Abstract
Transmission electron microscopy (TEM) is an important analysis technique to visualize (bio)macromolecules and their assemblies, including collagen fibers. Many protocols for TEM sample preparation of collagen involve one or more washing steps to remove excess salts from the dispersion that could hamper analysis when dried on a TEM grid. Such protocols are not standardized and washing times as well as washing solvents vary from procedure to procedure, with each research group typically having their own protocol. Here, we investigate the influence of washing with water, ethanol, but also methanol and 2‐propanol, for both mineralized and unmineralized collagen samples via a protocol based on centrifugation. Washing with water maintains the hydrated collagen structure and the characteristic banding pattern can be clearly observed. Conversely, washing with ethanol results in dehydration of the fibrils, often leading to aggregation of the fibers and a less obvious banding pattern, already within 1 min of ethanol exposure. As we show, this process is fully reversible. Similar observations were made for methanol and propanol. Based on these results, a standardized washing protocol for collagenous samples is proposed.
Keywords: collagen mineralization, sample preparation, transmission electron microscopy
Washing of collagen containing samples for electron microscopy with ethanol, methanol and 2‐propanol leads to dehydration and collapse of the collagen fibers with a vague banding pattern, while washing with water restores the initial structure.

1. INTRODUCTION
The organization of biomineralized tissues are often visualized with optical and/or electron microscopy. For example, the structure of nacre, consisting of calcium carbonate disks that are stacked via a brick‐and‐mortar motif (Meyers, Chen, Lin, & Seki, 2008; Nudelman, Chen, Goldberg, Weiner, & Addadi, 2007), and the arrangement of magnetite crystals in chiton teeth (Wang et al., 2013) were elucidated using scanning electron microscopy (SEM). For collagen‐containing samples, transmission electron microscopy (TEM) is often employed to assess mineralization inside the collagen fibers (Deshpande & Beniash, 2008; Olszta et al., 2007).
The intrafibrillar mineralization of collagen in vitro has been studied extensively to develop bone tissue engineering materials as well as to elucidate the mechanisms behind bone formation (Oosterlaken, Vena, & de With, 2021b). Different research groups have established their own in vitro collagen mineralization protocols (Oosterlaken et al., 2021a) and also for TEM sample preparation no standardized protocols are available, as these depend on the collagen source used. For example, larger collagen structures, such as pieces of bone or sponges, often require fixation followed by embedding in a polymer resin and subsequent sectioning via microtomy to be able to visualize the collagen fibers in TEM (Lausch, Quan, Miklas, & Sone, 2013; Niu et al., 2013b; Niu et al., 2013a), while dispersed collagen fibers are deposited on the TEM grid after mineralization (Olszta et al., 2007). Collagen can also be self‐assembled and mineralized on the TEM grids directly (Deshpande & Beniash, 2008).
Although TEM sample preparation varies depending on the collagen source used, the procedure generally involves one or more washing steps. Washing removes excess salts that are present in the mineralization medium, which could crystallize on the grid upon drying and thereby complicate analysis. Washing or rinsing with water seems to be the most logical and mostly employed procedure to remove the water‐soluble salts from the dispersion or collagen scaffold (Bradt, Mertig, Teresiak, & Pompe, 1999; Deshpande & Beniash, 2008; Jiao et al., 2016; Niu et al., 2017). However, despite its use as a chemical dehydrant, prior to embedding a collagenous structure in polymer resin (Gu et al., 2010; Niu et al., 2011) ethanol is also often used to rinse the samples (Ping et al., 2015; Xu et al., 2020). The use of methanol (Thula et al., 2011a) and 1‐propanol (Zhou et al., 2014) is occasionally reported. In some cases, multiple washing steps with different solvents are carried out (Olszta, Douglas, & Gower, 2003; Thula et al., 2011b). Clearly, differences in sample preparation may have a significant effect on the observable morphology of collagen and, therefore, effects of sample preparation need to be carefully evaluated.
Here, we investigate the influence of washing with water and with methanol, ethanol and 2‐propanol for dispersions containing collagen, prior to deposition of the sample on the TEM grid.
2. RESULTS
A washing protocol was established, based on centrifugation, in this paper sometimes also referred to as “spin‐concentration.” In the first step, the dispersion of collagen fibers was centrifuged for 2 min, after which the supernatant was removed. In the second step, fresh solvent, either one of the alcohols or water, was added and the collagen fibers are redispersed. Subsequently, the dispersion was centrifuged, and the supernatant removed. This step was repeated three times in total. The washing procedure was finalized by adding fresh solvent to the collagen fibers. TEM grids were prepared directly after the final step. Following this procedure, the effect of different washing procedures on the collagenous structure was investigated.
When imaging unmineralized collagen after different washing procedures in TEM in the dry state (hereafter: dry TEM), remarkable changes between the samples were observed (Figure 1).
FIGURE 1.
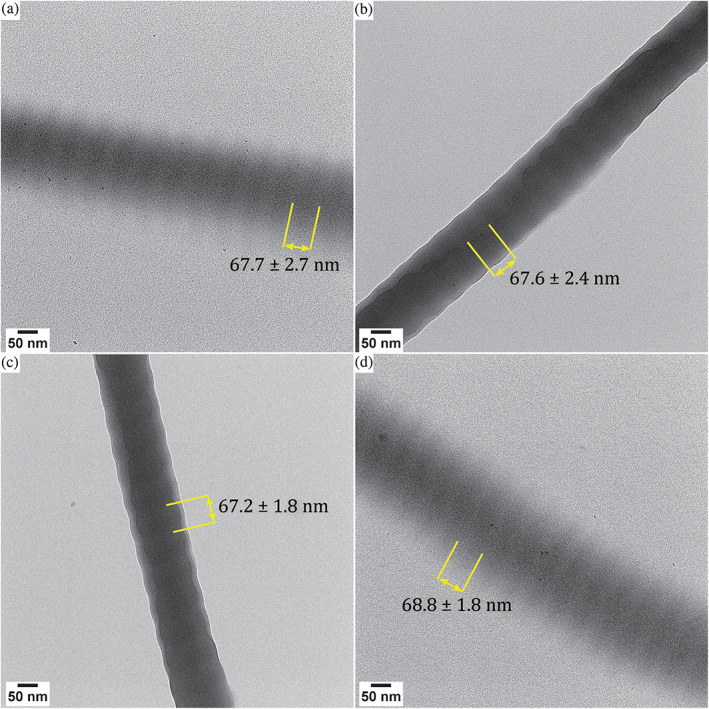
Effect of washing dispersed collagen fibers with different solvents. Dry TEM images of collagen washed with A. 3× MilliQ water, B. 3× ethanol, C. 3× MilliQ water, but dispersed in ethanol in the final step of the procedure, D. 3× ethanol, but dispersed in MilliQ water in the final step of the procedure. The D‐band spacing is indicated, which was measured via the intensity profile for 14 periods. The sample standard deviation is indicated with ±. In all cases, the number of spacings used is 14
When washing with water (Figure 1a), the banding pattern of the collagen is clearly observable, and the edges of the fiber are smooth. When washing with ethanol, however, the banding pattern is less clearly preserved (Figure 1b). Adjusting the contrast and brightness settings of the TEM image does not change the visibility of the banding pattern. The edges of the fiber display clear ridges, indicating a contraction of the collagen. Furthermore, although some aggregation is sometimes observed for collagen samples dispersed in water, aggregation of the fibers is more pronounced when the sample is washed with ethanol. This can already be observed in the aqueous dispersion that is used for TEM sample preparation, which becomes more transparent while large flocs of collagen form in the ethanol dispersion. Moreover, larger structures of aggregated fibers are present on the TEM grids, hampering imaging of single fibers.
The effects of using ethanol are noticeable almost instantaneously. When washing the samples with water three times but using ethanol to redisperse the fibers during the final washing step, aggregates are formed and the collagenous structures also appear contracted (Figure 1c). Thus, contraction with ethanol occurs within 1 min. These effects are fully reversible in a similar time frame, as dispersing the product in MilliQ water after three washing steps with ethanol leads to pristine‐looking and well‐separated fibers (Figure 1d), of which the banding pattern can be clearly observed. Similar observations were made for washing with methanol (Figure 2a,b) or 2‐propanol (Figure 2c,d). Like for ethanol, washing with methanol or 2‐propanol leads to contracted and aggregated fibers after 1 min exposure, which is reversible upon exposure to water for 1 min.
FIGURE 2.

Effect of washing dispersed collagen fibers with different solvents. Dry TEM images of collagen washed with A. 3× MilliQ water, but dispersed in methanol in the final step of the procedure, B. 3× methanol, but dispersed in MilliQ water in the final step of the procedure, C. 3× MilliQ water, but dispersed in 2‐propanol in the final step of the procedure, D. 3× 2‐propanol, but dispersed in MilliQ water in the final step of the procedure. The D‐band spacing is indicated, which was measured via the intensity profile for 14 periods. The sample standard deviation is indicated with ±. In all cases, the number of spacings used is 14
As washing steps are generally used to remove excess salts from collagen mineralization media, a similar set of experiments was performed on mineralized collagen fibers. Following a coprecipitation procedure, in which a mixture of collagen, poly(aspartic acid), Fe3+ and Fe2+ was continuously titrated with KOH until pH 9 was reached, collagen was mineralized with extrafibrillar goethite (α‐FeOOH) after a mineralization time of 400 days (13 months) (Oosterlaken, van Rijt, et al., 2021a). These mineralized fibers were exposed to the same washing procedure as described earlier (Figure 3).
FIGURE 3.
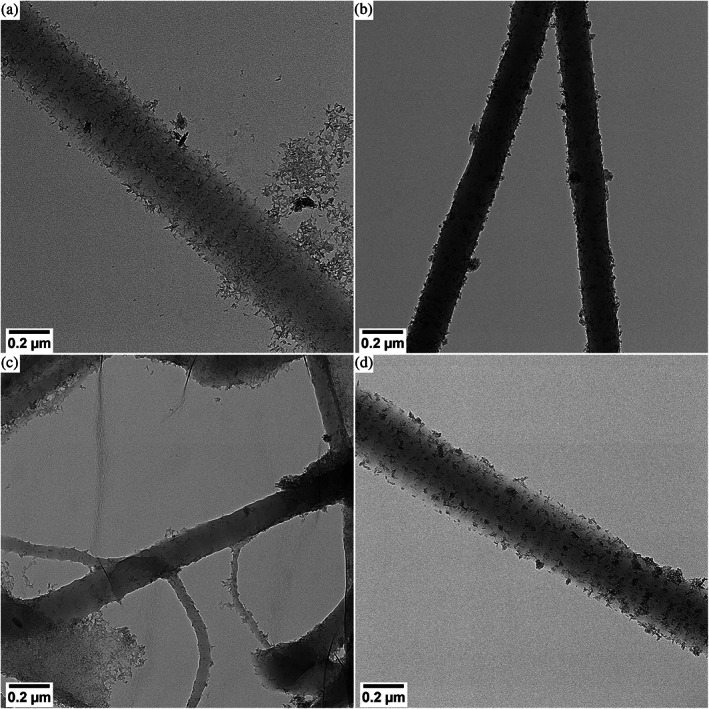
Effect of washing collagen fibers mineralized with goethite. Dry TEM images of collagen mineralized via a coprecipitation reaction and washed with A. 3× MilliQ water, B. 3× ethanol, C. 3× MilliQ water, but dispersed in ethanol in the final step of the procedure, D. 3× ethanol, but dispersed in MilliQ water in the final step of the procedure
The results observed for mineralized collagen fibers are similar to those of nonmineralized collagen. Washing with water results in separated fibers where banding is visible due to the decoration with particles (Figure 3a), whereas washing with ethanol results in aggregated structures in which the characteristic banding pattern is hardly visible (Figure 3b). Also, for mineralized fibers, the contraction upon exposure to ethanol occurs in less than 1 min (Figure 3c) but is reversible in a similar time frame (Figure 3d) upon exposure to water.
3. DISCUSSION
The washing of collagen samples is a procedure often used in sample preparation, though the details between different protocols vary. Here, we have performed a systematic study towards the effects of methanol, ethanol, 2‐propanol and water as washing solvents, on both mineralized and unmineralized collagen fibers in dispersion.
Dehydration with ethanol (and other lower alcohols) is a well‐known phenomenon for collagen, with ascending concentrations of ethanol often being employed before embedding the collagen structure in a polymer resin, as the presence of water might lead to poor polymerization of the resin (Ayar, 2016). Weiner and Traub (1986) already reported that the collagen structure appeared differently when it had been in contact with ethanol, like observed in our experiments. This altered appearance is due to ethanol‐induced shrinkage of the gap region exclusively (Jee et al., 2016), resulting from a rearrangement of the collagen monomers upon mixing with ethanol. From molecular dynamics simulations, it was established that no ethanol infiltration occurs in the overlap region (Jee et al., 2016), and thus, only the gap region contracts. Based on our observations, methanol and 2‐propanol also lead to a contraction of the gap region exclusively and thus we expect that methanol and 2‐propanol are also not infiltrating into the overlap region of the collagen.
The ethanol‐induced shrinkage of the collagen gap region does not result in a change in the characteristic D‐banding pattern of the collagen. For samples that were washed with water only, the D‐band is 67 nm, with the gap and overlap regions being approximately 40 and 27 nm, respectively. For samples washed in ethanol the same D‐band spacing was observed and also for methanol and 2‐propanol, no significant changes in D‐band spacing were measured. Thus, dehydration with methanol, ethanol or 2‐propanol only results in a contraction in diameter but not in length of the gap region, giving rise to the ribbed edges, but does not affect the characteristic D‐band period.
The dehydration effects resulting from dispersion in in either of the solvents used here are fully reversible. For ethanol this is not surprising, as ethanol can only remove up to 50% of the loosely‐bound and free water layers from the gap region, while it does not affect the more tightly bound water layers (Jee et al., 2016). Especially the tightly bound first water layer is very important in maintaining the helical structure of the collagen molecules, but this layer is unaffected by the ethanol treatment (Jee et al., 2016). As such, even though a collapse of the macroscopic structure is observed, the helical structure of the molecules is fully maintained, and the collagen structure can be restored when redispersing the sample in water. Even though methanol has stronger hydrogen bonding with water molecules (Beta & Sorensen, 2005), methanol is not able to remove all water from the collagen, as also for methanol the effects are fully reversible.
The method applied here, in which a sample is spin‐concentrated and redispersed in fresh solvent, is a facile method to remove excess salts from the dispersion, though it should be noted that salt crystals were occasionally observed, even in washed samples. This method can be easily applied, even in a glovebox. Obviously, for samples that are not in dispersion, other washing methods might be more suitable. For example, washing of TEM grids could be performed by placing the TEM grid with the side on which the sample was deposited down on top of a water droplet, which can be performed multiple times if desired. For larger collagen scaffolds that need to be embedded for microtomy, exposure to ethanol cannot be avoided, but it should be kept in mind that the collagenous structure becomes dehydrated and its appearance will be different compared to hydrated samples.
4. CONCLUSION
In this paper, the washing of collagenous samples to remove excess salts prior to TEM sample preparation is investigated. Washing of both mineralized and unmineralized samples was performed via centrifugation and redispersion of the sample in fresh solvent, either ethanol or water, in multiple washing steps.
Using MilliQ water to wash the samples leads to well‐dispersed collagen fibers with a clearly observable banding pattern, whereas washing with ethanol results in dehydrated, contracted, and aggregated fibers of which the banding pattern is much less apparent. This contraction takes place within 1 min, but it is reversible in a similar time frame by redispersing the sample in water. Due to the dehydration effects of ethanol, even though reversible, it is recommended to wash collagenous samples with water only prior to TEM sample preparation, if compatible with respect to follow‐up experimental procedures.
The washing method based on spin‐concentration as described here is a relatively facile and reliable method to remove excess salts from the collagen dispersion. The procedure can even be performed inside a glovebox without undue difficulties. Moreover, this washing method can be used to adjust the collagen concentration if needed, as the sample can be concentrated or diluted by adding less or more solvent during the final step of the procedure.
5. EXPERIMENTAL SECTION
5.1. Materials
Resorbable collagen tapes (RCT resorbable collagen tape, 2.5 × 7.5 cm2, Bovine Collagen type I) were acquired from Henry Schein Dental. Ferrous chloride tetrahydrate (FeCl2∙4H2O), ferric chloride hexahydrate (FeCl3∙6H2O), potassium hydroxide pellets, and poly(aspartic acid) (pAsp, poly‐[α,β]‐dl‐aspartic acid sodium salt, M w 2,000–11,000) were purchased from Sigma–Aldrich. Ethanol (TechniSolv, >99.5% purity) was acquired from VWR Chemicals. Methanol (Analytical Reagent, >99.8% purity) and 2‐propanol (Analytical Reagent, >99.8% purity) were acquired from Biosolve. All reagents were used without further purification. Collagen tapes were crushed under liquid nitrogen before use. MilliQ water was de‐aerated under argon flow for at least 1 hr and subsequently under nitrogen flow for another 15 min. All solutions and dispersions were prepared using de‐aerated MilliQ water.
5.2. Mineralization of collagen
Mineralization of collagen was performed inside a wet MBraun MB 200B glovebox under nitrogen atmosphere ([O2] < 5 ppm, unless stated otherwise). Titration experiments were performed at room temperature with a Metrohm Titrando 901 automated titration set‐up, controlled by a computer running the software program Tiamo 2.5, and equipped with a glass pH electrode (Metrohm article number 6.0234.100) and a Dosino 10 mL dosing device.
For the mineralization of collagen via a coprecipitation procedure, a solution of ferric chloride (4 mM) and ferrous chloride (2 mM) was titrated with 0.07 M KOH at a rate of 0.01 mL min−1 until pH 9 was reached. For experiments in the presence of pAsp, 7 mg mL−1 pAsp (12 mM aspartic acid groups) was added to the iron solution. For experiments in the presence of dispersed collagen, the iron solution was added to the collagen to reach a final collagen concentration of 5 mg mL−1. The dispersion was left standing for 400 days (13 months), after which TEM samples were prepared.
5.3. TEM
TEM samples were prepared by washing the samples by centrifuging the sample at 14,500 rpm for 2 min in an Eppendorf Mini Spin Plus. In the first step, the dispersion of collagen fibers was centrifuged and the supernatant was removed. In a second step, the washing solvent, either MilliQ water or ethanol (>99.5%), was added to the sample to redisperse the collagen fibers in. Subsequently, the sample was centrifuged and the supernatant removed. This step was repeated three times in total. After the fourth centrifugal cycle, the product was redispersed in the solvent that was used for the third washing step, unless stated otherwise.
The TEM grids, continuous carbon 200 mesh gold support, were surface plasma treated for 40 s using a Cressington 208 carbon coater prior to use. After washing, a 20 μL sample was deposited on a TEM grid and left to dry on filter paper inside the MBraun glovebox under nitrogen atmosphere. TEM imaging was performed on a Tecnai T20 G2 (Thermo Fisher Scientific), operating at 200 kV and equipped with a LaB6 filament. The images were acquired on a 4 k × 4 k CETA CMOS camera (Thermo Fisher Scientific).
ACKNOWLEDGMENTS
This work was supported by a TopPunt grant (Bi‐Hy, 718.016.003) of the Dutch Research Council (NWO).
Oosterlaken, B. M. , Friedrich, H. , & de With, G. (2022). The effects of washing a collagen sample prior to TEM examination. Microscopy Research and Technique, 85(1), 412–417. 10.1002/jemt.23915
Review Editor: Alberto Diaspro
Funding information Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Grant/Award Number: 718.016.003
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ayar, M. K. (2016). A review of ethanol wet‐bonding: Principles and techniques. European Journal of Dentistry, 10(1), 155–159. 10.4103/1305-7456.175687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beta, I. A. , & Sorensen, C. M. (2005). Quantitative information about the hydrogen bond strength in dilute aqueous solutions of methanol from the temperature dependence of the Raman spectra of the decoupled OD stretch. Journal of Physical Chemistry A, 109, 7850–7853. [DOI] [PubMed] [Google Scholar]
- Bradt, J.‐H. , Mertig, M. , Teresiak, A. , & Pompe, W. (1999). Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chemistry of Materials, 11(10), 2694–2701. 10.1021/cm991002p [DOI] [Google Scholar]
- Deshpande, A. S. , & Beniash, E. (2008). Bioinspired synthesis of mineralized collagen fibrils. Crystal Growth and Design, 8(8), 3084–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, L.‐S. , Kim, J. , Kim, Y. K. , Liu, Y. , Dickens, S. H. , Pashley, D. H. , … Tay, F. R. (2010). A chemical phosphorylation‐inspired design for type I collagen biomimetic remineralization. Dental Materials, 26(11), 1077–1089. 10.1016/j.dental.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee, S. E. , Zhou, J. , Tan, J. , Breschi, L. , Tay, F. R. , Gregoire, G. , … Jang, S. S. (2016). Investigation of ethanol infiltration into demineralized dentin collagen fibrils using molecular dynamics simulations. Acta Biomaterialia, 36, 175–185. 10.1016/j.actbio.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Jiao, K. , Niu, L. N. , Ma, C. F. , Huang, X. Q. , Pei, D. D. , Luo, T. , … Tay, F. R. (2016). Complementarity and uncertainty in intrafibrillar mineralization of collagen. Advanced Functional Materials, 26(38), 6858–6875. 10.1002/adfm.201602207 [DOI] [Google Scholar]
- Lausch, A. J. , Quan, B. D. , Miklas, J. W. , & Sone, E. D. (2013). Extracellular matrix control of collagen mineralization in vitro. Advanced Functional Materials, 23(39), 4906–4912. 10.1002/adfm.201203760 [DOI] [Google Scholar]
- Meyers, M. A. , Chen, P.‐Y. , Lin, A. Y.‐M. , & Seki, Y. (2008). Biological materials: Structure and mechanical properties. Progress in Materials Science, 53(1), 1–206. 10.1016/j.pmatsci.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Niu, L. N. , Jee, S. E. , Jiao, K. , Tonggu, L. , Li, M. , Wang, L. , … Tay, F. R. (2017). Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality. Nature Materials, 16, 370. 10.1038/nmat4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, L.‐N. , Jiao, K. , Qi, Y.‐P. , Yiu, C. K. Y. , Ryou, H. , Arola, D. D. , … Tay, F. R. (2011). Infiltration of silica inside fibrillar collagen. Angewandte Chemie International Edition, 50(49), 11688–11691. 10.1002/anie.201105114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, L. N. , Jiao, K. , Ryou, H. , Diogenes, A. , Yiu, C. K. Y. , Mazzoni, A. , … Tay, F. R. (2013b). Biomimetic silicification of demineralized hierarchical collagenous tissues. Biomacromolecules, 14(5), 1661–1668. 10.1021/bm400316e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, L. N. , Jiao, K. , Ryou, H. , Yiu, C. K. Y. , Chen, J.‐H. , Breschi, L. , … Tay, F. R. (2013a). Multiphase intrafibrillar mineralization of collagen. Angewandte Chemie International Edition, 52(22), 5762–5766. 10.1002/anie.201210259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman, F. , Chen, H. H. , Goldberg, H. A. , Weiner, S. , & Addadi, L. (2007). Spiers memorial lecture: Lessons from biomineralization: Comparing the growth strategies of mollusc shell prismatic and nacreous layers in Atrina rigida . Faraday Discussions, 136, 9–25. 10.1039/b704418f [DOI] [PubMed] [Google Scholar]
- Olszta, M. J. , Cheng, X. G. , Jee, S. S. , Kumar, R. , Kim, Y. Y. , Kaufman, M. J. , … Gower, L. B. (2007). Bone structure and formation: A new perspective. Materials Science and Engineering R‐Reports, 58(3–5), 77–116. 10.1016/j.mser.2007.05.001 [DOI] [Google Scholar]
- Olszta, M. J. , Douglas, E. P. , & Gower, L. B. (2003). Scanning electron microscopic analysis of the mineralization of type I collagen via a polymer‐induced liquid‐precursor (PILP) process. Calcified Tissue International, 72(5), 583–591. 10.1007/s00223-002-1032-7 [DOI] [PubMed] [Google Scholar]
- Oosterlaken, B. M. , van Rijt, M. M. J. , Joosten, R. R. M. , Bomans, P. H. H. , Friedrich, H. , & de With, G. (2021a). Time‐resolved cryo‐TEM study on the formation of iron hydroxides in a collagen matrix. ACS Biomaterials Science & Engineering, 7, 3123–3131. 10.1021/acsbiomaterials.1c00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterlaken, B. M. , Vena, M. P. , & de With, G. (2021b). In vitro mineralization of collagen. Advanced Materials, 33(16), e2004418. 10.1002/adma.202004418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping, H. , Xie, H. , Su, B.‐L. , Cheng, Y.‐B. , Wang, W. , Wang, H. , … Fu, Z. (2015). Organized intrafibrillar mineralization, directed by a rationally designed multi‐functional protein. Journal of Materials Chemistry B, 3(22), 4496–4502. 10.1039/c5tb00386e [DOI] [PubMed] [Google Scholar]
- Thula, T. T. , Rodriguez, D. E. , Lee, M. H. , Pendi, L. , Podschun, J. , & Gower, L. B. (2011a). In vitro mineralization of dense collagen substrates: A biomimetic approach toward the development of bone‐graft materials. Acta Biomaterialia, 7(8), 3158–3169. 10.1016/j.actbio.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thula, T. T. , Svedlund, F. , Rodriguez, D. E. , Podschun, J. , Pendi, L. , & Gower, L. B. (2011b). Mimicking the nanostructure of bone: Comparison of polymeric process‐directing agents. Polymers, 3(1), 10–35. 10.3390/polym3010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Nemoto, M. , Li, D. , Weaver, J. C. , Weden, B. , Stegemeier, J. , … Kisailus, D. (2013). Phase transformations and structural developments in the radular teeth of Cryptochiton Stelleri . Advanced Functional Materials, 23, 2908–2917. [Google Scholar]
- Weiner, S. , & Traub, W. (1986). Organization of hydroxyapatite crystals within collagen fibrils. FEBS Letters, 206(2), 262–266. [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Nudelman, F. , Eren, E. D. , Wirix, M. J. M. , Cantaert, B. , Nijhuis, W. H. , … Sommerdijk, N. (2020). Intermolecular channels direct crystal orientation in mineralized collagen. Nature Communications, 11(1), 5068. 10.1038/s41467-020-18846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B. , Niu, L. N. , Shi, W. , Zhang, W. , Arola, D. D. , Breschi, L. , … Tay, F. R. (2014). Adopting the principles of collagen biomineralization for intrafibrillar infiltration of yttria‐stabilized zirconia into three‐dimensional collagen scaffolds. Advanced Functional Materials, 24(13), 1895–1903. 10.1002/adfm.201302920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


