test was performed by ACT Solutions Corp (Newark, DE). The survey included four different sensory methods, that is check‐all‐that‐apply (CATA) questions, preference, ranking and rating tests (Figure 3).
Figure 3.

(a) and (b) Panel study survey page 1 and 2, respectively.
In vitro dermal irritation potential of alkenones was evaluated previously by Consumer Product Testing Company, Inc. (Fairfield, NJ). Results indicated that alkenones are safe for topical application and not expected to cause irritation in vivo 30.
Differential scanning analysis
Melting point was determined using differential scanning calorimetry (DSC) analysis. Using a Mettler MT 5 microbalance (Mettler Toledo, Columbus, OH), a 8‐mg sample was sealed in an aluminium crucible. DSC was performed at a 10°C min−1 ramp from 0 to 200°C using a DSC Q20 (TA Instruments, New Castle, DE) attached to a F25‐ME refrigerated/heating circulator (Julabo, Allentown, PA). Nitrogen gas was purged at a rate of 50 mL min−1. TA Universal analysis software was used to obtain the scans.
Stability
Stability of the lipsticks was monitored at two temperatures, room temperature (25°C) and an elevated temperature (45°C) in stability cabinets for 12 weeks. Samples were checked visually and tested instrumentally at day 1, weeks 4, 8 and 12 in their final containers.
Data analysis
Differences in the lipstick hardness, stiffness, firmness and pay‐off were evaluated using one‐way ANOVA followed by Tukey’s multiple comparison test using SPSS Statistics 21 software (IBM, Armonk, NY). A P value less than 0.05 was taken as the minimal degree of statistical significance.
Results
Hardness and stiffness
Lipstick hardness is an essential characteristic; lipsticks must not bend, crumble, crack or break during application. Hardness depends primarily on the type and amount of waxes in the formulation, as well as the oil:wax ratio. In our samples, the oil:wax ratio was constant, only a single wax was different in each lipstick.
Hardness (force needed to bend) of L1 and L4 did not change significantly over the 12 weeks at 25°C; however, L2 and L3 became softer over time at room temperature (P < 0.05) (Figure 4a). The difference in hardness between the control (L1) and L4 at 25°C was notable throughout the testing period; L4 was statistically significantly softer and easier to bend (P < 0.05). Two commercial lipsticks were tested as well (C1 and C2) at both temperatures as controls to understand how our lipsticks compared to commercially available lipsticks. Testing of C1 and C2 was only performed in a single timepoint. Hardness of C1 and C2 at 25°C fluctuated greatly, C1 was similar to L4, and C2 was similar to L1‐L3. Hardness of all of our lipsticks was considered acceptable since the values were similar to the commercial products. At 45°C, hardness of all lipsticks was lower, as expected. Higher temperature softens waxes; therefore, the lipsticks became softer. Similar to 25°C, lipsticks at 45°C had similar hardness throughout the testing period. An interesting observation was that the hardness of L4 at 45°C started high, compared to L1, and then significantly decreased over the 12 weeks (P < 0.05). No other change was observed. L2 was the softest at 45°C. It can be summarized that alkenones made the lipsticks softer at 25°C, this difference was not as noticeable at 45°C.
Figure 4.
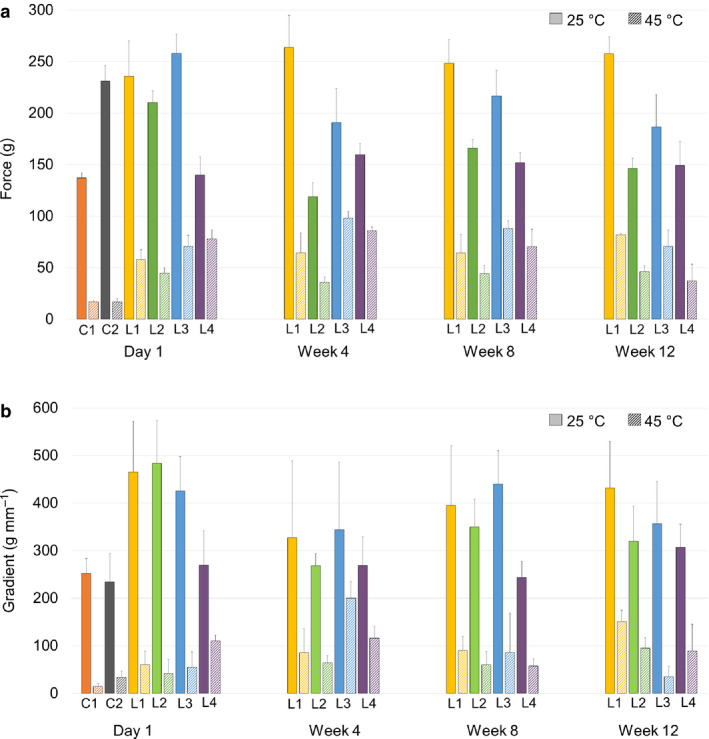
(a) Hardness of lipsticks during stability testing. (b) Stiffness of lipsticks during stability testing.
Stiffness refers to how easily the lipstick can be bent. A stiff sample lacks flexibility and is hard to bend. At 25°C, the stiffness of L4 was the lowest out of L1‐L4 throughout the testing period, and it was also the closest to the commercial lipsticks (Figure 4b). The values for L1‐L3 were higher, meaning they were less flexible. At 45°C, L4 was the least flexible on Day 1, but over the weeks, all lipsticks containing alkenones became less stiff than the control. Results indicate that alkenones made the lipsticks more flexible, especially in a higher concentration when used as an alternative for candelilla wax.
Needle penetration test
Firmness is a characteristic that needs to be balanced with softness. If a lipstick is too firm, it is difficult to apply and may feel waxy and dragging, especially at lower temperatures. If a lipstick is too soft, it may have an undesirably too high pay‐off, leading to a sticky sensation and faster product usage 31.
Firmness of the lipsticks remained consistent throughout the testing period at 25°C. L4 was the firmest compared to all other lipsticks (P < 0.05) (Figure 5). C1 and C2 were the least firm, suggesting that they were softer than our lipsticks. At 45°C, all lipsticks became softer and easier to penetrate, as expected – similar to bending. At elevated temperature, the firmness of most lipsticks remained relatively consistent, except for L4, which became significantly softer over the testing period (P < 0.05). Alkenones did not affect firmness markedly in a lower concentration; however, they increased firmness significantly in a higher concentration.
Figure 5.
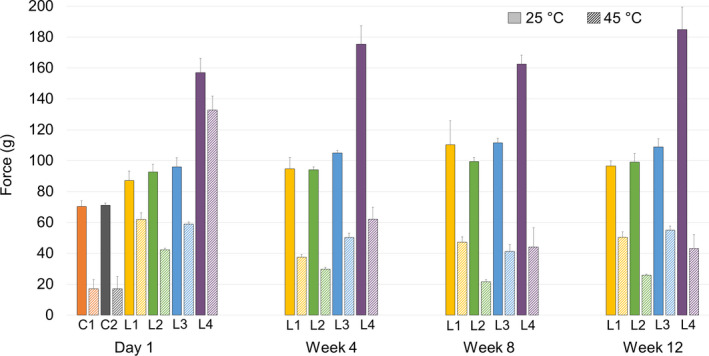
Firmness of lipsticks during the stability study.
Pay‐off
Pay‐off is often used as an indirect measure of how well a product works from a consumer’s perspective. When choosing lipsticks, consumers usually swatch samples to see the colour and test pay‐off. Too high of a pay‐off can lead to product build‐up on the lips and a waxy sensation. Too low of a pay‐off demands that consumers apply the product in multiple layers to achieve the desired colour and coverage. Friction is the resisting force that arises when one surface slides over another 29. A lipstick that generates a lot of friction feels ‘sticky’ and loses some of the glide and easy application that consumers’ desire.
As for pay‐off to fabric, C2, L1 and L4 were lower pay‐off lipsticks, whereas C1, L2 and L3 had higher amounts transferred (P < 0.05) (Table 2). In the case of pay‐off to skin, both C1 and C2 and L4 had a lower pay‐off, and L1‐L3 had higher values. From our lipsticks, L2 and L3 transferred the highest amounts, whereas L4 transferred the lowest amount to both fabric and skin. This difference is probably related to interaction of the waxes and oils in the lipsticks 32. Interestingly, whereas L4 transferred the lowest amount of product to both fabric and skin, it still had the highest colour intensity on the skin after the same number of strokes (determined visually, Figure 6b). These results imply that L4 would be the most desired because it had a low amount of product transferred, but still achieved the most intensive colour on skin, which can translate into slower product consumption.
Table 2.
Pay‐off to fabric and skin (average ± SD).
| Pay‐off to fabric (mg) | Pay‐off to skin (mg) | |
|---|---|---|
| C1 | 71 ± 4 | 10 ± 2 |
| C2 | 61 ± 3 | 7 ± 1 |
| L1 | 61 ± 3 | 14 ± 2 |
| L2 | 77 ± 4 | 19 ± 1 |
| L3 | 75 ± 2 | 16 ± 1 |
| L4 | 57 ± 2 | 10 ± 1 |
Figure 6.

(a) Lipsticks formulated and tested in this study, L1 to L4 from left to right. (b) Visual representation of pay‐off to skin after three strokes.
The kinetic friction force decreased over the cycles either dramatically (≥30%) or slightly (<30%) (Figure 7). The highest average friction was observed for L1 and L2. Decrease in friction was very high for L2 (32% change between cycle 1 and 3), whereas it was negligible for L4. There was not a clear target for the extent of decrease based on the commercial products; the decrease for C1 was 32%, whereas for C2 it was only 6%. Pay‐off most likely affected the friction during application. Lipsticks with higher pay‐off values showed higher change in friction. L4 had the lowest pay‐off to both fabric and skin, and it had the lowest overall change in friction as well. The minimal change in friction implies that L4 was easy to glide from the beginning, whereas our other lipsticks were more dragging first and then became smoother to apply.
Figure 7.
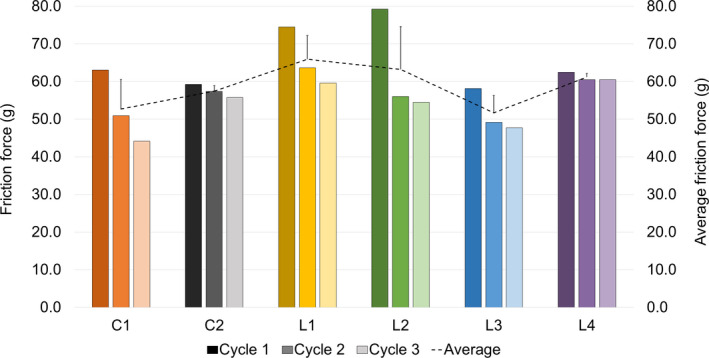
Friction results during three cycles for each lipstick. The x axis shows the numbers for the individual cycles, whereas the y axis indicates the average of the three cycles. Error bars represent the standard deviation of the average friction force.
Consumer study
In the container, consumers found all lipsticks, except for L3, evenly coloured (Figure 6a). L1, L2 and L4 were scored as matte, whereas L3 was glossy/shiny in the container according to the participants. When applied to the skin, all lipsticks were described as glossy/shiny and evenly coloured. In the preference test, L4 was selected as the most preferred lipstick by 71% of the participants, whereas L2 and L3 were at second place. No consumer indicated they would purchase/use L1. In the ranking test, consumers were asked to rank samples based on the pay‐off to skin. Overall ranking of the lipsticks was the following: L4 > L2 > L3 > L1. In the rating test, most consumers (64%) liked L4 the most, followed by L3, then L1 and L2. The commercial products were not included in the consumer study because we did not know the pigment content of those lipsticks. Colour intensity of the commercial lipsticks was higher, they probably contained more pigments than our lipsticks. Since colour is determined by the amount of pigments used in a lipstick, including all of them in the consumer study would not have been a fair comparison.
Overall, consumer study results indicated that participants felt L4 had the best coverage and colour intensity (was the most pigmented) on the skin and was soft, smooth and the easiest to apply. These results are in good correlation with our instrumental pay‐off and friction (Figure 7) and colour intensity results (Figure 6b). Visually we also found L4 to have the most pigmented colour on skin from the same number of strokes. It should be noted that all of our lipsticks contained the same pigment solid content; therefore, any difference in colour on the skin can be attributed to the composition.
DSC
The melting point of the lipsticks is displayed in Table 3. The DSC thermogram of each lipstick exhibited melting range with one or more characteristic endothermic melting peak(s) within the range. The melting point of our control (L1) was higher and similar to C1 and C2, whereas L2‐L4 had lower melting points. Although alkenones had a very similar melting point range as the comparator waxes, lipsticks with alkenones had lower melting points overall. A lower melting point may affect the stability of lipsticks as those with lower melting points may also soften at a lower temperature, leading to a product that is more sensitive to accidental exposure to higher temperature (e.g. leaving a lipstick in a hot car).
Table 3.
Melting point of lipsticks tested in this study
| Ingredient | Melting point range (°C) | Melting peak (°C) |
|---|---|---|
| Lipstick 1 | 35.9–85.2 | 60.3 |
| Lipstick 2 | 44.8–86.3 | 53.2 |
| Lipstick 3 | 38.64–82.63 | 50.6 |
| Lipstick 4 | 39.7–85.3 | 53.5 |
| Commercial lipstick 1 | 44.4–76.6 | 57.5 |
| Commercial lipstick 2 | 36.6–88.5 | 59.4 |
Stability study
All lipsticks remained stable at 25°C and 45°C, except for L2 which started sweating at week 8 at 45°C. Although differences were seen in the melting point of lipsticks with and without alkenones – lipsticks with alkenones had a lower melting point –, no significant negative effect of alkenones was observed on the stability of lipsticks.
Conclusions
In this study, alkenones were used as an alternative to three commonly used waxes in lipsticks, and the effect of alkenones on the quality and performance of lipsticks was evaluated.
Alkenones influenced each characteristic evaluated. In general, lipsticks with alkenones (L2‐L4) became softer, easier to bend than the control (L1). L2‐L4 was more flexible than the control. In terms of firmness (measured via the needle penetration test), lipsticks were similar to the control, except for L4, which was significantly firmer. The effect on pay‐off was not consistent; no trends were observed. L2 and L3 had higher pay‐off to skin and fabric than the control. L4 had the lowest amount transferred, but it still had the highest colour intensity on skin. Alkenones influenced friction (glide) positively, the average friction decreased for L2‐L4 compared to L1. The lowest friction (i.e. best glide) was shown in L4. Melting point of the lipsticks was lower when there was alkenones in the mixture.
Overall, L4, the lipstick containing the highest amount of alkenones in combination with microcrystalline wax, ozokerite and carnauba wax was found to have the most desirable attributes, including ease of bending, high level of firmness, low pay‐off in terms of amount, high colour intensity on skin and lower friction (i.e. better glide). The consumer study revealed that consumers liked L4 the most overall.
Acknowledgements
The authors would like to thank Texture Technologies for the technical assistance provided during this project. This research was funded by the Washington Research Foundation and a private donor from friends of the Woods Hole Oceanographic Institution, grant number N‐127244.
Paper was presented as a poster presentation at the American Chemical Society Central Regional Meeting in Midland, MI held on June 4‐8, 2019.
References
- 1. Global lipstick sales set to sparkle through to 2019 . Cosmetics Business. (2016). Available online: https://www.cosmeticsbusiness.com/news/article_page/Global_lipstick_sales_set_to_sparkle_through_to_2019/115753 (accessed on July 19, 2019).
- 2. Pucker up : US lip cosmetics market set to reach $1.4 billion this year. Mintel. (2014). Available online: https://www.mintel.com/press-centre/beauty-and-personal-care/color-cosmetics-sales-trends. (accessed on June 2, 2019).
- 3. Shahbandeh, M. Statistics & Facts on the U.S. Cosmetics and Makeup Industry. (2018). Available online: https://www.statista.com/topics/1008/cosmetics-industry/ (accessed on August 22, 2019).
- 4. More, A. Lipstick Market 2019 Global Industry Forecasts Analysis, Company Profiles, Competitive Landscape and Key Regions Analysis Available at Research Reports World. (2019). Available online: https://www.marketwatch.com/press-release/lipstick-market-2019-global-industry-forecasts-analysis-company-profiles-competitive-landscape-and-key-regions-analysis-available-at-research-reports-world-2019-04-03 (accessed on July 19, 2019).
- 5. Choudhury, T. The Physical Chemistry of Cosmetic Formulations. Happi. (2008). Available online: https://www.happi.com/contents/view_features/2008-02-29/the-physical-chemistry-of-cosmetic-formulatio.
- 6. Sandewicz, R. and Finkenaur, G. Lipstick. In: Chemistry and Manufacture of Cosmetics. ( Schlossman, M. , ed.), pp. 433‐56. 2. 4 ed. Allured Business Media, Carol Stream, IL: (2009). [Google Scholar]
- 7. Toro‐Vazquez, J.F. , Charó‐Alonso, M.A. , Pérez‐Martínez, J.D. and Morales‐Rueda, J.A. 6 ‐ Candelilla Wax as an Organogelator for Vegetable Oils—An Alternative to Develop Trans‐free Products for the Food Industry. In: Edible Oleogels ( Marangoni, A.G. and Garti, N. , eds.), pp. 119–48. AOCS Press, Urbana, IL: (2011). [Google Scholar]
- 8. Carnauba, organic . Available online: https://www.kosterkeunen.com/product/carnauba-organic/ (accessed on September 12, 2019).
- 9. Candelilla Wax and Substitution Options . Available online: https://www.brenntag.com/media/documents/bsi/product_data_sheets/life_science/koster_kuenen_food_waxes/candelilla_wax_alternatives.pdf (accessed on September 12, 2019).
- 10. Guzman, D. Demand for natural wax increases 2008. Available online: https://www.icis.com/explore/resources/news/2008/08/18/9149059/demand-for-natural-wax-increases/ (accessed on August 22, 2019).
- 11. Global Candelilla Wax Industry 2016 Market Research Report. (2016). Available online: https://www.marketresearchstore.com/report/global-candelilla-wax-industry-2016-market-research-report-63520.
- 12. Shahbandeh, M. Unit sales of the leading lipstick brands in the United States in 2018 (in millions). Statista. (2018). Available online: https://www.statista.com/statistics/503275/lipstick-brands-unit-sales-in-the-us/ (accessed on August 31, 2019).
- 13. O’Lenick, A.J. Jr . Naturals and Organics in Cosmetics: Trends and Technology. Allured Business Media, Carol Stream, IL: (2010). [Google Scholar]
- 14. McIntosh, K. , Smith, A. , Young, L.K. et al. Alkenones as a promising green alternative for waxes in cosmetics and personal care products. Cosmetics. 5, 34 (2018). [Google Scholar]
- 15. Volkman, J.K. , Eglinton, G. , Corner, E.D.S. and Forsberg, T.E.V. Long‐chain alkenes and alkenones in the marine coccolithophorid Emiliania huxleyi. Phytochemistry. 19, 2619–22 (1980). [Google Scholar]
- 16. Eltgroth, M.L. , Watwood, R.L. and Wolfe, G.V. Production and cellular localization of neutral long‐chain lipids in the haptophyte algae Isochrysis galbana and Emiliana huxleyi . J. Phycol. 41, 1000–9 (2005). [Google Scholar]
- 17. Offredo, H. Marine ingredients for skin care: an ocean of resources. In: Harry's Cosmetology. ( Rosen, M.R. ed.), 2. Chemical Publishing Co., Inc., Los Angeles, CA: (2015). [Google Scholar]
- 18. Brown, M.R. , Mular, M. , Miller, I. , Farmer, C. and Trenerry, C. The vitamin content of microalgae used in aquaculture. J. Appl. Phycol. 11, 247–55 (1999). [Google Scholar]
- 19. Singh, S. , Kate, B.N. and Banerjee, U.C. Bioactive compounds from cyanobacteria and microalgae: an overview. Crit. Rev. Biotechnol. 25, 73–95 (2005). [DOI] [PubMed] [Google Scholar]
- 20. Mourelle, M.L. , Gómez, C.P. and Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics. 4, 46 (2017). [Google Scholar]
- 21. O’Neil, G.W. , Williams, J.R. , Craig, A.M. , Nelson, R.K. , Gosselin, K.M. and Reddy, C.M. Accessing monomers, surfactants, and the queen bee substance by acrylate cross‐metathesis of long‐chain alkenones. J. Am. Oil. Chem. Soc. 94, 831–40 (2017). [Google Scholar]
- 22. O’Neil, G.W. , Culler, A.R. , Williams, J.R. et al. Production of jet fuel range hydrocarbons as a coproduct of algal biodiesel by butenolysis of long‐chain alkenones. Energ. Fuel. 29, 922–30 (2015). [Google Scholar]
- 23. O'Neil, G.W. , Yen, T.Q. , Leitch, M.A. et al. Alkenones as renewable phase change materials. Renew. Energ. 134, 89–94 (2019). [Google Scholar]
- 24. Huynh, A. , Abou‐Dahech, M.S. , Reddy, C.M. , O’Neil, G.W. , Chandler, M. and Baki, G. Alkenones, a renewably sourced, biobased wax as an SPF booster for organic sunscreens. Cosmetics. 6, 11 (2019). [Google Scholar]
- 25. O'Neil, G.W. , Williams, J.R. , Wilson‐Peltier, J. , Knothe, G. and Reddy, C.M. Experimental protocol for biodiesel production with isolation of alkenones as coproducts from commercial isochrysis algal biomass. J. Vis. Exp. 112, 54189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Microcrystalline waxes UL Prospector . Available online: https://www.ulprospector.com/documents/1344958.pdf?bs=4672&b=532260&st=1&r=na&ind=personalcare (accessed on August 29, 2019).
- 27. Ozokerite wax Koster Keunen . Available online: https://www.kosterkeunen.com/product/ozokerite-wax/ (accessed September 2, 2019).
- 28. Candelilla, U.L. Prospector. Available online: https://www.ulprospector.com/documents/1413120.pdf?bs=4672&b=637093&st=1&sl=80492103&crit=a2V5d29yZDpbY2FuZGVsaWxsYSB3YXhd&k=candelillawax&r=na&ind=personalcare (accessed on September 2, 2019).
- 29. Smewing, J. (2014) Available online: https://textureanalysisprofessionals.blogspot.com/2014/11/texture-analysis-in-action-lipstick.html (accessed August 24, 2019).
- 30. Final report. Consumer Product Testing Company, Inc.; (2018).
- 31. Lip Balm Smackdown. Texture Technologies Corp. (2012).
- 32. Hui, W.N. , Tamburic, S. , Grant‐Ross, P. , Cottin, P. and Corger, D. Lip‐smacking results: mixture design ‘pays off’ to optimize wax/oil lipstick ratio. Cosmet. Toiletries. 132, 48–68 (2017). [Google Scholar]


