Abstract
Background
Because of the widespread use of oral contraceptives (OCs) and the devastating effects of depression both on an individual and a societal level, it is crucial to understand the nature of the previously reported relationship between OC use and depression risk. Insight into the impact of analytical choices on the association is important when interpreting available evidence. Hence, we examined the association between adolescent OC use and subsequent depression risk in early adulthood analyzing all theoretically justifiable models.
Methods
Data from the prospective cohort study TRacking Adolescents’ Individual Lives Survey, among women aged 13–25 years were used. Adolescent OC use (ages 16–19 years) was used as a predictor and major depressive disorder (MDD) in early adulthood (ages 20–25 years), as assessed by the Diagnostic and Statistical Manual of Mental Disorders‐IV oriented Lifetime Depression Assessment Self‐Report and the Composite International Diagnostic Interview, was used as an outcome. A total of 818 analytical models were analyzed using Specification Curve Analysis in 534 adolescent OC users and 191 nonusers.
Results
Overall, there was an association of adolescent OC use and an episode of MDD in early adulthood [median odds ratio (OR)median = 1.41; ORmin = 1.08; ORmax = 2.18, p < .001], which was driven by the group of young women with no history of MDD (ORmedian = 1.72; ORmin = 1.21; ORmax = 2.18, p < .001).
Conclusions
In summary, adolescent OC use was associated with a small but robust increased risk for experiencing an episode of MDD, especially among women with no history of MDD in adolescence. Understanding the potential side effects of OCs will help women and their doctors to make informed choices when deciding among possible methods of birth control.
Keywords: Oral contraceptive use, adolescence, risk factors, major depressive disorder
Introduction
The invention of oral contraceptive (OC) has been liberating for women and contributed substantially to the dramatic increase in women’s education and participation in the labor market (Sonfield, Hasstedt, Kavanaugh, & Anderson, 2013). Worldwide, more than 150 million women use OC (United Nations, 2019) and about 50% of women in modern societies do so during their teenage years (Anderl, Li, & Chen, 2020). Although OCs are generally considered safe, several recent large‐scale observational studies suggest that OC use, especially during adolescence, may increase a woman’s risk for depressive symptoms and even major depressive disorder (MDD) (Anderl et al., 2020; Skovlund, Morch, Kessing, & Lidegaard, 2016; de Wit et al., 2020). However, other studies reported no or even protective effects of OC use on depression risk (Cheslack‐Postava, Keyes, Lowe, & Koenen, 2015; McKetta & Keyes, 2019). Due to these seemingly conflicting findings, the putative relationship remains disputed. However, because of the widespread use of OCs and the devastating effects of MDD on the individual as well as the societal level (Wittchen et al., 2011), it is crucial to understand the nature of the relationship between OC use and MDD risk.
One understudied factor of the relationship between OC use and depression are potential enduring effects of OCs years after use, as most previous studies have focused on examining its short‐term effects. The only published study that has specifically focused on long‐term effects found that adolescent OC users more often had an MDD years later, even after cessation of use (Anderl et al., 2020). However, use of OCs was assessed retrospectively and the study—like most studies in the field—relied on cross‐sectional data, limiting the possibility to control for important baseline differences between users and nonusers of OC and to establish the direction of the observed association between OC use and depression risk. Prospective studies can overcome these limitations and are critical for establishing the direction of the observed association between OC use and depression risk. In this study, we therefore set out to overcome the limitations of previous studies by addressing the question of whether adolescent OC use predicts later episodes of MDD in a large prospective data set.
In addition, we opted for conducting a specification curve analysis (SCA) (Simonsohn, Simmons, & Nelson, 2020) to account for the multitude of theoretically justifiable operationalizations and analytic choices naturally available in large data sets (Orben & Przybylski, 2019), which can lead to contradicting findings (Botvinik‐Nezer et al., 2020). To illustrate, a prior cross‐sectional study investigating the relationship between adolescent OC use and depression reported a wide range of effect sizes for the relationship between ever use of OC and ever having a depressive disorder (odds ratios ranged between 1.1 and 1.9), depending on the specific covariates that the analysis adjusted for (McKetta & Keyes, 2019). SCA is a transparent analytical approach that allows to implement every theoretically justifiable analytical pathway in parallel and interpret their combined results as one entity. Using SCA also provides a way to explore which specific clusters of analytic decisions result in divergent patterns of findings. We expected that across specifications, adolescent OC use would predict higher risk for MDD in early adulthood.
Methods
Study population
Data were derived from the Dutch population survey TRAILS (TRacking Adolescents' Individual Lives Survey), a large prospective cohort study designed to investigate the psychological, social, and physical development of adolescents. The national ethical ‘Central Committee on Research Involving Human Subjects’ approved the study design. Children were recruited from primary schools in 2001 and 2002 (de Winter et al., 2005). Exclusion criteria were serious health or language problems that would hamper participation in the study. The participants (children) consented to participate in the study and one of their parents gave informed consent. In total, 2,230 children (mean age 11.1 ± 0.6 SD years; 1,137 girls) were enrolled in the study. After study entry, children were followed up at mean (SD) ages of 13.6 (0.5) (T2, baseline), 16.3 (0.7) (T3), 19.1 (0.6) (T4), 22.3 (0.3) (T5), and 25.6 (0.6) (T6) years (Oldehinkel et al., 2015). For readability, we refer here to ‘baseline’ instead of T2, and to ages 16, 19, and 25 instead of T3, T4, and T6, respectively. For this study, we selected all biologically female participants with the following exceptions: We excluded participants (1) who had not completed the Lifetime Depression Assessment Self‐Report (LIDAS) (Bot et al., 2017) at age 25 (T6; n = 356); (2) who had no available data on current OC use for both age 16 (T3) and age 19 (T4; n = 18); (3) who could not be conclusively classified as either adolescent users or nonusers because they had missing data at one assessment and reported that they were not currently using OCs at the other (n = 32); (4) who were using sex steroids other than OC at age 16 or 19 (n = 0); and (5) who had discrepancies between the OC use question and the medication list (n = 6). This resulted in a final sample of 725 women.
Specification curve analyses
The SCA consisted of three steps (Simonsohn et al., 2020). First, all theoretically justified specifications that could be used to relate adolescent OC use and MDD in young adulthood were identified. Second, the specifications were analyzed and the results were plotted. Third, (in)consistency of the resulting specification curve with the null‐hypothesis (H0) that adolescent OC use did not predict higher risk for MDD was determined using an inferential specification curve.
Step 1. Identifying specifications
The amount of specifications was the product of all justifiable options regarding the four core elements of the model: the sample, the predictor, the outcome, and potential sets of covariates. All specifications are listed in Table S1.
Sample
First, the analyses were performed on the entire sample of 725 women. To focus on first‐onset MDD (Vrijen, Hartman, & Oldehinkel, 2019), the analyses were subsequently repeated on the sample of 583 participants who had no history of MDD at or before age 19. For the details on the determination of MDD, see the corresponding header.
Predictor: adolescent oral contraceptive use
All women who reported that they were currently using OCs at age 16, at age 19, or both were classified as ‘adolescent OC users’. All women who reported that they were not, at both age 16 and age 19, were classified as ‘adolescent non users’. OC use was assessed with the question: ‘Do you use an oral contraceptive pill?’ and as part of a medication list provided by the participants. At age 13, OC use was unlikely and hence this assessment was used as baseline (de Wit et al., 2020).
Outcome: major depressive disorder in young adulthood
The World Health Organization Composite International Diagnostic Interview (CIDI) version 3.0 (Kessler et al., 2004) at age 19 and the LIDAS (Bot et al., 2017) at age 25 were used to determine episodes of MDD after age 19. The CIDI is a structured diagnostic interview assessing DSM‐IV mental disorders, which has been shown to have good reliability and validity (Haro et al., 2006; Kessler et al., 2004). When a lifetime MDD was endorsed, the age of onset was interrogated. The LIDAS (Bot et al., 2017) is a self‐report instrument that includes all nine DSM‐IV symptoms’ criteria for MDD, supplemented with items on impairment of functioning. When at least five of nine symptoms, including one core symptom, were present and hampered functioning for at least 2 weeks somewhere in the past, a lifetime MDD was diagnosed. The LIDAS has been shown to have adequate sensitivity and specificity in measuring lifetime MDD as assessed with the CIDI (Bot et al., 2017).
Combined data from the CIDI and LIDAS data were used to construct a set of justifiable outcome variables. Following previous work, we used the 12‐month MDD prevalence at age 25 as our first outcome variable (Anderl et al., 2020). This variable should be least affected by recall bias, but has a small detection window (1 year) and may hence miss some episodes. Therefore, we created a second outcome variable in which all years between the assessments at age 19 and 25 were used to define any new episodes of MDD (Vrijen et al., 2019). For this outcome variable, women whose data at ages 19 and 25 were inconsistent (n = 11) were excluded. As the data on the number of episodes collected at age 25 were not specific enough to establish the timing of episodes in case of multiple recurrences, women who had already experienced two or more episodes at age 19 were excluded as well (n = 23). Third, to test the associations of OC use with first‐onset MDD in early adulthood, we used lifetime MDD at age 25 based on LIDAS in the subsample of women with no history of MDD at age 19 (Skovlund et al., 2016).
Selection of covariates
Potential covariates were selected from variables available in the dataset that had previously been suggested to entirely or partially account for the observed association between OC use and depression—either empirically or theoretically—in addition to basic demographic information. Age at baseline, ethnicity, and socioeconomic status (SES) were included in all but the crude model as fixed covariates; history of MDD before age 19 was included as additional fixed covariate in all models analyzing the entire sample. In addition to these fixed covariates, non‐fixed covariates were entered into the models one by one and in combinations of two. This enabled us to examine whether differences in OC users and nonusers could confound the association, whereas preventing overprediction of the model by limiting the number of covariates added. Some of non‐fixed covariates were time‐variant [body mass index (BMI), menstruation‐related pain, acne, virginity, smoking, and stress], while other were time‐invariant (age at menarche, sexual orientation, OC use at age 25, and depressive symptoms at baseline). The combinations of two involved either entering both variables to the model, or adding the multiplicative interaction of two time‐variant (within the same age, i.e., age 13, 16, and 19) or time‐invariant covariates. For details on the determination of covariates, see Appendix S1.
Step 2: Implementing specifications
We tested a total of 818 models between adolescent OC use and development of MDD in early adulthood, using logistic regressions in R (version 1.2.1335). The specifications were ranked by their ORs and plotted in a specification curve (Figure 1).
Figure 1.
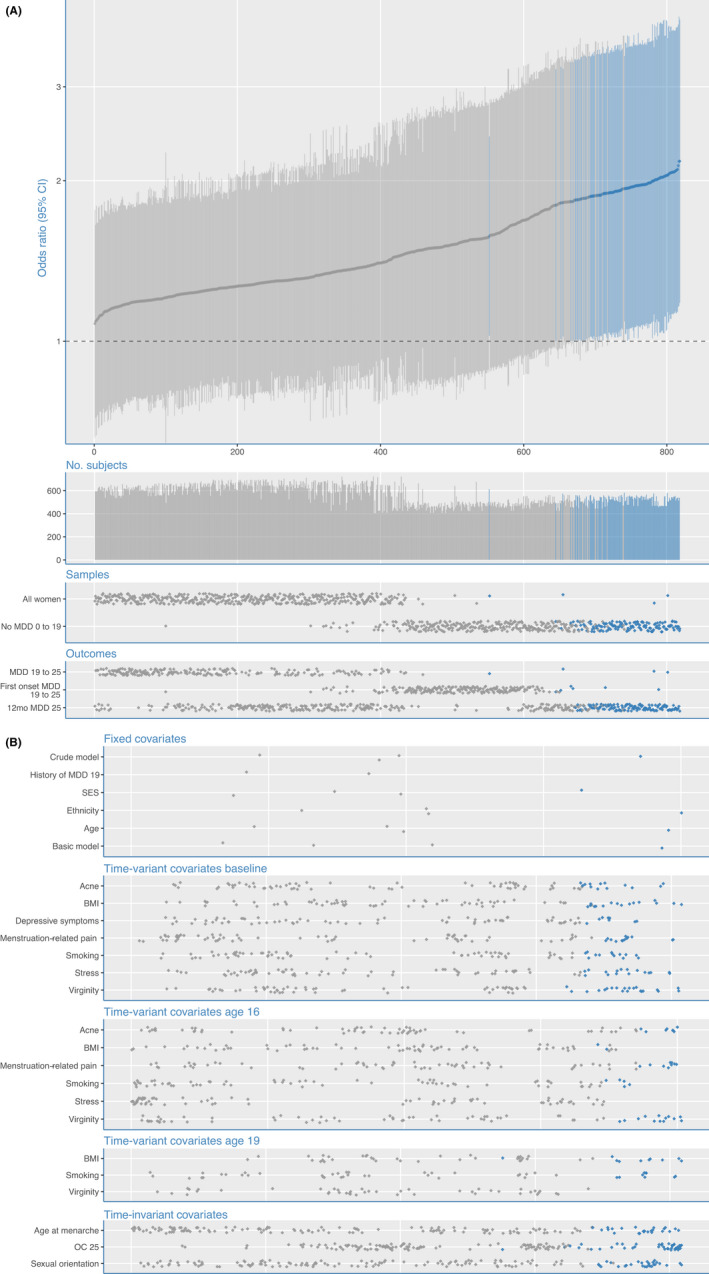
Specification curve analysis for the association of adolescent OC use with major depressive disorder (MDD) in early adulthood. (A) The specification curve panel shows the odds ratio (OR) with 95% confidence interval (CI). The thick gray line depicts the ORs for adolescent OC use for developing MDD during early adulthood across all 818 implemented model specifications (sorted by effect size). The thinner vertical lines depict the corresponding 95%CI. ORs with 95%CIs are colored blue in the case that they are significant, or gray in the case that they are not significant. The 'No. subjects' panel shows the number of participants included in the specific analyses, ranging from 411 to 725. The 'Samples' panel depicts which sample was used in each of the model specifications. Finally, the 'Outcomes' panel shows which of the outcome measure was used in each of the specifications. (B) Specification of which covariates were used in each of the analyses from the specification curve analysis for the association of adolescent OC use with major depressive disorder (MDD) in early adulthood. A gray or blue dot indicates that the variable of the current row was included in a specification. Together, the dots add up to the 818 specifications. A single specification may have any covariate or combination of two covariates (interaction or sum) at the specific time point (i.e., baseline, 16, or 19), where OC use at age 25, age at menarche, and sexual orientation were included at each of the time points. Each specification included the basic adjustments for socioeconomic status, ethnicity, and age (basic model). History of MDD at age 19 was included in all specifications in the subgroup of women who were not diagnosed with an MDD before age 19
Step 3: Joint statistical inferences
The hypothesis was tested using the resampling approach for non‐experimental data described by Simonsohn et al. (2020). In particular, the null was forced on the data by creating 818 new outcome variables—one for each specification—by subtracting the respective empirically estimated effect of the predictor variable (i.e., adolescent OC use) on the empirical outcome variable (i.e., MDD in adulthood). We then sampled, with replacement, 725 rows of data (corresponding to the empirical sample size) and repeated this procedure 1,000 times, thereby creating 1,000 new data sets under resampling for which the null hypothesis was known to be true. Finally, we ran 1,000 SCA, one for each resampled data set under the null, using the same 818 specifications identified for the empirical data set and their corresponding new outcome variables. Significance was then computed in three ways: (1) by counting the number of times that the empirically observed median OR (without resampling under the null), estimated across all specifications, was smaller than or equal to the median OR of each single resampled specification curve under the null and dividing this number by 1,000; (2) by counting the number of times that the share of significant effects (all effects with p < .05) in the predicted direction in the empirical specifications was smaller or equal to the share of significant effects in the predicted direction of each single resampled specification curve under the null, again dividing this number by 1,000; and (3) by averaging the Z values associated with all p‐values of each specification curve (called Stouffer’s method) and counting for how many this value was equal or less extreme for the empirical specification curve compared with each of the resampled ones for which the null is known to be true, following the procedure described in (Simonsohn et al., 2020), and again dividing this number by 1,000. Because subtracting the effect size from the binary empirical outcome variable results in non‐binary outcome variables under the forced null, SCA for inferential tests were conducted using ordinary least squares regression for both the empirical and the resampled data.
Results
Data from 411 to 725 women, depending on the underlying sample and number of missing data points, were included in the 818 models. Most of the participating young women (73.7%) used OCs at least once during adolescence. Descriptive statistics of adolescent OC users and nonusers at baseline and during ages 16 and 19 are shown in Table 1. Nonusers had a higher mean SES than users, were less likely to be of Dutch ethnicity, were more likely to be virgins, and were less likely to have smoked at least once. However, there were no differences in history of MDD during adolescence [33 (17.3%) vs. 109 (20.4%)] and also no differences in depressive symptoms at baseline [0.29 (0.27) vs. 0.33 (0.29)]. In accordance with common sense and prior literature (Burcusa & Iacono, 2007), the 12‐month prevalence of MDD at age 25 was substantially higher in women with a history of MDD at age 19 [48 of 142 women (33.8%)] compared with women with no such diagnosis [92 of 583 women (15.9%)], p < .001. There were no differences in the lifetime prevalence of comorbid psychiatric disorders between adolescent OC users and nonusers at the age of 25. Only panic and anxiety disorders were somewhat more prevalent in adolescent OC users than in nonusers (4.5% and 7.7% vs. 1.0% and 3.7%, respectively) but these differences were not statistically significant. For details, see Table S2.
Table 1.
Sociodemographic and clinical characteristics depending on history of oral contraceptive use during adolescence of 725 women
| No adolescent OC use | Adolescent OC use | p | |
|---|---|---|---|
| N = 191 | N = 534 | ||
| Age at baseline, mean ± SD (years) | 13.4 ± 0.5 | 13.6 ± 0.5 | .003 |
| Dutch ethnicity, n (%) | 159 (83.2) | 498 (93.3) | <.001 |
| SES, mean ± SD | 0.27 ± 0.74 | 0.07 ± 0.76 | .002 |
| Age at menarche, mean ± SD (years) | 12.9 ± 1.2 | 12.7 ± 1.1 | .009 |
| BMI, mean (kg/m2) | |||
| Baseline | 19.2 ± 3.2 | 19.2 ± 3.0 | .93 |
| Age 16 | 21.6 ± 3.3 | 21.7 ± 2.9 | .70 |
| Age 19 | 22.8 ± 4.0 | 23.2 ± 3.8 | .31 |
| Menstrual pain, n (%) | |||
| Baseline | 55 (31.3) | 207 (43.3) | .005 |
| Age 16 | 89 (52.0) | 301 (69.4) | <.001 |
| Acne, n (%) | |||
| Baseline | 68 (37.6) | 178 (36.9) | .93 |
| Age 16 | 72 (42.1) | 196 (45.2) | .53 |
| Virginity, n (%) | |||
| Baseline | 180 (98.9) | 494 (95.0) | <.001 |
| Age 16 | 166 (86.9) | 249 (51.3) | <.001 |
| Age 19 | 126 (66.3) | 69 (13.2) | <.001 |
| Smoking, n (%) | |||
| Baseline | 1 (0.5) | 21 (4.0) | .01 |
| Age 16 | 11 (5.8) | 95 (19.5) | <.001 |
| Age 19 | 21 (11.0) | 127 (24.2) | <.001 |
| Stressful experiences, mean ± SD | |||
| Baseline | 2.6 ± 2.5 | 3.0 ± 2.8 | .05 |
| Age 16 | 3.7 ± 2.5 | 4.2 ± 2.6 | .01 |
| Heterosexuality at age 19, n (%) | 180 (95.2) | 487 (93.3) | .07 |
| OC use, n (%) | |||
| Age 16 | — | 222 (47.4) | — |
| Age 19 | — | 501 (96.0) | — |
| Age 25 | 71 (37.4) | 255 (48.9) | .007 |
| History of MDD at age 19, n (%) | 33 (17.3) | 109 (20.4) | .40 |
| Depressive symptoms at baseline | 0.29 ± 0.27 | 0.33 ± 0.29 | .11 |
Data are percentages or mean ± standard deviation (SD). p‐Values are based on two‐tailed chi‐squared tests for dichotomous variables or t‐tests for continuous variables. MDD, major depressive disorder; OC, oral contraceptive; SES, socioeconomic‐status.
Figures 1A and 1B show the results of the SCA on the association between adolescent OC use and MDD in early adulthood. Notably, all ORs were larger than 1, indicating a higher MDD risk for adolescent OC users, with a median effect size of OR = 1.41. However, effect sizes varied considerably across specifications (ORmin = 1.08; ORmax = 2.18). The majority of the higher effect sizes were based on the subsample of women without a history of MDD at age 19 (ORmedian = 1.72; ORmin = 1.21; ORmax = 2.18). The variation in effect sizes between models was predominantly (79.5%) explained by the choice of sample; choice of outcome measure accounted for 15.4% and choices regarding the inclusion of specific covariates for 2.8% of the variation in effect sizes.
The results of the resampling tests showed that not a single one of the 1,000 median ORs were equally large or larger in the resampled specification curves than the median OR of the empirical specification curve (median OR = 1.41, p < .001). Similarly, not a single one of the 1,000 resampled specification curves had an equal or larger share of significant effects than the empirical specification curve (16.6% of the empirical models showed significant effects, p < .001) or a more extreme average Z value across all specifications than the empirical specification curve, p < .001. Qualitatively similar but quantitatively stronger effects were found when we restricted the analyses to the subgroup of women who had a first onset of MDD after the age of 19 years. Not a single one of the 1,000 median ORs were equally large or larger in the resampled specification curves than the median OR of the empirical specification curve (median OR = 1.72, p < .001) and neither the share of significant p‐values across models (34.4% of the empirical models showed significant effects, p < .001) nor the average Z value (p < .001) was equally or more extreme in any of the 1,000 resampled specification curves than in the empirical specification curve.
Discussion
The present study is the first to examine long‐term associations of OC use during adolescence with MDD in adulthood in a large, prospective cohort study. Overall, we found that women who had used OCs during adolescence showed an increased risk to experience an episode of MDD up to 6 years later. This finding was most pronounced in women who had not suffered from MDD before or during adolescence.
Generally, our findings converge with several recent studies suggesting that adolescent OC use is associated with increased risk for MDD (Anderl et al., 2020; Skovlund et al., 2016). However, while all ORs were consistently above 1 in all 818 models we ran, the effect sizes varied considerably (ORs between 1.1 and 2.2), depending on the specific sample, outcome variable, and control variables selected in the model. This supports the notion that if several plausible alternative operationalizations of key measures of interest are available in a given data set, partially arbitrary choices can severely impact the apparent outcomes and conclusions of a study (Botvinik‐Nezer et al., 2020).
Notably, the relationship between adolescent OC use and MDD risk was less strong for women with a history of MDD at age 19. Apparently, adolescent OC use does not contribute strongly to a heightened risk for a future MDD episode if this risk is already heightened by a previous episode [also known as the scar hypothesis (Burcusa & Iacono, 2007)]. Alternatively or additionally, it may reflect a healthy survivor bias: adolescents who suffer from MDD—whether triggered by OC use—might have been more likely to stop using OCs after a short period of time. Hence, these women may have been incorrectly classified as nonusers, which would have falsely caused an overestimation of the MDD risk in the nonuser group. This misclassification bias may have directed odds ratios toward the null in the total group of 725 women.
Pre‐existing differences between OC users and nonusers have been proposed to explain observed relationships between OC use and MDD risk. However, in this sample only 2.8% of the variance in the specification curve (e.g., the variance in the effect sizes) was explained by the selection of covariates. This suggests that, although factors such as SES, prior sexual activity, depressive symptoms, and OC use at age 25 might be predictors of future MDD, they did not confound the relationship between OC use and risk for MDD to a large extent in our sample of 725 women.
The present study is observational, so experimental studies will be needed to test whether the observed association is causal. Previous randomized controlled trials have overall not found a consistent link between OC use and depression (or mood, more broadly) (Lundin et al., 2017). However, to our knowledge, they all focused on short‐term effects and none of them restricted their sample to first‐time users. If adolescent OC use indeed increases depression risk in the long term, as the present study and two other studies suggest (Anderl et al., 2020; Skovlund et al., 2016), the (often large) subset of women who had used OCs during adolescence would already be at a heightened risk for MDD and contribute to the treatment‐unrelated variance. In consequence, researchers would be less likely to find differences between the placebo and the active treatment groups and therefore unintentionally underestimate the effects of OC use on depression risk.
There are several plausible reasons why adolescent OC use may be more likely to increase risk for depression than adult OC use. First, important emotion‐related regions of the brain, such as the amygdala, prefrontal cortex, and hippocampus, are still maturing during adolescence and may therefore be particularly sensitive to sex hormone–related changes during this period (Cahill, 2019). Neurobiological studies support this idea, as sex hormones are capable of directly influencing gene expression in the cell nuclei in the brain. Because of these sex steroid receptors, sex hormones during adolescence can not only have transient but also enduring effects (Del Río et al., 2018; Gillies & McArthur, 2010; Schmidt et al., 2000; Vigil et al., 2016; Woolley, 2007). Second, adolescence may be a critical time for women to develop susceptibility for depression, as the sex gap in the incidence of depression finds its origin during this period (Hankin et al., 1998). Finally, adolescent OC use has been linked to alterations in physiological stress responses both concurrently and prospectively (Bouma, Riese, Ormel, Verhulst, & Oldehinkel, 2009; Sharma et al., 2020). Because aberrant stress reactivity is a known risk factor for MDD (LeMoult, 2020), enduringly altered stress reactivity through adolescent OC use may be an additional potential route through which adolescent OC use may increase later vulnerability to MDD.
A major strength of our study is that SCA was used as a transparent way to investigate the relationship between adolescent OC use and later MDD risk. Furthermore, the availability of data from a large, prospective cohort study, allowing us to control for baseline differences between OC users and nonusers. Finally, this is the first prospective study in which MDD was assessed with standardized and validated assessments. Our study also had some limitations. First, we assessed the presence but not the duration of OC use. Although this ruled out recall bias, it is possible that some of the participants started and stopped using OC between two assessments and were therefore incorrectly classified as nonusers. However, if anything, this should have weakened the observed association, rather than caused spurious associations between OC use and MDD risk. Second, the specific hormonal contraceptive formulations used by women in this study was mostly unknown. Third, generalizability of results to other countries might be limited owing to differences in the acceptability of and access to contraception across societies and differences in population characteristics such as ethnicity (Higgins & Smith, 2016). For example, unlike Dutch adolescents, access to no‐cost contraception is limited in the United States (Anticonceptiemiddelen (Zvw), 2019; Committee opinion no. 615: Access to contraception, 2015; Insurance Coverage of Contraceptives, 2019). This is reflected in the user rate differences between the countries. In this Dutch study, 73.7% of the women used oral contraception during their teenage years, while 45.0% of the women included in a study from the United States did so (Anderl et al., 2020). Such differences have implications for group characteristics of women using OCs and, hence, possibly also with associated outcomes.
Observational data preclude causal inference. Hence, until data from long‐term experimental studies confirm the association, we do not suggest limiting OC use to counterbalance this risk for MDD. Access to safe and effective methods of birth control is a basic human right, and many women benefit from OC use as it improves dysmenorrhea and reproductive autonomy (de Wit et al., 2020). At the same time, MDD has a high impact on the functioning of the individual and the people around them (Wittchen et al., 2011), and therefore, awareness of the possible existence of increased odds for MDD among OC‐using adolescents is critical to the well‐being of women.
Conclusion
In this large prospective cohort study, we found evidence that women who had used OCs during adolescence were more likely to have a first MDD episode as an adult. This finding was found across a multitude of analytic choices and not primarily accounted for by (pre‐existing) differences between adolescent OC users and nonusers. This pattern of results may suggest that adolescence is a vulnerable developmental period during which OC use may increase women’s likelihood to develop MDD later in life.
Authorship
All authors contributed to the conception and design of the article, and the interpretation of the data. C.A., A.d.W., and E.G. conducted the analysis of the data. C.A. and A.d.W. drafted the article, but all authors revised the draft critically for important intellectual content, and approved the final version to be published. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supporting information
Appendix S1. Details of the covariates included in the 818 specifications.
Table S1. Details of the 818 analytic specifications—included outcomes, fixed covariates, and time‐variant/invariant covariates in all women and the subgroup of women with no history of MDD.
Table S2. Lifetime presence of psychiatric comorbidities at age 25 depending on history of oral contraceptive use during adolescence of 725 women.
Acknowledgements
This research is part of the TRacking Adolescents' Individual Lives Survey (TRAILS). Participating centers of TRAILS include the University Medical Center and University of Groningen, the University of Utrecht, the Radboud Medical Center Nijmegen, and the Parnassia group, all in the Netherlands. TRAILS has been financially supported by various grants from the Netherlands Organization for Scientific Research NWO (Medical Research Council program grant GB‐MW 940‐38‐011; ZonMW Brainpower grant 100‐001‐004; ZonMw Risk Behavior and Dependence grants 60‐60600‐97‐118; ZonMw Culture and Health grant 261‐98‐710; Social Sciences Council medium‐sized investment grants GB‐MaGW 480‐01‐006 and GB‐MaGW 480‐07‐001; Social Sciences Council project grants GB‐MaGW 452‐04‐314 and GB‐MaGW 452‐06‐004; NWO large‐sized investment grant 175.010.2003.005; NWO Longitudinal Survey and Panel Funding 481‐08‐013 and 481‐11‐001; NWO Vici 016.130.002 and 453‐16‐007/2735; NWO Gravitation 024.001.003), the Dutch Ministry of Justice (WODC), the European Science Foundation (EuroSTRESS project FP‐006), the European Research Council (ERC‐2017‐STG‐757364 and ERC‐CoG‐2015‐681466), Biobanking and Biomolecular Resources Research Infrastructure BBMRI‐NL (CP 32), the Gratama foundation, the Jan Dekker foundation, the participating universities, and Accare Centre for Child and Adolescent Psychiatry. The authors are grateful to everyone who participated in this research or worked on this project to make it possible. This work was also supported by a Feodor Lynen Research Fellowship from the Alexander von Humboldt‐Foundation awarded to C.A. (DEU 1187856 FLF‐P), and a Canadian Institutes of Health Research Project Grant (PJT‐155935) awarded to F.S.C. and C.A. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors have declared that they have no competing or potential conflicts of interest.
Conflict of interest statement: No conflicts declared.
Data availability statement
Data is available upon request (see www.trails.nl).
Key points.
Oral contraceptive (OC) use has been associated with an increased risk for depression in some, but not all, studies.
To provide insight into the impact of analytical choices on these prior findings, all theoretically justifiable models were analyzed.
The findings suggest that there is a small but robust predictive association between adolescent OC use and increased risk for first‐onset major depressive disorder in early adulthood.
If this association is true, currently available results from randomized controlled trials likely underestimate the effect of hormonal contraceptives on mood because none of these have restricted their sample to first‐time users.
We suggest users and doctors to be aware of the possible existence of increased odds for major depressive disorder among OC‐using adolescents.
References
- Anderl, C. , Li, G. , & Chen, F.S. (2020). Oral contraceptive use in adolescence predicts lasting vulnerability to depression in adulthood. Journal of Child Psychology and Psychiatry, 61, 148–156. [DOI] [PubMed] [Google Scholar]
- Anticonceptiemiddelen (Zvw) . (2019). National Health Care Institute. Available from: https://www.zorginstituutnederland.nl/Verzekerde+zorg/anticonceptiemiddelen‐zvw
- Bot, M. , Middeldorp, C.M. , de Geus, E.J.C. , Lau, H.M. , Sinke, M. , van Nieuwenhuizen, B. , … & Penninx, B.W.J.H. (2017). Validity of LIDAS (LIfetime Depression Assessment Self‐report): A self‐report online assessment of lifetime major depressive disorder. Psychological Medicine, 47, 279–289. [DOI] [PubMed] [Google Scholar]
- Botvinik‐Nezer, R. , Holzmeister, F. , Camerer, C.F. , Dreber, A. , Huber, J. , Johannesson, M. , … & Schonberg, T. (2020). Variability in the analysis of a single neuroimaging dataset by many teams. Nature, 582, 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma, E.M. , Riese, H. , Ormel, J. , Verhulst, F.C. , & Oldehinkel, A.J. (2009). Adolescents' cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology, 34, 884–893. [DOI] [PubMed] [Google Scholar]
- Burcusa, S.L. , & Iacono, W.G. (2007). Risk for recurrence in depression. Clinical Psychology Review, 27, 959–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, L. (2019). How does hormonal contraception affect the developing human adolescent brain? Current Opinion in Behavioral Sciences, 23, 131–135. [Google Scholar]
- Cheslack‐Postava, K. , Keyes, K.M. , Lowe, S.R. , & Koenen, K.C. (2015). Oral contraceptive use and psychiatric disorders in a nationally representative sample of women. Archives of Women's Mental Health, 18, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee opinion no (2015). 615: Access to contraception. Obstetrics and Gynecology, 125, 250–255. [DOI] [PubMed] [Google Scholar]
- de Winter, A.F. , Oldehinkel, A.J. , Veenstra, R. , Brunnekreef, J.A. , Verhulst, F.C. , & Ormel, J. (2005). Evaluation of non‐response bias in mental health determinants and outcomes in a large sample of pre‐adolescents. European Journal of Epidemiology, 20, 173–181. [DOI] [PubMed] [Google Scholar]
- de Wit, A.E. , Booij, S.H. , Giltay, E.J. , Joffe, H. , Schoevers, R.A. , & Oldehinkel, A.J. (2020). Association of use of oral contraceptives with depressive symptoms among adolescents and young women. Journal of American Medical Association Psychiatry, 77, 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Río, J.P. , Alliende, M.I. , Molina, N. , Serrano, F.G. , Molina, S. , & Vigil, P. (2018). Steroid hormones and their action in women's brains: The importance of hormonal balance. Front Public Health, 6, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies, G.E. , & McArthur, S. (2010). Estrogen actions in the brain and the basis for differential action in men and women: A case for sex‐specific medicines. Pharmacological Reviews, 62, 155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmacher Institute . (2019). Insurance Coverage of Contraceptives. New York: Author. [Google Scholar]
- Hankin, B.L. , Abramson, L.Y. , Moffitt, T.E. , Silva, P.A. , McGee, R. , & Angell, K.E. (1998). Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10‐year longitudinal study. Journal of Abnormal Psychology, 107, 128–140. [DOI] [PubMed] [Google Scholar]
- Haro, J.M. , Arbabzadeh‐Bouchez, S. , Brugha, T.S. , de Girolamo, G. , Guyer, M.E. , Jin, R. , … & Kessler, R.C. (2006). Concordance of the composite international diagnostic interview version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health surveys. International Journal of Methods in Psychiatric Research, 15, 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J.A. , & Smith, N.K. (2016). The sexual acceptability of contraception: Reviewing the literature and building a new concept. Journal of Sex Research, 53, 417–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R.C. , Abelson, J. , Demler, O. , Escobar, J.I. , Gibbon, M. , Guyer, M.E. , … & Zheng, H. (2004). Clinical calibration of DSM‐IV diagnoses in the World Mental Health (WMH) version of the World Health Organization (WHO) Composite International Diagnostic Interview (WMHCIDI). International Journal of Methods in Psychiatric Research, 13, 122–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult, J. (2020). From stress to depression: Bringing together cognitive and biological science. Current Directions in Psychological Science, 29, 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin, C. , Danielsson, K.G. , Bixo, M. , Moby, L. , Bengtsdotter, H. , Jawad, I. , … & Sundström Poromaa, I. (2017). Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle‐A double‐blind, placebo‐controlled randomized trial. Psychoneuroendocrinology, 76, 135–143. [DOI] [PubMed] [Google Scholar]
- McKetta, S. , & Keyes, K.M. (2019). Oral contraceptive use and depression among adolescents. Annals of Epidemiology, 29, 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel, A.J. , Rosmalen, J.G.M. , Buitelaar, J.K. , Hoek, H.W. , Ormel, J. , Raven, D. , … & Hartman, C.A. (2015). Cohort profile update: The TRacking Adolescents’ Individual Lives Survey (TRAILS). International Journal of Epidemiology, 44(1), 76–76n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orben, A. , & Przybylski, A.K. (2019). The association between adolescent well‐being and digital technology use. Nature Human Behaviour, 3, 173–182. [DOI] [PubMed] [Google Scholar]
- Schmidt, B.M. , Gerdes, D. , Feuring, M. , Falkenstein, E. , Christ, M. , & Wehling, M. (2000). Rapid, nongenomic steroid actions: A new age? Frontiers in Neuroendocrinology, 21, 57–94. [DOI] [PubMed] [Google Scholar]
- Sharma, R. , Smith, S.A. , Boukina, N. , Dordari, A. , Mistry, A. , Taylor, B.C. , … & Ismail, N. (2020). Use of the birth control pill affects stress reactivity and brain structure and function. Hormones and Behavior, 124, 104783. [DOI] [PubMed] [Google Scholar]
- Simonsohn, U. , Simmons, J.P. , & Nelson, L.D. (2020). Specification curve analysis. Nature Human Behaviour, 4, 1208–1214. [DOI] [PubMed] [Google Scholar]
- Skovlund, C.W. , Morch, L.S. , Kessing, L.V. , & Lidegaard, O. (2016). Association of hormonal contraception with depression. Journal of the American Medical Association Psychiatry, 73, 1154–1162. [DOI] [PubMed] [Google Scholar]
- Sonfield, A. , Hasstedt, K. , Kavanaugh, M. , & Anderson, R. (2013). The social and economic benefits of women’s ability to determine whether and when to have children. New York: Guttmacher Institute. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division . (2019). Contraceptive use by method 2019. Data Booklet (ST/ESA/SER.A/435). Available from: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2019_contraceptiveusebymethod_databooklet.pdf
- Vigil, P. , Del Río, J.P. , Carrera, B. , ArÁnguiz, F.C. , Rioseco, H. , & Cortés, M.E. (2016). Influence of sex steroid hormones on the adolescent brain and behavior: An update. Linacre Quarterly, 83, 308–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijen, C. , Hartman, C.A. , & Oldehinkel, A.J. (2019). Reward‐related attentional bias at age 16 predicts onset of depression during 9 years of follow‐up. Journal of the American Academy of Child Adolescent Psychiatry, 58, 329–338. [DOI] [PubMed] [Google Scholar]
- Wittchen, H.U. , Jacobi, F. , Rehm, J. , Gustavsson, A. , Svensson, M. , Jönsson, B. , … & Steinhausen, H.‐C. (2011). The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology, 21, 655–679. [DOI] [PubMed] [Google Scholar]
- Woolley, C.S. (2007). Acute effects of estrogen on neuronal physiology. Annual Review of Pharmacology and Toxicology, 47, 657–680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Details of the covariates included in the 818 specifications.
Table S1. Details of the 818 analytic specifications—included outcomes, fixed covariates, and time‐variant/invariant covariates in all women and the subgroup of women with no history of MDD.
Table S2. Lifetime presence of psychiatric comorbidities at age 25 depending on history of oral contraceptive use during adolescence of 725 women.
Data Availability Statement
Data is available upon request (see www.trails.nl).
Key points.
Oral contraceptive (OC) use has been associated with an increased risk for depression in some, but not all, studies.
To provide insight into the impact of analytical choices on these prior findings, all theoretically justifiable models were analyzed.
The findings suggest that there is a small but robust predictive association between adolescent OC use and increased risk for first‐onset major depressive disorder in early adulthood.
If this association is true, currently available results from randomized controlled trials likely underestimate the effect of hormonal contraceptives on mood because none of these have restricted their sample to first‐time users.
We suggest users and doctors to be aware of the possible existence of increased odds for major depressive disorder among OC‐using adolescents.


