Abstract
Objectives
To perform a systematic review and network meta‐analysis to compare the efficacy and safety of currently available treatments for the management of metastatic hormone‐sensitive prostate cancer (mHSPC), as there has been a paradigm shift with the use of next‐generation androgen receptor inhibitors (ARIs) and docetaxel.
Methods
Multiple databases were searched for articles published before May 2020 according to the Preferred Reporting Items for Systematic Review and Meta‐analysis extension statement for network meta‐analysis. Studies comparing overall/progression‐free survival (OS/PFS) and/or adverse events (AEs) in patients with mHSPC were eligible.
Results
Nine studies (N = 9960) were selected, and formal network meta‐analyses were conducted. Abiraterone (hazard ratio [HR] 0.83, 95% credible interval [CrI] 0.76–0.90), docetaxel (HR 0.90, 95% CrI 0.82–0.98), and enzalutamide (HR 0.85, 95% CrI 0.73–0.99) were associated with significantly better OS than androgen‐deprivation therapy (ADT), and abiraterone emerged as the best option. Abiraterone (HR 0.71, 95% CrI 0.67–0.76), apalutamide (HR 0.73, 95% CrI 0.65–0.81), docetaxel (HR 0.84, 95% CrI 0.78–0.90), and enzalutamide (HR 0.67, 95% CrI 0.63–0.71) were associated with significantly better PFS than ADT, and enzalutamide emerged as the best option. Abiraterone (HR 0.85, 95% CrI 0.78–0.93), apalutamide (HR 0.87, 95% CrI 0.77–0.98), and enzalutamide (HR 0.80, 95% CrI 0.73–0.88) were significantly more effective than docetaxel. Regarding AEs, apalutamide was the likely best option among the three ARIs. In patients with low‐volume mHSPC, enzalutamide was the best option in terms of OS and PFS.
Conclusions
All three ARIs are effective therapies for mHSPC; apalutamide was the best tolerated. All three seemed more effective than docetaxel. These findings may facilitate individualised treatment strategies and inform future comparative trials.
Keywords: metastatic hormone‐sensitive prostate cancer, androgen receptor inhibitors, docetaxel, network meta‐analysis, #ProstateCancer, #PCSM
Abbreviations
- ADT
androgen‐deprivation therapy
- AE
adverse event
- ALT
alanine aminotransferase
- AR(I)
androgen receptor (inhibitor)
- AST
aspartate aminotransferase
- CrI
credible interval
- (m)CRPC
(metastatic) castration‐resistant prostate cancer
- HR
hazard ratio
- (m)HSPC
(metastatic) hormone‐sensitive prostate cancer
- NMA
network meta‐analysis
- OS
overall survival
- PFS
progression free survival
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RCT
randomised controlled trial
- STAMPEDE
Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy
Introduction
Prostate cancer is the most common solid cancer and the second most common cause of cancer‐related death in men [1]. Of the estimated 175 000 new cases of prostate cancer in the USA diagnosed in 2019, ~6% will present with de novo metastatic disease and a significant number of patients will develop metastasis despite prior therapy with curative intent [1, 2, 3, 4, 5]. Systemic androgen‐deprivation therapy (ADT) has been the standard primary treatment strategy in patients with advanced prostate cancer. However, despite adequate therapy, the disease eventually progresses to a castration‐resistant prostate stage in most patients [6, 7].
Although ADT was the only systemic treatment option available for metastatic hormone‐sensitive prostate cancer (mHSPC) for many years, clinicians now have access to various life‐prolonging therapies that can be combined with ADT. These include docetaxel, abiraterone acetate, enzalutamide, and apalutamide, which all show significant survival benefits when combined with ADT compared to ADT alone [8, 9, 10, 11, 12]. However, data directly comparing the effectiveness and safety of these agents to inform optimal treatment decisions and guideline recommendations are limited. Treatment selection remains an individualised decision with factors such as disease burden, cost, toxicity, performance status, access to drug, and patient as well as physician preference playing a role [13]. Several network meta‐analyses (NMAs) have been performed to summarise the evidence from indirect comparisons [14, 15, 16, 17]. However, these NMAs did not include all possible comparisons, as they were limited to docetaxel and abiraterone or they did not include recently published data, or they analysed a heterogeneous population (including not only metastatic disease but also non‐metastatic advanced disease). Indeed, long‐term results of the Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDEClinicalTrials.gov identifier: NCT00268476) and LATITUDE (ClinicalTrials.gov identifier: NCT01715285) trials were recently reported [18, 19]. Therefore, we conducted a systematic review of all clinical trials assessing treatment with next‐generation androgen receptor inhibitors (ARIs) and docetaxel chemotherapy for mHSPC with ADT as the control arm, and performed NMAs to indirectly compare the efficacy and safety of these agents. These data may help inform and guide clinicians in their shared decision‐making with their patients regarding the best individualised treatment.
Methods
The protocol has been registered in the International Prospective Register of Systematic Reviews database (PROSPERO: CRD42020185965).
Search Strategy
The systematic review and NMA of randomised controlled trials (RCTs) comparing systemic therapies for mHSPC were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) extension statement for NMAs [20]. The PubMed, Web of Science, and Scopus databases were searched to identify reports published before May 2020 on systemic therapy for mHSPC. The following keywords were used in our search strategy: (prostate carcinoma OR prostate cancer OR prostatic carcinoma OR prostatic cancer) AND (metastatic OR M1 OR advanced) AND (castration sensitive OR castration naive OR hormone sensitive OR hormone naive) AND (Randomized). Furthermore, we also reviewed clinical trial registries and relevant abstracts presented at major conferences including the American Society of Clinical Oncology and the European Society for Medical Oncology. The primary outcome of interest was overall survival (OS), and the secondary outcomes were progression free survival (PFS) and adverse events (AEs). The detailed database search strategy is presented in Appendix S1.
Initial screening was performed independently by two investigators based on the titles and abstracts of the article to identify ineligible reports. Reasons for exclusions were noted. Potentially relevant reports were subjected to a full‐text review, and the relevance of the reports was confirmed after data extraction. Disagreements were resolved via consensus with the co‐authors.
Inclusion and Exclusion Criteria
Studies were included if they investigated patients with mHSPC (Patients) who had undergone systemic therapy (Intervention) compared with those treated with ADT or another systemic therapy (Comparison) to assess the differential effects of treatment on OS, PFS, and AEs (Outcome) in randomised studies only. We excluded observational studies, reviews, letters, editorials, meeting abstracts, replies from authors, and case reports. We did not apply language restrictions. References of all papers included were scanned for additional studies of interest.
Data Extraction
Two investigators independently extracted the following information from the included articles: first author’s name, publication year, number of patients, agents, treatment dosage, age, inclusion criteria, subsequent therapy, disease volume, follow‐up duration, oncological outcomes, and AE outcomes. Subsequently, the hazard ratios (HRs) and 95% CIs associated with OS and PFS, and AE rate were retrieved. All HRs were derived from Cox models. All discrepancies regarding data extraction were resolved by consensus with the co‐authors.
Risk of Bias Assessment
The risk of bias of each study was assessed according to The Cochrane Collaboration’s tool for assessing risk of bias [21]. This tool assesses selection bias (random sequence generation and allocation concealment), performance bias, detection bias, attrition bias, reporting bias, and other sources of bias (Fig. S1). The risk of bias of each study was assessed independently by two authors.
Statistical Analyses
The PFS was defined as the time from treatment initiation to radiological or clinical progression, or death. We did not include biochemical progression. The OS was defined as the time from treatment initiation to death from any cause. For each outcome, we conducted an NMA using random and fixed effect models for direct and indirect treatment comparisons [22, 23]. When assessing PFS and OS, contrast‐based analyses were applied with estimated differences in the log HR and the standard error calculated from the published HRs and CIs [24]. Relative treatment effects were presented as HR and 95% credible interval (CrI) [22]. For OS and PFS, subgroup analyses were conducted among high‐ and low‐volume disease. High‐volume disease was defined as the presence of metastases involving the viscera, or in the absence of visceral lesions, four or more bone lesions, one or more of which must have been in a bony structure beyond the vertebral column and pelvic bone, according to ChemoHormonal therapy versus Androgen Ablation Randomized Trial for Extensive Disease in prostate cancer (CHAARTED) criteria [8]. For AEs, arm‐based analyses were performed to estimate the odds ratios (ORs) and 95% CrI from the available raw data presented in the selected manuscripts [22]. We also estimated the relative ranking of the different treatments for each outcome using a P‐score, which can be considered a frequentist analogue to the surface under the cumulative ranking curves [25, 26]. Network plots were used to illustrate the connectivity of the treatment networks in terms of OS, PFS, and AEs. Heterogeneity was assessed using I 2 when more than one trial was available for a given comparison. All statistical analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) and Stata/MP 14.2 (Stata Corp., College Station, TX, USA); P < 0.05 was considered to indicate statistical significance.
Certainty of Evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool to evaluate each outcome for certainty or quality of evidence with the levels of evidence divided into ‘very low’, ‘low’, ‘moderate’, or ‘high’ (Table S1) [27, 28]. The evaluation of evidence certainty began as high, and by evaluating the limitations in risk of bias, inconsistency, imprecision, indirectness, and publication bias, the certainty of the evidence could be rated down.
Results
Study Selection and Characteristics
Our initial search identified 896 publications, and a total of 789 publications remained after elimination of the duplicates. A total of 747 articles were excluded after screening the titles and abstracts, and full‐text reviews were performed on 42 articles (Fig. 1). According to the selection criteria, we identified nine articles comprising 9960 patients for inclusion in our systematic review and NMA [8, 9, 10, 11, 12, 18, 19, 29, 30, 31, 32, 33, 34, 35]. The data extracted from the nine studies are outlined in Table 1. These studies were published between 2013 and 2019 and included 4994 patients treated with ADT, 3596 patients treated with next‐generation ARIs, and 1370 patients treated with docetaxel.
Fig. 1.
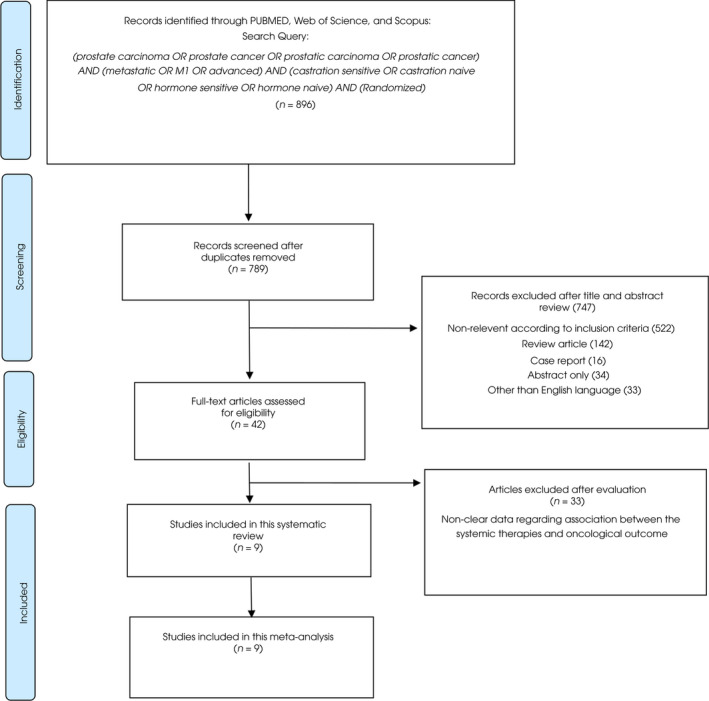
The PRISMA flow chart, detailing the article selection process.
Table 1.
Study characteristics of the nine studies.
| ARCHES | CHARRTED | ENZAMET | GETUG‐AFU15 | LATITUDE | STAMPEDE (Arm C, G) | STAMPEDE (Arm G) | STAMPEDE (Arm B, C, E) | TITAN | |
|---|---|---|---|---|---|---|---|---|---|
| Author | Armstrong | Kyriakopoulos | Davis | Gravis | Fizazi | Sydes | James | Clarke | Chi |
| Year | 2019 | 2018 | 2019 | 2016 | 2019 | 2018 | 2017 | 2019 | 2019 |
| Agents | Enzalutamide + ADT | Docetaxel + ADT | Enzalutamide + ADT | Docetaxel + ADT | Abiraterone + ADT | Abiraterone + ADT | Abiraterone + ADT | Docetaxel + ADT | Apalutamide + ADT |
| Dosage | 160 mg | 75 mg/m2 | 160 mg | 75 mg/m2 | 1000 mg | 1000 mg | 1000 mg | 75 mg/m2 | 240 mg |
| Control | Placebo + ADT | ADT | NSAA + ADT | ADT (including plus NSAA) | Placebo + ADT | Docetaxel + ADT | ADT | ADT | Placebo + ADT |
| Inclusion criteria | mHSPC | mHSPC | mHSPC | mHSPC |
High‐risk mHSPC Gleason ≥8 Bone meta ≥3 Visceral meta |
mHSPC or high‐risk locally advanced PC | mHSPC or high‐risk locally advanced PC | mHSPC | mHSPC |
| N | 1150 | 790 | 1125 | 385 | 1199 | 566 | 1917 | 1086 | 1152 |
| N (treatment) | 574 | 397 | 563 | 192 | 597 | 377 | 960 | 362 | 525 |
| N (control) | 576 | 393 | 562 | 193 | 602 | 189 | 957 | 724 | 527 |
| Age, years, median (range) |
70 (46−92) 70 (42−92) |
64 (36−88) 63 (39−91) |
69.2 (63.2−74.5) 69.0 (63.6−74.5) |
63 (57−68) 64 (58−70) |
67.3 66.8 |
66 (61−70) 66 (62−71) |
67 (63−72) 67 (62−72) |
65 (60−71) 65 (60−70) |
69 (45−94) 68 (43−90) |
| de novo disease, % |
73.0 72.0 |
72.8 73.0 |
57.7 58.2 |
67.7 66.2 |
100 100 |
92.8 96.8 |
93.7 96.0 |
95.9 95.2 |
82.1 85.0 |
| Docetaxel | Prior docetaxel were permitted (17.9%/17.7%) | No use | Concurrently docetaxel were permitted (44.3%/45.1%) | No use | No use | No use | No use | No use | Prior docetaxel were permitted (11.0%/10.4%) |
| Subsequent therapy, % | NR | NR |
67 85 |
30 57 |
NR |
53 58 |
68 80 |
38 61 |
|
| Disease volume (high/low), % |
62/38 65/35 |
66/34 64/36 |
52/48 53/47 |
48/52 47/53 |
82/18 78/22 |
NR |
46/54 57/43 |
54/46 57/43 |
62/38 64/36 |
| OS, months, median |
NRE/NRE HR 0.81 95% CI 0.53–1.25 |
57.6/47.2 HR 0.72 95% CI 0.59–0.89 |
NRE/NRE HR 0.67 95% CI 0.52–0.86 |
39.8/35.1 HR 0.78 95% CI 0.56–1.09 |
53.3/36.5 HR 0.66 95% CI 0.56–0.78 |
NR HR 1.13 95%CI 0.77–1.66 |
NR HR 0.61 95% CI 0.49–0.75 |
39.9/35.2 HR 0.81 95% CI 0.69‐0.95 |
NRE/NRE HR 0.67 95% CI 0.51–0.89 |
| PFS, months, median |
rPFS NRE/19 HR 0.39 95% CI 0.30–0.50 |
cPFS 33.0/19.8 HR 0.62 95% CI 0.51–0.75 |
cPFS NR HR 0.40 95% CI 0.33–0.49 |
rPFS 22.9/15.3 HR 0.69 95% CI 0.55–0.87 |
rPFS 33.0/14.8 HR 0.47 95% CI 0.39–0.55 |
rPFS NR HR 0.69 95% CI 0.50–0.95 |
rPFS 33.0/14.8 HR 0.69 95% CI 0.59–0.81 |
rPFS 33.0/14.8 HR 0.69 95% CI 0.59–0.81 |
rPFS NRE/22.1 HR 0.48 95% CI 0.39–0.60 |
| Follow‐up, months, median | 14.4 | 53.7 | 34 | 83.9 | 30.4 | 48 | 40 | 78.2 | 22.9 |
c, clinical; meta, metastasis; NR, not reported; NRE, not reached; NSAA, non‐steroidal antiandrogen; PC, prostate cancer; r, radiographic.
NMA
The networks of eligible comparisons are graphically represented in network plots in terms of OS and PFS (Fig. S2).
OS
An NMA assessing five different agents was conducted for the primary outcome of OS. Compared with ADT, abiraterone, docetaxel, and enzalutamide resulted in significantly improved OS (HR 0.83, 95% CrI 0.76–0.90; HR 0.90, 95% CrI 0.82–0.98; and HR 0.85, 95% CrI 0.73–0.99, respectively; Fig. 2A). According to treatment ranking analysis, abiraterone had the highest likelihood of providing the maximal OS (P‐score, 0.7666), closely followed by apalutamide and enzalutamide (P‐score, 0.6718 and 0.6283, respectively), and then followed by docetaxel (P‐score, 0.4170; Table 2). Heterogeneity was low in this analysis (I 2 = 0%).
Fig. 2.
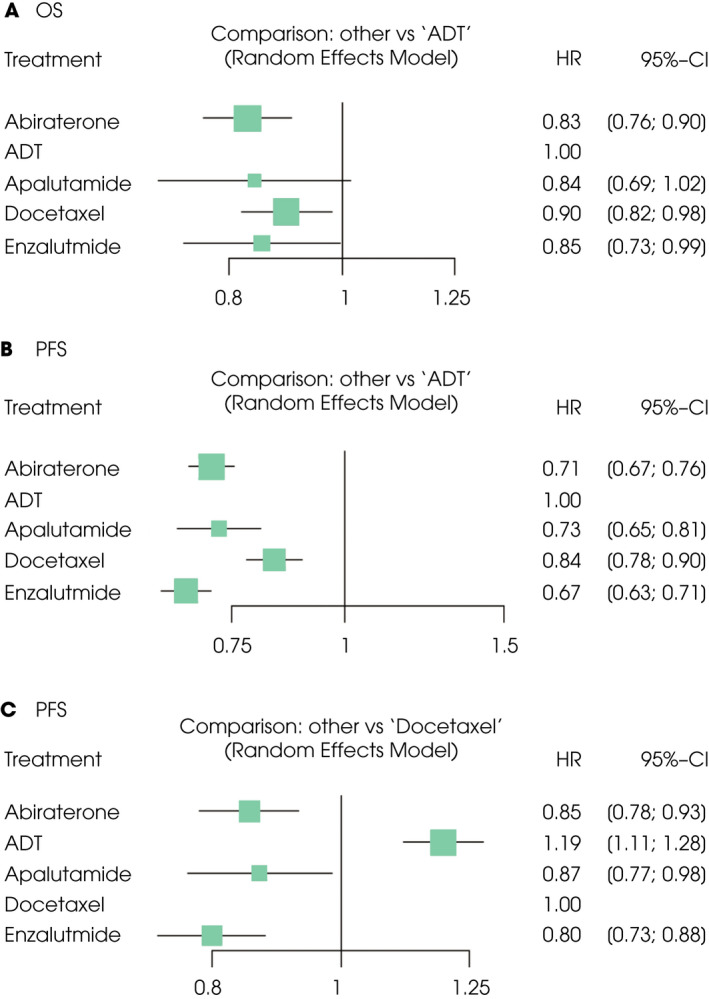
Forest plots showing the association of systemic therapy in mHSPC. (A) OS, (B) PFS (vs ADT), (C) PFS (vs docetaxel).
Table 2.
Analysis of the treatment ranking (OS and PFS).
| Treatment | P‐score (fixed) | P‐score (random) |
|---|---|---|
| OS | ||
| Abiraterone | 0.7666 | 0.7666 |
| Apalutamide | 0.6718 | 0.6718 |
| Enzalutamide | 0.6283 | 0.6283 |
| Docetaxel | 0.4170 | 0.4170 |
| ADT | 0.0163 | 0.0163 |
| PFS | ||
| Enzalutamide | 0.9613 | 0.9613 |
| Abiraterone | 0.6704 | 0.6704 |
| Apalutamide | 0.6148 | 0.6148 |
| Docetaxel | 0.2535 | 0.2535 |
| ADT | 0.0000 | 0.0000 |
PFS
An NMA assessing five different agents was conducted for the secondary outcome of PFS. Compared with ADT, abiraterone, apalutamide, docetaxel, and enzalutamide resulted in significantly improved PFS (HR 0.71, 95% CrI 0.67–0.76; HR 0.73, 95% CrI 0.65–0.81; HR 0.84, 95% CrI 0.78–0.90; and HR 0.67, 95% CrI 0.63–0.71, respectively; Fig. 2B). Compared with docetaxel, abiraterone, apalutamide, and enzalutamide resulted in significantly improved PFS (HR 0.85, 95% CrI 0.78–0.93; HR 0.87, 95% CrI 0.77–0.98; and HR 0.80, 95% CrI 0.73–0.88, respectively; Fig. 2C). According to treatment ranking analysis, enzalutamide had the highest likelihood of providing the maximal PFS (P‐score: 0.9613; Table 2). Heterogeneity was low in this analysis (I 2 = 0%).
AEs
An NMA assessing four different agents was conducted for the various outcomes of AEs (including any AEs, Grade ≥3 AEs, and serious AEs). None of the three next‐generation ARIs were associated with a significantly higher likelihood of toxicity defined as any AEs, Grade ≥3 AEs, or serious AEs compared to ADT (Fig. 3A–C). According to treatment ranking analysis, apalutamide was highly likely to have the lowest rate of all AE outcomes compared to abiraterone and enzalutamide (Table S2). Available AE profiles are summarised in Fig. S3.
Fig. 3.
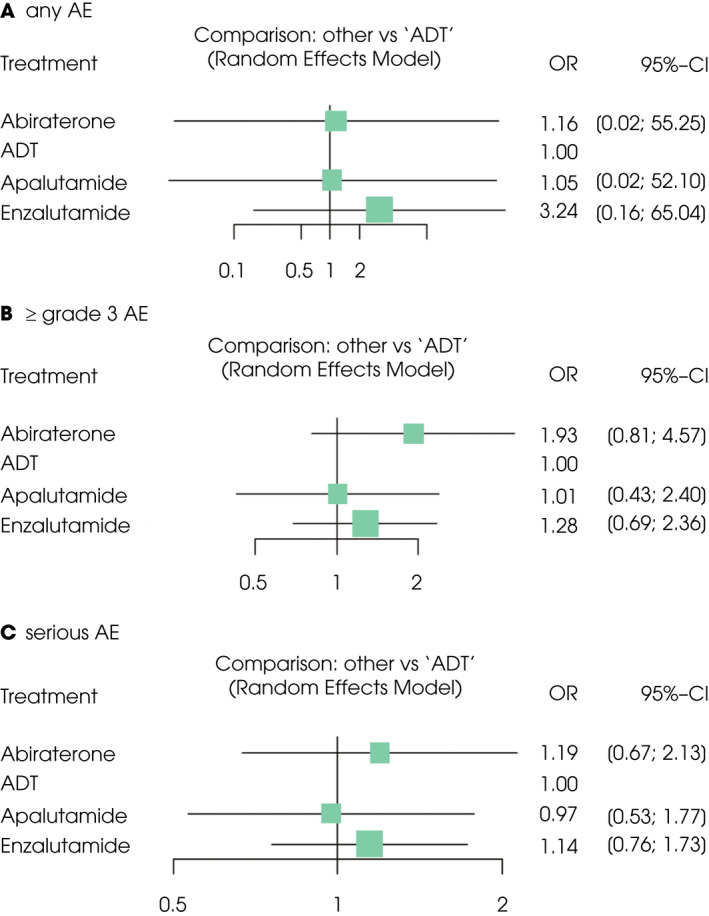
Forest plots showing the association of systemic therapy in mHSPC. (A) Any AEs rate, (B) Grade ≥3 AEs rate, (C) serious AEs rate.
High‐Volume Disease (OS and PFS)
In patients with high‐volume mHSPC, abiraterone and docetaxel resulted in significantly improved OS compared with ADT (HR 0.79, 95% CrI 0.73–0.87; and HR 0.86, 95% CrI 0.77–0.95, respectively; Fig. S4A). According to treatment ranking analysis, abiraterone had the highest likelihood of providing the maximal OS (P‐score: 0.8536; Table S3). Compared with ADT, abiraterone, apalutamide, docetaxel, and enzalutamide resulted in significantly improved PFS (HR 0.71, 95% CrI 0.67–0.76; HR 0.76, 95% CrI 0.67–0.86; HR 0.80, 95% CrI 0.73–0.87; and HR 0.70, 95% CrI 0.65–0.76, respectively; Fig. S4B). Compared with docetaxel, enzalutamide resulted in significantly improved PFS (HR 0.88, 95% CrI 0.78–0.99; Fig. S4C). According to treatment ranking analysis, enzalutamide had the highest likelihood of providing the maximal PFS (P‐score: 0.8668), closely followed by abiraterone (P‐score: 0.7841; Table S3).
Low‐Volume Disease (OS and PFS)
In patients with low‐volume mHSPC, only enzalutamide resulted in significantly improved OS compared with ADT (HR 0.69, 95% CrI 0.55–0.87; Fig. S5A). According to treatment ranking analysis, enzalutamide had the highest likelihood of providing the maximal OS (P‐score: 0.9079; Table S3). Compared with ADT, enzalutamide, apalutamide, and abiraterone resulted in significantly improved PFS (HR 0.58, 95% CrI 0.51–0.65; HR 0.64, 95% CrI 0.53–0.78; and HR 0.67, 95% CrI 0.58–0.78, respectively; Fig. S5B). Compared with docetaxel, enzalutamide, apalutamide, and abiraterone resulted in significantly improved PFS (HR 0.67, 95% CrI 0.55–0.81; HR 0.74, 95% CrI 0.57–0.96; HR 0.77, 95% CrI 0.63–0.95, respectively; Fig. S5C). According to treatment ranking analysis, enzalutamide had the highest likelihood of providing the maximal PFS (P‐score: 0.9521; Table S3).
Discussion
We conducted a systematic review of systemic therapy agents that have been evaluated in RCTs for the treatment of patients with mHSPC. Furthermore, we performed an NMA to indirectly compare their safety and efficacy. This approach generated several findings of interest.
First, all three ARIs appear to be more efficacious than docetaxel with regards to PFS, while OS associated with each agent did not differ significantly. Second, apalutamide was the best tolerated of the three ARIs with the rate of AEs being similar to that with ADT. Third, enzalutamide emerged as the most likely best treatment option regarding OS and PFS in patients with low‐volume mHSPC.
These represent important developments, given that earlier NMAs did not include recently published data, and/or included heterogeneous populations in their analyses (not only mHSPC but advanced HSPC) [14, 15, 16, 17]. Therefore, we focussed on patients with mHSPC only. Moreover, we included recently reported, long‐term results from the STAMPEDE and LATITUDE trials [18, 19]. We also excluded the STAMPEDE trial from our AE analysis, which provided only limited information on AEs reported in patients with metastasis. We employed these strict inclusion and exclusion criteria to make the populations included as homogeneous and comparable as possible. In addition, we did not include biochemical progression as a definition of progression in our analyses to allow comparisons between docetaxel and ARIs. Thus, on these points, we believe our NMA will be found of more value than the two recently published NMAs [16, 17] in facilitating individualised treatment selection, while our NMA is similar in its focus on docetaxel, abiraterone, enzalutamide, and apalutamide.
In agreement with earlier NMAs [15, 16, 17, 36], our updated NMA demonstrated that ARIs are superior to docetaxel in improving PFS. One major reason for better PFS benefits with ARIs over those with docetaxel in these studies could be that while docetaxel was discontinued after six courses of treatment, ARIs were continued indefinitely. The fact that this did not necessarily translate into OS benefits, however, may be accounted for by various factors, including the subsequent treatment and the RCTs, among these studies, which had insufficient follow up for OS assessment. It is also suggested that metastasis‐free survival could serve as a surrogate for OS in patients with localised prostate cancer [37], while there is insufficient evidence for the direct correlation between PFS and OS in patients with mHSPC; that PFS predicts OS in patients with metastatic castration‐resistant prostate cancer (mCRPC) treated with abiraterone [38] or enzalutamide [39] and that progression within 6 months of treatment with docetaxel is the best surrogate for OS in patients with mHSPC treated with docetaxel [40]. Taken together, it may be thus concluded that the superior PFS benefits with ARIs over those with docetaxel reflect the high clinical utility.
The STAMPEDE trial directly compared ADT plus docetaxel and ADT plus abiraterone in men with mHSPC (arms C and G) based on its multi‐arm design and showed an advantage of ADT plus abiraterone over ADT plus docetaxel for PFS but not for OS [34]. While being consistent with that in this NMA, this finding needs to be interpreted with caution, as the trial was not designed to directly compare these two therapies and might be underpowered to detect the differences that there could have been between the arms. Moreover, many patients treated with docetaxel had very limited salvage CRPC options compared to those treated with abiraterone, simply due to time considerations in licensing, which led to the two therapies being used in different ways [34]. Moreover, abiraterone is being used until patients develop CRPC, often for so many years until all options have been exhausted for CRPC, while, in contrast, docetaxel is being given as an 18‐week course treatment, which leaves still many options for patients who develop CRPC [34].
While every effort was made to include non‐heterogeneous populations, the population characteristics may have differed significantly between the studies, thus making the trials evaluated less comparable. Notably, caution should be exercised in interpreting the data on abiraterone from the LATITUDE trial [9, 18], which enrolled only those with high‐risk mHSPC, unlike the majority of RCTs evaluated in our present study, which enrolled all patients with mHSPC. Indeed, the efficacy of abiraterone may have been unfairly estimated in comparison with the other treatments in the LATITUDE trial. Our present analysis also focussed on patients with mHSPC in the STAMPEDE trial, which included not only patients with mHSPC but patients with high‐risk locally advanced prostate cancer. Moreover, the ENZAMET (ClinicalTrials.gov identifier: NCT02446405) trial involved the use of non‐steroidal antiandrogen therapy with ADT in the control arm, where, given the small survival benefit thought likely with complete androgen blockade, the results may therefore have weighed against enzalutamide [10, 41]. In addition, the optimal sequence of chemotherapy and the use of an oral antiandrogen remain controversial. Approval of docetaxel in the mHSPC setting led to its use in the ARCHES (ClinicalTrials.gov identifier: NCT02677896), ENZAMET, and TITAN (ClinicalTrials.gov identifier: NCT02489318) trials [10, 11, 12]. However, the timing of initiation of docetaxel varied widely between the trials, with docetaxel initiated before enzalutamide or apalutamide in the ARCHES and TITAN trials, used concurrently with enzalutamide in the ENZAMET trial, and not used at all in the LATITUDE trial. Because docetaxel was not yet approved when LATITUDE was accruing, prior or concurrent docetaxel use was not permitted. Furthermore, the proportions of patients with high‐volume disease (46–92% in the treatment group vs 47–78% in the control group), patients with relapsed/de novo disease (de novo disease, 57.7–100% in the treatment group vs 58.2–100% in the control group), and patients receiving subsequent treatments (30–68% in the treatment group vs 57–85%) varied widely between the RCTs evaluated. This is among the considerations to be borne in mind when interpreting the findings from our present NMA that indirectly compared these studies.
In our present NMA, apalutamide was the best tolerated of the three ARIs. Molecularly and mechanistically similar to enzalutamide, it antagonises the ligand‐binding domain of the AR with potent affinity, thereby preventing AR nuclear translocation [42]. Enzalutamide and apalutamide have low affinity for the gamma‐aminobutyric acid type A receptor in the brain. However, the steady‐state level of apalutamide is fourfold lower than that of enzalutamide, suggesting lower seizurogenic potential and fewer CNS AEs, such as fatigue. Indeed, the reported rates of fatigue in the TITAN trial were numerically much lower than those in the ENZAMET trial [10, 12]. Moreover, these agents differ substantially in their toxicity profile. In our present study, docetaxel was associated with the highest risk of fatigue, neuropathy, and myelosuppression including neutropenia, anaemia, and thrombocytopenia. Of the AEs related to the hepatic enzymes, docetaxel and abiraterone were associated with a higher risk of increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT). To focus on Grade ≥3 AEs related to the hepatic enzymes, abiraterone was associated with the highest risk of elevated AST and ALT. Moreover, abiraterone had the highest risk of hypertension, and apalutamide had the highest risk of cardiovascular disease, dizziness, and rash. Enzalutamide had a relatively high risk of cardiovascular disease, dizziness, fall, hypertension, and rash. Furthermore, unlike abiraterone and docetaxel, apalutamide and enzalutamide required no additional steroid administration and were deemed safer to use than the other agents in patients with poorly controlled diabetes or heart failure. Finally, these agents also differed in their mode of administration (intravenous or oral) and duration of treatment that could also have different AE implications.
Volume of disease is an important aspect in determining the optimal treatment [13]. In the present study, enzalutamide emerged as the best option in terms of OS and PFS benefits in patients with low‐volume mHSPC. In the ENZAMET and ARCHES trials, the treatment effect of enzalutamide was lower in patients with high‐volume disease compared to those with low‐volume disease [10, 11]. Low‐volume disease generally represents an earlier stage of disease than high‐volume disease and involves fewer AR‐independent cells that may thus be targeted more effectively with ARIs [43], potentially leading to the proportionally strategy’s superiority of ARIs over chemotherapy in low‐volume disease compared to high‐volume disease.
There are several other factors than just survival or AEs that could influence the choice of agents for patients with mHSPC and therefore the decision should still be individually tailored to each patient. First, the present analysis does not include any information on quality of life (QoL), and this should be an important consideration from a patient’s viewpoint. The literature reports that patients on ADT plus docetaxel initially have inferior QoL while on treatment compared with ADT alone, but this is shown to improve over time and outweigh that with ADT alone [44]. By contrast, the LATITUDE trial reported that patients on abiraterone had a consistently better QoL compared with those receiving ADT alone at most time points measured [45]. Enzalutamide plus ADT allowed men with mHSPC to maintain high‐functioning health‐related QoL and a low symptom burden in the ARCHES trial [46], while apalutamide was shown to be comparable to ADT alone from a QoL perspective in the TITAN trial [47]. Of all studies included in the present NMA, the STAMPEDE trial is the only RCT that compared docetaxel and abiraterone for their QoL impact [48]. Focussing on global QoL over 2 years, the study showed that the QoL scores were 3.9 points higher in those treated with abiraterone and that abiraterone was associated with a consistently better global QoL, as at the pre‐defined time points of 12 and 24 weeks, and 2 years [48]. Second, the drug cost that varies from one agent to another is among the factors to consider in the decision‐making process. A recent study of ADT alone, ADT plus docetaxel, and ADT plus abiraterone demonstrated that although abiraterone is potentially more effective than docetaxel, it was not a cost‐effective option [49]. Likewise, docetaxel was shown to be superior to abiraterone in cost‐effectiveness studies comparing these agents in patients with mHSPC [50, 51]. Furthermore, in a study comparing ADT and ADT plus ARIs (apalutamide, enzalutamide, darolutamide) in patients with nonmetastatic CRPC, apalutamide and ADT was shown to be most likely to be cost‐effective [52]. Thus, while too high a cost may be a barrier to the spread of these treatments and may limit the development of newer compounds, unfortunately, cost‐analysis was not part of the trials included in the present analysis and made it difficult to address the cost considerations [16].
Despite the comprehensive nature of the present systematic review, there are some limitations that should be considered when interpreting the results. First, although indirect treatment comparison analyses have been used and validated to compare outcomes from RCTs, this approach falls short of a direct (head‐to‐head) treatment comparison. In the absence of more extensive prospective comparative trial data, NMAs have only a limited role in facilitating appropriate patient selection for the different treatment options available, a task that remains yet to be fulfilled. Thus, the findings of our present study remain yet to be validated in direct and well‐designed comparative trials. Second, the present NMA was based on the reporting quality of the trials, which may have been affected by several types of biases, thus limiting the validity of the overall findings. Third, because of the inherent limitations of published data, it was impossible to perform a meta‐analysis of adjusted effect estimates. Finally, the differing subsequent therapies implemented across the treatment arms in the trials evaluated may have influenced OS. For example, 78.6% of patients in the ENZAMET trial received life‐prolonging agents as a subsequent therapy compared with 60.1% and 47.4% of patients in the TITAN and LATITUDE trials, respectively [9, 10, 12]. In addition, the OS data from some trials, such as the ARCHES trial, were immature, and study outcomes may vary considerably pending their final analyses [11]. In addition to these limitations, high‐ and low‐volume disease may not be accurately distinguished based solely on conventional imaging modalities because of their limited sensitivity. Thus, the patients analysed in the present study should be deemed to have high‐volume metastatic disease rather than low‐volume disease. The endpoint of radiographic progression on conventional imaging is currently losing favour as the use of prostate‐specific membrane antigen positron emission tomography imaging increases in this disease stage.
Conclusion
In the present systematic review and NMA of systemic therapies for patients with mHSPC, we indirectly compared data from phase III clinical trials. ARIs were identified as effective treatment options, providing both PFS and OS benefits. In addition, apalutamide appeared to have the best tolerability profile of all ARIs. However, it should also be noted that the median follow‐up of patients receiving apalutamide was much shorter and the probability of associated AEs was therefore smaller. On the other hand, enzalutamide had the highest likelihood of providing the maximum PFS and OS benefits in patients with low‐volume mHSPC. These findings may provide guidance to patients with mHSPC and clinicians in their treatment decisions in conjunction with other personalised medicine aspects.
Conflict of Interest
We certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript are the following: Shahrokh Shariat owns or co‐owns the following patents: Methods to determine prognosis after therapy for prostate cancer. Granted 2002‐09‐06. Methods to determine prognosis after therapy for bladder cancer. Granted 2003‐06‐19. Prognostic methods for patients with prostatic disease. Granted 2004‐08‐05. Soluble Fas: urinary marker for the detection of bladder transitional cell carcinoma. Granted 2010‐07‐20. He has a consulting or advisory role for the following: Astellas, Astra Zeneca, Bayer, BMS, Cepheid, Ferring, Ipsen, Jansen, Lilly, MSD, Olympus, Pfizer, Pierre Fabre, Roche, Sanochemia, Sanofi, Takeda, Urogen, Wolff. The other authors made no disclosures.
Author Contributions
Project development: K. Mori, P.I. Karakiewicz, S. Egawa, S.F. Shariat. Data collection: K. Mori, H. Mostafaei. Data analysis: K. Mori, H. Mostafaei. Manuscript writing/editing: K. Mori, H. Mostafaei, R. Sari Motlagh, B. Pradere, F. Quhal, E. Laukhtina, V.M. Schuettfort, G. Kramer, M. Abufaraj, P.I. Karakiewicz, T. Kimura, S. Egawa, S.F. Shariat.
Supporting information
Fig. S1. Risk of bias summary of the included studies for network meta‐analysis.
Fig. S2. Network plots showing the association of systemic therapy in mHSPC.
Fig. S3. Forest plots (adverse events profile).
Fig. S4. Forest plots (high‐volume disease).
Fig. S5. Forest plots (low‐volume disease).
Table S1. Certainty of the evidence for each outcome based on the GRADE approach.
Table S2. Analysis of the treatment ranking.
Table S3. Analysis of the treatment ranking.
Appendix S1. The detailed database search strategy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34 [DOI] [PubMed] [Google Scholar]
- 2. Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis 2016; 19: 395–7 [DOI] [PubMed] [Google Scholar]
- 3. Bill‐Axelson A, Holmberg L, Garmo H et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014; 370: 932–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walz J, Gallina A, Perrotte P et al. Clinicians are poor raters of life‐expectancy before radical prostatectomy or definitive radiotherapy for localized prostate cancer. BJU Int 2007; 100: 1254–8 [DOI] [PubMed] [Google Scholar]
- 5. Shariat SF, Kattan MW, Vickers AJ, Karakiewicz PI, Scardino PT. Critical review of prostate cancer predictive tools. Future Oncol 2009; 5: 1555–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kluth LA, Shariat SF, Kratzik C et al. The hypothalamic‐pituitary‐gonadal axis and prostate cancer: implications for androgen deprivation therapy. World J Urol 2014; 32: 669–76 [DOI] [PubMed] [Google Scholar]
- 7. Mottet N, Van den Bergh RC, Briers E. EAU guidelines: prostate cancer 2019. Eur Urol 2019; 76: 868–73 31350069 [Google Scholar]
- 8. Sweeney CJ, Chen YH, Carducci M et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer. N Engl J Med 2015; 373: 737–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fizazi K, Tran NP, Fein L et al. Abiraterone plus prednisone in metastatic, castration‐sensitive prostate cancer. N Engl J Med 2017; 377: 352–60 [DOI] [PubMed] [Google Scholar]
- 10. Davis ID, Martin AJ, Stockler MR et al. Enzalutamide with standard first‐line therapy in metastatic prostate cancer. N Engl J Med 2019; 381: 121–31 [DOI] [PubMed] [Google Scholar]
- 11. Armstrong AJ, Szmulewitz RZ, Petrylak DP et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone‐sensitive prostate cancer. J Clin Oncol 2019; 37: 2974–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chi KN, Agarwal N, Bjartell A et al. Apalutamide for Metastatic, castration‐sensitive prostate cancer. N Engl J Med 2019; 381: 13–24 [DOI] [PubMed] [Google Scholar]
- 13. Kinsey EN, Zhang T, Armstrong AJ. Metastatic hormone‐sensitive prostate cancer. Cancer J 2020; 26: 64–75 [DOI] [PubMed] [Google Scholar]
- 14. Wallis CJ, Klaassen Z, Bhindi B et al. Comparison of abiraterone acetate and docetaxel with androgen deprivation therapy in high‐risk and metastatic hormone‐naïve prostate cancer: a systematic review and network meta‐analysis. Eur Urol 2018; 73: 834–44 [DOI] [PubMed] [Google Scholar]
- 15. Tan PS, Aguiar P Jr, Haaland B, Lopes G. Addition of abiraterone, docetaxel, bisphosphonate, celecoxib or combinations to androgen‐deprivation therapy (ADT) for metastatic hormone‐sensitive prostate cancer (mHSPC): a network meta‐analysis. Prostate Cancer Prostatic Dis 2018; 21: 516–23 [DOI] [PubMed] [Google Scholar]
- 16. Marchioni M, Di Nicola M, Primiceri G et al. New antiandrogen compounds compared to docetaxel for metastatic hormone sensitive prostate cancer: results from a network meta‐analysis. J Urol 2020; 203: 751–9 [DOI] [PubMed] [Google Scholar]
- 17. Sathianathen NJ, Koschel S, Thangasamy IA et al. Indirect comparisons of efficacy between combination approaches in metastatic hormone‐sensitive prostate cancer: a systematic review and network meta‐analysis. Eur Urol 2020; 77: 365–72 [DOI] [PubMed] [Google Scholar]
- 18. Fizazi K, Tran NP, Fein L et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high‐risk metastatic castration‐sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double‐blind, phase 3 trial. Lancet Oncol 2019; 20: 686–700 [DOI] [PubMed] [Google Scholar]
- 19. Clarke NW, Ali A, Ingleby FC et al. Addition of docetaxel to hormonal therapy in low‐ and high‐burden metastatic hormone sensitive prostate cancer: long‐term survival results from the STAMPEDE trial. Ann Oncol 2019; 30: 1992–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hutton B, Salanti G, Caldwell DM et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–84 [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Altman DG, Gotzsche PC et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Res Synth Methods 2012; 3: 285–99 [DOI] [PubMed] [Google Scholar]
- 23. Dias S, Welton NJ, Sutton AJ, Ades AE. NICE Decision Support Unit Technical Support Documents. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta‐Analysis of Randomised Controlled Trials. London National Institute for Health and Care Excellence (NICE). Copyright © 2014 National Institute for Health and Clinical Excellence, unless otherwise stated. All rights reserved, 2014 [PubMed]
- 24. Woods BS, Hawkins N, Scott DA. Network meta‐analysis on the log‐hazard scale, combining count and hazard ratio statistics accounting for multi‐arm trials: a tutorial. BMC Med Res Methodol 2010; 10: 54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta‐analysis works without resampling methods. BMC Med Res Methodol 2015; 15: 58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. J Clin Epidemiol 2011; 64: 163–71 [DOI] [PubMed] [Google Scholar]
- 27. Atkins D, Eccles M, Flottorp S et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE Working Group. BMC Health Serv Res 2004; 4: 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guyatt G, Oxman AD, Akl EA et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–94 [DOI] [PubMed] [Google Scholar]
- 29. Gravis G, Fizazi K, Joly F et al. Androgen‐deprivation therapy alone or with docetaxel in non‐castrate metastatic prostate cancer (GETUG‐AFU 15): a randomised, open‐label, phase 3 trial. Lancet Oncol 2013; 14: 149–58 [DOI] [PubMed] [Google Scholar]
- 30. Gravis G, Boher J‐M, Joly F et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long‐term survival analysis of the randomized phase 3 GETUG‐AFU15 trial. Eur Urol 2016; 70: 256–62 [DOI] [PubMed] [Google Scholar]
- 31. James ND, Sydes MR, Clarke NW et al. Addition of docetaxel, zoledronic acid, or both to first‐line long‐term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016; 387: 1163–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. James ND, de Bono JS, Spears MR et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017; 377: 338–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kyriakopoulos CE, Chen YH, Carducci MA et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer: long‐term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018; 36: 1080–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sydes MR, Spears MR, Mason MD et al. Adding abiraterone or docetaxel to long‐term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi‐arm, multi‐stage platform protocol. Ann Oncol 2018; 29: 1235–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoyle AP, Ali A, James ND et al. Abiraterone in "high‐" and "low‐risk" metastatic hormone‐sensitive prostate cancer. Eur Urol 2019; 76: 719–28 [DOI] [PubMed] [Google Scholar]
- 36. Feyerabend S, Saad F, Li T et al. Survival benefit, disease progression and quality‐of‐life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone‐sensitive prostate cancer: a network meta‐analysis. Eur J Cancer 2018; 103: 78–87 [DOI] [PubMed] [Google Scholar]
- 37. Xie W, Regan MM, Buyse M et al. Metastasis‐free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol 2017; 35: 3097–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morris MJ, Molina A, Small EJ et al. Radiographic progression‐free survival as a response biomarker in metastatic castration‐resistant prostate cancer: COU‐AA‐302 results. J Clin Oncol 2015; 33: 1356–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rathkopf DE, Beer TM, Loriot Y et al. Radiographic progression‐free survival as a clinically meaningful end point in metastatic castration‐resistant prostate cancer: the PREVAIL randomized clinical trial. JAMA Oncol 2018; 4: 694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marco B, Martini A, Pfail J et al. Surrogate endpoints for overall survival for patients with metastatic hormone‐sensitive prostate cancer in the CHAARTED trial. Eur Urol Open Sci 2020; 20: S142 [DOI] [PubMed] [Google Scholar]
- 41. Schmitt B, Bennett C, Seidenfeld J, Samson D, Wilt T. Maximal androgen blockade for advanced prostate cancer. Cochrane Database Syst Rev 2000; 2: CD001526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clegg NJ, Wongvipat J, Joseph JD et al. ARN‐509: a novel antiandrogen for prostate cancer treatment. Can Res 2012; 72: 1494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nelson PS. Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol 2012; 30: 644–6 [DOI] [PubMed] [Google Scholar]
- 44. Morgans AK, Chen YH, Sweeney CJ et al. Quality of life during treatment with chemohormonal therapy: analysis of E3805 chemohormonal androgen ablation randomized trial in prostate cancer. J Clin Oncol 2018; 36: 1088–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chi KN, Protheroe A, Rodríguez‐Antolín A et al. Patient‐reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration‐naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol 2018; 19: 194–206 [DOI] [PubMed] [Google Scholar]
- 46. Stenzl A, Dunshee C, De Giorgi U et al. Effect of enzalutamide plus androgen deprivation therapy on health‐related quality of life in patients with metastatic hormone‐sensitive prostate cancer: an analysis of the ARCHES randomised, placebo‐controlled, phase 3 study. Eur Urol 2020; 78: 603–14 [DOI] [PubMed] [Google Scholar]
- 47. Agarwal N, McQuarrie K, Bjartell A et al. Health‐related quality of life after apalutamide treatment in patients with metastatic castration‐sensitive prostate cancer (TITAN): a randomised, placebo‐controlled, phase 3 study. Lancet Oncol 2019; 20: 1518–30 [DOI] [PubMed] [Google Scholar]
- 48. Rush HL, Cook AD, Brawley CD et al. Comparative quality of life in patients randomized contemporaneously to docetaxel or abiraterone in the STAMPEDE trial. J Clin Oncol 2020; 38: 14. [Google Scholar]
- 49. Sathianathen NJ, Alarid‐Escudero F, Kuntz KM et al. A cost‐effectiveness analysis of systemic therapy for metastatic hormone‐sensitive prostate cancer. Eur Urol Oncol 2019; 2: 649–55 [DOI] [PubMed] [Google Scholar]
- 50. Chiang CL, So TH, Lam TC, Choi HC. Cost‐effectiveness analysis of Abiraterone Acetate versus Docetaxel in the management of metastatic castration‐sensitive prostate cancer: Hong Kong's perspective. Prostate Cancer Prostatic Dis 2020; 23: 108–15 [DOI] [PubMed] [Google Scholar]
- 51. Aguiar PN Jr, Tan PS, Simko S et al. Cost‐effectiveness analysis of abiraterone, docetaxel or placebo plus androgen deprivation therapy for hormone‐sensitive advanced prostate cancer. Einstein (São Paulo) 2019; 17: eGS4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Riaz IB, Almutairi A, Lang DK et al. Cost‐effectiveness of novel antiandrogens (AAs) for treatment of nonmetastatic castrate‐resistant prostate cancer (nmCRPC). J Clin Oncol 2020; 38: 5583. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Risk of bias summary of the included studies for network meta‐analysis.
Fig. S2. Network plots showing the association of systemic therapy in mHSPC.
Fig. S3. Forest plots (adverse events profile).
Fig. S4. Forest plots (high‐volume disease).
Fig. S5. Forest plots (low‐volume disease).
Table S1. Certainty of the evidence for each outcome based on the GRADE approach.
Table S2. Analysis of the treatment ranking.
Table S3. Analysis of the treatment ranking.
Appendix S1. The detailed database search strategy.


