Abstract
The recent development of simple, rapid genotyping techniques for Campylobacter species has enabled investigation of the determinative epidemiology of these organisms in a variety of situations. In this study we have used the technique of fla typing (PCR-restriction fragment length polymorphism analysis of the flaA and flaB genes) to identify the sources of strains contaminating the carcasses of five campylobacter-positive and two campylobacter-negative broiler flocks during abattoir processing. The results confirmed that, in the United Kingdom, individual broiler flocks are colonized by a limited number of subtypes of Campylobacter jejuni or C. coli. In some but not all cases, the same subtypes, isolated from the ceca, contaminated the end product as observed in carcass washes. However, the culture methodology, i.e, use of direct plating or enrichment, affected this subtype distribution. Moreover, the number of isolates analyzed per sample was limited. fla typing also indicated that some campylobacter subtypes survive poultry processing better than others. The extent of resistance to the environmental stresses during processing varied between strains. The more robust subtypes appeared to contaminate the abattoir environment, surviving through carcass chilling, and even carrying over onto subsequent flocks. From these studies it is confirmed that some campylobacter-negative flocks reach the abattoir but the carcasses from such flocks are rapidly contaminated by various campylobacter subtypes during processing. However, only some of these contaminating subtypes appeared to survive processing. The sources of this contamination are not clear, but in both negative flocks, campylobacters of the same subtypes as those recovered from the carcasses were isolated from the crates used to transport the birds. In one case, this crate contamination was shown to be present before the birds were loaded.
Campylobacter jejuni is a common cause of human acute bacterial enteritis worldwide (14). A recent community-based intestinal infectious disease study estimates an incidence of up to 500,000 cases per annum in England and Wales alone (15). Although the sources of infection are still debatable, the handling and/or consumption of poultry meat is considered a significant risk for human infection (11). Up to 90% of broiler chicken flocks in the United Kingdom are colonized with Campylobacter jejuni at the time of slaughter (5), and the colonization of young chickens under experimental conditions may be as high as 1010 CFU per g of cecal contents (4). Therefore, the cross-contamination of broiler carcasses by spilled gut contents at slaughter and evisceration presents a potential hygiene problem in abattoirs. This may be particularly significant when campylobacter-free flocks follow colonized flocks through the processing plant.
The tracing of pathogens through the food chain requires appropriate methods for strain differentiation. This is particularly important with a bacterium as ubiquitous in the environment as C. jejuni. Such methods need to be highly sensitive, discriminatory, and reliable. Recently, several genotyping methods have been developed for campylobacters (reviewed in reference 16). For poultry campylobacters, methods such as fla typing (restriction fragment length polymorphism [RFLP] analysis of the PCR products of the fla genes) have the advantages of low levels of nontypeability, acceptable levels of discriminatory power, and cost-effectiveness (10).
In this study, five campylobacter-positive and two campylobacter-negative broiler flocks were followed through commercial abattoirs. The campylobacter status of the birds at slaughter was determined by culture of cecal contents. The carcasses were sampled at various stages throughout processing. Selected transport crates, carrying the birds to the abattoir, as well as some processing machinery and the scald water were also sampled. Selected campylobacter isolates were genotyped by fla typing (1) and, in some cases, by pulsed-field gel electrophoresis (PFGE) in order to detect changes in subtypes contaminating the carcasses before and after processing.
MATERIALS AND METHODS
Bacteriological samples.
Five commercial poultry flocks previously shown to be campylobacter-positive approximately 7 days prior to slaughter were sampled on three separate visits to three different commercial abattoirs. Two of the visits each involved sampling of two flocks; flock 3 was subsequent to flock 2, and flock 4 was subsequent to flock 5. Two additional flocks (flocks 6 and 7) from a consistently campylobacter-negative farm and shown to be campylobacter negative 7 days prior to slaughter were also sampled in two separate visits to one further commercial poultry abattoir. Each flock sampled comprised between 10,000 and 20,000 birds at slaughter.
For each flock, the ceca from 10 birds were removed postevisceration and surface sterilized by heat searing, and the contents were sampled aseptically with swabs. Swab samples were also collected from obvious fecal contamination of the crates in which the birds from each flock were transported, in each case immediately prior to crate washing. For one of the negative flocks, samples were also taken from crates prior to transportation to the farm and after transportation but before bird loading and prior to crate washing. Up to five poultry carcasses from each flock were also sampled by carcass rinsing according to Simonsen (12) with 500 ml of maximum recovery diluent (Oxoid Ltd., Basingstoke, U.K.) at various stages during processing, including following slaughter, defeathering, evisceration, and chilling.
In addition, for some flocks, water samples were collected from the scald tank (15 ml, of which 1 ml was analyzed) and samples were taken from the external surface of the defeathering machine.
Bacteriological culture.
Swabs of the cecal contents were incubated in 10 to 18 ml of Exeter broth medium (7) for 48 h at 37°C, and swabs from the crates and defeathering machine were incubated in 100 ml of Exeter broth medium for 48 h at 37°C under microaerobic conditions. A sample of 50 μl was then removed for plating on mCCDA medium (Oxoid Ltd.) containing selective antibiotics (13) with actidione (100 μg/ml) and cefoperazone (30 μg/ml). The plates were incubated microaerobically at 37°C for 48 h. For the carcass rinses from flocks 2 to 7, samples were cultured either following enrichment or by direct plating. Carcass rinses from flock 1 were cultured following enrichment only. For enrichment, 1 ml of carcass rinse fluid was diluted in 18 ml of Exeter broth and then cultured as above. For direct plating, 50 μl of carcass rinse fluid was plated directly onto mCCDA medium.
All campylobacters isolated were identified to species level by standard microbiological procedures (3), including microaerobic growth at 42°C, hippurate and indoxylacetate hydrolysis, and catalase and oxidase activities. At least one colony from each sample was stored in glycerol broth (10% [vol/vol] glycerol in 1% [wt/vol] proteose peptone) at −80°C for subsequent molecular subtyping.
Molecular subtyping.
All the strains isolated from the scald tanks and defeathering machines were analyzed for all flocks. One or two colonies were analyzed per positive cecal sample for flocks 1 to 3 and flocks 4 and 5, respectively. One colony per positive carcass wash cultured directly or following enrichment was also analyzed.
PCR-RFLP of the flaA and flaB genes (fla typing) was performed according to the technique of Ayling et al. (1) except that two separate digestion reactions were carried out using the restriction enzymes DdeI and HinfI. The fla types were designated numerically based on profile patterns obtained for DdeI and HinfI (i.e., strains of fla type 1.1 have profile number 1 for DdeI and profile number 1 for HinfI). The profile patterns for both enzymes were compared against a database of over 2,000 strains. A selected number of isolates were also typed by PFGE using the enzymes SmaI and KpnI (6).
RESULTS
A limited number of genotypically distinct campylobacter subtypes were isolated from the ceca of each campylobacter-positive flock investigated (Table 1); one subtype from flocks 2 and 5, two subtypes from flocks 3 and 4, and three subtypes from flock 1. The subtypes isolated from each flock were unique except for flocks 2 and 3. fla type 1.1 was isolated from the ceca of both these flocks. In the case of mixed colonization generally, one subtype dominated the ceca in birds from each flock.
TABLE 1.
Genotype of campylobacter isolates and colonies recovered from ceca, carcasses, and poultry house during processing of campylobacter-positive flocks
| Sample |
fla types detected (no. of isolates positive/no. tested)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Flock 1, enriched | Flock 2
|
Flock 3
|
Flock 4
|
Flock 5
|
|||||
| Direct | Enriched | Direct | Enriched | Direct | Enriched | Direct | Enriched | ||
| Ceca | 1.13 (1/8) | NDa | 1.1 (4/4) | ND | 1.1 (8/9) | ND | 2.5 (14/18) | ND | 1.7 (20/20) |
| 1.4 (5/8) | 3.7 (1/9) | 1.12 (4/18) | |||||||
| 1.6 (2/8) | |||||||||
| Crates, postload | ND | ND | 1.1 (2/2) | ND | 3.7 (2/5) | ND | 1.14 (2/4) | ND | 2.5 (5/5) |
| 2.5 (2/5) | 2.5 (2/4) | ||||||||
| 1.1 (1/5) | |||||||||
| Postslaughter | 1.13 (4/4) | 2.5 (1/2) | 2.5 (1/1) | 1.1 (2/5) | 1.1 (4/5) | 2.5 (1/5) | 2.5 (5/5) | 1.7 (5/5) | 2.5 (5/5) |
| 1.12 (1/2) | 1.14 (2/5) | 1.14 (1/5) | 1.12 (4/5) | ||||||
| 2.7 (1/5) | |||||||||
| Postdefeathering | 1.13 (3/3) | 2.5 (1/2) | 2.5 (1/1) | 1.1 (5/5) | 1.1 (5/5) | 2.5 (2/5) | 2.5 (5/5) | 1.7 (5/5) | 2.5 (5/5) |
| 1.12 (1/2) | 1.12 (3/5) | ||||||||
| Postevisceration | 1.13 (5/5) | 1.12 (2/2) | 1.1 (5/5) | 1.1 (4/5) | 2.5 (4/5) | 2.5 (5/5) | 2.5 (5/5) | 2.5 (5/5) | |
| 1.7 (1/5) | 1.12 (1/5) | ||||||||
| Postchilling | 1.3 (4/4) | 1.12 (2/2) | 2.5 (2/2) | 1.1 (1/5) | 1.1 (5/5) | 2.5 (3/5) | 2.5 (5/5) | 1.7 (3/5) | |
| 1.14 (1/5) | 1.12 (2/5) | 2.5 (2/5) | 2.5 (5/5) | ||||||
| 3.7 (3/5) | |||||||||
| Plucking machine | 1.13 (1/1) | ND | ND | ND | ND | ND | 2.5 (3/3) | ND | 1.12 (1/4) |
| 2.5 (2/4) | |||||||||
| 1.6 (1/4) | |||||||||
| Scald tank | 1.13 (1/1) | ND | 1.12 (1/1) | ND | 1.1 (1/1) | ND | 2.5 (4/4) | 1.7 (4/4) | |
ND, not done.
In four of the five campylobacter-positive flocks sampled, at least one of the subtypes colonizing the ceca was recovered from the carcass washings postslaughter. The exception was flock 2, which appeared to be colonized by only one subtype (fla type 1.1), and this subtype was never recovered from any postslaughter carcass.
It was clear that the fla types of strains recovered from the carcass washings were influenced by the use of enrichment or direct plating cultural techniques. For example, in flock 5, fla type 1.7 was isolated from the postslaughter carcasses by direct culture but not following enrichment. However, this subtype was found in the ceca using the enrichment techniques. Because of these observations, comparisons of subtypes from carcasses were made with all the subtypes observed from each sample regardless of the recovery technique used.
Subtypes that were the identical fla type as isolated from the ceca were also recovered from the scald water in four of five flocks and from the abattoir processing machinery in two of three flocks. Occasionally, however, subtypes in addition to those observed in the ceca contaminated the carcasses and the machinery.
In most flocks, changes in fla type distribution occurred during processing. Such changes were most evident after chilling. For example, in flock 1, the minor subtype isolated from the ceca (fla type 1.13), contaminated the carcasses right through processing until postchilling. Only strains of fla type 1.3 were found on the chilled carcasses. This difference in subtypes recovered was confirmed by PFGE.
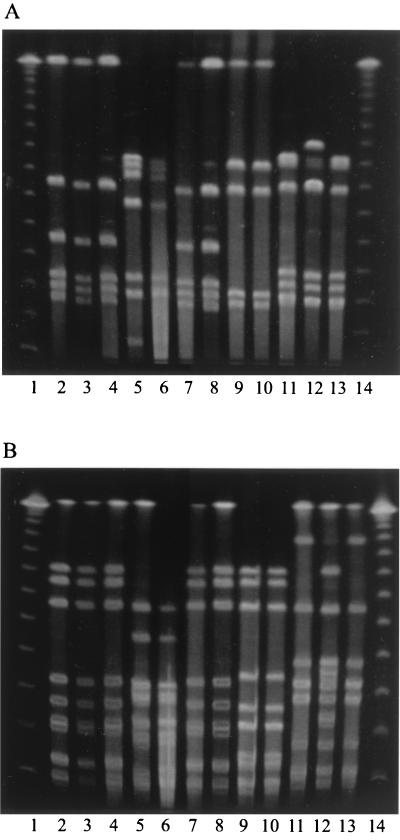
On two occasions, two sequential flocks in the abattoir were sampled (flocks 2 and 3 and flocks 4 and 5). As flocks 2 and 3 were colonized with strains of the same fla type (1.1), it was not possible to determine by this typing method whether any cross-contamination from flock 2 to flock 3 occurred. However, by PFGE using SmaI and KpnI, the strains of fla type 1.1 from these two flocks were distinctly different, and there was no evidence of carryover from flock 2 to carcasses of flock 3. Flocks 4 and 5 were colonized with strains of quite different fla types (Fig. 1). The dominant subtype isolated from the first flock (flock 4) contaminated areas of the abattoir, including the crate washing section and the plucking machine, prior to processing of the subsequent flock (flock 5). The carcasses of this second flock were contaminated with this subtype. However, campylobacters recovered from the scald tank were of the same fla type as those isolated from the second flock only.
FIG. 1.
PFGE profiles of DNA cut with SmaI (A) and KpnI (B) from representative strains from flocks 4 and 5. Isolates with fla type 2.5 (lanes 7 and 8) and fla type 1.12 from the ceca and carcasses of birds in flock 4 (lanes 9 and 10), isolates with fla type 1.7 from the ceca and carcasses of birds in flock 5 (lanes 11 to 13), isolates of fla type 2.5 (lanes 2 and 4) and fla type 1.14 (lanes 5 and 6) from the crates in which birds from flock 4 were transported, and isolates of fla type 2.5 from the crates, after washing, in which birds from flock 5 were transported (lane 3). Lanes 1 and 14, λ size ladder.
Two flocks (flocks 6 and 7) from a consistently campylobacter-negative farm were sampled 7 days before slaughter. Both of the flocks were campylobacter negative at that point. The ceca from birds of both flocks were also culture negative at slaughter (Table 2). In flock 7, strains of one fla type (9.18) dominated the carcasses at all stages postslaughter and heavily contaminated the carcasses postchilling. This subtype was recovered from the crates postloading and was even recovered from the crate wash following unloading of the birds at the abattoir. This subtype was also recovered, albeit as a minority population, from these crates before and after transportation to the farm prior to chicken collection.
TABLE 2.
fla types of strains isolated from the ceca, carcasses, and crates of Campylobacter-negative flocks
| Sample |
fla types (no. of isolates positive/no. tested)
|
|
|---|---|---|
| Flock 6 | Flock 7 | |
| Ceca | Negative | Negative |
| Postslaughter | 1.13 (7/17) | 9.18 (4/4) |
| 1.1 (5/17) | ||
| 7.8 (5, 17) | ||
| Postdefeathering | 1.13 (1/4) | 9.18 (1/1) |
| 1.18 (3/4) | ||
| Postevisceration | 7.18 (5/5) | 9.18 (4/4) |
| Postchilling | Negative | 9.18 (10/11) |
| 3.14 (1/11) | ||
| Crates on arrival at farm | NDa | 10.19 (6/10) |
| 4.4 (3/10) | ||
| 9.18 (1/10) | ||
| Crates on arrival at abattoir | 1.11 (2/21) | 9.18 (13/13) |
| 1.12 (14/21) | ||
| 1.6 (2/21) | ||
| 7.18 (1/21) | ||
| 6.2 (1/21) | ||
| 1.1 (1/21) | ||
| Crate wash | ND | 4.4 (2/5) |
| 9.18 (3/5) | ||
ND, not done.
The cecal contents of birds from flock 6 were also negative. The carcasses of this flock became contaminated during processing with campylobacters of four distinct fla types. Only 2 of 21 isolates analyzed from the transport crates at postload had fla types (7.18 and 1.1) the same as those recovered from the carcasses immediately postslaughter. An additional four fla types isolated from the crates were not detected in carcass rinses.
DISCUSSION
Campylobacteriosis is a significant food-borne disease worldwide (14). The major source of infection is thought to be poultry meat contaminated from the intestinal contents of asymptomatically colonized chickens. To date, the development of intervention strategies to control this infection have largely focused at the farm level (11). Unfortunately, these strategies have been largely ineffective.
Little is known about the fate of campylobacter populations following chicken slaughter. All the microbiological evidence indicates that campylobacters cannot grow naturally outside the host gut environment. As campylobacters are relatively susceptible to stresses such as heat and atmospheric oxygen, the numbers of viable organisms should therefore decline in any environmental situation. This is supported by evidence that the total bacterial burden on poultry carcasses declines during processing (2, 9). Jacobs-Reitsma (8) has suggested that improved procedures might further reduce campylobacter contamination during poultry processing. Such procedures could include the use of counterflow multistage water systems during scalding, the decontamination of equipment, and the “disinfection” of carcasses with antibacterial agents. In addition, the slaughter and processing of known campylobacter-free flocks before any campylobacter-positive flocks was recommended.
C. jejuni comprises an extremely diverse population. This diversity is apparent using both phenotypic and genotypic characteristics and is reflected in a broad spectrum of subtypes (16). The population of campylobacters colonizing broilers comprises a large number of subtypes detectable using genetic methods such as flatyping (1). During processing of poultry carcasses, contaminating campylobacters experience a range of stresses, including rapid changes in temperature, osmolarity, oxygen tension, and nutrient deprivation, all of which can affect bacterial viability. A bacterium's ability to detect, respond to, and survive the variety of environmental stresses experienced during meat processing is dependent on the presence and expression of a number of stress response genes. Such differential properties may well be subtype specific and consequently may affect the diversity of the campylobacter population contaminating poultry carcasses at retail. To investigate this hypothesis, changes in the subtypes of campylobacter isolates recovered from carcasses throughout the processing of seven poultry flocks were identified.
Previous studies have shown that, at least in the United Kingdom, individual poultry flocks, when positive, are colonized by a limited number of campylobacter subtypes (1). These observations were confirmed in this study. In all the positive flocks investigated, three or fewer subtypes were detected. Because the number of clones subtyped per flock was limited, it is possible that other, possibly minor, subtypes were also present. However, in some of the flocks investigated in other studies (unpublished data), up to 150 colonies were tested from 20 birds. In these cases the types detected from such extensive sampling reflected those observed using the smaller sampling regimens. It is also clear from these studies that the method of recovery can influence the subtypes of strains observed. In our studies, two recovery techniques were used, direct plating and prior enrichment. Enrichment is considered the optimal method for recovery of campylobacters under stress conditions, such as from poultry carcasses. However, in several cases strain types were recoverable by direct plating from the carcasses but were not observed by enrichment, suggesting that enrichment preferentially selects some strains. This selection was not so obvious in the cultures from cecal contents, presumably because the organisms were present in larger numbers and in a relatively unstressed state. This observation raises the issue of choice of recovery technique for campylobacter strains for molecular epidemiological purposes. Our results suggest that further investigations are required but that multiple techniques may be necessary to obtain a clear picture of the subtypes present in any mixed campylobacter population.
Sampling during processing confirmed that the entrance of a positive flock resulted in contamination of the abattoir environment, including the scald tank and defeathering equipment. Campylobacters from the ceca of the birds also contaminated the carcasses. However, interestingly, some subtypes survived all the processing stages, while others apparently survived only to the chilling stage. Finally, some subtypes were never recovered from the carcasses despite being present in the ceca. This suggests that there is a considerable differential in the ability of campylobacter strains to survive different environmental stresses. Such possibilities need to be tested using larger numbers of colonies per flock and extending the work to in vitro models of survival.
From the subtyping of strains from flocks 4 and 5, it appears that some campylobacter fla types (in this case fla type 2.5) are so robust that they can carry over onto the carcasses of subsequent flocks. This observation was confirmed by using PFGE. This particular subtype caused a heavy and persistent contamination of the abattoir environment, including the crate washing area. This strain was also identified as the causative agent in an accidentally acquired case of campylobacteriosis during the visit to the abattoir in a member of the sampling team (Toszeghy et al., submitted for publication).
As mentioned previously (8), it has been suggested that the “logistical” slaughtering of campylobacter-negative flocks at the beginning of the day may reduce carcass contamination. It is clear from our study and those of others (10) that some campylobacter-negative flocks reach the abattoir. The results from this study demonstrate that the carcasses of such birds can quickly become contaminated during processing by various subtypes of campylobacter. However, as before, only some of these contaminating strains survived abattoir processing. The absence of campylobacters recoverable from the chilled carcasses of one of the campylobacter-negative flocks may reflect a relatively low exposure during the processing stages. Alternatively environmentally stressed campylobacters from previous flocks may have survived poorly on or adhered poorly to the poultry carcasses. In the two negative flocks studied, at least two of the subtypes contaminating the carcasses were isolated from the crates in which the birds were collected for transportation. In one case this crate contamination was identified as the crates reached the farm and before they were loaded with the birds. This is the first substantial evidence that contaminated crates entering the poultry farms constitute a source of contamination on poultry carcasses following processing. Crate washing to remove campylobacter contamination is well recognized as being largely ineffectual. The use of high-power hoses or the reuse of water from other parts of the abattoir ensures widespread distribution rather than reduction of organisms. Alternative decontamination procedures or the use of disposable crate linings must be seriously considered if negative carcasses are to be obtained from negative flocks.
The results from this work have clearly shown the benefits of molecular typing, in particular fla typing, in investigating the sources in the abattoir environment and survival of campylobacter contamination on poultry carcasses. Unfortunately, such subtyping techniques are both expensive and time-consuming. This constrains the number of isolates which can be reasonably analyzed from each sample and consequently may influence some of the conclusions drawn. Nevertheless, similar observations have been made in recent complementary studies elsewhere in the United Kingdom (J. Slater and T. J. Humphrey, unpublished observations). Future studies focusing on individual flocks and a greater depth of isolate analysis per sample will be undertaken to confirm these conclusions.
In particular, this study has shown that campylobacter strains, both those colonizing the poultry gut and those present in the abattoir environment, vary in their capacity to survive during processing, presumably as a consequence of differing abilities to adapt to environmental stresses. Comparison of the phenotypic and genotypic properties of these strains will open the way to investigation of the mechanisms of survival in campylobacters using in vitro modeling methods. Strain differences, if associated with virulence properties, could have significant implications for assessing the risk of human campylobacteriosis. Furthermore, the deliberate colonization of chickens with strains highly susceptible to environmental stresses may competitively exclude the environmentally persistent types and prevent contamination of poultry carcasses with more persistent and therefore potentially hazardous strains.
ACKNOWLEDGMENTS
This work was funded by a grant from the Ministry of Agriculture, Fisheries and Food, Great Britain (FS3303).
We thank Anne Ridley for help and advice in parts of this study.
REFERENCES
- 1.Ayling R D, Woodward M J, Evans S, Newell D G. Restriction fragment length polymorphism of polymerase chain reaction products applied to the differentiation of poultry campylobacters for epidemiological investigations. Res Vet Sci. 1996;60:168–172. doi: 10.1016/s0034-5288(96)90013-2. [DOI] [PubMed] [Google Scholar]
- 2.Berndtson E, Tivemo M, Engvall A. Distribution and numbers of Campylobacter in newly slaughtered broiler chickens and hens. Int J Food Microbiol. 1992;15:45–50. doi: 10.1016/0168-1605(92)90134-o. [DOI] [PubMed] [Google Scholar]
- 3.Bolton F J, Wareing D R A, Skirrow M B, Hutchingson D N. Identification and biotyping of campylobacters. In: Board G R, Jones D, Skinner F A, editors. Identification methods in applied and environmental microbiology. Society for Applied Microbiology Technical Series No. 29. Oxford, U.K: Blackwell Scientific Publications; 1992. pp. 151–161. [Google Scholar]
- 4.Cawthraw S A, Wassenaar T M, Ayling R, Newell D G. Increased colonization potential of Campylobacter jejuni strain 81116 after passage through chickens and its implification on the rate of transmission within flocks. Epidemiol Infect. 1996;117:213–215. doi: 10.1017/s0950268800001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans S J. Epidemiological studies of Salmonella and Campylobacter in poultry. Ph.D. thesis. London, U.K: London University; 1997. [Google Scholar]
- 6.Gibson J, Lorenz E, Owen R J. Lineages within Campylobacter jejuni defined by numerical analysis of pulsed-field gel electrophoretic DNA profiles. J Med Microbiol. 1997;46:157–163. doi: 10.1099/00222615-46-2-157. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey T J, Martin K W, Mason M J. Isolation of Campylobacter species from non-clinical samples. PHLS Microbiol Dig. 1998;13:86–88. [Google Scholar]
- 8.Jacobs-Reitsma W. Campylobacter in the food supply. In: Nachamkin I, Blaser M J, editors. Campylobacter. 2nd ed. Washington, D.C.: ASM Press; 2000. pp. 467–481. [Google Scholar]
- 9.Mead G C, Hudson W R, Hinton M H. Effect of changes in processing to improve hygiene control on contamination of poultry carcasses with campylobacter. Epidemiol Infect. 1995;115:495–500. doi: 10.1017/s0950268800058659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newell D G, Frost J A, Duim B, Wagenaar J A, Madden R H, van der Plas J, On S L W. New developments in the subtyping of Campylobacter species. In: Nachamkin I, Blaser M J, editors. Campylobacter. 2nd ed. Washington, D.C.: ASM Press; 2000. pp. 27–44. [Google Scholar]
- 11.Newell D G, Wagenaar J A. Poultry infections and their control at the farm level. In: Nachamkin I, Blaser M J, editors. Campylobacter. 2nd ed. Washington, D.C.: ASM Press; 2000. pp. 497–509. [Google Scholar]
- 12.Simonsen B. Methods for determining the microbial counts of ready-to-cook poultry. World Poult Sci J. 1971;27:368. [Google Scholar]
- 13.Skirrow M B. Campylobacter enteritis: a “new” disease. Br Med J. 1977;2:9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 15.Tompkins D S, Hudson M J, Smith H, Brett M M, Owen R J, Brazier J S, Cumberland P, King V, Cook P E. A study of intestinal infectious disease in England: microbiological findings in cases and controls. Commun Dis Public Health. 1999;2:108–113. [PubMed] [Google Scholar]
- 16.Wassenaar T M, Newell D G. Genotyping of Campylobacter spp. Appl Environ Microbiol. 2000;66:1–9. doi: 10.1128/aem.66.1.1-9.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]