Abstract
Aim
We investigated the timing of survival differences and effects on morbidity for foetuses alive at maternal admission to hospital delivered at 22 to 26 weeks’ gestational age (GA).
Methods
Data from the EXPRESS (Sweden, 2004–07), EPICure‐2 (England, 2006) and EPIPAGE‐2 (France, 2011) cohorts were harmonised. Survival, stratified by GA, was analysed to 112 days using Kaplan‐Meier analyses and Cox regression adjusted for population and pregnancy characteristics; neonatal morbidities, survival to discharge and follow‐up and outcomes at 2–3 years of age were compared.
Results
Among 769 EXPRESS, 2310 EPICure‐2 and 1359 EPIPAGE‐2 foetuses, 112‐day survival was, respectively, 28.2%, 10.8% and 0.5% at 22–23 weeks’ GA; 68.5%, 40.0% and 23.6% at 24 weeks; 80.5%, 64.8% and 56.9% at 25 weeks; and 86.6%, 77.1% and 74.4% at 26 weeks. Deaths were most marked in EPIPAGE‐2 before 1 day at 22–23 and 24 weeks GA. At 25 weeks, survival varied before 28 days; differences at 26 weeks were minimal. Cox analyses were consistent with the Kaplan‐Meier analyses. Variations in morbidities were not clearly associated with survival.
Conclusion
Differences in survival and morbidity outcomes for extremely preterm births are evident despite adjustment for background characteristics. No clear relationship was identified between early mortality and later patterns of morbidity.
Keywords: epidemiology, extreme preterm birth, international comparisons, neonatal, perinatal, survival analysis
Key notes.
Survival for births at 22–26 weeks' gestation varies internationally, but little is known about the causes behind these differences.
Large differences in survival of extremely preterm births occur during labour and the first hours and days after birth, particularly at the lowest gestational ages, which are not explained by population baseline or maternal and foetal characteristics.
A major possible explanation for these differences is variation in societal choices between countries.
1. BACKGROUND
Care and outcomes of extremely preterm birth, including ethical decisions, were recently identified by an international Delphi consensus panel including healthcare professionals, patient representatives and policy makers as a top priority theme for investigation. 1 Because of the high risk of long‐term health consequences among survivors of birth before 27 weeks of gestation, most notably neurosensory impairment, understanding how care around birth and during initial hospitalisation can be improved to optimise survival without major morbidity is of major interest.
Survival of babies born at 22 to 26 completed weeks of gestational age (GA) varies widely both within and between countries; this variation has been primarily attributed to ethical decisions regarding the institution of active management around the time of birth. 2 , 3 , 4 However, variation could be due to methodological differences such as the choice of baseline population, 5 whether subject identification is by specialist centres or conducted geographically, or through differential recording of live and stillbirths. 6 Differences in 28‐day survival have been demonstrated in aggregated population level registries 7 but, given the challenges, few studies evaluate confounding due to population factors concerning complications of pregnancy. Even less is known about relationships between obstetric and neonatal care and longer term impairments: It is feared that active management may result in a larger proportion of survivors with disability, but reports are contradictory, and high‐quality comparative data are scarce. Detailed data are not available in population registries, and many neonatal registers only follow the infants until final discharge from hospital. 8 Questions, therefore, persist about how varying management strategies might affect longer term health of survivors.
In order to compare and contrast management and outcomes, we combined data from three prospectively collected, national population‐based European cohort studies of extremely preterm births carried out in Sweden, England and France. The aim of this study was to analyse country‐level differences using harmonised populations and variables, thus ensuring data consistency and enabling detailed investigation of outcomes at different time points. We compared survival patterns over time and sought to understand associations with population characteristics at discharge and follow‐up in early childhood. The study was carried out as part of the RECAP preterm project, which aims to create a harmonised database of very preterm European birth cohorts to optimise the use of population data for research and innovation. 1
2. METHODS
2.1. Data sources
Data from three European population‐based cohorts investigating births below 27 weeks' GA were included. The EXPRESS study comprised births in Sweden between 1 April 2004 and 31 March 2007. 9 Surviving children were followed until 1 year of age and, at 2.5 years corrected age, neurodevelopmental outcomes of 415 out of 481 children known to be alive were assessed by paediatric specialists, ophthalmologists and neuropsychologists; information was available for a further 41 following medical chart review. 10 EPICure‐2 studied all births in England between 1 January and 31 December 2006. 11 At 3 years corrected age, of 1029 survivors, 576 children were evaluated by trained paediatricians, data were available from attending paediatrician reports for a further 191, and questionnaire responses were obtained from 523 parents. 12 The EPIPAGE‐2 study included births at 22 to 26 weeks' GA in France between 28 March and 31 December 2011. 13 At 2 years corrected age, among the 544 survivors, attending physician reports were available for clinical assessment of 450 children with case review by an independent committee, and parent report data were available for 313 infants. 14
2.2. Populations included
We included foetuses alive at maternal admission to hospital, which were subsequently born between 22 and 26 completed weeks' GA from the three cohorts (i.e., up to 26 weeks and 6 days). Foetuses delivered following termination of pregnancy performed in accordance with national regulatory guidance were excluded (647 in EPICure‐2 15 and 188 in EPIPAGE‐2 13 ; terminations of pregnancy were not permitted beyond 22 weeks' GA in Sweden); we did not exclude babies with antenatally diagnosed congenital malformations.
2.3. Data harmonisation
Variable definitions from data dictionaries of each cohort were examined in detail to ensure data compatibility: Only variables harmonised across all three cohorts were included (Supplementary Appendix S1, Table S1). We included maternal age (years), presence of diabetes or hypertension before pregnancy, parity (nulliparous or not), pre‐eclampsia, any antenatal steroid administration, placental abruption, type of labour onset (spontaneous, induced or none), level of neonatal care provided at the delivery hospital, mode of delivery (vaginal or Caesarean), infant sex, GA at delivery (in completed weeks), birth weight (grams) and the presence of congenital abnormalities (categorised in accordance with cohort guidelines). It was not possible to harmonise all variables across the cohorts due to discrepancies in variable definitions (maternal smoking, other pre‐existing maternal illnesses, preterm prolonged rupture of membranes, presence of chorioamnionitis, use of tocolysis and maternal receipt of antibiotics antenatally) or because data were not collected in at least one of the cohorts (socioeconomic classification, maternal ethnicity, and Apgar scores). In addition to age at death for babies that did not survive, we harmonised information on neonatal morbidities: highest grade of intraventricular haemorrhage (using the classification of Papile et al.), cystic periventricular leucomalacia (de Vries et al.), surgical treatment for patent ductus arteriosus or necrotising enterocolitis, bronchopulmonary dysplasia (classified as none or mild; moderate; and severe) and both stage and treatment of retinopathy of prematurity (using the international classification).
As developmental follow‐up occurred at different ages using two different developmental tests (the Bayley Scales of Infant and Toddler Development Third Edition, and Ages and Stages Questionnaire Third Edition), we could not directly harmonise data from neurocognitive assessments. However, we report blindness and deafness as binary variables and functional motor level based broadly on the Gross Motor Functional Classification System as no disability or, for those with cerebral palsy: mild disability (GMFCS level 1); moderate disability (GMFCS level 2); and severe disability (GMFCS levels 3 to 5).
2.4. Outcomes
The primary outcome was survival to 112 days (16 weeks) of postnatal age. Survival to this age was chosen as it is unlikely to be misclassified and to ensure the majority of postnatal deaths were captured while allowing early differences to be clearly visualised. We also report neonatal morbidity levels among survivors to hospital discharge, post‐discharge mortality and neurosensory impairment identified at follow‐up at 2 to 3 years corrected age. We assumed that differences in the rates of impairment between follow‐up at 2, 2.5 and 3 years of age were negligible as moderate‐severe impairments like those examined here have previously been shown to be stable at a population level through to middle childhood. 16
2.5. Statistical analysis
Following harmonisation, we compared characteristics of mothers and foetuses alive at maternal admission to hospital between the three cohorts. Breakdowns by GA at delivery, sex, birth weight (<500 grams, and 500 grams or greater) and presence of congenital anomalies are presented alongside neonatal morbidity rates for children discharged alive from hospital; we include a count of the total number of severe morbidities (defined as intraventricular haemorrhage ≥grade 3, cystic periventricular leucomalacia, surgically treated necrotising enterocolitis, retinopathy of prematurity ≥stage 3 and severe bronchopulmonary dysplasia) among survivors at discharge. Survival status and sensorimotor disabilities (vision, hearing and functional motor level) are presented for survivors to follow‐up. Differences were examined using the chi‐squared test. As lower numbers of babies were born at 22 and 23 weeks, and survival in the EPIPAGE‐2 cohort is known to be extremely poor at these gestations, 13 we combined data for births below 24 weeks in all analyses.
Survival to 112 days of age was examined according to week of gestation at birth using Kaplan‐Meier analysis on an individual foetus/infant basis with baselines of (a) foetuses alive at maternal admission to hospital, (b) live births, (c) babies surviving to 1 hour of age, (d) 24 hours (1 day) of age, (e) 7 days of age and (f) 28 days of age. Differences between the cohorts were examined using the log‐rank test. We verified these results using unadjusted Cox proportional hazards regression conducted for each week of gestation. We then performed Cox proportional hazards regression adjusted for baseline maternal and pregnancy factors, multiple birth, infant sex and birth weight. We did not adjust for obstetric interventions, place or mode of delivery as these have been demonstrated 9 , 17 , 18 to be related to perinatal decision‐making and are, therefore, intermediate variables between our exposure, the country of each cohort, and survival and other outcomes. Furthermore, the organisation of perinatal care and use of obstetric interventions, in particular Caesarean delivery, vary greatly across countries for reasons that are unrelated to care of extremely preterm birth. We included clustering at the level of the mother using the sandwich estimator in all regression models to account for effects from women with multiple births. The full range of data available was used in the Cox models, with survival status right‐censored at cohort follow‐up (2.5 years for EXPRESS, 3 years for EPICure‐2 and 2 years for EPIPAGE‐2) for surviving children. As missing data among included covariates were minimal, we only performed complete case analyses. The proportional hazards assumption was assessed by examining chi‐squared tests of scaled Schoenfeld residuals.
All analyses were performed in R (version 3.5.2) and reported according to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (Supplementary Appendix S1, Table S2) using 95% confidence intervals (95% CI).
2.6. Sensitivity analyses
Because outcomes may differ between singleton and multiple births, we repeated the adjusted Cox proportional hazards models using births from singleton pregnancies only.
3. RESULTS
In EXPRESS, 769 of 1011 (76.1%) foetuses were alive at admission for delivery (687 women), in EPICure‐2, 2310 of 3132 (73.8%) foetuses were alive at admission for delivery (1649 women), and in EPIPAGE‐2, 1359 of 2205 (61.6%) foetuses were alive at admission for delivery (1135 women).
3.1. Maternal characteristics
Women were more often younger (under 25 years old) and primiparous in EPICure‐2 compared with either of the other two cohorts (p < 0.001 for both variables for the differences between the cohorts). Although there were no differences in the prevalence of pre‐existing diabetes or hypertension, more women were diagnosed with pre‐eclampsia in EXPRESS. Full results are shown in Table 1.
TABLE 1.
Baseline data for 3876 mothers with a live foetus at admission to hospital in the EXPRESS (Sweden, 2004–06), EPICure‐2 (England, 2006) and EPIPAGE‐2 (France, 2011) cohorts
| Variable Levels | EXPRESS | EPICure‐2 | EPIPAGE‐2 | p‐value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Maternal age (years) | |||||||
| <25 | 108 | 15.8 | 575 | 28.3 | 274 | 23.8 | <0.001 |
| 25–34 | 384 | 56.3 | 1028 | 50.6 | 668 | 58.1 | |
| ≥35 | 190 | 27.9 | 429 | 21.1 | 208 | 18.1 | |
| Missing | 5 | – | 4 | – | 3 | – | |
| Parity | |||||||
| Nullipara | 270 | 39.3 | 979 | 48.6 | 481 | 42.1 | <0.001 |
| Para 1+ | 417 | 60.7 | 1034 | 51.4 | 661 | 57.9 | |
| Missing | 0 | – | 23 | – | 11 | – | |
| Pre‐existing diabetes | |||||||
| No | 644 | 98.8 | 2010 | 98.8 | 1118 | 99.1 | 0.709 |
| Yes | 8 | 1.2 | 24 | 1.2 | 10 | 0.9 | |
| Missing | 35 | – | 2 | – | 25 | – | |
| Pre‐existing hypertension | |||||||
| No | 662 | 96.4 | 1978 | 97.2 | 1117 | 96.9 | 0.579 |
| Yes | 25 | 3.6 | 58 | 2.8 | 36 | 3.1 | |
| PIH/PE | |||||||
| No | 613 | 89.2 | 1906 | 93.6 | 1071 | 92.9 | <0.001 |
| Yes | 74 | 10.8 | 130 | 6.4 | 82 | 7.1 | |
| Onset of labour | |||||||
| Spontaneous | 518 | 76.5 | 1709 | 84.4 | 872 | 77.4 | <0.001 |
| Induced | 12 | 1.8 | 83 | 4.1 | 43 | 3.8 | |
| None | 147 | 21.7 | 234 | 11.5 | 211 | 18.7 | |
| Missing | 10 | – | 10 | – | 27 | – | |
Percentages may not equal 100 due to rounding.
Abbreviations: PE: pre‐eclampsia; PIH: pregnancy‐induced hypertension.
3.2. Foetal characteristics
Characteristics of the 4438 foetuses alive at maternal admission to hospital are shown in Table 2, with a breakdown by GA group in Supplementary Appendix S1, Tables S4 and S5. The proportion of births from multiple pregnancies varied from 22.0% in EXPRESS to 25.5% in EPICure‐2 and 31.2% in EPIPAGE‐2, whereas placental abruption was more commonly diagnosed in EXPRESS. Foetuses in EXPRESS and EPICure‐2 were more likely to be exposed to antenatal steroids compared with those in EPIPAGE‐2 and to be born in a tertiary unit in EXPRESS and EPIPAGE‐2, and by Caesarean delivery in EXPRESS. Differences between cohorts were seen in the GA distribution (p = 0.002) but not in birth weight distribution or sex ratios; congenital anomalies were more frequently reported in EXPRESS compared with EPICure‐2 or EPIPAGE‐2.
TABLE 2.
Baseline data for 4438 foetuses alive at maternal admission to hospital from the EXPRESS (Sweden, 2004–06), EPICure‐2 (England, 2006) and EPIPAGE‐2 (France, 2011) cohorts
| Variable Levels | EXPRESS | EPICure‐2 | EPIPAGE‐2 | p‐value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Level of neonatal care at delivery hospital | |||||||
| 1 | 20 | 2.6 | 217 | 9.5 | 87 | 6.4 | <0.001 |
| 2 | 152 | 19.9 | 757 | 33.2 | 290 | 21.3 | |
| 3 | 590 | 77.4 | 1304 | 57.2 | 982 | 72.3 | |
| Missing | 7 | – | 32 | – | 0 | – | |
| Exposure to antenatal steroids | |||||||
| No | 125 | 17.0 | 649 | 28.3 | 610 | 46.7 | <0.001 |
| Yes | 612 | 83.0 | 1641 | 71.7 | 695 | 53.3 | |
| Missing | 32 | – | 20 | – | 54 | – | |
| Placental abruption | |||||||
| No | 641 | 88.8 | 2137 | 93.0 | 1278 | 96.1 | <0.001 |
| Yes | 81 | 11.2 | 161 | 7.0 | 52 | 3.9 | |
| Missing | 47 | – | 12 | – | 29 | – | |
| Multiple birth | |||||||
| No | 600 | 78.0 | 1721 | 74.5 | 935 | 68.8 | <0.001 |
| Yes | 169 | 22.0 | 589 | 25.5 | 424 | 31.2 | |
| Delivery type | |||||||
| Vaginal | 413 | 53.7 | 1800 | 78.1 | 933 | 70.4 | <0.001 |
| Caesarean | 356 | 46.3 | 505 | 21.9 | 392 | 29.6 | |
| Missing | 0 | – | 5 | – | 34 | – | |
| Gestational age at delivery (weeks) | |||||||
| 22 | 89 | 11.6 | 262 | 11.3 | 171 | 12.6 | 0.002 |
| 23 | 120 | 15.6 | 413 | 17.9 | 195 | 14.3 | |
| 24 | 146 | 19 | 493 | 21.3 | 246 | 18.1 | |
| 25 | 205 | 26.7 | 548 | 23.7 | 325 | 23.9 | |
| 26 | 209 | 27.2 | 594 | 25.7 | 422 | 31.1 | |
| Missing | 0 | – | 0 | – | 0 | – | |
| Sex | |||||||
| Male | 420 | 54.7 | 1218 | 52.8 | 732 | 54.0 | 0.609 |
| Female | 348 | 45.3 | 1088 | 47.2 | 624 | 46.0 | |
| Missing | 1 | – | 4 | – | 3 | – | |
| Birth weight (g) | |||||||
| <500 | 91 | 12 | 260 | 11.3 | 142 | 10.7 | 0.678 |
| ≥500 | 668 | 88 | 2031 | 88.7 | 1180 | 89.3 | |
| Missing | 10 | – | 19 | – | 37 | – | |
| Congenital anomalies | |||||||
| No | 690 | 89.7 | 2190 | 97.8 | 1335 | 98.4 | <0.001 |
| Yes | 79 | 10.3 | 50 | 2.2 | 22 | 1.6 | |
| Missing | 0 | – | 70 | – | 2 | – | |
Percentages may not equal 100 due to rounding.
Abbreviation: g, grams.
3.3. Kaplan‐Meier analysis
Kaplan‐Meier survival curves for babies born at 22–23 weeks (Figure 1) highlight that survival was substantially higher in EXPRESS than either EPICure‐2 or EPIPAGE‐2; only two of 366 babies born in EPIPAGE‐2 below 24 weeks gestation survived to 112 days, and one of these subsequently dying before hospital discharge. Differences in outcome between EXPRESS and EPICure‐2 are also apparent using each of the baseline populations; for example, of babies surviving to 28 days who were born at 22–23 weeks, only 78% (73 of 93 babies) survived in EPICure‐2 compared to 97% (59 of 61) babies in EXPRESS. At 24 weeks (Figure 2), survival varied between the three cohorts based on the populations of foetuses alive at the maternal delivery admission, live births and of the babies who survived to 1 and 24 hours of age, being highest in EXPRESS and lowest in EPIPAGE‐2. From 24 hours, survival in EPICure‐2 and EPIPAGE‐2 converged but remained lower than in EXPRESS. Survival was higher in all three countries at 25 weeks of gestation (Figure 3): with differences between cohorts less marked and no differences among babies who survived to 28 days postnatal age. At 26 weeks (figure 4), there were small but consistent early differences between cohorts up to 24 h of age, but no important differences thereafter.
FIGURE 1.
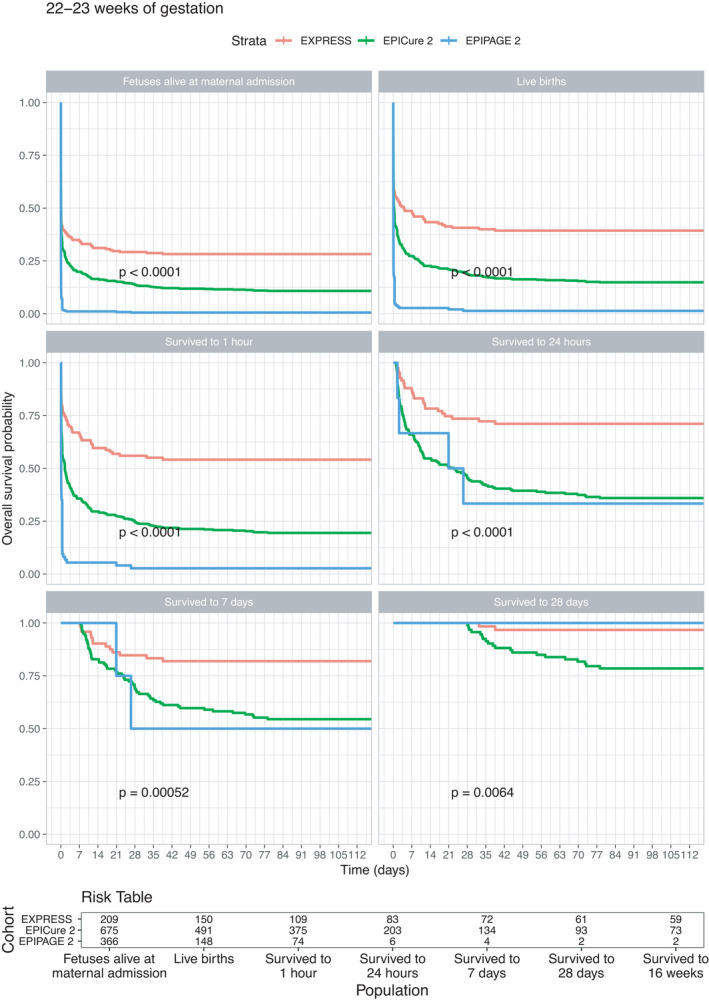
Survival curves for the EXPRESS (Sweden, 2004–07), EPICure‐2 (England, 2006) and EPIPAGE‐2 (France, 2011) cohorts for foetuses born at 22–23 completed weeks of gestational age
FIGURE 2.

Survival curves for the EXPRESS (Sweden, 2004–07), EPICure‐2 (England, 2006) and EPIPAGE‐2 (France, 2011) cohorts for foetuses born at 24 completed weeks of gestational age
FIGURE 3.
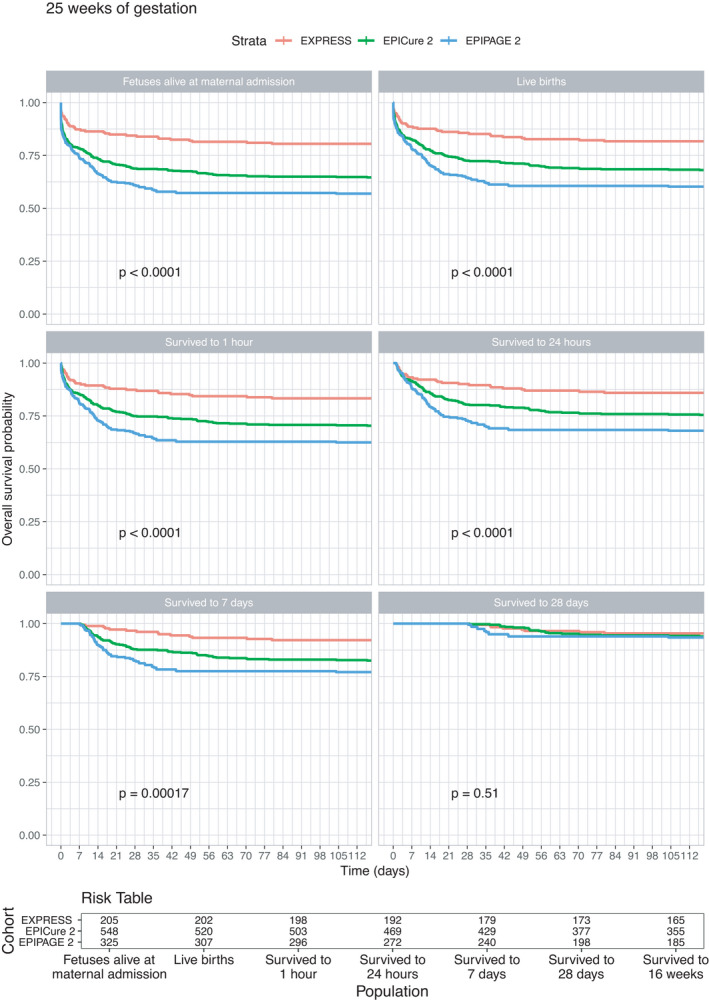
Survival curves for the EXPRESS (Sweden, 2004–07), EPICure‐2 (England, 2006) and EPIPAGE‐2 (France, 2011) cohorts for foetuses born at 25 completed weeks of gestational age
FIGURE 4.
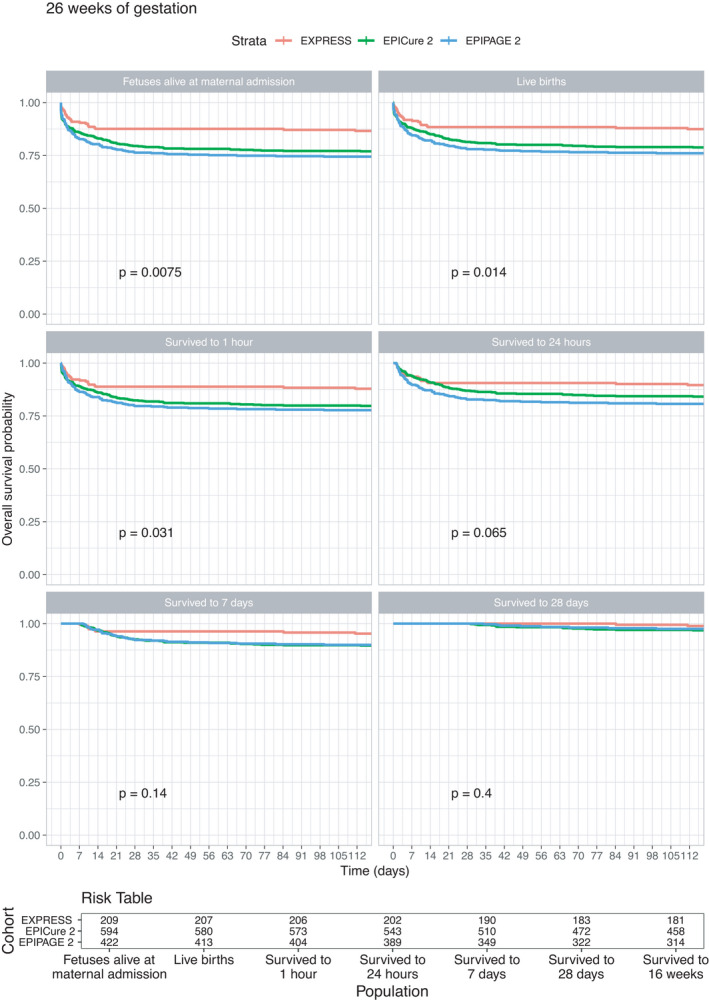
Survival curves for the EXPRESS (Sweden, 2004–07), EPICure‐2 (England, 2006) and EPIPAGE‐2 (France, 2011) cohorts for foetuses born at 26 completed weeks of gestational age
3.4. Cox regression
Hazard ratios (HRs) were consistent with results from Kaplan‐Meier analyses (Table 3). Following adjustment for baseline maternal and offspring characteristics, differences between the cohorts became even more pronounced at 22–23 weeks of gestation, but at 24, 25 and 26 weeks, there were few differences between adjusted and unadjusted results. The assessment of proportionality (Supplementary Appendix S1, Table S3) demonstrated that some variations in HRs existed across the time points used for the baseline populations. At 22–23 weeks, these were most apparent in HRs for EPICure‐2 up to 1 hour of age, and at 24 weeks in EPICure‐2 up to 1 day of age and across the whole time span for EPIPAGE‐2. There were few apparent differences between the three cohorts at 25 or 26 weeks.
TABLE 3.
Hazard ratios for mortality of babies born extremely preterm in the EPICure‐2 (England, 2006) and EPIPAGE‐2 (France, 2011) cohorts in comparison with EXPRESS (Sweden, 2004–07) according to different baseline populations; unadjusted results and results adjusted for maternal age, parity, pre‐existing diabetes and hypertension, pre‐eclampsia, placental abruption, spontaneous labour, multiple pregnancy, sex and birth weight
| GA | Population | Unadjusted HRs | Adjusted HRs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | EPICure‐2 | EPIPAGE‐2 | n | EPICure‐2 | EPIPAGE‐2 | ||||||
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | ||||
| 22–23 | Foetuses alive at maternal admission | 1250 | 1.42 | (1.15–1.75) | 3.76 | (2.99–4.71) | 1141 | 1.44 | (1.15–1.80) | 3.91 | (3.04–5.02) |
| Live births | 789 | 1.73 | (1.33–2.24) | 4.62 | (3.39–6.29) | 751 | 1.75 | (1.31–2.34) | 5.07 | (3.57–7.19) | |
| Survived to 1 hour | 558 | 2.48 | (1.81–3.41) | 7.20 | (4.80–10.80) | 537 | 2.45 | (1.75–3.44) | 7.65 | (4.82–12.13) | |
| Survived to 1 day | 292 | 2.84 | (1.80–4.48) | 3.71 | (1.61–8.60) | 287 | 2.79 | (1.74–4.45) | 4.66 | (1.74–12.47) | |
| Survived to 7 days | 210 | 2.76 | (1.48–5.16) | 4.41 | (1.60–12.17) | 208 | 2.87 | (1.47–5.62) | 4.66 | (1.79–12.13) | |
| Survived to 28 days | 156 | 4.46 | (1.56–12.74) | 8.42 | (1.34–52.79) | 155 | 5.93 | (1.65–21.30) | 14.55 | (2.70–78.25) | |
| 24 | Foetuses alive at maternal admission | 885 | 2.29 | (1.69–3.08) | 3.97 | (2.85–5.51) | 840 | 2.10 | (1.53–2.88) | 3.59 | (2.52–5.10) |
| Live births | 771 | 2.06 | (1.50–2.82) | 3.16 | (2.21–4.52) | 739 | 1.94 | (1.39–2.69) | 2.94 | (2.01–4.30) | |
| Survived to 1 hour | 686 | 2.46 | (1.76–3.43) | 2.83 | (1.91–4.18) | 661 | 2.29 | (1.61–3.24) | 2.51 | (1.65–3.81) | |
| Survived to 1 day | 567 | 2.43 | (1.63–3.62) | 2.48 | (1.54–4.01) | 548 | 2.21 | (1.47–3.33) | 2.09 | (1.26–3.46) | |
| Survived to 7 days | 487 | 2.64 | (1.63–4.27) | 2.06 | (1.12–3.76) | 470 | 2.58 | (1.57–4.25) | 1.83 | (0.96–3.49) | |
| Survived to 28 days | 394 | 2.97 | (1.41–6.22) | 1.46 | (0.53–4.07) | 381 | 2.92 | (1.36–6.25) | 1.46 | (0.51–4.19) | |
| 25 | Foetuses alive at maternal admission | 1078 | 2.02 | (1.44–2.84) | 2.56 | (1.79–3.64) | 1040 | 1.96 | (1.36–2.81) | 2.45 | (1.68–3.57) |
| Live births | 1029 | 1.90 | (1.33–2.72) | 2.46 | (1.69–3.57) | 994 | 1.86 | (1.27–2.73) | 2.31 | (1.55–3.44) | |
| Survived to 1 hour | 997 | 1.93 | (1.32–2.82) | 2.54 | (1.71–3.77) | 964 | 1.88 | (1.24–2.83) | 2.39 | (1.56–3.66) | |
| Survived to 1 day | 933 | 1.86 | (1.23–2.81) | 2.49 | (1.62–3.84) | 902 | 1.89 | (1.22–2.94) | 2.42 | (1.53–3.83) | |
| Survived to 7 days | 848 | 2.34 | (1.39–3.96) | 3.10 | (1.78–5.39) | 821 | 2.19 | (1.25–3.87) | 2.93 | (1.63–5.28) | |
| Survived to 28 days | 748 | 1.48 | (0.73–3.00) | 1.52 | (0.68–3.38) | 725 | 1.60 | (0.70–3.66) | 1.85 | (0.73–4.64) | |
| 26 | Foetuses alive at maternal admission | 1225 | 1.70 | (1.15–2.51) | 1.85 | (1.24–2.76) | 1172 | 1.76 | (1.16–2.66) | 1.92 | (1.26–2.93) |
| Live births | 1200 | 1.64 | (1.10–2.47) | 1.82 | (1.20–2.76) | 1147 | 1.74 | (1.13–2.66) | 1.88 | (1.22–2.90) | |
| Survived to 1 hour | 1183 | 1.62 | (1.07–2.45) | 1.72 | (1.12–2.65) | 1131 | 1.73 | (1.12–2.69) | 1.78 | (1.13–2.78) | |
| Survived to 1 day | 1134 | 1.43 | (0.90–2.27) | 1.70 | (1.06–2.72) | 1085 | 1.53 | (0.94–2.50) | 1.76 | (1.07–2.88) | |
| Survived to 7 days | 1049 | 1.82 | (1.01–3.30) | 1.63 | (0.87–3.06) | 1003 | 1.68 | (0.88–3.19) | 1.43 | (0.74–2.77) | |
| Survived to 28 days | 977 | 1.58 | (0.65–3.82) | 1.05 | (0.39–2.83) | 932 | 1.48 | (0.53–4.16) | 0.81 | (0.28–2.37) | |
Abbreviations: CI: confidence interval; GA, gestational age (weeks) at delivery; HR, hazard ratio.
3.5. Sensitivity analyses
Among singleton pregnancies, results were generally consistent with the main analyses with similar effect sizes albeit wider confidence intervals. Compared with EXPRESS, there were no differences at 25 weeks after 7 days of age for EPICure‐2 or EPIPAGE‐2, and at 26 weeks after 1 hour for EPICure‐2 and 1 day for EPIPAGE‐2. Full results are shown in Supplementary Appendix S1, Tables S10 and S11.
3.6. Neonatal outcomes at discharge
Overall, 2088 children were discharged alive from hospital: among live births, 497 of 702 (70.8%) in the EXPRESS cohort, 1040 of 2017 (51.6%) in EPICure‐2 and 551 of 1046 (52.7%) in EPIPAGE‐2. The prevalence of neonatal morbidities varied between the three cohorts, as shown in Table 4. For example, the highest grades of intraventricular haemorrhage and surgically treated necrotising enterocolitis were reported in EPICure‐2, whereas the highest rates of surgically treated patent ductus arteriousus were reported by EXPRESS. EPIPAGE‐2 had the lowest rates of stage 3 or higher retinopathy, cystic periventricular leukomalacia and moderate or severe bronchopulmonary dysplasia. Few children who survived to discharge had more than one severe neonatal morbidity, although this figure was notably higher in EPICure‐2. Severity of both bronchopulmonary dysplasia and retinopathy of prematurity increased with decreasing GA (Supplementary Appendix S1, Tables S6 and S7) but, in general, neonatal outcomes were consistent across GA groups.
TABLE 4.
Data from the EXPRESS (Sweden, 2004–06), EPICure‐2 (England, 2006) and EPIPAGE‐2 (France, 2011) cohorts for babies who were discharged alive from hospital
| Variable Levels | EXPRESS | EPICure‐2 | EPIPAGE‐2 | p‐value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Gestational age at delivery (weeks) | |||||||
| 23 | 58 | 11.7 | 69 | 6.6 | 1 | 0.2 | <0.001 |
| 24 | 95 | 19.1 | 178 | 17.1 | 58 | 10.5 | |
| 25 | 164 | 33 | 345 | 33.2 | 181 | 32.8 | |
| 26 | 180 | 36.2 | 448 | 43.1 | 311 | 56.4 | |
| Sex | |||||||
| Male | 270 | 54.3 | 505 | 48.6 | 280 | 50.8 | 0.105 |
| Female | 227 | 45.7 | 535 | 51.4 | 271 | 49.2 | |
| Birth weight (g) | |||||||
| <500 g | 18 | 3.6 | 12 | 1.2 | 4 | 0.7 | <0.001 |
| ≥500 g | 479 | 96.4 | 1028 | 98.8 | 547 | 99.3 | |
| Congenital anomalies | |||||||
| No | 442 | 88.9 | 1004 | 98.9 | 545 | 98.9 | <0.001 |
| Yes | 55 | 11.1 | 11 | 1.1 | 6 | 1.1 | |
| Missing | 0 | – | 25 | – | 0 | – | |
| Highest grade of IVH | |||||||
| 0 | 310 | 62.9 | 490 | 47.3 | 286 | 51.9 | <0.001 |
| 1 | 89 | 18.1 | 170 | 16.4 | 93 | 16.9 | |
| 2 | 45 | 9.1 | 188 | 18.2 | 126 | 22.9 | |
| 3 | 26 | 5.3 | 54 | 5.2 | 29 | 5.3 | |
| 4 | 23 | 4.7 | 133 | 12.9 | 17 | 3.1 | |
| Missing | 4 | – | 5 | – | 0 | – | |
| Cystic PVL | |||||||
| No | 469 | 94.4 | 976 | 94.3 | 538 | 97.6 | 0.008 |
| Yes | 28 | 5.6 | 59 | 5.7 | 13 | 2.4 | |
| Missing | 0 | – | 5 | – | 0 | – | |
| PDA treated surgically | |||||||
| No | 362 | 72.8 | 867 | 83.9 | 406 | 76.0 | <0.001 |
| Yes | 135 | 27.2 | 166 | 16.1 | 128 | 24.0 | |
| Missing | 0 | – | 7 | – | 17 | – | |
| NEC treated surgically | |||||||
| No | 476 | 96.9 | 961 | 92.4 | 526 | 95.5 | <0.001 |
| Yes | 15 | 3.1 | 79 | 7.6 | 25 | 4.5 | |
| Missing | 6 | – | 0 | – | 0 | – | |
| Bronchopulmonary dysplasia | |||||||
| None/mild | 142 | 31.3 | 323 | 31.1 | 304 | 60.8 | <0.001 |
| Moderate | 199 | 43.9 | 291 | 28.0 | 64 | 12.8 | |
| Severe | 112 | 24.7 | 425 | 40.9 | 132 | 26.4 | |
| Missing | 44 | – | 1 | – | 51 | – | |
| Treated ROP | |||||||
| No | 124 | 52.5 | 474 | 74.1 | 330 | 94.0 | <0.001 |
| Yes | 112 | 47.5 | 166 | 25.9 | 21 | 6.0 | |
| Missing | 261 | – | 400 | – | 200 | – | |
| ROP stage | |||||||
| 0 | 137 | 27.8 | 393 | 38.0 | 186 | 52.2 | <0.001 |
| 1 | 75 | 15.2 | 198 | 19.2 | 77 | 21.6 | |
| 2 | 112 | 22.8 | 229 | 22.2 | 65 | 18.3 | |
| 3 | 162 | 32.9 | 209 | 20.2 | 27 | 7.6 | |
| 4 | 3 | 0.6 | 3 | 0.3 | 1 | 0.3 | |
| 5 | 3 | 0.6 | 1 | 0.1 | 0 | 0.0 | |
| Missing | 5 | – | 7 | – | 195 | – | |
| Total number of neonatal morbidities | |||||||
| 0 | 231 | 46.5 | 410 | 39.4 | 348 | 63.2 | <0.001 |
| 1 | 180 | 36.2 | 373 | 35.9 | 165 | 29.9 | |
| 2 | 68 | 13.7 | 188 | 18.1 | 35 | 6.4 | |
| 3 | 16 | 3.2 | 62 | 6.0 | 3 | 0.5 | |
| 4 | 2 | 0.4 | 7 | 0.7 | 0 | 0.0 | |
| Missing | 0 | – | 0 | – | 0 | – | |
Total neonatal morbidities: IVH ≥ grade III, PVL, surgically treated NEC, ROP ≥ stage 3, severe bronchopulmonary dysplasia.
Abbreviations: IVH, intraventricular haemorrhage; NEC, necrotising enterocolitis; PDA, patent ductus arteriosus; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity.
3.7. Post‐discharge outcomes
Death between discharge and initial follow‐up was similar across the three cohorts: 2.2% in EXPRESS (five children had no data available due to emigration or having a protected identity), 1.1% in EPICure‐2 and 1.3% in EPIPAGE‐2 (p = 0.178). At 2 to 3 years of age, among children with available data, there were similar proportions with blindness or deafness in the three cohorts. The proportions of children categorised with severe motor impairment (GMFCS levels 3 to 5) were similar in EXPRESS and EPIPAGE‐2 but markedly higher in EPICure‐2, and there was also an excess of children diagnosed with mild cerebral palsy (GMFCS level 1) in EPICure‐2 compared with EXPRESS and EPIPAGE‐2; full details are in Table 5. Results were again broadly similar across the gestational age range (Supplementary Appendix S1, Tables S8 and S9).
TABLE 5.
Data for babies from the EXPRESS (Sweden, 2004–06), EPICure‐2 (England, 2006) and EPIPAGE‐2 (France, 2011) cohorts who were discharged alive from hospital and survived to follow‐up
| Variable Levels | EXPRESS | EPICure‐2 | EPIPAGE‐2 | p‐value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Survived to follow‐up* | |||||||
| Yes | 481 | 97.8 | 1029 | 98.9 | 544 | 98.7 | 0.178 |
| No | 11 | 2.2 | 11 | 1.1 | 7 | 1.3 | |
| Blind | |||||||
| No | 451 | 99.1 | 569 | 99 | 418 | 99.5 | 0.615 |
| Yes | 4 | 0.9 | 6 | 1.0 | 2 | 0.5 | |
| Missing | 26 | – | 454 | – | 124 | – | |
| Deaf | |||||||
| No | 442 | 99.8 | 574 | 99.8 | 441 | 100 | 0.632 |
| Yes | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | |
| Missing | 38 | – | 454 | – | 103 | – | |
| Functional motor impairment level (GMFCS) | |||||||
| None | 423 | 93.0 | 591 | 78.7 | 418 | 93.1 | <0.001 |
| Mild (GMFCS 1) | 13 | 2.9 | 90 | 12.0 | 11 | 2.4 | |
| Moderate (GMFCS 2) | 13 | 2.9 | 27 | 3.6 | 12 | 2.7 | |
| Severe (GMFCS 3–5) | 6 | 1.3 | 43 | 5.7 | 8 | 1.8 | |
| Missing | 26 | – | 278 | – | 95 | – | |
Abbreviations: GMFCS, Gross Motor Functional Classification System.
*Five children from EXPRESS had emigrated or had a protected identity at 2.5 years of age, hence survival information was not available; they have been excluded from this table.
4. DISCUSSION
This comparative analysis of three population‐based extremely preterm birth cohorts found important differences in survival using different baseline populations up to and including that of babies who survived to 28 days of age. At 22–23 and 24 weeks of gestation, differences were evident even when comparing survival among the populations of babies surviving to 28 days of age, at 25 weeks, differences existed to 7 days of age and, at 26 weeks, they were evident up to 24 hours of age. EXPRESS had the highest survival and the best motor outcomes at follow‐up; survival was generally better in EPICure‐2 than EPIPAGE‐2, but the proportion with neonatal morbidities and children categorised with cerebral palsy was more frequent.
4.1. Strengths and limitations
International comparisons of outcomes for extremely preterm births are difficult. This study's strengths are that we compared three large national prospective population‐based cohorts using individual patient‐level data with harmonisation of both inclusion criteria and individual variables, thus enabling us to overcome pitfalls such as discrepancies in population baseline or variable definitions 19 that may have affected other comparisons. 20 Minimal missing data for the included variables (including none for survival) and effective statistical methods provide confidence that remaining differences are not explained by included characteristics. We were, however, limited in the variables we could harmonise. There was no common socioeconomic measure ‐ although previously deprivation has only been related to rates of preterm birth and not to perinatal survival 21 ‐ nor was there common information about maternal ethnicity or country of birth. We lacked indicators relating to condition at birth such as the CRIB, CRIB‐II or Apgar scores, nor did we have information about delivery room or neonatal practices, and we could not compare cognitive outcomes at follow‐up.
Despite careful harmonisation, some potential differences remained among included variables. For example, we did not have detailed information relating to how gestational age was determined, and we know that there are differences between countries. In England and France, determination is usually with first‐trimester ultrasound, although it also occurs using time since last menstrual period, whereas in Sweden, at the time of the EXPRESS study, dating was performed around 18 weeks. This could be less accurate, leading to increased variance around the estimated gestation but would be unlikely to alter the mean. When we compared relationships between GA and birth weight, we found no differences between cohorts (data not shown). Another difference related to congenital anomalies, which were higher in EXPRESS. National policies on termination of pregnancy varied between the countries. In both EPICure‐2 and EPIPAGE‐2, a majority of terminations are performed for foetal anomalies 15 , 22 ; this might imply higher survival rates than EXPRESS, but we found the inverse. We believe the different rates seen for congenital anomalies are most likely due to residual differences in harmonisation, as shown in Supplementary Appendix S1, Table S1, hence why they are not related to survival.
4.2. Interpretation
We provide strong evidence of survival differences between the three cohorts. Furthermore, testing of the proportional hazards assumption demonstrated variation in HRs over time. Such variation can be assessed using an interaction for time in the models or, as we did, by varying the baseline population. HRs decreased for all GA groups as the starting point became later, indicating that the most important differences occur early on. We investigated model differences by examining p‐values for individual HRs and the overall model. Compared with EXPRESS, variation was related more to differences with EPIPAGE‐2 than EPICure‐2. Importantly, differences persisted beyond just the antenatal or delivery‐room periods and into the first days and weeks after birth. This supports the hypothesis that variations in survival are not only due to decisions occurring antenatally and around the time of birth, but that they might evolve over time and relate to adverse events occurring at later time points in the neonatal period and infancy.
Neonatal morbidity patterns are more difficult to explain. Some might be expected: the higher proportions of survivors at 22 to 24 weeks in EXPRESS and EPICure‐2 could be linked to increased need for more aggressive ventilatory management, thus explaining the higher proportion of moderate‐severe bronchopulmonary dysplasia, periventricular leukomalacia and retinopathy stages 3–5 seen in these cohorts. The pattern of rising retinopathy of prematurity and falling mortality is similar to that seen in the recent oxygen saturation targeting trials, 23 but the close relationship between the prevalence of retinopathy and gestational age means that retinopathy becomes more frequent as survival increases. However, increased survival of less mature babies also leads to more retinopathy independently of oxygen targets. Lower levels of surgically treated necrotising enterocolitis in EXPRESS and EPIPAGE‐2 might be associated with increased use of individual rooms in Sweden and France, perhaps mediated by the availability of maternal breast milk. 24 A more recent cohort ‐ the EXPRESS 2 ‐ study found neonatal morbidities in 22–24 week infants were reduced by half, underlining the potential role of neonatal management. 25 However, we did not have data available to investigate these hypotheses.
Following discharge from hospital, survival did not differ between the cohorts, but numbers are low and consequently the power to examine this is lacking: the higher post‐discharge mortality seen in EXPRESS thus may be either due to chance or related to Swedish policy. Morbidity status at 2–3 years of age is also difficult to interpret as there were no clear relationships between survival and levels of morbidity. Few children suffer from blindness or deafness hence again, no statistical differences were seen. Knowledge about functional motor status was most complete in EXPRESS, with impairment rates lower than the other cohorts; however, substantial data were missing in both EPICure‐2 and EPIPAGE‐2. The excess numbers of children diagnosed with cerebral palsy in EPICure‐2 compared to the other two cohorts is noteworthy. This might be related to missing data or to differences between countries in end‐of‐life decision‐making. It could also reflect different approaches to diagnosis: In EPICure‐2, categorisations were made by the research team based on recorded neurological assessments by trained assessors 12 ; for EXPRESS and EPIPAGE‐2, diagnoses were provided by paediatricians or paediatric neurologists. 10 , 14 Differences in follow‐up age may also have had an impact.
4.3. Generalisability
Using harmonised inclusion criteria and definitions, this study confirms previous research documenting wide variation in survival for extremely preterm births. It extends our knowledge by showing that these survival differences, while most marked perinatally, persist over time during the first hours, days and weeks after birth, particularly for the most preterm babies, and that population characteristics or methodological differences do not contribute to explaining them. The results also demonstrate that the relationships between ‘early selection’ and later outcomes are complex ‐ even in terms of survival, higher mortality around birth does not appear to identify a subset of babies who are subsequently more likely to live; the relationships with neonatal morbidity and longer term neurosensory outcomes are likewise complex.
We attribute these findings to variations in decision‐making attitude and believe that such variation between countries is likely to have remained similar over time. At a societal level, different decisions are reached regarding factors such as the centralisation of care, provision of parental support, and availability and type of follow‐up programs. For example, the lower rate of delivery in level 3 units in the EPICure‐2 cohort may have contributed to higher rates of cerebral palsy than in the EXPRESS and EPIPAGE‐2 cohorts. The risk of intraventricular haemorrhage, the single most important cause of cerebral palsy in extremely preterm infants, has been reported to increase if infants below 28 weeks were born outside a tertiary hospital in England. 26 However, this relationship is complex as seen by highly contrasting survival in the EXPRESS and EPIPAGE‐2 cohorts where there were similar proportions of births in hospitals with a level 3 neonatal unit (77.4% and 72.3%, respectively). In Sweden, England and France, management practices since the inception of these cohorts have evolved towards less aggressive handling at birth, avoidance of mechanical ventilation where possible, more effective ventilation and better parent engagement, including skin‐to‐skin care and family rooms in the intensive care unit. Combinations of subtle improvements may lead to improvements in survival: EXPRESS 2 recently reported improved survival compared with the first EXPRESS study, 25 optimum antenatal care combined with active postnatal management has been associated with improved survival, 27 and previous studies (including based on data from EXPRESS and EPIPAGE‐2) demonstrate that more active treatment is associated with higher survival. 2 , 3 , 4 The impact of these practices on long‐term morbidity outcomes is less clear, however. This is reflected in differences between guidelines for perinatal management of extremely preterm birth recently published in Sweden, 28 England 29 and France. 30 Further investigation, including cognitive and quality of life outcomes also, is warranted and should take into consideration practices which may explain variation such as that we identified.
5. CONCLUSION
Differences between the EXPRESS, EPICure‐2 and EPIPAGE‐2 cohorts in the survival of extremely preterm births to 112 days chronological age are evident despite the use of different baseline populations and adjustment for maternal and foetal characteristics. Neonatal morbidities and neurological outcomes at 2 to 3 years of age also varied between the three cohorts. While it is possible these findings could be explained by socioeconomic or ethnic discrepancies, this explanation is unlikely to account for all the variation seen. One of the major remaining explanations for these differences is the variation in societal choices between the three countries.
CONFLICT OF INTERESTS
All authors have completed ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). ASM, JZ, KK, ESD, SvB, SJ, VB, VP and PYA report grants from H2020/European Union during the conduct of this study; MN reports grants from Stockholm County Council and Karolinksa Institutet (ALF 2020‐0443) during the conduct of this study, and personal fees from AbbVie and Chiesi Pharma AB; and NM reports grants from the Medical Research Council during the conduct of the study, and personal fees from Novartis, Takeda and RSM Consulting outside the submitted work. All other authors report no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years.
Supporting information
Supplementary Material
Morgan AS, Zeitlin J, Källén K, et al. Birth outcomes between 22 and 26 weeks' gestation in national population‐based cohorts from Sweden, England and France. Acta Paediatr. 2022;111:59–75. 10.1111/apa.16084
Funding information
This project was funded by the European Union's Horizon 2020 Research and Innovation Program (RECAP Preterm Project, grant no. 733280) (ASM, JZ, KK, ESD, SvB, SJ, VP, VB and PYA). ASM additionally received part funding from the Fondation pour la Recherche Médicale (reference SPF20160936356), and NM receives part funding from the Department of Health’s NIHR Biomedical Research Centre’s funding scheme at UCLH/UCL. The EXPRESS cohort study was supported by the Swedish Research Council grants 2006‐3855 and 2009‐4250, the Uppsala‐Örebro Regional Research Council grant RFR‐10324, a grant from the Research Council South East Region of Sweden, and grants to Researchers in the Public Health Care from the Swedish government. Financial support was also provided through a regional agreement between the University of Umeå and Västerbotten County Council and through a regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet. The study also received support from The ‘Lilla Barnets Fond’ Children’s fund, the Evy and Gunnar Sandberg and the Birgit and Håkan Ohlsson Foundations, and from the Marie Curie Individual Intra‐European Fellowship within the EU FP6 Framework Program. The EPICure‐2 cohort was funded by the Medical Research Council (G0401525). The EPIPAGE‐2 cohort has been funded with support from the French Institute of Public Health Research/Institute of Public Health and its partners: the French Health Ministry, the National Institute of Health and Medical Research (INSERM), the National Institute of Cancer, and the National Solidarity Fund for Autonomy (CNSA); the National Research Agency, through the French EQUIPEX programme of investments in the future (reference ANR‐11‐EQPX‐0038); the PREMUP Foundation; and Fondation de France (reference 00050329). The funders had no role in study design, data collection, data analysis, data interpretation, decision to publish or preparation of the manuscript.
REFERENCES
- 1. Zeitlin J, Sentenac M, Morgan AS, et al. Priorities for collaborative research using very preterm birth cohorts, Archives of Disease in Childhood. Fetal Neonatal Ed. 2020;105(5):538‐544. 10.1136/archdischild-2019-317991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rysavy MA, Li L, Bell EF, et al. Between‐hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801‐1811. 10.1056/NEJMoa1410689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Serenius F, Blennow M, Marsal K, Sjors G, Kallen K. Intensity of perinatal care for extremely preterm infants: outcomes at 2.5 years. Pediatrics. 2015;135(5):e1163‐e1172. 10.1542/peds.2014-2988 [DOI] [PubMed] [Google Scholar]
- 4. Morgan AS, Foix L’Helias L, Diguisto C, et al. Intensity of perinatal care, extreme prematurity and sensorimotor outcome at 2 years corrected age: evidence from the EPIPAGE‐2 cohort study. BMC Med. 2018;16(1):227. 10.1186/s12916-018-1206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larroque B, Breart G, Kaminski M, et al. Survival of very preterm infants: epipage, a population based cohort study, Archives of Disease in Childhood. Fetal Neonatal Ed. 2004;89(2):F139‐F144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith LK, Blondel B, Zeitlin J. Producing valid statistics when legislation, culture and medical practices differ for births at or before the threshold of survival: report of a European workshop. BJOG Int J Obstetr Gynaecol. 2020;127(3):314‐318. 10.1111/1471-0528.15971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith LK, Morisaki N, Morken N‐H, et al. An international comparison of death classification at 22 to 25 weeks' gestational age. Pediatrics. 2018;142(1):e20173324. 10.1542/peds.2017-3324 [DOI] [PubMed] [Google Scholar]
- 8. Shah PS, Lui K, Reichman B, et al. The International Network for Evaluating Outcomes (iNeo) of neonates: evolution, progress and opportunities. Transl Pediatr. 2019;8(3):170‐181. 10.21037/tp.2019.07.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fellman V, Hellstrom‐Westas L, Norman M, et al. One‐year survival of extremely preterm infants after active perinatal care in Sweden. JAMA, J Am Med Assoc. 2009;301(21):2225‐2233. 10.1001/jama.2009.771 [DOI] [PubMed] [Google Scholar]
- 10. Serenius F, Källén K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309(17):1810. 10.1001/jama.2013.3786 [DOI] [PubMed] [Google Scholar]
- 11. Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ. 2012;345:e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ancel P‐Y, Goffinet F, Kuhn P, et al. Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011: results of the EPIPAGE‐2 cohort study. JAMA Pediatrics. 2015;169(3):230‐238. 10.1001/jamapediatrics.2014.3351 [DOI] [PubMed] [Google Scholar]
- 14. Pierrat V, Marchand‐Martin L, Arnaud C, et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks' gestation in France in 2011: EPIPAGE‐2 cohort study. BMJ. 2017;358:j3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Draper ES, Alfirevic Z, Stacey F, Hennessy E, Costeloe K. An investigation into the reporting and management of late terminations of pregnancy (between 22 +0 and 26 +6 weeks of gestation) within NHS hospitals in England in 2006: the EPICure preterm cohort study. BJOG Int J Obstetr Gynaecol. 2012;119(6):710‐715. 10.1111/j.1471-0528.2012.03285.x [DOI] [PubMed] [Google Scholar]
- 16. Johnson S, Marlow N. Charting the survival, health and development of extremely preterm infants: epicure and beyond. Paediatr Child Health. 2016;26(11):498‐504. 10.1016/j.paed.2016.08.003 [DOI] [Google Scholar]
- 17. Morgan AS, Marlow N, Draper ES, Alfirevic Z, Hennessy EM, Costeloe K. Impact of obstetric interventions on condition at birth in extremely preterm babies: evidence from a national cohort study. BMC Pregnancy Childbirth. 2016;16(1):390. 10.1186/s12884-016-1154-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeitlin J, Manktelow BN, Piedvache A, et al. Use of evidence based practices to improve survival without severe morbidity for very preterm infants: results from the EPICE population based cohort. BMJ. 2016;354:i2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torchin H, Morgan AS, Ancel P‐Y. International comparisons of neurodevelopmental outcomes in infants born very preterm. Semin Fetal Neonatal Med. 2020;25(3):101109. 10.1016/j.siny.2020.101109 [DOI] [PubMed] [Google Scholar]
- 20. Helenius K, Sjors G, Shah PS, et al. Survival in very preterm infants: an international comparison of 10 national neonatal networks. Pediatrics. 2017;140(6):e20171264. 10.1542/peds.2017-1264 [DOI] [PubMed] [Google Scholar]
- 21. Smith LK, Draper ES, Manktelow BN, Field DJ. Socioeconomic inequalities in survival and provision of neonatal care: population based study of very preterm infants. BMJ. 2009;339:b4702. 10.1136/bmj.b4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monier I, Lelong N, Ancel P‐Y, et al. Indications leading to termination of pregnancy between 22(+0) and 31(+6) weeks of gestational age in France: a population‐based cohort study. Eur J Obstet Gynecol Reprod Biol. 2019;233:12‐18. 10.1016/j.ejogrb.2018.11.021 [DOI] [PubMed] [Google Scholar]
- 23. Askie LM, Darlow BA, Finer N, et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the Neonatal Oxygenation Prospective Meta‐analysis Collaboration. JAMA. 2018;319(21):2190‐2201. 10.1001/jama.2018.5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Veenendaal NR, Heideman WH, Limpens J, et al. Hospitalising preterm infants in single family rooms versus open bay units: a systematic review and meta‐analysis. Lancet Child Adolesc Health. 2019;3(3):147‐157. 10.1016/S2352-4642(18)30375-4 [DOI] [PubMed] [Google Scholar]
- 25. Norman M, Hallberg B, Abrahamsson T, et al. Association between year of birth and 1‐year survival among extremely preterm infants in Sweden during 2004‐2007 and 2014‐2016. JAMA. 2019;321(12):1188‐1199. 10.1001/jama.2019.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helenius K, Longford N, Lehtonen L, Modi N, Gale C. Association of early postnatal transfer and birth outside a tertiary hospital with mortality and severe brain injury in extremely preterm infants: observational cohort study with propensity score matching. BMJ. 2019;367:l5678. 10.1136/bmj.l5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks' gestation. JAMA Network Open. 2018;1(6):e183235. 10.1001/jamanetworkopen.2018.3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Domellof M, Pettersson K. [Guidelines for threatening premature birth will provide better and more equal care]. Lakartidningen. 2017;114:EEYI. [PubMed] [Google Scholar]
- 29. Mactier H, Bates SE, Johnston T, et al. Perinatal management of extreme preterm birth before 27 weeks of gestation: a framework for practice, Archives of Disease in Childhood. Fetal Neonatal Ed. 2020;105(3):232‐239. 10.1136/archdischild-2019-318402 [DOI] [PubMed] [Google Scholar]
- 30. Ancel P‐Y, Breart G, Bruel H, et al. Propositions for perinatal care at extremely low gestational ages – working group on “extremely low gestational ages” for SFMP, CNGOF, and SFN. Gynecologie, Obstetrique Fertilite Senologie. 2020;48(12):850‐857. 10.1016/j.gofs.2020.09.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


