Abstract
The Junior Adverse Drug Event Manager (J‐ADEM) team is a multifaceted intervention focusing on real‐life education for medical students that has been shown to assist healthcare professionals in managing and reporting suspected adverse drug reactions (ADRs) to the Netherlands Pharmacovigilance Centre Lareb. The aim of this study was to quantify and describe the ADRs reported by the J‐ADEM team and to determine the clinical potential of this approach. The J‐ADEM team consisted of medical students tasked with managing and reporting ADRs in hospitalized patients. All ADRs screened and reported by J‐ADEM team were recorded anonymously, and categorized and analysed descriptively. From August 2018 through January 2020, 209 patients on two wards in an academic hospital were screened for ADR events. The J‐ADEM team reported 101 ADRs. Although most ADRs (67%) were first identified by healthcare professionals and then reported by the J‐ADEM team, the team also reported an additional 33 not previously identified serious ADRs. In 10% of all reported ADRs, the J‐ADEM team helped optimize ADR treatment. The ADR reports were largely well‐documented (78%), and ADRs were classified as type A (66%), had a moderate or severe severity (85%) and were predominantly avoidable reactions (69%). This study shows that medical students are able to screen patients for ADRs, can identify previously undetected ADRs and can help optimize ADR management. They significantly increased (by 300%) the number of ADR reports submitted, showing that the J‐ADEM team can make a valuable clinical contribution to hospital care.
Keywords: medical education, pharmacotherapy, pharmacovigilance, reporting ADRs
What is already known about this subject
Managing adverse drug reactions (ADRs) is an important task of healthcare professionals, but is greatly overlooked.
Numerous strategies to improve the reporting of ADRs have been tried over the years with varying success.
A Junior Adverse Drug Event Manager (J‐ADEM) program has been found feasible to assist healthcare professionals in their ADR managing tasks, but clinical outcomes remain unknown.
What this study adds
Medical students, as J‐ADEMs, can significantly increase the number of ADR reports made in a hospital setting and help optimize ADR treatment plans.
Healthcare professionals should be more vigilant about reporting serious ADRs in hospitalized elderly patients on high‐risk medication.
By integrating a J‐ADEM program into the clinical pharmacology training, it can be realized without a major investment in time or money, students will have a clinically relevant role while learning, patients will receive more attention for potential side effects and prescribers' awareness for pharmacovigilance is increased.
1. INTRODUCTION
Managing adverse drug reactions (ADRs) is an important task of healthcare professionals, especially since 5‐20% of patients visiting the emergency department attend because of an ADR 1 , 2 , 3 and 11.9% of hospitalized patients experience an ADR during their stay. 3 Because ADRs adversely affect patients' quality of life 4 and delayed detection of ADRs can be harmful and postpone discharge, we should be more vigilant about ADRs and their treatment. Despite this importance more than half of all ADRs are not diagnosed at admission 1 , 2 , 3 and only 6% of all ADRs are reported to the competent authority. 5 The spontaneous reporting of suspected ADRs by healthcare professionals and patients is an important source of information about possible ADRs, information which could improve drug safety. 5 , 6
The underdetection and underreporting of ADRs have been related to a poor awareness of ADRs and a limited knowledge of pharmacology on the part of healthcare professionals. 5 , 7 , 8 Numerous strategies to improve the reporting of ADRs have been tried over the years, with varying success. 9 The most successful interventions are educational activities, modification of reporting forms or reporting procedures, incentives (economic or other), assistance with reporting or improved feedback to reporters. 9 , 10 , 11 While most single or retrospective interventions have been less successful, some multifaceted interventions, especially those focusing on real‐life education, have increased awareness and pharmacovigilance knowledge and improved ADR reporting rates significantly. 10 , 12
The Junior Adverse Drug Event Manager (J‐ADEM) program provides under‐ and postgraduates with real‐life education. Medical students assist healthcare professionals in detecting, managing and reporting ADRs to the Netherlands Pharmacovigilance Centre Lareb. After analysis of the report, the pharmacovigilance centre provides the J‐ADEM team with feedback, which is passed on to physicians and patients. This process educates both students and healthcare professionals in pharmacovigilance and ADR reporting. While our low‐cost and promising intervention has proven feasible for patients, physicians and students, little is known about the clinical value of this approach. 13 Therefore, the aim of this study was to quantify and describe the ADRs reported by the J‐ADEM team to determine the clinical potential of this approach.
2. METHODS
This prospective observational study was set up to evaluate the clinical results of a J‐ADEM team in a tertiary academic hospital. The J‐ADEM team and setting have been described in an earlier study. 13 This team systematically screen patients for suspected serious or unrecognized ADRs, and patients could also be referred to the team when suspecting an ADR at admission or during hospitalization.
3. J‐ADEM TEAM AND PROCEDURE
The J‐ADEM team consisted of two medical students (first‐year bachelor to third‐year master) who participated as part of the Learner Centered Student Run Clinic VUmc (LC‐SRC). 14 , 15 The LC‐SRC is an extracurricular program dedicated to pharmacotherapy and medication safety initiatives in which students participate on a voluntary basis. 16 , 17 , 18
The J‐ADEM team procedure consisted of four consecutive steps (Figure 1), all performed by the students and taking approximately 3 hours to complete the full process for one patient. This was done in two ways. First, (1) the physicians, nurses, pharmacists and/or the pharmacotherapy team (a multidisciplinary team focusing on medication safety by performing medication reviews, optimizing prescribing and educating healthcare professionals 19 ) could report suspected ADRs to the team by providing the patient's initials, personal identification number and short description of the suspected ADR; (2) the J‐ADEM team also screened the records of patients who were most recently admitted to the two wards for suspected ADRs. Second, the patient's electronic healthcare records were analysed and a thorough medication and side‐effect interview with the patient was carried out. Third, all suspected ADRs were reported to the Netherlands Pharmacovigilance Centre Lareb. The last step provided patient and physician feedback received from the Netherlands Pharmacovigilance Centre Lareb and this information was uploaded into the patient's Electronic Patient Record (EPR). A full description can be found in an earlier feasibility study.
FIGURE 1.
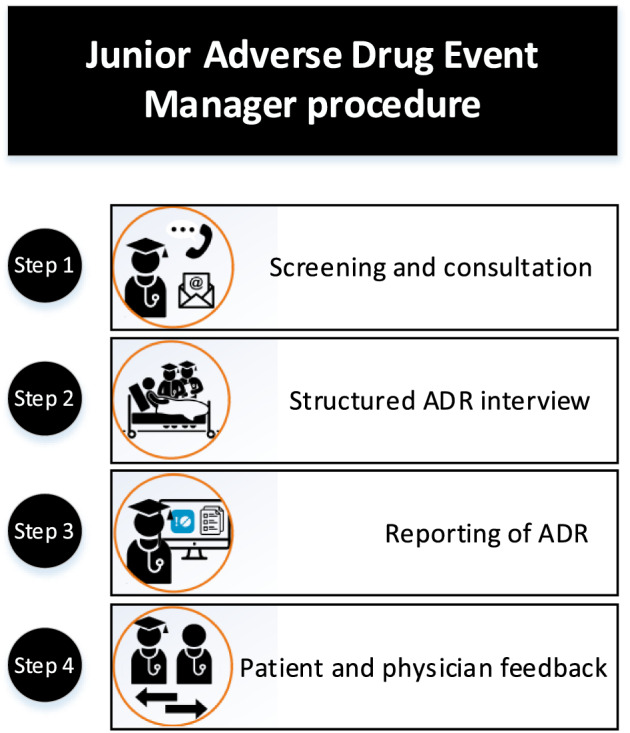
The Junior Adverse Drug Event Manager procedure. The first step consisted of identifying all patients with potential ADRs by screening or being consulted by a healthcare professional. The second step consisted of reviewing the patient's electronic patient record (EPR) and performing a thorough medication and side effect interview with the patient. The third step consisted of reporting the ADR to the Netherlands Pharmacovigilance Center Lareb and handling all follow‐up questions. The final step consisted of providing the attending physician with feedback received from Lareb and uploading this information into the patient's EPR
4. MEASUREMENTS
If a patient was screened for an ADR, basic patient characteristics (age and sex), drug details (including Anatomical Therapeutic Chemical (ATC) code), the nature of the reaction (including MedDRA class) and the outcome were recorded. If a patient was suspected to have an ADR, a clinical pharmacologist (MR) classified the ADR according to Rawlins and Thomson, 20 and established causality according to the WHO‐UMC 21 scale and Naranjo algorithm, 22 ADR severity according to the modified Hartwig and Siegel scale, 23 and preventability according to the modified Schumock and Thornton scale. 24 ADRs were defined according to Hallas. 25 After the ADR report was sent, the quality of the clinical documentation was scored using the ClinDoc algorithm. 26
4.1. Ethical aspects
This study did not fall under the scope of the Dutch Medical Research Involving Human Subjects Act (reference number 2018.097). Physician and patient participation was voluntary and based on informed consent. The ethics review board of the Netherlands Association for Medical Education reviewed the protocol regarding the students' participation and approved this study (ID: 826).
4.2. Data analysis
All data were imported in SPSS Statistics 26 (IBM Corp., Armonk, New York, USA). Descriptive statistics were used to report frequencies, percentages and medians (with range) of ADRs screened and reported by the J‐ADEM team. The “number needed to screen to report 1 ADR” was calculated to show how many drugs or disorders needed to be screened by the J‐ADEMs to report one ADR to the Netherlands Pharmacovigilance Centre Lareb. This number is calculated by dividing the number of screened drugs or disorders by the number of reported drugs or disorders. Differences in the ADR reporting frequency before and after the J‐ADEM program were compared using the Mann‐Whitney U test (α = 0.5 and P < .05).
5. RESULTS
From August 2018 to January 2020 (18 months), 209 patients were screened. In total, the J‐ADEM team reported 101 ADRs in patients on two wards (internal medicine and otorhinolaryngology) in one academic hospital; 25 ADRs were reported on the remaining 21 wards. This was a significant 300% increase (P ≤ .05) in the number of ADRs reported relative to the 31 reports made in the entire hospital in the previous 18 months (February 2017‐August 2018) (Table 1A). Most ADR reports concerned patients on the internal medicine ward (76%), followed by the otorhinolaryngology ward (16%) and outpatient clinics (9%) (Table 1B). The mean age of patients with a suspected ADR was 75 years (range 23‐98 years) and 59% were female (Table 1D).
TABLE 1.
Adverse drug reaction reports and baseline characteristics
| A. Total reports in hospital | |||
|---|---|---|---|
| Time period | Total number of reports | Number of reports without J‐ADEMs | Number of reports by J‐ADEMs |
| Before J‐ADEMs: Feb 2017‐July 2018 | 31 | 31 | Na |
| During J‐ADEMs: Aug 2018‐Jan 2020 | 126 | 25 | 101 |
| B. Total reports per ward/outpatient clinic during intervention period | |||
|---|---|---|---|
| Ward/outpatient clinic | Total number of reports | Number of reports without J‐ADEMs | Number of reports by J‐ADEMs |
| Internal medicine | 80 | 3 | 77 |
| Otorhinolaryngology | 16 | 0 | 16 |
| Outpatient clinics | ≥8 | ? | 8 |
| C. Identification of patients with potential ADRs | ||||
|---|---|---|---|---|
| Ward/outpatient clinic | First identified by: | Number of patients screened | Number of ADR reports | ADR reporting chance |
| All wards/outpatient clinic | J‐ADEMS | 121 | 33 | 27% |
| Pharmacotherapy team | 45 | 30 | 67% | |
| Physicians | 43 | 38 | 88% | |
| Internal medicine ward only | J‐ADEMS | 94 | 27 | 29% |
| Pharmacotherapy team | 35 | 21 | 60% | |
| Physicians | 33 | 29 | 88% | |
| D. Baseline characteristics | ||
|---|---|---|
| Patients screened for an ADR (n = 209) | Patients with a reported ADR (n = 101) | |
| Age (yr), median (range) | 74 (30‐88) | 75 (23‐98) |
| Male, n (%) | 90 (43) | 41 (41) |
| Days in hospital when screened, median (range) | 17 (3‐52) | 17 (4‐58) |
| Total days in hospital, median (range) | 31 (9‐56) | 36 (12‐56) |
| Medications, n (per patient) | 2386 (11) | 1426 (14) |
| Disorders on problem list, n (per patient) | 753 (3.6) | 473 (4.7) |
| In‐hospital deaths, n (%) | 4 (1.9) | 3 (3.0) |
1A: Total number of adverse drug reaction (ADR) reports before and after the start of the Junior Adverse Event Manager (J‐ADEM) program. 1B: Number of ADR reports categorized by ward/outpatient clinic. 1C: Categorization of the ADRs in the way they were identified. The “ADR reporting chance” was the likelihood an ADR was reported when a patient was screened or referred to by either the pharmacotherapy team or a physician and was calculated by the total number of ADR reports divided by the number of patients screened. 1D: Baseline characteristics of patients screened for an ADR and who had an ADR reported.
5.1. ADR signal characteristics
Of the 209 patients screened by the J‐ADEM team, 88 suspected of having an ADR had been identified by healthcare professionals and 121 were identified by the J‐ADEM team by randomly screening patients on the various wards. The J‐ADEM team identified and reported 33 not previously recognized ADRs in the 121 patients. In these patients, the incidence of the ADR being the cause of hospital admission or of the ADR occurring during the first days of hospital stay was 27% (internal medicine ward = 29%, ENT‐specialist ward = 22%) (Table 1C).
Of the 88 patients with ADRs identified by healthcare professionals, the ADRs of 45 patients were identified by the local pharmacotherapy team 19 and the ADRs of the remaining 43 patients were identified by attending physicians. In total, 68 ADRs (77%) were reported to the Netherlands Pharmacovigilance Center (Table 1C). In 20 cases, the J‐ADEM team concluded the events were not related to the drug.
5.2. ADR screening characteristics
During the program, a total of 2386 drugs (mean 11 per patient) and 753 medication‐related problems (mean 3.6 per patient) were screened. The J‐ADEM team most frequently screened patients with “blood and lymphatic system disorders” (n = 211), “gastrointestinal disorders” (n = 133) and “psychiatric disorders” (n = 72). “General disorders and administration site conditions” and “skin and subcutaneous tissue disorders” were most likely to be a symptom of an ADR (number needed to screen 2.5 and 3, respectively) (Table 2A). Gastrointestinal (n = 591), cardiovascular (n = 496) and over‐the‐counter drugs (n = 283) were most frequently assessed for causing ADRs, whereas it was found that “immunomodulatory drugs and chemotherapeutics”, “cardiovascular drugs” and “blood and lymphatic drugs” were most frequently found to cause an ADR (number needed to screen 4.0, 12.1 and 12.6, respectively) (Table 2B).
TABLE 2.
Characteristics of disorders and drugs screened by the Junior Adverse Event Manager team
| A. Disorders by organ class (MedDRA classification) | Screened | Reported | Number needed to screen to report 1 ADR |
|---|---|---|---|
| General disorders and administration site conditions | 30 | 12 | 2 .5 |
| Nervous system disorders | 55 | 14 | 3.9 |
| Gastrointestinal disorders | 133 | 13 | 10.2 |
| Skin and subcutaneous tissue disorders | 12 | 4 | 3.0 |
| Respiratory, thoracic and mediastinal disorders | 62 | 6 | 10.3 |
| Psychiatric disorders | 72 | 18 | 4.0 |
| Musculoskeletal and connective tissue disorders | 32 | 4 | 8.0 |
| Blood and lymphatic system disorders | 211 | 16 | 13.2 |
| Eye disorders | 3 | 0 | ‐ |
| Ear, nose and throat disorders | 62 | 0 | ‐ |
| Renal and urinary disorders | 58 | 11 | 5.3 |
| Vascular disorders | 23 | 3 | 7.7 |
| Total | 753 | 101 | 7.5 |
| B. Type of drug (ATC classification) | Screened | Reported | Number needed to screen to report 1 ADR |
|---|---|---|---|
| A. Gastrointestinal | 591 | 10 | 59.1 |
| B. Blood and lymphatic | 101 | 8 | 12.6 |
| C. Cardiovascular | 496 | 41 | 12.1 |
| D. Skin and subcutaneous tissue | 62 | 0 | ‐ |
| G. Renal and urinary | 43 | 2 | 21.5 |
| H. Hormones | 121 | 5 | 24.2 |
| J. Antibiotics and antiparasitics | 215 | 14 | 15.4 |
| L. Immunomodulatory and chemotherapeutics | 16 | 4 | 4.0 |
| M. Musculoskeletal and connective tissue | 72 | 2 | 36.0 |
| N. Nervous system and senses | 215 | 12 | 17.9 |
| R. Pulmonary drugs | 171 | 2 | 85.5 |
| V. Over‐the‐counter drugs | 283 | 1 | 283 |
| Total | 2386 | 101 | 23.6 |
2A: Overview of the number of disorders screened (according to MedDRA classification), the number of reports per disorder and the reporting chance. 2B: Overview of the number of drugs screened (according to ATC classification), the number of reports per drug class and the reporting chance.
5.3. ADR report characteristics
In total, 67 of 101 reported ADRs were classified as type A ADRs according to the classification of Rawlins and Thompson, and were classified as moderate to severe in severity (n = 86). Causality assessment, using the Naranjo algorithm and World Health Organisation ‐ Uppsala Monitoring Centre causality algorithm, classified most admissions as having a “probable” association with medication. No admissions were classified “unlikely” or “doubtful” (Table 3). The avoidability and preventability of hospital admissions related to an ADR were assessed using the method of Hallas et al and the Schumock and Thornton scale. Both methods indicated that only 31 or 26 (31%/26%) ADRs were unavoidable, whereas 70 or 75 (69%/74%) were classified as “possible or definitely avoidable or preventable” (Table 3).
TABLE 3.
Characteristics of the 101 adverse drug reactions (ADRs) and ADR reports in this study
| Features | Parameters | Percentage (%) |
|---|---|---|
| Causality (Naranjo algorithm) | Doubtful | … |
| Possible | 45 | |
| Probable | 53 | |
| Definite | 2 | |
| Causality (WHO‐UMC causality) | Unclassifiable/unclassified | … |
| Unlikely | … | |
| Possible | 42 | |
| Probable | 55 | |
| Certain | 3 | |
| Severity (Hartwig and Siegel scale) | Mild | 15 |
| Moderate | 67 | |
| Severe | 18 | |
| Avoidability (Hallas et al) | Unavoidable | 31 |
| Possible avoidable | 46 | |
| Definitely avoidable | 23 | |
| Preventability (Schumock and Thornton scale) | Nonpreventable | 26 |
| Probably preventable | 49 | |
| Definitely preventable | 25 | |
| Report of a serious adverse drug reaction | 84 | |
| Drug in the report had an off‐label use | 4 | |
| Drug in the report was under additional monitoring | 9 | |
| ClinDoc scores (quality of the ADR report) | ||
| Scores >75% (well documented) | 72 | |
| Scores 46‐74% (moderately documented) | 27 | |
| Scores <45% (poorly documented) | 1 | |
5.4. ADR reporting quality
It was only possible to analyse the ADR reports submitted by the J‐ADEM team and not those submitted before the study began. With ClinDoc scores higher than 75%, 79 of the 101 (78%) ADR reports were considered to be well documented (Table 3).
5.5. ADR special reports
Not only did the J‐ADEM team detect and report ADRs to the Netherlands Pharmacovigilance Center, they also helped optimize ADR treatment plans in 10% (n = 10/101) of the patient cases.
Case 1
A 76‐year‐old patient was hospitalized because of recurring massive nose bleeds while on acetylsalicylic acid and solifenacin. Because of the severity of the nose bleeds, the acetylsalicylic acid was discontinued by the attending physician, even though there was a valid indication for the drug. After a thorough medication and side‐effect interview with the patient, the J‐ADEM team concluded the solifenacin was responsible for the nose bleeds. The patient had had extremely dry mucous membranes in his nose since the start of solifenacin treatment and had applied Vaseline with a pointy ear stick in his nose on a daily basis, which caused the repeated nose bleeds. After solifenacin was discontinued and acetylsalicylic acid restarted, the patient no longer had any nose bleeds and was discharged.
Case 2
A 64‐year‐old patient was hospitalized for recurring hematomas. She was taking acenocoumarol (vitamin K antagonist) and other nonrelevant prescription drugs. After investigating her past and current prescription drugs, the J‐ADEM team found she had also been using miconazole cream (for athlete's foot) recurrently at 1‐month intervals. This over‐the‐counter drug usage was not known by her physicians and had increased the International normalized ratio to 6.2 by inhibiting CYP2C9, thereby causing the hematomas. The patient was advised to stop miconazole and a different antifungal ointment was prescribed, after which the recurring hematomas disappeared.
6. DISCUSSION
This study shows that medical students can have an important clinical role in detecting, managing and reporting ADRs in a hospital setting. Students participating in the J‐ADEM team detected 33 additional serious ADRs not detected at admission or which occurred during hospitalization. By performing an in‐depth side‐effect medication interview, in 10% of the cases the students could provide physicians with an ADR treatment plan and significantly increased (by 300%) the number of ADR reports submitted to the appropriate authority.
To our knowledge, this is the first initiative involving students that supports healthcare professionals in their detection, management and reporting of ADRs. In our earlier J‐ADEM study 13 we found that students, patients and healthcare professionals find the team feasible, that students gain valuable pharmacovigilance competences and that this program provides students with the most realistic form of pharmacovigilance training.
The major advantage of the J‐ADEM team approach compared with other physician‐ and pharmacy‐led interventions is that it saves time, is relatively low cost and enables the detection and management of ADRs. While physicians take on average 30‐40 minutes to report an ADR, 27 it takes them less than 5 minutes per patient to supervise students. In our study, this saved over 60 working hours that could be spent more efficiently. Compared with other ADR reporting initiatives, such as administrative or computerized coding systems, the J‐ADEM team approach is probably more sensitive in detecting ADRs. 28 By gathering prospective detailed ADR information from the electronic patient record, assisting healthcare professionals in accurate ADR diagnosis and occasionally advising on ADR management/treatment, the J‐ADEM team is a real clinical support team rather than merely an administrative solution.
The incidence of ADRs was high (27%) in our study participants. This could be explained by their relatively old age (mean age 75 years), the setting (tertiary academic hospital) and the fact that patients were screened during hospitalization (median length of stay was 17 days). This enabled us to detect ADRs which were the cause of the hospital admission and which occurred during hospitalization. This high incidence of patients with ADRs in hospital wards is in line with previous studies of elderly patients. 3 , 29 The finding that more than a quarter of the patients had an ADR at or during hospitalization should prompt healthcare professionals and specific pharmacotherapy teams 19 to be more alert to ADRs, and the J‐ADEM team could have a role in this. This is especially true for immunomodulatory drugs, chemotherapeutics, anticoagulants/antiplatelets and cardiovascular drugs being a frequent cause of the ADRs. Since general disorders and administration site conditions, skin and subcutaneous tissue disorders, and psychiatric and nervous system disorders were symptoms most often classified as an ADR, they should function as red flags.
Our study had some strengths and limitations. The 18‐month prospective design allowed accurate recording of both the drug history and symptoms, and the assessment of causality. A second strength is the low implementation and running costs of the J‐ADEM program since most work was done by medical students who were only supervised by a clinical pharmacologist at two points during the entire process. This efficient way of managing ADRs in a clinical setting reduces costs and has learning benefits for both medical students and healthcare professionals (clinical pharmacologists). Lastly, the J‐ADEM program increases in the motivation and awareness of physicians with regard to the detection and reporting of ADRs. Since learning and feedback are incorporated in the J‐ADEM procedure, physicians will also see the benefit of reporting ADRs.
The main limitation of our study is that ADRs are necessarily identified on the basis of clinical judgment. To overcome this, the attending physician and a clinical pharmacologist jointly made a clinical judgment about potential ADRs, and all suspected ADRs were assessed with regard to causality using two validated instruments (Naranjo and WHO), which showed a high degree of agreement (96%). Nevertheless, it is impossible to be absolutely certain of a causal link between a drug and an ADR. A second limitation is that not every ADR report submitted by the J‐ADEM team is equally clinically relevant for detecting new signs of ADRs or further strengthening signals for drugs under additional monitoring. For instance, a patient hospitalized with dehydration because of a dosage increase in loop‐diuretics could be classified as a serious ADR, although the relevance of such a report could be argued. Nonetheless, all physicians agreed that the reports were necessary; moreover, healthcare professionals in the Netherlands are obliged to report serious ADRs to the Netherlands Pharmacovigilance Centre Lareb. A final limitation could be the modest campaign strategy at the start of the program. Although some presentations were held and contact information was sent out, a larger ADR awareness campaign might lead to the identification of more signs/symptoms of ADRs.
Taking these strengths and limitations into account, we conclude that the J‐ADEM team program is not only feasible but also has significant clinical value in the reporting and management of ADRs in a hospital setting. Although absolute numbers are still relatively small and a longer follow‐up is needed, the concept of a (student‐run) J‐ADEM team has potential for hospitals because the underreporting of ADRs, especially in hospital settings, is a universal problem. The J‐ADEM team can not only help in reporting previously detected ADRs at minimal expense, but also in detecting and managing previously undetected ADRs. With an ADR incidence of 20‐30% in our patient population, active screening is a promising manner to detect, report and manage previously unrecognized ADRs.
As with many successful initiatives, the challenge is to keep the project running after the study has ended. Because the program is integrated in clinical pharmacology training and is low cost, the J‐ADEM team has been incorporated into daily practice in our hospital without additional cost. Although this program is intended to provide medical students with valuable educational and hands‐on experience, it could also include interns and be incorporated into the formal medical curricula, thereby giving these students a valuable role in real‐life clinical pharmacovigilance.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
M.O.R, J.T and M.v.A contributed to the design of the project, M.O.R. performed the measurements, M.O.R, J.T, M.C.R, and M.v.A. were involved in planning and supervised the work, M.O.R. and J.T. processed the experimental data and performed the analysis, M.O.R and M.C.R. drafted the manuscript and designed the figures. All authors discussed the results and commented on the manuscript.
ACKNOWLEDGMENTS
The authors want to thank the VU University Medical Centre (former name of the Amsterdam University Medical Centers, location VUmc) for awarding the Junior Adverse Drug Event Managers the VUmc Innovation prize in 2018, Mrs R. Harting, Mrs S. van der Hoorn, Mrs M. Koeman, Mrs S. Mahmoud, Mrs A. Mahraoui and Mr F. Mocking as student coordinators of the Junior‐Adverse Drug Event Managers, and all students participating in the learner‐centred student‐run program. The project was funded with a VU University Medical Centre Innovation prize.
Reumerman MO, Tichelaar J, Richir MC, van Agtmael MA. Medical students as junior adverse drug event managers facilitating reporting of ADRs. Br J Clin Pharmacol. 2021;87(12):4853–4860. 10.1111/bcp.14885
Principal investigator: The authors confirm that the Principal Investigator for this paper is Michiel van Agtmael and that he had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Roulet L, Asseray N, Dary M, Chiffoleau A, Potel G, Ballereau F. Implementing a clinical pharmacy survey of adverse drug events in a French emergency department. Int J Clin Pharmacol. 2012;34(6):902‐910. [DOI] [PubMed] [Google Scholar]
- 2. Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296(15):1858‐1866. [DOI] [PubMed] [Google Scholar]
- 3. Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 2015;38(5):437‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rolfes L, van Hunsel F, Taxis K, van Puijenbroek E. The impact of experiencing adverse drug reactions on the patient's quality of life: a retrospective cross‐sectional study in the Netherlands. Drug Saf. 2016;39(8):769‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hazell L, Shakir SA. Under‐reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385‐396. [DOI] [PubMed] [Google Scholar]
- 6. Miguel A, Azevedo LF, Lopes F, Freitas A, Pereira AC. Methodologies for the detection of adverse drug reactions: comparison of hospital databases, chart review and spontaneous reporting. Pharmacoepidemiol Drug Saf. 2013;22(1):98‐102. [DOI] [PubMed] [Google Scholar]
- 7. Lopez‐Gonzalez EH, MT. Figueiras A. Determinants of under‐reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32(1):19‐31. [DOI] [PubMed] [Google Scholar]
- 8. Dormann H, Criegee‐Rieck M, Neubert A, et al. Lack of awareness of community‐acquired adverse drug reactions upon hospital admission: dimensions and consequences of a dilemma. Drug Saf. 2003;26(5):353‐362. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez‐Gonzalez C, Lopez‐Gonzalez E, Herdeiro MT, Figueiras A. Strategies to improve adverse drug reaction reporting: A critical and systematic review. Drug Saf. 2013;36(5):317‐328. [DOI] [PubMed] [Google Scholar]
- 10. Pagotto C, Varallo F, Mastroianni P. Impact of educational interventions on adverse drug events reporting. Int J Technol Assess Health Care. 2013;29(4):410‐417. [DOI] [PubMed] [Google Scholar]
- 11. Ribeiro‐Vaz I, Silva AM, Costa Santos C, Cruz‐Correia R. How to promote adverse drug reaction reports using information systems ‐ a systematic review and meta‐analysis. BMC Med Inform Decis Mak. 2016;16(1). 10.1186/s12911-016-0265-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reumerman M, Tichelaar J, van Eekeren R, van Puijenbroek EP, Richir MC, van Agtmael MA. The potential of training specialist nurses in real‐life reporting of adverse drug reactions to reduce the level of underreporting by current healthcare professionals. Eur J Clin Pharmacol. 2021;Epub ahead of print. 10.1007/s00228-021-03138-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reumerman M, Tichelaar J, Richir MC, van Agtmael MA. Medical students as adverse drug event managers, learning about side effects while improving their reporting in clinical practice. Naunyn Schmiedebergs Arch Pharmacol. 2021;Epub ahead of print. 10.1007/s00210-021-02060-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schutte T, Tichelaar J, Dekker RS, et al. Motivation and competence of participants in a learner‐centered student‐run clinic: an exploratory pilot study. BMC Med Educ. 2017;17(1). 10.1186/s12909-017-0856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schutte T, Tichelaar J, Donker E, Richir MC, Westerman M, van Agtmael MA. Clarifying learning experiences in student‐run clinics: a qualitative study. BMC Med Educ. 2018;18(1):244. 10.1186/s12909-018-1352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reumerman MO, Richir MC, Domela Nieuwenhuis PM, et al. The clinical and educational outcomes of an inter‐professional student‐led medication review team, a pilot study. Eur J Clin Pharmacol. 2021;77(1):117‐123. 10.1007/s00228-020-02972-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schutte T, Tichelaar J, Reumerman MO, et al. Feasibility and educational value of a student‐run pharmacovigilance programme: a prospective cohort study. Drug Saf. 2017;40(5):409‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schutte TPK, Richir M, Donker E, et al. Opportunities for students to prescribe: an evaluation of 185 consultations in the student‐run cardiovascular risk management programme. Basic Clin Pharmacol Toxicol. 2018;Feb;122(2):299‐302. [DOI] [PubMed] [Google Scholar]
- 19. Mahomedradja RF, Sigaloff KCE, Bekema JK, et al. The pharmacotherapy team: A novel strategy to improve appropriate in‐hospital prescribing using a participatory intervention action method. Br J Clin Pharmacol. 2021;87(2):565‐576. 10.1111/bcp.14418 (1365‐2125 [Electronic]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rawlins MD, Thompson JW. Pathogenesis of adverse drug reactions. In: Davies DM, ed. Textbook of adverse drug reactions Oxford, Vol. 10. Oxford University Press; 1977. [Google Scholar]
- 21. The use of the WHO‐UMC system for standardized case causality assessment . World Health Organization (WHO) — Uppsala Monitoring Centre. http://www.who-umc.org/Graphics/24734.pdf. Accessed November 23, 2020.
- 22. Naranjo C, Busto U, Sellers E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239‐245. [DOI] [PubMed] [Google Scholar]
- 23. Hartwig SC, Siegel J, Schneider P. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229‐2232. [PubMed] [Google Scholar]
- 24. Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27(6). [PubMed] [Google Scholar]
- 25. Hallas J, Harvald B, Gram LF, et al. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med. 1990;228(2):83‐90. [DOI] [PubMed] [Google Scholar]
- 26. Oosterhuis I, Rolfes L, Ekhart C, Muller‐Hansma A, Härmark L. First experiences with a tool to measure the level of clinical information present in adverse drug reaction reports. Expert Opin Drug Saf. 2018;17(2):111‐115. 10.1080/14740338.2018.1400008 [DOI] [PubMed] [Google Scholar]
- 27. Sørup FKH, Jacobsen CB, Jimenez‐Solem E. Increasing the number of spontaneous ADE reports in a Danish region: a retrospective analysis. Pharmaceu Med. 2015;29(4):211‐217 [Google Scholar]
- 28. Parameswaran Nair N, Chalmers L, Peterson GM, Bereznicki BJ, Curtain CM, Bereznicki LR. Prospective identification versus administrative coding of adverse drug reaction‐related hospitalizations in the elderly: A comparative analysis. Pharmacoepidemiol Drug Saf. 2018;27(11):1281‐1285. 10.1002/pds.4667 [DOI] [PubMed] [Google Scholar]
- 29. Parameswaran Nair N, Chalmers L, Bereznicki BJ, et al. Adverse drug reaction‐related hospitalizations in elderly Australians: a prospective cross‐sectional study in two Tasmanian hospitals. Drug Saf. 2017;40(7):597‐606. 10.1007/s40264-017-0528-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


