Abstract
The association between the use of vitamin K antagonists (VKAs) and cancer risk reduction remains unclear. We aimed to assess the association between the use of VKAs or direct oral anticoagulants (DOACs) and the incidence of cancer in a large cohort of patients with atrial fibrillation (AF) by means of a population‐based, propensity‐weighted cohort study using population‐wide databases including patients diagnosed with nonvalvular AF (NVAF) followed for up of 5 years (median 2.94 years). We created two cohorts based on the initiation therapy (VKA or DOAC). Initiation with VKA or DOAC was defined as filling a prescription with no previous exposure in the preceding 12 months. Cancer diagnoses of any type and for specific tumors (lung, colon, prostate, bladder, and breast). We included 39,989 patients, 31,200 (78.0%) in the VKA cohort. Incidence rate for any cancer was 12.45 per 1,000 person‐year in the DOAC cohort vs. 14.55 in the VKA cohort (adjusted hazard ratio (HR): 1.16, 95% confidence interval (CI): 1.02–1.32). In secondary outcomes, no differences were found for specific types of cancer, such as lung (HR: 1.28, CI: 0.89–1.83), colon (HR: 0.84, CI: 0.62–1.13), prostate (HR: 1.40, CI: 0.94–2.10), bladder (HR: 1.07, CI: 0.76–1.52), and breast (HR: 1.05, CI: 0.66–1.69). Sensitivity analyses yielded similar results. Subgroup analyses also produced consistent findings, except for men, for whom VKA was associated with a lower risk of colon cancer (HR: 0.68, 95% CI: 0.48–0.96). Our results do not confirm a chemoprotective effect of VKA when compared with DOAC in a large, real‐world cohort of patients with NVAF followed for up to 5 years.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Observational evidence with regard to the potential chemoprotective effect of vitamin K antagonists (VKAs) is unclear and may be affected by several confounding biases.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Whether patients with atrial fibrillation (AF) treated with VKA are protected against cancer when compared with patients treated with non‐VKA (direct oral anticoagulant (DOAC)) agents.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ In this population‐based, propensity‐weighted cohort study comprising 39,989 patients with AF that initiated oral anticoagulant treatment followed for up to 5 years, we found no risk reduction of cancer incidence in patients using VKA compared with DOAC users. Several sensitivity and subgroup analyses produced comparable results.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Our findings do not confirm a chemoprotective effect of VKA as a protective factor against cancer when compared with DOAC in patients with AF.
Vitamin K antagonists (VKAs), such as warfarin, phenprocoumon, or acenocoumarin (the most prescribed VKA in Spain), are traditionally used as oral anticoagulant (OAC) agents and are widely prescribed worldwide, mainly for atrial fibrillation (AF), valvular heart disease, and venous thromboembolism. In the last decade, non‐VKA oral anticoagulants, namely dabigatran, rivaroxaban, apixaban, and edoxaban, that directly inhibit thrombin or factor‐X have been commercialized (direct oral anticoagulants (DOACs)). In several clinical trials and observational studies, DOAC appears to be at least as effective and safe to VKA, 1 , 2 , 3 with some advantages and disadvantages. 4 They have rapidly replaced VKA in their approved indications and, currently, DOAC is the treatment of choice for patients with nonvalvular AF (NVAF) in most developed countries.
Different in vitro and animal experimental models have proposed the antitumorigenic potential of VKA. 5 , 6 , 7 A clear molecular mechanism by which VKA would affect cancer development has not been identified yet, but it has been postulated that VKA inhibits the AXL receptor signaling (a vitamin K‐dependent receptor of the tyrosine‐kinase family associated with immunity and cancer), consequently enhancing natural killer cell antitumor activity. 7 In humans, the evidence of VKA antitumorigenic effect remains inconclusive. Some studies suggest the existence of a generalized antitumor effect 8 , 9 others find limited effects in some types of tumors (essentially prostate cancer), 10 , 11 , 12 whereas others do not find a relationship between VKA exposure cancer risk reduction 13 , 14 , 15 (including prostate cancer 16 , 17 , 18 ), or even suggest an increased risk of some cancer types. 19 Clinical trials and observational studies are heterogeneous regarding study populations or time of VKA exposure, or are affected by insufficient sample sizes or common biases present in causal observational studies, such as confounding by selection, immortal‐time bias, lack of active comparators, and inadequate adjustment of potential confounding. Two recent meta‐analyses provide an accurate picture of this heterogeneity and of the reported opposite conclusions about VKA effectiveness, notably regarding prostate cancer risk reduction. 17 , 20
In this context, and given that OAC therapy can entail severe risks, the clinical use of VKA as a cancer preventive therapy cannot be recommended, until well‐designed and adequately powered randomized clinical trials confirm its efficacy and its risk‐benefit balance. Nevertheless, well‐designed real‐world data studies can provide relevant information about the antitumorigenic effect of VKA when compared with other OAC agents, and this evidence may be useful for guidance on treatment choice (VKA vs. DOAC) in patients requiring oral anticoagulation.
Our aim was to examine the association between VKA or DOAC use and cancer incidence, overall and for some specific cancers, in a large, population‐based cohort of patients with NVAF that initiate OAC treatment.
METHODS
Study design
This population‐based retrospective cohort included all patients aged 40 years and over with NVAF or atrial flutter who initiated oral anticoagulant therapy (with either a VKA or a DOAC) from November 1, 2011, to December 31, 2015. Patients were followed up to December 31, 2016.
Data sources
Data were obtained from the VHS Integrated Databases (VID). The VID is the result of the linkage, by means of a single personal identification number, of a set of publicly owned, population‐based healthcare, clinical and administrative electronic databases in Valencia, which has provided comprehensive information for the region’s 5 million inhabitants since 2008. It includes sociodemographic and administrative data (sex, age, and nationality) as well as healthcare information, such as diagnoses, procedures, laboratory data, pharmaceutical prescriptions, and dispensing (including brand and generic name, formulation, strength, and dosing schedule/regimen), hospitalizations, mortality, healthcare utilization, and public health data. Additionally, the VID includes a set of specific associated databases with population‐wide information on significant care areas, such as rare diseases, vaccines, imaging data, or the regional Cancer Information System, from which information on cancer incidence was retrieved. 21 , 22
Setting
The study was conducted in the region of Valencia, namely in the Valencia Health System (VHS), an extensive network of public hospitals and primary healthcare centers, which is part of the Spanish National Health System, 23 funded and mostly provided by the Valencia Regional Government, free at the point of care (except for some co‐payments for out‐of‐hospital medication), and almost universal, covering about 97% of the region’s population (~ 5 million inhabitants, equivalent to 10% of the Spanish population or 1% of the European population).
Population and inclusion/exclusion criteria
All patients aged 40 years and over with a diagnosis of NVAF or atrial flutter, according to the diagnosis code of the corresponding version of the International Classification of Diseases Clinical Modification (ICD‐9‐CM: 427.31; ICD‐10‐ES: I48) who initiated therapy for stroke prevention in NVAF with an OAC medication (warfarin, acenocoumarin, dabigatran, rivaroxaban, or apixaban) from November 1, 2011 (date of market launch of the first DOAC for NVAF in Spain) to December 31, 2015, were initially included, and follow‐up was available up to the date of data extraction (December 31, 2016). Patients without anticoagulant treatment for stroke prevention in NVAF in the 12 months preceding the index prescription (index date) were defined as therapy initiators (naïve patients), and thus were included in the study; non‐naïve patients were excluded. People without pharmaceutical/health coverage by the VHS (mainly some Spanish central government employees whose prescriptions are reimbursed by civil service insurance mutualities, and thus not included in the pharmacy databases of the VHS), and patients not registered in the municipal census (non‐residents or temporary residents) were excluded due to limitations on follow‐up. Other exclusion criteria were age under 40 years old, presence of valvular disease (in Spain, DOACs are not licensed to treat valvular heart disease) or presence of a cancer diagnosis in the 24 months before the index date (see Table S1 for codes used). Finally, patients were divided into two groups according to the choice of initial prescription, resulting in the VKA and DOAC cohorts (see Figure 1 Flowchart).
Figure 1.
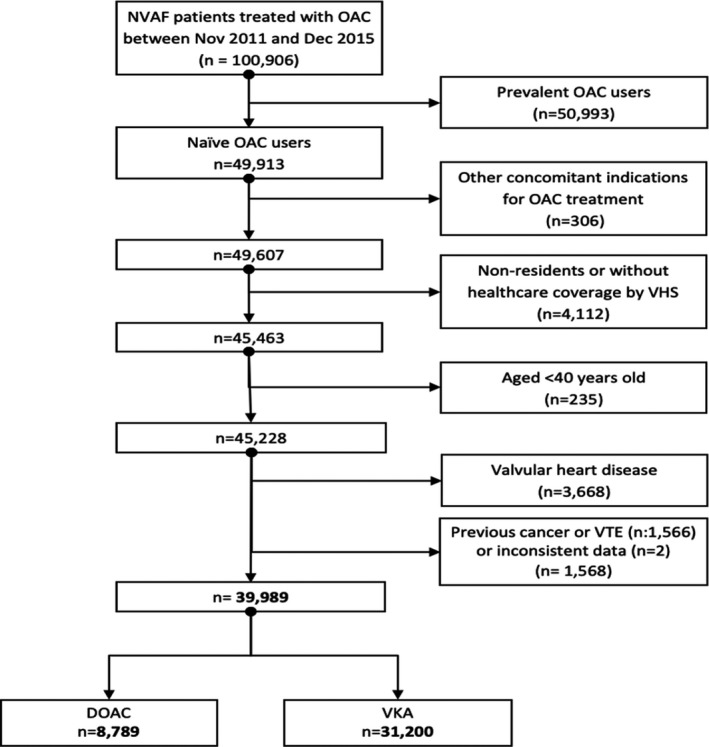
Study population. DOAC, direct oral anticoagulant; NVAF, nonvalvular atrial fibrillation; OAC, oral anticoagulant; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Main end point
A combined end point of diagnosis of any incident malignant neoplasm during the follow‐up period, except nonmelanoma skin malignancy. See Table S2 for codes used.
Secondary end points
Diagnosis of the types of cancers with a higher observed incidence during that follow‐up period in the region include: lung, colon, prostate, bladder, and breast cancer. 24 See Table S2 for codes used.
Follow‐up
Follow‐up of the cohorts for the identification of end points started at the index date and lasted until censoring or the end of the follow‐up period. In the main intention‐to‐treat analyses, follow‐up was censored in the occurrence of any incident cancer diagnosis included in our primary and secondary end points, death or loss of coverage (individuals leaving the region, mainly). In secondary per‐protocol analysis, we included all patients with at least 150 days covered with medication over a period of 180 days or less since therapy initiation. Patients who discontinued or switched were followed up (in their original group in the case of switching) for up to 12 months from the date of discontinuation or switching. Discontinuation was defined as having at least 60 days consecutive days without medication; switching was defined as discontinuation of VKA treatment and initiation of DOAC treatment lasting for at least 2 months, or vice versa. Covariates
We used a 12‐month look‐back period since the index date to define the baseline sociodemographic, clinical, and lifestyle characteristics of the population. Sociodemographic data included age, sex, country of origin, vulnerability status, and income. Cancer risk factors included chronic obstructive pulmonary disease, inflammatory bowel disease, rheumatologic diseases, previous organ transplantation, gastritis, polyps, and lifestyle factors, such as tobacco and alcohol use. Other clinical conditions included were congestive heart failure, hypertension, diabetes, liver and renal disease, previous ischemic stroke or transient ischemic attack, coronary artery disease, gastrointestinal bleeding, venous thromboembolism or pulmonary embolism (VTE‐PE), dementia, and depression (see Table S3 for codes used).
Ethics
The study protocol was classified by the Spanish Drugs and Medical Devices Agency as a “Post‐authorization study with designs other than prospective follow‐up” (Ref. FIS‐ACO‐2018‐01) and received ethics approval by the Research Ethics Committee of the “Hospital Clínico‐Universitario de Valencia” (Ref. F‐CE‐GEva −14 v1.2; October 26, 2018). As usual in anonymized real‐world data studies, patient consent was waived. According to the EU General Data Protection Regulation and Spanish law, data accessed by researchers rely on pseudo‐anonymized, non‐traceable codes that do not allow the identification of individual patients.
Statistical analysis
We used an intention‐to‐treat approach for our main analyses. First, we described the characteristics of the cohorts (means for continuous variables and frequencies for categorical variables). Second, we performed a time‐to‐event analysis from the data of therapy initiation to the first incident cancer or censoring event. Third, we used a stepwise Cox proportional hazards regression with an entry and removal alpha significance level of 0.2 and 0.1, respectively, using DOAC as the primary reference, to assess the risk of cancer in the VKA cohort compared with the DOAC cohort. To deal with potential confounding by indication, we used an inverse probability treatment‐weighting estimation (IPTW). Stabilized weights were derived to obtain estimates representing population average treatment effects. 25 , 26 The underlying propensity models were estimated using a logistic regression analysis and including all available covariates (see Table S3 ) to minimize confounding. Covariate balance between the weighted exposure cohorts was assessed using standardized mean differences, with standardized differences < 0.10 suggesting adequate balance. 27 Incidence rates by 1,000 person‐years in both cohorts as well as crude and adjusted hazard ratios (HRs) for risk of cancer in VKA patients when compared with DOAC patients were presented as main results. We also plotted Kaplan–Meier survival curves for the primary and secondary end points over the observation window of 5 years. We then carried on several secondary, sensitivity analyses. Fourth, we performed an additional IPTW analysis after weight truncation (all weights with value below the 2.5th percentile and above the 97.5th percentile were set equal to the values of the 2.5th and 97.5th percentiles). Fifth, we conducted a competing risk regression analysis (using the Fine and Gray method) with mortality handled as a competing event. Sixth, we performed per‐protocol analyses, using IPTW and IPTW with truncation. Finally, we analyzed associations stratified by gender and for specific subgroups, such as patients with AF (excluding patients with flutter), patients 65 years and over, and patients initiating from August 1, 2013 (date of market launch of apixaban for NVAF in Spain; date from which all drugs under assessment in this study were available for prescription).
RESULTS
After exclusion criteria, we identified a study population of 39,989 patients, 31,200 (78.0%) in the VKA cohort and 8,789 in the DOAC cohort. Patients starting treatment with VKA were older than DOAC initiators (75.4 years old vs. 74.4, P < 0.001), with a higher proportion of women (48.3% vs. 46.8%, P < 0.001), of those born in Spain (91.3% vs. 88.2%, P < 0.001) and of people with low income (76.9% vs. 71.6% of patients earning < 18,000 euros/year, P < 0.001). They showed a higher prevalence of heart failure (18.5% vs. 15.7%, P < 0.05), hypertension (80.4% vs. 77.1%, P < 0.05), diabetes (35.9% vs. 31.4%, P < 0.001), renal failure (13.8% vs. 9.6%, P < 0.001), VTE‐PE (7.5% vs. 5.2%, P < 0.001), and previous organ transplantation (0.6% vs. 0.2%, P < 0.001), but less inflammatory bowel disease (0.6% in the VKA cohort vs. 0.9% in the DOAC cohort, P = 0.005), coronary disease (18.1% vs. 18.5%, P < 0.001), previous stroke or transient ischemic attack (20% vs. 21.8%, P < 0.001), and dementia (6.8% vs. 8.2%, P < 0.001). After inverse probability weighting, the standardized differences were < 0.2 for all covariates, resulting in a cohort with a comparable distribution of baseline covariates between groups (see Table 1 ). Propensity score weight distribution is available at Supplementary Material Figure S4 . The median follow‐up time was 2.94 years and was similar although slightly longer in the VKA cohort than in the DOAC cohort (VKA: 3.05 years, interquartile range: 1.96–4.10, DOAC: 2.62 years, interquartile range: 1.68–3.65).
Table 1.
Patients’ characteristics at baseline and weighted characteristics after IPTW
| Patients’ characteristics n = 39,992 | Weighted populations | |||||||
|---|---|---|---|---|---|---|---|---|
|
DOAC n = 8,789 |
VKA n = 31,200 |
DOAC | VKA | Standardized diff. | ||||
| Mean | SE | Mean | SE | Mean | Mean | Before | After | |
| Age (age when first started treatment) | 74.42 | 0.12 | 75.36 | 0.05 | 75.33 | 75.17 | 0.09 | −0.015 |
| N | % | N | % | % | % | |||
| Sex (female) | 4,112 | 46.8 | 15,054 | 48.3 | 48.3 | 48.0 | 0.029 | −0.007 |
| Country | ||||||||
| Africa | 60 | 07 | 160 | 0.5 | 0.6 | 0.5 | −0.022 | −0.015 |
| America | 75 | 0.8 | 213 | 0.7 | 0.8 | 0.7 | −0.020 | −0.017 |
| Spain | 7,755 | 88.2 | 28,488 | 91.3 | 88.5 | 91.2 | 0.102 | 0.089 |
| Europe | 739 | 8.4 | 1,699 | 5.4 | 8.2 | 5.5 | −0.117 | −0.107 |
| Other | 160 | 1.8 | 640 | 2.1 | 1.8 | 2.1 | 0.017 | 0.020 |
| Vulnerability | ||||||||
| No risk | 8,087 | 92.0 | 28,655 | 91.8 | 92 | 91.8 | −0.006 | −0.005 |
| Unemployed | 169 | 1.9 | 560 | 1.8 | 1.7 | 1.9 | −0.009 | 0.014 |
| Irregular foreigner | 2 | 0,02 | 14 | 0.04 | 0 | 0 | 0.012 | 0.013 |
| Without resources | 218 | 2,5 | 772 | 2.5 | 2.5 | 2.5 | 0.000 | −0.004 |
| Undefined | 4 | 0,1 | 11 | 0.04 | 0 | 0 | −0.005 | −0.006 |
| Missing | 309 | 3,5 | 1,188 | 3.8 | 3.7 | 3.7 | 0.016 | 0.000 |
| Income | ||||||||
| < 18,000 | 6,294 | 71,6 | 23,996 | 76.9 | 70.5 | 76.8 | 0.121 | 0.098 |
| 18,000–100,000 | 1,836 | 20,9 | 4,722 | 15.1 | 20 | 15.3 | −0.150 | −0.122 |
| > 100,000 | 75 | 0,9 | 63 | 0.2 | 0.8 | 0.2 | −0.090 | −0.078 |
| Without resources | 309 | 3,5 | 1,211 | 3.9 | 3.6 | 3.9 | 0.019 | 0.016 |
| Unknown | 275 | 3,1 | 1,208 | 3.9 | 3.2 | 3.9 | 0.040 | 0.036 |
| Cancer risk factors | ||||||||
| Smoking | 899 | 10,2 | 3,395 | 10.9 | 10.7 | 10.7 | 0.021 | 0.003 |
| Gastritis | 530 | 6,0 | 1,867 | 6.0 | 6.1 | 6 | −0.002 | −0.002 |
| Intestinal polyps | 146 | 1,7 | 500 | 1.6 | 1.6 | 1.6 | −0.005 | −0.002 |
| COPD | 439 | 5,0 | 1,715 | 5.5 | 5.4 | 5.4 | 0.023 | 0.000 |
| Inflammatory bowel disease | 79 | 0,9 | 191 | 0.6 | 0.7 | 0.7 | −0.032 | 0.002 |
| Alcohol use | 178 | 2,0 | 678 | 2.2 | 2.2 | 2.1 | 0.004 | −0.003 |
| Comorbidities | ||||||||
| Rheumatologic diseases | 193 | 2.2 | 729 | 2.3 | 2.3 | 2.3 | 0.009 | −0.002 |
| Previous organ transplant | 18 | 0.2 | 175 | 0.6 | 0.5 | 0.5 | 0.058 | 0.004 |
| Coronary heart disease | 1,471 | 18.5 | 5,781 | 18.1 | 18.2 | 17.9 | 0.047 | −0.007 |
| VTE‐PE | 458 | 5.2 | 2,330 | 7.5 | 7.0 | 7.0 | 0.093 | −0.002 |
| Gastrointestinal bleeding | 355 | 4.0 | 1,284 | 4.1 | 4.1 | 4.1 | 0.004 | −0.005 |
| Heart failure | 1,382 | 15.7 | 5,756 | 18.5 | 18.2 | 17.9 | 0.072 | −0.009 |
| Hypertension | 6,775 | 77.1 | 25,090 | 80.4 | 79.9 | 79.7 | 0.082 | −0.005 |
| Diabetes | 2,756 | 31.4 | 11,188 | 35.9 | 35.1 | 34.9 | 0.095 | −0.004 |
| Liver disease | 615 | 7.0 | 2,274 | 7.3 | 7.2 | 7.2 | 0.011 | 0.000 |
| Renal disease | 839 | 9.7 | 4,315 | 13.8 | 12.9 | 12.9 | 0.134 | 0.001 |
| Previous stroke or TIA | 1,912 | 21.7 | 6,255 | 20.1 | 20.7 | 20.5 | −0.042 | −0.006 |
| Dementia | 717 | 8.2 | 2,129 | 6.8 | 7.2 | 7.1 | 0.031 | −0.005 |
| Depression | 1,194 | 13.6 | 4,285 | 13.7 | 13.8 | 13.7 | −0.051 | −0.002 |
COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; IPTW, inverse probability treatment‐weighting; TIA, transient ischemic attack; VKA, vitamin K antagonist; VTE‐PE, venous thromboembolism or pulmonary embolism.
In the main analysis and for the primary end point, the incidence rate for any cancer was 12.45 per 1,000 person‐years in the DOAC cohort, vs. 14.55 in the VKA cohort (crude HR: 1.19; adjusted HR: 1.16, 95% confidence interval (CI): 1.02–1.32). For secondary end points, no differences were found for specific types of cancer, such as lung (HR: 1.28, CI: 0.89–1.83), colon (HR: 0.84, CI: 0.62–1.13), prostate (HR: 1.40, CI: 0.94–2.10), bladder (HR: 1.07, CI: 0.76–1.52), and breast (HR: 1.05, CI: 0.66–1.69; see Table 2 and Figure 2 ). Kaplan–Meier survival curves for the main and secondary end points show the trends in the occurrence of events in both groups over time (see Figure 3 ). Secondary sensitivity analyses, including weight truncation, the per protocol approach, and the competing risks analysis yielded similar results (see Table 3 ). In subgroup analyses, men showed a significant reduction in the risk of colon cancer (HR: 0.68; 95% CI: 0.48–0.96). All other differences were nonsignificant for both primary and secondary outcomes (see Table S5 ).
Table 2.
Incidence rates by 1,000 person‐years in both cohorts as well as crude and adjusted HRs and 95% CI) for risk of cancer in VKA patients compared to DOAC patients
| Cancer | Group | Person‐time | Failures | Rate | 95% CI | Crude HR | 95% CI | Adj. HR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | DOAC | 23,051.44 | 287 | 12.45 | 11.09 | 13.98 | 1.19 | 1.05 | 1.35 | 1.16 | 1.02 | 1.32 |
| VKA | 91,751.50 | 1335 | 14.55 | 13.79 | 15.35 | |||||||
| Lung | DOAC | 23,493.70 | 36 | 1.53 | 1.11 | 2.12 | 1.31 | 0.92 | 1.87 | 1.28 | 0.89 | 1.83 |
| VKA | 93,846.79 | 185 | 1.97 | 1.71 | 2.28 | |||||||
| Colon | DOAC | 23,452.44 | 56 | 2.39 | 1.84 | 3.10 | 0.88 | 0.65 | 1.19 | 0.84 | 0.62 | 1.13 |
| VKA | 93,789.97 | 192 | 2.05 | 1.78 | 2.36 | |||||||
| Prostate | DOAC | 12,423.58 | 29 | 2.33 | 1.62 | 3.36 | 1.34 | 0.90 | 1.99 | 1.40 | 0.94 | 2.10 |
| VKA | 48,502.67 | 152 | 3.13 | 2.67 | 3.67 | |||||||
| Bladder | DOAC | 23,480.53 | 40 | 1.70 | 1.25 | 2.32 | 1.10 | 0.78 | 1.56 | 1.07 | 0.76 | 1.52 |
| VKA | 93,815.44 | 172 | 1.83 | 1.58 | 2.13 | |||||||
| Breast | DOAC | 11,054.97 | 22 | 1.99 | 1.31 | 3.02 | 1.06 | 0.67 | 1.68 | 1.05 | 0.66 | 1.69 |
| VKA | 45,197.77 | 95 | 2.10 | 1.72 | 2.57 | |||||||
Adj, adjusted; CI, confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; VKA, vitamin K antagonist.
HR reference group: DOAC cohort.
Figure 2.
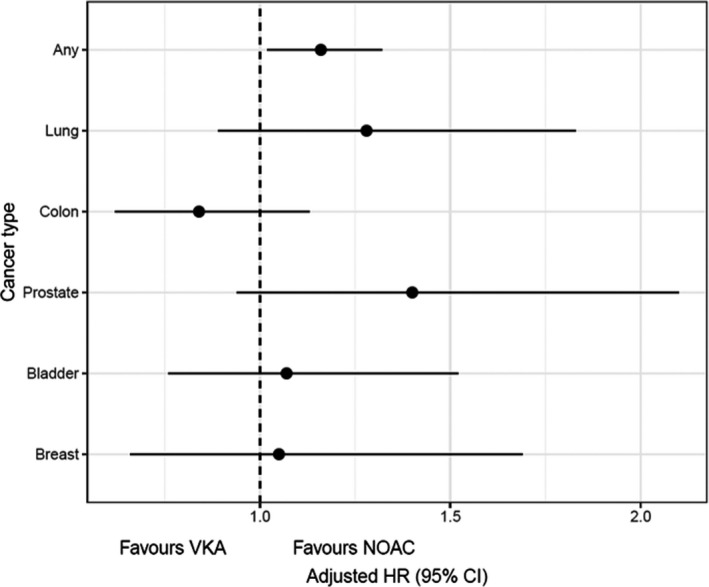
Cancer incidence in VKA initiators vs. DOAC initiators. Adjusted hazard ratios. CI, confidence interval; HR, hazard ratio; NOAC, nonvalvular atrial fibrillation; VKA, vitamin K antagonist.
Figure 3.
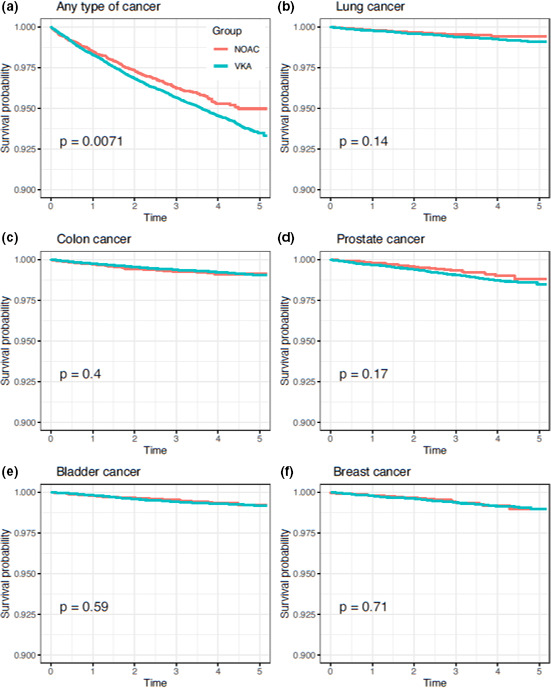
Kaplan Meier survival curves for the primary and secondary end point over the 5‐year observation period. NOAC, nonvalvular atrial fibrillation; VKA, vitamin K antagonist. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 3.
Results of main analysis, main analysis with truncation, and secondary per protocol analyses and competing risk analysis (adjusted HRs and 95% CIs are shown)
| Cancer type | Secondary analyses | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main analysis | Main analysis with truncation | Per protocol analysis | Per protocol with truncation | Competing risks analysis | |||||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||||
| Any | 1.16 | 1.02 | 1.32 | 1.15 | 1.02 | 1.31 | 1.18 | 1.04 | 1.34 | 1.17 | 1.03 | 1.34 | 1.17 | 1.03 | 1.33 |
| Lung | 1.28 | 0.89 | 1.83 | 1.26 | 0.88 | 1.81 | 1.29 | 0.90 | 1.86 | 1.28 | 0.89 | 1.83 | 1.28 | 0.89 | 1.83 |
| Colon | 0.84 | 0.62 | 1.13 | 0.83 | 0.61 | 1.12 | 0.85 | 0.63 | 1.15 | 0.84 | 0.63 | 1.14 | 0.85 | 0.63 | 1.14 |
| Prostate | 1.40 | 0.94 | 2.10 | 1.37 | 0.92 | 2.05 | 1.42 | 0.95 | 2.10 | 1.40 | 0.94 | 2.09 | 0.85 | 0.57 | 1.26 |
| Bladder | 1.07 | 0.76 | 1.52 | 1.06 | 0.75 | 1.50 | 1.09 | 0.77 | 1.54 | 1.08 | 0.76 | 1.53 | 1.08 | 0.76 | 1.53 |
| Breast | 1.05 | 0.66 | 1.69 | 1.06 | 0.66 | 1.69 | 1.09 | 0.68 | 1.73 | 1.11 | 0.69 | 1.76 | 0.87 | 0.40 | 1.92 |
Patients included in per protocol analyses: 8,387 in the DOAC cohort, 22,813 in the VKA cohort Median follow‐up in per‐protocol analyses: 2.62 years in the DOAC cohort (IQR: 1.72–3.62), 3.02 in the VKA cohort (IQR: 1.98–4.05).
CI, confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; IQR, interquartile range; VKA, vitamin K antagonist.
DISCUSSION
In this large, population‐base study comparing the risk of cancer incidence in patients with NVAF initiating treatment with either VKA or DOAC and followed up for up to 5 years with a median follow‐up of almost 3 years, we could not ascertain a chemoprotective effect of VKA when compared with DOAC. In fact, we found a slight increase in the overall risk of cancer in VKA users when compared with DOAC users; however, and despite adjusting for differences between groups, this increased risk could be explained, at least partially, by remaining unmeasured confounding. In any case, our results do not support the alleged antitumorigenic effect of VKA mediated by vitamin K‐dependent factors. Potential mechanisms for an antitumor effect of vitamin K (and, consequently, a possible tumorigenic effect of VKA) have been proposed and advocated for refs. 8, 28; however, in the light of our findings, consideration should be given to the possibility that uncontrolled confounding may be impacting observational findings, despite the use of large cohorts and adjustment techniques.
A diversity of experimental and observational studies carried on in different settings have assessed the association between VKA use and cancer, showing a marked heterogeneity in designs used, populations included, and directionality of results found, leading to conflicting conclusions. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 Starting from this point, we took a series of different methodological decisions to overcome the main weaknesses of previous studies. First, by using a “new‐user” active comparator design, we aimed to obtain two groups of patients who newly initiate medication. In this way, we avoid the comparison of patients with intrinsically discrepant risk profiles, as happens when using a nonuser comparator (for instance, VKA vs. the general population) or when comparing new to prevalent users. By doing so, some specific biases, such as immortal‐time bias or healthy‐user bias, are minimized, and a more homogeneous population with more balanced (measured and unmeasured) risks is generated at baseline. 29 Second, we restricted both cohorts to patients with NVAF (new oral anticoagulants are not authorized for treating valvular heart disease in our setting), and we excluded those cases where treatment could respond to the initial symptoms of a cancer not yet diagnosed (VTE), to further increase the homogeneity of the cohorts and to minimize the effect of differential treatments. Third, patients were exposed to VKA and followed up for large periods of time, which is consistent with the levels of exposure to VKA theoretically required by the proposed VKA antitumor mechanisms of action. Fourth, we performed several sensitivity and subgroup analyses that yielded, overall, comparable results to those of our main analysis. Fifth, we used data from VID, which is a valuable source of real‐world data, including the regional Cancer Information System.
Our study is subject to some limitations. First, we followed up patients for a maximum of 5 years (median follow‐up: 2.94 and 25% of patients in the VKA cohort followed up for > 4 years), which could be perceived as a relatively short period of time to assess the chemo‐preventive potential of VKA when compared with DOAC. We estimated Kaplan–Meier survival curves for the main and secondary end points to observe the evolution of events in time in DOAC and VKA patients. With our data, Kaplan–Meier curves do not suggest the presence of a delayed potential chemoprotective effect when compared with DOAC within the 5‐year total observation period (see Figure 3 ). Even so, the length of period required by VKA to exert a potential protective effect is uncertain, and our results should be extrapolated with caution. Second, patients using VKAs are more regularly in contact with the healthcare system than DOAC patients to control their International Normalized Ratio (INR), especially after initiation, where dose adjustment can be extremely frequent. Detection bias could occur if this leads to opportunistic screening and detection of prevalent cancers in the VKA cohort. In our region, however, the control of INR is performed by nurses in the primary care setting. These visits are exclusively devoted to INR control with no additional analyses or procedures performed, therefore it would be extremely rare that prevalent cancer cases are detected during INR consultations. In addition, Kaplan–Meier survival curves overlap after initiation, which is inconsistent with a phenomenon of early cancer detection in VKA patients when compared with DOAC patients (see Figure 3 ). However, VKA patients also interact more frequently with the healthcare system as they are older, less wealthy, and more fragile than DOAC patients (see Table 1 ). Even when controlling this potential differential effect with IPTW, we still cannot rule out the presence of residual uncontrolled confounding. Third, VID gather real‐world clinical practice data containing information as registered by health professionals during routine clinical practice, which are not specifically prepared for research. In this sense, studies based on real‐world clinical information like VID may be subject to well‐known information biases due to absent registration or differing data‐recording practices. We addressed this problem, inherent to any study using data from routine clinical practice, by performing different sensitivity analyses. Nevertheless, diagnostic accuracy for hospital discharge diagnoses due to severe conditions (such as our main clinical outcomes) and mortality in our study is very high, as we used data from the regional Cancer Information System and the regional mortality registry. In addition, prescription and dispensation information (the essential data for defining exposure) is also fully accurate in VID as it is used for billing purposes. Fourth, due to the nature of the study, the presence of indication bias could be expected. To address this problem, we used IPTW adjusted models. Fifth, we accounted for many relevant covariates in the adjustment, but we may have missed some potentially relevant information that could be mediating among patient characteristics, exposure, and effect (for instance, INR control, cancer screening, use of concomitant medication, or adherence to OAC medication), and thus we cannot rule out the existence of residual confounding. In fact, this could explain, partially at least, the slight increase that can be observed in the overall risk of cancer (composite end point) associated to VKA vs. DOAC. However, we performed several sensitivity analyses, which yielded similar results to those of the main analysis remained, suggesting a limited potential for further adjustment, and confirming the robustness of our findings. Additionally, with regard to the potential mediator effect of medication nonadherence, previous evidence shows that adherence to both VKA and DOAC treatment is remarkably high in our setting. 30 Sixth, secondary per‐protocol analyses could be affected by additional bias, such as immortal time bias or allocation bias. As patients can switch or discontinue treatment over time or exit the cohort due to death or other reasons, secondary per‐protocol results should be interpreted with caution. However, the consistency between the results obtained in both the intention‐to‐treat and the secondary per‐protocol approaches suggest marginal or no bias. In addition, switching between VKA and DOAC as well as OAC treatment discontinuation have been reported to be exceptionally low in our setting. 30 , 31 Finally, our analyses comprised only patients with NVAF using OAC agents, therefore we cannot deny (or confirm) the possibility of an antitumorigenic class effect of anticoagulant medication.
CONCLUSIONS
Our study is the first to assess the relative risk of cancer in a large, real‐world cohort of patients with NVAF initiating with VKA vs. DOAC. Over a 5‐year observation period with a median follow‐up of 2.94 years, VKA did not show a chemoprotective effect when compared with DOAC. In this sense, our findings would not support the recommendation of a preferent use of VKA vs. DOAC based on a hypothetical antitumorigenic effect. Adequately sized and designed randomized clinical trials should be undertaken to confirm our findings.
FUNDING
No funding was received for this work.
CONFLICTS OF INTEREST
The FISABIO Foundation (a non‐profit research institution depending on the Valencia Health System) had a research collaboration agreement with Daiichi‐Sankyo to conduct real‐world research about the quality of INR control in patients with atrial fibrillation treated with VKA (2017‐2018).
AUTHOR CONTRIBUTIONS
A.G. wrote the manuscript. A.I., S.P., and G.S. designed the research. A.I., S.P., G.S., I.H., and A.F. conducted the research. All authors analyzed the data.
Supporting information
Supplementary Material
DATA AVAILABILITY STATEMENT
The datasets presented in this article are not readily available due to legal restrictions on sharing the data set, as regulated by the Valencia regional government by means of legal resolution by the Valencia Health Agency (2009/13312) ,which forbids the dissemination of data to third parties (accessible at: http://www.san.gva.es/documents/152919/157920/resolucionsolicituddatos.pdf). Upon request, authors can get access to the databases to verify the accuracy of the analysis or the reproducibility of the study. Requests to access the data sets should be directed to Management Office of the Data Commission in the Valencia Health Agency (email: solicitud_datos@gva.es; telephone numbers: +34 961‐928207; +34 961‐928198).
References
- 1. Ruff, C.T. et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 383, 955–962 (2014). [DOI] [PubMed] [Google Scholar]
- 2. López‐López, J.A. et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta‐analysis, and cost effectiveness analysis. BMJ 359, j5058 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruins Slot, K.M. & Berge, E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst. Rev. 3, CD008980 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steinberg, B.A. & Piccini, J.P. Anticoagulation in atrial fibrillation. BMJ 348, g2116 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryan, J.J. , Ketcham, A.S. & Wexler, H. Warfarin treatment of mice bearing autochthonous tumors: effect on spontaneous metastases. Science 162, 1493–1494 (1968). [DOI] [PubMed] [Google Scholar]
- 6. Paolino, M. et al. The E3 ligase Cbl‐b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 507, 508–512 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirane, A. et al. Warfarin blocks Gas6‐mediated Axl activation required for pancreatic cancer epithelial plasticity and metastasis. Cancer Res. 75, 3699–3705 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haaland, G.S. , Falk, R.S. , Straume, O. & Lorens, J.B. Association of warfarin use with lower overall cancer incidence among patients older than 50 years. JAMA Intern. Med. 177, 1774–1780 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulman, S. & Lindmarker, P. Incidence of cancer after prophylaxis with warfarin against recurrent venous thromboembolism. Duration of anticoagulation trial. N. Engl. J. Med. 342, 1953–1958 (2000). [DOI] [PubMed] [Google Scholar]
- 10. Pottegård, A. , Friis, S. & Hallas, J. Cancer risk in long‐term users of vitamin K antagonists: a population‐based case‐control study. Int. J. Cancer 132, 2606–2612 (2013). [DOI] [PubMed] [Google Scholar]
- 11. Pengo, V. et al. Long‐term use of vitamin K antagonists and incidence of cancer: a population‐based study. Blood 117, 1707–1709 (2011). [DOI] [PubMed] [Google Scholar]
- 12. Tagalakis, V. , Tamim, H. , Blostein, M. , Collet, J.P. , Hanley, J.A. & Kahn, S.R. Use of warfarin and risk of urogenital cancer: a population‐based, nested case‐control study. Lancet Oncol. 8, 395–402 (2007). [DOI] [PubMed] [Google Scholar]
- 13. Ahern, T.P. , Pedersen, L. , Sværke, C. , Rothman, K.J. , Sørensen, H.T. & Lash, T.L. The association between vitamin K antagonist therapy and site‐specific cancer incidence estimated by using heart valve replacement as an instrumental variable. Am. J. Epidemiol. 174, 1382–1390 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blumentals, W.A. , Foulis, P.R. , Schwartz, S.W. & Mason, T.J. Does warfarin therapy influence the risk of bladder cancer? Thromb. Haemost. 91, 801–805 (2004). [DOI] [PubMed] [Google Scholar]
- 15. Taliani, M.R. et al. Incidence of cancer after a first episode of idiopathic venous thromboembolism treated with 3 months or 1 year of oral anticoagulation. J. Thromb. Haemost. 1, 1730–1733 (2003). [DOI] [PubMed] [Google Scholar]
- 16. Kinnunen, P.T. , Murtola, T.J. , Talala, K. , Taari, K. , Tammela, T.L. & Auvinen, A. Warfarin use and prostate cancer risk in the Finnish randomized study of screening for prostate cancer. Scand. J. Urol. 50, 413–419 (2016). [DOI] [PubMed] [Google Scholar]
- 17. Kristensen, K.B. , Jensen, P.H. , Skriver, C. , Friis, S. & Pottegård, A. Use of vitamin K antagonists and risk of prostate cancer: meta‐analysis and nationwide case‐control study. Int. J. Cancer 144, 1522–1529 (2019). [DOI] [PubMed] [Google Scholar]
- 18. Blanc‐Lapierre, A. , Weiss, D. & Parent, M.É. Use of oral anticoagulants and risk of prostate cancer: a population‐based case‐control study in Montreal, Canada. Cancer Causes Control 25, 1159–1166 (2014). [DOI] [PubMed] [Google Scholar]
- 19. Ji, J. , Zöller, B. , Giaccia, A. , Haile, R. , Sundquist, J. & Sundquist, K. Risk of breast cancer among patients with bioprosthetic or mechanical valve replacement: a population‐based study in Sweden. Breast Cancer Res. Treat. 154, 369–375 (2015). [DOI] [PubMed] [Google Scholar]
- 20. Luo, J.D. , Luo, J. , Lai, C. , Chen, J. & Meng, H.Z. Is use of vitamin K antagonists associated with the risk of prostate cancer?: a meta‐analysis. Medicine (Baltimore) 97, e13489 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. García‐Sempere, A. et al. Data resource profile: the Valencia health system integrated database (VID). Int. J. Epidemiol. 49, 740–741e (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spanish Society for Medical Oncology . Las cifras del cáncer en España 2021 [Cancer figures in Spain 2021]. Online. https://seom.org/images/Cifras_del_cancer_en_Espnaha_2021.pdf (2019). Accessed July 23, 2021.
- 23. Martin‐Moreno, J.M. , Alonso, P. , Claveria, A. , Gorgojo, L. & Peiró, S. Spain: a decentralised health system in constant flux. BMJ 338, b1170 (2009). [DOI] [PubMed] [Google Scholar]
- 24. Valencia Government . Estrategia contra el cáncer de la Comunidad Valenciana 2019‐2022 [Strategy against cancer of the Valencian Community 2019‐2022]. Online. https://socvalped.com/wp‐content/uploads/2019/06/EstrategiaCancer20190121.pdf (2020). Accessed July 23, 2021.
- 25. Cole, S.R. & Hernán, M.A. Adjusted survival curves with inverse probability weights. Comput. Methods Prog. Biomed. 75, 45–49 (2004). [DOI] [PubMed] [Google Scholar]
- 26. Westreich, D. , Cole, S.R. , Schisterman, E.F. & Platt, R.W. A simulation study of finite‐sample properties of marginal structural Cox proportional hazards models. Statist. Med. 31, 2098–2109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat. Med. 28, 3083–3107 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dahlberg, S. , Ede, J. & Schött, U. Vitamin K and cancer. Scand. J. Clin. Lab. Invest. 77, 555–567 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Ray, W.A. Evaluating medication effects outside of clinical trials: new‐user designs. Am. J. Epidemiol. 158, 915–920 (2003). [DOI] [PubMed] [Google Scholar]
- 30. Hurtado‐Navarro, I. , García‐Sempere, A. , Rodríguez‐Bernal, C. , Santa‐Ana‐Tellez, Y. , Peiró, S. & Sanfélix‐Gimeno, G. Estimating adherence based on prescription or dispensation information: impact on thresholds and outcomes. a real‐world study with atrial fibrillation patients treated with oral anticoagulants in Spain. Front. Pharmacol. 3, 1353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. García‐Sempere, A. et al. Quality of INR control and switching to non‐vitamin K oral anticoagulants between women and men with atrial fibrillation treated with vitamin K antagonists in Spain. A population‐based, real‐world study. PLoS One 14, e0211681 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The datasets presented in this article are not readily available due to legal restrictions on sharing the data set, as regulated by the Valencia regional government by means of legal resolution by the Valencia Health Agency (2009/13312) ,which forbids the dissemination of data to third parties (accessible at: http://www.san.gva.es/documents/152919/157920/resolucionsolicituddatos.pdf). Upon request, authors can get access to the databases to verify the accuracy of the analysis or the reproducibility of the study. Requests to access the data sets should be directed to Management Office of the Data Commission in the Valencia Health Agency (email: solicitud_datos@gva.es; telephone numbers: +34 961‐928207; +34 961‐928198).


