Abstract
Background and purpose
A large proportion of headache sufferers do not routinely seek medical care. App‐based technologies permit the collection of real‐world data over time and between countries that can help assess true burden of headache. This study used a mobile phone application to collect information on the real‐world burden of self‐diagnosed headache and to describe its impact on daily life in headache sufferers who do not routinely seek medical advice.
Methods
This retrospective, non‐interventional, cross‐sectional study analysed self‐reported data from users of the ‘Migraine Buddy’ app. The main objective was to describe self‐reported characteristics of headache and migraine (triggers, duration, frequency), treatment patterns and impact on daily activity in headache sufferers from Australia, Brazil, France, Germany and Japan. Data including demographics, self‐diagnosed episode type (headache/migraine), duration, potential triggers and impact on daily activity are reported. All analyses were exploratory and performed per country.
Results
Self‐reported data were collected from 60,474 users between August 2016 and August 2018. Approximately 90% of users were females; >60% were aged 24–45 years. Over one‐third of users reported having two to five episodes of headache or migraine per month; impact included impaired concentration, being slower and missing work or social activities. Variations across countries were observed; within countries, episode characteristics were very similar for self‐diagnosed headache versus migraine.
Conclusions
Headache tracking was used to describe the experience, impact and self‐management approaches of migraine and headache sufferers in a real‐world setting. Headache disorders present a range of important issues for patients that deserve more study and reinforce the need for better approaches to management.
Keywords: headache, headache impact, headache triggers, real‐world evidence
Headache tracking with an app was used to describe the experience, impact and self‐management approaches of migraine and headache sufferers in a real‐world setting. Headache disorders present a range of important issues for patients that deserve more study and reinforce the need for better approaches to management.

BACKGROUND
Headache disorders comprise one of the most common global health challenges with only a minority of those affected being diagnosed and managed adequately [1, 2]. Migraine is the most common of these disorders in terms of global disability, and the most common cause of disability in people under the age of 50 [2, 3]. Despite considerable advances in the understanding of migraine and substantial advances in treatment, under‐treatment remains a global issue [1, 4, 5]. Coupled with the large cost burden on healthcare, there is a pressing need to improve headache management [6, 7].
Self‐diagnosis and self‐management of headache disorders is common, and some studies suggest that up to three‐quarters of headache sufferers do not take medical advice about treatment [8, 9, 10, 11]. Furthermore, simple lifestyle factors, such as the effects of stress and exercise or sleep disturbance, often go unaddressed in real‐world clinical practice, although they are widely discussed in the context of headache and may provide valuable insights into its pathophysiology [12, 13, 14]. Typically, clinicians have used paper‐based or electronically deployed versions of disability measures, such as the migraine disability assessment questionnaire [15], the headache impact test [16], the migraine‐specific quality of life questionnaire [17] or the migraine physical function impact diary [18], to gain insights into the effects of headache disorders on patient function.
Technological advances in hand‐held and personal devices have revolutionized many aspects of research. Trigger factors, long pursued for a potential to ameliorate migraine, have been recently explored using smartphone applications [19, 20, 21]. Indeed, mobile devices and applications are now widely used in clinical trials, for monitoring patients and most recently for the delivery of care [22, 23, 24].
This study aimed to collect information on characteristics and treatment patterns of self‐diagnosed headache in a real‐world setting and to describe the impact of headache and migraine on the quality of life of headache sufferers across six countries. The study used a mobile phone application for data collection; users who were experiencing an episode of headache or migraine could record the episode and associated data by answering questions in the application. The study focused on individuals who were using self‐medication including over‐the‐counter medication and non‐pharmaceutical approaches for treating their headache.
METHODS
Study design, population, inclusion criteria
This retrospective, non‐interventional, cross‐sectional study analysed self‐reported data from users of the ‘Migraine Buddy’ smartphone application (available at https://migrainebuddy.com/; hereafter referred to as Migraine Buddy app). Data were collected from users of the Migraine Buddy app (Healint Ltd) in Australia, Brazil, France, Germany and Japan between 1 August 2016 and 31 August 2018 (Figure [Link], [Link]).
App users were not specifically recruited or selected from the general population. App users were offered to opt out of data collection; if they did not opt out, descriptive information was collected through the app. Only users who did not opt out and had at least 45 days of activity were included in the descriptive summaries and other analysis. By downloading, accessing or using the Migraine Buddy app, or providing information to Healint in connection with the app, users agreed to the terms and conditions of the Healint policy of data collection and disclosure. As part of individual data protection, no user‐identifiable information was collected or stored. Institutional Review Board review or approval was not required.
Study objective
The main objective of the study was to perform a descriptive analysis of reported primary headache and migraine characteristics (triggers, duration, frequency), treatment patterns and impact on daily activity in headache sufferers outside the medical practice across five countries: Australia, Brazil, France, Germany and Japan.
Variables and epidemiological measurements
Baseline demographics, country and location were collected when first using the app and could be skipped by the user. For each new episode of migraine or headache, the following variables were collected sequentially: timing and duration (date and time of onset and stop; overlapping records were not permitted), type of episode (self‐diagnosed), highest pain level (self‐reported on a Likert scale from 0 to 10) and where pain started, potential triggers, user's ability to predict episode, symptoms, impact of the episode on the user's daily life, and use of medication or non‐pharmacological relief.
Reported data
Data summarized included the following epidemiological measures: demographic variables (age, gender); headache profile (episode type, headache days, headache frequency/chronicity, episode duration [recorded to the minute], episode intensity); triggers (nutrition, daily stressors, sensory, medication, physical, menstruation, psychological, weather, sleep, unknown); burden (impairment due to headache/migraine/other episode type and presence or absence of recorded symptoms or affected activities during an episode, number of good/bad days).
Statistical analysis
All analyses were exploratory. All eligible users meeting the inclusion criteria were included. Data were analysed separately for each country.
Qualitative variables were described by number of observed values, absolute and relative frequencies and number of missing values (users with missing data were not included in the percentage calculation). Quantitative variables were described by number of observed values and either by mean, standard deviation and 95% confidence intervals of the mean or by median, 1st and 3rd quartiles, minimum and maximum, depending on the nature of the variable.
Data were analysed for each country individually; no analysis was conducted for the entire population (non‐country specific).
RESULTS
Self‐reported data were collected from 60,474 users via the Migraine Buddy app in Australia (N = 6091), Brazil (N = 9222), France (N = 23,498), Germany (N = 10,807) and Japan (N = 10,856) between 1 August 2016 and 31 August 2018 (Figure 1). Data capturing details of headache or migraine episodes (type, frequency, duration, triggers, burden and non‐medical treatment) were collected to a comparable level of detail across countries. Reported episodes in Australia included 23,229 episodes of headache and 39,710 episodes of migraine; in Brazil 14,430 episodes of headache and 26,269 episodes of migraine; in France 35,560 episodes of headache and 97,703 episodes of migraine; in Germany 41,297 episodes of headache and 72,758 episodes of migraine; in Japan 29,418 episodes of headache and 79,381 episodes of migraine (Figure 1).
FIGURE 1.

Users and headache/migraine episodes included in the analysis. Each individual user could report more than one type of cephalea (headache, migraine, other [not shown])
Data obtained in each country were analysed separately for each self‐reported episode type: headache and migraine. Notably, each user could self‐report both types of episodes; these users were included in each part of the analysis.
Headache
The number of headache episodes and the number of users reporting them are presented in Figure 1. Demographics of users reporting headache were similar across countries. Gender was reported by the majority of users; amongst users with known gender, around 90% were females (Figure 1). Age was frequently not reported; age reporting was highest in Brazil, where 58% of users reported their age, and lowest in Japan, where only 16% of users reported their age. The majority of users with known age across five countries were between 25 and 45 years old (Figure 2a).
FIGURE 2.

Age distribution of users reporting headache (a) and migraine (b) across all countries. Exact percentages are shown for the four largest age groups (18 to ≤25 years, 25 to ≤35 years, 35 to ≤45 years and 45 to ≤55 years)
Amongst headache subtypes, tension‐type headache was the most commonly reported, followed by cluster headache (Table S1). Mean episode duration was between 11.6 h (in Japan) and 17.3 h (in Australia) (Table S1). Across countries, 57%–67% of users reported having less than two episodes of headache per month, and 29%–36% reported having two to five episodes of headache per month (Table S1 and Figure 3a).
FIGURE 3.
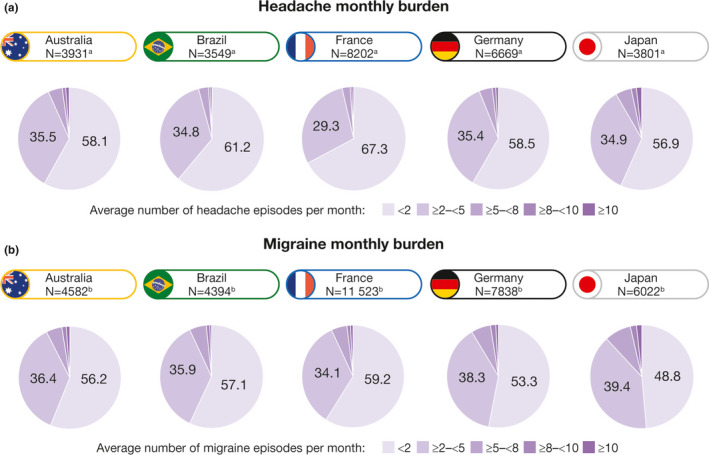
Monthly burden of headache (a) and migraine (b) across all countries. aNumber of users who reported headache episodes. bNumber of users who reported migraine episodes
The most frequently reported headache triggers across countries were neck pain, stress and lack of sleep; however, reporting of some triggers varied across countries (Figure 4a).
FIGURE 4.

Summary of headache triggers across all countries. Headache (a) and migraine episodes (b) are separated
The effects of headache on everyday activities were similar across countries (Figure 5a). In 10%–25% of episodes, users reported no effect on daily activities. Nausea/vomiting was reported in every fifth episode in Australia, Brazil, France and Germany and in 3% of headache episodes in Japan (Figure 5a). Many users reported being slower at their daily activities (at home/at work); 7%–25% missed social activities, and 13%–21% missed family time as a result of their headache (Figure 5b). In Australia, Brazil and Germany, more than half of users found it hard to concentrate during a headache episode (Figure 5b).
FIGURE 5.

Headache burden. Summary of headache burden across all countries, presented for all headache episodes (a) and for all users who reported headache (b). Arbitrary categories of headache burden include no burden (daily activity not affected), moderate burden and high burden
The most popular non‐medication methods for relief were sleeping, drinking water, staying indoors, being in a dark room and drinking coffee (Table S2).
Migraine
The number of migraine episodes and the number of users reporting them were generally higher than the corresponding numbers for headache (Figure 1); however, gender and age of users reporting migraine were similar to those with headache and generally similar across countries (Figures 1 and 2b). The majority of users with known age were between 18 and 45 years old in Australia, Brazil, France and Germany; in Japan, almost a fifth of users reporting migraine were between 45 and 55 years old (Figure 2b).
Mean episode duration was between 11.2 h (in Japan) and 16.7 h (in Australia) (Table S3). Across countries, 49%–59% of users reported having fewer than two episodes of migraine per month, and 34%–39% reported having two to five episodes of migraine per month (Table S3 and Figure 3b).
Stress and lack of sleep were frequently reported as migraine triggers; the variation of triggers across countries was more pronounced than for headache episodes (Figure 4b).
The effects of migraine on everyday activities were similar across countries (Figure 6a). The proportion of episodes in which users reported no effect on daily activities ranged from 6% in Australia to 21% in Japan. Nausea/vomiting was reported in approximately every third episode in Australia, Brazil, France and Germany and in 5% of migraine episodes in Japan (Figure 6a). Many users reported being slower at their daily activities (at home/at work); 11%–41% missed social activities, and 20%–35% missed family time as a result of their migraine (Figure 6b). In Australia, Brazil and Germany, more than half of users found it hard to concentrate during a migraine episode (Figure 6b).
FIGURE 6.

Migraine burden. Summary of migraine burden across all countries, presented for all migraine episodes (a) and for all users who reported migraine (b). Arbitrary categories of migraine burden include no burden (daily activity not affected), moderate burden and high burden
The most popular non‐medication methods for relief were sleeping, drinking water, staying indoors/at home and staying in a dark room (Table S4).
DISCUSSION
This retrospective real‐world study analysed self‐reported data of 60,474 headache sufferers across five countries in a 2‐year period. Results obtained in Australia, Brazil, France, Germany and Japan showed similar overall trends. To our knowledge, this is one of the largest global studies on the impact and self‐management of headache. Importantly, the captured real‐world data illustrate how burdensome headache and migraine are from the sufferers’ perspective. Notably, the app used in this study did not include a question on a possible previous diagnosis by a doctor, and the collected data specifically reflect the users’ subjective self‐diagnosis of their headache. The data support collecting further insights into headache self‐management and sufferers’ access to various approved treatment options.
The distribution of users by age, with the majority 25–45 years old, the episode frequency of 2 per month, and episode duration reflected well the current knowledge of the epidemiology of headache and migraine [1, 25]. Although a higher prevalence of headache has been noted amongst women than men [3, 26, 27], the proportion of women in this study was much higher than would be expected from other epidemiological data. Interestingly, other studies with app‐based data collection reported recruiting a high proportion of women (81%–94%), suggesting that the study design/tools may influence gender imbalance [20, 23, 24].
This study documents the substantial burden of headache and migraine: one‐third of users had two to four episodes a month, each lasting on average 11–17 h. Episodes brought about a substantial cognitive impact, with users being slower at performing their daily tasks and missing social activities or family time. Although some variability was detected across countries, within each country headache and migraine episodes were remarkably similar in terms of frequency, duration, pain intensity, potential triggers and life impact.
One of the main study limitations was selection bias: to be eligible for inclusion in this study, users must have been active on the app for at least 45 days. Users were only required to have opened or logged into the app to be classified as active. Some active users may not have recorded any episodes during the study period. Conversely, users were more likely to use the app if headache/migraine was an important part of their life. This may have introduced bias to the frequency of episodes (users of the app may have been more likely to have headaches than the general population) and to the perception of headache symptoms.
Further study limitations include incorrect self‐diagnosis, recall bias (episode data could be added in the app retrospectively) and lost or missing data. Users were self‐selected in the absence of a medically confirmed diagnosis. Users may also have selected the app on the basis of self‐defined ‘migraine’ and perceived severity or frequency that drove the user to seek non‐medical support. Data were captured from individuals who were sufficiently technically minded to download and use the app, which could introduce bias to the age distribution. As mentioned above, gender imbalance was also observed; however, gender representation was similar to other studies reporting data from app users [20, 23, 24].
The use of headache/migraine diaries is recommended by the International Headache Society for diagnostic and treatment decision purposes [28], and electronic diaries are increasingly used in clinical practice to monitor outcomes [29]. Alongside recording recommended features, such as pain intensity and headache duration, some studies used electronic diaries to record premonitory (preceding the attack) symptoms or triggers and postdromal (after‐attack) symptoms that reflect the impact of headache on participants’ daily activities [30, 31, 32], some of which, such as neck pain, may be premonitory symptoms misinterpreted as a trigger. Whilst app‐collected data offer very considerable advantages in terms of broadly collected, large datasets, they suffer the limitation of phenotypic clarity in contrast to a direct clinical history. To some extent, diagnostic overlap is inevitable between headache and migraine, since patients often under‐report mild symptoms even in direct questioning. Crucially, app‐based data are ideally deployed when broader sampling is desired whilst clinic‐based sampling offers a depth in endophenotyping that can feed into app development. Six years ago a systematic review identified 38 headache‐tracking mobile phone applications that are used by headache sufferers themselves to monitor their headache and migraine episodes [21]. A recent study used Migraine Buddy app to capture the burden and treatment patterns of migraine in European users with more than four episodes per month [33]. Although the studied population was different from that used in our study, and no direct comparison can be made, the authors also leveraged patient‐reported data collected through the app to identify the impact and burden of migraine [33]. According to the World Health Organization report, half of headache sufferers never come into contact with health professionals [1]. The self‐monitoring approach based on the use of personal devices will be instrumental to gain knowledge on this population and will provide a unique opportunity to capture the sufferers’ concerns beyond those that are currently visible to clinicians.
The experience, impact and self‐management approaches of migraine and headache sufferers in the real‐world setting are described in our study using headache tracking. The results highlight the impact of headache on the users’ cognitive and social wellbeing. Further studies are needed to identify specific evidence‐based measures that can help accurately capture the overall impact of headache or migraine on the individual's cognitive function. Once identified, these measures should be further validated in prospective studies before they can be used to assess the burden, life impact and treatment efficacy in headache and migraine.
CONFLICT OF INTEREST
PJG reports, over the last 36 months, grants and personal fees from Amgen and Eli‐Lilly and Company, grant from Celgene, personal fees from Alder Biopharmaceuticals, Aeon Biopharma, Allergan, Biohaven Pharmaceuticals Inc., Clexio, Electrocore LLC, eNeura, Epalex, GlaxoSmithKline, Impel Neuropharma, Lundbeck, MundiPharma, Novartis, Pfizer, Sanofi, Santara Therapeutics, Teva Pharmaceuticals, Trigemina Inc.; WL Gore, personal fees from MedicoLegal work, Massachusetts Medical Society, Up‐to‐Date, Oxford University Press and Wolters Kluwer, and a patent on the magnetic stimulation for headache assigned to eNeura without fee. LC, CE‐B, IIT, SH, CA‐B and AS are employees of Sanofi.
AUTHOR CONTRIBUTIONS
Peter J. Goadsby: Conceptualization (equal); investigation (equal); methodology (equal); writing—original draft (equal); writing—review and editing (equal). Luminita Constantin: Conceptualization (equal); funding acquisition (equal); methodology (equal); writing—original draft (equal); writing—review and editing (equal). Caty Ebel‐Bitoun: Conceptualization (equal); funding acquisition (equal); investigation (equal); writing—review and editing (equal). Iva Igracki Turudic: Conceptualization (equal); funding acquisition (equal); writing—review and editing (equal). Simon Hitier: Conceptualization (equal); formal analysis (equal); methodology (equal); writing—review and editing (equal). Caroline Amand‐Bourdon: Conceptualization (equal); funding acquisition (equal); writing—review and editing (equal). Andrew Stewart: Conceptualization (equal); funding acquisition (equal); writing—review and editing (equal).
ETHICAL APPROVAL
The study was conducted in accordance with the ethical principles laid out in the Declaration of Helsinki. This was a non‐interventional, retrospective analysis of data collected via individual self‐reports through a mobile phone app. Participants were offered to opt out of data collection; if they did not opt out, descriptive information was collected, anonymized and analysed. Due to the retrospective and non‐interventional nature of the study, ethics board approval was not warranted.
Supporting information
Fig S1
Appendix S1
ACKNOWLEDGEMENTS
The authors are grateful to the study participants for their contribution to the study.
Goadsby PJ, Constantin L, Ebel‐Bitoun C, et al. Multinational descriptive analysis of the real‐world burden of headache using the Migraine Buddy application. Eur J Neurol. 2021;28:4184–4193. 10.1111/ene.15037
Funding information
This study was funded by Sanofi. The funding body was involved in the development and approved the study design, supervised data collection and analysis, and was involved in the data interpretation. Final decisions on data interpretation remained with the authors. Editorial support was provided by Olga Ucar and Nichola Cruickshanks of inScience Communications, Springer Healthcare Ltd, UK, and was funded by Sanofi. All authors critically revised the manuscript for intellectual content.
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to study data and related documents (including, for example, the clinical study report, study protocol with any amendments, statistical analysis plan, and dataset specifications). Participant‐level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
REFERENCES
- 1. World Health Organisation and Lifting the Burden . Atlas of headache disorders and resources in the world. Geneva: World Health Organisation; 2011. https://www.who.int/mental_health/management/who_atlas_headache_disorders.pdf [Google Scholar]
- 2. GBD 2019 Diseases Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204‐1222. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. G. B. D. Neurology Collaborators . Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459‐480. 10.1016/S1474-4422(18)30499-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goadsby PJ. Primary headache disorders: five new things. Neurol Clin Pract. 2019;9(3):233‐240. 10.1212/CPJ.0000000000000654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goadsby PJ, Holland PR, Martins‐Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553‐622. 10.1152/physrev.00034.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gustavsson A, Svensson M, Jacobi F, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(10):718‐779. 10.1016/j.euroneuro.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 7. Mahon R, Huels J, Hacking V, et al. Economic evaluations in migraine: systematic literature review and a novel approach. J Med Econ. 2020;23(8):864‐876. 10.1080/13696998.2020.1754840 [DOI] [PubMed] [Google Scholar]
- 8. McCrone P, Seed PT, Dowson AJ, et al. Service use and costs for people with headache: a UK primary care study. J Headache Pain. 2011;12(6):617‐623. 10.1007/s10194-011-0362-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brusa P, Allais G, Scarinzi C, et al. Self‐medication for migraine: a nationwide cross‐sectional study in Italy. PLoS One. 2019;14(1):e0211191. 10.1371/journal.pone.0211191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki N, Ishikawa Y, Gomi S, et al. Prevalence and characteristics of headaches in a socially active population working in the Tokyo metropolitan area—surveillance by an industrial health consortium. Intern Med. 2014;53(7):683‐689. 10.2169/internalmedicine.53.1700 [DOI] [PubMed] [Google Scholar]
- 11. Viana M, Khaliq F, Zecca C, et al. Poor patient awareness and frequent misdiagnosis of migraine: findings from a large transcontinental cohort. Eur J Neurol. 2020;27(3):536‐541. 10.1111/ene.14098 [DOI] [PubMed] [Google Scholar]
- 12. Houle TT, Butschek RA, Turner DP, Smitherman TA, Rains JC, Penzien DB. Stress and sleep duration predict headache severity in chronic headache sufferers. Pain. 2012;153(12):2432‐2440. 10.1016/j.pain.2012.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Varkey E, Hagen K, Zwart JA, Linde M. Physical activity and headache: results from the Nord‐Trondelag Health Study (HUNT). Cephalalgia. 2008;28(12):1292‐1297. 10.1111/j.1468-2982.2008.01678.x [DOI] [PubMed] [Google Scholar]
- 14. Karsan N, Goadsby PJ. Biological insights from the premonitory symptoms of migraine. Nat Rev Neurol. 2018;14(12):699‐710. 10.1038/s41582-018-0098-4 [DOI] [PubMed] [Google Scholar]
- 15. Lipton RB, Stewart WF, Sawyer J, Edmeads JG. Clinical utility of an instrument assessing migraine disability: the Migraine Disability Assessment (MIDAS) questionnaire. Headache. 2001;41(9):854‐861. [PubMed] [Google Scholar]
- 16. Kosinski M, Bayliss MS, Bjorner JB, et al. A six‐item short‐form survey for measuring headache impact: the HIT‐6. Qual Life Res. 2003;12(8):963‐974. 10.1023/a:1026119331193 [DOI] [PubMed] [Google Scholar]
- 17. Cole JC, Lin P, Rupnow MF. Validation of the Migraine‐Specific Quality of Life Questionnaire version 2.1 (MSQ v. 2.1) for patients undergoing prophylactic migraine treatment. Qual Life Res. 2007;16(7):1231‐1237. 10.1007/s11136-007-9217-1 [DOI] [PubMed] [Google Scholar]
- 18. Hareendran A, Mannix S, Skalicky A, et al. Development and exploration of the content validity of a patient‐reported outcome measure to evaluate the impact of migraine—the migraine physical function impact diary (MPFID). Health Qual Life Outcomes. 2017;15(1):224. 10.1186/s12955-017-0799-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peris F, Donoghue S, Torres F, Mian A, Wöber C. Towards improved migraine management: determining potential trigger factors in individual patients. Cephalalgia. 2017;37(5):452‐463. 10.1177/0333102416649761 [DOI] [PubMed] [Google Scholar]
- 20. Park J‐W, Chu MK, Kim J‐M, Park S‐G, Cho S‐J. Analysis of trigger factors in episodic migraineurs using a smartphone headache diary applications. PLoS One. 2016;11(2):e0149577. 10.1371/journal.pone.0149577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hundert AS, Huguet A, McGrath PJ, Stinson JN, Wheaton M. Commercially available mobile phone headache diary apps: a systematic review. JMIR Mhealth Uhealth. 2014;2(3):e36. 10.2196/mhealth.3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perry B, Herrington W, Goldsack JC, et al. Use of mobile devices to measure outcomes in clinical research, 2010–2016: a systematic literature review. Digit Biomark. 2018;2(1):11‐30. 10.1159/000486347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bandarian‐Balooch S, Martin PR, McNally B, Brunelli A, Mackenzie S. Electronic‐diary for recording headaches, triggers, and medication use: development and evaluation. Headache. 2017;57(10):1551‐1569. 10.1111/head.13184 [DOI] [PubMed] [Google Scholar]
- 24. Minen MT, Torous J, Raynowska J, et al. Electronic behavioral interventions for headache: a systematic review. J Headache Pain. 2016;17:51. 10.1186/s10194-016-0608-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . WHO fact sheet: Headache disorders 2016 8 April 2016.
- 26. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343‐349. 10.1212/01.wnl.0000252808.97649.21 [DOI] [PubMed] [Google Scholar]
- 27. Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. J Headache Pain. 2010;11(4):289‐299. 10.1007/s10194-010-0217-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Headache Classification Committee of the International Headache Society (IHS), The International Classification of Headache Disorders. Cephalalgia. 2018;38(1):1‐211. 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 29. Stinson JN, Huguet A, McGrath P, et al. A qualitative review of the psychometric properties and feasibility of electronic headache diaries for children and adults: where we are and where we need to go. Pain Res Manag. 2013;18(3):142‐152. 10.1155/2013/369541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moloney MF, Aycock DM, Cotsonis GA, Myerburg S, Farino C, Lentz M. An internet‐based migraine headache diary: issues in internet‐based research. Headache. 2009;49(5):673‐686. 10.1111/j.1526-4610.2009.01399.x [DOI] [PubMed] [Google Scholar]
- 31. Giffin NJ, Lipton RB, Silberstein SD, Olesen J, Goadsby PJ. The migraine postdrome: an electronic diary study. Neurology. 2016;87(3):309‐313. 10.1212/WNL.0000000000002789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Viana M, Sances G, Terrazzino S, Sprenger T, Nappi G, Tassorelli C. When cervical pain is actually migraine: an observational study in 207 patients. Cephalalgia. 2018;38(2):383‐388. 10.1177/0333102416683917 [DOI] [PubMed] [Google Scholar]
- 33. Vo P, Paris N, Bilitou A, et al. Burden of migraine in Europe using self‐reported digital diary data from the Migraine Buddy© application. Neurology and Therapy. 2018;7(2):321‐332. 10.1007/s40120-018-0113-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Appendix S1
Data Availability Statement
Qualified researchers may request access to study data and related documents (including, for example, the clinical study report, study protocol with any amendments, statistical analysis plan, and dataset specifications). Participant‐level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies and process for requesting access can be found at https://www.clinicalstudydatarequest.com.


