Abstract
Objectives
To determine the predictive and prognostic value of a panel of systemic inflammatory response (SIR) biomarkers relative to established clinicopathological variables in order to improve patient selection and facilitate more efficient delivery of peri‐operative systemic therapy.
Materials and Methods
The preoperative serum levels of a panel of SIR biomarkers, including albumin–globulin ratio, neutrophil–lymphocyte ratio, De Ritis ratio, monocyte–lymphocyte ratio and modified Glasgow prognostic score were assessed in 4199 patients treated with radical cystectomy for clinically non‐metastatic urothelial carcinoma of the bladder. Patients were randomly divided into a training and a testing cohort. A machine‐learning‐based variable selection approach (least absolute shrinkage and selection operator regression) was used for the fitting of several multivariable predictive and prognostic models. The outcomes of interest included prediction of upstaging to carcinoma invading bladder muscle (MIBC), lymph node involvement, pT3/4 disease, cancer‐specific survival (CSS) and recurrence‐free survival (RFS). The discriminatory ability of each model was either quantified by area under the receiver‐operating curves or by the C‐index. After validation and calibration of each model, a nomogram was created and decision‐curve analysis was used to evaluate the clinical net benefit.
Results
For all outcome variables, at least one SIR biomarker was selected by the machine‐learning process to be of high discriminative power during the fitting of the models. In the testing cohort, model performance evaluation for preoperative prediction of lymph node metastasis, ≥pT3 disease and upstaging to MIBC showed a 200‐fold bootstrap‐corrected area under the curve of 67.3%, 73% and 65.8%, respectively. For postoperative prognosis of CSS and RFS, a 200‐fold bootstrap corrected C‐index of 73.3% and 72.2%, respectively, was found. However, even the most predictive combinations of SIR biomarkers only marginally increased the discriminative ability of the respective model in comparison to established clinicopathological variables.
Conclusion
While our machine‐learning approach for fitting of the models with the highest discriminative ability incorporated several previously validated SIR biomarkers, these failed to improve the discriminative ability of the models to a clinically meaningful degree. While the prognostic and predictive value of such cheap and readily available biomarkers warrants further evaluation in the age of immunotherapy, additional novel biomarkers are still needed to improve risk stratification.
Keywords: muscle‐invasive bladder cancer, non‐muscle invasive bladder cancer, bladder cancer, biomarker, adjuvant chemotherapy, systemic therapy, transitional cell carcinoma, #utuc, #uroonc
Introduction
Radical cystectomy (RC) and neoadjuvant cisplatin‐based combination chemotherapy (NAC), in eligible patients, is the standard‐of‐care treatment for carcinoma invading bladder muscle (MIBC) [1, 2]. However, because of the heterogeneous nature of urothelial carcinoma of the bladder (UCB), as well as the high rate of occult micrometastases, approximately half of all MIBC patients will ultimately succumb to their disease despite apparently successful extirpative surgery [3, 4, 5, 6]. Unfortunately, appropriate patient selection for delivery of peri‐operative systemic therapy remains difficult, as there is discrepancy between clinical stage and final pathological stage and only postoperative pathological features offer the highest prognostic value [7, 8, 9, 10, 11, 12]. Accurate preoperative identification of patients who are most likely to experience occult metastasis due to ≥pT3 disease or lymph node metastasis (LNM) would be helpful in order to facilitate more efficient delivery of neoadjuvant systemic therapy. This is of special importance, as the current one one‐size‐fits‐all approach seems suboptimal for most healthcare providers worldwide, and its absolute net benefit in overall survival (OS) of only 5%, together with its non‐negligible adverse events, contribute to the underutilization of NAC [12, 13]. Similarly, a more accurate postoperative prognosis of survival outcomes could allow improved patient selection with respect to adjuvant systemic therapies in patients who did not receive NAC [14, 15]. Because the biological behaviour of tumours varies among individuals, there is an unmet need to provide reliable risk stratification tools as there is still a lack of clinically useful biomarkers that add sufficient value to outcome prediction methods [7, 9, 16, 17, 18, 19, 20, 21, 22].
We and others have previously reported that systemic inflammatory response (SIR) biomarkers, such as albumin–globulin ratio (AGR), NLR, modified Glasgow prognostic score (mGPS), MLR and De Ritis ratio, are associated with adverse pathological features and eventual disease recurrence and progression in UCB [23, 24, 25, 26]. Despite promising results on conventional uni‐ and multivariable analyses, none of these biomarkers demonstrated the ability to increase the discriminative power of predictive and prognostic models fitted with well‐established clinicopathological variables. For biomarkers to be of clinical significance, they must offer unique additional predictive and prognostic information, demonstrated by meaningfully improving the performance of reference models constructed without the novel biomarker [17]. Although the value of singular biomarkers so far remains limited in UCB, it has previously been suggested that a panel of biomarkers can significantly improve outcome prognosis [7, 27, 28, 29].
We, therefore, hypothesized that a panel of readily available blood‐based SIR biomarkers could improve outcome prediction in patients treated with RC for UCB. Using a machine‐learning‐based variable selection approach, we analysed a large, well‐established, international multicentre database to determine the most effective predictors and create the most informative, yet parsimonious model with respect to several clinically important outcome variables. To fully determine the discriminative ability of the finally included SIR biomarkers, their additional predictive and prognostic value was separately assessed in a comprehensive model performance evaluation.
Methods
Subjects/Patients
This retrospective study included patients who underwent RC for treatment of clinically non‐metastatic UCB from 12 participating international medical institutions between 1979 and 2012. All cases were histologically confirmed UCB with only a minor secondary variant component, if any. The preoperative serum levels of AGR, neutrophil–lymphocyte ratio (NLR), mGPS, MLR and the De Ritis ratio were measured in 4199 patients within 4 weeks of RC. The biomarkers and the respective thresholds have previously been described in detail [23, 24, 25, 26]. The study was approved by the local ethics committee. No patient received NAC or radiotherapy. The extent of lymph node dissection and the choice of urinary diversion were at the surgeon’s discretion. Patients with known autoimmune, chronic inflammatory or haematological disorders, as well as patients with any concomitant second malignancy other than UCB, concomitant upper urinary tract carcinoma, or missing data were excluded.
All surgical specimens were processed according to standard pathological procedures as previously described [8]. All cases were histologically confirmed UCB with only a minor variant component, if any. All tumours were staged according to the American Joint Committee on Cancer Staging Manual (8th edition) TNM classification and graded according to the 1973 WHO grading system. The presence of concomitant carcinoma in situ (CIS) was defined as the presence of CIS in conjunction with a tumour other than CIS [29]. Pelvic lymph nodes were examined grossly, and all lymphoid tissue was submitted for histological examination. Positive soft tissue surgical margins were defined as the presence of tumour at inked areas of soft tissue on the RC specimen [30]. Urethral or ureteric margins were not considered as soft tissue surgical margins. Lymphovascular invasion was defined as the unequivocal presence of tumour cells within an endothelium‐lined space without underlying muscular walls [31].
Adjuvant chemotherapy was typically administered within 3 months of RC at the discretion of the treating physician and according to international guideline recommendations at the time. Clinical and radiological follow‐up was performed in accordance with institutional protocols. For most patients, physical examination, radiological imaging and urine cytology were obtained every 3 months for 2 years, then semiannually between the second and the fifth year. After 5 years, annual follow‐up was performed. Tumour recurrence was defined as locoregional recurrence or distant metastasis on radiological imaging. Cause of death was abstracted from medical charts end/or from death certificates [32]. Patient data were collected and stored in a common anonymized dataset.
Statistical Analyses
In order to simulate external validation and to perform a true performance assessment, we randomly divided patients into a training cohort (n = 2100) and a testing cohort (n = 2099). Patients’ characteristics in the training set and testing set, as well as the distribution of SIR biomarkers, were compared using Wilcoxon's rank‐sum test, the chi‐squared test of independence, the Kruskal–Wallis test or Fisher's exact test, as appropriate. Three separate logistic regression models were fitted that focused on preoperative prediction of upstaging to pT2 disease in patients that were staged cTis/cTa or cT1, LNM and ≥pT3 disease at the time of RC, respectively. Furthermore, two separate Cox models were fitted for postoperative prognosis of recurrence‐free survival (RFS) and cancer‐specific survival (CSS).
For fitting of these models, the least absolute shrinkage and selection operator (LASSO) approach and 10‐fold cross‐validation were used to determine the most significant predictors from all available variables. During the LASSO procedure, the absolute value of the regression coefficients of the assessed variables is continuously reduced through the use of a penalty. Using this penalty, which is the sum of the absolute size of the regression coefficients multiplied by a tuning parameter (λ), some coefficients are shrunk to zero. The corresponding variables hold little predictive value and can be neglected during the fitting of the model. The optimal weight of λ was determined by a 10‐fold cross‐validation in the training set. For this purpose, the C‐index or area under the curve (AUC) across the cross‐validation folds was calculated for increments of λ. The weight of λ that minimizes deviation in the cross‐validation is given by λmin; however, the weight of λ that empirically has been shown to create the most parsimonious yet informative model is λ1.se, defined as the value of λ within one standard deviation of the minimum mean cross‐validated error [33]. Variables whose LASSO coefficient were not equal to zero at λ1.se were extracted and used during fitting of the prognostic models. This cross‐validation process minimizes risk of overfitting and is a way of assessing how a model will perform in an independent dataset. In summary, the LASSO procedure allows a machine‐learning‐based variable selection for the fitting of prognostic and predictive models. It has been suggested to be particularly well suited for variables that show high levels of multicollinearity, as would be expected for SIR biomarkers [34, 35].
The selected variables were then used to fit separate multivariable logistic regression and Cox models. Discriminative ability of these models was assessed by calculating the AUC of receiver‐operating characteristic curves or C‐index (Harrell's concordance index, an approximation of the AUC in censored data) for both the training and the testing cohort. To assess the additional discriminative power of the biomarkers, reference models were fitted that did not include the previously selected SIR biomarkers. AUCs were statistically compared using DeLong’s test. Calibration plots graphically explored the association between predicted probabilities and the observed proportions. Goodness‐of‐fit of logistic regression models was tested using the Hosmer–Lemeshow test, while goodness‐of‐fit of Cox regression models was tested using the Grønnesby‐and‐Borgan test. Validation was performed using 200 bootstrap re‐samples as a means of calculating the most unbiased predictive accuracy. Based on the prognostic and predictive models, separate nomograms were created to guide clinical decision‐making. Finally, decision‐curve analysis was used to evaluate the clinical net benefit of the models for both the training and testing cohort. All reported P values were two‐sided, and statistical significance was set at 0.05. All statistical analyses were performed using R (Version 4.0.3, Vienna, Austria, 2020).
Results
Overall, 4199 patients were included in the analysis. With the exception of a higher rate of administration of adjuvant chemotherapy in the testing cohort (23% vs 19%; P < 0.001), patient characteristics as well as the distribution of SIR biomarkers were similar in both cohorts (Table S1). There was no statistically significant correlation between the assessed SIR biomarkers (P > 0.05 for all). The median (interquartile range) follow‐up of all surviving patients was 41.9 (18.1–84.3) months. The 5‐year RFS, CSS and OS were 61.2% (95% CI 59.5–62.9), 67.3% (95% CI 65.6–69) and 56.3% (95% CI 54.6–58), respectively.
Overall, our database included 1527 patients (36.3%) who were staged to have non‐muscle‐invasive disease before RC; upstaging to MIBC occurred in 43.7% of these cases.
Model Performance Evaluation and Validation
For all outcome variables, at least one SIR biomarker was selected by the machine‐learning process to have high discriminative power during the fitting of the models (Figs 1, 2, 3, 4, 5). A high NLR was found to be the most effective predictor of LNM and upstaging to MIBC in the testing cohort (odds ratio 2.26, P < 0.001 and odds ratio 1.97, P < 0.001, respectively). In the testing cohort, model performance evaluation for preoperative prediction of LNM, ≥pT3 disease and upstaging to MIBC showed a 200‐fold bootstrap‐corrected AUC of 67.3%, 73% and 65.8%, respectively. For postoperative prognosis of CSS and RFS, a 200‐fold bootstrap corrected C‐index of 73.3% and 72.2%, respectively, was found.
Fig. 1.
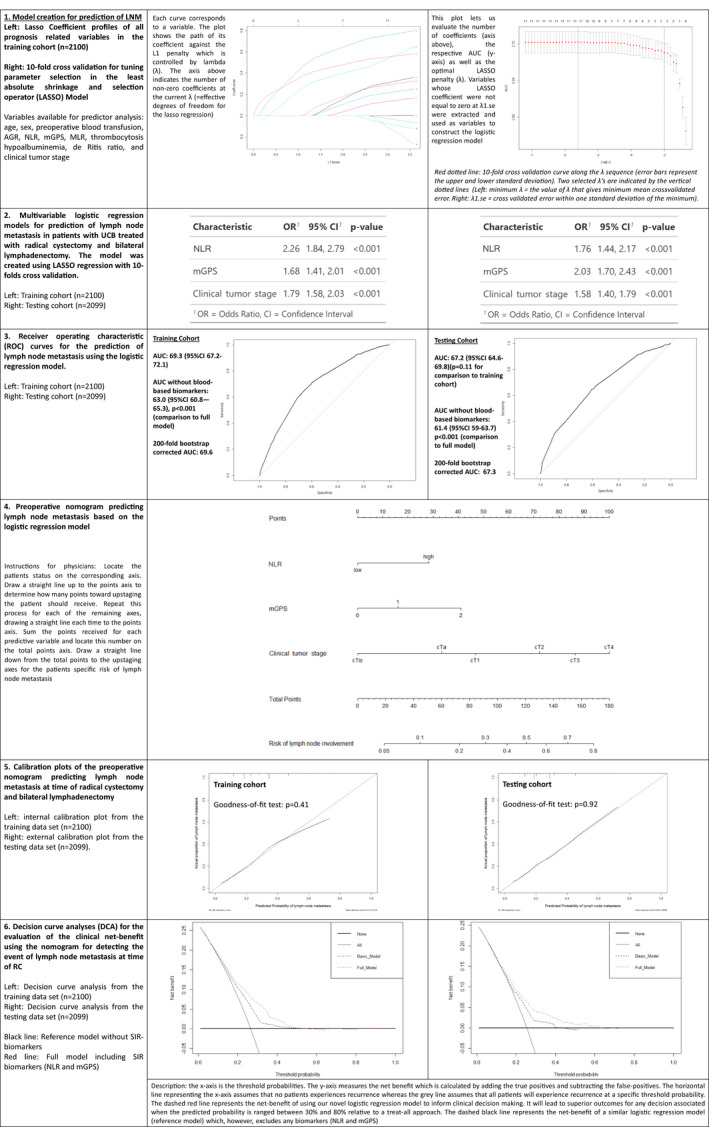
Model creation and performance evaluation for the prediction of lymph node involvement in 4199 patients treated with radical cystectomy for urothelial carcinoma of the bladder. 1. Model creation through least absolute shrinkage and selection operator regression analysis. 2. Logistic regression analysis. 3. Receiver‐operating characteristic curves and model performance evaluation. 4. Nomogram based on the logistic regression model. 5. Model calibration curves. 6. Decision‐curve analyses. AGR, albumin–globulin ratio; AUC, area under the curve; mGPS, modified Glasgow prognostic score; MLR, monocyte‐lymphocyte ratio; NLR, neutrophil‐lymphocyte ratio; OR, odds ratio; UCB, urothelial carcinoma of the bladder.
Fig. 2.

Model creation and performance evaluation for prediction of ≥pT3 disease in 4199 treated with radical cystectomy for urothelial carcinoma of the bladder. 1. Model creation through LASSO regression analysis. 2. Logistic regression analysis. 3. Receiver operating characteristic curves and model performance evaluation. 4. Nomogram based on the logistic regression model. 5. Model calibration curves. 6. Decision‐curve analyses. AGR, albumin–globulin ratio; AUC, area under the curve; mGPS, modified Glasgow prognostic score; MLR, monocyte‐lymphocyte ratio; NLR, neutrophil‐lymphocyte ratio; OR, odds ratio; UCB, urothelial carcinoma of the bladder.
Fig. 3.
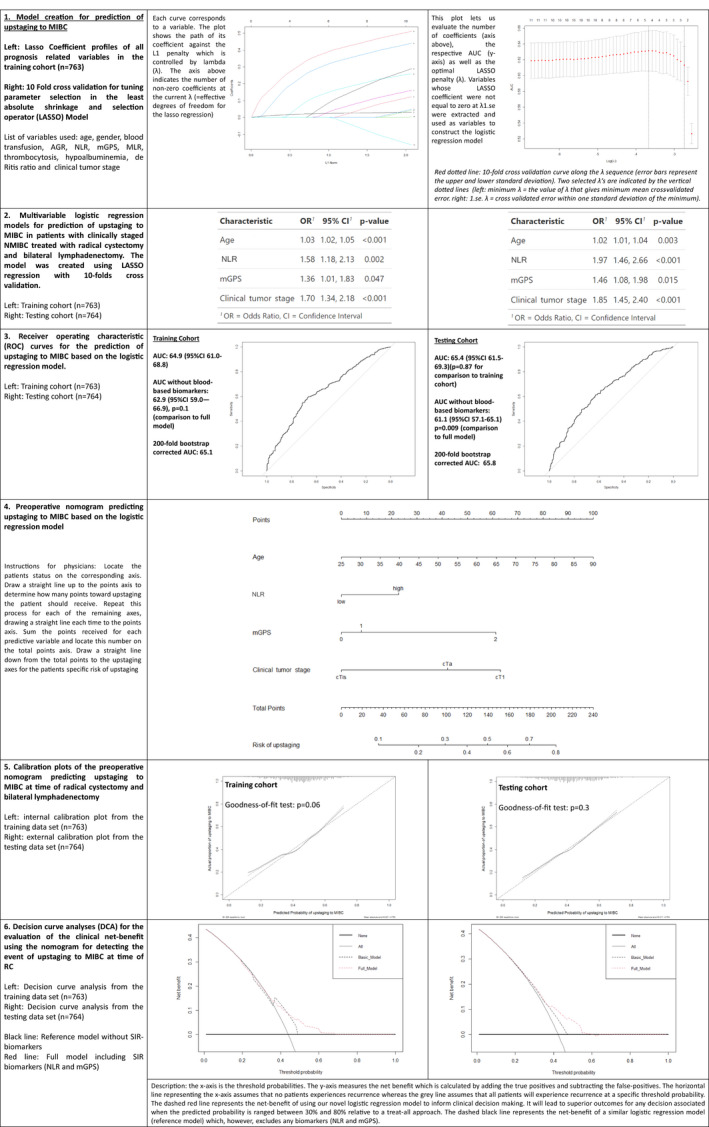
Model creation and performance evaluation for prediction of upstaging to muscle‐invasive bladder cancer in 1527 patients treated with radical cystectomy for urothelial carcinoma of the bladder staged cT1, cTa, or cTis. 1. Model creation through LASSO regression analysis. 2. Logistic regression analysis. 3. Receiver operating characteristic curves and model performance evaluation. 4. Nomogram based on the logistic regression model. 5. Model calibration curves. 6. Decision‐curve analyses. AGR, albumin–globulin ratio; AUC, area under the curve; mGPS, modified Glasgow prognostic score; MLR, monocyte‐lymphocyte ratio; NLR, neutrophil‐lymphocyte ratio; OR, odds ratio; UCB, urothelial carcinoma of the bladder.
Fig. 4.

Model creation and performance evaluation for prediction of two‐year recurrence‐free survival in 4199 treated with radical cystectomy for urothelial carcinoma of the bladder. 1. Model creation through LASSO regression analysis. 2. Cox regression analysis and performance evaluation. 3. Nomogram based on the Cox regression model. 4. Model calibration curves. 5. Time‐dependent AUC. 6. Decision curve analyses. AGR, albumin–globulin ratio; AUC, area under the curve; mGPS, modified Glasgow prognostic score; MLR, monocyte–lymphocyte ratio; NLR, neutrophil‐lymphocyte ratio; OR, odds ratio; UCB, urothelial carcinoma of the bladder.
Fig. 5.
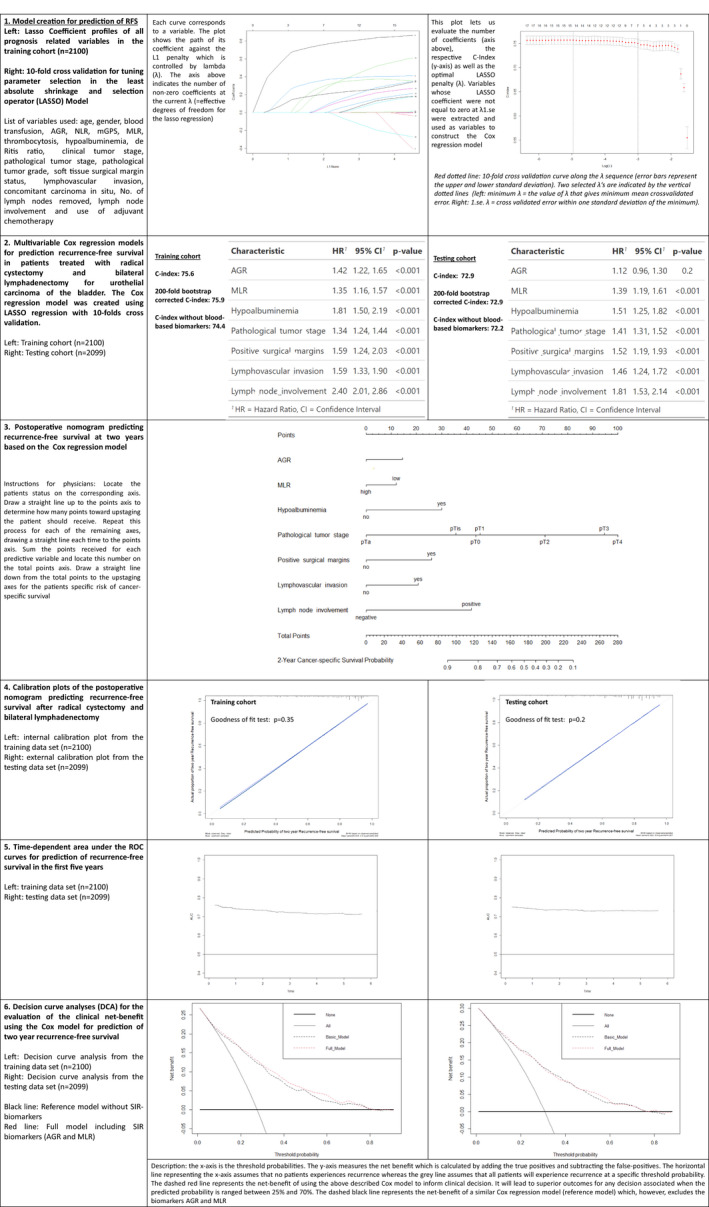
Model creation and performance evaluation for prediction of two‐year cancer‐specific survival in 4199 treated with radical cystectomy for urothelial carcinoma of the bladder. 1. Model creation through LASSO regression analysis. 2. Cox regression analysis and performance evaluation. 3. Nomogram based on the Cox regression model. 4. Model calibration curves. 5. Time‐dependent AUC. 6. Decision curve analyses. AGR, albumin–globulin ratio; AUC, area under the curve; mGPS, modified Glasgow prognostic score; MLR, monocyte–lymphocyte ratio; NLR, neutrophil‐lymphocyte ratio; OR, odds ratio; UCB, urothelial carcinoma of the bladder.
Performance Evaluation without the Selected SIR Biomarkers
For prediction of LNM, exclusion of the SIR biomarkers resulted in a significant decrease in the AUC in both the training and testing cohorts (−6.3% training cohort vs −5.8% testing cohort; P < 0.001). For prediction of upstaging to MIBC, exclusion of both SIR biomarkers did not result in a significant decrease in AUC in the training cohort (−2.0%; P = 0.1). However, exclusion of both biomarkers led to a statistically significant reduction in AUC in the testing cohort (−4.3%, P = 0.009). The exclusion of the SIR biomarkers for prediction/prognosis of ≥pT3 disease, CSS and RFS resulted in a decrease in AUC/C‐index (i.e. under 3%) for all cohorts.
Nomogram and Model Calibration
Assessment of the nomogram axes indicated that all demonstrated a wide range of predicted probabilities; however, for none of the models did an SIR biomarker contribute the highest number of risk points. The calibration plots showed that the models demonstrated near‐optimal agreement between prediction by the model and actual outcome observation. In accordance with that, the goodness‐of‐fit tests were insignificant for all cohorts. For the two Cox models, time‐dependent AUC plots demonstrate a stable model performance over a period of 5 years (Figs 4, 5).
Decision‐Curve Analysis
For prediction of LNM, decision‐curve analysis showed that the model offers a clinical net benefit relative to the treat‐all approach at a threshold of between 30% and 50%; the addition of the SIR biomarkers increased the net benefit relative to a reference model across this threshold range. This range group contained 706 patients (33.6%) in the training cohort and 408 patients (19.4%) in the testing cohort. For prediction of upstaging, addition of the two included SIR biomarkers resulted in a net benefit gain of up to 10% in patients with an upstaging threshold probability of between 50% and 60%. This range group contained 245 patients (11.7%) in the training cohort and 83 patients (3.95%) in the testing cohort. For prediction of ≥pT3 disease as well as for prognosis of CSS and RFS, addition of the SIR biomarkers to a reference model resulted in no relevant net benefit gain across any threshold probability.
Discussion
Accurate identification of patients who are at a seemingly high risk of disease recurrence, despite a presumed adequate surgical treatment with curative intent, remains a healthcare challenge in the treatment of UCB [36]. Ideal biomarkers could detect occult micrometastases and therefore offer potential to improve survival outcomes by facilitating more accurate patient selection for intensified peri‐operative systemic therapy [28]. However, despite major advances in the molecular profiling of UCB, its heterogeneity still hampers the establishment of a clinically useful, reproducible, readily available, cheap and accurate biomarker [7, 9, 16, 17, 18, 19, 20, 21, 22]. The lack of clinically useful biomarkers also hinders the emergence of new therapeutic approaches such as bladder preservation strategies, which require precise risk stratification [37]. Through the use of a machine‐learning‐based approach, we were able to select the most valuable predictors with respect to several clinically relevant outcomes from a large collection of variables. For all analyzed outcome variables, blood‐based SIR biomarkers were chosen for the fitting of the most parsimonious, yet informative, models. Even though several other prognostic and predictive models as well as nomograms have been developed, the current model possesses multiple characteristics that distinguishes it from comparable tools. It is indeed, the first to feature a panel of cheap, reproducible and ready‐available SIR biomarkers.
As calibration and validation of nomograms are paramount before implementation in clinical practice, we performed a statistically rigorous evaluation of all models [14]. Indeed, our models showed nearly perfect calibration properties. The large sample size of over 4000 patients and the study's multicentre nature render the nomograms highly generalizable. Furthermore, the nomograms demonstrate a wide range of predicted probabilities. Finally, the inclusion of a maximum of three readily available SIR biomarkers offers a very low level of complexity for our nomograms, suggesting that they are easily reproducible. Due to the large number of available patients for this retrospective analysis, we aimed to imitate external validation by splitting our cohort into two equal‐sized large groups of patients. While true external validation with separate cohorts remains the best assessment of a model's accuracy and a crucial step before transferring the models into clinical practice [14], we found that all results from the training cohort could be reproduced in the testing cohort.
The comparison of our prognostic and predictive models, however, to similar reference models that excluded any SIR biomarkers revealed that the addition of the selected SIR biomarkers only offered a minimal additional increase in discriminative ability. The highest increase was found in the prediction of LNM through the addition NLR and mGPS to the model (increase in AUC: 5.8% in the testing cohort). For all other models, the increase in discriminative ability through the addition of the selected SIR biomarkers was far below 3%. Similarly, on decision‐curve analysis, most models failed to offer any relevant net benefit in comparison to a reference model or only offered a minor benefit for a small group of patients. However, the accuracy of our models was similar during both internal and external validation and was comparable to previously reported nomograms that only included clinicopathological variables [15, 38, 39, 40, 41]. Nevertheless, we failed to reach a clinically superior predictive performance, as clinical applicability of a nomogram has been previously been proposed only for those who exhibit AUC/C‐indices >0.75 after external validation [42]. As we used a sophisticated machine‐learning‐based approach for the development of our model, the ultimate reason for our negative findings is probably that the analysed SIR biomarkers simply do not hold enough unique information to improve the discriminative ability of the models and that they are too unspecific to be used in cancer‐related outcome prediction.
While next‐generation sequencing has revealed several genetic alterations in UCB with a potential to become candidate biomarkers, their implementation into clinical practice remains hindered by a lack of large and properly powered external validation studies [28, 43]. External validation, however, is frequently not successful and/or attempted due to costs, intratumour heterogeneity, absence of a standardized approach and the overall complexity of next‐generation sequencing [7]. As UCB is more than the sum of genetic alterations, the complete landscape of features contributing to this disease, including environmental, hereditary, behavioural and epigenetic factors, needs to be explored. Consequently, models that focus on the combination of a greater variety of data (e.g. a combinatorial approach to identifying genetic and non‐genetic biomarkers from different sources), improved imaging techniques combined with the use of artificial intelligence holds the highest potential to generate externally reproducible results thereby coming a quantum leap closer to improving individual patient care [44]. In this setting, SIR biomarkers might yet prove useful for outcome prediction, especially with respect to novel immunotherapies and their ease of procurement and high sample homogeneity [7]. Our approach of testing the incremental predictive accuracy of such biomarkers beyond that already provided by established risk factors could serve as a benchmark for the evaluation of novel biomarkers and thus guide their clinical implementation.
Although the present study uses a statistically rigorous validation and calibration process, it has several limitations. First and foremost are the limitations inherent to any retrospective data collection, especially with respect to any potential selection bias and/or attrition bias. Second is the wide temporal range and the lack of data with respect to the use of NAC or novel therapies such as Programmed Death‐Ligand 1 or Fibroblast Growth Factor Receptor 3 inhibitors. The multi‐institutional nature of our study could be interpreted as a potential limitation, as it may ignore differences that might distinguish the contributing centres. However, this approach is less likely to transfer bias due to varying community practice. Finally, our results are limited by the singular testing and the failure to control for additional potential risk factors (e.g. smoking, therapies before RC such as intravesical instillations or use of re‐transurethral resection of bladder tumour, occupational exposure).
In conclusion, although our machine‐learning approach for fitting of the model with the highest discriminative ability incorporated several previously validated, cheap and readily available SIR biomarkers, these failed to improve the discriminative ability of the models by a prognostically and clinically meaningful margin. While the prognostic and predictive value of such biomarkers warrants further evaluation in the age of immunotherapy, novel biomarkers are still needed to improve risk stratification. We established a framework for validation of promising biomarkers in a phased, structured biomarker assessment.
Conflicts of Interest
All authors have no conflict of interest.
Abbreviations
- AGR
albumin–globulin ratio
- AUC
area under the curve
- CIS
carcinoma in situ; CSS, cancer‐specific survival
- LASSO
least absolute shrinkage and selection operator
- LNM
lymph node metastasis
- mGPS
modified Glasgow prognostic score
- MLR
monocyte–lymphocyte ratio
- MIBC
carcinoma invading bladder muscle
- NAC
neoadjuvant cisplatin‐based combination chemotherapy
- NLR
neutrophil–lymphocyte ratio
- OS
overall survival
- RC
radical cystectomy
- RFS
recurrence‐free survival
- SIR
systemic inflammatory response
- UCB
urothelial carcinoma of the bladder
Supporting information
Table S1. Association of the preoperative blood‐based systemic inflammatory response biomarkers with clinicopathologic characteristics in 4199 patients treated with radical cystectomy for urothelial carcinoma of the bladder.
Acknowledgements
Victor Schuettfort, Benjamin Pradere and Ekaterina Laukhtina are supported by the EUSP Scholarship of the European Association of Urology (EAU). Nico C. Grossmann is supported by the Zurich Cancer League.
References
- 1. Witjes J, Bruins M, Cathomas R et al. EAU guidelines on muscle‐invasive and metastatic bladder cancer. EAU Guidelines 2019 (2019 Edn)
- 2. Grossman HB, Natale RB, Tangen CM et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003; 349: 859–66 [DOI] [PubMed] [Google Scholar]
- 3. Ploussard G, Shariat SF, Dragomir A et al. Conditional survival after radical cystectomy for bladder cancer: evidence for a patient changing risk profile over time. Eur Urol 2014; 66: 361–70 [DOI] [PubMed] [Google Scholar]
- 4. Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle‐invasive bladder cancer: long‐term results of the BA06 30894 trial. J Clin Oncol 2011; 29: 2171–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moschini M, Afferi L, Gandaglia G et al. Prediction of the need for an extended lymphadenectomy at the time of radical cystectomy in patients with bladder cancer. Eur Urol Focus 2020. [DOI] [PubMed] [Google Scholar]
- 6. Mir MC, Marchioni M, Zargar H et al. Nomogram predicting bladder cancer‐specific mortality after neoadjuvant chemotherapy and radical cystectomy for muscle‐invasive bladder cancer: results of an international consortium. Eur Urol Focus 2020. [DOI] [PubMed] [Google Scholar]
- 7. Soria F, Krabbe LM, Todenhofer T et al. Molecular markers in bladder cancer. World J Urol 2019; 37: 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Svatek RS, Shariat SF, Novara G et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int 2011; 107: 898–904 [DOI] [PubMed] [Google Scholar]
- 9. Mari A, Campi R, Tellini R et al. Patterns and predictors of recurrence after open radical cystectomy for bladder cancer: a comprehensive review of the literature. World J Urol 2018; 36: 157–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Novara G, Svatek RS, Karakiewicz PI et al. Soft tissue surgical margin status is a powerful predictor of outcomes after radical cystectomy: a multicenter study of more than 4,400 patients. J Urol 2010; 183: 2165–70 [DOI] [PubMed] [Google Scholar]
- 11. Svatek RS, Shariat SF, Lasky RE et al. The effectiveness of off‐protocol adjuvant chemotherapy for patients with urothelial carcinoma of the urinary bladder. Clin Cancer Res 2010; 16: 4461–7 [DOI] [PubMed] [Google Scholar]
- 12. Schiffmann J, Sun M, Gandaglia G et al. Suboptimal use of neoadjuvant chemotherapy in radical cystectomy patients: a population‐based study. Can Urol Assoc J 2016; 10: E82–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Advanced Bladder Cancer Meta‐analysis C . Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta‐analysis of individual patient data advanced bladder cancer (ABC) meta‐analysis collaboration. Eur Urol 2005; 48: 202–6 [DOI] [PubMed] [Google Scholar]
- 14. Shariat SF, Margulis V, Lotan Y, Montorsi F, Karakiewicz PI. Nomograms for bladder cancer. Eur Urol 2008; 54: 41–53 [DOI] [PubMed] [Google Scholar]
- 15. Karakiewicz PI, Shariat SF, Palapattu GS et al. Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol 2006; 176(4 Pt 1): 1354–61 [DOI] [PubMed] [Google Scholar]
- 16. Shariat SF, Chade DC, Karakiewicz PI et al. Combination of multiple molecular markers can improve prognostication in patients with locally advanced and lymph node positive bladder cancer. J Urol 2010; 183: 68–75 [DOI] [PubMed] [Google Scholar]
- 17. Shariat SF, Lotan Y, Vickers A et al. Statistical consideration for clinical biomarker research in bladder cancer. Urol Oncol 2010; 28: 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Motterle G, Andrews JR, Morlacco A, Karnes RJ. Response to neoadjuvant chemotherapy in bladder cancer. Eur Urol Focus 2020; 6: 642–9 [DOI] [PubMed] [Google Scholar]
- 19. Rink M, Chun FK, Dahlem R et al. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: a prospective study. Eur Urol 2012; 61: 810–7 [DOI] [PubMed] [Google Scholar]
- 20. Xylinas E, Robinson BD, Kluth LA et al. Association of T‐cell co‐regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur J Surg Oncol 2014; 40: 121–7 [DOI] [PubMed] [Google Scholar]
- 21. Mir C, Shariat SF, van der Kwast TH et al. Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: a large multi‐institutional study. BJU Int 2011; 108: 24–30 [DOI] [PubMed] [Google Scholar]
- 22. Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Correlation of cyclin D1 and E1 expression with bladder cancer presence, invasion, progression, and metastasis. Hum Pathol 2006; 37: 1568–76 [DOI] [PubMed] [Google Scholar]
- 23. Mori K, Miura N, Mostafaei H et al. Prognostic value of preoperative hematologic biomarkers in urothelial carcinoma of the bladder treated with radical cystectomy: a systematic review and meta‐analysis. Int J Clin Oncol 2020; 25: 1459–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'Andrea D, Moschini M, Gust KM et al. Lymphocyte‐to‐monocyte ratio and neutrophil‐to‐lymphocyte ratio as biomarkers for predicting lymph node metastasis and survival in patients treated with radical cystectomy. J Surg Oncol 2017; 115: 455–61 [DOI] [PubMed] [Google Scholar]
- 25. Laukhtina E, Mostafaei H, D'Andrea D et al. Association of De Ritis ratio with oncological outcomes in patients with non‐muscle invasive bladder cancer (NMIBC). World J Urol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kimura S, D’ Andrea D, Soria F et al. Prognostic value of modified Glasgow prognostic score in non‐muscle‐invasive bladder cancer. Urol Oncol 2019; 37: 179.e19–28 [DOI] [PubMed] [Google Scholar]
- 27. Bensalah K, Montorsi F, Shariat SF. Challenges of cancer biomarker profiling. Eur Urol 2007; 52: 1601–9 [DOI] [PubMed] [Google Scholar]
- 28. Ilijazi D, Abufaraj M, Hassler MR, Ertl IE, D'Andrea D, Shariat SF. Waiting in the wings: the emerging role of molecular biomarkers in bladder cancer. Expert Rev Mol Diagn 2018; 18: 347–56 [DOI] [PubMed] [Google Scholar]
- 29. Shariat SF, Kim J, Raptidis G, Ayala GE, Lerner SP. Association of p53 and p21 expression with clinical outcome in patients with carcinoma in situ of the urinary bladder. Urology 2003; 61: 1140–5 [DOI] [PubMed] [Google Scholar]
- 30. Xylinas E, Rink M, Robinson BD et al. Impact of histological variants on oncological outcomes of patients with urothelial carcinoma of the bladder treated with radical cystectomy. Eur J Cancer 2013; 49: 1889–97 [DOI] [PubMed] [Google Scholar]
- 31. Shariat SF, Khoddami SM, Saboorian H et al. Lymphovascular invasion is a pathological feature of biologically aggressive disease in patients treated with radical prostatectomy. J Urol 2004; 171: 1122–7 [DOI] [PubMed] [Google Scholar]
- 32. Rink M, Fajkovic H, Cha EK et al. Death certificates are valid for the determination of cause of death in patients with upper and lower tract urothelial carcinoma. Eur Urol 2012; 61: 854–5 [DOI] [PubMed] [Google Scholar]
- 33. Krstajic D, Buturovic LJ, Leahy DE, Thomas S. Cross‐validation pitfalls when selecting and assessing regression and classification models. J Cheminform 2014; 6: 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010; 33: 1–22 [PMC free article] [PubMed] [Google Scholar]
- 35. Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective. J R Stat Soc Ser B Stat Methodol 2011; 73: 273–82 [Google Scholar]
- 36. Alkhateeb SS, Neill M, Bar‐Moshe S et al. Long‐term prognostic value of the combination of EORTC risk group calculator and molecular markers in non‐muscle‐invasive bladder cancer patients treated with intravesical Bacille Calmette‐Guerin. Urol Ann 2011; 3: 119–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schuettfort VM, Pradere B, Quhal F et al. Incidence and outcome of salvage cystectomy after bladder sparing therapy for muscle invasive bladder cancer: a systematic review and meta‐analysis. World J Urol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakagawa T, Taguchi S, Uemura Y et al. Nomogram for predicting survival of postcystectomy recurrent urothelial carcinoma of the bladder. Urol Oncol 2017; 35: 457.e15–21 [DOI] [PubMed] [Google Scholar]
- 39. Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006; 24: 3967–72 [DOI] [PubMed] [Google Scholar]
- 40. Di Trapani E, Sanchez‐Salas R, Gandaglia G et al. A nomogram predicting the cancer‐specific mortality in patients eligible for radical cystectomy evaluating clinical data and neoadjuvant cisplatinum‐based chemotherapy. World J Urol 2016; 34: 207–13 [DOI] [PubMed] [Google Scholar]
- 41. Karakiewicz PI, Shariat SF, Palapattu GS et al. Precystectomy nomogram for prediction of advanced bladder cancer stage. Eur Urol 2006; 50: 1254–60 [DOI] [PubMed] [Google Scholar]
- 42. Bianco FJ Jr. Nomograms and medicine. Eur Urol 2006; 50: 884–6 [DOI] [PubMed] [Google Scholar]
- 43. Tripathi A, Grivas P. The utility of next generation sequencing in advanced urothelial carcinoma. Eur Urol Focus 2020; 6: 41–4 [DOI] [PubMed] [Google Scholar]
- 44. Schuettfort VM, Pradere B, Rink M, Comperat E, Shariat SF. Pathomics in urology. Curr Opin Urol 2020; 30: 823–31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association of the preoperative blood‐based systemic inflammatory response biomarkers with clinicopathologic characteristics in 4199 patients treated with radical cystectomy for urothelial carcinoma of the bladder.


