Summary
Background
Alcohol is a main cause of preventable deaths and frequently leads to the development of alcohol‐related liver disease. Due to the lack of diagnostics, patients are commonly diagnosed after developing clinical manifestations. Recently, the biomarker PRO‐C3 was shown to accurately identify fibrosis due to non‐alcoholic fatty liver disease.
Aim
To assess the diagnostic accuracy of PRO‐C3, the ADAPT score and best‐performing non‐patented serological test to detect advanced alcohol‐related liver fibrosis.
Methods
We enrolled 426 patients with alcohol overuse in a prospective biopsy‐controlled study. We evaluated the accuracy of PRO‐C3 and the PRO‐C3‐based algorithm ADAPT to detect advanced liver fibrosis.
Results
The accuracy of PRO‐C3 was good with an AUROC of 0.85 (95% CI 0.79‐0.90). The best‐performing non‐patented test was the Forns index with an AUROC of 0.83 (95% CI 0.78‐0.89). The ADAPT algorithm performed better as compared to both the Forns index and PRO‐C3 alone with an AUROC = 0.88 (95% CI 0.83‐0.93).
Conclusion
PRO‐C3 is a new marker with high accuracy to detect advanced alcohol‐related liver fibrosis. The diagnostic accuracy of PRO‐C3 can be further improved by using the ADAPT algorithm in which the test outperforms currently available non‐patented serological fibrosis markers. The study is registered in the Odense Patient Data Exploratory Network (OPEN) under study identification numbers OP_040 (https://open.rsyd.dk/OpenProjects/da/openProject.jsp?openNo=40) and OP_239 (https://open.rsyd.dk/OpenProjects/openProject.jsp?openNo=239&lang=da).
PRO‐C3 and ADAPT algorithm accurately identify patients with advanced fibrosis due to alcohol related liver disease

1. INTRODUCTION
Prgressive fibrosis due to alcohol‐related (ALD) and non‐alcoholic fatty liver disease (NAFLD) is the core process leading to the development of cirrhosis and increasingly drives the development of end‐stage liver disease and liver‐related death in the Western world. 1 Due to the epidemic burden of alcohol overuse, the majority of patients are handled in the primary care setting in which assessment of liver fibrosis is rarely performed. 2 , 3 As a result, ALD is frequently diagnosed in a disease stage with advanced fibrosis and after developing clinical manifestations. 4 Standard liver function tests (LFT) are already widely used to assess liver injury by general practitioners, but these tests do not reflect the severity of fibrosis. 5 Adding an accurate fibrosis biomarker on top of the LFTs complements current clinical practice and bears the potential to enter clinical practice, as this strategy does not require implementation of new technology in the primary care setting. Liver biopsies are due to their small size prone to sampling errors and there is considerable intra‐ and interobserver disagreement when assessing the severity of fibrosis. 6 Serological markers may prove to be a more objective measurement of the global content of fibrosis in the liver in the future. 7 However, a biomarker to detect liver fibrosis, prognosticate patients and evaluate efficacy of interventions remains an unmet need. 8
Type III collagen, one of the major scar tissue‐related collagens, is highly upregulated during hepatic fibrogenesis. 9 PRO‐C3, a systemic marker of type III collagen formation and fibroblast activity, has shown promising utility to detect fibrosis stage, progression rate and treatment response in patients with chronic liver disease. 10 , 11 , 12 , 13 Recently, it was demonstrated that both PRO‐C3 alone and when incorporated in an algorithm, known as the ADAPT score, had high diagnostic accuracy to detect NAFLD‐related advanced liver fibrosis. 14 In the aforementioned study, PRO‐C3 had an AUROC of 0.81 (95% CI 0.74‐0.87) for the detection of advanced fibrosis. Integrating PRO‐C3 with the widely available parameters age, diabetes and platelets into the ADAPT score further increased the AUROC to 0.86 (95% CI 0.79‐0.91). The potential of PRO‐C3 and ADAPT to detect alcohol‐related liver fibrosis (ALF) has not previously been evaluated. Despite similarities between NAFLD and ALD, there are differences in the morphology and the abundance of specific collagen types in the extracellular matrix (ECM), which may impact the performance of a fibrosis biomarker. 15 , 16 In the current study, we thus sought to validate the previous finding from NAFLD in the setting of ALD. More specifically, we wanted to (a) explore the association between PRO‐C3 and ALF, (b) evaluate the accuracy of PRO‐C3, the ADAPT score and best‐performing non‐patented serological test to detect advanced ALF and (c) evaluate the diagnostic performance of PRO‐C3 to detect advanced ALF, in different subpopulations.
2. METHODS AND MATERIALS
The study was performed as a prospective biopsy‐controlled single‐centre study. The study was approved by the ethics committee of the Region of Southern Denmark (S‐20120071, S‐20160021). The study adheres to the 2013 Helsinki Declaration and is registered in the Odense Patient Data Exploratory Network (OPEN) under study identification numbers OP_040 (https://open.rsyd.dk/OpenProjects/da/openProject.jsp?openNo=40) and OP_239 (https://open.rsyd.dk/OpenProjects/openProject.jsp?openNo=239&lang=da). This report follows the Liver‐Fibro STARD checklist. 17
The liver biopsies were performed percutaneously with a 17‐G Menghini suction needle (Hepafix). Biopsies were considered to be of adequate quality in the absence of cirrhosis if they were >10 mm length and contained >5 portal tracts. A single experienced pathologist evaluated the biopsies according to the Kleiner fibrosis stage and non‐alcoholic fatty liver disease activity score (NAS‐CRN). 18 According to the Kleiner fibrosis stage, F0 is no fibrosis, F1 is perisinusoidal or portal/periportal fibrosis, F2 is perisinusoidal fibrosis in combination with portal/periportal fibrosis, F3 is bridging fibrosis, and F4 is cirrhosis. The NAS CRN is a semi‐quantitative score of steatosis (0‐3), ballooning (0‐2) and lobular inflammation (0‐3). The ADAPT score was calculated using the previously published formula
where Diabetes is coded as 0 if absent and 1 if present. 14 FIB4 was calculated using the formula from the original publication. 19
2.1. Biomarker quantification
Type III collagen formation was assessed in serum samples by using the ELISA‐based PRO‐C3 assay from Nordic Bioscience, Herlev, Denmark as previously described. 20
2.2. Study Population
We enrolled 426 patients with prior or current alcohol overuse for more than 1 year defined as >24 g per day for women and >36 g per day for men. Additional criteria were age 18‐75 years and informed consent to undergo a liver biopsy. We consecutively recruited participants from two municipal alcohol rehabilitation centres, through advertisement in newspapers and from three liver clinics in the Region of Southern Denmark. All participants were informed in oral and writing prior to inclusion. All patients were considered to have a significant risk of alcohol‐related liver disease that justified performing a liver biopsy. We revised the criteria in January 2016. Thereafter, we avoided performing a liver biopsy in patients with a liver stiffness below 6.0 kPa, as none of these patients had severe fibrosis, and instead, we categorized these individuals as not suffering from advanced fibrosis without performing a liver biopsy. 21
Exclusion criteria were decompensated liver disease with clear clinical signs of cirrhosis, severe alcoholic hepatitis, debilitating disease with an expected survival less than 1 year, concurrent liver disease including hepatitis B and C, hepatic congestion or inability to comply with the study protocol. All investigations were performed on the same day according to standard operating procedure after an overnight of fasting. Blood samples from 154 healthy gender‐ and aged‐matched participants were used to determine the concentration of PRO‐C3 in healthy individuals.
2.3. Statistical analysis
We used summary statistics to describe patient characteristics. Differences between continuous variables were tested by using an unpaired Students t‐test or a Mann‐Whitney test as appropriate. Differences between categorical variables were tested using Chi‐squared test. Kruskal‐Wallis and a post‐hoc Dunn´s test were used to identify statistical difference in PRO‐C3 between fibrosis stages. By performing a multivariate logistic regression model using forced entry, we identified factors independently associated with the presence of advanced ALF. Variables with a P ≤ 0.05 in univariate analysis were included in the final multivariate analysis. The diagnostic accuracy of PRO‐C3 was evaluated by AUROC. We used Delong test to compare AUROC between PRO‐C3, ADAPT and FIB4 scores. Recently published cut‐off values of 15.6 ng/ml for PRO‐C3 and 6.3287 for the ADAPT score to detect advanced fibrosis were used to calculate sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for each test. 14 We developed a risk prediction score based on PRO‐C3 by using a logistic regression model. We used Hosmer‐Lemeshow goodness of fit with 10 quantiles and plotting of the observed and predicted values to evaluate the calibrations. P ≤ 0.05 were considered significant. We used stata 15 (StataCorp) for the statistical analyses.
3. RESULTS
We included 426 participants as described in the flow chart in Figure 1. Patients were randomly assigned to a test or validation cohort. The characteristics of the enrolled participants in the total, test‐ and validation cohorts are shown in Table 1. In general, participants in the test‐ and validation cohort did not differ statistically in core parameters.
FIGURE 1.
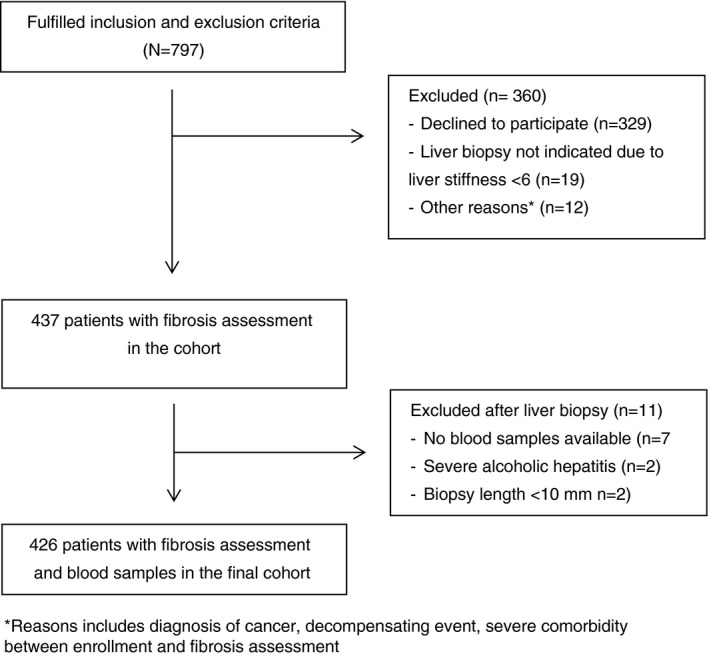
Study flow chart
TABLE 1.
Characteristics of participants
| Participants | All (N = 426) | Test (N = 213) (50%) | Validation (N = 213) (50%) | P‐value a |
|---|---|---|---|---|
| Gender (male) a | 325 (76%) | 166 (78%) | 159 (75%) | 0.425 |
| Age (years) | 56.5 ± 10.5 | 56.4 ± 10.1 | 56.7 ± 10.9 | 0.641 |
| BMI (kg/m2) | 27.6 (±5.3) | 27.1 ± 5.3 | 28.2 ± 5.4 | 0.976 |
| Diabetes | 59 (14%) | 29 (14%) | 36 (14%) | 0.888 |
| Smoking (current) | 237 (56%) | 132 (63%) | 105 (50%) | 0.010 |
| Alcohol history | ||||
| Heavy drinking ≥10 years b | 276 (69%) | 135 (68%) | 141 (71%) | 0.514 |
| Abstinent at inclusion | 178 (42%) | 92 (43%) | 86 (41%) | 0.555 |
| Daily alcohol intake in active drinkers (beverage/day) | 4 (±6) | 4 (±7) | 4 (±6) | 0.769 |
| Histological features | ||||
| Fibrosis stage (F0/F1/F2/F3/F4) | 34/124/100/24/48 | 15/62/45/12/33 | 19/62/55/12/15 | 0.085 |
| Lobular inflammation grade (0/1/2/3) | 79/148/77/25 | 40/73/40/14 | 39/75/37/11 | 0.932 |
| Ballooning grade (0/1/2) | 171/104/54 | 78/55/34 | 93/49/20 | 0.074 |
| Steatosis grade (0/1/2/3) | 145/77/71/36 | 72/38/40/17 | 73/39/31/19 | 0.754 |
| Steatohepatitis | 109 (32.9%) | 58 (35%) | 51 (31%) | 0.531 |
| NAFLD activity score | 3 (±3) | 3 (±3) | 2 (±3) | 0.241 |
| TE (kPa) | 6.3 (±6.1) | 6.3 (±7.1) | 6.2 (±5.8) | 0.673 |
| Paraclinical status | ||||
| ALT (U/L) | 31 (±27) | 32 (±27) | 30 (±25) | 0.513 |
| AST (U/L) | 32 (±23) | 34 (±29) | 32 (±22) | 0.156 |
| GGT (U/L) | 66 (±146) | 72 (±146) | 64 (±131) | 0.651 |
| AP (U/L) | 79 (±40) | 81 (±45) | 78 (±35) | 0.118 |
| INR (U/L) | 1 (±0.2) | 1 (±0.2) | 1 (±0.2) | 0.443 |
| Albumin (g/L) | 43 (±5) | 43 (±5) | 43 (±4) | 0.212 |
| Platelets (×109/L) | 234 (±98) | 234 (±104) | 234 (±92) | 0.456 |
| PRO‐C3 (ng/ml) | 12.9 (±10.3) | 13.2 (±10.5) | 12.7 (±9.6) | 0.713 |
Counts are presented as N (%). Continuous data are presented as mean ± SD, non‐normal distributed data are presented as median ± IQR.
Abbreviations: ALT, alanine aminotransferase; AP, Alkaline phosphatase; BMI, Body mass index; GGT, gamma‐glutamyltransferase; INR, International Normalized Ratio; TE: transient elastography.
P‐value reports equality test between test‐ and validation cohorts.
Defined as >24 g/day for women and >36 g/day for men.
3.1. PRO‐C3 is highly associated with the severity of ALF
The median concentration of PRO‐C3 was 12.9 (±10.3) ng/ml in the total cohort, 13.2 (±10.5) ng/ml in the test cohort and 12.7 (±9.6) ng/ml in the validation cohort (P = 0.713 Mann‐Whitney test). PRO‐C3 strongly correlated with Kleiner fibrosis stage in the total cohort (rho = 0.61, P = 0.000). Kruskal‐Wallis confirmed that the concentration of PRO‐C3 differed significantly between Kleiner fibrosis stages (P = 0.000). Post hoc Dunns test confirmed statistically significant differences in PRO‐C3 concentrations between all consecutive stages apart from stage 2 fibrosis vs stage 3 fibrosis (P = 0.481). Dotplot of PRO‐C3 serum concentration related to Kleiner fibrosis stage is depicted in Figure 2. We subsequently performed a logistic regression model to validate the association between advanced fibrosis and PRO‐C3 when adjusted for various clinical variables. As seen in Table 2, PRO‐C3 remained independently associated with advanced fibrosis (OR = 1.07, 95% CI 1.04‐1.10, P = 0.000).
FIGURE 2.
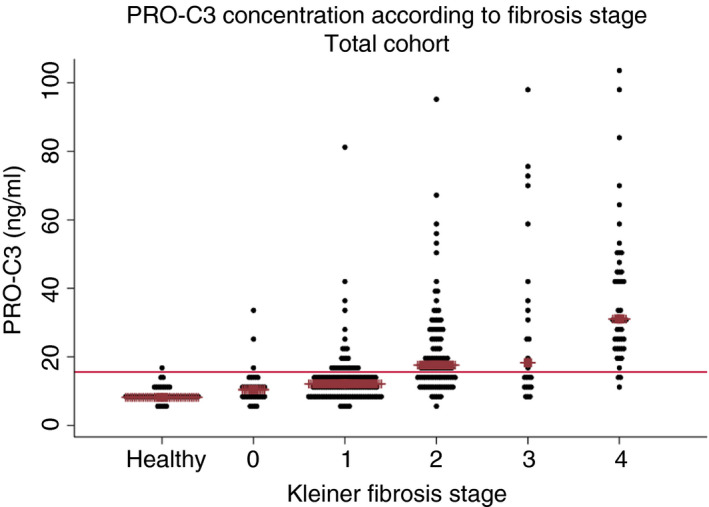
PRO‐C3 concentration in serum according to Kleiner fibrosis stage. Dotplot of the serum concentration of PRO‐C3 in the healthy population group and its relation to the Kleiner fibrosis stage in the total cohort of patients with current or prior alcohol overuse. The brown line indicates the median value
TABLE 2.
Association of study variables with advanced fibrosis in a logistic regression model
| Variable | Univariate analysis, odds ratio (95% CI) | P |
Multivariate analysis Odds ratio (95% CI) |
P |
|---|---|---|---|---|
| Gender* | 0.74 (0.39‐1.42) | 0.379 | ||
| Age | 1.03 (1.01‐1.06) | 0.018 a | 1.00 (0.96‐1.04) | 0.957 |
| BMI | 1.01 (0.96‐1.06) | 0.677 | ||
| Diabetes | 2.21 (1.18‐4.16) | 0.014 a | 1.93 (0.83‐4.50) | 0.127 |
| Smoking |
Smoker: 1.09 (0.50‐2.37) Ex‐smoker: 1.83 (0.80‐4.21) |
0.823 0.152 |
||
| Abstinent at inclusion | 1.15 (0.68‐1.94) | 0.598 | ||
| Daily alcohol intake (beverage/day) | 0.99 (0.97‐1.01) | 0.400 | ||
| ALT | 1.00 (0.99‐1.00) | 0.275 | ||
| AST | 1.00 (1.00‐1.01) | 0.123 | ||
| GGT | 1.00 (1.00‐1.00) | 0.014 a | 1.00 (0.99‐1.00) | 0.162 |
| AP | 1.01 (1.01‐1.02) | 0.000 a | 1.01 (1.00‐1.01) | 0.260 |
| Platelets | 0.99 (0.98‐0.99) | 0.000 a | 0.99 (0.99‐1.00) | 0.000 a |
| PRO‐C3 | 1.07 (1.05‐1.10) | 0.000 a | 1.07 (1.04‐1.10) | 0.000 a |
| Creatinine | 0.99 (0.97‐1.00) | 0.101 | ||
| Lobular inflammation grade (0/1/2/3) |
I1 4.08 (1.52‐10.95) I2 6.30 (2.25‐17.64) I3 11.6 (3.50‐38.67) |
0.005 a 0.000 a 0.000 a |
I1 2.06 (0.60‐7.05) I2 1.40 (0.33‐5.96) I3 0.76 (0.12‐4.77) |
0.250 0.650 0.770 |
| Ballooning grade (0/1/2) |
B1 3.47 (1.75‐6.92) B2 13.00 (6.11‐ 27.64) |
0.000 a 0.000 a |
B1 2.25 (0.97‐5.26) B2 8.70 (2.71‐ 27.92) |
.060 .000 a |
| Steatosis grade (0/1/2/3) |
S1 2.07 (1.09‐3.94) S2 1.33 (0.66‐2.68) S3 0.74 (0.26‐2.08) |
0.026 a 0.423 0.566 |
S1 1.40 (0.56‐3.48) S2 0.62 (0.21‐1.86) S3 0.15 (0.03‐0.70) |
0.469 0.397 0.015 a |
Abbreviations: ALT, alanine aminotransferase; AP: alkaline phosphatase; AST, aspartate transaminase; BMI, body mass index; GGT, gamma‐glutamyltransferase; INR, international normalized ratio.
Statistical significant P value < 0.05.
Male gender used as reference.
3.2. Accuracy of PRO‐C3 to detect advanced ALF
The PRO‐C3 had good diagnostic accuracy to detect advanced ALF with an AUROC of 0.85 (95% CI 0.79‐0.90) in the total cohort, as seen in Table 3. When analysing the test and validation cohorts, the AUROC was 0.86 (95% CI 0.79‐0.92) in the former and 0.83 (95% CI 0.75‐0.92 P = 0.7056) in the latter. ROC curves are seen in Figure 3. When applying the suggested cut‐off value of 15.6 ng/ml to detect advanced fibrosis, the sensitivity of PRO‐C3 in the total cohort was 81%, specificity 73% and PPV 38% and NPV 95%. The corresponding results from the test and validation cohorts are seen in Table 3.
TABLE 3.
Diagnostic test results
| PRO‐C3 | ADAPT | Forns | |
|---|---|---|---|
| Total cohort | |||
| Prevalence, n (%) | 72 (17) | 72 (17) | 71 (17) |
| AUROC (95% CI) | 0.85 (0.79‐0.90) | 0.88 (0.83‐0.93) | 0.83 (0.78‐0.89) |
| Cut‐off | 15.6 | 6.3287 | 6.9 |
| Correctly classifies, n (%) | 318 (75) | 338 (79) | 358 (85) |
| TP/FP/FN/TN | 58/94/14/260 | 62/78/10/276 | 48/39/24/310 |
| Sensitivity (%) | 81 (70‐89) | 86 (76‐93) | 67 (55‐77) |
| Specificity (%) | 73 (69‐78) | 78 (73‐82) | 89 (85‐92) |
| PPV (%) | 38 (30‐46) | 44(36‐53) | 55 (44‐66) |
| NPV (%) | 95 (92‐97) | 97 (94‐98) | 93 (90‐95) |
| Pre‐test odds | 0.20 | 0.20 | 0.21 |
| LR (+) | 3.03 (2.47‐3.73) | 3.91 (3.15‐4.85) | 5.97 (4.26‐8.36) |
| LR (−) | 0.27 (0.17‐0.43) | 0.18 (0.1‐0.32) | 0.38 (0.27‐0.52) |
| Test cohort | |||
| Prevalence n (%) | 45 (21) | 45 (21) | 55 (21) |
| AUROC (95% CI) | 0.86 (0.79‐0.92) | 0.91 (0.86‐0.96) | 0.83 (0.76‐0.90) |
| Cutoff | 15.6 | 6.3287 | 6.9 |
| Correctly classifies n (%) | 164 (77) | 168 (79) | 180 (85) |
| TP/FP/FN/TN | 36/40/9/128 | 39/39/6/129 | 30/17/15/150 |
| Sensitivity (%) | 80 (65‐99) | 87 (73‐95) | 67 (51‐80) |
| Specificity (%) | 76 (69‐82) | 77 (70‐83) | 90 (84‐94) |
| PPV (%) | 47 (36‐59) | 50 (39‐62) | 64 (49‐77) |
| NPV (%) | 93 (98‐97) | 96 (91‐98) | 91 (85‐95) |
| Pre‐test odds | 0.27 | 0.27 | 0.27 |
| LR (+) | 3.36 (2.47‐4.57) | 3.73 (2.77‐5.03) | 6.55 (3.99‐10.8) |
| LR (−) | 0.26 (0.15‐0.47) | 0.17 (0.08‐0.37) | 0.37 (0.25‐0.56) |
| Validation cohort | |||
| Prevalence n (%) | 27 (13) | 27 (13) | 27 (13) |
| AUROC (95% CI) | 0.83 (0.75‐0.92) | 0.85 (0.75‐0.94) | 0.84 (0.75‐0.92) |
| Cutoff | 15.6 | 6.3287 | 6.9 |
| Correctly classifies, n (%) | 154 (72) | 170 (80) | 178 (85) |
| TP/FP/FN/TN | 22/54/5/132 | 23/39/4/147 | 18/22/9/160 |
| Sensitivity (%) | 82 (62‐94) | 85 (66‐96) | 67 (46‐84) |
| Specificity (%) | 71 (64‐77) | 79 (73‐85) | 88 (82‐92) |
| PPV (%) | 29 (19‐41) | 37 (25‐50) | 45 (29‐62) |
| NPV (%) | 96 (92‐99) | 97 (93‐99) | 85 (90‐98) |
| Pre‐test odds | 0.15 | 0.15 | 0.15 |
| LR (+) | 2.81 (2.1‐3.74) | 4.06 (2.95‐5.6) | 5.12 (3.43‐8.86) |
| LR (−) | 0.26 (0.12‐0.58) | 0.19 (0.08‐0.47) | 0.38 (0.22‐0.65) |
Abbreviations: AUROC, area under the receiver operating characteristics curve; FN, false negative; FP, false positive; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive.
FIGURE 3.
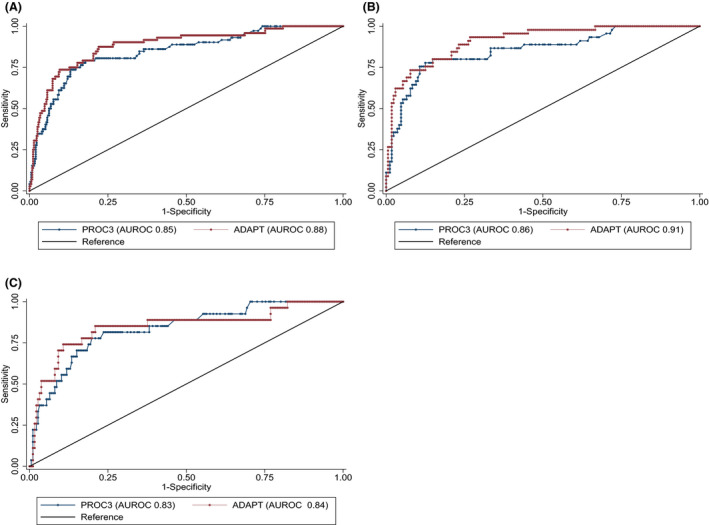
Receiver operating characteristic for PRO‐C3 and ADAPT score to diagnose advanced fibrosis and cirrhosis. Receiver operating characteristics curves for PRO‐C3 and ADAPT algorithms to detect advanced liver fibrosis in the total cohort (A), test cohort (B) and validation cohort (C)
PRO‐C3 was not well calibrated (Hosmer‐Lemeshow chi square = 21.86, P = 0.005). Risk prediction and calibration plots are seen in Figure 4. Misclassifications were mainly driven by false‐positive results. We subsequently performed a logistic regression model to identify risk factors for being falsely classified as having advanced ALF with the variables diabetes, alcohol consumption, gender, age, steatosis, ballooning, lobar inflammation, GGT, plates and biopsy length. Only the degree of steatosis, ballooning, lobular inflammation and concentration of GGT were associated with an increased risk to be falsely classified as having advanced ALF (data not shown). In a subsequent subgroup analysis, the AUROC was significantly lower among patients classified as with GGT >260 U/L compared to the group with GGT below the threshold (AUROC = 0.70 vs AUROC = 0.86, P = 0.034). In contrast, neither drinking status (abstinent vs alcohol consuming), diabetes (diabetic vs non‐diabetic) nor a high ALT level (comparing ALT levels above or below 49 U/L) reduced the AUROC significantly as depicted in Figure 5. Likewise, obesity did not impact the diagnostic accuracy (Supporting Information). Further subgroup analysis was performed to mimic the diagnostic accuracy of PRO‐C3 in a primary care setting. Patients were divided into a low‐risk group if recruited from municipal alcohol rehabilitation clinics or advertisements and a high‐risk group if they were recruited from hospital liver clinics. The negative predictive value was 98% (95‐100) when PRO‐C3 was used to exclude advanced fibrosis in the low‐risk group with a disease prevalence of 7%. The positive predictive value of PRO‐C3 dropped to 22% (12‐36) in this setting. The full analysis based on referral is available in the Supporting Information.
FIGURE 4.
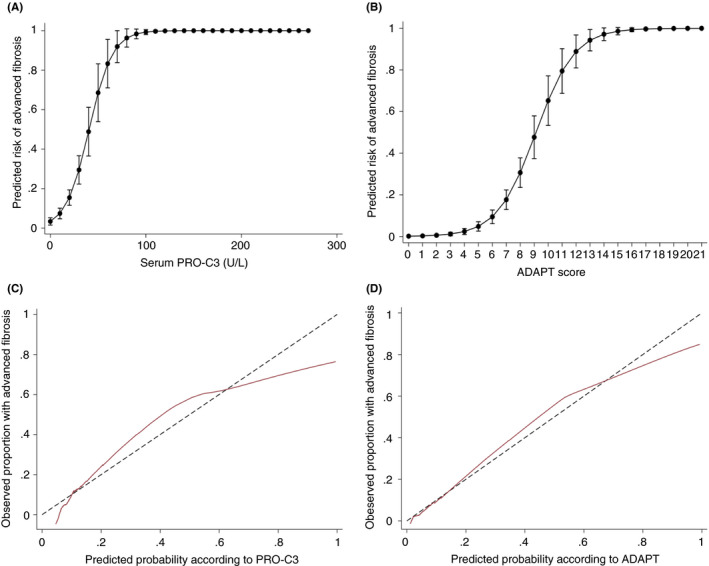
Risk prediction and calibration curves according to serum PRO‐C3. (A and B) Risk‐prediction curves to evaluate the probability of advanced fibrosis according to the serum concentration of PRO‐C3 and the ADAPT score. (C and D) Calibration slopes for PRO‐C3 and the ADAPT score in the total cohort. The marron line graphs the agreement between predicted probability of advanced fibrosis on the x‐axis and observed proportion with advanced fibrosis on the y‐axis. The perfect calibration with 100% agreement is marked with a black dashed line
FIGURE 5.

Receiver operating characteristic for PRO‐C3 in subgroups. ROC curves for the detection of advanced fibrosis by PRO‐C3 in four subgroups. (A) Abstinent vs. alcohol using participants (B) Diabetic vs. non‐diabetics participants (C) Participants with an ALT level above or below 49 U/L (D) Participants with an GGT level above or below 260 U/Ls
3.3. Diagnostic accuracy of the ADAPT algorithm to detect advanced ALF
The median ADAPT score was 5.5098 (±4.7929) in the total cohort. ADAPT had higher diagnostic accuracy compared to PRO‐C3 alone in the total cohort with AUROC = 0.88 (95% CI 0.83‐0.93, P = 0.010). The corresponding AUROC of ADAPT was 0.91 (95% CI 0.86‐0.96) in the test cohort and 0.85 (95% CI 0.75‐0.94, P = 0.230) in the validation cohort (Table 3). The reported optimal cut‐off value of 6.3287 for ADAPT to detect advanced fibrosis was used. 14 By applying this cut‐off value, the sensitivity was 86%, specificity 78%, PPV 44% and NPV 97% in the total cohort. The results from the test and validation cohorts are reported in Table 3. Results when using optimized cut‐offs and rule‐in and rule‐out criteria are available in the Supporting Information.
3.4. Head‐to‐head comparison of diagnostic accuracy with other serological fibrosis markers
Results of the head‐to‐head comparison with non‐patented serological fibrosis markers are seen in Table 4. Forns index was the best‐performing non‐patented biomarker with an AUROC of 0.83 (95% CI 0.78‐0.89). The ADAPT score, but not PRO‐C3, performed significantly better than the Forn index in the total cohort. When using the recommended cut‐off value to detect advanced fibrosis, the sensitivity of the Forns index was 67%, specificity 89%, PPV 55% and NPV 93% (Table 3).
TABLE 4.
Accuracy of diagnostic tests for liver fibrosis in the total cohort of patients with alcohol‐related liver disease
| P value for AUROC comparison with PROC3 | P value for AUROC comparison with ADAPT | Brier score Advanced fibrosis (≥F3) |
AUROC Advanced fibrosis (≥F3) |
|
|---|---|---|---|---|
| ADAPT | 0.010 a | — | 0.089 a | 0.88 (0.84‐0.93) |
| PRO‐C3 | — | 0.010 a | 0.105 a | 0.85 (0.79‐0.90) |
| Forns index | 0.682 a | 0.043 a | 0.106 a | 0.83 (0.78‐0.89) |
| FIB‐4 | 0.416 a | 0.013 a | 0.128 a | 0.81 (0.75‐0.87) |
| GGT‐to‐platelet ratio | 0.053 a | 0.000 a | 0.137 a | 0.79 (0.74‐0.84) |
| Age‐platelet index | 0.086 | 0.000 a | 0.112 | 0.78 (0.71‐0.84) |
| APRI score | 0.058 | 0.000 a | 0.138 | 0.78 (0.72‐0.84) |
| AST:ALT ratio | 0.003 a | 0.000 a | 0.128 | 0.73 (0.66‐0.79) |
Abbreviations: APRI, aspartate transaminase‐platelet ratio; FIB‐4,fibrosis‐4 index.
Statistical significant P value < 0.05.
4. DISCUSSION
In this study, we measured PRO‐C3 in a large cohort of patients suffering from the full spectrum of ALF. Our main findings were: (a) PRO‐C3 was significantly associated with the degree of ALF, also after adjustment for a variety of parameters. (b) The diagnostic accuracy of PRO‐C3 as a stand‐alone marker to detect advanced ALF was good, but did not differ significantly when compared to the Forns index. (c) A high level of GGT was associated with increased risk of being wrongly classified as having advanced ALF and reduced the diagnostic accuracy of PRO‐C3. (d) Combining PRO‐C3 with available clinical parameters into the ADAPT score increased diagnostic accuracy to an excellent level in the total cohort and outperformed all non‐patented serological fibrosis markers including the Forns index.
Our results validate the recent study based on NAFLD patients, that PRO‐C3 and the ADAPT algorithm can be used to detect advanced liver fibrosis. In the previous study, the AUROC for identification of advanced liver fibrosis by PRO‐C3 was 0.81 (95% CI 0.74‐0.87) and increased to 0.86 (95% CI 0.79‐0.91) by the ADAPT algorithm. Due to the epidemic in fatty liver disease from alcohol and obesity, there is, from a management point of view, a lack of biomarkers to rule out advanced fibrotic liver disease in a population with low disease prevalence. Interestingly, a PRO‐C3 concentration below the threshold of 15.6 ng/ml almost abolished the risk of having advanced ALF and could potentially be used as a first diagnostic tool to rule out disease in the primary care setting.
We identified GGT as an independent risk factor for being falsely classified as having advanced ALF and GGT should be taken into account when interpreting PRO‐C3. A high concentration of GGT likewise increased the risk of false‐positive results when elastography was used to assess the degree of liver fibrosis. 21 The mechanism by which GGT affects diagnostic accuracy of PRO‐C3 and elastography is not completely understood. A change in GGT probably reflects subtle alcohol‐induced rearrangements in the hepatic parenchyma, leading to an increase in stiffness and/or release of ECM proteins into the blood stream, without a concurrent effect on the fibrosis stage.
Some limitation of the study should be mentioned. The optimal cut‐off values for detecting advanced fibrosis were based on the NAFLD setting. Disease aetiology may impact ECM remodelling processes which could potentially impact the optimal cut‐off values for PRO‐C3. 22 However, fibrosis in the setting of NAFLD and ALD shares commonalities, as both diseases at an early stage lead to deposition of ECM in a perisinusoidal and pericellular (chicken wire) pattern. 23 Harmful alcohol consumption may coexist with and then impair the outcome of other prevalent chronic liver diseases including NAFLD. 24 Biomarkers and algorithms should have the robustness to identify liver fibrosis in patients with mixed clinical phenotypes, if they should translate into clinical practice. Difference in disease spectrum impacts the diagnostic accuracy of a test and complicates comparison between different cohorts. 25
In conclusion, our results validate PRO‐C3 as biomarker with high diagnostic accuracy to detect liver fibrosis. The diagnostic accuracy can be further increased by incorporating clinical and biochemical parameters into the ADAPT algorithm, which outperforms currently available non‐patented serological fibrosis assessment markers.
AUTHORSHIP
Guarantor of the article: BSM.
Author contributions: BSM: study concept and design, acquisition and statistical analysis of data, interpretation of data, drafting of manuscript. MT: acquisition and analysis of data, interpretation of data, critical revision of the manuscript for important intellectual content. SDE: acquisition of data, interpretation of data, critical revision of the manuscript for important intellectual content. MJN, NSG, DJL, JT: analysis and interpretation of data, critical revision of the manuscript for important intellectual content. MK, LM: acquisition of data, critical revision of the manuscript for important intellectual content. MAK: conceptualized the study, interpretation of data, critical revision of the manuscript for important intellectual content. AK: conceptualized the study, interpretation of data, critical revision of the manuscript for important intellectual content, obtained funding. All authors approved the final version of the manuscript.
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the support from the European Union’s Horizon 2020 research and innovation programme under grant agreement number 668031, The Novo Nordisk Foundation’s Challenge Programme, Innovation Fund Denmark former The Danish Advanced Technology Foundation, Region of Southern Denmark.
Declaration of personal interests: MAK, MJN, NSG, DJL are full‐time employees at Nordic Bioscience A/S. MAK, MJN and DJL are the original inventors and patentholders for PRO‐C3. The remaining authors declare nothing to disclose This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement number 668031, The Novo Nordisk Foundation’s Challenge Programme, Innovation Fund Denmark former The Danish Advanced Technology Foundation, Region of Southern Denmark.
Madsen BS, Thiele M, Detlefsen S, et al. PRO‐C3 and ADAPT algorithm accurately identify patients with advanced fibrosis due to alcohol‐related liver disease. Aliment Pharmacol Ther. 2021;54:699–708. 10.1111/apt.16513
The Handling Editor for this article was Professor Gideon Hirschfield, and it was accepted for publication after full peer‐review.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kim D, Li AA, Gadiparthi C, et al. Changing trends in etiology‐based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155:1154–1163.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–168. [DOI] [PubMed] [Google Scholar]
- 3. Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953–1997. [DOI] [PubMed] [Google Scholar]
- 4. Dam Fialla A, Schaffalitzky de Muckadell OB, Touborg LA. Incidence, etiology and mortality of cirrhosis: a population‐based cohort study. Scand J Gastroenterol. 2012;47:702–709. [DOI] [PubMed] [Google Scholar]
- 5. Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davison BA, Harrison SA, Cotter G, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322–1332. [DOI] [PubMed] [Google Scholar]
- 7. Sanyal AJ, Harrison SA, Ratziu V, et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology. 2019;70:1913–1927. [DOI] [PubMed] [Google Scholar]
- 8. Younossi ZM, Loomba R, Anstee QM, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karsdal MA, Nielsen SH, Leeming DJ, et al. The good and the bad collagens of fibrosis – their role in signaling and organ function. Adv Drug Deliv Rev. 2017;121:43–56. [DOI] [PubMed] [Google Scholar]
- 10. Praktiknjo M, Lehmann J, Nielsen MJ, et al. Acute decompensation boosts hepatic collagen type III deposition and deteriorates experimental and human cirrhosis. Hepatol Commun. 2018;2:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nielsen MJ, Veidal SS, Karsdal MA, et al. Plasma Pro‐C3 (N‐terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 2015;35:429–437. [DOI] [PubMed] [Google Scholar]
- 12. Karsdal MA, Henriksen K, Nielsen MJ, et al. Fibrogenesis assessed by serological type III collagen formation identifies patients with progressive liver fibrosis and responders to a potential antifibrotic therapy. Am J Physiol Gastrointest Liver Physiol. 2016;311:G1009–G1017. [DOI] [PubMed] [Google Scholar]
- 13. Karsdal MA, Hjuler ST, Luo YI, et al. Assessment of liver fibrosis progression and regression by a serological collagen turnover profile. Am J Physiol Gastrointest Liver Physiol. 2019;316:G25–G31. [DOI] [PubMed] [Google Scholar]
- 14. Daniels SJ, Leeming DJ, Eslam M, et al. ADAPT: An algorithm incorporating PRO‐C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology. 2019;69:1075–1086. [DOI] [PubMed] [Google Scholar]
- 15. Lackner C, Tiniakos D. Fibrosis and alcohol‐related liver disease. J Hepatol. 2019;70:294–304. [DOI] [PubMed] [Google Scholar]
- 16. Nakano M, Fukusato T. Histological study on comparison between NASH and ALD. Hepatol Res. 2005;33:110–115. [DOI] [PubMed] [Google Scholar]
- 17. Boursier J, de Ledinghen V, Poynard T, et al. An extension of STARD statements for reporting diagnostic accuracy studies on liver fibrosis tests: the Liver‐FibroSTARD standards. J Hepatol. 2015;62:807–815. [DOI] [PubMed] [Google Scholar]
- 18. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 19. Vallet‐Pichard A, Mallet V, Nalpas B, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. [DOI] [PubMed] [Google Scholar]
- 20. Nielsen MJ, Nedergaard AF, Sun S, et al. The neo‐epitope specific PRO‐C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res. 2013;5:303–315. [PMC free article] [PubMed] [Google Scholar]
- 21. Thiele M, Detlefsen S, Sevelsted Møller L, et al. Transient and 2‐dimensional shear‐wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology. 2016;150:123–133. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen MJ, Karsdal MA, Kazankov K, et al. Fibrosis is not just fibrosis ‐ basement membrane modelling and collagen metabolism differs between hepatitis B‐ and C‐induced injury. Aliment Pharmacol Ther. 2016;44:1242–1252. [DOI] [PubMed] [Google Scholar]
- 23. Epstein FH, Lieber CS. Biochemical and molecular basis of alcohol‐induced injury to liver and other tissues. N Engl J Med. 1988;319:1639–1650. [DOI] [PubMed] [Google Scholar]
- 24. Fuster D, Samet JH. Alcohol use in patients with chronic liver disease. N Engl J Med. 2018;379:1251–1261. [DOI] [PubMed] [Google Scholar]
- 25. Poynard T, Halfon P, Castera L, et al. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem. 2007;53: 1615–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


