Abstract
Hematopoietic cell transplantation (HCT) brings important alterations in erythropoiesis and iron metabolism. Hepcidin, which regulates iron metabolism, increases in iron overload or inflammation and decreases with iron deficiency or activated erythropoiesis. Erythroferrone (ERFE) is the erythroid regulator of hepcidin. We investigated erythropoiesis and iron metabolism after allogeneic HCT in 70 patients randomized between erythropoietin (EPO) treatment or no EPO, by serially measuring hepcidin, ERFE, CRP (inflammation), soluble transferrin receptor (sTfR, erythropoiesis), serum iron and transferrin saturation (Tsat; iron for erythropoiesis) and ferritin (iron stores). We identified biological and clinical factors associated with serum hepcidin and ERFE levels. Serum ERFE correlated overall with sTfR and reticulocytes and inversely with hepcidin. Erythroferrone paralleled sTfR levels, dropping during conditioning and recovering with engraftment. Inversely, hepcidin peaked after conditioning and decreased during engraftment. Erythroferrone and hepcidin were not significantly different with or without EPO. Multivariate analyses showed that the major determinant of ERFE was erythropoiesis (sTfR, reticulocytes or serum Epo). Pretransplant hepcidin was associated with previous RBC transfusions and ferritin. After transplantation, the major determinants of hepcidin were iron status (ferritin at all time points and Tsat at day 56) and erythropoiesis (sTfR or reticulocytes or ERFE), while the impact of inflammation was less clear and clinical parameters had no detectable influence. Hepcidin remained significantly higher in patients with high compared to low pretransplant ferritin. After allogeneic HCT with or without EPO therapy, significant alterations of hepcidin occur between pretransplant and day 180, in correlation with iron status and inversely with erythroid ERFE.
1. INTRODUCTION
Erythropoiesis and iron metabolism are closely related, as iron is critical for the production of red blood cells, and erythropoiesis is an essential actor in the regulation of hepcidin, the regulator of iron metabolism. 1
Hepcidin limits iron absorption by the gut and iron release by macrophages and hepatocytes through binding to and degradation of ferroportin, the iron exporter protein, or through occlusion of its central cavity. 2 , 3 , 4 , 5 Hepcidin synthesis is up‐regulated by iron overload and inflammation leading to reduced iron availability, and down‐regulated by iron deficiency, anemia, erythropoiesis and hypoxia, thereby better meeting iron requirements during erythrocyte production. 3 , 5
Iron regulates hepcidin production through Bone Morphogenetic Protein‐2 and 6 (BMP‐2 and BMP‐6), acting via their receptors and co‐receptors including hemojuvelin (HJV). This induces a phosphorylation cascade leading to activation of the SMAD1/5/8 complex that binds to SMAD4, translocates into the nucleus, binds BMP response elements and induces HAMP gene transcription. 5 , 6 , 7 Matriptase‐2, a protease encoded by the TMPRSS6 gene, causes reduced activation of the BMP‐SMAD pathway upon BMP binding to its receptor and HJV. 5 , 8 Mutations in matriptase thus induce large excesses of hepcidin leading to Iron‐Resistant Iron Deficiency Anemia. 9 Iron also regulates hepcidin expression by activating the SMAD pathway through the HFE/TFR1/TFR2 complex independently of BMP. 5 , 10
Inflammation‐based regulation is mainly dependent on inflammatory cytokines such as interleukin‐6 (IL‐6), which upregulates hepcidin expression by activating the IL‐6 receptor‐JAK2‐STAT3 pathway, the later binding to STAT3‐responsive elements in the hepcidin promoter to induce its transcription. 5 , 11 , 12 In addition, the BMP‐SMAD pathway is also involved in hepcidin production during inflammation. 12
Erythropoiesis inhibits hepcidin expression more efficiently than anemia or hypoxia. 13 A rapid, persistent drop in hepcidin levels was observed after injection of erythropoietin (EPO) in mice, 14 healthy volunteers 15 and patients with renal failure. 16 In 2008, Pinto demonstrated the regulation of hepcidin expression by EPO, in a dose‐dependent manner, suggesting the contribution of a transcription factor in the response of hepcidin to EPO. 17 Later, Kautz identified the erythroid regulator of iron metabolism in erythroferrone (ERFE), establishing the link between EPO and hepcidin. 18 Erythroferrone is released by erythroblasts through STAT‐5 activation upon erythropoietin stimulation and mediates inhibition of hepcidin production when erythropoiesis is stimulated by EPO in mice 18 and humans, 19 but matriptase is also essential for regulation of hepcidin by erythropoiesis. 8 Animal models of inflammation and thalassemia have demonstrated that this negative erythropoietic regulator dominates the positive effect of inflammation 20 or iron overload 21 in hepcidin regulation. We also previously showed that, after autologous hematopoietic cell transplantation (HCT), the major determinants of hepcidin production were iron stores and erythropoietic activity while inflammation exerted a minor role. 22
Hematopoietic cell transplantation offers the opportunity to recapitulate the development of erythropoietic activity from newly developed precursors through fully recovered erythropoiesis. We previously demonstrated that HCT is associated with Epo deficiency 23 , 24 , 25 , 26 , 27 , 28 , 29 and that HCT‐associated anemia is exquisitely sensitive to EPO therapy after autologous 23 , 30 , 31 and allogeneic 31 , 32 HCT. Efficacy of EPO therapy is further enhanced by iron supplementation, 30 , 33 , 34 , 35 , 36 , 37 , 38 without significant short‐term or long‐term toxicity, 33 , 39 as now recommended in published guidelines. 40
Here we aimed to broaden our knowledge of erythropoiesis and iron metabolism during the first 6 months after allogeneic HCT. We investigated longitudinally serum levels of: CRP (marker of inflammation); soluble transferrin receptor (sTfR, quantitative marker of erythropoietic activity); serum iron and transferrin saturation (Tsat; markers of iron availability for erythropoiesis) and ferritin (marker of iron stores); hepcidin (regulator of iron metabolism); and ERFE (erythroid regulator of hepcidin). We compared their evolution with or without erythropoietic stimulating agents (ESA) according to two different schedules. We also examined biological and clinical factors correlated with serum hepcidin and ERFE levels measured at different time points during follow‐up.
2. METHODS
2.1. Study population
We previously published 41 the results of a prospective trial in which 119 patients undergoing allogeneic HCT with peripheral blood stem cells (PBSC) for malignant or non‐malignant diseases at the University Hospital of Liège were randomized between treatment with weekly EPO (Neorecormon, kindly provided by Roche) (n = 57) or no EPO treatment (n = 62).
There were three cohorts of patients, that is those undergoing myeloablative (MA) conditioning who started EPO at day 28 post‐transplant (control arm = 19; EPO arm = 15), and those undergoing nonmyeloablative (NMA) conditioning with EPO started at either day 1 (control arm = 25; EPO arm = 23) or day 28 (control arm = 18; EPO arm = 19). Complete blood counts, Tsat and ferritin were measured routinely and biobanking serum samples were collected prospectively before conditioning and weekly thereafter through day 180 post‐transplant. The trial was approved by the Ethics Committee of the University Hospital of Liège under number 2003/59 and patients (or his/her guardian if of minor age) signed an informed consent for the clinical study, as well as to collect and analyze blood samples. The primary endpoint of achieving a normal Hb value ≥13 g/dL was reached by 63.1% in the EPO arm after a median time of 90 days versus 8.1% in the control arm (p < 0.001).
Among these 119 patients, we excluded those who experienced death, relapse or graft rejection and those given donor lymphocyte infusion the first 100 days after transplantation. Patients without sufficient stored samples were also excluded. Consequently, 70 patients were included in the current study, 35 patients in the EPO arm and 35 in the control arm. There were 24 patients in cohort one, 25 in cohort two and 21 in cohort three. In the EPO arm, four patients received 300 mg of IV iron saccharate (Venofer) for functional iron deficiency (Tsat < 20%): one patient in cohort one received IV iron at day 120, one in cohort 2 at day 87, and two in cohort three at day 120 or 130, respectively. Iron was omitted in patients with severe iron overload (serum ferritin > 2500 μg/L without inflammation or liver damage). Control patients never received IV iron. Clinical and biological characteristics of patients are described in Table 1 and Table S1, respectively. Post‐transplant complications, including infections and acute or chronic GVHD, were recorded.
TABLE 1.
Patient clinical characteristics
| Clinical characteristics | All (n = 70) | Control arm (n = 35) | EPO arm (n = 35) | p value | |
|---|---|---|---|---|---|
| Age (years) | M ± SD | 49 ± 13 | 48 ± 13 | 51 ± 13 | NS |
| Sex | Male | 44 (63%) | 23 (66%) | 21 (60%) | NS |
| Female | 26 (37%) | 12 (34%) | 14 (40%) | NS | |
| Performance status (PS) | 0 | 11 (16%) | 6 (17%) | 5 (14%) | NS |
| 1 | 54 (77%) | 26 (74%) | 28 (80%) | NS | |
| 2 | 5 (7%) | 3 (9%) | 2 (6%) | NS | |
| Disease | Acute leukemia | 20 (29%) | 9 (26%) | 11 (31%) | NS |
| MDS or MPN | 12 (17%) | 9 (26%) | 3 (9%) | NS | |
| Lymphoid malignancy | 36 (51%) | 16 (45%) | 20 (57%) | NS | |
| Other | 2 (3%) | 1 (3%) | 1 (3%) | NS | |
| Disease Risk Index | Low | 17 (24%) | 7 (20%) | 10 (29%) | NS |
| Intermediate | 34 (49%) | 17 (48%) | 17 (48%) | NS | |
| High | 17 (24%) | 9 (26%) | 8 (23%) | NS | |
| Very high | 2 (3%) | 2 (6%) | 0 | NS | |
| Number treatment lines | 0 | 7 (10%) | 5 (14%) | 2 (6%) | NS |
| 1 | 22 (31%) | 8 (23%) | 14 (40%) | NS | |
| 2 or 3 | 30 (43%) | 16 (46%) | 14 (40%) | NS | |
| ≥4 | 11 (16%) | 6 (17%) | 5 (14%) | NS | |
| Pretransplant RBC transfusions M ± SD | 17 ± 21 | 14 ± 17 | 21 ± 24 | NS | |
| Pretransplant platelet transfusions M ± SD | 14 ± 18 | 10 ± 13 | 17 ± 22 | NS | |
| Comorbidity score (HCT‐CI) 43 | 0 | 48 (69%) | 24 (69%) | 24 (69%) | NS |
| 1–2 | 13 (18%) | 7 (20%) | 6 (17%) | NS | |
| ≥3 | 9 (13%) | 4 (11%) | 5 (14%) | NS | |
| Transplant number | First transplant | 43 (61%) | 19 (54%) | 24 (69%) | NS |
| 2nd or 3rd transplant | 27 (39%) | 16 (46%) | 11 (31%) | NS | |
| CD34+ cell dose (106/kg) (all PBSC) M ± SD | 4.60 ± 2.77 | 4.61 ± 2.85 | 4.58 ± 2.73 | NS | |
| Conditioning | Myeloablative (MA) | 24 (34%) | 14 (40%) | 10 (29%) | NS |
| Nonmyeloablative (NMA) | 46 (66%) | 21 (60%) | 25 (71%) | NS | |
| Cohort | MA, EPO at day 28 | 24 (34%) | 14 (40%) | 10 (29%) | NS |
| NMA, EPO at day 0 | 25 (36%) | 11 (31%) | 14 (40%) | NS | |
| NMA, EPO at day 28 | 21 (30%) | 10 (29%) | 11 (31%) | NS | |
| ATG | No | 65 (93%) | 32 (91%) | 33 (94%) | NS |
| Yes | 5 (7%) | 3 (9%) | 2 (6%) | NS | |
| GVHD prophylaxis | Tacrolimus‐based | 17 (24%) | 8 (23%) | 9 (26%) | NS |
| Ciclosporin‐based | 46 (66%) | 21 (60%) | 25 (71%) | NS | |
| Methotrexate +/− other IS | 7 (10%) | 6 (17%) | 1 (3%) | NS | |
| Donor relationship and HLA | Related 10/10 | 25 (36%) | 14 (40%) | 11 (31%) | NS |
| Related HLA‐mismatched | 2 (3%) | 2 (6%) | 0 | NS | |
| Unrelated 10/10 | 25 (36%) | 12 (34%) | 13 (38%) | NS | |
| Unrelated HLA‐mismatched | 18 (25%) | 7 (20%) | 11 (31%) | NS | |
| ABO compatibility | Identical | 33 (47%) | 12 (34%) | 21 (60%) | NS |
| Major mismatch | 11 (16%) | 3 (9%) | 8 (23%) | NS | |
| Minor mismatch | 7 (10%) | 3 (9%) | 4 (11%) | NS | |
| Major + minor mismatch | 19 (27%) | 17 (48%) | 2 (6%) | NS | |
| Donor sex match | F donor > M recipient | 15 (21%) | 6 (17%) | 9 (26%) | NS |
| Other | 55 (79%) | 29 (83%) | 26 (74%) | NS | |
| Acute GVHD | No | 27 (39%) | 11 (31%) | 16 (46%) | NS |
| Yes | 43 (61%) | 24 (69%) | 19 (54%) | NS | |
| Chronic GVHD | No | 8 (11%) | 5 (14%) | 3 (9%) | NS |
| Yes | 62 (89%) | 30 (86%) | 32 (91%) | NS | |
| Infection before day 180 | No | 6 (9%) | 2 (6%) | 2 (6%) | NS |
| Yes | 64 (91%) | 33 (94%) | 33 (94%) | NS | |
Abbreviations: ATG, Anti‐thymocyte globulin; F, female; GVHD, graft‐versus‐host disease; HLA, human leukocyte antigen; IS, Immunosuppressive drug; IV, intravenous; M, mean; M, male; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NS, not significant; PBSC, peripheral blood stem cells; SD, standard deviation.
2.2. Laboratory measurements
Laboratory data were monitored from prior to administration of the conditioning regimen (day −10) until day 180 post‐transplant, partly on fresh blood and partly on serum samples stored at −80°C until analysis.
Complete blood counts (hemoglobin (Hb), white blood cells (WBC) and differential, platelets (Plt), percentage and absolute reticulocyte counts (Ret)) and percentage of hypochromic red cells, 38 , 43 , 45 C‐reactive protein (CRP), creatinine, liver function tests (bilirubin, alanine amino‐transferase (ASAT), lactate dehydrogenase (LDH), albumin and uric acid. Parameters of iron metabolism (serum iron (SeFe), transferrin saturation (Tsat) and ferritin were measured weekly using standard laboratory techniques. The ferritin peak was defined as the highest value between day 0 and day 100 post‐transplant.
Soluble transferrin receptor (sTfR), a quantitative measure of total erythropoietic activity, was measured weekly on stored samples by a commercially available ELISA (R&D, Minneapolis, MN), with normal values ranging from 3000 to 7000 μg/L, as previously reported. 43 , 46 , 47 , 48 , 49
Serum hepcidin‐25 was quantified on serum samples stored before conditioning and on days 0, 7, 14, 28, 60, 100 and 180 , by a liquid chromatography coupled to triple quadrupole mass spectrometry method validated, among others, by the MS‐8 lab of our institution. 50
Serum erythroferrone was measured on stored samples at the same time points by a specific ELISA kit (Intrinsic Lifesciences, The Bio Iron Company, USA, SKU# ERF‐001) in 62 patients (31 controls and 31 in EPO arm). The kit has a detection range of 0.16–10 ng/mL.
Serum Epo levels were measured by a commercially available radioimmunoassay (Incstar Corp., Stillwater, MN), as previously published, 51 , 52 before starting EPO administration, that is at day 28 post‐transplant.
2.3. Statistical analyses
Results were expressed as mean ± standard deviation (SD) unless otherwise stated. Some parameters underwent a logarithm or a square root transformation to normalize their distribution (logarithm for Fer, SeFe, Tsat, sTfR, CRP, creatinine, bilirubin, ASAT, LDH, Hepcidin, Epo and ferritin peak; square root for WBC and differential, platelets and reticulocytes).
Student t tests were used to compare the means of biological parameters between the control and EPO arms at each predetermined time over a follow‐up of 6 months in the three cohorts and in the cumulative group. A generalized linear mixed model (GLMM) was used to compare their evolution with respect to time and treatment arm.
Pearson R correlations were used to assess the relations between biological parameters.
Univariate and multivariate linear regression models were used to explain hepcidin and ERFE at each time point with respect to covariates. All variables whose p value was <0.15 in univariate analysis were included in multivariate analysis using the stepwise method. All p values <0.05 were considered as statistically significant. Clinical parameters (age, sex, diagnosis, Disease‐risk Index (DRI), HCT‐CI comorbidity index, performance status (PS), conditioning, donor type, graft type, ABO, platelet transfusions, CMV status, GVHD prophylaxis, acute and chronic GVHD, infections and EPO treatment) were tested in univariate analyses but did not qualify for multivariate analyses.
In multivariate analyses, we reported r‐square value (R2) to give a global appreciation of the accuracy of the model, as well as beta‐coefficients for significant individual parameters.
Statistical analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC, USA) and graphs were done using R version 3.6.1. or GraphPad Prism version 7 (GraphPad Software, San Diego, CA).
3. RESULTS
3.1. Post‐transplant course of iron and erythropoietic parameters
First, we analyzed the means of the various parameters over time between the control and EPO arms.
Figure 1A represents the evolution of serum ERFE. After an initial rapid decrease during conditioning until day 7, fast recovery occurred in both arms until day 56, after which ERFE stabilized in the control arm while fluctuating with EPO doses in the EPO arm. Generalized linear mixed model (GLMM) confirmed the effect of time on ERFE levels (p = 0.017) but not the effect of treatment arm (control vs. EPO, p = 0.85) in the whole cohort nor in the different subgroups (MA with EPO at day 28; NMA with EPO at day 0 and NMA with EPO at day 28). The difference between the two arms was maximum on day 56 without being significant (4.53 ± 9.23 ng/mL in controls vs. 7.16 ± 15.29 ng/mL in EPO arm; p = 0.09).
FIGURE 1.
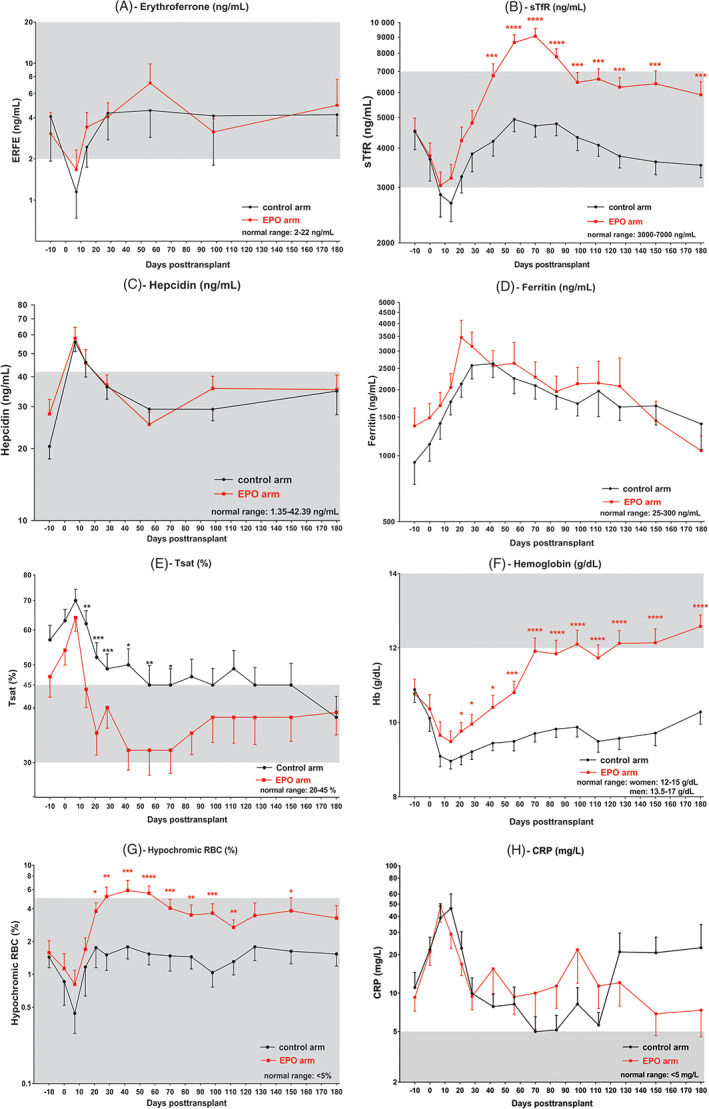
Temporal evolution of mean ± SEM. (A) erythroferrone, (B) sTfR, (C) hepcidin, (D) ferritin, (E) transferrin saturation, (F) hemoglobin, (G) hypochromic red blood cells and (H) CRP following HCT (day 0), in the control (in black, n = 35) and EPO (in red, n = 35) arms. *: <0.05; **: <0.01; ***: <0.001; ****: <0.0001. The gray zone represents the reference interval in normal subjects [Color figure can be viewed at wileyonlinelibrary.com]
Figure 1B displays the evolution sTfR, which was quite similar to that of ERFE: a rapid drop after conditioning until day 7, followed by a sharp increase with engraftment in both arms. However, the EPO arm had significantly higher sTfR levels at each time point from day 42 to day 180 post‐transplant. Note, GLMM confirmed the effect of post‐transplant time (p < 0.001) and treatment arm (p = 0.001) on sTfR values.
Figure 1C shows serum hepcidin over time in the control and EPO arms, with the curve following an inverse kinetics to that of ERFE. Before HCT, hepcidin values were 20.5 ± 13.8 and 27.98 ± 25.06 ng/mL in the control and EPO arms, respectively. Hepcidin peaked 7 days after HCT, followed by a progressive decrease until day 56 and stabilization thereafter. GLMM confirmed the effect of time (p < 0.001) but not treatment arm (p = 0.69) on hepcidin levels.
Figure 1 also describes the post‐transplant course of ferritin (D), transferrin saturation (E), hemoglobin (F), hypochromic red blood cells (G) and CRP (H), while reticulocyte counts (A), albumin (B), LDH (C) and ASAT (D) are displayed in Figure S1. Ferritin peaked 28–42 days after HCT in the two arms, before slowly decreasing to values below those measured at baseline in the EPO arm but remaining higher than at baseline in the control arm. The Tsat level was highest on day 7, then decreased until day 56, with a significantly larger decrease in the EPO arm (p = 0.01). The difference between the two arms was most pronounced on day 21 (52% ± 25% in control vs. 35% ± 22% in EPO arm, p = 0.013). Note, GLMM confirmed the effect of time (p < 0.001) and treatment arm (p = 0.014) on Tsat values. After their initial drop following conditioning, Hb and percentages of hypochromic red cells increased much more significantly in the EPO arm (effect of time and treatment arm confirmed in GLMM, p < 0.001). Thus, CRP peaked during aplasia and changed more randomly thereafter when few patients were infected at the same time point. GLMM demonstrated the effect of time on CRP levels (p < 0.001) but showed no effect of treatment arm (p = 0.56).
Serum hepcidin levels have been shown to correlate positively with serum ferritin 5 and this was confirmed pretransplant in our study. Therefore, we examined the evolution of serum hepcidin and sTfR (Figure 2) in patients separated into two groups based on their pretransplant ferritin value. The cutoff for ferritin levels was their median values (≤ or >820 ng/mL). Baseline hepcidin was significantly higher in patients with higher pretransplant ferritin (13.9 ± 8.6 vs. 4.4 ± 23.6 ng/mL, p < 0.001) and remained so throughout the posttransplant course (p < 0.001) (Figure 2A). When we investigated the course of serum hepcidin in the control and EPO arms separately, there was a small effect of EPO therapy (significant at day 56) in patients with low baseline ferritin (Figure 2B) but not in those with high pre‐transplant ferritin levels (Figure 2C).
FIGURE 2.
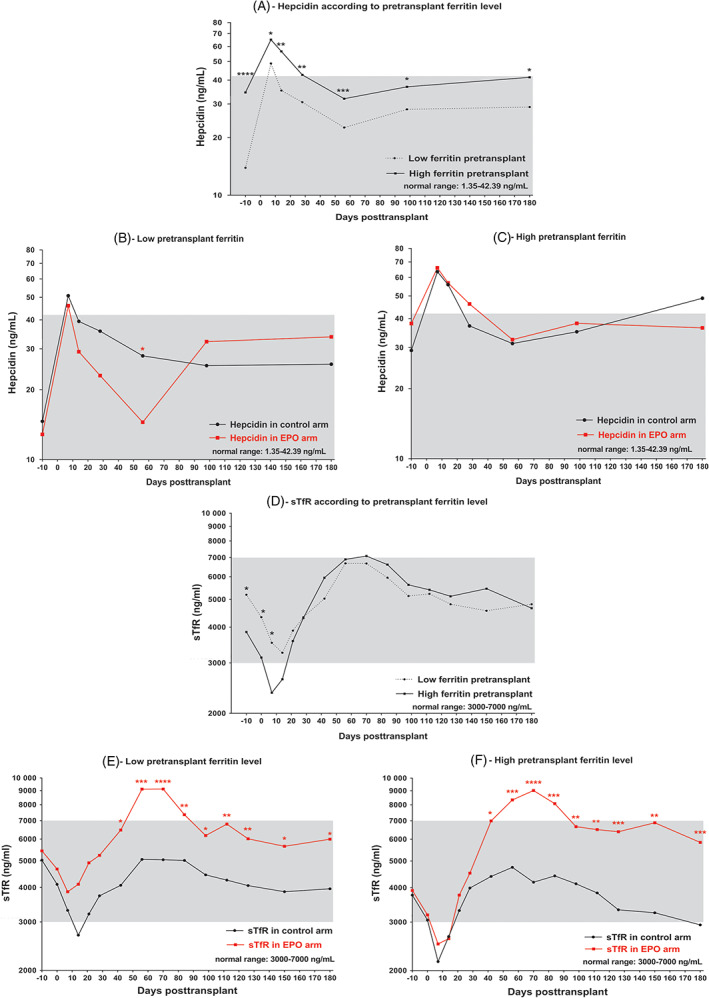
Temporal evolution of (A‐C) mean hepcidin or (D‐F) sTfR following HCT (day 0) according to pretransplant ferritin level (the cutoff for pretransplant ferritin level was the median value of 820 ng/mL), (A,D), in all patients as well as in (B,E), patients with pretransplant ferritin ≤ or > (C,F) the median value of 820 ng/mL in the control (in black, n = 35) and EPO (n = 35, in red) arms. *: <0.05; **: <0.01; ***: <0.001; ****: <0.0001. The gray zone represents the reference interval in normal subjects [Color figure can be viewed at wileyonlinelibrary.com]
Patients with high baseline ferritin were more likely to have myeloid than lymphoid malignancies (p = 0.008), had received more red cell (28 ± 25 vs. 8 ± 9, p < 0.001) and platelet (20 ± 22 vs. 7 ± 9, p < 0.001) transfusions and had significantly lower baseline sTfR values (5193 ± 3327 vs. 3849 ± 2644 ng/mL, p = 0.014) (Figure 2D). During erythropoietic recovery, sTfR increments were observed in the two arms and were clearly stronger in patients receiving EPO therapy, with no major difference between those with low (Figure 2E) or high (Figure 2F) baseline ferritin. Because of a wider range of values, baseline or later ERFE values did not significantly differ between patients with low or high ferritin.
As type of conditioning probably influences erythropoiesis, we compared the means of the different parameters after myeloablative compared to nonmyeloablative conditioning. Figure S2 illustrates the evolution of serum ERFE (A), sTfR (B), hepcidin (C), ferritin (D), Tsat (E), hemoglobin (F), hypochromic red blood cells (G) and CRP (H) according to conditioning. The overall pattern of evolution remained similar with the two types of conditioning, but the magnitude of change was less prominent after nonmyeloablative than after myeloablative conditioning, particularly in the early post‐transplant period. So, ERFE at day 7 (p = 0.049) and sTfR from day 0 until day 14 (p = 0.004 to < 0.001) were significantly lower after myeloablative conditioning, resulting into more anemia (p = 0.007) until day 112. Hepcidin at days 7 (p = 0.0003) and 14 (p = 0.04), ferritin from day 0 until day 70 (p = 0.02 to 0.0007) and Tsat at many time points (p = 0.02 to < 0.001) were higher in patients undergoing myeloablative compared to nonmyeloablative conditioning. In the nonmyeloablative cohorts, EPO therapy was started on day 0 in cohort two or on day 28 in cohort three. Changes induced by EPO treatment were quite comparable in their kinetics and range, although a little delayed in patients started on day 28.
Next, we analyzed correlations between ERFE and hepcidin at a given time point and corresponding values at previous and subsequent time, and investigated factors associated with serum hepcidin and ERFE levels overall as well as at each individual time point.
3.2. Factors associated with serum hepcidin levels
In univariate analyses, hepcidin at any time point correlated well with values at nearly all later time points (p < 0.001). All hepcidin values also correlated with preceding or subsequent ferritin levels (r = 0.39 to 0.74, p < 0.001). Hepcidin also correlated with Tsat (except at day 7; r = 0.25 to 0.36, p = 0.04 to 0.002), and inversely with sTfR (from day −10 to day 28, r = −0.43 to −0.48, p < 0.001), ERFE (on day 14, r = −0.29, p = 0.02), Hb (from day −10 to day 28, r = −0.25 to −0.52, p = 0.04 to < 0.001) and reticulocytes (from day −10 to day 28, r = −0.26 to −0.40, p = 0.03 to < 0.001) at early time points. Negative correlations were also observed between hepcidin and platelets (from day −10 to day 100: r = −0.25 to −0.42, p = 0.04 to < 0.001), WBC (from day −10 to day 14: r = −0.27 to −0.32, p = 0.03 to 0.007), CRP (from day −10 to day 14: r = −0.23 to −0.45, p = 0.046 to < 0.001), but not serum Epo at day 28, creatinine, uric acid, ASAT, bilirubin or LDH. Figure 3 displays the most significant overall correlations, comprising all time points, between hepcidin and ferritin (Figure 3A), Tsat (Figure 3B), ERFE (Figure 3C), sTfR (Figure 3D), reticulocytes (Figure 3E) or Hb (Figure 3F). Finally, based on the median number of four RBC transfusions received between days 0 and 100, we compared hepcidin levels in patients receiving 0–4 versus >4 RBC transfusions. Hepcidin was significantly higher in patients who received >4 RBC transfusions (Figure S3).
FIGURE 3.
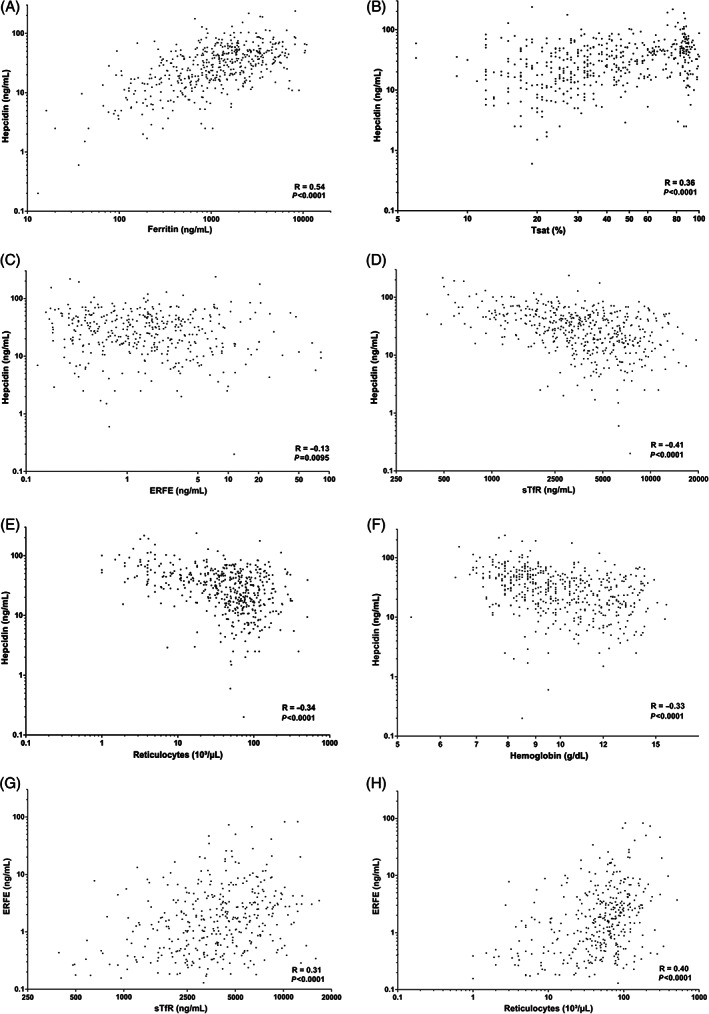
Correlations between same day hepcidin or ERFE and parameters of iron metabolism and erythropoiesis in the whole group of patients, each of them being sampled at all time points after allogeneic HCT. [A‐F] (A) Hepcidin correlated strongly with ferritin (R = 0.54, p < 0.001), (B) Tsat (R = 0.36, p < 0.001), (C) ERFE (R = −0.13, p = 0.01), (D) sTfR (R = −0.41, p < 0.001), (E) reticulocytes (R = −0.34, p < 0.001) or (F) Hb (R = −0.33, p < 0.001). (G) ERFE correlated strongly with sTfR (R = 0.31, p < 0.001) or (H) reticulocytes (R = 0.40, p < 0.001)
In multivariate linear regressions, the number of RBC transfusions (β‐coefficient = 0.11, p = 0.02) and pretransplant ferritin (β‐coefficient = 0.29, p = 0.001) explained 52% of the variation of pretransplant hepcidin (R2 = 0.52, p < 0.001). After transplantation, iron (ferritin at all time points except day 7 (β‐coefficient between 0.38 and 0.65, p < 0.001), Tsat at day 56 (β‐coefficient = −0.62, p = 0.001)) and erythropoietic parameters (reticulocytes at day 7 (β‐coefficient = −0.10, p = 0.004) and 100 (β‐coefficient = −0.05, p = 0.05), ERFE at day 14 (β‐coefficient = −0.23, p < 0.001), and sTfR at days 28 (β‐coefficient = −0.27, p = 0.02) and 56 (β‐coefficient = −0.38, p = 0.006)) were the most predictive factors of hepcidin levels (R2 = 0.36 to 0.60, p < 0.001). Age, sex, diagnosis, DRI, HCT‐CI, PS, conditioning, donor type, graft type, ABO, platelet transfusions, CMV status, GVHD prophylaxis, GVHD, infections and EPO treatment did not contribute to this prediction.
3.3. Factors associated with serum ERFE levels
In univariate analyses, ERFE at any time point correlated only with ERFE values at the next two time points. During conditioning and engraftment until day 14, all ERFE values correlated with preceding, same day or subsequent sTfR levels (r = 0.27 to 0.45, p = 0.03 to 0.001) and reticulocyte counts (except day 28; r = 0.27 to 0.61, p = 0.05 to < 0.001) and the correlations were strong on same day samples considering all time points together for sTfR (r = 0.31, p < 0.001, Figure 3G) and reticulocytes (r = 0.40, p < 0.001, Figure 3H). After engraftment, ERFE correlated negatively with Hb (r = −0.38, p = 0.002 on day 100; r = −0.35, p = 0.01 on day 180). The overall correlation between same day ERFE and hepcidin was weak (r = −0.13, p = 0.01, Figure 3C). We also observed correlations between ERFE and LDH (from day 14 to day 180: r = 0.28 to 0.39, p = 0.03 to 0.009), WBC (from day 7 to day 14: r = 0.25 to 0.45, p = 0.05 to 0.0003), platelets (on days 100 (r = −0.28, p = 0.03) and 180 (r = −0.33, p = 0.03)), but not with ferritin, CRP, renal or liver parameters. ERFE was higher in males compares to females overall (p = 0.03), but this was true only in the control arm (p = 0.01) and not in the EPO arm. Erythroferrone was significantly lower in patients with >4 RBC transfusions between days 0 and 100, but only at days 7 and 14 (Figure S3). This may be explained by the fact that patients with low transfusion needs were less anemic (11.5 ± 2.0 vs. 10.0 ± 2.0 g/dL) before transplantation and more frequently received nonmyeloablative conditioning (only four myeloablated patients among the 36 patients with <4 transfusions) and therefore had less suppression of erythropoiesis.
In multivariate analyses, the major determinant of ERFE during conditioning and engraftment (R2 = 0.18 to 0.39, p = 0.0008 to < 0.001) was erythropoietic activity as measured by sTfR (at days 7 (β‐coefficient = 0.69, p < 0.001) or 100 (β‐coefficient = 0.73, p = 0.003)), reticulocytes (at days −10 (β‐coefficient = 0.16, p = 0.001), 14 (β‐coefficient = 0.14, p = 0.004) or 100 (β‐coefficient = 0.20, p < 0.0001)) or day 28 serum Epo (β‐coefficient = 0.45, p = 0.0032). This is also illustrated by the relatively parallel kinetics of ERFE and sTfR levels (Figure 1). At day 180, ERFE correlated mostly with neutrophils (β‐coefficient = 0.75, p = 0.004) and negatively with Tsat (β‐coefficient = −0.88, p = 0.001). Note, ERFE correlated with male sex pretransplant (β‐coefficient = 0.68, p = 0.03) and at day 14 (β‐coefficient = 0.81, p = 0.02). The other clinical parameters listed for hepcidin did not contribute to prediction of ERFE values.
4. DISCUSSION
Few studies have examined the significance of serum hepcidin in the context of allogeneic HCT 53 , 54 , 55 , 56 , 57 and none has analyzed in detail the interplay between iron metabolism and erythropoietic activity in this setting. In 2014, Kautz identified erythroferrone (ERFE), an erythropoietic regulator of iron mobilization and absorption through hepcidin suppression. 18 Subsequently, studies demonstrated increased serum ERFE low serum hepcidin in subjects living at high altitude and healthy subjects after EPO injection. 58 , 59 This prompted us to investigate ERFE regulation and role in the pathophysiology of iron metabolism and erythropoiesis after allogeneic HCT. A randomized trial of EPO therapy 41 provided a further opportunity to investigate the interactions between iron, inflammation and erythropoietic activity over time after transplantation.
After destruction of erythroid precursors following conditioning, marrow erythroid activity is strongly suppressed. 24 , 60 Indeed, we observed that erythropoiesis decreased consistently, and as expected more severely so after myeloablative than nonmyeloablative conditioning. Both myelosuppression and defective Epo production could contribute, as we previously showed that endogenous Epo remained appropriate for the degree of anemia after nonmyeloablative conditioning, but rapidly became inadequately low after myeloablative transplantation. 25 Hemoglobin, reticulocytes, hypochromic red cells and sTfR decreased sharply and, in addition, we demonstrated for the first time that this also led to a significant drop in ERFE levels. Suppression of erythropoiesis resulted in a significant peak in Tsat values. The close relationship between erythropoietic activity and serum ERFE is also illustrated by significant correlations between ERFE and sTfR or reticulocytes, as well as by the relative parallelism between sTfR and ERFE evolutions from before conditioning through 6 months post‐transplant. Although sTfR and ERFE have similar time course (Figure 1A,B), there is a striking difference between the response of sTfR and ERFE to exogenous EPO. The EPO therapy per se did not translate into further enhancement of ERFE as shown in healthy subjects. 59 One likely explanation is that sTfR (but not ERFE) also responds to EPO‐induced functional iron deficiency, as illustrated by decreasing transferrin saturation and the appearance of hypochromic red cells (Figure 1E,G), sensed by erythroblasts. Moreover, it is possible that, contrarily to EPO therapy in normal individuals with stable erythropoiesis, EPO treatment in the setting of a dynamically changing erythropoietic environment after allogeneic HCT, with large inter‐patient variations, may not produce further statistically significant modifications in ERFE production rates. Finally, it could be speculated that nascent erythroblasts in an altered environment could have an inappropriate secretion of ERFE upon EPO injection or that still unknown inhibitory factors of ERFE secretion may operate in the particular setting of HCT, but this remains to be investigated.
We also observed a strong surge of ferritin values culminating on day 42 in the control arm and day 28 in the EPO arm. This ferritin peak has been observed previously 61 , 62 and may be explained in part by inactive erythropoiesis resulting in decreased marrow demand for iron, Tsat elevation by non‐utilization of serum iron, and further iron deposition in tissues. However, the ferritin peak was delayed relative to engraftment. To explain that, other factors must be involved, such as conditioning‐induced inflammation and hepatic cytolysis. 63 Indeed there was an early, moderate, rise in ASAT and a later increase of LDH (Figure S1C,D), but a relationship with the ferritin course is not evident. On the other hand, CRP also peaked very early (Figure 1H) but its normalization did not prevent ferritin from further increasing. CRP remained stable after nonmyeloablative conditioning (Figure S2H), but this did not prevent ferritin from raising in this setting also (Figure S2D). Serum albumin decreased until day 14 and slowly recovered thereafter (Figure S1B), also preceding ferritin changes. We have not measured other, more specific, markers of inflammation. Of course, iron overload worsens post‐transplant with RBC transfusions, but only four patients received IV iron and none before day 87, excluding IV iron as a culprit. On the other hand, compared to histocompatible HCT, mice given histoincompatible T cells show a loss of expression of hepatic hepcidin, enhancing iron absorption, rising serum iron and contributing to liver iron overload after HCT. 64 Therefore, the pathophysiology of ferritin kinetics after transplantation remains very complex and this study lacks the power to dissect all these potential explanatory pathways. Hence, we are engaged in another study specifically addressing this issue in a group of >500 patients.
Pretransplant hepcidin was strongly associated with the number of previous RBC transfusions and ferritin. Paralleling the kinetics of ferritin and Tsat and inversely following those of ERFE and sTfR, hepcidin increased significantly over baseline and peaked at day 7. This increase could be explained by hepcidin upregulation from inflammation and iron overload through the BMP complex and SMAD pathway 5 and through downregulation of ERFE production following post‐conditioning myelosuppression. 19 This also supports the experimental evidence that suppression of hepcidin during anemia requires erythropoietic activity. 22 , 65 Kanda 54 attributed a similar ferritin peak after autologous HCT to inflammation because of a concomitant elevation of serum IL‐6 levels (not measured in our study), but, although CRP also peaked at day 7, we did not observe any correlation between hepcidin and CRP values.
After day 7, hepcidin progressively returned to baseline, despite persistence of very high ferritin values. Although this was similar with or without EPO therapy, it suggests that engraftment and erythropoietic recovery were capable of a certain degree of regulation of hepcidin production. Hepcidin at all time points correlated positively with ferritin and Tsat and inversely with sTfR, ERFE, Hb and reticulocytes. In addition, there was a strong overall correlation between hepcidin and ferritin (r = 0.54, p < 0.001) and a much weaker negative correlation with ERFE (r = −0.13, p = 0.01). Furthermore, multivariate disclosed very strong associations between hepcidin and ferritin from day 14 through day 180 after HCT, and weaker associations with erythropoietic markers. This could indicate that, contrarily to thalassemia where negative erythropoietic regulators dominate positive iron regulators of hepcidin production, 21 the reverse is true after allogeneic HCT even with EPO therapy. This apparently contradicts our previous study after autologous HCT, 22 where EPO therapy induced stronger inhibition of hepcidin production than in the current study. However, separate analyses of patients with low and high baseline ferritin (Figure 2) showed that EPO therapy modulated hepcidin levels when iron burden was low (as in autologous HCT), but also indicated that the hepcidin‐inducing ability of iron overload in heavily transfused patients with myeloid malignancies 66 was capable to annihilate erythroid regulation of hepcidin by EPO therapy. Likewise, hepcidin was significantly higher and ERFE lower in patients receiving > 4 RBC transfusions between days 0 and 100. These were patients who arrived more anemic at transplantation and received more frequently myeloablative conditioning, but this additional iron burden also contributed to higher hepcidin values.
Furthermore, similar sTfR kinetics in patients with low or high baseline ferritin suggests that this is not due to an impact of iron burden on response to EPO (Figure 2E,F). Serum sTfR depends on the number of cells bearing TfR receptors (elevated with increased erythropoietic activity, for instance with EPO therapy (Figure 2D)) and on TfR receptor density on cells (elevated in iron deficiency but not inflammation). Thus sTfR is a good quantitative marker of erythropoietic activity (high positive correlation between sTfR and Hb, Figure S4) and, to a lesser extent, iron status. 67
In conclusion, we investigated hepcidin regulation in the setting of allogeneic HCT with a variety of parameters of iron metabolism and erythropoiesis. The kinetics and correlations of ERFE, hepcidin and sTfR after transplantation confirm the strong regulatory effects of erythropoiesis and iron status on post‐transplant hepcidin. However, contrary to other situations, the stimulatory effect of iron overload appears to dominate over the suppressive effect of erythropoietic activity on hepcidin production. Inflammation did not appear to exert a major effect on hepcidin. However, besides CRP, which is not well coordinated among the overall patient population as it may vary abruptly in individual patients, inflammation was only assessed by a limited number of less specific markers. For example, Kanda demonstrated an elevation of serum IL‐6 levels concomitantly with the ferritin peak. 54 Another limitation in this study is potential collinearity in multivariable analyses. Indeed, some explanatory variables are highly correlated with each other, and therefore only some remain significant in multivariate analyzes.
The study also supports the role of erythropoiesis in ERFE production. 18 , 59 Investigations of hepcidin and ERFE, in addition to the more classical parameters of iron metabolism and erythropoiesis, may shed additional light on the interplay between erythropoiesis and iron metabolism during allogeneic HCT as well as in other settings.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was funded in part by grants from the FNRS (Fonds National de la Recherche Scientifique)‐Télévie and The Léon Fredericq Foundation at the University of Liège in Belgium. MP is PhD student and FB senior research associate at the FNRS.
Pirotte M, Fillet M, Seidel L, Jaspers A, Baron F, Beguin Y. Erythroferrone and hepcidin as mediators between erythropoiesis and iron metabolism during allogeneic hematopoietic stem cell transplant. Am J Hematol. 2021;96(10):1275‐1286. 10.1002/ajh.26300
Funding information FNRS (Fonds National de la Recherche Scientifique)‐Télévie, Grant/Award Number: 7.6529.18; The Léon Fredericq Foundation at the University of Liège in Belgium
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Beguin Y, Aapro M, Ludwig H, Mizzen L, Osterborg A. Epidemiological and nonclinical studies investigating effects of iron in carcinogenesis: a critical review. Crit Rev Oncol Hematol. 2014;89(1):1‐15. [DOI] [PubMed] [Google Scholar]
- 2. Qiao B, Sugianto P, Fung E, et al. Hepcidin‐induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012;15(6):918‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105(2):260‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aschemeyer S, Qiao B, Stefanova D, et al. Structure‐function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. 2018;131(8):899‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117(17):4425‐4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canali S, Wang CY, Zumbrennen‐Bullough KB, Bayer A, Babitt JL. Bone morphogenetic protein 2 controls iron homeostasis in mice independent of Bmp6. Am J Hematol. 2017;92(11):1204‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meynard D, Kautz L, Darnaud V, Canonne‐Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478‐481. [DOI] [PubMed] [Google Scholar]
- 8. Nai A, Rubio A, Campanella A, et al. Limiting hepatic bmp‐Smad signaling by matriptase‐2 is required for erythropoietin‐mediated hepcidin suppression in mice. Blood. 2016;127(19):2327‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jaspers A, Caers J, Le Gac G, Ferec C, Beguin Y, Fillet G. A novel mutation in the CUB sequence of matriptase‐2 (TMPRSS6) is implicated in iron‐resistant iron deficiency anaemia (IRIDA). Br J Haematol. 2013;160(4):564‐565. [DOI] [PubMed] [Google Scholar]
- 10. Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin‐induced hepcidin expression. Cell Metab. 2009;9(3):217‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armitage AE, Eddowes LA, Gileadi U, et al. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118(15):4129‐4139. [DOI] [PubMed] [Google Scholar]
- 12. Mayeur C, Lohmeyer LK, Leyton P, et al. The type I BMP receptor Alk3 is required for the induction of hepatic hepcidin gene expression by interleukin‐6. Blood. 2014;123(14):2261‐2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Q, Davidoff O, Niss K, Haase VH. Hypoxia‐inducible factor regulates hepcidin via erythropoietin‐induced erythropoiesis. J Clin Invest. 2012;122(12):4635‐4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sasaki Y, Noguchi‐Sasaki M, Yasuno H, Yorozu K, Shimonaka Y. Erythropoietin stimulation decreases hepcidin expression through hematopoietic activity on bone marrow cells in mice. Int J Hematol. 2012;96(6):692‐700. [DOI] [PubMed] [Google Scholar]
- 15. Ashby DR, Gale DP, Busbridge M, et al. Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica. 2010;95(3):505‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weiss G, Theurl I, Eder S, et al. Serum hepcidin concentration in chronic haemodialysis patients: associations and effects of dialysis, iron and erythropoietin therapy. Eur J Clin Invest. 2009;39(10):883‐890. [DOI] [PubMed] [Google Scholar]
- 17. Pinto JP, Ribeiro S, Pontes H, et al. Erythropoietin mediates hepcidin expression in hepatocytes through EPOR signaling and regulation of C/EBPalpha. Blood. 2008;111(12):5727‐5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arezes J, Foy N, McHugh K, et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018;132(14):1473‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lasocki S, Millot S, Andrieu V, et al. Phlebotomies or erythropoietin injections allow mobilization of iron stores in a mouse model mimicking intensive care anemia. Crit Care Med. 2008;36(8):2388‐2394. [DOI] [PubMed] [Google Scholar]
- 21. Nemeth E. Hepcidin and β‐thalassemia major. Blood. 2013;122(1):3‐4. [DOI] [PubMed] [Google Scholar]
- 22. Jaspers A, Baron F, Willems E, et al. Serum hepcidin following autologous hematopoietic cell transplantation: an illustration of the interplay of iron status, erythropoiesis and inflammation. Haematologica. 2014;99(3):e35‐e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beguin Y, Baron F, Fillet G. Influence of marrow erythropoietic activity on serum erythropoietin levels after autologous hematopoietic stem cell transplantation. Haematologica. 1998;83(12):1076‐1081. [PubMed] [Google Scholar]
- 24. Beguin Y, Oris R, Fillet G. Dynamics of erythropoietic recovery following bone marrow transplantation: role of marrow proliferative capacity and erythropoietin production in autologous versus allogeneic transplants. Bone Marrow Transplant. 1993;11(4):285‐292. [PubMed] [Google Scholar]
- 25. Baron F, Fillet G, Beguin Y. Erythropoiesis after nonmyeloablative stem‐cell transplantation is not impaired by inadequate erythropoietin production as observed after conventional allogeneic transplantation. Transplantation. 2002;74(12):1692‐1696. [DOI] [PubMed] [Google Scholar]
- 26. Beguin Y, Clemons GK, Oris R, Fillet G. Circulating erythropoietin levels after bone marrow transplantation: inappropriate response to anemia in allogeneic transplants. Blood. 1991;77(4):868‐873. [PubMed] [Google Scholar]
- 27. Beguin Y, Yerna M, Loo M, Weber M, Fillet G. Erythropoiesis in multiple myeloma: defective red cell production due to inappropriate erythropoietin production. Br J Haematol. 1992;82(4):648‐653. [DOI] [PubMed] [Google Scholar]
- 28. Cazzola M, Guarnone R, Cerani P, Centenara E, Rovati A, Beguin Y. Red blood cell precursor mass as an independent determinant of serum erythropoietin level. Blood. 1998;91(6):2139‐2145. [PubMed] [Google Scholar]
- 29. Beguin Y, Clemons GK, Pootrakul P, Fillet G. Quantitative assessment of erythropoiesis and functional classification of anemia based on measurements of serum transferrin receptor and erythropoietin. Blood. 1993;81(4):1067‐1076. [PubMed] [Google Scholar]
- 30. Beguin Y, Maertens J, De Prijck B, et al. Darbepoetin‐alfa and intravenous iron administration after autologous hematopoietic stem cell transplantation: a prospective multicenter randomized trial. Am J Hematol. 2013;88(12):990‐996. [DOI] [PubMed] [Google Scholar]
- 31. Locatelli F, Zecca M, Pedrazzoli P, et al. Use of recombinant human erythropoietin after bone marrow transplantation in pediatric patients with acute leukemia: effect on erythroid repopulation in autologous versus allogeneic transplants. Bone Marrow Transplant. 1994;13(4):403‐410. [PubMed] [Google Scholar]
- 32. Baron F, Sautois B, Baudoux E, Matus G, Fillet G, Beguin Y. Optimization of recombinant human erythropoietin therapy after allogeneic hematopoietic stem cell transplantation. Exp Hematol. 2002;30(6):546‐554. [DOI] [PubMed] [Google Scholar]
- 33. Jaspers A, Baron F, Maertens J, et al. Long‐term safety follow‐up of a randomized trial of darbepoetin alpha and intravenous iron following autologous hematopoietic cell transplantation. Am J Hematol. 2015;90(7):E133‐E134. [DOI] [PubMed] [Google Scholar]
- 34. Cavill I, Auerbach M, Bailie GR, et al. Iron and the anaemia of chronic disease: a review and strategic recommendations. Curr Med Res Opin. 2006;22(4):731‐737. [DOI] [PubMed] [Google Scholar]
- 35. Aapro M, Österborg A, Gascón P, Ludwig H, Beguin Y. Prevalence and management of cancer‐related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol. 2012;23(8):1954‐1962. [DOI] [PubMed] [Google Scholar]
- 36. Beguin Y. Prediction of response to optimize outcome of treatment with erythropoietin. Semin Oncol. 1998;25(3 Suppl 7):27‐34. [PubMed] [Google Scholar]
- 37. Beguin Y. Prediction of response and other improvements on the limitations of recombinant human erythropoietin therapy in anemic cancer patients. Haematologica. 2002;87(11):1209‐1221. [PubMed] [Google Scholar]
- 38. Beguin Y, Loo M, R'Zik S, et al. Early prediction of response to recombinant human erythropoietin in patients with the anemia of renal failure by serum transferrin receptor and fibrinogen. Blood. 1993;82(7):2010‐2016. [PubMed] [Google Scholar]
- 39. Jaspers A, Baron F, Servais S, et al. Erythropoietin therapy after allogeneic hematopoietic cell transplantation has no impact on long‐term survival. Am J Hematol. 2015;90(9):E197‐E199. [DOI] [PubMed] [Google Scholar]
- 40. Aapro M, Beguin Y, Bokemeyer C, et al. Management of anaemia and iron deficiency in patients with cancer: ESMO clinical practice guidelines. Ann Oncol. 2018;29(Suppl 4):iv271. [DOI] [PubMed] [Google Scholar]
- 41. Jaspers A, Baron F, Willems E, et al. Erythropoietin therapy after allogeneic hematopoietic cell transplantation: a prospective, randomized trial. Blood. 2014;124(1):33‐41. [DOI] [PubMed] [Google Scholar]
- 42. Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664‐3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)‐specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912‐2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bovy C, Tsobo C, Crapanzano L, et al. Factors determining the percentage of hypochromic red blood cells in hemodialysis patients. Kidney Int.. 1999;56(3):1113‐1119. [DOI] [PubMed] [Google Scholar]
- 45. Bovy C, Gothot A, Delanaye P, Warling X, Krzesinski JM, Beguin Y. Mature erythrocyte parameters as new markers of functional iron deficiency in haemodialysis: sensitivity and specificity. Nephrol Dial Transplant. 2007;22(4):1156‐1162. [DOI] [PubMed] [Google Scholar]
- 46. Beguin Y. The soluble transferrin receptor: biological aspects and clinical usefulness as quantitative measure of erythropoiesis. Haematologica. 1992;77(1):1‐10. [PubMed] [Google Scholar]
- 47. R'zik S, Beguin Y. Serum soluble transferrin receptor concentration is an accurate estimate of the mass of tissue receptors. Exp Hematol. 2001;29(6):677‐685. [DOI] [PubMed] [Google Scholar]
- 48. R'zik S, Loo M, Beguin Y. Reticulocyte transferrin receptor (TfR) expression and contribution to soluble TfR levels. Haematologica. 2001;86(3):244‐251. [PubMed] [Google Scholar]
- 49. Cazzola M, Beguin Y, Bergamaschi G, et al. Soluble transferrin receptor as a potential determinant of iron loading in congenital anaemias due to ineffective erythropoiesis. Br J Haematol. 1999;106(3):752‐755. [DOI] [PubMed] [Google Scholar]
- 50. Diepeveen LE, Laarakkers CMM, Martos G, et al. Provisional standardization of hepcidin assays: creating a traceability chain with a primary reference material, candidate reference method and a commutable secondary reference material. Clin Chem Lab Med. 2019;57(6):864‐872. [DOI] [PubMed] [Google Scholar]
- 51. Beguin Y, Lipscei G, Oris R, Thoumsin H, Fillet G. Serum immunoreactive erythropoietin during pregnancy and in the early postpartum. Br J Haematol. 1990;76(4):545‐549. [DOI] [PubMed] [Google Scholar]
- 52. Corazza F, Beguin Y, Bergmann P, et al. Anemia in children with cancer is associated with decreased erythropoietic activity and not with inadequate erythropoietin production. Blood. 1998;92(5):1793‐1798. [PubMed] [Google Scholar]
- 53. Kanda J, Mizumoto C, Kawabata H, et al. Clinical significance of serum hepcidin levels on early infectious complications in allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(8):956‐962. [DOI] [PubMed] [Google Scholar]
- 54. Kanda J, Mizumoto C, Kawabata H, et al. Serum hepcidin level and erythropoietic activity after hematopoietic stem cell transplantation. Haematologica. 2008;93(10):1550‐1554. [DOI] [PubMed] [Google Scholar]
- 55. Eisfeld AK, Westerman M, Krahl R, et al. Highly elevated serum Hepcidin in patients with acute myeloid leukemia prior to and after allogeneic hematopoietic cell transplantation: does this protect from excessive parenchymal iron loading? Adv Hematol. 2011;2011:491058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sakamoto S, Kawabata H, Kanda J, et al. High pretransplant hepcidin levels are associated with poor overall survival and delayed platelet engraftment after allogeneic hematopoietic stem cell transplantation. Cancer Med. 2017;6(1):120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li X, Xu F, Karoopongse E, et al. Allogeneic transplantation, Fas signaling, and dysregulation of hepcidin. Biol Blood Marrow Transplant. 2013;19(8):1210‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ganz T, Jung G, Naeim A, et al. Immunoassay for human serum erythroferrone. Blood. 2017;130(10):1243‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Robach P, Gammella E, Recalcati S, et al. Induction of erythroferrone in healthy humans by micro‐dose recombinant erythropoietin or high‐altitude exposure. Haematologica. 2020;106:384‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vellenga E, Sizoo W, Hagenbeek A, Löwenberg B. Different repopulation kinetics of erythroid (BFU‐E), myeloid (CFU‐GM) and T lymphocyte (TL‐CFU) progenitor cells after autologous and allogeneic bone marrow transplantation. Br J Haematol. 1987;65(2):137‐142. [DOI] [PubMed] [Google Scholar]
- 61. Fingrut W, Law A, Lam W, et al. Post‐transplant ferritin level predicts outcomes after allogeneic hematopoietic stem cell transplant, independent from pre‐transplant ferritin level. Ann Hematol. 2021;100:789‐798. [DOI] [PubMed] [Google Scholar]
- 62. Or R, Matzner Y, Konijn AM. Serum ferritin in patients undergoing bone marrow transplantation. Cancer. 1987;60(5):1127‐1131. [DOI] [PubMed] [Google Scholar]
- 63. Gordon LI, Brown SG, Tallman MS, et al. Sequential changes in serum iron and ferritin in patients undergoing high‐dose chemotherapy and radiation with autologous bone marrow transplantation: possible implications for treatment related toxicity. Free Radic Biol Med. 1995;18(3):383‐389. [DOI] [PubMed] [Google Scholar]
- 64. Bair S, Spaulding E, Parkkinen J, et al. Transplantation of allogeneic T cells alters iron homeostasis in NOD/SCID mice. Blood. 2009;113(8):1841‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730‐3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre‐transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95(3):476‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta. 2003;329(1–2):9‐22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


