Abstract
Aim
To assess the efficacy and safety of insulin degludec/liraglutide (IDegLira) versus insulin degludec (degludec) in Chinese people with type 2 diabetes (T2D) treated with basal insulin.
Materials and Methods
In DUAL II China, a randomized, double‐blinded, multicentre, treat‐to‐target trial, Chinese adults with T2D and HbA1c of 7.5% or more on basal insulin and metformin, with or without other oral antidiabetic drugs (OADs), were randomized 2:1 to 26 weeks of treatment with either IDegLira (max. dose 50 U degludec/1.8 mg liraglutide) or degludec (max. 50 U/day), respectively, combined with metformin. At 26 weeks, superiority of IDegLira over degludec was assessed for change in HbA1c (primary endpoint), and body weight and number of severe or blood glucose (BG)‐confirmed hypoglycaemic episodes (confirmatory secondary endpoints).
Results
Overall, 453 participants were randomized to IDegLira (n = 302) or degludec (n = 151). Superiority was confirmed for IDegLira over degludec in HbA1c change (−1.9% vs. −1.0%, respectively, estimated treatment difference [ETD] [95% confidence interval]: −0.92% [−1.09; −0.75], P < .0001), body weight change (−0.7 vs. +0.4 kg, respectively, ETD [95% CI]: −1.08 kg [−1.63; −0.52], P = .0002) and severe or BG‐confirmed hypoglycaemia (estimated rate ratio [95% CI]: 0.53 [0.30; 0.94], P = .0297). The odds of achieving HbA1c less than 7.0% without hypoglycaemia and/or weight gain were greater with IDegLira than degludec (P < .0001 for all). Daily insulin dose at 26 weeks was lower for IDegLira (34.3 U) than degludec (37.4 U) (P = .0014). No unexpected safety signals were observed.
Conclusions
IDegLira may be an efficacious and well‐tolerated treatment intensification option for Chinese people with T2D uncontrolled on basal insulin and OADs.
Keywords: Chinese, insulin, insulin degludec/liraglutide, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D) is a growing concern in China. In 2019, 116 million Chinese people were living with diabetes; this is predicted to reach 147 million by 2045, the vast majority with T2D. 1 Chinese people with T2D exhibit notably distinct clinical characteristics from global populations, with more impaired β‐cell function, 2 and experience a greater contribution of postprandial glucose (PPG) excursions to hyperglycaemia. 3 , 4 However, despite lower average body mass index (BMI) at diagnosis compared with international cohorts, 2 , 5 approximately two‐thirds of Chinese people with T2D are overweight or obese. 6
The Chinese Diabetes Society guidelines recommend that people with T2D should be treated with oral antidiabetic drug (OAD) monotherapy (such as metformin, α‐glucosidase inhibitors [AGIs], or insulin secretagogues) if not achieving a target HbA1c of 7.0% or less with lifestyle changes, 7 and with combination therapy with an OAD or injectable medication if targets are still not attained. Either insulin (basal or premixed) or a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) should be initiated if glycaemic control is not achieved with OADs, 7 and bolus insulin can be added to basal insulin therapy or premixed insulin can be intensified if this fails to achieve euglycaemia. 7
Achieving glycaemic control is central to improving long‐term outcomes in diabetes, and treatment intensification is often necessary because of the progressive nature of T2D. However, treatment inertia is common, with many perceived barriers that may delay initiation and intensification of insulin therapy, in particular when this involves regimens with multiple insulins or multiple daily injections. 8 These perceived barriers include fear of weight gain and hypoglycaemia, complex and burdensome treatment regimens, as well as concerns around adherence and fear of injections, among others. 8 Therapies that address these concerns, therefore, have considerable clinical importance.
Insulin degludec/liraglutide (IDegLira) is a fixed‐ratio combination of insulin degludec (degludec) and the GLP‐1 RA liraglutide, which is administered subcutaneously once daily. 9 IDegLira has been extensively studied in the global DUAL clinical trial programme and has been approved for the management of T2D in Europe 9 and the United States. 10 The global DUAL clinical trial programme has found that use of IDegLira offers efficacious glycaemic control across the clinical spectrum of people with T2D. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 In the global DUAL II trial, involving basal insulin‐treated participants, IDegLira provided superior reductions in HbA1c and body weight over 26 weeks compared with degludec, with similar levels of confirmed hypoglycaemia and at similar doses to degludec. 12
Given the differences between Chinese and global populations with T2D, data from randomized trials are important to confirm the efficacy of IDegLira in Chinese people. As part of the regulatory approval process in China, two DUAL China trials and a single‐dose pharmacokinetics study have recently been completed. In the DUAL I China trial, the safety and efficacy of IDegLira were compared with each of its monocomponents in insulin‐naïve Chinese people with T2D who were not controlled with OADs. DUAL II China, presented here, was undertaken to confirm the superiority of IDegLira over degludec, both in combination with metformin, in reducing HbA1c after 26 weeks in Chinese people with T2D inadequately controlled with basal insulin and metformin, with or without one other OAD. The secondary objectives of DUAL II China were to confirm the superiority of IDegLira versus degludec using confirmatory endpoints and to compare the overall efficacy and safety after 26 weeks using supportive endpoints.
2. MATERIALS AND METHODS
2.1. Trial design
This was a 26‐week, randomized, double‐blinded, multicentre, treat‐to‐target, active‐controlled, phase 3 trial (NCT03175120, Figure S1). The trial was conducted at 40 sites across mainland China and Hong Kong from May 2017 to July 2019. The total trial duration was 32 weeks, with a 2‐week or longer screening period, 26‐week treatment period, and follow‐up contacts at weeks 27 and 30 (Figure S1).
The trial was conducted in accordance with the Declaration of Helsinki 19 and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice Guidelines. 20 The trial protocol was reviewed and approved according to local regulations by appropriate health authorities and independent ethics committees or institutional review boards. Written informed consent was obtained from all individuals prior to trial participation.
2.2. Inclusion/exclusion criteria
The full eligibility criteria are included in Table S1. Male or female participants aged 18 years or older with clinically diagnosed T2D, BMI of 24 kg/m2 or higher, and an HbA1c of 7.5% or more, were included in the trial. Per protocol, baseline HbA1c requirements varied later into enrolment, based on the median HbA1c once 50% of participants had been randomized, to achieve a target median HbA1c of 8.5%.
Eligible participants had been treated for 90 days or longer prior to screening with basal insulin and metformin, with or without one other of the following OADs: AGIs, sulphonylureas, glinides, or thiazolidinediones. Additionally, participants had received stable doses for 60 days or longer of 1500 mg or more of metformin (or maximum tolerated dose), and 20 to 50 U/day of a basal insulin.
2.3. Stratification and randomization
Eligible participants were randomized 2:1 to IDegLira or degludec, respectively, both in combination with metformin, using an interactive web‐based response system. Stratification was implemented between both groups to ensure similar proportions of participants had received pretrial treatment with either metformin and basal insulin or metformin, basal insulin, and one other OAD.
2.4. Treatment and dose titration
Participants received subcutaneous IDegLira or degludec once daily, both in combination with metformin; other OADs were discontinued at randomization. The recommended starting doses were 16 dose steps for IDegLira (i.e. 16 units [U] degludec/0.6 mg liraglutide) and 16 U for degludec. Doses were titrated twice weekly for both IDegLira and degludec to a target fasting plasma glucose (FPG) of 4 to 5 mmol/L, based on the mean of the three previous self‐measured plasma glucose (SMPG) values and the titration algorithm (Table S2).
2.5. Trial endpoints
The primary endpoint in the trial was the HbA1c change from baseline after 26 weeks of treatment. Confirmatory secondary endpoints included: change from baseline in body weight at 26 weeks and the number of treatment‐emergent severe or blood glucose (BG)‐confirmed (<3.1 mmol/L) hypoglycaemic episodes during 26 weeks of treatment. The primary endpoint and confirmatory secondary endpoints were assessed for the superiority of IDegLira over degludec.
Supportive secondary endpoints after 26 weeks included: total actual daily insulin dose; responders for HbA1c less than 7.0% and responders for HbA1c of 6.5% or less; composite endpoints of HbA1c responders (both <7.0% and ≤6.5%) without weight gain and/or hypoglycaemia; change from baseline in FPG, mean of the nine‐point SMPG profile, postprandial increment (premeal to 90 minutes postmeal at breakfast, lunch, and dinner), and waist circumference; and the fasting lipid profile.
Secondary safety endpoints included the number of treatment‐emergent adverse events, severe or BG‐confirmed symptomatic hypoglycaemic episodes, nocturnal severe or BG‐confirmed hypoglycaemic episodes, and hypoglycaemic episodes, as per the American Diabetes Association 2013 definitions. 21 Change from baseline after 26 weeks was also assessed for pulse and blood pressure, as well as laboratory tests including urinalysis. Hypoglycaemia definitions are shown in Table S3, and prespecified adverse events (AEs) assessed by an independent event‐adjudication committee (EAC) are shown in Table S4.
2.6. Statistical analysis
The primary endpoint was analysed in the full analysis set (FAS) using an analysis of covariance (ANCOVA) model with treatment and previous antidiabetic treatment as fixed effects and baseline HbA1c as a covariate. Missing values after 26 weeks of treatment were imputed by last observation carried forward (LOCF) using HbA1c values at, and after, baseline. The superiority of IDegLira over degludec was confirmed if the upper limit of the 95% confidence interval (CI) for the estimated mean treatment difference (ETD) was less than 0%.
The robustness of the primary endpoint result was assessed in various prespecified sensitivity analyses, including analysis of different datasets (per protocol and completers), a mixed model for repeated measurements, and a reference‐based multiple imputation.
A hierarchical testing procedure was used to control the overall type 1 error on a two‐sided 5% level. If superiority of IDegLira over degludec was confirmed in the primary analysis, the secondary confirmatory tests were performed for superiority of IDegLira over degludec with a fixed sequence (change in body weight, followed by the number of treatment‐emergent severe or BG‐confirmed hypoglycaemic episodes).
Change from baseline in body weight was analysed using an ANCOVA model and superiority was confirmed if the upper limit of the two‐sided 95% CI for the ETD (IDegLira vs. degludec) was strictly below zero. The number of hypoglycaemic events was analysed using a negative binomial regression model with a log‐link function and the logarithm of the exposure time as offset. The model included treatment and previous antidiabetic treatment as fixed effects. Superiority for severe or BG‐confirmed episodes was confirmed if the upper limit of the two‐sided 95% CI for the estimated rate ratio (IDegLira vs. degludec) was strictly below one.
Continuous supportive efficacy endpoints were analysed separately using an ANCOVA model with treatment and previous antidiabetic treatment as fixed effects and corresponding baseline value as a covariate. For total actual daily insulin dose, baseline HbA1c was also included as a covariate, and for lipid variables, both end‐of‐trial and baseline values were log‐transformed prior to the analysis. A linear mixed effects model was fitted to the nine‐point SMPG profile data. For HbA1c responder and composite responder endpoints, a logistic regression model was used. For all secondary endpoints, missing values were imputed by LOCF.
The trial sample size was determined by use of a t statistic, assuming a two‐sided test size of 5%, a mean difference between treatments (IDegLira vs. degludec) in HbA1c change from baseline of −0.4%, and a standard deviation (SD) of 1.2%. Accounting for these assumptions and based on 2:1 randomization, the sample size was determined to be 300 participants in the IDegLira group and 150 in the degludec group; in total, 450 randomized participants. This provided a power of 91.4% for confirmation of the primary endpoint in the FAS.
3. RESULTS
3.1. Participant disposition
In total, 555 participants were screened, of whom 453 were eligible for inclusion and randomized to IDegLira (n = 302) or degludec (n = 151) (Figure S2). Of those randomized to IDegLira, 301 were exposed to the trial medication; all participants randomized to degludec were exposed. In total, 429 (94.7%) participants completed the trial: 290 (96%) of participants receiving IDegLira and 139 (92.1%) receiving degludec.
3.2. Baseline characteristics
Baseline characteristics were similar between treatment groups (Table 1). Overall, 60.5% of participants were male; mean (SD) age was 54.7 (9.9) years, BMI was 27.4 (3.1) kg/m2, diabetes duration was 11.46 (6.0) years, and HbA1c was 8.94% (1.19%) (Table 1).
TABLE 1.
Baseline characteristics
| Characteristic | IDegLira (n = 302) | Degludec (n = 151) | Total (n = 453) |
|---|---|---|---|
| Age, y | 54.5 (9.8) | 55.3 (10.0) | 54.7 (9.9) |
| Sex, n (%) | |||
| Female | 119 (39.4) | 60 (39.7) | 179 (39.5) |
| Male | 183 (60.6) | 91 (60.3) | 274 (60.5) |
| Body weight, kg | 76.8 (13.0) | 74.3 (11.4) | 76.0 (12.5) |
| BMI, kg/m2 | 27.5 (3.3) | 27.0 (2.9) | 27.4 (3.1) |
| Duration of diabetes, y | 11.52 (5.9) | 11.33 (6.3) | 11.46 (6.0) |
| HbA1c, % | 8.93 (1.20) | 8.96 (1.17) | 8.94 (1.19) |
| FPG, mmol/L | 9.84 (2.8) | 9.59 (2.8) | 9.75 (2.8) |
| Height, m | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) |
| OAD use during screening period, n (%) | |||
| Metformin only | 141 (46.7) | 74 (49.0) | 215 (47.5) |
| Metformin + sulphonylurea | 44 (14.6) | 22 (14.6) | 66 (14.6) |
| Metformin + glinide | 20 (6.6) | 10 (6.6) | 30 (6.6) |
| Metformin + α‐glucosidase inhibitors | 89 (29.5) | 44 (29.1) | 133 (29.4) |
| Metformin + TZD | 8 (2.6) | 1 (0.7) | 9 (2.0) |
| Basal insulin use during screening period, n (%) | |||
| Insulin detemir | 33 (10.9) | 17 (11.3) | 50 (11.0) |
| Insulin glargine | 218 (72.2) | 111 (73.5) | 329 (72.6) |
| Insulin isophane | 46 (15.2) | 23 (15.2) | 69 (15.2) |
| Insulin zinc protamine | 5 (1.7) | 0 (0) | 5 (1.1) |
| Basal insulin dose during screening, all insulins (units) | 25.3 (6.2) | 24.5 (5.7) | 25.1 (6.0) |
Note: Full analysis set. Data are mean (SD) unless otherwise stated. Baseline refers to week 0 except for height, which was measured at screening. The duration of diabetes is calculated as the time from date of diagnosis to the randomization date.
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose; IDegLira, insulin degludec/liraglutide; OAD, oral antidiabetic drug; SD, standard deviation; TZD, thiazolidinedione.
The most commonly used pretrial basal insulin was insulin glargine (72.6%) (Table 1). In addition to metformin and basal insulin, the most common class of pretrial OAD was an AGI (Table 1). The mean daily insulin dose across all basal insulins at screening was 25.1 U/day.
3.3. Primary endpoint
3.3.1. Change in HbA1c
Superiority was confirmed for IDegLira, compared with degludec, for change in HbA1c after 26 weeks. Mean observed HbA1c was reduced by −1.9% with IDegLira (from 8.9% to 7.1%) compared with −1.0% with degludec (from 9.0% to 8.0%). The ETD (95% CI) was −0.9% (−1.1%; −0.8%), P < .0001 (Figure 1A). Results from the prespecified sensitivity analyses were consistent with the primary endpoint and are shown in Table S5.
FIGURE 1.
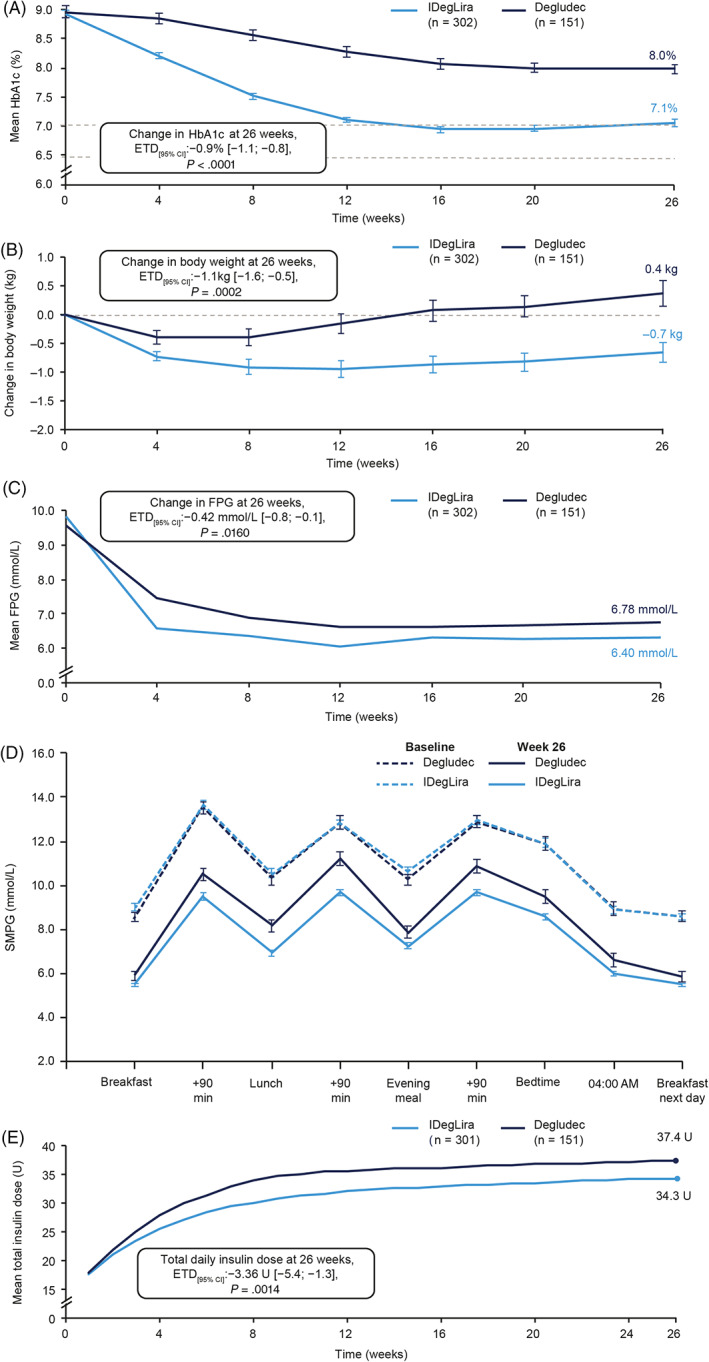
Mean (A) HbA1c, (B) body weight, (C) fasting plasma glucose over 26 weeks of treatment, (D) SMPG profile at baseline and week 26, and (E) mean total daily insulin dose over 26 weeks. (A) Adapted from Pei Y et al., Abstract #690 of the 56th EASD Annual Meeting of the European Association for the Study of Diabetes. Diabetologia 2020;63:1‐485. ©2020 Springer. (A), (B), (C), and (D): full analysis set. (E): safety analysis set. Data are observed means (±SEM), except for week 26 (LOCF), which are estimated means (±SEM). Estimated means and treatment differences were analysed using ANCOVA models. SMPG was assessed with a glucose meter as plasma equivalent values of capillary blood glucose, with missing values imputed by LOCF. ANCOVA, analysis of covariance; CI, confidence interval; ETD, estimated treatment difference; FPG, fasting plasma glucose; IDegLira, insulin degludec/liraglutide; LOCF, last observation carried forward; SEM, standard error of the mean; SMPG, self‐measured plasma glucose
3.4. Secondary endpoints
3.4.1. Body weight
Superiority in body weight change was confirmed for IDegLira over degludec, with a weight loss of −0.7 kg versus a weight gain of 0.4 kg, respectively (ETD [95% CI]: −1.1 kg [−1.6; −0.5], P = .0002) (Figure 1B). Mean observed body weight reduced from 76.8 to 76.2 kg in the IDegLira group and increased from 74.3 to 74.7 kg in the degludec group. Significantly greater reductions in waist circumference at 26 weeks were also seen with IDegLira versus degludec (ETD [95% CI]: −0.87 cm [−1.64; −0.09], P = .0279).
3.4.2. Hypoglycaemia
Hypoglycaemic events occurring during the trial are shown in Table S6. Superiority for severe or BG‐confirmed hypoglycaemia over 26 weeks was confirmed for IDegLira over degludec (estimated rate ratio [95% CI], 0.5 [0.3; 0.9], P = .0297) (Figure S3). Events occurred in 11.3% of participants in the IDegLira group and 14.6% in the degludec group, at rates of 25.0 versus 48.3 events per 100 participant‐years of exposure (PYE), respectively (Table S6). There were no differences between groups in nocturnal severe or BG‐confirmed hypoglycaemia (Table S6).
3.4.3. Fasting plasma glucose
Mean observed FPG was reduced by −3.44 versus −2.81 mmol/L for IDegLira versus degludec at 26 weeks, respectively, with a significantly greater reduction with IDegLira (ETD [95% CI]: −0.42 mmol/L [−0.75; −0.08], P = .0160) (Figure 1C).
3.4.4. Nine‐point SMPG
Reductions in nine‐point SMPG at all time periods in the profile were significantly greater for IDegLira than degludec (P < .05 for all), except for before breakfast the following day (Figure 1D). Participants treated with IDegLira experienced significantly larger reductions from baseline in mean nine‐point SMPG at 26 weeks compared with degludec (ETD [95% CI]: −0.98 mmol/L [−1.29; −0.66], P < .0001). Additionally, the mean postprandial increment at breakfast and the mean of all meals were smaller in the IDegLira versus degludec groups (P < .05 for both; Table 2); however, there were no significant differences at lunch or dinner.
TABLE 2.
Mean prandial increments in the nine‐point SMPG profile at baseline and week 26
| Mean prandial increment, mmol/L | IDegLira | Degludec | ETD [95% CI] IDegLira–degludec | P value | ||
|---|---|---|---|---|---|---|
| Baseline | Week 26 | Baseline | Week 26 | |||
| All meals | 3.04 | 3.12 | 3.39 | 3.61 | −0.40 [−0.74; −0.07] | .0189 |
| Breakfast | 4.61 | 3.99 | 4.98 | 4.89 | −0.77 [−1.28; −0.26] | .0030 |
| Lunch | 2.24 | 2.76 | 2.55 | 2.89 | −0.07 [−0.59; 0.46] | >.05 |
| Dinner | 2.27 | 2.60 | 2.64 | 2.93 | −0.24 [−0.72; 0.23] | >.05 |
Note: Full analysis set. The change from baseline after 26 weeks was analysed using an ANCOVA model with treatment and previous antidiabetic medication as fixed factors and corresponding baseline value as covariate. Missing values were imputed with LOCF. P values were based on a two‐sided test of no difference, without correction for multiplicity.
Abbreviations: ANCOVA, analysis of covariance; CI, confidence interval; degludec, insulin degludec; ETD, estimated treatment difference; IDegLira, insulin degludec/liraglutide; LOCF, last observation carried forward; SMPG, self‐measured plasma glucose.
3.4.5. HbA1c responder endpoints
At 26 weeks, the odds of achieving HbA1c less than 7.0% were significantly greater in the IDegLira group compared with the degludec group (51.0% vs. 15.2% of participants, respectively; Figure 2), as were the odds of achieving an HbA1c of 6.5% or less (31.8% vs. 6.6%, respectively; Figure S4).
FIGURE 2.
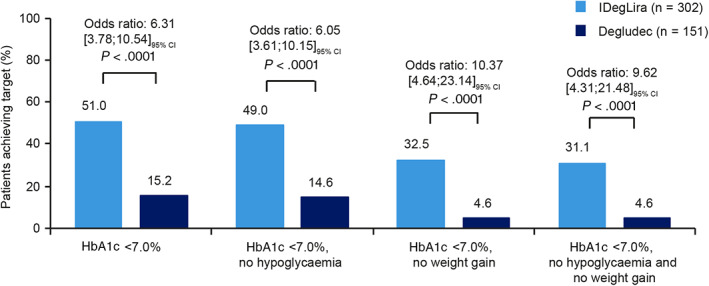
Responder endpoints for HbA1c less than 7.0%, with composite endpoints of reaching HbA1c targets without weight gain and/or without treatment‐emergent severe or BG‐confirmed symptomatic hypoglycaemic episodes at 26 weeks. Full analysis set. Treatment‐emergent severe or BG‐confirmed symptomatic hypoglycaemic episodes during the last 12 weeks of treatment. BG, blood glucose; CI, confidence interval; degludec, insulin degludec; IDegLira, insulin degludec/liraglutide
3.4.6. Composite endpoints
Results of the composite endpoints are shown in Figure 2. A greater proportion of participants treated with IDegLira versus degludec achieved the triple composite endpoint of HbA1c less than 7.0% without weight gain and hypoglycaemia after 26 weeks (31.1% vs. 4.6%, estimated odds ratio: 9.62 [4.31; 21.48], P < .0001), or without hypoglycaemia or weight gain alone (P < .0001 for both). Similarly, greater proportions of the IDegLira group achieved composite responder endpoints for an HbA1c of 6.5% or less without hypoglycaemia and/or weight gain, compared with the degludec group (Figure S4).
3.4.7. Actual daily total insulin dose
Mean actual daily total insulin dose at 26 weeks was significantly lower with IDegLira (34.3 U) versus degludec (37.4 U) (ETD [95% CI]: −3.4 U [−5.4; −1.3], P = .0014; Figure 1E). This equated to a mean end‐of‐trial actual daily insulin dose of 0.45 versus 0.50 U/kg, respectively.
3.4.8. Fasting lipid profiles
A greater reduction was observed in total cholesterol with IDegLira versus degludec (P = .0281; Table S7). There were no significant differences in other lipid variables.
3.5. Safety
3.5.1. Adverse events
AEs during the trial are summarized in Table 3. A similar proportion of participants experienced one or more AE in both the IDegLira (71.8%) and degludec (72.8%) groups, while rates of AEs were higher with IDegLira than degludec (422.1 vs. 308.7 events per 100 PYE, respectively). AEs determined to be “possibly” or “probably” related to trial medication occurred at rates of 152.1 versus 73.8 per 100 PYE in the IDegLira and degludec groups, respectively. Two participants in both groups experienced AEs leading to permanent trial medication discontinuation. The most frequently reported AEs (occurring in >5% of trial participants) are shown in Table 3.
TABLE 3.
Treatment‐emergent adverse events
| Events | IDegLira (N = 301) | Degludec (N = 151) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | % | E | R | N | % | E | R | |
| AEs | 216 | 71.8 | 641 | 422.1 | 110 | 72.8 | 230 | 308.7 |
| AEs possibly or probably related to treatment | 136 | 45.2 | 231 | 152.1 | 47 | 31.2 | 55 | 73.8 |
| Most frequent AEs (≥5% of participants) | ||||||||
| Infections and infestations | ||||||||
| Upper respiratory tract infection | 38 | 12.6 | 45 | 29.6 | 27 | 17.9 | 32 | 43.0 |
| Gastrointestinal disorders | ||||||||
| Diarrhoea | 22 | 7.3 | 31 | 20.4 | 2 | 1.3 | 2 | 2.7 |
| Metabolism and nutrition | ||||||||
| Decreased appetite | 21 | 7.0 | 23 | 15.1 | 3 | 2.0 | 3 | 4.0 |
| Eye disorders | ||||||||
| Diabetic retinopathy | 35 | 11.6 | 35 | 23.1 | 11 | 7.3 | 11 | 14.8 |
| Investigations | ||||||||
| Lipase increased | 36 | 12.0 | 38 | 25.0 | 3 | 2.0 | 3 | 4.0 |
| SAEs | 13 | 4.3 | 15 | 9.9 | 7 | 4.6 | 7 | 9.4 |
| SAEs possibly or probably related to treatment | 2 | 0.7 | 3 | 2.0 | 1 | 0.7 | 1 | 1.3 |
| MACE | 3 | 1.0 | 4 | 2.6 | 2 | 1.3 | 2 | 2.7 |
| Acute myocardial infarction | 1 | 0.3 | 2 | 1.3 | 0 | ‐ | ‐ | ‐ |
| Stroke | 2 | 0.7 | 2 | 1.3 | 2 | 1.3 | 2 | 2.7 |
Note: Safety analysis set.
Abbreviations: %, percentage of individuals; AE, adverse event; degludec, insulin degludec; E, number of events; IDegLira, insulin degludec/liraglutide; MACE, major adverse cardiovascular events; N, number of individuals; R, rate of events per 100 participant‐years of exposure; SAE, serious adverse event.
Gastrointestinal (GI) AEs were more common with IDegLira (80.33 events per 100 PYE) than with degludec (14.76 per 100 PYE). Most GI AEs were mild; among them, diarrhoea was most commonly reported.
Diabetic retinopathy was more commonly reported with IDegLira compared with degludec (Table 3). All events were of moderate or mild severity and non‐serious; all were reported during routine eye examinations at the end‐of‐trial visit.
No elevations of serum calcitonin were reported, or any EAC‐confirmed events of thyroid disease. Serum lipase elevation was seen more frequently in the IDegLira than in the degludec groups (Table 3). No elevations in serum amylase concentrations occurred in the degludec group; these occurred at a rate of 6.58 events per 100 PYE in the IDegLira group.
3.5.2. Serious AEs
In total, 22 serious AEs (SAEs) occurred in 20 participants; 15 events in participants treated with IDegLira versus seven events in those receiving degludec (respective rates, 9.9 vs. 9.4 events per 100 PYE; Table 3). The majority of SAEs were adjudicated as “unlikely” to be related to the trial product.
Overall, six EAC‐confirmed major adverse cardiovascular events (MACE) occurred during the trial, four in the IDegLira group and two in the degludec group; event rates for MACE were similar between treatment groups (Table 3). There were no EAC‐confirmed episodes of pancreatitis or neoplasms and no deaths occurred during the trial.
3.5.3. Vital signs
Significantly greater reductions in systolic blood pressure were observed with IDegLira compared with degludec, but not in diastolic blood pressure (Table S8). Change from baseline in pulse rate was significantly greater with IDegLira compared with degludec (Table S8).
4. DISCUSSION
In this randomized trial, the efficacy and safety of IDegLira versus degludec were assessed in Chinese people with T2D inadequately controlled with metformin and basal insulin ±OAD over 26 weeks.
Compared with degludec, treatment with IDegLira provided significantly greater improvements in glycaemic control and body weight, at a lower insulin dose, with fewer severe or BG‐confirmed hypoglycaemic episodes and no unexpected safety or tolerability concerns.
Superiority in change from baseline in HbA1c with IDegLira was confirmed over degludec, meeting the trial's primary objective. The magnitude of the HbA1c reduction in DUAL II China was consistent with previous findings from the global DUAL II trial, wherein participants receiving IDegLira experienced mean reductions of −1.9% over 26 weeks. 12 Significant reductions were also observed in FPG and the mean postprandial increment of all meals and after breakfast, suggesting improvement of the GLP‐1 RA component (liraglutide) versus degludec alone against both aspects of hyperglycaemia. This may be particularly relevant in Chinese individuals, given the increased contribution of raised PPG to hyperglycaemia relative to international populations because of carbohydrate‐rich diets in China, which are high in glycaemic index and load. 4 , 22
Superiority was also confirmed for IDegLira over degludec with respect to change in body weight, consistent with previous findings with IDegLira. 12 The smaller magnitude of weight loss with IDegLira observed in this trial (−0.7 kg), compared with the global DUAL II trial (−2.7 kg), 12 may be attributable in part to the lower baseline body weight of the Chinese trial population than the DUAL II population (76.8 vs. 95.4 kg, respectively). Additionally, a greater proportion of participants on IDegLira versus degludec attained treatment goals (HbA1c <7.0% and ≤6.5%) without weight gain in DUAL II China.
IDegLira was similarly confirmed to be superior in regard to the rate of severe or BG‐confirmed hypoglycaemia versus degludec, in line with the trend reported in the global DUAL II trial. 12 Fear of hypoglycaemia is commonly identified by both people with diabetes and clinicians as a significant contributor to treatment inertia and may be an important management consideration when selecting an intensification of diabetes therapy. The high rates of participants achieving HbA1c targets of less than 7.0% and 6.5% or less without hypoglycaemia in this trial suggest that use of IDegLira may help to address the limiting factor of fear of hypoglycaemia to the optimization of glycaemic control.
Reported AEs during the trial were in line with the known safety and tolerability profiles of the monocomponents of IDegLira, degludec, and liraglutide. GI side effects were more common with IDegLira compared with degludec, as might be expected from the known safety profile of the GLP‐1 RA drug class, but these were generally mild, transient, and early in treatment. IDegLira was generally well tolerated, with very few participants discontinuing IDegLira because of AEs overall.
The key strengths of this trial include its large size and randomized, double‐blinded trial design. High rates of trial completion were also observed, suggesting a robust dataset. The eligibility criteria, which may not be fully representative of the real‐world population with T2D, and the controlled trial setting, may be considered limitations; as with many randomized controlled trials, these factors may impede the generalizability of results to routine clinical practice.
A further limitation was the capping of the maximum dose of degludec at 50 U. This was necessary to allow assessment of the liraglutide component of IDegLira (maximum dose 50 U/1.8 mg), but may also limit the generalizability of our findings to real‐world clinical practice. However, few participants reached the maximum permitted dose of degludec over 26 weeks, and the limit was also supported by expert consensus in China, which recommends treatment intensification with prandial insulin if glycaemic control is not achieved with 0.6 U/kg/day of basal insulin. 23 Taken together, it is likely that 50 U/day of basal insulin encompassed the requirements of most participants and therefore probably had a minimal effect on trial outcomes.
In conclusion, compared with degludec, treatment with IDegLira over 26 weeks resulted in superior HbA1c and FPG reductions, with weight loss benefits, fewer hypoglycaemic episodes, and an acceptable safety profile. These findings show that IDegLira may be an efficacious and well‐tolerated treatment option for the intensification of insulin therapy in Chinese people with T2D who are inadequately controlled on basal insulin and OADs.
CONFLICT OF INTEREST
YP, XD, DL, JL, ML, YL, XX, and YM have no conflicts of interest to declare. BA was a Novo Nordisk employee and shareholder at the time of manuscript development. BL, LL, and TN are Novo Nordisk employees and shareholders.
AUTHOR CONTRIBUTIONS
All authors confirm that they meet the International Committee of Medical Journal Editors uniform requirements for authorship and that they have contributed to: collection of data, critical analysis and interpretation of the data, drafting/critically revising the article, and sharing in the final responsibility for the content of the manuscript and the decision to submit it for publication. YP is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data.
Supporting information
Appendix S1. Supporting information
ACKNOWLEDGEMENTS
Medical writing and editorial support for the development of this manuscript, under the direction of the authors, was provided by Chloe Harrison, MBBS, and Izabel James, MBBS, of Ashfield MedComms, an Ashfield Health company, and was funded by Novo Nordisk. Parts of this trial were presented as a poster at the European Association for the Study of Diabetes, 56th Annual Meeting, 22‐25 September 2020, Virtual Meeting.
Pei Y, Agner BR, Luo B, et al. DUAL II China: Superior HbA1c reductions and weight loss with insulin degludec/liraglutide (IDegLira) versus insulin degludec in a randomized trial of Chinese people with type 2 diabetes inadequately controlled on basal insulin. Diabetes Obes Metab. 2021;23(12):2687‐2696. doi: 10.1111/dom.14522
Funding information Novo Nordisk
DATA AVAILABILITY STATEMENT
The participant‐level analysis data sets for the research presented in the publication are available from the corresponding author on reasonable request.
REFERENCES
- 1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 2. Editorial . Diabetes in China: mapping the road ahead. Lancet Diabetes Endocrinol. 2014;2:923. [DOI] [PubMed] [Google Scholar]
- 3. Venn BS, Williams SM, Mann JI. Comparison of postprandial glycaemia in Asians and Caucasians. Diabet Med. 2010;27:1205‐1208. [DOI] [PubMed] [Google Scholar]
- 4. Kang X, Wang C, Lifang L, et al. Effects of different proportion of carbohydrate in breakfast on postprandial glucose excursion in normal glucose tolerance and impaired glucose regulation subjects. Diabetes Technol Ther. 2013;15:569‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia. 2018;61:1249‐1260. [DOI] [PubMed] [Google Scholar]
- 6. Hou X, Lu J, Weng J, et al. Impact of waist circumference and body mass index on risk of cardiometabolic disorder and cardiovascular disease in Chinese adults: a national diabetes and metabolic disorders survey. PLoS One. 2013;8:e57319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35:e3158. [DOI] [PubMed] [Google Scholar]
- 8. Russell‐Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20:488‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Novo Nordisk . Xultophy® Summary of Product Characteristics. 2014. https://www.ema.europa.eu/en/documents/product-information/xultophy-epar-product-information_en.pdf. Accessed July 28, 2021.
- 10. Novo Nordisk Xultophy® Prescribing Information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208583s008s010s011lbl.pdf. Accessed July 28, 2021.
- 11. Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open‐label, randomised, 26‐week, treat‐to‐target trial in insulin‐naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885‐893. [DOI] [PubMed] [Google Scholar]
- 12. Buse JB, Vilsboll T, Thurman J, et al. Contribution of liraglutide in the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37:2926‐2933. [DOI] [PubMed] [Google Scholar]
- 13. Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP‐1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther. 2017;8:101‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodbard HW, Bode BW, Harris SB, et al. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin‐naive people with type 2 diabetes: the DUAL IV trial. Diabet Med. 2017;34:189‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lingvay I, Perez Manghi F, Garcia‐Hernandez P, et al. Effect of insulin glargine up‐titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315:898‐907. [DOI] [PubMed] [Google Scholar]
- 16. Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal‐bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41:1009‐1016. [DOI] [PubMed] [Google Scholar]
- 17. Philis‐Tsimikas A, Billings LK, Busch R, et al. Superior efficacy of insulin degludec/liraglutide versus insulin glargine U100 as add‐on to sodium‐glucose co‐transporter‐2 inhibitor therapy: a randomized clinical trial in people with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21:1399‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller E, Doshi A, Gron R, et al. IDegLira improves patient‐reported outcomes while using a simple regimen with fewer injections and dose adjustments compared with basal‐bolus therapy. Diabetes Obes Metab. 2019;21:2643‐2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Medical Association . Declaration of Helsinki. Ethical principles for medical research involving human subjects: last amended by the 59th WMA General Assembly, Seoul 2008. https://www.wma.net/what‐we‐do/medical‐ethics/declaration‐of‐helsinki/doh‐oct2008/. Accessed July 28, 2021.
- 20. International Conference on Harmonisation Guideline . Integrated addendum to ICH e6(r1): guideline for good clinical practice ICH. E6(R2). 2016. https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf. Accessed July 28, 2021.
- 21. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab. 2013;98:1845‐1859. [DOI] [PubMed] [Google Scholar]
- 22. Su Q, Liu J, Li P, Qian L, Yang W. Relative contribution of fasting and postprandial blood glucose in overall glycemic control: post hoc analysis of a phase IV randomized trial. Diabetes Ther. 2018;9:987‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ji L, Lu J, Zhu D, et al. Chinese expert recommendation on basal insulin treatment in adult type 2 diabetes mellitus. Chin J Diabetes. 2017;25:2‐9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information
Data Availability Statement
The participant‐level analysis data sets for the research presented in the publication are available from the corresponding author on reasonable request.


