Abstract
Background
Treatment of animal models with ataxia telangiectasia (A‐T) with nicotinamide riboside (NR) improved their neurological outcome and survival.
Objective
The aim of this study is to investigate the effects of NR in patients with A‐T.
Methods
In this open‐label, proof‐of‐concept study, 24 patients with A‐T were treated with NR during four consecutive months. The effects of NR on ataxia, dysarthria, quality of life, and laboratory parameters were analyzed.
Results
During treatment, ataxia scores improved; mean total Scale for the Assessment and Rating of Ataxia and International Cooperative Ataxia Rating Scale scores decreased to 2.4 and 10.1 points, respectively. After NR withdrawal, ataxia scores worsened. In immunodeficient patients, the mean serum IgG concentration increased substantially until the end of the study period with 0.52 g/L. Untargeted metabolomics analysis revealed increased plasma levels of NR metabolites and purine nucleosides during treatment. Adverse effects did not occur.
Conclusions
Treatment with NR is tolerated well and associated with improvement in ataxia and serum immunoglobulin concentrations in patients with A‐T. © 2021 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: ataxia telangiectasia, A‐T mutated gene, nicotinamide riboside
Ataxia telangiectasia (A‐T) is a neurodegenerative disorder with immunodeficiency and cancer predisposition.1, 2 Patients with “variant A‐T” have a milder phenotype, without immunodeficiency but with cancer predisposition. 3 To date, therapy for A‐T is restricted to symptomatic treatment, leaving patients with a greatly reduced life expectancy.1, 4, 5
A‐T is caused by variants of the A‐T mutated (ATM) gene, encoding the ATM protein. ATM plays a central role in vital cellular processes like DNA repair, oxidative stress responses, and energy metabolism.6, 7, 8, 9 Nicotinamide adenine dinucleotide (NAD+) is an essential cofactor for many of these processes, and NAD+ deficiency plays a role in disease mechanisms underlying DNA repair disorders.10, 11, 12 ATM‐deficient mice have neuronal NAD+ deficiency, in particular in the cerebellum. 13 Treatment of A‐T animal models with nicotinamide riboside (NR), a precursor of NAD+, improved their neurological disorder and survival impressively. 14
NR has been approved as a dietary supplement. 15
Given the experimental evidence and needs of patients with A‐T, we decided to perform the clinical trial as described here.
Patients and Methods
In this single‐center, interventional, open‐label, proof‐of‐concept study, 24 patients with A‐T were treated with NR (25 mg/kg bodyweight per day) during four consecutive months and subsequently followed during a 2‐month period without treatment. During the 6‐month study period, clinical and laboratory parameters were measured. Statistical analysis was performed using SPSS 25 for Windows. Group‐level results are presented as means. To assess the differences in the individual outcome measures between various time points, we applied linear mixed model analyses. Detailed information about the methods is presented in Supplementary File 1.
Results
All 34 patients with A‐T known in our center received information about the study; 24 of them (15 men, 9 women) were included. Ten patients (5 with classic A‐T, mean age 18.6 [standard deviation, SD: 13.7] years, and 5 with variant A‐T, mean age 42.6 [SD: 10.3] years) did not participate because they did not want to (n = 6), could not be reached (n = 2), or were too young (n = 2). The mean age of the participating patients was 17.5 (SD: 15.0) years; 17 were children (age < 18 years). Eighteen patients had the classic phenotype (mean age 10.3 [SD: 6.0] years) and 6 had variant A‐T (mean age 39.6 [SD: 11.8] years). None of the patients had ever used NR before. Most patients used medications, such as intravenous immunoglobulins and antibiotic prophylaxis; none of them had a recent medication change.
Clinical Outcome Measures
Mean total ataxia scores (Scale for the Assessment and Rating of Ataxia [SARA] 16 and International Cooperative Ataxia Rating Scale [ICARS] 17 ) improved during treatment with NR; this effect disappeared after NR withdrawal. No other differences were observed in clinical scores during the study period. The results of the clinical outcome measures are presented in Table 1.
TABLE 1.
Clinical effects and effects on serum immunoglobulins of nicotinamide riboside in patients with A‐T
| Clinical scales | T0 | T1 | T4 | T6 | T4–T0 | 95% CI | T6 –T4 | 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SARA (mean) n=22 | SD | SD | SD | SD | ||||||||||
| Total score | 21.3 | 9.2 | 19.6 | 8.6 | 19.6 | 8.4 | 23.1 | 8.4 | –2.4 | –4.0 | –0.9 | 3.9 | 2.5 | 5.3 |
| Gait | 4.8 | 2.5 | 4.7 | 2.7 | 4.9 | 2.7 | 5.3 | 2.3 | 0.1 | –0.3 | 0.6 | 0.4 | –0.04 | 0.8 |
| Stance | 4.1 | 2.0 | 3.9 | 1.8 | 4.3 | 2.1 | 4.3 | 2 | 0.1 | –0.3 | 0.5 | 0.04 | –0.3 | 0.4 |
| Sitting | 1.7 | 1.2 | 1.3 | 0.7 | 1.2 | 0.9 | 1.9 | 1.3 | –0.5 | –0.9 | –0.2 | 0.7 | 0.5 | 1.0 |
| Speech disturbance | 2.6 | 1.3 | 2.1 | 1.3 | 2.4 | 1.1 | 2.4 | 1.1 | –0.2 | –0.8 | 0.2 | 0 | ||
| Finger chase | 1.8 | 0.7 | 1.7 | 0.7 | 1.2 | 0.4 | 2.3 | 0.8 | –0.6 | –0.9 | –0.3 | 1.0 | 0.7 | 1.4 |
| Nose‐finger test | 2.1 | 0.8 | 1.9 | 0.7 | 1.8 | 1.1 | 2.3 | 0.8 | –0.3 | –0.8 | 0.1 | 0.5 | –0.07 | 1.1 |
| Fast alternating hand movements | 2.2 | 0.9 | 2.2 | 0.9 | 2.1 | 1.1 | 2.4 | 0.8 | –0.1 | –0.3 | 0.1 | 0.3 | –0.05 | 0.6 |
| Heel‐shin slide | 2.3 | 1.4 | 2.0 | 1.2 | 2.1 | 1.5 | 2.3 | 1.1 | –0.2 | –0.7 | 0.3 | 0.2 | –0.4 | 0.8 |
| ICARS (mean) n=22 | ||||||||||||||
| Total score | 58.3 | 23.0 | 50.6 | 21.9 | 49.7 | 20.8 | 61.5 | 20.4 | –10.1 | –13.2 | –6.8 | 12.7 | 9 | 16.4 |
| Posture/gait subscale | 22.6 | 10.5 | 20.8 | 10.7 | 23.1 | 10.6 | 24.1 | 9.2 | –0.2 | –2.0 | 1.6 | 1.4 | 0.2 | 2.7 |
| Kinetic subscale | 28.5 | 10.7 | 23.5 | 9.1 | 21 | 10.1 | 30.2 | 9.8 | –8 | –10 | –6 | 9.8 | 6.7 | 12.9 |
| Speech subscale | 3.6 | 1.7 | 2.9 | 1.3 | 2.8 | 1.5 | 3.1 | 1.3 | –0.8 | –1.4 | –0.2 | 0.4 | –0.2 | 1 |
| Oculomotor subscale | 3.8 | 1.8 | 3.5 | 2.0 | 2.8 | 1.6 | 4.0 | 1.5 | –1.1 | –1.5 | –0.7 | 1.3 | 0.9 | 1.8 |
| 9‐HPT (mean) | ||||||||||||||
| 9 pegs for time R‐hand (s) (n=18) | 75.4 | 46.6 | 74.1 | 56.7 | 77.4 | 44.5 | 81.1 | 44.4 | 1.9 | –8.3 | 12.2 | 1.0 | –6.8 | 8.7 |
| 9 pegs for time L‐hand (s) (n=18) | 93.5 | 56.3 | 80.1 | 45.5 | 88.6 | 55.0 | 94.6 | 53.3 | –4.4 | –23.6 | 13.8 | 3.7 | –7.9 | 15.5 |
| Number of pegs in 50 s R‐hand (n=6) | 2.0 | 1.3 | 3.2 | 2.6 | 2.7 | 2.9 | 2.0 | 1.9 | 0.7 | –1.1 | 2.4 | –0.8 | –3.0 | 1.5 |
| Number of pegs in 50 s L‐hand (n=6) | 1.7 | 1.9 | 2.3 | 2.2 | 2.3 | 2.0 | 1.4 | 1.7 | 0.7 | –0.6 | 1.9 | –0.8 | –2.4 | 0.7 |
| RDA/P‐RDA (mean) n=22 | ||||||||||||||
| Severity on function scale | 2.6 | 0.7 | 2.6 | 0.7 | 2.6 | 0.9 | 2.6 | 0.9 | 0.1 | –0.5 | 0.3 | 0.05 | –0.2 | 0.3 |
| Severity on activity scale | 1.8 | 0.7 | 1.7 | 0.6 | 2.0 | 0.7 | 1.9 | 0.8 | 0.1 | –0.1 | 0.4 | –0.05 | –0.3 | 0.2 |
| MPV (db) | 95.7 | 7.6 | 98.4 | 7.0 | 95 | 5.6 | 94.9 | 7.9 | ‐0.7 | –3.4 | 2.0 | –0.2 | –2.0 | 1.9 |
| MPT (s) | 6.0 | 3.7 | 7.7 | 5.1 | 6.4 | 4.5 | 6.7 | 5.2 | 0.6 | –0.7 | 1.8 | 0.3 | –0.4 | 1.0 |
| FFR LH (ST) | 14.6 | 6.4 | 18.7 | 6.4 | 15.4 | 6.3 | 15.1 | 5.6 | 1.0 | –1.8 | 3.9 | –0.2 | –2.4 | 2.1 |
| FFR HL (ST) | 13.7 | 5.5 | 15.5 | 5.9 | 14.2 | 5.7 | 15.2 | 6.7 | 0.7 | –1.8 | 3.2 | 1.1 | –1.2 | 3.4 |
| MRR (syl/s) | 3.7 | 0.9 | 3.5 | 0.9 | 3.7 | 1.1 | 3.7 | 0.9 | 0.1 | –0.3 | 0.5 | 0.1 | –0.1 | 0.4 |
| ICS (mean) n=24 | ||||||||||||||
| Total ICS | 3.9 | 0.5 | 3.8 | 0.5 | 3.7 | 0.5 | 3.7 | 0.5 | –0.2 | –0.1 | 0.4 | –0.01 | 0.9 | –0.2 |
| HRQOL (mean) n=24 | ||||||||||||||
| Mobility | 2.3 | 0.6 | 2.2 | 0.6 | 2.3 | 0.6 | 2.6 | 0.5 | –0.1 | –0.4 | 0.2 | 0.3 | 0.1 | 0.6 |
| Self‐care | 2.4 | 0.8 | 2.3 | 0.7 | 2.4 | 0.7 | 2.4 | 0.7 | –0.03 | –0.3 | 0.2 | 0.05 | –0.1 | 0.2 |
| Usual activities | 2.1 | 0.7 | 1.9 | 0.5 | 2.1 | 0.6 | 2.1 | 0.7 | –0.03 | –0.3 | 0.2 | 0.1 | –0.2 | 0.4 |
| Pain/discomfort | 1.6 | 0.5 | 1.3 | 0.5 | 1.5 | 0.5 | 1.4 | 0.5 | –0.1 | –0.3 | 0.1 | –0.1 | –0.2 | 0.1 |
| Anxiety/depression | 1.3 | 0.5 | 1.2 | 0.4 | 1.4 | 0.5 | 1.3 | 0.5 | 0.05 | –0.1 | 0.2 | –0.1 | –0.2 | 0.01 |
| VAS | 69.3 | 17.4 | 73.1 | 13.9 | 73.6 | 14.6 | 67.2 | 13.1 | 4.6 | –2.0 | 11.4 | –7.1 | –11.5 | –2.7 |
| Serum immunoglobulin levels in patients with classic A‐T | ||||||||||||||
| IgG | ||||||||||||||
| Patients with IgG replacement (n=6) | 9.56 | 3.5 | 10.89 | 4.1 | 12.42 | 4.7 | 11.09 | 2.6 | ||||||
| Patients without IgG replacement (n=12) | 7.68 | 1.9 | 8.07 | 2.0 | 7.95 | 2.0 | 8.3 | 2.0 | 0.28 | –0.07 | 0.62 | 0.35 | 0.05 | 0.65 |
| IgA | ||||||||||||||
| Patients with IgA deficiency (n=7) | <0.04 | 0 | <0.04 | 0 | <0.04 | 0 | <0.04 | 0 | ||||||
| Patients without IgA deficiency (n=11) | 0.98 | 0.4 | 1.15 | 0.6 | 1.10 | 0.6 | 0.97 | 0.5 | 0.12 | –0.05 | 0.29 | –0.13 | –0.25 | –0.01 |
| IgM | ||||||||||||||
| All patients (n=18) | 1.20 | 0.8 | 1.28 | 0.9 | 1.27 | 0.9 | 1.25 | 1.0 | 0.04 | –0.08 | 0.15 | 0.03 | –0.13 | 0.19 |
Mean serum IgA and IgM concentrations are reported for all patients with classic A‐T, whereas mean serum IgG concentrations are reported only for patients without immunoglobulin replacement therapy. All concentrations are in grams per liter. Bold differences and 95% CI indicate that 0 is not included in the 95% CI.
Abbreviations: A‐T, ataxia telangiectasia; CI, confidence interval; SARA, Scale for the Assessment and Rating of Ataxia (0–40 points; higher score indicates more severe ataxia); SD, standard deviation; ICARS, International Cooperative Ataxia Rating Scale (0–100 points; higher score indicates more severe ataxia); 9‐HPT, 9‐hole peg board test; RDA, Radboud Dysarthria Assessment; P‐RDA, Pediatric Radboud Dysarthria Assessment; MPV, maximum phonation volume; db, decibel; MPT, maximum phonation time; FFR, fundamental frequency range; LH, low‐to‐high pitch; HL, high‐to‐low pitch; ST, semitunes; MRR, maximum repetition rate; syl/s, syllables/second; ICS, intelligibility in context score; HRQOL, health‐related quality of life; VAS, Visual Analogue Scale (0–100).
Laboratory Measurements (Except Metabolomics)
Table 1 includes the mean serum immunoglobulin concentrations in patients with classic A‐T. In these patients serum IgG increased during the total study period. In patients with variant A‐T, mean serum IgG, IgA, and IgM concentrations were normal at baseline and during the study period, and no differences were observed (data not shown). No clinically relevant improvements were observed in any other routine laboratory parameter during the use of NR.
Untargeted Metabolomics
Samples taken from 23 patients were available for metabolomics analyses. NR metabolites showed increased signal intensities at the end of the treatment period compared to baseline in all 23 patients (Fig. 1A). Similarly, the concentrations of purine nucleosides, especially adenosine, guanosine, and inosine, clearly increased during treatment with NR (Fig. 1B).
FIG. 1.
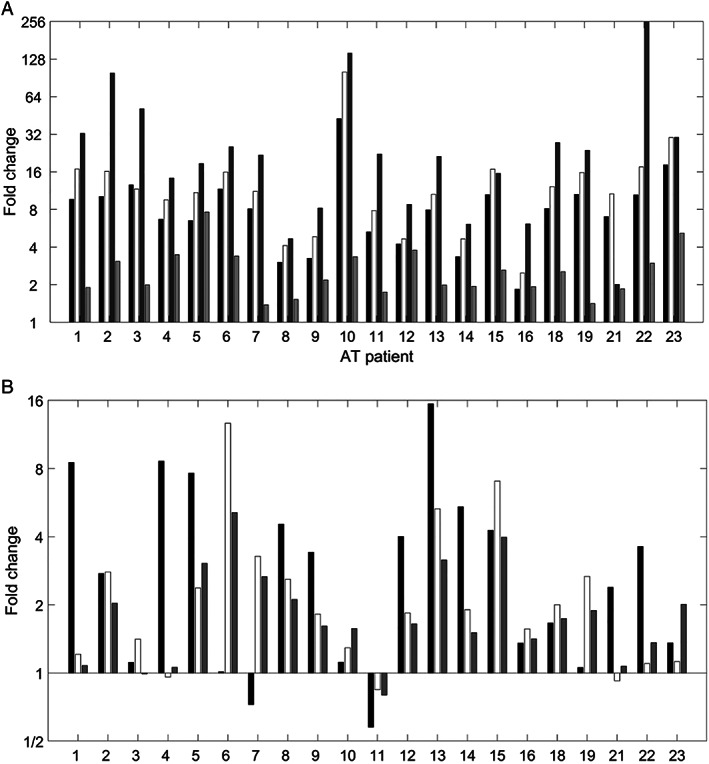
Fold changes of plasma levels of NR (nicotinamide riboside) metabolites and purine nucleosides after 4‐month treatment with NR. (A) Fold changes of four metabolites are given for individual patients: N1‐methyl‐2‐pyridone‐5‐carboxamide (first bar, black), N1‐methyl‐4‐pyridone‐5‐carboxamide (second bar, white), N1‐methylnicotinamide (third bar, dark gray), and nicotinamide (fourth bar, light gray). (B) Fold changes of adenosine (first bar, black), guanosine (second bar, white), and inosine (third bar, dark gray) are given for every single patient.
Discussion
In this study, we showed improvement on two ataxia rating scales during treatment with NR and loss of this effect on withdrawal, suggesting a transient, symptomatic treatment effect of NR in A‐T. The results of SARA and ICARS indicated that, at baseline, ataxia was present in all patients (minimal total scores: 2 and 10, respectively). Although the precise rate of progression of ataxia in A‐T has never been studied longitudinally in a large cohort of patients, one would anticipate an increase—instead of a decrease—in SARA and ICARS scores during the study period if NR had not had a positive effect. This assumption is substantiated by observations in similar neurodegenerative disorders like Friedreich's ataxia.18, 19 During the washout period, we observed substantial increases in the total ataxia scores and some subscales of ICARS and SARA, also pointing toward a beneficial effect of NR during the treatment period.
Remarkably, NR resulted in more improvement in ataxia on ICARS compared to SARA. Possible explanations for this finding are that ICARS is more detailed and contains more domains that interrogate different brain areas, in particular oculomotor disturbances, although no specific changes were detected in that subscale.
SARA and ICARS can be used in young children but have not been validated below the age of 12 years. 20 Furthermore, children often had lower ataxia scores compared to adults, and patients with variant A‐T had lower ataxia scores compared to patients with the classic phenotype. Nevertheless, when we adjusted the mixed model analyses for age or A‐T phenotype, as well as for sex, we noticed that these three characteristics did not contribute to the observed effects of NR.
Dysarthria was present in all patients at baseline, and large changes did not occur in its severity or in any of the maximum performance tasks during the study, although we did observe improvements in dysarthria in the subscale of ICARS. This apparent contradiction may be explained by the fact that the maximum performance tasks examine the upper limits of speech performance, whereas ICARS and SARA measure normal speech production. No changes in health‐related quality of life were observed using the EuroQol‐5D questionnaire, and also the Visual Analogue Scale scores remained unchanged. These findings contrasted with the improvements in the ataxia rating scales and with the functional improvements that patients reported during the study visits. Possibly, the effects are too small to measure, or the tests may simply lack specificity for the study population. Patients with classic A‐T have decreased serum concentrations of immunoglobulins, but patients with variant A‐T have normal immune functions.1, 2, 3, 4 To study the effects of NR on the immune system in classic A‐T patients, we excluded patients with immunoglobulin replacement therapy when analyzing IgG, because this therapy determines the serum IgG concentration and may thus mask a potential therapeutic effect of NR. For the IgA analyses, we excluded patients with IgA deficiency (serum IgA < 0.04 g/L), hypothesizing that the molecular mechanism that leads to IgA deficiency would not easily be restored by NR. Despite the low numbers of remaining patients in the different groups (see Table 1), we found increases in serum immunoglobulins in these patients. Mean serum IgG concentrations increased by 7% during the total study period, whereas IgA and IgM seemed to increase during treatment only. The longer‐lasting effect of NR on the concentration of IgG compared to IgA and IgM may be explained by the much‐longer serum half‐life of IgG (23 vs. 6 and 5 days, respectively). 21 Unfortunately, the effects of NR on immunological features in A‐T animal models have not been reported. 14
No relevant changes in any of the other laboratory parameters, including known biomarkers for A‐T like AFP and lymphocyte counts, were encountered during the treatment period in this trial. Simultaneously, no indications were found for adverse effects of NR.
Untargeted metabolomics analysis was used to study the biochemical effects of NR treatment. NR metabolites substantially increased during treatment (see Fig. 1), providing evidence for medication compliance, uptake, and metabolism of NR. Interestingly, untargeted metabolomics analysis also revealed an effect of NR treatment on purine metabolism, which we could confirm using targeted analyses. In particular, within‐patient increases in the purine nucleosides, adenosine, guanosine, and inosine, were observed on treatment with NR, starting from normal baseline levels to reach mildly elevated levels compared to reference ranges. We hypothesize that this effect is caused by competition at the level of purine nucleoside phosphorylase, the enzyme that is responsible for the breakdown of the purine nucleosides as well as the conversion of NR to nicotinamide. 22 To the best of our knowledge, this effect of NR on purine metabolism has never been reported before. Further investigation is needed to assess the clinical relevance of this biochemical response.
The main limitation of our study is that it is an open‐label study rather than a randomized, placebo‐controlled trial. In the absence of any data on biological activity and clinical and laboratory effects of NR in patients with A‐T and given the limited number of patients eligible for study, we chose this study design. The relatively small sample size hampered the possibility for adequate dose finding, whereas the lack of a control group prevented us from ruling out placebo effects in the clinical scales and self‐reported outcomes. This may have caused overestimation both in perceived improvements during treatment and in reported progression of ataxia during the washout period. The stability of the positive effects on the ataxia rating scales during treatment and the lack of effects in other scales, however, may indicate that placebo effects cannot explain the full extent of our clinical findings. Importantly, the laboratory results indicate the presence of biological effects of NR in A‐T. Notwithstanding its limitations, we are convinced that an explorative study with a rather simple, relatively affordable, and noninvasive design was necessary before a large multicenter and international, placebo‐controlled trial can be initiated. Therefore, the present study opens the way for further research to corroborate our findings and to investigate if treatment with NR will influence the disease course of A‐T beneficially in the long run.
Full financial disclosures for the previous 12 months
B.P.C.W. reports grants from Radboud University Medical Centre, Gossweiler Foundation, Hersenstichting, ZonMW, uniQure, other from uniQure, outside the submitted work.
Supporting information
APPENDIX S1. Supporting Information
Acknowledgments
We are grateful to the families who participated in this study. Furthermore, we thank the Twan Foundation (Veenendaal, the Netherlands) and A‐T Children's Project (Coconut Creek, Florida, USA) for their financial and moral support. We also thank our colleagues from the multidisciplinary A‐T team of the Radboud University Medical Center for the pleasant cooperation and their contribution to make this study possible. We thank A. Regev for sharing his early experiences with NR with us. We thank P. Kulkarni for bioinformatic support and S. de Boer and E. van der Heeft for technical assistance. This research made use of metabolomics infrastructure that is part of the NWO‐funded Netherlands X‐omics initiative, project 184.034.019. Several authors (B.P.C.W. and M.A.A.P.W.) on this publication are members of the European Reference Network for Rare Neurological Diseases—Project ID No. 739510.
Appendix A.
Specific roles in the project and manuscript preparation. These should include but not be restricted to:
(1) Research project: A. Conception, B. Organization, C. Execution, D. interpretation data; (2) Statistical analysis: A. Design, B. Execution, C. Review and critique; (3) Manuscript preparation: A. Writing of the first draft, B. Review and critique.
| Name | Location | Contribution |
|---|---|---|
| Stefanie J.G. Veenhuis, MD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: A, B, C, D 2: A, B 3: A |
| Nienke J. van Os, MD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: A, B, C, D 2: A, B 3: A |
| Anjo J.W.M. Janssen, PhD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: C 2 3: B |
| Marjo H.J.C. van Gerven, MSc | Radboud University Medical Center, Nijmegen, The Netherlands |
1: C 2 3: B |
| Karlien L.M. Coene, PhD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: B, C, D 2 3: B |
| Udo Engelke, PhD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: D 2 3: B |
| Ron A. Wevers, PhD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: D 2 3: B |
| Gerjen H. Tinnevelt, PhD | Radboud University, Nijmegen, The Netherlands |
1: D 2 3: B |
| Rob ter Heine, PhD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: A 2 3: B |
| Bart P.C. van de Warrenburg, MD, PhD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: A, B, D 2 3: B |
| Corry M.R. Weemaes, MD, PhD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: A 2 3: B |
| Nel Roeleveld, PhD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: A 2: A, B, C 3: B |
| Michèl A.A.P. Willemsen, MD, PhD | Radboud University Medical Center, Nijmegen, The Netherlands |
1: A, B, C, D 2 3: B |
Relevant conflicts of interest/financial disclosures: All authors have nothing to disclose.
Funding agencies: Ataxia Telangiectasia Children's Project (Coconut Creek, Florida, USA) and Twan Foundation (Veenendaal, the Netherlands).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Chun HH, Gatti RA. Ataxia–telangiectasia, an evolving phenotype. DNA Repair (Amst) 2004;3(8–9):1187–1196. [DOI] [PubMed] [Google Scholar]
- 2. Boder E, Sedgwick RP. Ataxia‐telangiectasia; a familial syndrome of progressive cerebellar ataxia, oculocutaneous telangiectasia and frequent pulmonary infection. Pediatrics 1958;21(4):526–554. [PubMed] [Google Scholar]
- 3. Schon K, van Os NJ, Oscroft N, et al. Genotype, extrapyramidal features, and severity of variant ataxia‐telangiectasia. Ann Neurol 2019;85(2):170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Os NJH, Haaxma CA, van der Flier M, et al. Ataxia‐telangiectasia: recommendations for multidisciplinary treatment. Dev Med Child Neurol 2017;59(7):680–689. 10.1111/dmcn.13424 [DOI] [PubMed] [Google Scholar]
- 5. Lavin MF, Yeo AJ, Kijas AW, et al. Therapeutic targets and investigated treatments for ataxia‐telangiectasia. Expert Opin Orphan Drugs 2016;4(12):1263–1276. 10.1080/21678707.2016.1254618 [DOI] [Google Scholar]
- 6. Savitsky K, Bar‐Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI‐3 kinase. Science 1995;268(5218):1749–1753. [DOI] [PubMed] [Google Scholar]
- 7. Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 2013;14(4):197–210. [PubMed] [Google Scholar]
- 8. Guleria A, Chandna S. ATM kinase: much more than a DNA damage responsive protein. DNA Repair (Amst) 2016;39:1–20. [DOI] [PubMed] [Google Scholar]
- 9. Cheema AK, Timofeeva O, Varghese R, et al. Integrated analysis of ATM mediated gene and protein expression impacting cellular metabolism. J Proteome Res 2011;10(5):2651–2657. [DOI] [PubMed] [Google Scholar]
- 10. Yoshino J, Baur JA, Imai SI. NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab 2018;27(3):513–528. 10.1016/j.cmet.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chi Y, Sauve AA. Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Curr Opin Clin Nutr Metab Care 2013;16(6):657–661. 10.1097/MCO.0b013e32836510c0 [DOI] [PubMed] [Google Scholar]
- 12. Weidele K, Beneke S, Burkle A. The NAD(+) precursor nicotinic acid improves genomic integrity in human peripheral blood mononuclear cells after X‐irradiation. DNA Repair (Amst) 2017;52:12–23. 10.1016/j.dnarep.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 13. Stern N, Hochman A, Zemach N, et al. Accumulation of DNA damage and reduced levels of nicotine adenine dinucleotide in the brains of Atm‐deficient mice. J Biol Chem 2002;277(1):602–608. 10.1074/jbc.M106798200 [DOI] [PubMed] [Google Scholar]
- 14. Fang EF, Kassahun H, Croteau DL, et al. NAD(+) replenishment improves lifespan and Healthspan in ataxia telangiectasia models via Mitophagy and DNA repair. Cell Metab 2016;24(4):566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr 2008;28:115–130. [DOI] [PubMed] [Google Scholar]
- 16. Schmitz‐Hubsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 2006;66(11):1717–1720. 10.1212/01.wnl.0000219042.60538.92 [DOI] [PubMed] [Google Scholar]
- 17. Trouillas P, Takayanagi T, Hallett M, et al. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. J Neurol Sci 1997;145(2):205–211. [DOI] [PubMed] [Google Scholar]
- 18. Reetz K, Dogan I, Costa AS, et al. Biological and clinical characteristics of the European Friedreich's ataxia consortium for translational studies (EFACTS) cohort: a cross‐sectional analysis of baseline data. Lancet Neurol 2015;14(2):174–182. [DOI] [PubMed] [Google Scholar]
- 19. Metz G, Coppard N, Cooper JM, et al. Rating disease progression of Friedreich's ataxia by the international cooperative ataxia rating scale: analysis of a 603‐patient database. Brain 2013;136(1):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brandsma R, Lawerman TF, Kuiper MJ, et al. Reliability and discriminant validity of ataxia rating scales in early onset ataxia. Develop Med Child Neurol 2017;59(4):427–432. [DOI] [PubMed] [Google Scholar]
- 21. Waldmann TA, Strober W, BLAESE RM. Metabolism of immunoglobulins. Prog Immunol 1971;891–903. 10.1016/B978-0-12-057550-3.50072-7 [DOI] [Google Scholar]
- 22. Hayat F, Migaud ME. Nicotinamide riboside–amino acid conjugates that are stable to purine nucleoside phosphorylase. Org Biomol Chem 2020;18(15):2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


