Abstract
Computed tomography (CT)‐derived body metrics such as skeletal muscle index (SMI), psoas muscle index (PMI), and subcutaneous fat area index (ScFI) are measurable components of sarcopenia, frailty, and nutrition. While these body metrics are advocated in adults for predicting postoperative outcomes after liver transplantation (LT), little is known about their value in pediatric populations. This study assessed the relation between preoperative CT‐based body metrics and postoperative short‐term outcomes in pediatric LT recipients. Patients aged 0‐18 years who underwent a primary LT were retrospectively included (n = 101; median age 0.5 years; range 0.2‐17.1). SMI, PMI, and ScFI were derived from preoperative axial CT slices. Postoperative outcomes and complications within 90 days were correlated with the CT‐based body metrics. To classify postoperative infections, the Clavien‐Dindo (CD) classification was used. Subgroup analyses were performed for age groups (<1, 1‐10, and >10 years old). An optimal threshold for test performance was defined using Youden’s J‐statistic and receiver operating characteristic curve as appropriate. ScFI was significantly (P = 0.001) correlated with moderate to severe postoperative infections (CD grade 3‐5) in children aged <1 year, with the optimal ScFI threshold being ≤27.1 cm2/m2 (sensitivity 80.4% and specificity 77.8%). A weak negative correlation between SMI and the total duration of hospital stay (R = −0.3; P = 0.01) and intensive care unit (ICU) stay (R = −0.3; P = 0.01) was observed in children aged <1 year. No other associations between CT‐based body metrics and postoperative outcomes were shown. In children aged <1 year with cirrhotic liver disease undergoing LT, preoperative CT‐based body metrics were correlated with moderate to severe postoperative infections (ScFI) and with longer duration of hospital and ICU stay (SMI), and thus can be considered important tools for pre‐LT risk assessment.
Abbreviations
- AFI
total abdominal fat index
- BMI
body mass index
- CD
Clavien‐Dindo
- CSMA
cross‐sectional skeletal muscle area
- CT
computed tomography
- DDLT
deceased donor liver transplantation
- DICOM
Digital Imaging and Communications in Medicine
- ICU
intensive care unit
- IQR
interquartile range
- LDLT
living donor liver transplantation
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- MUAC
mid‐upper arm circumference
- PELD
Pediatric End‐Stage Liver Disease
- PMI
psoas muscle index
- ScFI
subcutaneous fat area index
- SMI
skeletal muscle index
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- tPMSA
total psoas muscle surface area
- TSFT
triceps skinfold thickness
- VFI
visceral fat area index
Children requiring a liver transplantation (LT) are generally chronically ill and have end‐stage liver disease.( 1 ) Because of altered nutrient absorption and processing, higher metabolism, and reduced intake, many children suffer from malnutrition.( 2 , 3 , 4 ) Malnutrition is independently associated with a higher rate of severe postoperative infections and with longer hospital stay in children undergoing LT.( 5 , 6 ) Necessity for nasogastric tube feeding or parenteral feeding, often along with growth failure, is also an important indicator for poorer outcome.( 7 , 8 )
Children waiting for LT may also suffer from frailty, which consists of multisystemic physiological decline and increased vulnerability to stressors associated with chronic illness, and is closely related to malnutrition.( 9 ) Sarcopenia is considered a measurable component of frailty, defined as low muscle quality and quantity, low strength, and poor physical performance caused by chronic illness, malnutrition, or aging.( 10 ) Although measurement of sarcopenia is clinically often performed by determining the mid‐upper arm circumference (MUAC), computed tomography (CT)‐based body metrics may be superior in reflecting body composition and nutritional status.( 11 , 12 , 13 )
A recent study showed that psoas muscle index (PMI), skeletal muscle index (SMI), and fat indices could be reliably determined even in young children (median age, 4.6 months) with biliary atresia.( 13 ) Recent pediatric studies have also shown potential clinical application for PMI and total psoas muscle surface area (tPMSA) but did not investigate SMI or fat indices.( 14 , 15 , 16 , 17 ) In adults undergoing LT, sarcopenia diagnosed by CT‐based body metrics is advocated as an independent predictor of higher waitlist and posttransplantation mortality and higher perioperative complication rates.( 18 , 19 ) However, because of differences in body composition, findings from adult studies cannot be readily extrapolated to children, and thus, further pediatric studies are needed.
Therefore, this study aimed to determine the relation between preoperative CT‐based body metrics and postoperative short‐term clinical outcomes in pediatric LT recipients with cirrhotic liver disease.
Patients and Methods
Patients
This study was approved by the local research ethics committee (registry number 201800080), and informed consent was waived.
All pediatric (<18 years old) LT recipients, treated between 2003 and 2019 in our national pediatric LT center, were eligible for the study (n = 265). Patients were excluded in 2 phases; first, if a portal venous phase abdominal CT scan was not available (n = 75), the CT scan was performed >6 months before LT (n = 60), CT slice thickness was >3 mm (n = 0), or in case of a previous LT (n = 12). Second, children with noncirrhotic liver disease (eg, acute hepatic failure, hepatoblastoma) were also excluded (n = 16). In total, 101 patients were included. The flowchart of the study population can be found in Fig. 1. Data collection and reporting of analysis were performed according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.( 20 )
FIG. 1.
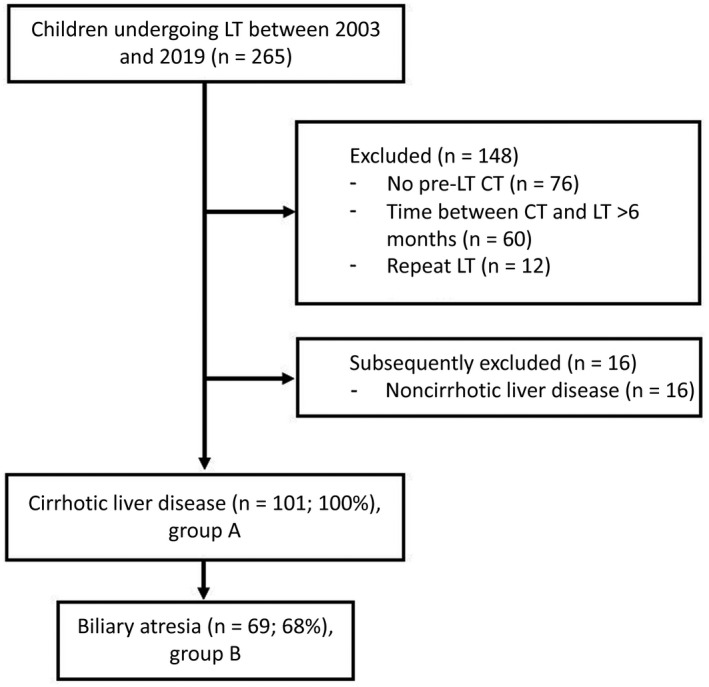
Flowchart of the study population.
Data Collection and Postoperative Short‐Term Clinical Outcomes
Data were collected from our prospectively maintained institutional database and retrospectively analyzed. Collected demographic and anthropometric data at the time of the preoperative CT included age, sex, weight, length, growth failure (<−2 z score length or weight for age and sex,( 21 )) body mass index (BMI), primary disease requiring LT, Pediatric End‐Stage Liver Disease (PELD) score (aged under 12 years) and Model for End‐Stage Liver Disease (MELD) score (all ages), international normalized ratio, bilirubin level (μmol/L), albumin level (g/L), creatinine level (μmol/L), living donor or deceased donor liver transplantation (LDLT or DDLT), and type of LT (full size or split liver). Data were available and complete for all patients, with the exception of the MELD score in 4 patients.
General postoperative outcomes and complications retrieved from the medical records concerned length of total postoperative hospital stay, length of intensive care unit (ICU) stay, death <90 days after LT, and graft failure or acute rejection <90 days after LT (necessitating re‐LT, increase of immunosuppressive treatment, or resulting in death).
Surgical postoperative complications comprised biliary leakage or anastomotic stenosis <90 days after LT (requiring surgery or percutaneous transhepatic cholangiography with balloon dilatation or stenting), and vascular anastomotic stenosis or thrombosis (hepatic artery, portal vein, liver veins) <90 days after LT requiring treatment.
Postoperative infection severity <90 days after LT was classified according to the Clavien‐Dindo (CD) classification.( 22 ) CD grades were clustered for CD 0‐2 (no or low‐grade infection) and CD 3‐5 (moderate to severe infection or death).
CT‐Based Body Metrics Assessment
All CT scans were performed according to a standard clinical protocol with acquisition parameters adjusted to patient weight. CT images were obtained from our Picture Archiving and Communication System, anonymized, and stored in DICOM (Digital Imaging and Communications in Medicine) format. Measurements included tPMSA (cm2), cross‐sectional skeletal muscle area (CSMA; cm2), total abdominal fat area (cm2), visceral fat area (cm2), and subcutaneous fat area (cm2) on 3‐mm axial slices at vertebral level L3, in accordance with previous publications,( 23 , 24 , 25 ) using in‐house developed software (Sarcomeas version 0.56; Supporting Fig. 1). This software automatically delineated the geometric regions of interest using Hounsfield units, followed by manual correction of any inconsistencies by a pediatric radiologist (M.V.V., 5 years of dedicated pediatric radiology experience). All geometric regions of interest were subsequently checked by a musculoskeletal radiologist (A.R.V., 8 years of dedicated musculoskeletal radiology experience, and corrected in consensus with M.V.V).
tPMSA (cm2) was converted to PMI using the formula PMI (cm2/m2) = tPMSA (cm2)/(patient length [m])2. This allowed for interpatient comparison corrected for patient length as a measure of body proportions.( 26 ) CSMA, total abdominal fat area, visceral, and subcutaneous fat areas were corrected similarly, resulting in SMI (cm2/m2), total abdominal fat index (AFI; cm2/m2), visceral fat area index (VFI; cm2/m2), and subcutaneous fat area index (ScFI; cm2/m2).
Analysis
Because mesenteric and retroperitoneal fat measurements were frequently impaired by fat stranding, we assessed the significance of this fat stranding. Therefore, CT slices were categorized into a 5‐point visual scale and graded as follows: 1) not affected; 2) mild (<50% mesenteric fat stranding); 3) moderate (≥50% mesenteric but not retroperitoneal edema); 4) significant (mesenteric and retroperitoneal fat stranding); and 5) severe fat stranding. If the percentage of patients with moderate or severe fat stranding was above 25%, we considered these data not suitable for further analysis.
Assessment of the relation between preoperative CT‐based body metrics (PMI, SMI, AFI, VFI, ScFI) and postoperative short‐term clinical outcomes was performed for all children with cirrhotic liver disease (group A) and for those with biliary atresia alone (group B).
Because body metrics vary according to age, analyses were performed in 3 age groups: <1‐year old (babies and infants), 1‐10 years old (mobilizing toddlers and prepubertal children), and >10 years old (peri/postpubertal children).
CT‐based body metrics were also analyzed according to type of LT and growth failure (−2 standard deviation for age). Correlation between CT‐based body metrics and MELD scores was determined using previously published MELD score divisions: <15, 15‐20, and >20.( 27 )
Statistics
Statistics were performed using SPSS for Windows (version 26; IBM, New York, NY). The level of significance was set at α < 0.05. Continuous variables were summarized using median and interquartile range (IQR).
To assess whether subgroups needed to be made because of potential variations of body metrics during the time interval between CT and LT, a goodness‐of‐fit linear regression was performed for all body metrics, and body metrics were compared between 0‐3 months’ and 4‐6 months’ interval time.
In case of 2 groups with non‐normally distributed continuous data, the Mann‐Whitney U test was used. Specifically, postoperative complications were analyzed for dependence on indexed body metrics using the Mann‐Whitney U test. Spearman’s rank correlation test was performed to determine correlations between CT‐based body metrics and continuous postoperative variables. This was also done for BMI and z score for height and weight compared with the body metrics.
A receiver operating characteristic curve was constructed and an area under the curve was calculated for significant findings related to postoperative infection (CD 0‐2 versus 3‐5). When significant, the optimal threshold for test performance was assessed using Youden’s J‐statistic to predict severe postoperative infection based on body metrics.
The z scores for sarcopenia were determined for children aged 1‐16 years, based on the available reference values provided by Lurz et al.,( 25 ) with sarcopenia defined as a z score of <−2. Reference z scores for children aged <1 year or >16 years are currently not available. Postoperative outcomes were compared between the subsequent sarcopenic and nonsarcopenic groups.
This study was conducted according to the ethical standards established by the 1964 Declaration of Helsinki and later amendments and prevailing national regulations and guidelines.
Results
Baseline Characteristics
Group A—all children with cirrhotic liver disease—consisted of 101 children (male‐to‐female ratio, 42:59; median age, 0.5 years; IQR, 5.3, range, 0.2‐17.1 years) and group B—biliary atresia—consisted of 69 children (median age, 0.4 years). Further baseline characteristics are presented in Table 1. The distribution of specific liver diseases can be found in Supporting Table 1.
TABLE 1.
Demographic Data and CT‐Based Body Metrics of Patients with Cirrhotic Liver Disease and the Biliary Atresia Subgroup
| Demographics and Metrics | Patients With Cirrhosis (Group A) | Patients With Biliary Atresia (Group B) |
|---|---|---|
| Age at CT, years | 0.50 (5.3) | 0.39 (0.2) |
| Number | 101 | 69 |
| Sex, male:female | 42:59 | 22:47 |
| tPMSA, cm2 | 3.4 (2.7) | 3.2 (0.9) |
| PMI, cm2/m2 | 5.9 (2.9) | 6.7 (2.5) |
| CSMA, cm2 | 25.9 (25.9) | 24.10 (5.6) |
| SMI, cm2/m2 | 48.0 (16.6) | 51.4 (12.2) |
| Subcutaneous fat area, cm2 | 17.7 (13.7) | 15.9 (11.1) |
| ScFI, cm2/m2 | 31.4 (22.5) | 35.0 (22.9) |
| MELD | 19 (7) | 18.5 (7) |
| MELD subgroups (<15, 15‐20, >20, missing) | 23, 39, 25, 4 | 13, 34, 21, 1 |
| PELD | 10 (9.8) | 10 (9.5) |
| Bilirubin, μmol/L | 146 (181.5) | 141.5 (129.5) |
| International normalized ratio | 1.3 (0.4) | 1.3 (0.4) |
| Albumin, g/L | 34 (8) | 34 (6) |
| Creatinine, μmol/L | 18 (12.8) | 16 (7) |
| Growth failure* | 23/87 (26.4) | 17/60 (28.3) |
| Body mass index | 16.1 (3.4) | 17.6 (3.2) |
| LDLT | 52 | 43 |
| DDLT | 49 | 26 |
| Full size | 21 | 8 |
| Split liver | 80 | 61 |
Data are presented as n, n/all (%), or median (IQR).
Missing data not included.
The median time interval between CT and LT was 2.9 months (IQR, 2.5 months). The goodness‐of‐fit linear regression for the time interval between CT and LT was R 2 < 0.02 for all indices. In addition, there were no differences between CT‐based body metrics in the interval time group 0‐3 months compared with 4‐6 months, regardless of age groups. Therefore, no further subgroups were made regarding the time interval between CT and LT.
Intra‐Abdominal Fat Stranding
Intra‐abdominal fat stranding occurred in >25% of the total study population (Supporting Table 2), and therefore, AFI and VFI were excluded from subsequent analyses. Moderate and higher grades of intra‐abdominal fat stranding were significantly more frequent in children aged <1 year (P < 0.001). In none of the patients subcutaneous fat stranding was observed, and thus, ScFI was included for further analysis.
CT‐Based Body Metrics Assessment
tPMSA, PMI, CSMA, SMI, subcutaneous fat area, and ScFI for groups A and B are given in Table 1.
PMI, SMI, and ScFI were significantly higher in children aged <1 year (P < 0.001) compared with the other age subgroups (Table 2 provides the CT‐based body metrics for group A according to age subgroups).
TABLE 2.
CT‐Based Body Metrics in Patients With Cirrhotic Liver Disease (Group A) Categorized According to Age Subgroups
| Metrics | <1 Year (n = 59) | 1‐10 Years (n = 28) | ≥10 Years (n = 14) |
|---|---|---|---|
| Age at CT, years | 0.4 (0.2) | 3.9 (5.5) | 12.4 (5.6) |
| Sex, male:female | 21:38 | 13:15 | 8:6 |
| Growth failure* | 14/51 (27.5) | 4/23 (17.4) | 5/13 (38.5) |
| Interval between CT and LT, months | 3.2 (2.3) | 2.7 (2.5) | 2.3 (3.1) |
| tPMSA, cm2 | 3.1 (1.1) | 5.1 (2.9) | 9.9 (5.7) |
| PMI, cm2/m2 | 7.1 (2.1) † | 4.8 (1.4) | 4.7 (2.6) |
| CSMA, cm2 | 23.4 (3.9) | 42.1 (26.6) | 77.5 (31.7) |
| SMI, cm2/m2 | 53.2 (11.1) † | 40.4 (11.4) | 38.5 (9.9) |
| Subcutaneous fat area, cm2 | 15.5 (9.3) | 22.9 (18.7) | 58.4 (40.3) |
| ScFI, cm2/m2 | 36.3 (21.8) † | 28.6 (22.9) | 23.6 (25.6) |
Data are presented as n/all (%) or median (IQR).
Defined as −2 standard deviation weight and/or length for age.
Significant at P < 0.001: index higher compared with other age subgroups.
No significant differences were observed when comparing body metrics between DDLT and LDLT, full size and split liver, and children with or without growth failure, neither in group A or B, nor in age‐dependent subanalyses.
PMI did not correlate with z score for weight, z score for height, or BMI. SMI showed a weak negative correlation with z score for height (R = 0.3; P = 0.002), but not for z score for weight or BMI. ScFI correlated moderately with BMI (R = 0.4; P < 0.001) and had a weak correlation with z score for weight (R = 0.3; P = 0.004); there was no correlation with z score for height.
Postoperative Outcomes and Complications
In group A, among children aged <1 year, a significant but weak negative correlation was observed between SMI and the total length of hospital stay (R = −0.3; P = 0.01) and ICU stay (R = −0.3; P = 0.01), indicating that lower SMI correlated with longer hospital and ICU stay. No correlations between SMI and hospital or ICU stay were demonstrated in other age groups, and no correlations were demonstrated for PMI or ScFI.
No differences in PMI, SMI, or ScFI were demonstrated for general and surgical postoperative outcomes and (noninfectious) complications, neither in group A or B, nor in the age‐based subgroups (Table 3).
TABLE 3.
CT‐Based Body Metrics in Patients With Cirrhotic Liver Disease (Group A) Categorized According to Postoperative Outcomes for Both All Ages and Children Aged <1 Year
| Metrics | n | All Ages | n | Aged <1 Year | ||||
|---|---|---|---|---|---|---|---|---|
| PMI, cm2/m2 | SMI, cm2/m2 | ScFI, cm2/m2 | PMI, cm2/m2 | SMI, cm2/m2 | ScFI, cm2/m2 | |||
| Death | ||||||||
| Yes | 5 | 6.1 (1.6) | 49.5 (11.8) | 26.4 (14.1) | 4 | 6.6 (1.3) | 55.6 (11.3) | 30.0 (13.3) |
| No | 96 | 6.1 (2.0) | 49.4 (12.4) | 33.6 (18.9) | 55 | 7.0 (1.9) | 54.0 (7.2) | 37.7 (19.9) |
| Graft failure or rejection | ||||||||
| Yes | 9 | 5.5 (1.1) | 47.5 (10.4) | 42.4 (35.0) | 4 | 5.8 (1.3) | 51.6 (6.0) | 58.4 (42.2) |
| No | 92 | 6.1 (2.0) | 49.4 (12.4) | 32.3 (16.0) | 55 | 7.1 (1.9) | 55.2 (11.4) | 35.3 (16.2) |
| Biliary complications | ||||||||
| Yes | 17 | 6.6 (2.1) | 50.3 (8.7) | 38.6 (14.9) | 12 | 7.2 (2.3) | 52.9 (8.3) | 39.6 (8.8) |
| No | 84 | 5.9 (1.9) | 49.1 (12.8) | 32.1 (19.0) | 47 | 6.9 (1.8) | 55.5 (11.8) | 36.2 (21.2) |
| Vascular complications | ||||||||
| Yes | 20 | 6.5 (1.4) | 51.3 (7.5) | 40.3 (24.5) | 17 | 6.6 (1.3) | 51.7 (5.5) | 40.9 (23.9) |
| No | 81 | 5.9 (2.1) | 48.8 (13.1) | 31.4 (16.3) | 42 | 7.1 (2.0) | 56.3 (12.6) | 35.2 (17.2) |
| Total | 101 | 59 | ||||||
Data are presented as mean (standard deviation).
Distribution of severity of postoperative infections in group A according to the CD classification is presented in Table 4. The majority of children had no or low‐grade infection (84.2%; CD 0‐2). Moderate to severe postoperative infections (CD 3‐4) were present in 15.8% of children. No children died from infection (CD 5) during the first 90 postoperative days.
TABLE 4.
Postoperative Infections Within 90 Days Stratified According to the Clavien‐Dindo Classification, Group A
| Age Groups | All Ages | <1 Year | 1‐10 Years | ≥10 Years |
|---|---|---|---|---|
| Clavien‐Dindo grade | ||||
| 0 | 30 (29.7) | 14 (23.3) | 11 (39.3) | 5 (35.7) |
| 1‐2 | 55 (54.5) | 36 (61.0) | 14 (50.0) | 5 (35.7) |
| 3‐4 | 16 (15.8) | 9 (15.0) | 43(10.7) | 4 (28.6) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 101 | 59 | 28 | 14 |
Data are presented as n (%).
Table 5 lists the differences in body metrics for CD 0‐2 compared with CD 3‐5 in group A for all ages and in age subgroups. ScFI in group A was significantly (P = 0.001) higher in children aged <1 year for CD 0‐2 compared with CD 3‐5, and a similar result was observed when combining all ages (P = 0.02). In group B, ScFI was also significantly higher in children aged <1 year (P = 0.001), and for all ages together (P = 0.01), when comparing CD 0‐2 with CD 3‐5. No associations between CD and PMI or SMI were observed.
TABLE 5.
Differences in Body Metrics According to Severity of Postoperative Infections <90 Days After Liver Transplantation, Group A
| Clavien‐Dindo | All Ages | <1 Year | 1‐10 Years | ≥10 Years | ||||
|---|---|---|---|---|---|---|---|---|
| 0‐2 | 3‐5 | 0‐2 | 3‐5 | 0‐2 | 3‐5 | 0‐2 | 3‐5 | |
| PMI | 6.2 (2.6) | 5.5 (3.3) | 7.1 (2.0) | 7.4 (3.9) | 4.9 (1.5) | 4.6 (1.1) | 3.9 (2.5) | 5.6 (3.4) |
| SMI | 50.1 (16.9) | 45.6 (12.6) | 53.8 (9.6) | 48.0 (10.6) | 40.8 (12.2) | 38.9 (14.3) | 37.2 (7.1) | 43.4 (28.7) |
| ScFI | 33.2 (20.7) | 16.7 (27.4)* | 38.3 (19.2) | 13.3 (21.4) † | 28.3 (20.7) | 40.5 (37.3) | 27.0 (24.2) | 16.5 (48.5) |
Data are presented as median (IQR).
Significant at P = 0.017.
Significant at P = 0.001.
The area under the curve for ScFI in children aged <1 year (group A) stratified according to postoperative infection severity (CD 0‐2 versus CD 3‐5) was 0.9. Based on Youden’s J‐statistic of 0.6, an ScFI threshold of ≤27.1 cm2/m2 yielded a sensitivity of 80.4% and specificity of 77.8% for the prediction of severe postoperative infections (CD grade 3‐5).
Sarcopenia at Ages 1‐16 Years
When applying the reference values for z scores provided by Lurz et al.( 25 ) to group A, 19 (55.6%) children aged 1‐16 years (n = 34) had a tPMSA <−2 z score, indicating sarcopenia. There were no significant differences in postoperative outcomes between the resulting sarcopenic and nonsarcopenic groups.
MELD and PELD Scores
CT‐based body metrics did not differ significantly between different MELD score categories. In addition, there was no significant correlation between CT‐based body metrics and the MELD or PELD score (Supporting Table 3).
Discussion
This study aimed to determine the relation between body metrics (PMI and SMI as measurements of sarcopenia, and ScFI as a measurement of subcutaneous fat storage) derived from preoperative abdominal CT scans and postoperative short‐term clinical outcomes and complications in children with cirrhotic liver disease undergoing LT. While these body metrics are advocated in adults for predicting postoperative outcomes,( 4 , 10 ) studies on tPMSA and PMI in children have shown varying results, and SMI and ScFI have not been previously studied.( 14 , 15 , 16 , 17 ) In the current study, in children <1 year ScFI emerged as a useful marker to identify the risk of moderate to severe postoperative infections, and in these children a high SMI correlated with a shorter hospital and ICU stay. However, PMI and SMI were of no other apparent value in predicting other short‐term postoperative outcomes. Nevertheless, these results suggest an additional value of CT‐derived body metrics in pre‐LT risk assessment.
A possible hypothesis for the association between low ScFI and infections could be that low amounts of subcutaneous fat represent malnutrition. It is imperative to diagnose and treat malnutrition in children undergoing LT, both because of the established association between malnutrition and immunological alterations leading to infection,( 28 ) and because of the poor growth and recurrent hospitalization after LT.( 2 ) Our suggested ScFI cutoff value of ≤27.1 cm2/m2 in children aged under 1 year for assessing postoperative infection risk illustrates how body metrics may aid in individualizing nutritional strategies before LT. Besides, it further underlines the importance of recognizing preoperative malnutrition.
SMI, PMI, and ScFI were higher in children aged <1 year. This could be the result of a better nutritional status, but could also be age dependent. Age‐dependent differences of body metrics have been reported previously. In one pediatric study investigating percentual body fat mass, this peaked in infancy between 0.5 and 3‐6 months in a multicomponent model,( 29 ) whereas in another study for triceps skinfold thickness (TSFT) measurements a bimodal peak at age <1 year and 12 years was demonstrated.( 30 ) In terms of sarcopenia body metrics, Mangus et al.( 3 ) found that healthy children below 5 years and older than 12 years had a higher PMI compared with those aged 5‐12 years, and ScFI was higher at ages 0‐12 years compared with 13‐18 years. Thus, age‐dependent variations of CT‐based body metrics are evident, and for each body metric normal values are needed.
Defining body composition and malnutrition in children may be challenging. BMI does not differentiate between fat‐free mass and fat mass; therefore, large nutritional deficits may be missed when children lose muscle mass and gain fat at the same time.( 3 , 12 , 31 ) We showed an absence of correlation between BMI and PMI/SMI and only moderate correlation with ScFI. This further underlines the notion that BMI is not suitable for the assessment of muscle mass and fat stores. Subcutaneous fat measurements by determining the MUAC and TSFT are conventionally used to measure body composition and nutritional status,( 12 ) but edema and hypoalbuminemia may bias these measurements.( 11 , 12 ) Thus, CT‐based body metric assessment seems a logical next step. Although our study only demonstrated a limited correlation between CT‐based sarcopenia body metrics and postoperative outcomes, it may still be beneficial to combine CT‐based body metrics with MUAC and TSFT to ultimately provide a multifactorial model of frailty, body composition, and malnutrition. In addition, CT‐based body metrics suggesting sarcopenia despite extensive attempts to improve nutritional status in children requiring an LT could be used in optimizing LT waitlist placement or LDLT planning.
A possible concern regarding the structural implementation of CT‐based body metrics is the associated radiation exposure. In this study measurements were done on CT studies performed as part of the pre‐LT workup, and no additional radiation exposure occurred. However, if repeated indices are necessary during follow‐up or surveillance, the radiation burden of repeat abdominal CTs could become substantial. In this scenario, 2‐mm single‐slice CT may provide accurate measurements,( 32 ) while significantly decreasing the radiation dose. Alternatively, measurement may also be feasible on MRI,( 14 ) albeit more costly than CT. Last, ultrasound measurements of the psoas muscles have shown moderate to good correlation with PMI in adults,( 33 ) although SMI and ScFI could not be measured, and this requires further research in children.
The primary limitation of this study was the lack of reference values for body metrics in healthy children in the literature. Available studies providing some normal values consistently lack children under the age of 1 year because they are based on children with acute pathology (eg, appendicitis( 2 , 24 , 25 )). tPMSA reference values for children aged 1‐16 years are available,( 25 ) but validated reference values for other (indexed) body metrics, and at all ages, are needed.
A second limitation was the lack of availability of MUAC and TSFT in our study. Boster et al.( 14 ) showed a moderate correlation between MUAC and tPMSA; however, further research is necessary to better understand the differences and complementary value of MUAC and TSFT compared with CT‐based body metrics.
In conclusion, low ScFI derived from transverse abdominal CT slices is significantly associated with moderate to severe postoperative infections in children aged <1 year undergoing LT for cirrhotic liver disease, and in children undergoing LT ScFI seems to be the only reliable CT‐based body fat marker. In addition, a high SMI in children aged <1 year is significantly correlated with a shorter hospital stay. Thus, CT‐based body metrics provide important information to estimate body composition before LT and can be used to assess the risk of post‐LT moderate to severe infection.
Supporting information
Fig S1
Table S1
Table S2
Table S3
Potential conflict of interest: Nothing to report.
References
- 1. Cuenca AG, Kim HB, Vakili K. Pediatric liver transplantation. Semin Pediatr Surg 2017;26:217‐223. [DOI] [PubMed] [Google Scholar]
- 2. Mager DR, Hager A, Ooi PH, Siminoski K, Gilmour SM, Yap JYK. Persistence of sarcopenia after pediatric liver transplantation is associated with poorer growth and recurrent hospital admissions. J Parenter Enteral Nutr 2019;43:271‐280. [DOI] [PubMed] [Google Scholar]
- 3. Mangus RS, Bush WJ, Miller C, Kubal CA. Severe sarcopenia and increased fat stores in pediatric patients with liver, kidney, or intestine failure. J Pediatr Gastroenterol Nutr 2017;65:579‐583. [DOI] [PubMed] [Google Scholar]
- 4. Ooi PH, Hager A, Mazurak VC, Dajani K, Bhargava R, Gilmour SM, Mager DR. Sarcopenia in chronic liver disease: impact on outcomes. Liver Transpl 2019;25:1422‐1438. [DOI] [PubMed] [Google Scholar]
- 5. Pawlowska J. The importance of nutrition for pediatric liver transplant patients. Clin Exp Hepatol 2016;2:105‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barshes NR, Chang IF, Karpen SJ, Carter BA, Goss JA. Impact of pretransplant growth retardation in pediatric liver transplantation. J Pediatr Gastroenterol Nutr 2006;43:89‐94. [DOI] [PubMed] [Google Scholar]
- 7. Hammad A, Kaido T, Aliyev V, Mandato C, Uemoto S. Nutritional therapy in liver transplantation. Nutrients. 2017;9:1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Utterson EC, Shepherd RW, Sokol RJ, Bucuvalas J, Magee JC, McDiarmid SV, et al. Biliary atresia: clinical profiles, risk factors, and outcomes of 755 patients listed for liver transplantation. J Pediatr 2005;147:180‐185. [DOI] [PubMed] [Google Scholar]
- 9. Laube R, Wang H, Park L, Heyman JK, Vidot H, Majumdar A, et al. Frailty in advanced liver disease. Liver Int 2018;38:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 10. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bakshi N, Singh K. Nutrition assessment and its effect on various clinical variables among patients undergoing liver transplant. Hepatobiliary Surg Nutr 2016;5:358‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collins L, Beaumont L, Cranston A, Savoie S, Nayiager T, Barr R. Anthropometry in long‐term survivors of acute lymphoblastic leukemia in childhood and adolescence. J Adolesc Young Adult Oncol 2017;6:294‐298. [DOI] [PubMed] [Google Scholar]
- 13. Grutters LA, Pennings JP, Bruggink JLM, Viddeleer AR, Verkade HJ, de Kleine RHJ, de Haas RJ. Body composition of infants with biliary atresia: anthropometric measurements and computed tomography‐based body metrics. J Pediatr Gastroenterol Nutr 2020;71:440‐445. [DOI] [PubMed] [Google Scholar]
- 14. Boster JM, Browne LP, Pan Z, Zhou W, Ehrlich PF, Sundaram SS. Higher mortality in pediatric liver transplant candidates with sarcopenia. Liver Transpl 2021;27:808‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jitwongwai S, Lertudomphonwanit C, Junhasavasdikul T, Fuangfa P, Tanpowpong P, Gesprasert G, Treepongkaruna S. Low psoas muscle index as an unfavorable factor in children with end‐stage liver disease undergoing liver transplantation. Pediatr Transplant 2021;25:e13996. [DOI] [PubMed] [Google Scholar]
- 16. Takeda M, Sakamoto S, Uchida H, Shimizu S, Yanagi Y, Fukuda A, et al. Impact of sarcopenia in infants with liver transplantation for biliary atresia. Pediatr Transplant 2021;25:e13950. [DOI] [PubMed] [Google Scholar]
- 17. Woolfson JP, Perez M, Chavhan GB, Johara FT, Lurz E, Kamath BM, Ng VL. Sarcopenia in children with end‐stage liver disease on the transplant waiting list. Liver Transpl 2021;27:641‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JN. Systematic review and meta‐analysis of the impact of computed tomography‐assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant 2016;16:2277‐2292. [DOI] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344‐349. [DOI] [PubMed] [Google Scholar]
- 21. Rodd C, Metzger DL, Sharma A, Canadian Pediatric Endocrine Group Working Committee for National Growth Charts . Extending World Health Organization weight‐for‐age reference curves to older children. BMC Pediatr 2014;14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Ann Surg 2009;250:187‐196. [DOI] [PubMed] [Google Scholar]
- 23. Dedhia PH, White Y, Dillman JR, Adler J, Jarboe MD, Teitelbaum DH, et al. Reduced paraspinous muscle area is associated with post‐colectomy complications in children with ulcerative colitis. J Pediatr Surg 2018;53:477‐482. [DOI] [PubMed] [Google Scholar]
- 24. Lopez JJ, Cooper JN, Albert B, Adler B, King D, Minneci PC. Sarcopenia in children with perforated appendicitis. J Surg Res 2017;220:1‐5. [DOI] [PubMed] [Google Scholar]
- 25. Lurz E, Patel H, Lebovic G, Quammie C, Woolfson JP, Perez M, et al. Paediatric reference values for total psoas muscle area. J Cachexia Sarcopenia Muscle 2020;11:405‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chae MS, Kim Y, Kim J, Ko YR, Jung JY, Choi HJ, et al. Perioperative changes in the psoas muscle index in patients undergoing ABO‐incompatible living‐donor liver transplantation: a single‐center experience. Transplant Proc. 2018;50:3656‐3660. [DOI] [PubMed] [Google Scholar]
- 27. van der Doef HPJ, van Rheenen PF, van Rosmalen M, Rogiers X, Verkade HJ, for pediatric liver transplantation centers of Eurotransplant . Wait‐list mortality of young patients with Biliary atresia: competing risk analysis of a eurotransplant registry‐based cohort. Liver Transpl 2018;24:810‐819. [DOI] [PubMed] [Google Scholar]
- 28. Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition—a systematic review. PLoS One 2014;9:e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butte NF, Hopkinson JM, Wong WW, Smith EOB, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res 2000;47:578‐585. [DOI] [PubMed] [Google Scholar]
- 30. Tanner JM, Whitehouse RH. Revised standards for triceps and subscapular skinfolds in British children. Arch Dis Child 1975;50:142‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barone M, Viggiani MT, Avolio AW, Iannone A, Rendina M, Di Leo A. Obesity as predictor of postoperative outcomes in liver transplant candidates: review of the literature and future perspectives. Dig Liver Dis 2017;49:957‐966. [DOI] [PubMed] [Google Scholar]
- 32. Zopfs D, Theurich S, Große Hokamp N, Knuever J, Gerecht L, Borggrefe J, et al. Single‐slice CT measurements allow for accurate assessment of sarcopenia and body composition. Eur Radiol 2020;30:1701‐1708. [DOI] [PubMed] [Google Scholar]
- 33. Kobayashi K, Maruyama H, Kiyono S, Ogasawara S, Suzuki E, Ooka Y, et al. Application of transcutaneous ultrasonography for the diagnosis of muscle mass loss in patients with liver cirrhosis. J Gastroenterol 2018;53:652‐659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Table S2
Table S3


