Abstract
Background
Consideration of the huge burden both of tuberculosis (TB) and diabetes mellitus (DM) in China as a major public health issue, research focused on the relationship between DM and TB was needed.
Methods
An observational study was conducted (2015‐2018) in regional representative TB and lung disease hospitals in China. All the adult patients newly diagnosed of pulmonary TB were consecutively recruited in this study.
Results
A total of 1417 patients newly diagnosed pulmonary TB was recruited in this research, 312 (22.02%) of them had the history of type 2 DM. Majority of patients were with fatigue, loss of weight and mild anaemia in TB‐DM group compared with TB‐NDM group (58.3% vs 47.5%, p = .001; 8.21 ± 6.2 vs 5.74 ± 4.0 kg, p < .001, 88.9% vs 77.6% p = .021). TB‐DM patients were with higher the proportion of TB severity score ≥3, compared with TB‐NDM patients, but the distributions of drug susceptibility testing (DST) analysis were not significantly different between the two groups of patients. Remarkably, the sign of central shadow of pulmonary lobe distribution and cavity in TB‐DM group presented significantly higher rate than it in TB‐NDM group. Multivariable logistic regression showed that high uric acid level was an independent risk factor for thick wall cavity in TB‐DM patients (OR 2.81, 95% CI 1.24‐6.40), haemoptysis (OR 2.43, 95% CI 1.10‐5.38) and chest pain (OR 5.22, 95% CI 1.38‐19.70) were significantly associated with thick wall cavity.
Conclusions
The clinical features of TB‐DM patients are associated with cavities in CT scan, rather than DST results. It can help us recognition confounding variables, also may influence the treatment strategy and outcomes in TB‐DM patients.
Keywords: CT feature, diabetes mellitus, pulmonary tuberculosis
1. BACKGROUND
Tuberculosis (TB) is the leading cause of death due to infectious diseases in the worldwide. Although the number of new TB cases is decreasing each year, closely achieving goal of reverse the spread of the disease, this advancement is threatened by a rapid increasing burden of noncommunicable diseases in populations from TB‐endemic countries. 1 , 2 , 3 The rising epidemic of diabetes mellitus (DM) becomes one of the important global health challenges and could lead to an increase burden in TB. 2 , 4 The burden of both TB and DM has attracted much attention in the past few years as diabetes prevalence has increased remarkably in countries which had already afflicted with a huge burden of TB. Tackling co‐epidemic of diabetes and TB is important in both policy making and clinical practice.
Previous studies proved a strong relationship between DM and active TB, which demonstrated that DM was triple‐increased risk of TB infection among patients with DM than those without. 5 , 6 , 7 , 8 , 9 , 10 These are crucial considerations as DM is frequent associated with elder, obesity, underlying diseases, which can both affect TB risk and treatment outcomes. 11 , 12
With the evidence supporting the TB–DM relationship, there is a need for us in improving our understanding on the epidemiological and clinical features of TB patients combined with DM. Therefore, we performed a cross‐sectional study to assess the clinical manifestation and CT‐scan features of TB patients with DM among newly diagnosed TB patients in China.
2. METHODS
2.1. Study design
A national‐wide, multicentre, cross‐sectional study on new diagnosis of pulmonary TB patients was conducted from January 2015 to December 2018. The study was performed at eight hospitals from six provinces with TB endemic area of China. The names and the distribution of eight hospitals were listed in Table S1. Patients with suspicious symptoms of airway diseases were consecutively screened. After identification of positive Mycobacterium tuberculosis from sputum, patients were enrolled in this study. Patients meet the following aspects had been enrolled: newly diagnosed pulmonary TB; above 18 years old; confirmed by positive M. tuberculosis from sputum cultivation and examined by thoracic computed tomography (CT) scan. The exclusion criteria were patients had been diagnosed of TB previously or received anti‐TB therapy.
Clinical data was collected, including patients’ characteristics, condition disease, thoracic CT scan manifestation, sputum cultivation and drug susceptibility testing (DST), when patients were included in this research. The diagnosis of DM was recorded by self‐report or matching DM diagnosis criteria according to the American Diabetes Association guideline or under therapy of hypoglycaemic drugs. 13 Recruited TB patients were divided into two groups according to whether they were comorbid with DM. Protocol and materials of this research were reviewed and approved by Institutional Review Board in Beijing Hospital, China (protocol number: 2016BJYYEC‐014‐01).
2.2. Laboratory diagnosis and DST
Two sputum samples were obtained from each recruited patient for sputum cultivation and DST. DST of M. tuberculosis was performed by absolute concentration method on L‐J media following the guideline of WHO. The performance of laboratory procedures by investigators in each participant site was trained and supervised by TB national reference laboratory.
The definition of mono‐resistance (MR) referred to resistance to one first‐line anti‐TB drug only. The definition of poly‐drug resistance (PDR) referred to resistance to more than one first‐line anti‐TB drugs, other than both isoniazid and rifampicin. The definition of multidrug resistance (MDR) referred to resistance to at least both isoniazid and rifampicin, and the definition of extensive‐drug resistance (XDR) referred to resistance to not only isoniazid and rifampicin, but also three of the six classes of second‐line drugs.
2.3. CT scan and image assessments
All the enrolled patients were examined by thoracic CT scan when they were diagnosed of active pulmonary TB. The radiological presentations were reviewed separately by two radiologists and clinical specialists for pulmonary TB characteristics. The imaging assessments consisted of consolidation (nodules above 1 cm in diameters), central nodules of pulmonary lobule (between 2 and 6 cm in diameters); cavities (cavity with thick wall [>2 mm] and with thin wall [<2 mm]); unilateral pleural effusion; mediastinal hilar lymphadenopathy; bilateral military nodules; multiple morphological changes; Tree in bud pattern (segmental distribution); Distribution of features were described and recorded, including the posterior segment of the upper lobe tip and the dorsal segment of the lower lobe; and the signs of extensive thickening of tracheal wall and mediastinal lymphadenopathy.
2.4. Severity evaluation of TB
A modified version of TB severity score was used in order to evaluate severity of TB symptoms in study. According to the scoring system, the presence of each TB‐related symptom was given for one point, including cough, fever, weight loss, night sweats, anorexia, haemoptysis, and malaise. Six score category was ranged from 0 to 6, equalled from no symptom to all symptoms, which was a modified severity evaluation referenced by previous research. 14 Patients with a score value ≥3 were considered as suffering severe TB in this study.
2.5. Statistical analysis
Categorical variables were summarized as frequency (percentage) and continuous variables were presented as mean ± standard deviation (SD). Student's t test or (Fisher) Chi‐square test was used to test difference in continuous and categorical variables between two groups. A univariate logistic regression model was performed to identify the relationship between each risk factor/clinical feature and the CT presentation of lung cavity. The variables which achieved a significance level of 0.05 and those of our interests according to expert opinions or literature reviews were included in the multivariable stepwise logistic regression analysis. Collinearity diagnosis was performed before multivariable analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to quantify the associations. All analyses were performed using SPSS 25 software (Chicago, IL, USA). The p value less than .05 was considered to be statistically significant.
3. RESULTS
3.1. Demographic information in pulmonary TB patients with or without DM
A total of 1417 patients newly diagnosed of pulmonary TB were enrolled in this study, including 312 (22.02%) TB patients with DM and 1105 (77.98%) without DM (see flowchart in Figure 1). Clinical characteristics of the two groups of patients are shown in Table 1. A majority of TB patients comorbid with DM were male, compared to those without DM (male gender in TB‐DM group vs in TB‐NDM group; 80.4% vs 68.6%, p < .001). TB‐DM patients were older than TB‐NDM patients (54.2 ± 12.4 years vs 45.5 ± 19.1 years, p < .001). TB patients with DM had a higher BMI compared to TB patients without DM (21.91 ± 3.76 vs 19.66 ± 3.33, p < .001). Smoking status and alcoholic drinking history are similar between groups.
FIGURE 1.
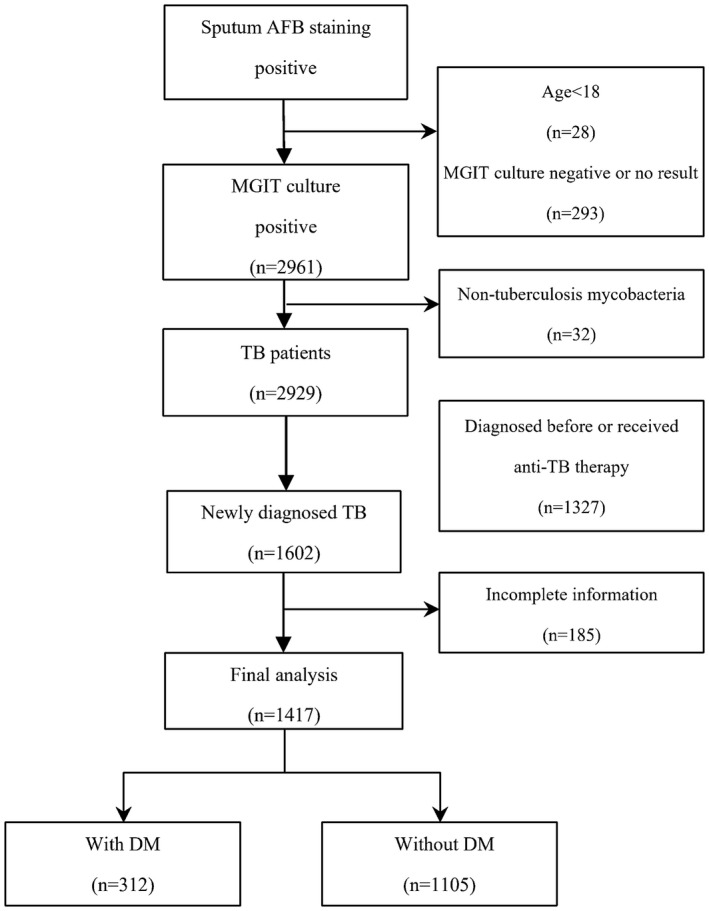
Flowchart for patient's enrollment. AFB, anti‐fast bacilli; MGIT, mycobacteria growth indicator tube; TB, tuberculosis; DM, diabetes mellitus
TABLE 1.
Clinical characteristics in TB patients with or without DM
| Parameters | TB DM (N = 312) | TB Non‐DM (N = 1105) | p value |
|---|---|---|---|
| Demographic features | |||
| Male (%) | 251 (80.4) | 758 (68.6) | <.001 |
| Age (SD) | 54.2 (12.4) | 45.5 (19.1) | <.001 |
| BMI (kg/m2) (SD) | 21.9 (3.8) | 19.7 (3.3) | <.001 |
| Smoking | |||
| Non‐smoker (%) | 178 (57.2) | 679 (61.4) | .1661 |
| Ever‐smoker (%) | 134 (42.9) | 427 (38.6) | |
| History of long‐term heavy drinking | |||
| Never (%) | 220 (70.7) | 835 (75.9) | .0638 |
| Ever (%) | 91 (29.3) | 265 (24.1) | |
| Symptoms and signs | |||
| Fever (%) | 139 (44.6) | 480 (43.6) | .755 |
| Night sweats (%) | 101 (32.4) | 345 (31.3) | .721 |
| Fatigue (%) | 182 (58.3) | 524 (47.5) | .001 |
| Cough (%) | 291 (93.3) | 1037 (94.0) | .63 |
| Haemoptysis (%) | 55 (17.6) | 185 (16.8) | .727 |
| Chest pain (%) | 29 (9.3) | 154 (14.0) | .028 |
| Dyspnea (%) | 52 (16.7) | 192 (17.4) | .755 |
| Weight loss (%) | 101 (32.4) | 346 (31.4) | .744 |
| Loss of weight (kg) (SD) | 8.21 (6.2) | 5.74 (4.0) | <.001 |
| TB severity score (SD) | 2.79 (1.3) | 2.65 (1.3) | .098 |
| TB severity score >3 (%) | 178 (57.2) | 559 (50.7) | .045 |
| Laboratory tests | |||
| Anaemia a | |||
| Mild (%) | 96 (88.9) | 337 (77.6) | .021 |
| Moderate (%) | 11 (10.2) | 95 (21.9) | |
| Severe (%) | 2 (0.9) | 2 (0.5) | |
| ALT | |||
| <40 IU/L (%) | 270 (98.5) | 908 (98.2) | .911 |
| 120‐200 IU/L (%) | 2 (0.7) | 8 (0.9) | |
| >200 IU/L (%) | 2 (0.7) | 9 (1.0) | |
| AST | |||
| <40 IU/L (%) | 279 (98.9) | 933 (98.6) | .919 |
| 120‐200 IU/L (%) | 2 (0.7) | 9 (1.0) | |
| >200 IU/L (%) | 1 (0.4) | 4 (0.4) | |
| Uric acid | |||
| Normal (%) | 276 (89.3) | 905 (82.6) | .005 |
| Elevated b (%) | 33 (10.7) | 191 (17.4) | |
Abbreviations: ALT, glutamic‐pyruvic transaminase; AST, glutamic oxaloacetic transaminase; BMI, body mass index; DM, diabetes mellitus; SD, standard deviation; TB, tuberculosis.
Mild anaemia refers to haemoglobin between 90 g/L and 120 g/L for male, and between 90 g/L and 110 g/L for female, respectively; moderate anaemia refers to haemoglobin between 60 and 89 g/L; severe anaemia refers to haemoglobin between 30 and 60 g/L.
Elevation refers to serum uric acid >420 µmol/L in male and >360 µmol/L in female.
3.2. Clinical manifestation in pulmonary TB patients with or without DM
Pulmonary TB patients were observed of typical manifestations of fever, cough, fatigue, chest pain, dyspnea and loss of weight. According to these symptoms, a majority of patients had fatigue and lost more weight in the TB‐DM group than the TB‐NDM group (58.3% vs 47.5%, p = .001; 8.21 ± 6.2 vs 5.74 ± 4.0 kg, p < .001). Laboratory tests showed anaemia was a prominent item in TB patients. The majority of patients had mild anaemia. The proportion of mild anaemia of patients in TB‐DM group was higher than it was in TB‐NDM group (88.9% vs 77.6% p = .021). The proportion of TB severity score ≥3 in TB‐DM group was higher than it in TB‐NDM groups (57.2% vs 50.7%, p = .045).
3.3. Drug resistance profile in TB patients
All the subjects recruited in this study were performed for DST results. The distributions of drug resistance phenotypes are similar between two groups, showed 26.0% of drug resistant in TB patients with DM and 23.3% of drug resistant in TB patients without DM. The rates of mono‐resistance, PDR, MDR and XDR between the two groups were comparable. Also, the cases of resistance of isoniazid, rifampin, levofloxacin and amikacin were counted (Table 2).
TABLE 2.
Drug resistance manifestation of TB patients with and without DM
| Parameters | DM (N = 312) | Non‐DM (N = 1105) | p value |
|---|---|---|---|
| Drug resistant TB (%) | 81 (26.0) | 258 (23.3) | .437 |
| Mono‐resistance (%) | 54 (66.7) | 154 (59.7) | .31 |
| PDR (%) | 2 (2.5) | 9 (3.5) | |
| MDR (%) | 15 (18.5) | 72 (27.9) | |
| XDR (%) | 10 (12.3) | 23 (8.9) | |
| Isoniazid (%) | 57 (18.3) | 190 (17.2) | .645 |
| Rifampin (%) | 36 (11.5) | 126 (11.4) | 1.00 |
| Levofloxacin (%) | 20 (6.4) | 44 (4.0) | .088 |
| Amikacin (%) | 8 (2.6) | 14 (1.3) | .119 |
Abbreviations: DM, diabetes mellitus; MDR, multi‐drug resistant; PDR, poly‐drug resistant; TB, tuberculosis; XDR, extensive‐drug resistant.
3.4. Assessment of imaging features on CT scan in pulmonary TB patients with or without DM
Compared with the TB‐NDM‐ patients, signs of central nodules of pulmonary lobe, cavities and extensive thickening of tracheal wall were more common in TB‐DM patients (p < .05). Among these features, the sign of central nodules of pulmonary lobe distribution and cavity in TB–DM patients were significantly higher than in TB‐NDM patients (42.9% vs 33.8%, 66.7% vs 58.4%), especially thick wall (wall thickness above 2 mm). Signs of extensive thickening of trachea wall were with higher ratio in TB‐DM patients than in TB‐NDM patients. Other signs including consolidation, unilateral pleural effusion, tree in bud pattern and so on, showed no statistically significant differences between the two groups (Table 3).
TABLE 3.
Radiological presentation (by computed tomography) in TB patients with or without DM
| Parameters | DM (N = 312) | Non‐DM (N = 1105) | p value |
|---|---|---|---|
| Consolidation (nodules above 1 cm in diameter) (%) | 213 (68.3) | 766 (69.4) | .698 |
| Central nodules of pulmonary lobule (between 2 to 6 mm in diameters) (%) | 134 (42.9) | 374 (33.8) | .003 |
| Cavities | 208 (66.7) | 645 (58.4) | .009 |
| Thick wall (wall thickness > 2 mm) (%) | 195 (62.5) | 595 (53.8) | .007 |
| Thin wall (wall thickness < 2 mm) (%) | 39 (12.5) | 117 (10.6) | .355 |
| Unilateral pleural effusion (%) | 51 (16.3) | 197 (17.8) | .554 |
| Mediastinal hilar lymphadenopathy (%) | 66 (21.2) | 246 (22.3) | .66 |
| Bilateral military nodules (%) | 18 (5.7) | 50 (4.5) | .37 |
| Multiple morphological changes (%) | 211 (67.6) | 763 (69.0) | .529 |
| Tree in bud sign (segmental distribution) (%) | 119 (38.15) | 373 (33.8) | .16 |
| Posterior segment of the upper lobe tip and the dorsal segment of the lower lobe distribution (%) | 161 (51.6) | 529 (47.9) | .238 |
| Extensive thickening of tracheal wall (%) | 37 (11.9) | 80 (7.2) | .014 |
Abbreviation: DM, diabetes mellitus.
3.5. Risk and associated factors of TB‐DM patient with presentation of cavities
As cavity on CT scan was observed a significant presentation among TB‐DM patients, multivariable regression was conducted to investigate risk factors of different types of cavities in TB patients with DM. Age, BMI and haemoptysis were related to cavity as the sign of CT scans among TB‐DM patients, high uric acid level was an independent risk factor for thick wall cavity in TB‐DM patients, and some symptoms, especially haemoptysis, chest pain were significantly associated with thick wall cavity (Table 4). Whereas fatigue and amikacin resistance were significantly associated with thin wall cavity.
TABLE 4.
Multivariable analysis for TB‐DM patients with CT presentation of cavities
| Cavity of either kind | Thick wall cavity | Thin wall cavity | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) b | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.98 (0.96‐1.00) | .0330 | 0.97 (0.95‐0.99) | .0052 | ||
| BMI | 0.89 (0.82‐0.96) | .0043 | 0.86 (0.79‐0.94) | .0011 | ||
| Fever | 0.54 (0.31‐0.94) | .0283 | ||||
| Night sweat | 0.31 (0.13‐0.78) | .0131 | ||||
| Fatigue | 6.12 (2.24‐16.73) | .0004 | ||||
| Haemoptysis | 2.26 (1.05‐4.84) | .0369 | 2.43(1.10‐5.38) | .0282 | ||
| Chest pain | 5.22 (1.38‐19.70) | .0147 | ||||
| Weight loss | 0.51 (0.28‐0.95) | .0324 | ||||
| Elevated uric acid a | 2.81 (1.24‐6.40) | .0136 | ||||
| Amikacin resistance | 9.63 (1.90‐48.85) | .0062 | ||||
Abbreviations: BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; OR, odds ratio.
Refers to serum uric acid >420 µmol/L in male and >360 µmol/L in female.
Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multivariate logistic regression models (stepwise framework) with non‐missing data.
4. DISCUSSION
The high prevalence of type 2 DM threatens the advanced controlling made in reducing TB burden worldwide. DM brings a significant threaten to public, by increasing the risk of TB, particularly in countries where both diseases are prevalent. Much more concern was raised by experts about the merging epidemics of DM and TB. 15 , 16 , 17 Therefore, there is an urgent need for a systematic assessment of the association between DM and TB. DM was associated with an increased risk of TB which had been proved by previous studies, but they have not fully elaborated the influence of DM on TB clinical manifestation. It was still puzzled for clinicians that the sequential orders of DM and TB: whether DM caused TB or whether TB led to the clinical manifestations of DM. 18 , 19 , 20 TB disease severity was categorized by clinical scores, which have been previously reported and performed in clinical research. 14 Here, we summarize the clinical manifestations of DM on TB, including clinical symptoms, radiological (presence of cavitation) and microbiological (sputum cultivation positivity) information. In this research, the combination of TB symptoms, radiographic finding in CT scan (cavity) and presence of positive sputum cultivation at TB diagnosis was considered as the feature of TB patients with DM, especially for sign of cavity on CT scan. Although high uric acid level was reached statistical significance as an independent risk factor for thick‐wall cavity, their clinical significance was not adequately discussed. Specific CT scan features can distinguish TB‐DM patients from other patients. If patients were presenting thick‐wall cavity with smear positive, they would continue producing TB and it would be necessary that the prolonging of treatment period in order to eradication of the TB cavity. Analysis using the combination of symptoms with radiological and microbiological data revealed that TB‐DM patients, which can help the physicians stratify of patients between TB and TB‐DM. Although some items just showed as risk factor in statistical significance, the CT sign of TB‐DM patients themselves were much more important. The clinical features of TB‐DM individuals associated with cavities in CT scan and TB cavities in lung may reduce the ratio of sputum negative conversion, also may influence the treatment strategy and outcomes in TB‐DM patients.
At the initial time of TB diagnosis and treatment we noticed a high proportion of new adult subjects with pulmonary TB also suffering DM, which is nearly 22% in cross‐sectional study. 21 Among those identified with DM, a quarter did not have a previous diagnosis of DM and nearly a third were not receiving DM treatment. People with DM may become special targets for interventions and efforts which should make to diagnose, detect, and treat DM, which may benefit to TB control. In this research, the clinical features of TB‐DM patients are associated with cavities in thoracic CT scan. According to the duration of clinical symptoms and radiographic abnormalities, cavities were broadly classifying by two types: an acute or subacute process (<12 weeks) and a chronic process (≥12 weeks). 22 It also can be influenced by immune status due to the interaction of pathogens and host, like mycobacterium TB and DM patients. Smear or cultivation positive TB patients with cavities in thoracic CT scan could continuously expelling M. tuberculosis, which need early identification and treatment, in order to prevention and control of transmission. This study still has strength. It thoroughly assesses clinical features of TB patients with DM. Thoracic CT scan used in pulmonary TB could show information in detail which will be informative to physicians in this area. There are also some limitations of this study. First, as this was a multi‐centered cross‐sectional study without following up. Visiting for these patients could help us understand the relationship of TB and DM in a longitude observation. Second, we did not consider the disease duration from onset to diagnosis, which may influence the feature in CT scan, for example the incidence of cavitation usually showed in chronic pulmonary TB disease. 23 , 24
5. CONCLUSIONS
Diabetes mellitus was common comorbidity in newly diagnosed pulmonary TB patients in China. The clinical features of TB‐DM patients are associated with cavities in CT scan, rather than DST results. It can help us to recognize confounding variables and also may influence the treatment strategy and outcomes in TB‐DM patients.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Yanming Li and Xunliang Tong contributed to the conception and design of the study, acquisition of the data, and interpretation of the results and drafted the manuscript. He Wang, Yixuan Liao, Xuefeng Zhong and Yang Ju contributed to the acquisition of the mycological data and revision of the manuscript for important intellectual content. Yimeng Song, Yuanchun Li and Yue Zhang contributed to the acquisition of the bacteriological data and revision of the manuscript for important intellectual content. Dingyi Wang and Guohui Fan performed the statistical analysis and revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol and materials were reviewed and approved by Institutional Review Board in Beijing Hospital, China (protocol number: 2016BJYYEC‐014‐01).
CONSENT FOR PUBLICATION
Not applicable.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors thank Academician Chen Wang for his guidance and assistance with this work.
Tong X, Wang D, Wang H, et al. Clinical features in pulmonary tuberculosis patients combined with diabetes mellitus in China: An observational study. Clin Respir J. 2021;15:1012–1018. 10.1111/crj.13405
Funding information
This project was supported by grant 81400037 from the National Natural Science Foundation of China
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article.
REFERENCES
- 1. Degner NR, Wang JY, Golub JE, Karakousis PC. Metformin use reverses the increased mortality associated with diabetes mellitus during tuberculosis treatment. Clin Infect Dis. 2018;66:198‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al‐Rifai RH, Pearson F, Critchley JA, Abu‐Raddad LJ. Association between diabetes mellitus and active tuberculosis: a systematic review and meta‐analysis. PLoS One. 2017;12(11):e0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Global Status Report on Noncommunicable Diseases 2014. Geneva, Switzerland: WHO; 2015. [DOI] [PubMed] [Google Scholar]
- 4. Global, regional, and national disability‐adjusted life‐years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England). 2016;388:1603‐1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim SJ, Hong YP, Lew WJ, Yang SC, Lee EG. Incidence of pulmonary tuberculosis among diabetics. Tuber Lung Dis. 1995;76(6):529‐533. [DOI] [PubMed] [Google Scholar]
- 6. Pablos‐Mendez A, Blustein J, Knirsch CA. The role of diabetes mellitus in the higher prevalence of tuberculosis among Hispanics. Am J Public Health. 1997;87(4):574‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alisjahbana B, van Crevel R , Sahiratmadja E, et al. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis. 2006;10(6):696‐700. [PubMed] [Google Scholar]
- 8. Perez A, Brown HS 3rd, Restrepo BI. Association between tuberculosis and diabetes in the Mexican border and non‐border regions of Texas. Am J Trop Med Hyg. 2006;74(4):604‐611. [PMC free article] [PubMed] [Google Scholar]
- 9. Marks SM. Diabetes and tuberculosis, US National Health Interview Survey, 2000‐2005. Int J Tuberc Lung Dis. 2011;15(7):982‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baghaei P, Marjani M, Tabarsi P, et al. Impact of chronic renal failure on anti‐tuberculosis treatment outcomes. Int J Tuberc Lung Dis. 2014;18(3):352‐356. [DOI] [PubMed] [Google Scholar]
- 12. Kobashi Y, Mouri K, Yagi S, et al. Clinical features of immunocompromised and nonimmunocompromised patients with pulmonary tuberculosis. J Infect Chemother. 2007;13(6):405‐410. [DOI] [PubMed] [Google Scholar]
- 13. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42(Suppl 1):S13‐S28. [DOI] [PubMed] [Google Scholar]
- 14. Hella J, Cercamondi CI, Mhimbira F, et al. Anemia in tuberculosis cases and household controls from Tanzania: contribution of disease, coinfections, and the role of hepcidin. PLoS One. 2018;13(4):e0195985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Restrepo BI. Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances. Clin Infect Dis. 2007;45(4):436‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dixon B. Diabetes and tuberculosis: an unhealthy partnership. Lancet Infect Dis. 2007;7(7):444. [DOI] [PubMed] [Google Scholar]
- 17. Stevenson CR, Forouhi NG, Roglic G, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boucot KR, Dillon ES, Cooper DA, Meier P, Richardson R. Tuberculosis among diabetics: the Philadelphia survey. Am Rev Tuberc. 1952;65(1:2):1‐50. [PubMed] [Google Scholar]
- 19. Nichols GP. Diabetes among young tuberculous patients; a review of the association of the two diseases. Am Rev Tuberc. 1957;76(6):1016‐1030. [DOI] [PubMed] [Google Scholar]
- 20. Silwer H, Oscarsson PN. Incidence and coincidence of diabetes mellitus and pulmonary tuberculosis in a Swedish county. Acta Med Scand Suppl. 1958;335:1‐48. [PubMed] [Google Scholar]
- 21. Yang BR, Kang YA, Heo EY, et al. Regional differences in the incidence of tuberculosis among patients with newly diagnosed diabetes mellitus. Clin Respir J. 2018;12(4):1732‐1738. [DOI] [PubMed] [Google Scholar]
- 22. Gafoor K, Patel S, Girvin F, et al. Cavitary lung diseases: a clinical‐radiologic algorithmic approach. Chest. 2018;153(6):1443‐1465. [DOI] [PubMed] [Google Scholar]
- 23. Goto A, Komiya K, Kan T, et al. Factors associated with atypical radiological findings of pulmonary tuberculosis. PLoS One. 2019;14(7):e0220346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller WT, Miller WT Jr. Tuberculosis in the normal host: radiological findings. Semin Roentgenol. 1993;28(2):109‐118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
All data generated or analysed during this study are included in this published article.


