Abstract
Objective
To assess whether mind–body therapies are effective for relieving cancer‐related pain in adults, since at least one‐third of adults with cancer are affected by moderate or severe pain.
Methods
We searched for all randomized or quasi‐randomized controlled trials that included adults (≥18 years) with cancer‐related pain who were treated with mind–body therapies (mindfulness, hypnosis, yoga, guided imagery, and progressive muscle relaxation) in MEDLINE, Embase, CINAHL, Cochrane Central Register of Controlled Trials (CENTRAL), Science Citation Index, Web of Science, trials registers, and reference lists. The primary outcome was pain intensity. We calculated the standardized mean differences and 95% confidence intervals (CIs) and assessed the risk of bias.
Results
We identified 40 primary studies involving a total of 3569 participants. The meta‐analysis included 24 studies (2404 participants) and showed a significant effect of −0.39 (95% CI −0.62 to −0.16) with considerable heterogeneity (I 2 = 86.3%, p < 0.001). After we excluded four “outlier” studies in sensitivity analyses, the effect size remained significant but weaker. There was a high risk of bias in all studies, for example, performance bias due to lack of participant blinding. Patients in multiple settings were included but many studies were of low quality.
Conclusions
Mind–body therapies may be effective in improving cancer pain, but the quality of the evidence is low. There is a need for further high‐quality clinical trials.
Keywords: adults, cancer, mind–body therapies, oncology, pain, Psycho‐Oncology
1. BACKGROUND
Although 25 years have passed since the publication of World Health Organization (WHO) guidelines for cancer pain relief, 1 the prevalence of pain in patients with cancer is still high. A systematic review 2 concluded that, worldwide, with little improvement since 2007, 3 over one‐third of patients (39%) have pain after curative treatment, over half of patients (55%) have pain during anticancer treatment, and two‐thirds of patients (66%) have pain with advanced, metastatic, or terminal disease; overall, more than one‐third of patients (38%) graded their pain as moderate or severe (numerical rating scale score ≥5/10). The highest prevalence of pain occurred in patients with head/neck cancer, 3 and up to 80% of patients with bone metastases have pain. 4 Moreover, the worldwide prevalence of persons living after a diagnosis of cancer (accounting for around 5% of the US population) is increasing due to improvements in early detection, oncological treatments, and extension of life expectancy. 5 The management of cancer pain, therefore, remains an important challenge in the clinical setting. 1 Although healthcare practitioners often use opioid therapies for cancer pain, these pharmacological interventions have side effects. 4 Published guidelines in conventional 6 , 7 , 8 and in integrative medicine 9 , 10 suggest that more evidence is needed about treatments for cancer‐related pain, including non‐pharmacological interventions. For example, the American Society of Clinical Oncology guidelines for the management of chronic pain in survivors of adult cancers recommend a multimodality plan of care that balances pharmacological and non‐pharmacological techniques, the latter of which include mind–body therapies such as hypnosis or mindfulness. 11 The quality of the evidence is, however, considered intermediate in these guidelines, and there is a need for more robust evidence about whether mind–body therapies could help patients with cancer pain.
In the last decades, the Western world has developed a growing interest in several mind–body therapies stemming from ancient medical systems, mainly from Asia (traditional Chinese medicine, Ayurveda, etc.). These therapies include yoga, meditation, tai ji, and their variants, as well as other techniques or schools. The practice of mind–body techniques has existed since ancient times. Mind–body therapies are not expensive and have few negative side effects. 12 Nevertheless, there is no consensus on a standard definition of mind–body techniques, and some mind–body definitions partly overlap with the definition of complementary medicine (CM): according to the National Center for Complementary and Integrative Health, 13 “mind and body practices are a large and diverse group of techniques that are administered or taught to others by a trained practitioner or teacher.” In PubMed Medical Subject Headings (MeSH), 14 mind–body therapies are “treatment methods or techniques which are based on the knowledge of mind and body interactions. These techniques can be used to reduce the feeling of tension and effect of stress, and to enhance the physiological and psychological well‐being of an individual.”
In 2006, a systematic review on the efficacy of CM for cancer pain 15 concluded that there was a paucity of multi‐institutional randomized controlled trials (RCTs) evaluating CM interventions for cancer pain with adequate power, duration, and sham control. Hypnosis, imagery, support groups, acupuncture, and healing touch seemed promising, particularly in the short term. However, none could be recommended because of the paucity of rigorous trials, which also highlighted the need for methodologically strong RCTs to assess their effectiveness. 15 More recently, several systematic reviews have explored the effects of mind–body therapies on psychological stress and well‐being, chronic pain, and health‐related quality of life among women with breast cancer, 16 , 17 , 18 without, however, a specific focus on cancer‐related pain. This review is necessary on the basis that (1) pain management guidelines call for a multimodal approach, and (2) mind–body therapies offer a different mechanism of action than analgesic pain management. The objective of the present systematic review is to assess whether mind–body therapies are effective for relieving cancer‐related pain. Pain relief applies to different situations in oncological patients; we therefore decided to focus on cancer‐related pain.
2. METHODS
This review included intervention studies that involved adults aged 18 years and over with any cancer who were treated with mind‐body therapies for cancer pain. As there is no official list of mind‐body therapies, we had to create a specific list for this review that was based on lists built by reference centers and expert advice. In order to decrease confusion in the analysis, we decided not to include therapies that are not strictly mind‐body therapies. For example, aromatherapy is on the MeSH list, 14 although it is generally considered herbal medicine.
We developed a list of the mind–body interventions included in this systematic review (Table S1) on the basis of the following three information sources: (1) “Mind‐Body Therapies” in PubMed MeSH, 14 (2) “Mind‐Body Interventions” in the Cochrane Reviews related to Complementary Medicine, 19 and (3) “Mind and Body Practices” of the National Center for Complementary and Integrative Health. 20 All therapies from the three lists were considered and submitted for evaluation to a certified mindfulness instructor with long‐time experience in mind–body therapies. His role was to make sure that the list was coherent with clinical practice. At last, it was submitted to two overseas internationally recognized academic experts in CM.
The primary or secondary outcome of included studies had to be pain or use of analgesics (if reported). We adhered to the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) 21 and indicated and justified the derogations where necessary. We published the protocol in the PROSPERO register. 22
2.1. Search strategy
The initial search was performed in May 2018 in the following databases: MEDLINE, Embase, PubMed, PsychInfo, PsycArticles, CINAHL, Cochrane Central Register of Controlled Trials (CENTRAL), Science Citation Index, Web of Science, Google Scholar, Clinicaltrials.gov, and WHO International Clinical Trials Registry Platform (update May 2020). Besides electronic searches, we manually searched reference lists to identify additional eligible studies.
2.2. Eligibility
Eligible studies were RCTs and quasi‐RCTs that evaluated the effectiveness of mind–body techniques for cancer‐related pain occurring during or after specific cancer treatment in adults aged 18 years and over. The following mind–body therapies were included as the experimental interventions: meditation, mindfulness, qigong, hypnosis, autogenic training, suggestion, guided imagery, relaxation therapy, tai ji, and yoga (Table S1). “Breathing exercises” were not included as a mind–body intervention, since this broad category includes heterogeneous techniques already included in our search, such as yoga, qigong, and tai ji. We considered the following comparison groups: waitlist control, treatment as usual, no therapy, and any other active therapy (or exercise). Participants in both groups had to have been intended to receive similar modalities of anticancer and supportive therapy. Exclusion criteria were studies that focused on pain related to a specific medical or surgical procedure (such as biopsy, bone marrow transplantation, surgery) or neuropathic pain (a side effect of chemotherapy). We chose to exclude procedural pain, which extends on a different time interval, since acute pain has to be relieved during a short duration (a few hours or 2 days at most). It is likely that the mechanisms involved in alleviating pain on 2–12 weeks differ from the mechanisms acting against acute pain for 2 days. A separate review would thus be needed for procedural pain.
Neuropathic pain was not included in the present review, as it is different in many points than nociceptive pain. Neuropathic cancer pain is associated with poorer outcomes, 23 , 24 more oncological treatments, greater analgesic requirements (including strong opioids and adjuvant analgesics), and lower performance status than nociceptive pain. 24 Patients with neuropathic pain also reported worse physical, cognitive, and social functioning. 24 Moreover, the International Association for the Study of Pain emphasizes the importance of a correct diagnosis of the pain in cancer so that tailored treatment can optimize pain outcomes. 25 For these reasons and to decrease bias in interpretation of results, we considered that treatments of neuropathic pain in cancer patients should be evaluated separately.
Full text was sought from corresponding authors, where necessary. Studies for which the full text was not available despite the support of the library of the university hospital and direct requests to the corresponding authors were excluded because the data included in the abstract were insufficient for this systematic review. Two reviewers (ND and BB) independently screened all abstracts by using Covidence software. In addition, the reviewers could include only full texts in a language accessible to them (English, French, German) or at least with Latin characters (with the help of translation software where necessary).
2.3. Data abstraction and synthesis
Two reviewers (ND and MA) independently extracted and entered data from all included studies into Cochrane extraction sheets, and then into the “Characteristics of Included Studies Table” (Table 1). Disagreements were discussed with a third reviewer (BB) until a consensus was reached.
TABLE 1.
Design and main characteristics of the 40 studies
| First author year, country | Study design | Sample size (N); population | Intervention; N | Control, N | Time point/duration of intervention | Mean age | Women (%) | Follow‐up assessments (excluding baseline) | Funding sources | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Adair 2018, USA | Parallel pilot RCT | 40; head and neck cancer survivors | Hatha yoga; 20 | WL; 20 | >3 months post–cancer treatment/8 weeks | I: 65.0 (7.4); C: 61.8 (9.2) years | I: 26.7; C: 45.0 | 4 weeks | Private (non‐profit) institutions | VHNSS and BPI: see references |
| 8 weeks | ||||||||||
| Aguado 2012, USA | Parallel RCT | 221; newly diagnosed with cancer, scheduled to receive ≥4 cycles of intravenous CT | SSMT, including PMR and GIs; 109 | UCO; 111 | During chemotherapy; baseline visit (before chemotherapy cycle 1), then follow‐up visits 1, 2, and 3 | SSMT: Mean (SD) 57.5 (11.9); UCO 56.2 (12.0) | 73 in SSMT, 86 in UCO | Follow‐up visits: | Public | N = 220 after randomization |
| V1: before CT cycle 2 | ||||||||||
| V2: before CT cycle 3 | ||||||||||
| V3: before CT cycle 4 | ||||||||||
| Anderson 2006, USA | Parallel RCT | 57; patients with chronic cancer pain taking opioid medications | 1) relaxation; 16 | WL; 14 | Excluded if receiving pain‐modifying therapy (e.g., RT) or major surgery, or blood or BMT in past 30 days/2 weeks (practice at home) | 52 years (range 30–80) | 79 | T2: 2–3 weeks | Public | Only relaxation is really a mind–body intervention |
| 2) distraction; 13 | T3: 4–5 weeks | |||||||||
| 3) positive mood; 16 | T4: 8–9 weeks after baseline | |||||||||
| Bower 2015, USA | Parallel RCT | 71; early‐stage (0–III) BC, age ≤ 50 | Mindful awareness practices; 39 | WL; 32 | Cancer treatment completed/6 weeks | Mean: I: 46.1; C: 47.7 years | 100 | Post‐intervention | Public and private | Participants recruited from an earlier study (Ventura 2013) |
| 3‐month follow‐up (3 months after intervention) | ||||||||||
| Butler 2009, USA | Parallel RCT | 125; metastatic or locally recurrent BC | Group therapy with hypnosis plus education; 63 | Education‐only; 61 | 90‐min sessions for several times a day, duration 1 year | Mean (SD) I: 52.7 (10.5) years C: 53.1 (10.8) | 100 | Every 4 months for the first year and every 6 months thereafter | Public and private | Double intervention (supportive‐expressive therapy plus hypnosis) |
| Charalambous 2016, Cyprus | Parallel RCT | 236; (a) BC (T3N1M0) or prostate cancer (clinical stage T3a, Gleason score ≥ 8), (b) receiving chemotherapy, (c) experience of fatigue, pain, nausea and vomiting, anxiety, depression | GI and PMR; 120 | UC; 116 | 4 weekly supervised and daily unsupervised sessions of GI and PMR/4 weeks | Majority of participants: In 51–60 years age group (I: 41.3% and C: 36.5%). | 50 | Post‐intervention (4 weeks) | Public and private | No follow‐up after end of intervention; inability to blind patients (risk of placebo effect) |
| Chen 2015, Taiwan | Parallel RCT | 65; BC | Relaxation with GI; 32 after 1 exclusion | UC; 33 | 7 days after chemotherapy; inclusion criterion: received cyclophosphamide, epirubicin, and 5‐fluoro‐uracil chemotherapy for the first time. Each patient received 1 h of relaxation with GI before CT and 20 min daily at home for 7 days after CT (compact disk). Duration 7 days after CT | GI: 49.3 (9.6); C: 52.3 (11.6) years | 100 | Post‐intervention (7 days) | NR | |
| Cramer 2016, Germany | Parallel RCT, bicenter | 54; non‐metastatic colorectal cancer (stages I–III) | Traditional hatha yoga intervention (90 min once weekly); patients encouraged to practice yoga at home daily; 27 | UC; after week 22, offered the same yoga classes; 27 | Between 2 and 48 months post‐surgery prior to recruitment/10 weeks | Yoga: Mean 68.70 (9.13); control 67.81 (10.37) years | I: 37; C: 41 | Post‐intervention (10 weeks) | No external funding | Single item on pain in FACT |
| At 22 weeks | ||||||||||
| De Paolis, 2019; Italy | Multicenter parallel RCT | 104 hospice patients with terminal cancer | Single individual PMR–GI sessions of 20 min; 53 | UC; 51 | All patients admitted at least 48 h previously; otherwise NR/20 min | 71.83 (SD 11.57), range 41–99 | 51.92 | 24 h following the intervention | NR | Short intervention and follow‐up |
| Dikmen 2019, Turkey | Parallel RCT, 3 intervention groups | 80 participants with uterine, ovarian, and cervical cancers (grades I–III) | Reflexology (20), PMR (20), or both (20) | NR (probably UC); 20 | Patients treated with the second or third cycle of chemotherapy/8 weeks (16 home visits) | 56.36 (10.61) | 100 | 3rd, 8th, and 12th week | NR | 740 patients randomized, but 140 allocated to 1 of 4 groups |
| Ebell 2008, Germany | RCT, crossover | 32 (61 signed informed consent); routine cancer patients in a multidisciplinary pain unit | Treatment with instructions for self‐hypnosis in addition to pharmacological treatment; 15 | Pharmacological treatment alone; 17 | 4 weeks period 1, 4 weeks period 2 | NR | NR | Post‐intervention (4 weeks) | German cancer Society | Washout impossible with hypnosis |
| (8 weeks) | ||||||||||
| Eyigor 2018, Turkey | Parallel RCT | 42; BC | Hatha yoga 2 × 1 h/week; 22 | UC; 20 | Being free of any recurrent or progressive disease, having completed surgical treatment, RT, and/or CT/10 weeks | I: 52.3 (9.5); | 100 | Post‐intervention (10 weeks) | No external funds | |
| C: 51.5 (7.3) years | After 20 weeks | |||||||||
| Huberty 2019, USA | Parallel RCT | 62 enrolled; 48 completed; myeloproliferative neoplasm patients | Online yoga; 27 | WL; 21 | NR/12 weeks | I: 58.3 (9.3); C: 55.0 (11.4) | 93.8 | Week 7, 12, and 16 | Private | Yoga participation assessed (Clicky) |
| Johannsen 2016, Denmark | Parallel RCT | 129; BC with post‐treatment pain (≥3/10 intensity or burden) | MBCT; 67 | WL; 62 | ≥3 months after surgery, completed CT and/or RT/8 weeks | I: 56.8 (10.0); C: 56.7 (8.1) years | 100 | Post‐intervention | Private | Metastatic BC excluded |
| 3 months, | ||||||||||
| 6 months | ||||||||||
| Johns 2016, USA | Parallel RCT, pilot | 71; breast (n = 60) and colorectal (n = 11) cancer survivors (stages 0–III) with persistent CRF after completing CT and/or RT | MBSR; 35 | PES groups on CRF self‐management; 36 | Excluded if received any cancer treatment (i.e. CT, RT, or surgery) < 3 months or >5 years prior to enrollment/8 weeks | I: 56.9 (9.9); C: 56.4 (12.7) | 90.1 | Post‐intervention | Public and private | |
| 6 months | ||||||||||
| Kenne Sarenmalm 2017, Sweden | Parallel 3‐arm RCT | 177; BC | 1) MBSR (8 weeks self‐instructing MBSR + instructor and weekly group sessions); 62 | Non‐MBSR; 52 | After completion of adjuvant CT and/or RT, with or without endocrine therapy/8 weeks | 57.2 (SD 10.2) | 100 | 1 or 3 months after the intervention | Public + Swedish cancer Society | 11 dropouts after randomization Follow‐ups for MBSR and active controls: 1 month after intervention; similar time points of 3 months for non‐MBSR group |
| 2) active controls (8 weeks self‐instructing MBSR program); 52 | ||||||||||
| Kubo 2019, USA | Parallel RCT | 97 patients with a diagnosis of cancer and 31 caregivers | Mobile/online‐based mindfulness; 54 patients and 17 caregivers | WL; 43 patients and 14 caregivers | Currently receiving or had received chemotherapy, targeted therapies, or immunotherapy in the prior 6 months/8 weeks | I: 59.3 (14.1); C: 56.7 (14.7) patients | I: 62.3; C: 76.7 patients | Post‐intervention | Private | Feasibility study |
| Kumar 2013, India | Parallel RCT | 147, advanced‐stage (IIb–IV) BC | Standard along with Sudarshan Kriyas and Pranayam intervention; 78 | UC; 69 | Completed RT, CT, and surgery, and now in the follow‐up period for pain management/NR | I: 46.8 (9.4); C: 48.2 (9.4) | 100 | 3 months | Public | One 18‐h workshop spread over 3 days |
| 6 months | ||||||||||
| Kwekkeboom 2018, USA | RCT | 164; patients with metastatic or recurrent solid tumor cancer | Brief cognitive‐behavioral strategies intervention: Imagery, relaxation, and distraction exercises; 85 | Attention‐control: listened to cancer education recordings; 79 | Participants receiving outpatient chemotherapy/9 weeks | I: 58.44 (9.89); C: 58.61 (9.03) | I: 72; C: 75 | 3 weeks | Public | Pain, fatigue, and sleep disturbance symptom cluster |
| 6 weeks | ||||||||||
| 9 weeks | ||||||||||
| Kwekkeboom 2012, USA | Parallel RCT, pilot | 86; advanced lung, prostate, colorectal, or gynecological cancer | 12 relaxation, imagery, or distraction exercises delivered via an MP3 player; 43 | WL; 43 | During cancer treatment/2 weeks | I: 60.44 (10.76); C: 60.14 (11.54) | 59 | Post‐intervention | Public | Pain, fatigue, and sleep disturbance symptom cluster in cancer |
| Kwekkeboom 2008, USA | Parallel RCT, pilot crossover | 40; hospitalized patients with cancer‐related pain | Received 2 trials of PMR, 2 trials of analgesic imagery, Order 1 (PMR‐Imagery), n = 24; | Two trials of a control condition; the first trial of each day was always the control trial to prevent any potential carryover effect | Excluded postoperative pain/2‐day period, with subjects receiving 1 control trial and 2 trials of PMR or imagery each day | I (completers, n = 33): M = 46.45, (16.44); C (non‐completers, n = 7): 60.57, (9.61) | 55 | Post‐intervention (2 days) | Public | Not really a control group; design; randomized to the order of interventions |
| Order 2 (Imagery‐PMR), n = 16 | ||||||||||
| Lengacher 2009, USA | Parallel RCT | 84; BC (stages 0–III) | MBSR; 41 | UC; 43 | Within 18 months of treatment completion with surgery and adjuvant RT and/or CT/6 weeks (weekly 2‐h sessions) | 57.5 (SD 9.4) years | 100 | Post‐intervention | Public | |
| Lengacher 2016 a , USA | Parallel RCT | 322; BC (stages 0–III) | MBSR; 167 | UC; 155 | Post‐treatment/2‐h sessions once per week for 6 weeks | 56.6 (SD 9.7) | 100 | Post‐intervention | Public/state funds | Patients completed treatment (2 weeks to 2 years); BC stage IV excluded |
| 12 weeks | ||||||||||
| Lotzke 2016, Germany | Parallel RCT | 92; BC (stages I–III) | Yoga; 45 | Physical exercise; 47 | During (neo)adjuvant therapy/60‐min session over 12 weeks | 51.2 (SD 11.05) | 100 | 6 weeks, | No external funds | Patients undergoing cytotoxic (neo)adjuvant or endocrine adjuvant therapy |
| 25 weeks | ||||||||||
| Mendoza 2017, USA | Crossover RCT | 44; patients diagnosed with cancer (undergoing treatment or after treatment for cancer) | Valencia model of waking hypnosis with CBT; 22 | Education control; 22 | Patients under treatment or cancer survivors/4 sessions of 1 h each | 60.95 (range 29−85) | 89 | Post‐intervention and up to 3 months | Government | |
| Morishima 2019, Japan | Crossover | 56; cancer patients (breast, gastrointestinal, lung, urological, gynecological, and others) aged 40 –64 years | Laughter yoga; 26 | Routine care; 30 | During treatment/1 h every 2 weeks over 7 weeks | Median (interquartile range): 55 (48–61) versus 56 (52–62) | I: 77; C: 73 | Week 7 | Public | |
| Mozafari‐Motlagh 2019, Iran | Parallel RCT | 24; BC patients, > 6 months of diagnosis, stages II‐III | CBT integrated with mindfulness; 12 | Routine care; 12 | During treatment/8 weeks | Unspecific | 100 | Post‐intervention | None | |
| Nooner 2016, USA | Parallel RCT | 12; patients with hematologic malignancies or solid tumors | Relaxation, guided imagery, combined relaxation and guided imagery; 3 (for each group) | UC; 3 | During cancer treatment/60 days | 41 years (range = 27–63) | ≈45 | 1 month | Not reported | |
| 2 months | ||||||||||
| Oh 2008, Australia | Parallel RCT | 30; heterogeneous cancer patients | MQ; 15 | Control (UC); 15 | Unspecific/8 weeks (each session lasted 90 min) | 54 (SD 9, range 35‐75) years | 75 | NR (post‐intervention we assume) | Public university | |
| Peppone 2015, USA | Parallel RCT | 167; BC survivors receiving tamoxifen or aromatase inhibitors | Yoga; 75 | Control; 92 | BC survivors/4 weeks | Mean (standard error) 53.2 (0.86) in the control versus 55.1 (1.24) in the yoga group | 100 | During 1‐week post‐intervention | Public/state funds | No participation in yoga during the previous 3 months |
| Porter 2019, USA | Parallel RCT | 63; women with MBC | Mindful yoga; 43 | Support group; 20 | During treatment for MBC/8 weeks | 56.3 (SD 11.6) in yoga group; 59.4 (SD 11.3) in support group | 100 | Post‐intervention, and 3 and 6 months post‐intervention | Public and private | Pain is a secondary outcome; therefore, study under‐powered. The study was for feasibility and acceptability purposes |
| Rahmani 2014, Iran | Parallel RCT | 24; BC patients | Mindfulness; 12 | Control; 12 | Unspecific/8 sessions of 2 h length, thus 8‐week duration | 43.25 (SD 3.07 in the experimental group vs. 44.08 (SD 3.28) in the control group | 100 | 8 weeks (post‐intervention we assume) | Not reported | |
| Reinhardt 1999, Germany | Pilot parallel RCT | 28; patients with incurable, metastatic tumors of the pancreas, prostate, breast, and stomach with chronic pain | Relaxation therapy; 14 | No training; 14 | Incurable tumors/14 days | NR (range 36‐74 years) | 46 | Post‐intervention (14 days) | NR | |
| Song 2013, China | Parallel RCT | 100; postoperative BC patients | Relaxation techniques; 50 | Control (routine nursing care); 50 | Postoperative/respiratory frequency of 6 times/min or about 15 s each breath/duration NR | 43.6 (SD 12.7, range: 25‐70) years | 100 | NR | NR | |
| Spiegel 1983, USA | Parallel RCT | 54, primary carcinoma of the breast and documented metastases | Self‐hypnosis training; 30 | Control; 24 | 5–10 min of each self‐hypnosis exercise/duration NR | 54 (I); 55 (C) | 100 | Each 4 months for 1 year | Public/state funds | |
| Steindorf 2014, Germany | Parallel RCT | 160; BC (stages 0–III) | Relaxation; 80 | Resistance training; 80 | During adjuvant radiotherapy/60 min twice weekly for 12 weeks | 55.8 (SD 9.1) | 100 | 7 weeks (post‐RT, T1) and at week 13 (T2) | Public/state funds | The control intervention in this study is our intervention of interest |
| Teo 2020, Singapore and USA | Parallel RCT | 34 and 38; BC stage IV | CBT; 19 and 19 | WL group; 15 and 19 | During treatment/8 weeks | 60 or 55 | 100 | Post‐intervention | Public/private | Study in 2 countries |
| Vadiraja 2009; India | Parallel RCT | 88; stages I–III BC patients | Yoga; 44 | Supportive counseling; 44 | During adjuvant RT/at least 1 h 3 times/weekly for 6 weeks | Range 30–70 years | 100 | NR (post‐intervention we assume) | Public/state funds | |
| Vanderbyl 2017, Canada | Crossover RCT | 36; patients with advanced lung and gastrointestinal cancer | MQ; 11 | SET; 13 | Undergoing or eligible for chemotherapy/45‐min group sessions and 1 h every day at home/6 weeks | Mean (SD) MQ 66.1 (11.7), SET 63.7 (7.7) | ≈41.6 | NR, mean follow‐up time 27 months | Public/private Institutions | |
| Yagli 2015, Turkey | Parallel RCT | 20; elderly BC patients | Yoga group; 10 | Exercise group; 10 | During treatment/1 h weekly for 8 weeks | 65–70 years | 100 | NR (post‐intervention we assume) | NR |
Abbreviations: BC, breast cancer; BMT, bone marrow transplantation; BPI, Brief Pain Inventory; C, control group; CBT, cognitive–behavioral therapy; CRF, cancer‐related fatigue; CT, chemotherapy; FACT, Functional Assessment of Cancer Therapy; GI, guided imagery; I, intervention group; MBC, metastatic breast cancer; MBCT, mindfulness‐based cognitive therapy; MBSR, mindfulness‐based stress reduction program; MQ, medical qigong; NR, not reported; PES, psychoeducation/support; PMR, progressive muscle relaxation; RCT, randomized controlled trial; RT, radiotherapy; SD, standard deviation; SET, standard endurance and strength training; SSMT, self‐administered stress management training; UC, usual care; UCO, usual psychosocial care only; VHNSS, The Vanderbilt Head and Neck Symptom Survey; WL, waitlist.
Same study: Reich 2017.
2.4. Quality appraisal (risk of bias)
Two review authors (ND and MA) independently assessed risk of bias by using the Cochrane Risk of Bias Assessment Tool. 26 We assessed risk of bias for the following dimensions: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. We judged each field for every criterion as “low risk of bias” if requirements were adequately fulfilled, “high risk of bias” if requirements were not adequately fulfilled, or “unclear risk of bias” if data provided were insufficient for a judgment. 26 Disagreements were solved by consensus and involved a third reviewer (BB) where necessary.
2.5. Statistical analysis
We chose a two‐step approach in our study. The first step was to “evaluate the evidence” of mind–body interventions in a systematic review. The second step was to statistically synthesize the collected data in a meta‐analysis, which required more restrictive rules in terms of heterogeneity. In order to reduce the heterogeneity of the groups in the meta‐analysis, we performed it for studies with data on follow‐up measures of pain when the pain was generalized (not localized) and for studies with a follow‐up lasting at least 10 days.
Overall, 24 studies met these inclusion criteria and were included in the meta‐analysis. We included the follow‐up means and standard deviations (SD) and ignored baseline values. Since only RCTs (or quasi‐RCTs) were included, we considered baseline values to be similar in comparison groups (assuming that randomization had been efficiently performed). We pooled data from the outcomes of each study to provide an overall measure of the effect of mind–body therapies on cancer‐related pain. We expressed primary outcomes as standardized mean differences (SMDs) with 95% confidence intervals (CIs). The SMD expresses the size of the intervention effect in each study relative to the variability observed in that study. It can be used when all studies assess the same outcome but measure it in a variety of ways such as through different questionnaires. 26 We defined a negative SMD as indicating beneficial effects of the experimental intervention compared with the comparator intervention for pain. We inverted scores by subtracting the mean pain score from zero if studies reported a scale that ranged from 0 to 100, with 100 indicating “no pain at all,” or “optimal health.” 27 , 28 , 29 For crossover trials, 30 , 31 we used data only from the first period of intervention because the washout period may not be efficiently satisfied with mind–body interventions. We were unable to calculate the change scores from baseline because of missing data (namely, the SDs for changes from baseline or the corresponding correlation coefficients). We considered that imputing these parameters might be unreliable because of the variety of interventions, study participant characteristics, and outcome measurement scales in the included studies.
We analyzed data with STATA version 14.2 (StataCorp LP) and Cochrane Review Manager for risks of bias.
2.6. Dealing with missing data
In the case of missing outcome results for pain, no data were substituted. We attempted to obtain by email the missing results from trial authors or calculated the standard deviations from the 95% CIs or the standard errors (if reported). 28 , 32 , 33 , 34 , 35 , 36 We manually extracted outcome data from the published figures if the data were not available in the tables. 30 , 32 , 37 , 38
2.7. Assessment of heterogeneity
We assessed statistical heterogeneity between studies by using the chi‐square test. To avoid the interpretation of a non‐significant result of the chi‐square test as evidence of no heterogeneity, we used a significance level of 0.10, as recommended by Cochrane guidelines. 39 We also used the I 2 statistic to categorize the magnitude of heterogeneity with the following levels: I 2 = 0%–24%: low heterogeneity, I 2 = 25%–49%: moderate heterogeneity, I 2 = 50%–74%: substantial heterogeneity, and I 2 = 75%–100%: considerable heterogeneity. 40 Where heterogeneity was statistically significant, we used a random‐effects model to interpret the results. Potential sources of heterogeneity exist in the outcomes used (e.g., differences in methods of reporting pain), population (differences in cancer site and nature, or cause of pain, age, gender, etc.), and comparators used (e.g., active controls, waitlist). We analyzed all included studies to identify possible sources of heterogeneity. 41
2.8. Assessment of reporting biases
We generated funnel plots of effect estimates against their standard errors (on a reversed scale) by using Review Manager software (RevMan). We assessed the potential risk of publication bias through visual analysis of funnel plots, with approximately symmetrical funnel plots indicating low risk and asymmetrical funnel plots hinting at high risk of publication bias. 26 We also attempted to avoid publication bias by searching trial registries and conference proceedings for unpublished studies. We addressed duplicate publication bias by including studies with more than one publication only once. We addressed location bias and language bias by searching multiple databases and by including non‐English language journals.
2.9. Subgroup analysis, investigation of heterogeneity, and sensitivity analysis
We combined interventions into four main categories (mindfulness, hypnosis, yoga, and relaxation) in order to allow comparisons. We tested subgroup differences by using the chi‐square test for heterogeneity across subgroups and computed the I 2 statistic for subgroup differences as the percentage of variance between different subgroups due to genuine subgroup differences rather than to chance. 26 We performed subgroup and sensitivity analyses to explore possible reasons for the heterogeneity. For the sensitivity analysis, we performed the meta‐analysis after excluding four strongly positive studies.
3. RESULTS
3.1. Study and participant characteristics
3.1.1. Literature search yield
We identified 2437 potentially relevant records. After removal of duplicates, the final number was 1256. We then excluded 970 records on the basis of title or abstract screening. Thereafter, we searched for full texts for the remaining records and manually added potentially eligible studies from the references of retrieved full texts. We had to exclude studies without full text (n = 50, including 26 protocols, 5 narrative or editorial reviews, 4 conference abstracts, and 15 others) after attempts to find them and contacting their corresponding authors failed. Although 286 records were screened against our inclusion criteria at full‐text level, 246 of them were excluded for different reasons (see flow chart in Figure S1). Altogether, the screening process yielded 40 primary studies, with a total of 3569 participants.
3.2. Types of study designs, populations, and settings
The 40 studies were published between 1983 and 2020, including patients with early (19 studies) 28 , 30 , 31 , 38 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 or advanced cancer (13 studies). 32 , 33 , 34 , 35 , 37 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 The study by Oh et al. 65 included patients with cancer at any stage; in 7 studies, 27 , 29 , 36 , 66 , 67 , 68 , 69 the cancer stage was not reported. Ten studies had a mindfulness intervention, 28 , 29 , 42 , 47 , 48 , 49 , 51 , 53 , 59 , 62 13 studies had a yoga or assimilated 13 intervention (including laughter yoga, tai ji, and qigong), 32 , 34 , 36 , 45 , 46 , 50 , 52 , 55 , 56 , 63 , 64 , 65 , 66 4 studies had a hypnosis intervention, 30 , 33 , 57 , 67 and 13 studies a guided imagery and relaxation intervention. 27 , 31 , 35 , 37 , 38 , 43 , 44 , 54 , 58 , 60 , 61 , 68 , 69 One yoga intervention was online and home practice was monitored. 66 One study 29 included an active control group. The interventions lasted between 20 min 58 and 12 weeks 50 , 54 , 66 (or 1 year, 57 but the description was not completely clear). The number of participants ranged between 12 68 and 322. 49 The study countries were mainly the United States, but Europe, Asia, and Australia were also represented. The cancer type was most frequently breast (main focus in 23 studies) 28 , 29 , 31 , 32 , 33 , 34 , 42 , 43 , 44 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 62 , 69 and 21 studies included women only. 28 , 29 , 32 , 33 , 34 , 38 , 42 , 44 , 46 , 47 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 62 , 69 One study involved an intervention of resistance training. 54 This study included a relaxation control group, which was considered an intervention group in the present systematic review. Information on methods, participants, interventions, and outcomes is presented in Tables 1 and 2.
TABLE 2.
Outcomes and results of the 40 studies
| First author year, country | Outcome | 1° versus 2° | Outcome measures and scales | Intervention (N) | Control (n) | Statistical tests | Key conclusions of authors | Comments |
|---|---|---|---|---|---|---|---|---|
| Adair 2018, USA | Pain | 1° | 1) VHNSS–V2 General pain Score (range 0–10) | N = 15; | 20 | Preliminary efficacy data support further investigation of yoga | VHNSS and BPI: see references; no information on parallel use of pain medication; 5 missing intervention participants | |
| 1) Median (IQR) | 1) Median (IQR) | |||||||
| Baseline: 2.0 (0–4) | Baseline: 0.7 (0–4) | |||||||
| 4 weeks: 1.0 (0–3) | 4 weeks: 2.0 (0–5) | |||||||
| 8 weeks: 1.0 (0–3) | 8 weeks: 2.0 (0–6) | |||||||
| 2) BPI‐SF, including BPI pain Interference Score (range 0–10, worst) | 2) Median (IQR) | 2) Median (IQR) | ||||||
| Baseline: 0.43 (0–4) | Baseline: 0.00 (0–3) | |||||||
| 4 weeks: 0.14 (0–2) | 4 weeks: 0.57 (0–5) | |||||||
| 8 weeks: 0.00 (0–2) | 8 weeks: 0.14 (0–5) | |||||||
| Aguado 2012, USA | 1° | SF‐36 bodily pain (Medical Outcomes Study Short Form‐36), 0‐100 (100 more favorable) | 109 | 111 | Although NS, SSMT produced relatively greater improvements on bodily pain than UCO | N = 99 (I) and 102 (C) at V1; N = 97 (I) and 100 (C) at V2; N = 93 (I) and 101 (C) at V3; Check if adjusted results | ||
| Mean (SD) | Mean (SD) | |||||||
| Baseline: 64.5 (26.6) | Baseline: 63.4 (24.9) | |||||||
| V1: 72.7 (23.0) | V1: 69.3 (23.4) | |||||||
| V2: 74.9 (25.2) | V2: 72.3 (24.3) | |||||||
| V3: 74.0 (26.1) | V3: 73.1 (24.6) | |||||||
| Anderson 2006, USA | 1° | Pain intensity and interference, quality of life, mood, self‐efficacy. | Figure 3 (pain severity at T1–T4) and 4 (pain interference at T1–T4) | Pain severity | Missing SD; no measure of variance | Brief relaxation and distraction audiotape interventions produced immediate pain reductions but not longer‐term pain relief. | High dropout rate (25% before completing T2); 2 active controls | |
| The BPI asks patients to rate their pain for the last week at its “worst,” “least,” “average,” and “now” on a 0–10 (worst) scale. | Control (n = 13): | |||||||
| Stamped addressed postcards were used to record current pain intensity on a 0–10 scale. | Pain severity | T1) 6.3, T2) 5.1, T3) 5.6, T4) 6.0 | ||||||
| MDASI: brief measure of intensity of cancer symptoms | Relaxation (n = 16) | Positive mood (n = 15) | ||||||
| T1) baseline: 8.0 | T1) 7.7, T2) 7.3, T3) 7.0 T4) 7.4 | |||||||
| T2) second visit (2–3 weeks): 7.5 | Distraction (n = 13) | |||||||
| T3) 4–5 weeks: 7.3 | T1) 7.5, T2) 7.0, T3) 6.8 T4) 6.7 | |||||||
| T4) 8–9 weeks after baseline: 7.4 | Pain interference | |||||||
| Pain interference | Control (n = 13): | |||||||
| Relaxation (n = 16) | T1) 4.7, T2) 3.7, T3) 3.9, T4) 4.0 | |||||||
| T1) baseline: 5.9 | Positive mood (n = 15) | |||||||
| T2) second visit (2–3 weeks): 5.2 | T1) 5.2, T2) 4.6, T3) 4.8 T4) 4.8 | |||||||
| T3). 4–5 weeks: 5.7 | Distraction (n = 13) | |||||||
| T4) 8–9 weeks after baseline: 5.4 | T1) 5.0, T2) 4.6, T3) 5.0 T4) 5.0 | |||||||
| Bower 2015, USA | 1° | Musculoskeletal pain: BCPT Symptom Checklist (range 0–4, worst); 2° | 39 | 32 | P post‐intervention versus baseline (interaction): 0 = 0.444 | A brief mindfulness intervention may offer short‐term benefit and lead to improvements in psychological, behavioral, and biological outcomes (not pain). | Stanton 2005 on BCPT | |
| Mean (SD) | Mean (SD) | P 3 months follow‐up versus baseline (interaction): 0 = 0.881 | Post‐intervention: N = 65 | |||||
| Baseline: 1.31 (0.17) | Baseline: 1.56 (0.19) | At 3 months: total N = 59 | ||||||
| Post‐intervention: 1.27 (0.17) | Post‐intervention: 1.37 (0.19) | |||||||
| 3 months: 1.17 (0.18) | 3 months: 1.38 (0.19) | |||||||
| Butler 2009, USA | 1° | Pain level, pain rating scale (1–10, worst) | Current pain and suffering intensity at baseline: | Current pain and suffering intensity at baseline: | Effect sizes in slopes; Cohen's d: a positive effect size indicates that the group therapy condition had better results. | The experience of pain and suffering for patients with MBC can be successfully reduced with an intervention that includes hypnosis in a group therapy setting. | Data from the final assessment excluded from the slope if proximal to death (i.e., assessment closest to death and fell in the 4 [or 6]‐month window prior to death) | |
| Pain frequency: the number of days(0–7) that were affected by pain in a given week of a typical episode | Mean (SD) = 2.0 (1.5), N = 63 | Mean (SD) = 1.9 (1.4), N = 61 | ||||||
| Constant pain 0/1 (at least 6 months) | Slope = −0.002, P = 0.034, Cohen's d = 0.31 | |||||||
| Frequency of pain: | ||||||||
| Mean (SD) = 4.2 (3.2), N = 63 | ||||||||
| Slope = 0.32, P = 0.734, Cohen's d = −0.13 | Frequency of pain: | |||||||
| Constant pain: | Mean (SD) = 4.2 (3.1), N = 61 | |||||||
| Mean (SD) = 0.3 (0.5), N = 63 | Constant pain: | |||||||
| Slope = 0.06, P = 0.863, Cohen's d = −0.07 | Mean (SD) = 0.3 (0.5), N = 61 | |||||||
| Charalambous 2016, Cyprus | 104; | 104; | Chi‐square tests, independent t‐test, paired t‐test, and linear mixed models | The intervention was significantly more effective in improving pain outcomes in the intervention group compared with the control. | Linear Mixed Model of PAIN scale (1‐10) for the effect of intervention group: significant interaction intervention group × time: F = 13.55, P = 0.0003; symptom cluster | |||
| 1° | 1) Level of pain: 10‐point numeric scale (0 absence of pain and 10 worst experienced level) | 1) At baseline: | At baseline: | |||||
| Mean (SD) = 4.17 (1.47) | Mean (SD) = 3.55 (1.73) | |||||||
| Post‐intervention (N = 104): | Post‐intervention (N = 104): | |||||||
| Mean (SD) = 2.48 (1.35) | Mean (SD) = 4.80 (1.46) | |||||||
| 2° | 2) Pain in QLQ‐C30 score: 30‐item general questionnaire that assesses a wide range of functional outcomes and symptoms. Each question/item was scored on a numeric scale from 1 to 4 (1 = “not at all”; 2 = “a little”; 3 = “quite a bit”; 4 = “very much”). Pain numeric scale 0–100 (worst) | 2) At baseline: | 2) At baseline: | |||||
| Mean (SD) = 45.9 (26.1) | Mean (SD) = 44.9 (28.3) | |||||||
| Post‐intervention: | Post‐intervention: | |||||||
| Mean change = −11.3, paired t‐test p‐value < 0.0001 | Mean change = 1.0, paired t‐test p‐value = 0.0004 | |||||||
| Chen 2015, Taiwan | 2° | Pain in Symptom Distress Scale to measure the degree of patient discomfort during CT. Comprises 23 items that are rated with 5 grades: no problem to very serious (0–4). Higher score: higher number of symptoms | At baseline: | At baseline: | Chi‐square tests, Student's t tests, paired t tests, GEE analysis | 20 min of daily home relaxation with Guided imagery for 7 days has a significant effect on overall symptoms of distress, insomnia, bloating, numbness, anxiety, and depression on BC patients undergoing first‐time CT. | Beta (95% CI), GEE = 0.16 (−0.58‐0.26); SE = 0.22; pain scales (Table 2): Pretest–posttest differences | |
| Mean (SD) = 1.81 (0.78) | Mean (SD) = 1.91 (0.88) | |||||||
| Post‐intervention (N = 32): | Post‐intervention (N = 33): | |||||||
| Mean (SD) = 1.53 (0.57) | Mean (SD) = 1.79 (0.86) | |||||||
| Cramer 2016, Germany | 2° | Functional assessment of cancer Therapy, assessing colorectal cancer‐specific quality of life), including a pain scale (0–4, worst) | Mean (SD) | Mean (SD) | Raw data on pain | No effects of yoga on health‐related QoL. Given high attrition and low adherence, no definite conclusions can be drawn. | Low adherence; on average, patients attended only half of available yoga sessions and practiced only 1 h per week at home. High attrition rate. Results in e‐mail | |
| Baseline (N = 27): 0.37 (0.688) | Baseline (N = 26): 0.92 (1.262) | |||||||
| Post‐intervention (N = 20): 0.55 (0.759) | Post‐intervention (N = 22): 0.68 (1.041) | |||||||
| At 22 weeks (N = 22): 0.32 (0.568) | At 22 weeks (N = 22): 0.68 (0.945) | |||||||
| De Paolis 2019, Italy | Pain | 1° | Numerical rating scale (0–10, 10 worst); included in ESAS‐r multidimensional tool | Mean (SD) | Mean (SD) | t‐Test P < 0.0001; no group comparisons | Pain was significantly reduced both in the treated and the control group. | Very short intervention and follow‐up |
| Baseline 4.11 (2.05) | Baseline 4.51 (2.39) | |||||||
| Post‐intervention 2.28 (2.15) | Post‐intervention 3.96 (3.04) | |||||||
| Dikmen 2019, Turkey | Pain | 1° | BPI (0‐10, 10 worst) | Severity of pain | Severity of pain | t‐test or ANOVA | In the PMR alone group, pain severity decreased significantly (p < 0.05). Reflexology interventions are more effective than PMR exercises in pain management. However, the fact that the effect of pain on the daily lives of gynecological cancer patients was the lowest in the reflexology + PMR group suggested that when applied concomitantly, these interventions create a synergistic effect with better outcomes. | Results extracted from Figures 4 and 5 (manually).Higher pain severity at baseline in control group (Figure 4).No results for pain at week 12 for the control group (Figures 4 and 5) |
| Baseline: | Baseline: | |||||||
| P25–P50–P75: | P25–P50–P75: | |||||||
| 4.0–6.4–8.0 | 7.0–8.0–9.0 | |||||||
| At week 8: | At week 8: | |||||||
| 1.4–5.0–6.0 | 5.3–7.0–7.7 | |||||||
| Effect of pain on daily life: | Effect of pain on daily life: | |||||||
| Baseline: | Baseline: | |||||||
| P25–P50–P75: | P25–P50–P75: | |||||||
| 5.4–8.5–10.0 | 8.3–8.3–10.0 | |||||||
| At week 8: | At week 8: | |||||||
| 0.0–5.6–8.7 | 5.4–7.5–8.0 | |||||||
| Ebell 2008, Germany | Pain | 1° | VAS in a “pain diary” for a total of 10 weeks (0–100, 100 worst). Pain intensity and suffering from pain; self‐report on the “use of analgesics” and “number and character of self‐ hypnosis exercises” | Baseline: Mean (SD) | Baseline: Mean (SD) | NR | Statistically significant reduction of pain and suffering after the first 4 weeks for treatment A (with self‐hypnosis) in comparison to treatment B (without self‐hypnosis). | Results extracted manually from the figure |
| 73.8 (22.0) | 63.5 (16.1) | |||||||
| Post‐intervention (SD) | Post‐intervention (SD) | |||||||
| 58.1 (26.0) | 70.3 (16.1) | |||||||
| Eyigor 2018, Turkey | 1° | Shoulder pain intensity (VAS) | Shoulder: Mean (SD) | Shoulder: Mean (SD) | The delta (pre‐post treatment) does not significantly differ between the 2 groups. Shoulder p = .0.33; arm: p = 0.83 | When compared with the control group, there were no statistically significant differences between the 2 groups with respect to the parameters assessed at the end of week 10. Therefore, we could not conclude that yoga is more effective in reducing pain. (abstract: “Yoga was effective for alleviating shoulder and arm pain”). | Statistical analyses: Pre‐post at 20 weeks (no data for the control group, 18/20 missing) | |
| Arm pain intensity (VAS) | Pretreatment: 2.7 (2.7) | Pretreatment: 2.4 (3.2) | ||||||
| Range 0‐10 | 10 weeks: 1.3 (1.8) | 10 weeks: 1.1 (1.6) | ||||||
| 20 weeks: 0.6 (1.0) | Arm: | |||||||
| Arm: | Pretreatment: 2.7 (2.9) | |||||||
| Pretreatment: 2.6 (2.6) | 10 weeks: 2.0 (2.8) | |||||||
| 10 weeks: 1.4 (1.5) | ||||||||
| 20 weeks: 0.7 (1.5) | ||||||||
| Huberty 2019, USA | Pain intensity | 1° | NIH PROMIS measures included pain Intensity Short Form 3a (3‐item | Baseline: | Baseline: | Effect size (Cohen's d): difference in means between both groups divided by the pooled SD: Week 7: −0.34; week 12: −0.43; week 16: −0.51 | Small to moderate effect (0.2 small; 0.5 moderate) | |
| 45.1 (8.6) | 40.4 (9.0) | |||||||
| Change from baseline to: | Change from baseline to: | |||||||
| Week 7: −1.6 (5.8) | Week 7: 0.6 (7.5) | |||||||
| Week 12: −2.4 (7.0) | Week 12: 0.6 (6.6) | |||||||
| Week 16: −3.2 (7.3) | Week 16: 0.8 (8.4) | |||||||
| Johannsen 2016, Denmark | 1° | Pain (primary outcome): SF‐MPQ‐2, the present pain Intensity subscale (the McGill pain Questionnaire), and perceived pain intensity and pain burden (numeric rating scales). | Baseline: | Baseline: | Cohen's d time × group interaction | MBCT showed a statistically significant, robust, and durable effect on pain intensity. | SF‐MPQ‐2: 4 subscales (continuous, intermittent, neuropathic, affective); Significant time × group interactions: Use of pain medication also reported (2 items) | |
| SF‐MPQ‐2: 2.90 (1.64) | SF‐MPQ‐2: 3.31 (2.10) | |||||||
| MPQ PPI: 2.6 (0.7) | MPQ PPI: 2.9 (0.9) | |||||||
| Pain intensity: 5.5 (2.1) | Pain intensity: 5.3 (2.6) | |||||||
| Pain burden: 5.8 (1.8) | Pain burden: 6.5 (2.1) | |||||||
| Post‐intervention: | Post‐intervention: | |||||||
| SF‐MPQ‐2: 2.19 (1.35) | SF‐MPQ‐2: 3.11 (2.04) | |||||||
| MPQ PPI: 2.1 (0.9) | MPQ PPI: 2.8 (0.9) | |||||||
| Pain intensity: 4.0 (2.0) | Pain intensity: 5.3 (2.5) | |||||||
| Pain burden: 4.4 (1.8) | Pain burden: 5.7 (2.2) | |||||||
| Secondary outcomes were quality of life (world Health Organization‐5 well‐Being Index), psychological distress (the Hospital Depression and anxiety Scale), and self‐reported use of pain medication (6‐point response). | 3 months: | 3 months: | In addition, a statistically significant effect on self‐reported use of nonprescription pain medication was detected (but possible dropout bias). | |||||
| SF‐MPQ‐2: 2.16 (1.41) | SF‐MPQ‐2: 3.07 (2.03) | |||||||
| MPQ PPI: 2.0 (1.0) | MPQ PPI: 2.7 (0.6) | |||||||
| Pain intensity: 3.6 (2.1) | Pain intensity: 5.0 (2.4) | |||||||
| Pain burden: 4.1 (2.2) | Pain burden: 5.7 (2.4) | |||||||
| 6 months: | 6 months: | |||||||
| SF‐MPQ‐2: 2.29 (1.48)* | SF‐MPQ‐2: 3.18 (2.06) | |||||||
| MPQ PPI: 2.1 (0.9)* | MPQ PPI: 2.6 (0.9) | |||||||
| Pain intensity: 4.1 (1.9)* | Pain intensity: 5.1 (2.5) | |||||||
| Pain burden: 4.3 (2.4) | Pain burden: 5.8 (2.3) | |||||||
| Johns 2016, USA | 2° | Pain: PEG: 3‐item abbreviated version of BPI (range, 0–10, worst) | Baseline: Mean (SD): | Baseline: Mean (SD): | Significant group effect at post‐intervention (Cohen's d) | MBSR group reported moderate and significant reduction in pain at the end of intervention compared with PES participants. | Primary outcome: cancer‐related fatigue | |
| 3.95 (3.09) | 3.43 (2.80) | |||||||
| Post‐intervention: 2.10 (2.07)* | Post‐intervention: 2.73 (2.44) | |||||||
| At 6 months: 2.56 (3.00) | At 6 months: 2.37 (2.68) | |||||||
| Kenne Sarenmalm 2017, Sweden | 2° | SF‐36, bodily pain (0–100, optimal) | Baseline: Mean (SD) | Baseline | Change over time versus non‐MBSR group MBSR: P = 0.799; active controls: P = 0.526 | NR (improvements in depression, not in anxiety) | ||
| MBSR 65.2 (26.4) | 70 (23.1) | |||||||
| Active controls 70.9 (20.7) | Post‐intervention (at 3 months): | |||||||
| Post‐intervention (at 1 month): | 73.5 (27.1) | |||||||
| MBSR 71.4 (23.5) | ||||||||
| Active controls 74.4 (25.2) | ||||||||
| Kubo 2019, USA | Pain | 1° | PROMIS pain scales: pain intensity (0–10, 10 worst) and pain interference (8–40, 40 worst) | N = 40 patients | N = 32 patients | Intervention effect p = 0.08; effect size Cohen's d −0.427; Intervention effect p = 0.25; effect size Cohen's d −0.364 | Although the results were of borderline significance, patients in the intervention arm experienced greater improvements on the PROMIS pain base scale. | Compared with controls, participants who had practiced mindfulness at least 50% of the days showed greater improvements in PROMIS pain interference. |
| Pain intensity: | Pain intensity: | |||||||
| Baseline: 3.2 (2.4) | Baseline: 2.4 (2.2) | |||||||
| Post‐intervention 2.4 (2.1) | Post‐intervention: 2.5 (2.4) | |||||||
| Pain interference: | Pain interference: | |||||||
| Baseline: 19.2 (7.2) | Baseline: 18.1 (7.3) | |||||||
| Post‐intervention 16.8 (8.1) | Post‐intervention 18.3 (7.7) | |||||||
| Kumar 2013, India | Pain | 2° | Pain perception on 0–10 (worst) verbal scale of pain | Post‐intervention; mean (SD) | Post‐intervention; mean (SD) | NR | SK and P is an effective intervention in reducing stress and pain among advanced stage patients of BC. | Results extracted manually from the figure |
| 1.51 (0.82) | 2.67 (0.99) | |||||||
| Kwekkeboom 2018, USA | Pain, fatigue, and sleep disturbance symptom cluster | 2° | Pain, fatigue, and sleep disturbance symptom cluster, including 4 0–10 NRS ratings of pain (“now,” “worst,” “least,” and “usual” in the past week) | Week 3: Mean (95% CI) | Week 3: Mean (95% CI) | Analysis of covariance. One‐tailed tests for directional hypotheses. | Limited effects in this trial. It may provide some small therapeutic benefit for patients experiencing the cluster. | Attention control activities may mask symptom worsening. |
| 3.10 (2.52, 3.68) | 2.96 (2.38, 3.53) | |||||||
| Week 9: | Week 9: | |||||||
| 3.06 (2.37, 3.76) | 3.28 (2.53, 4.04) | |||||||
| Kwekkeboom 2012, USA | 1° but in a cluster | Symptom cluster severity and overall symptom interference with daily life. Pain severity was measured with 4 pain severity items from the BPI. Participants rated pain at its “worst,” “least,” and “average” in the last 24 h and pain “now” on a 0–10 NRS. A pain summary score was created by averaging the 4 ratings, with higher scores indicating more severe pain. | Pain severity: unadjusted: | Pain severity: unadjusted: | P < 0.01: Persons in the PC‐CB intervention group reported less pain severity at Time 2 (MAdj = 1.99, SE = 0.30) compared with those in the control group (M Adj = 3.23, SE = 0.37), F = 6.70, P = 0.006 (effect size partial η 2 = 0.093, CI η 2 > 0.021). | Significant differences in pain were observed between groups. | ||
| Baseline: Mean (SD) 1.97 (1.64) | Baseline: Mean (SD) 2.49 (1.88) | |||||||
| At 2 weeks: 1.65 (1.61) | At 2 weeks: 2.23 (1.96) | |||||||
| Kwekkeboom 2008, USA | 1° | One primary pain outcome (change in pain intensity) and 2 secondary pain outcomes (change in pain‐related distress and perceived control over pain). | PMR | Significantly greater change in pain intensity, distress, and control with PMR compared with control. Similar with analgesic imagery compared with control. No statistical tests were carried out comparing PMR to analgesic imagery. | The PMR and analgesic imagery interventions appeared to be helpful to some participants. Group means suggested that both cognitive‐behavioral strategies were significantly more effective in improving pain outcomes than the control condition. | Short‐term effects on pain (1 h), intervention of 2 days | ||
| Mean (SD): | Mean (SD): | |||||||
| Percentage change in: | Percentage change in: | |||||||
| Change in pain‐related distress. Participants were asked to rate their pain‐related distress (i.e. How distressing is your pain right now?) by using a 0 (no distress) to 10 (unbearable distress) NRS. | Pain intensity: 31 (32) | Pain intensity: 18 (27) | ||||||
| Distress: 26 (61) | Distress: 19 (38) | |||||||
| Control: 2.37 (0.90) | Control: 1.98 (0.91) | |||||||
| Perceived control over pain. The control subscale from the Survey of pain Attitudes: 5 statements about personal control over pain rated on a 5‐point scale (range 0–4) | Guided imagery: | Guided imagery: | ||||||
| Mean (SD): | Mean (SD): | |||||||
| Percentage change in: | Percentage change in: | |||||||
| Pain intensity: 31 (36) | Pain intensity: 8 (34) | |||||||
| Distress: 37 (43) | Distress: 16 (40) | |||||||
| Control: 2.51 (0.80) | Control: 2.26 (0.78) | |||||||
| Lengacher 2009, USA | 2° | QoL, using SF‐36 scales: Medical Outcomes Study Short‐Form General Health Survey: Bodily pain Scale (0‐100, more favorable) | Pain at 6 weeks: | Pain at 6 weeks: | Analysis of covariance Pearson correlation | MBSR(BC) significantly\.Iimproves QoL among BC survivors. The extent of practice influences its overall benefit. | Scores are normed to the general population (mean value of 50) (legend Table 2). | |
| Adjusted mean (95% CI) = 52.3 (50.4‐54.3), p = 0.15 | Adjusted mean (95% CI) = 50.3 (48.4‐52.2) | |||||||
| Non‐compliers (n = 12): Mean = 49.5 | ||||||||
| Compliers (n = 28): Mean = 53.5; p = 0.06 | ||||||||
| Correlation coefficient with pain: | ||||||||
| Total hours of practice: r = 0.38 (p < 0.05) | ||||||||
| Lengacher 2016, USA and Reich 2017, USA | Pain – Severity (BPI) | 1° | BPI (0–10, worst): Pain cluster with the SF‐36 pain scale and the BPI Severity Scale | 167; | 155; | Effect size (d) 95% CI; 0.02 (95% CI, −0.18 to 0.22); 0.19 (95% CI, −0.01 to 0.39) | MBSR(BC) works to improve symptom clusters, particularly for psychological and fatigue symptom clusters. | MDASI for fatigue assessment (among others) |
| Mean (SD) | Mean (SD) | |||||||
| Baseline: 11.42 (10.12) | Baseline: 9.69 (8.6) | |||||||
| Baseline: 11.30 (10.12) | Week 6: 8.28 (8.16) | |||||||
| Week 6: 9.59 (9.44) | Week 12: 8.66 (8.4) | |||||||
| Week 12: 8.46 (9.41) | ||||||||
| P*: 0.08 | ||||||||
| Pain – Interference (BPI) | Baseline: 18.04 (18.85) | Baseline: 15.13 (16.51) | 0.03 (95% CI, −0.17 to 0.24); 0.17 (95% CI, −0.04 to 0.37) | |||||
| Week 6: 14.16 (16.55) | Week 6: 12.52 (15.31) | |||||||
| Week 12: 17.89 (27.16) | Week 12: 20 (28.69) | |||||||
| P*: 0.12 | ||||||||
| Lotzke 2016, Germany | Pain | 2° | EORTC's Symptom Scales (0–100, worst) | 45; yoga | 47; physical exercise Mean (SD) t0 = −0.37 (30.46) | P = 0.721; I: p‐value (t 0 to t 1): 0.853; p‐value (t 0, t 1, t 2): 0.036; C: p‐value (t0 to t 1): 0.433; p‐value (t 0, t 1, t 2): 0.795; (I vs. C) P: t 1 = 0.684; t 2 = 0.347 | No significant improvement in most common symptoms from CT, “nausea and vomiting,” and “pain” | |
| Mean (SD) t 0 = 1.13 (29.28) | t 1 = 1.41 (33.48) | |||||||
| t 1 = 2.96 (30.41) | t 2 = −0.35 (23.17) | |||||||
| t 2 = −4.81 (20.90) | ||||||||
| Mendoza 2017, USA | Pain intensity | 1° | 0–10 (high score is apparently more pain) | 22; | 22; | Effect size (I vs. C): P = 0.038 versus 0.454; η 2 p: 0.13 versus 0.02 d = 0.38 versus −0.12 | The effect sizes for pretreatment to post‐treatment improved in the intervention compared with the control. | |
| Mean (SD): | Mean (SD): | |||||||
| Pretreatment: 52.98 (8.09) | Pretreatment: 51.91 (7.49) | |||||||
| Post‐treatment: 50.23 (6.21) | Post‐treatment: 52.50 (6.97) | |||||||
| Pain interference and pain catastrophizing | 2° | Pain Catastrophizing Scale: score of 30 means clinical level of catastrophizing; 6‐ item PROMIS pain Interference Short Form | Mean (SD): | Mean (SD): | Effect size (I vs. C): P = 0.004 versus 266; η 2 p: 0.30 versus 0.04 d = 0.65 versus 0.21 | |||
| Pretreatment: 14.13 (11.39) | Pretreatment: 11.73 (9.18) Post‐treatment: 9.81 (9.75) | |||||||
| Post‐treatment: 4.96 (6.49) | ||||||||
| Morishima 2019, Japan | Pain | 2° | QLQ‐C30 (0–100) | 26; mean (SD): | 30; mean (SD): | In week 3: −3.9 (95% CI: −16.4 to −0.5 points; P = 0.037). | Laughter yoga may improve specific domains of QoL and symptoms in cancer survivors. | |
| 15.4 (20.5) at baseline | 12.2 (19.5) at baseline | In week 7: −5.1 (95% CI: −12.9 to 2.7 points; P = 0.20). | ||||||
| Mozafari‐Motlagh 2019, Iran | Pain; pain self‐efficacy | 1° | BPI (0–10) | 12 | 12 | MS: | The intervention might be effective to reduce cancer pain. | |
| Stage: 176.33; F = 36.95 | ||||||||
| Stage × group: 200.83; F = 44.20 | ||||||||
| Error: 4.52 | ||||||||
| MS: | ||||||||
| Stage: 298.84; F = 71.77 | ||||||||
| Stage × group: 223.23; F = 58.41 | ||||||||
| Error: 2.02 | ||||||||
| Nooner 2016, USA | Pain | 1° | Eight‐item PROMIS pain Interference Short Form (upper scores indicate worse symptoms): 1 (not at all) to 5 (very much) | 3; Relaxation, guided imagery, relaxation and guided imagery: | 3; usual care: | None | The use of relaxation and guided imagery techniques are feasible interventions | Small sample size |
| No grouped result (only individual scores listed) | No grouped result | |||||||
| Oh 2008, Australia | Pain | 2° | QoL and symptom experience (fatigue, pain, and nausea and vomiting), as measured by the European Organization for Research and Treatment of cancer (EORTC QLQ‐C30, range 0–100, worst) questionnaire | 15; MQ | 15; usual care | Change Scores (Time 2 – Time 1) | Although no significant results due to small sample size, data suggest that MQ with usual medical treatment can enhance the QoL of cancer patients and reduce inflammation. | |
| Time 1: 16.7 | Time 1: 20.0 | Treatment: −4.2 (p = 0.563) | ||||||
| Time 2: 12.5 | Time 2: 23.3 | Control: 3.3 (p = 0.735) | ||||||
| Peppone 2015, USA | Pain | 1° | Symptom inventory (0–10 scale: [0 (pain not present) to 10 (worst pain ever)]; FACIT‐F–I have pain; negative values indicate improvement in symptoms (range 0–4, worst) | 75; yoga | 92; control | P: 0.094 odds ratio (95% CI) for pain improvement: 3.51 (1.17 to −10.47) | The intervention reduced general pain. | |
| FACIT‐F–I have pain: −0.18 | FACIT‐F–I have pain: 0.04 | |||||||
| % Improved | % Improved | |||||||
| FACIT‐F–pain: 57.1 | FACIT‐F–pain: 37.1 | |||||||
| Porter 2019, USA | Pain severity | 2° | BPI‐SF (0–10 scale, 10 worst): assesses worst, least, average, and interference | 43; mindful yoga | 20; support group | Difference yoga versus support (95% CI) | Little change over time; low level of symptoms at baseline; not powered to be informative about efficacy potential; small control group. | No statistical tests: Pilot feasibility study with small sample size |
| Pain severity, mean (95% CI) | Pain severity, mean (95% CI) | Post‐intervention: 0.1 (−0.8, 0.9) | ||||||
| Baseline 2.0 (1.6‐2.4) | Baseline 2.0 (1.6‐2.4) | At 3 months: 0.0 (−0.9, 0.9) | ||||||
| Post‐intervention: | Post‐intervention: | At 6 months: | ||||||
| 1.9 (1.3–2.4) | 1.8 (1.1–2.5) | −0.6 (−1.5, 0.3) | ||||||
| At 3 months: | At 3 months: | |||||||
| 2.4 (1.7, 3.0) | 2.3 (1.5, 3.2) | |||||||
| At 6 months: | At 6 months: | |||||||
| 2.1 (1.4, 2.8) | 2.7 (1.8, 3.5) | |||||||
| Rahmani 2014, Iran | Pain | 2° | Questionnaire Measuring the Global “Life Quality” in cancer patients (QLQ‐C30): 0‐100 (higher score means higher pain level) | 12; Group mindfulness | 12; control (no intervention) | Post‐test and follow‐up: P < 0.001 | The intervention is an effective method for decreasing the fatigue severity and improving global and specific life quality. | |
| Mean (SD): | Mean (SD): | |||||||
| Pre‐test: 68.05 (4.8) | Pre‐test: 75.0 (15.07) | |||||||
| Post‐test: 37.50 (10.3) | Post‐test: 73.61 (11.14) | |||||||
| Follow‐up: 50.00 (18.8) | Follow‐up: 83.33 (15.89) | |||||||
| Reinhardt 1999, Germany | 2° | Pain: assessed by using a VRS (1–6, very severe) for the pain of the previous day in the morning. | Results not interpretable | |||||
| Self‐report of the number of additional analgesic applications required per day | ||||||||
| Song 2013, China | Back pain | 2° | Rotterdam Symptom Scale: 30 items. Each item can be scored 1–5, as follows: 1, Never; 2, Occasionally; 3, Sometimes; 4, Frequently; 5, Always; Dichotomic: 1–2 versus 3–5 | 50; relaxation techniques | 50; control (routine nursing care) | χ 2 = 5.19; P = 0.023 | Progressive muscle relaxation may reduce pain. | |
| Before CT n (%): 20 (40.0) | Before CT n (%): 21 (42.0) | |||||||
| After CT n (%): 13 (26.0) | After CT n (%): 24 (48.0) | |||||||
| Spiegel 1983, USA | Pain frequency, duration, sensation, and suffering | 1° | Pain Rating Scale (0–10); more is worse. | 30; self‐hypnosis training | 24; control | df = 52; t, p: 2.5, p < 0.02; 2.17, p < 0.03; 0.05, p = NS; 1.30, p = NS | Better “pain control” in the intervention group compared with the control. | Pain sensation (F = 3.1, p < 0.05) |
| Mean (SE) | Mean (SE) | |||||||
| Sensation: 0.02 (0.26) | Sensation: 0.77 (0.17) | |||||||
| Suffering: −0.11 (0.23) | Suffering: 0.65 (0.26) | |||||||
| Frequency: 0.00 (0.11) | Frequency: 0.01 (0.17) | |||||||
| Duration: 0.20 (0.15) | Duration: 0.55 (0.23) | |||||||
| Steindorf 2014, Germany | Pain | 2° | Fatigue assessment Questionnaire (0–100 scale, worst); EORTC QLQ‐C30 | 75; relaxation (control) | 77; exercise | Adjusted mean change (95% CI); I: 3.4 (−1.6 to 8.4); C: −4.0 (−8.9 to 1.0) | The study showed that resistance exercise is safe, feasible, and efficacious in improving fatigue. | Improvement in pain in control group (exercise) |
| Mean (SD): | Mean (SD): | Adjusted between group difference (95% CI): −7.4 (−14.4 to −0.3) | ||||||
| Baseline: 30 (29) | Baseline: 29 (30) | P = 0.040 | ||||||
| Post‐intervention: 33 (32) | Post‐intervention: 26 (26) | |||||||
| Teo 2020, Singapore and USA | Pain severity | 1° | BPI (0–10 (0 = no pain, 10: worst): The 7‐item pain Disability Index (0–70) (high score: the more disability) | 44; CBT‐MV | 41; waitlist control | Mean scores change/pooled SD | The CBT‐MV protocol is likely to lead to important alleviation of symptom‐related outcomes. | |
| In USA | In USA | In USA: 0.12 | ||||||
| Mean (SD) | Mean (SD) | In Singapore: 0.05 | ||||||
| Pre: 2.38 (2.05) | Pre: 2.63 (2.25) | In USA: 0.03 | ||||||
| Post: 2.57 (2.08) | Post: 2.57 (1.79) | In Singapore: 0.21 | ||||||
| In Singapore | In Singapore | |||||||
| Pre: 1.20 (1.43) | Pre: 1.28 (2.20) | |||||||
| Post: 1.75 (1.77) | Post: 1.93 (2.18) | |||||||
| Pain disability | In USA | In USA | ||||||
| Pre: 22.07 (19.11) | Pre: 19.58 (15.55) | |||||||
| Post: 19.78 (18.82) | Post: 17.84 (11.70) | |||||||
| In Singapore | In Singapore | |||||||
| Pre: 14.43 (17.22) | Pre: 17.84 (20.29) | |||||||
| Post: 12.37 (13.97) | Post: 19.42 (17.55) | |||||||
| Vadiraja 2009, India | Pain | 2° | EORTC QLQ‐C30 (0–100 scale, worst) | 42; yoga therapy | 33; supportive counseling | ANOVA: Adjusted mean (95% CI): −18.36 (−32.39 to −4.32) | Significant reduction in fatigue, pain, insomnia, nausea, and vomiting on the EORTC QLQ symptom subscale in the yoga group compared with controls. | |
| ANOVA: | ANOVA: | Effect size: 0.14 | ||||||
| Pre: 33.74 (26.74) | Pre: 42.47 (28.49) | Physical distress: p = 0.34 | ||||||
| Post: 23.17 (27.10) | Post: 41.52 (32.57) | Psychological distress: p = 0.42 | ||||||
| RMANOVA: | RMANOVA: | Activity: p = −0.06 | ||||||
| Pre: 34.07 (27.96) | Pre: 42.04 (25.79) | |||||||
| Post: 24.44 (28.56) | Post: 41.38 (28.96) | |||||||
| Vanderbyl 2017, Canada | Pain | 2° | ESAS scale, 0–10 (worst) (e‐mail 12 Dec 2019) | 11; MQ | 13; SET | (P = 0.08): P exercise type: 0.67, P order of I: 0.03 | No improvements in anxiety, depression, or QoL. | Washout period might be insufficient (crossover RCT) |
| Mean (SD) change: | Mean (SD) change: | |||||||
| After first exercise: | After first exercise: | |||||||
| 1.0 (0.9) | −1.1 (1.9) | |||||||
| After completing both interventions (N = 19): | After completing both interventions (N = 19): | |||||||
| 0.5 (2.2) | 0.1 (2.7) | |||||||
| Yagli 2015, Turkey | Pain | 2° | The VAS [0 cm (not satisfied at all; 10 cm (very satisfied)]; Nottingham Health Profile (0‐100: low scores meant low effect of the complaint/case, whereas high scores meant high influence of the complaint/case). | 10; yoga program | 10; exercise group | P = 0.002; p = 0.008: NS p‐value for the difference between groups post‐treatment | Improvement in QoL, pain, fatigue, depression, and sleep disturbance in both yoga and exercise. | |
| Mean (SD) | Mean (SD) | |||||||
| Pretreatment (a): 7.93 (1.12) | Pre‐treatment (a): 8.30 (1.01) | |||||||
| Post‐treatment (b): | Post‐treatment (b): | |||||||
| 2.33 (0.98) | 2.16 (1.00) | |||||||
| Pretreatment (a): 63.37 (20.13) | Pre‐treatment (c): 62.97 (32.00) | |||||||
| Post‐treatment (b): | Post‐treatment (d): | |||||||
| 20.66 (14.58) | 24.51 (17.13) |
Abbreviations: 1°, primary; 2°, secondary; ANOVA, analysis of variance; BC, breast cancer; BCPT, Breast Cancer Prevention Trial; BPI, Brief Pain Inventory (4 direct measures and 7 measures on the consequences); BPI‐SF, Brief Pain Inventory‐Short Form; C, control group; CBT‐MV, cognitive behavioral therapy mindfulness and values‐guided principles; CI, confidence interval; CT, chemotherapy; d, Cohen's d; df, degrees of freedom; EORTC QLQ C30, 30‐item self‐assessment questionnaire of the European Organisation for Research and Treatment of Cancer; ESAS(‐r), Edmonton Symptom Assessment System (‐revised); GEE, generalized estimating equation; I, intervention group; IQR, interquartile range; MBC, metastatic breast cancer; MBCT, mindfulness‐based cognitive therapy; MBSR(BC), mindfulness‐based stress reduction for breast cancer program; MDASI, M; D, Anderson Symptom Inventory; MPQ PPI, McGill Pain Questionnaire Present Pain Intensity subscales; MQ, medical qigong; MS, mean squares; NR, not reported; NRS, numeric rating scale; NS, non‐significant; PC‐CB, patient‐controlled cognitive‐behavioral; PEG, pain; enjoyment, general activities; PES, psychoeducation/support; PMR, progressive muscle relaxation; QLQ‐C30, Questionnaire Measuring the Global “Life Quality” in Cancer Patients; QoL, quality of life; RMANOVA, repeated‐measures ANOVA; RCT, randomized controlled trial; SD, standard deviation; SE, standard error; SET, standard endurance and strength training; SF‐36, short‐form general health survey; SF‐MPQ‐2, Short Form McGill Pain Questionnaire 2; SSMT, self‐administered stress management training; t, student t test; UC, usual care; UCO, usual psychosocial care only; VAS, visual analog scale; VHNSS, Vanderbilt Head and Neck Symptom Survey; VRS, verbal rating scale.
*Statistically significant time × group interaction.
3.3. Characteristics of the outcome measures
Pain was the primary outcome in 20 studies (Table 2) and secondary in 17 studies, 28 , 29 , 32 , 34 , 36 , 42 , 45 , 48 , 50 , 53 , 54 , 55 , 56 , 61 , 63 , 65 , 69 often as a subscale of quality‐of‐life measures. 28 In a few studies, pain was studied in a cluster syndrome. 35 , 43 , 60 Different outcome scales were used for pain, the most frequently used being the visual analog scale or numerical pain rating scale (0–10 or 1–10) 30 , 32 , 33 , 35 , 43 , 46 , 52 , 56 , 57 , 58 , 67 and the Brief Pain Inventory (0–10). 34 , 37 , 38 , 48 , 49 , 51 , 60 , 62 , 64 One small study published no group results (only individual data) for pain. 68 One study published results that were not interpretable. 61 To our knowledge, no study reported any data on adverse effects. In addition, the study by Johannsen et al. reported outcome data on use of pain medication (as well as the study with uninterpretable results). 61
3.4. Quality of studies
The analysis of risk of bias yielded the following results (Figure 1). Among the 40 studies, 37 had a low risk of selection bias (random sequence generation), more than half had an unclear risk related to allocation concealment, 2 studies 30 , 69 had a high risk, and 16 studies had a low risk (Figure 1). All 40 studies had a high risk of performance bias due to lack of blinding of participants and personnel (which is not feasible for mind–body interventions). Almost all studies had a high risk of detection bias due to lack of blinding of outcome assessment. Almost half of the studies had a high risk of attrition bias due to incomplete outcome data. Almost all studies had an unclear risk of reporting bias due to potential selective outcome reporting, except for 4 studies with a previously published protocol or registration in a trial registry such as clinicaltrials.gov. 36 , 43 , 59 , 66 Almost all studies had an unclear risk of other bias.
FIGURE 1.
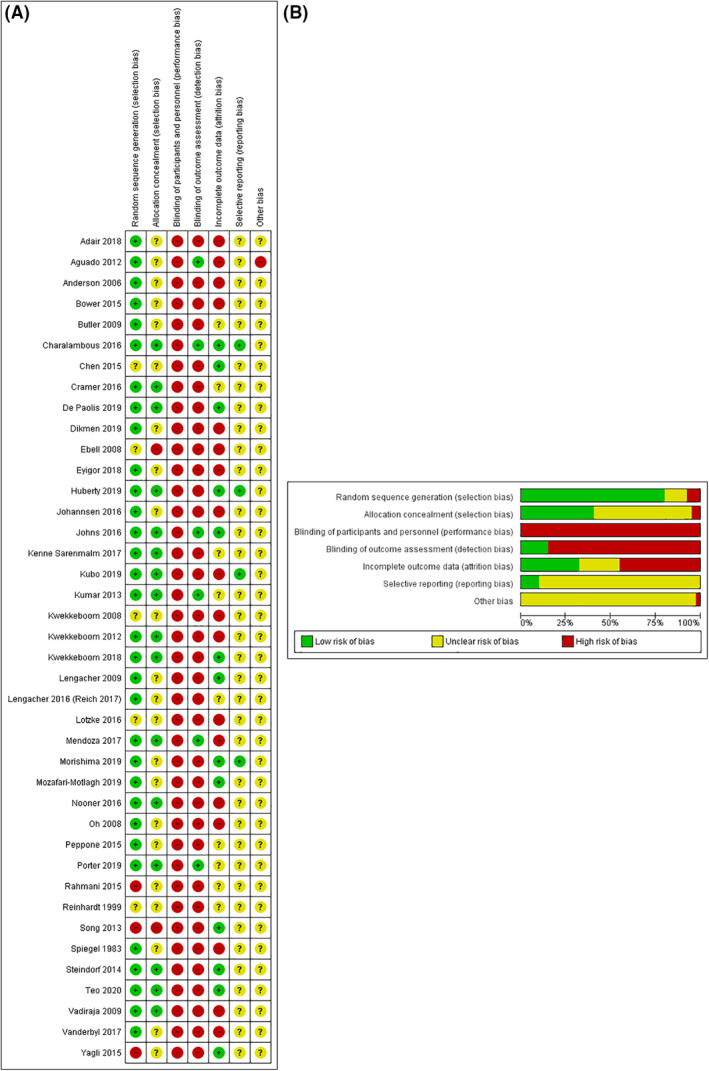
Risks of bias
3.5. Meta‐analysis
Of the 40 studies included in this systematic review, 24 were included in the meta‐analysis. The remaining 16 studies were excluded for the following reasons: mean and SD not available 37 , 51 , 65 , 68 (median and interquartile range only, 38 , 64 or effect size in slopes, 57 or mean change from baseline only 52 , 63 , 66 ), results not interpretable, 61 intervention lasting less than 10 days 31 , 44 , 58 or duration not reported, 69 and localized pain. 46 The follow‐up time was usually 8 weeks (i.e., in 9 of the 24 included studies). The meta‐analysis of the 24 studies showed a significant effect of −0.39 (95% CI −0.62 to −0.16) of mind–body therapies on cancer‐related pain (Figure 2).
FIGURE 2.
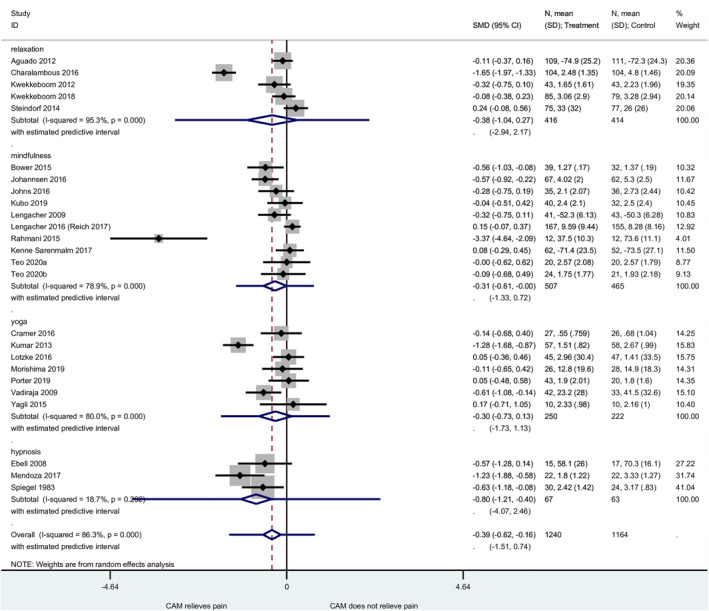
Meta‐analysis: forest plot with four main intervention categories (24 studies)
Heterogeneity was considerable (I 2 = 86.3%, p < 0.001). To explore the possible reasons for these heterogeneous results, we examined four studies with particularly positive results. 32 , 43 , 53 , 67 The study by Mendoza et al. on hypnosis 67 had several limitations and risks of bias, in particular a high risk of attrition bias (Figure 1) and little information about the randomization process and concealment of allocation. The study by Rahmani et al. on mindfulness 53 had a high risk of selection bias (random sequence generation in Figure 1) and an apparently weak quality. A small study with no sample size calculation, it was presented as a quasi‐experimental study but with randomization (yet without any description of the randomization method). The baseline values for pain were well above mid‐scale (68.1 for the intervention group and 75.0 for the control group on a scale of 100, with 100 being the worst). The study gave no information about blinding and little information about outcome assessment. 53 The protocol of the study by Charalambous et al. on guided imagery and progressive muscle relaxation (PMR) lacked detail. Besides the lack of blinding of the participants, there was no explanation for the strongly positive effect of guided imagery and PMR. 43 The study by Kumar et al. on yoga had a high risk of bias. The concealment of allocation was not clear. The article was relatively short and lacked detail, and the quality of English was poor. The baseline values for pain were not reported. We had to manually extract pain outcome results from a figure. 32
The meta‐analysis was performed after exclusion of these four positive studies, yielding a significant effect of SMD of −0.15 (95% CI −0.27 to −0.03), with moderate heterogeneity (I 2 = 41.8%, p = 0.024). The positive effect of mind–body interventions remained significant in this sensitivity analysis.
The four main intervention categories were compared in subgroup analyses. Although relaxation therapies and yoga showed non‐significant SMDs, mindfulness and hypnosis showed significant results that favored the intervention (mindfulness: SMD −0.31, 95% CI −0.61 to −0.00, with considerable heterogeneity, I 2 = 78.9%; hypnosis: SMD −0.80, 95% CI −1.21 to −0.40, with low heterogeneity, I 2 = 18.7%). Although the effect of mindfulness was just beyond statistical significance after exclusion of the highly positive study 53 (SMD −0.17, 95% CI −0.38 to 0.04), the effect of hypnosis remained statistically significant after this exclusion 67 (SMD −0.61, 95% CI −1.04 to −0.17). In the funnel plot (Figure S2), apart from the four outlier studies, 32 , 43 , 53 , 67 there was visual symmetry and no evidence of publication bias.
3.6. Studies not included in the meta‐analysis
Among the 16 studies of the systematic review that could not be included in the meta‐analysis, 1 study was about mindfulness, 51 1 was about hypnosis, 57 6 were about yoga 46 , 52 , 64 , 66 or qigong, 63 , 65 and 8 assessed relaxation (+‐ guided imagery). 31 , 37 , 38 , 44 , 58 , 61 , 68 , 69 These studies (including pilot or feasibility studies) had the following outcome results. The study on hypnosis 57 in patients with metastatic breast cancer showed statistically significant effect sizes in slopes, a result in accordance with the three studies on hypnosis included in the meta‐analysis. The study on cognitive behavioral therapy integrated with mindfulness, 51 which included 24 participants, concluded that the intervention might be effective to reduce cancer pain. The six studies on yoga or qigong obtained mixed results (yoga: two studies had a small to moderate effect size 66 or reduced general pain, 52 one study concluded that preliminary efficacy data supported further investigation of yoga, 64 and one study could not conclude that yoga is more effective than a control condition in reducing pain). 46 One medical qigong study showed no significant results and explained it a result of the small sample size (30 participants). 65 The second qigong study (with 36 participants, but 24 were compared) reported mixed results on pain. 63 Overall, yoga studies not included in the meta‐analysis reported results in accordance with the meta‐analyzed yoga studies.
The eight remaining studies on relaxation (+‐ guided imagery) showed only weak evidence for very small results (if any): one study reported immediate pain reductions but no longer term relief, 37 and another study 44 reported non‐significant effects on pain. One study (with a short intervention and follow‐up) reported pain reductions in both comparison groups, but did no group comparison. 58 One study reported that PMR alone significantly reduced pain, but not as much as reflexology did, and reported a probable synergistic effect of PMR and reflexology on pain. 38 One study (with a short intervention and follow‐up) reported that PMR and analgesic imagery interventions appeared to be helpful (and more effective than the control condition). 31 One pilot study concluded that relaxation and guided imagery techniques are feasible, but gave no group results on pain. 68 One study concluded that PMR may reduce pain. 69 In summary, the results of the eight studies on guided imagery and PMR are in line with the five studies included in the meta‐analysis. Overall, when analyzed in each of the four intervention groups, the results of the 16 studies excluded from the meta‐analysis are in line with the 24 meta‐analyzed studies.
4. DISCUSSION
4.1. Main findings
This systematic review on the effect of mind–body interventions on cancer‐related pain identified 40 studies (3569 participants). The meta‐analysis showed a significant effect of mind–body therapies on cancer‐related pain, with considerable heterogeneity. This positive effect remained significant, although lower, in a sensitivity analysis that excluded four outlier studies. The quality of evidence is, however, low.
4.2. Study limitations
As there is no definite list of mind–body therapies, we had to create our own list in this systematic review and meta‐analysis, excluding or including some therapies, which could have had an impact on the reported results. Moreover, we may have underestimated the risk of bias stemming from the lack of blinding of study participants, since pain intensity was reported by them, whether directly or via an assessor. There is a high risk of exaggeration of the effect on pain, which could reach 34%, according to a systematic review on binary outcomes. 70 Although the encouraging results of the effect of mind–body therapies could be explained by the placebo effect because of the impossibility of blinding the participants, it is unlikely that 100% of the effect is explained by the placebo effect. It is generally regarded as impossible to blind participants and personnel in mind–body studies, since no satisfying sham or placebo procedures have been developed for these interventions. Almost all of the studies included had a high risk of detection bias due to lack of blinding of outcome assessors. In addition, in trials that use a waitlist or usual care control condition, the reported benefits could be the results of nonspecific effects.
The present meta‐analysis included follow‐up data only, for mean pain intensity. Although the trials were randomized, as can be seen in Table 2, baseline data for pain sometimes differed between comparison groups (control and intervention). In addition, the largest study included in the meta‐analysis 49 (322 participants) reported baseline pain values that were above the theoretical maximum of 10 in the intervention group, which should raise awareness about quality issues. Furthermore, there were no outcome data on pain frequency or disability related to pain. 71 Our meta‐analysis included many studies with small sample sizes that were often limited by their design and the quality of their implementation and reporting. The considerable heterogeneity was not explained. A floor effect cannot be ruled out: effect sizes of therapies might be more discernible among patients with higher pain ratings at baseline.
In addition, our review bears its own limitations. We may have missed published or unpublished studies. We have indicated potential reporting errors in the studies included in our review, but we may also have misread or misinterpreted some results or explanations. Finally, assessing potential biases in studies is an exercise involving judgment.
4.3. Context
The systematic review from 2006 15 suggested that, at best, promising data existed for the ability of some CM therapies to positively affect cancer pain, the most promising being related to mind–body medicine. Although many RCTs have been conducted since then, systematic reviews that summarize the effects of CM therapies on cancer pain are scarce. A meta‐analysis on psycho‐social interventions (including relaxation and hypnosis) 72 reported medium‐size effects on cancer pain severity and interference. Although not only linked to cancer pain, another meta‐analysis 73 also showed that mind–body therapies were associated with moderate improvement of pain.
The mechanism of action of mind–body therapies probably has to do with the subjective perception of pain, which can be modulated by different factors such as temperature, circumstances, or context. 74 The positive effect of mindfulness can be explained by the use of highly standardized procedures in this intervention (especially the mindfulness‐based stress reduction [MBSR] program). MBSR is a structured program that extends for 8 weeks, requiring daily meditation practice at home of about 45 min. 75 To date, many studies have investigated the effects of the MBSR program, mainly for mental health outcomes such as depression, stress and anxiety, 76 quality of life, 28 , 77 , 78 and insomnia in patients with a cancer diagnosis. 79 Mindfulness diminishes affective responses to stress. 71 The changed pain perception, coupled with clinically meaningful improvements in cognitive or affective processes, suggests that MBSR participants learn a new way of processing pain. Although studies have assessed the biological effects of mind‐body therapies and MBSR, 80 the physiological mechanisms are not yet fully elucidated.
4.4. Clinical implications
Mind–body interventions could be an option proposed to patients with cancer‐related pain. Because adherence is lower with behavioral interventions than it is with pill‐taking, 81 and factors associated with better adherence to exercise therapies, such as motivation or functional limitations, have been identified among patients with cancer, 82 prescribing such therapies should be preceded by a discussion with the patient.
4.5. Implications for research
Further studies are needed to address uncertainties caused by inconsistencies in the body of evidence, deficiencies in power, and risks of bias.
5. CONCLUSIONS
Mind–body therapies may be effective in improving pain, but the quality of the evidence is low. The observed effects on pain are heterogeneous and the quality of the reporting of RCTs should be improved.
CONFLICT OF INTERESTS
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Nadia Danon, Pierre‐Yves Rodondi, and Bernard Burnand designed the systematic review. Nadia Danon, Muaamar Al‐Gobari, Pierre‐Yves Rodondi, and Bernard Burnand collected, analyzed, and interpreted the data. Nadia Danon, Muaamar Al‐Gobari, Pierre‐Yves Rodondi, and Bernard Burnand were involved in drafting the manuscript or critically revising it for important intellectual content.
Supporting information
FIGURE S1
FIGURE S2
TABLE S1
ACKNOWLEDGMENTS
This study was funded by the Pain Center and Center for Integrative and Complementary Medicine, Department of Anesthesiology, the Center for Primary Care and Public Health (Unisanté) and Cochrane Switzerland, University of Lausanne, Lausanne, Switzerland; and the Institute of Family Medicine, University of Fribourg, Fribourg, Switzerland. The funders had no role in the study conception, writing, interpretation of results, or the decision to submit the manuscript for publication.
We are grateful to Pierre Gallaz, certified mindfulness instructor (https://www.enpleineconscience.ch/a‐propos‐du‐formateur‐3/, Lausanne, Switzerland), Prof. Eran Ben‐Arye (MD, Director, Integrative Oncology Program, Lin, Carmel & Zebulun Medical Centers, Clalit Health Services, Israel; Associate Professor, Rappaport Faculty of Medicine, Technion‐Israel Institute of Technology, Israel), and Prof. Brent Bauer (MD, Director of the Mayo Clinic Complementary and Integrative Medicine Program, Mayo Clinic, Rochester, Minnesota, USA) for helping us set up and classify the list of mind‐body therapies. We also sincerely thank Thomas Brauchli, Sylvie Godel, and Alexandre Racine, librarians from the Lausanne University Hospital, for database searches, finding of numerous full texts, and support with EndNote software.
Open Access Funding provided by Universite de Lausanne.
Danon N, Al‐Gobari M, Burnand B, Rodondi P‐Y. Are mind–body therapies effective for relieving cancer‐related pain in adults? A systematic review and meta‐analysis. Psychooncology. 2022;31(3):345‐371. 10.1002/pon.5821
Systematic review registration number: PROSPERO 2018 CRD42018102741.
Contributor Information
Nadia Danon, Email: nadiadanon@yahoo.fr.
Pierre‐Yves Rodondi, Email: Pierre-Yves.Rodondi@unifr.ch.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Bennett MI, Eisenberg E, Ahmedzai SH, et al. Standards for the management of cancer‐related pain across Europe—a position paper from the EFIC Task Force on Cancer Pain. Eur J Pain. 2019;23(4):660‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van den Beuken‐van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan‐Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta‐analysis. J Pain Symptom Manag. 2016;51(6):1070‐1090. [DOI] [PubMed] [Google Scholar]
- 3. van den Beuken‐van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437‐1449. [DOI] [PubMed] [Google Scholar]
- 4. Running A, Seright T. Integrative oncology: managing cancer pain with complementary and alternative therapies. Curr Pain Headache Rep. 2012;16(4):325‐331. [DOI] [PubMed] [Google Scholar]
- 5. Mayer DK, Nasso SF, Earp JA. Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol. 2017;18(1):e11‐e18. [DOI] [PubMed] [Google Scholar]
- 6. Schatz AA, Oliver TK, Swarm RA, et al. Bridging the gap among clinical practice guidelines for pain management in cancer and sickle cell disease. J Natl Compr Cancer Netw. 2020;18(4):392‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(Suppl 4):iv166‐iv191. [DOI] [PubMed] [Google Scholar]
- 8. Portenoy RK. Treatment of cancer pain. Lancet. 2011;377(9784):2236‐2247. [DOI] [PubMed] [Google Scholar]
- 9. Greenlee H, Balneaves LG, Carlson LE, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. J Natl Cancer Inst Monogr. 2014;2014(50):346‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greenlee H, DuPont‐Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence‐based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67(3):194‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(27):3325‐3345. [DOI] [PubMed] [Google Scholar]
- 12. Cramer H, Ward L, Saper R, Fishbein D, Dobos G, Lauche R. The safety of yoga: a systematic review and meta‐analysis of randomized controlled trials. Am J Epidemiol. 2015;182(4):281‐293. [DOI] [PubMed] [Google Scholar]
- 13. National Center for Complementary and Integrative Health . Complementary, Alternative, or Integrative Health: What's in a Name? https://www.nccih.nih.gov/health/complementary‐alternative‐or‐integrative‐health‐whats‐in‐a‐name [Google Scholar]
- 14. U. S. National Library of Medicine . Mind‐Body Therapies – MeSH. https://www.ncbi.nlm.nih.gov/mesh/?term=mind‐body+therapies [Google Scholar]
- 15. Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: a systematic review. J Clin Oncol. 2006;24(34):5457‐5464. [DOI] [PubMed] [Google Scholar]
- 16. Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well‐being: a systematic review and meta‐analysis. JAMA Intern Med. 2014;174(3):357‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta‐analysis. Ann Behav Med. 2017;51(2):199‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health‐related quality of life, mental health and cancer‐related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1:CD010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The Cochrane Collaboration . Cochrane Reviews Related to Complementary Medicine. https://cam.cochrane.org/cochrane‐reviews‐related‐complementary‐medicine [Google Scholar]
- 20. National Center for Complementary and Integrative Health . Mind and Body Practices. https://www.nccih.nih.gov/health/mind‐and‐body‐practices [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danon N, Al‐Gobari M, Burnand B, Rodondi P‐Y. Are Mind‐Body Therapies Effective for Relieving Cancer‐Related Pain in Adults? https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=102741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Beuken‐van Everdingen MHJ, van Kuijk SMJ, Janssen DJA, Joosten EAJ. Treatment of pain in cancer: towards personalised medicine. Cancers (Basel). 2018;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rayment C, Hjermstad MJ, Aass N, et al. Neuropathic cancer pain: prevalence, severity, analgesics and impact from the European Palliative Care Research Collaborative‐Computerised Symptom Assessment study. Palliat Med. 2013;27(8):714‐721. [DOI] [PubMed] [Google Scholar]
- 25. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta‐analysis. Lancet Neurol. 2015;14(2):162‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins J, Green S. In: Julian PTH, Sally G, eds. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley‐Blackwell; 2011. [Google Scholar]
- 27. Aguado Loi CX, Taylor TR, McMillan S, et al. Use and helpfulness of self‐administered stress management therapy in patients undergoing cancer chemotherapy in community clinical settings. J Psychosoc Oncol. 2012;30(1):57‐80. [DOI] [PubMed] [Google Scholar]
- 28. Lengacher CA, Johnson‐Mallard V, Post‐White J, et al. Randomized controlled trial of mindfulness‐based stress reduction (MBSR) for survivors of breast cancer. Psycho‐Oncology. 2009;18(12):1261‐1272. [DOI] [PubMed] [Google Scholar]
- 29. Kenne Sarenmalm E, Mårtensson LB, Andersson BA, Karlsson P, Bergh I. Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer Med. 2017;6(5):1108‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ebell H. The therapist as a travelling companion to the chronically ill: hypnosis and cancer related symptoms. Contemp Hypn. 2008;25(1):46‐56. [Google Scholar]
- 31. Kwekkeboom KL, Wanta B, Bumpus M. Individual difference variables and the effects of progressive muscle relaxation and analgesic imagery interventions on cancer pain. J Pain Symptom Manag. 2008;36(6):604‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kumar N, Bhatnagar S, Velpandian T, et al. Randomized controlled trial in advance stage breast cancer patients for the effectiveness on stress marker and pain through Sudarshan Kriya and Pranayam. Indian J Palliat Care. 2013;19(3):180‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spiegel D, Bloom JR. Group therapy and hypnosis reduce metastatic breast carcinoma pain. Psychosom Med. 1983;45(4):333‐339. [DOI] [PubMed] [Google Scholar]
- 34. Porter LS, Carson JW, Olsen M, et al. Feasibility of a mindful yoga program for women with metastatic breast cancer: results of a randomized pilot study. Support Care Cancer. 2019;27(11):4307‐4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwekkeboom K, Zhang Y, Campbell T, et al. Randomized controlled trial of a brief cognitive‐behavioral strategies intervention for the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. Psycho‐Oncology. 2018;27(12):2761‐2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morishima T, Miyashiro I, Inoue N, et al. Effects of laughter therapy on quality of life in patients with cancer: an open‐label, randomized controlled trial. PLoS One. 2019;14(6):e0219065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson KO, Cohen MZ, Mendoza TR, Guo H, Harle MT, Cleeland CS. Brief cognitive‐behavioral audiotape interventions for cancer‐related pain: immediate but not long‐term effectiveness. Cancer. 2006;107(1):207‐214. [DOI] [PubMed] [Google Scholar]
- 38. Dikmen HA, Terzioglu F. Effects of reflexology and progressive muscle relaxation on pain, fatigue, and quality of life during chemotherapy in gynecologic cancer patients. Pain Manag Nurs. 2019;20(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 39. Higgins JPT, Green S, eds. Identifying and measuring heterogeneity. Cochrane Handbook for Systematic Reviews of Interventions Version 510 [updated March 2011]. The Cochrane Collaboration; 2011. [Google Scholar]
- 40. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paley CA, Johnson MI, Tashani OA, Bagnall AM. Acupuncture for cancer pain in adults. Cochrane Database Syst Rev. 2015;10:CD007753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Charalambous A, Giannakopoulou M, Bozas E, Marcou Y, Kitsios P, Paikousis L. Guided imagery and progressive muscle relaxation as a cluster of symptoms management intervention in patients receiving chemotherapy: a randomized control trial. PLoS One. 2016;11(6):e0156911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen SF, Wang HH, Yang HY, Chung UL. Effect of relaxation with guided imagery on the physical and psychological symptoms of breast cancer patients undergoing chemotherapy. Iran Red Crescent Med J. 2015;17(11):e31277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cramer H, Pokhrel B, Fester C, et al. A randomized controlled bicenter trial of yoga for patients with colorectal cancer. Psycho‐Oncology. 2016;25(4):412‐420. [DOI] [PubMed] [Google Scholar]
- 46. Eyigor S, Uslu R, Apaydin S, Caramat I, Yesil H. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A randomized, controlled, single‐blind trial. Complement Ther Clin Pract. 2018;32:40‐45. [DOI] [PubMed] [Google Scholar]
- 47. Johannsen M, O'Connor M, O'Toole MS, Jensen AB, Hojris I, Zachariae R. Efficacy of mindfulness‐based cognitive therapy on late post‐treatment pain in women treated for primary breast cancer: a randomized controlled trial. J Clin Oncol. 2016;34(28):3390‐3399. [DOI] [PubMed] [Google Scholar]
- 48. Johns SA, Brown LF, Beck‐Coon K, et al. Randomized controlled pilot trial of mindfulness‐based stress reduction compared to psychoeducational support for persistently fatigued breast and colorectal cancer survivors. Support Care Cancer. 2016;24(10):4085‐4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lengacher CA, Reich RR, Paterson CL, et al. Examination of broad symptom improvement resulting from mindfulness‐based stress reduction in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2016;34(24):2827‐2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lotzke D, Wiedemann F, Rodrigues Recchia D, et al. Iyengar‐Yoga compared to exercise as a therapeutic intervention during (neo)adjuvant therapy in women with stage I‐III breast cancer: health‐related quality of life, mindfulness, spirituality, life satisfaction, and cancer‐related fatigue. Evid Based Complement Altern Med. 2016;2016:5931816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mozafari‐Motlagh M‐R, Nejat H, Tozandehjani H, Samari A‐A. Effect of cognitive behavior therapy integrated with mindfulness on perceived pain and pain self‐efficacy in patients with breast cancer. J Nurs Midwifery Sci. 2019;6(2):51‐56. [Google Scholar]
- 52. Peppone LJ, Janelsins MC, Kamen C, et al. The effect of YOCAS© yoga for musculoskeletal symptoms among breast cancer survivors on hormonal therapy. Breast Cancer Res Treat. 2015;150(3):597‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rahmani S, Talepasand S. The effect of group mindfulness‐based stress reduction program and conscious yoga on the fatigue severity and global and specific life quality in women with breast cancer. Med J Islamic Repub Iran. 2015;29:175. [PMC free article] [PubMed] [Google Scholar]
- 54. Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer‐related fatigue and quality of life. Ann Oncol. 2014;25(11):2237‐2243. [DOI] [PubMed] [Google Scholar]
- 55. Vadiraja SH, Rao MR, Nagendra RH, et al. Effects of yoga on symptom management in breast cancer patients: a randomized controlled trial. Int J Yoga. 2009;2(2):73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yagli NV, Ulger O. The effects of yoga on the quality of life and depression in elderly breast cancer patients. Complement Ther Clin Pract. 2015;21(1):7‐10. [DOI] [PubMed] [Google Scholar]
- 57. Butler LD, Koopman C, Neri E, et al. Effects of supportive‐expressive group therapy on pain in women with metastatic breast cancer. Health Psychol. 2009;28(5):579‐587. [DOI] [PubMed] [Google Scholar]
- 58. De Paolis G, Naccarato A, Cibelli F, et al. The effectiveness of progressive muscle relaxation and interactive guided imagery as a pain‐reducing intervention in advanced cancer patients: a multicentre randomised controlled non‐pharmacological trial. Complement Ther Clin Pract. 2019;34:280‐287. [DOI] [PubMed] [Google Scholar]
- 59. Kubo A, Kurtovich E, McGinnis M, et al. A randomized controlled trial of mHealth mindfulness intervention for cancer patients and informal cancer caregivers: a feasibility study within an integrated health care delivery system. Integr Cancer Ther. 2019;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kwekkeboom KL, Abbott‐Anderson K, Cherwin C, Roiland R, Serlin RC, Ward SE. Pilot randomized controlled trial of a patient‐controlled cognitive‐behavioral intervention for the pain, fatigue, and sleep disturbance symptom cluster in cancer. J Pain Symptom Manag. 2012;44(6):810‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reinhardt U. [Investigations into synchronisation of heart rate and musical rhythm in a relaxation therapy in patients with cancer pain]. Forsch Komplementarmedizin. 1999;6(3):135‐141. [DOI] [PubMed] [Google Scholar]
- 62. Teo I, Vilardaga JP, Tan YP, et al. A feasible and acceptable multicultural psychosocial intervention targeting symptom management in the context of advanced breast cancer. Psycho‐Oncology. 2020;29(2):389‐397. [DOI] [PubMed] [Google Scholar]
- 63. Vanderbyl BL, Mayer MJ, Nash C, et al. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Support Care Cancer. 2017;25(6):1749‐1758. [DOI] [PubMed] [Google Scholar]
- 64. Adair M, Murphy B, Yarlagadda S, Deng J, Dietrich MS, Ridner SH. Feasibility and preliminary efficacy of tailored yoga in survivors of head and neck cancer: a pilot study. Integr Cancer Ther. 2018.1534735417753540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oh B, Butow P, Mullan B, Clarke S. Medical qigong for cancer patients: pilot study of impact on quality of life, side effects of treatment and inflammation. Am J Chin Med. 2008;36(3):459‐472. [DOI] [PubMed] [Google Scholar]
- 66. Huberty J, Eckert R, Dueck A, et al. Online yoga in myeloproliferative neoplasm patients: results of a randomized pilot trial to inform future research. BMC Altern Med. 2019;19(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mendoza ME, Capafons A, Gralow JR, et al. Randomized controlled trial of the Valencia model of waking hypnosis plus CBT for pain, fatigue, and sleep management in patients with cancer and cancer survivors. Psycho‐Oncology. 2017;26(11):1832‐1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nooner AK, Dwyer K, DeShea L, Yeo TP. Using relaxation and guided imagery to address pain, fatigue, and sleep disturbances: a pilot study. Clin J Oncol Nurs. 2016;20(5):547‐552. [DOI] [PubMed] [Google Scholar]
- 69. Song QH, Xu RM, Zhang QH, Ma M, Zhao XP. Relaxation training during chemotherapy for breast cancer improves mental health and lessens adverse events. Int J Clin Exp Med. 2013;6(10):979‐984. [PMC free article] [PubMed] [Google Scholar]
- 70. Hrobjartsson A, Thomsen AS, Emanuelsson F, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ. 2012;344:e1119‐e1119. [DOI] [PubMed] [Google Scholar]
- 71. Wells RE, O'Connell N, Pierce CR, et al. Effectiveness of mindfulness meditation vs headache education for adults with migraine: a randomized clinical trial. JAMA Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sheinfeld Gorin S, Krebs P, Badr H, et al. Meta‐analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol. 2012;30(5):539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Garland EL, Brintz CE, Hanley AW, et al. Mind‐body therapies for opioid‐treated pain: a systematic review and meta‐analysis. JAMA Intern Med. 2020;180(1):91‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I. The importance of context: when relative relief renders pain pleasant. Pain. 2013;154(3):402‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Crane RS, Brewer J, Feldman C, et al. What defines mindfulness‐based programs? The warp and the weft. Psychol Med. 2017;47(6):990‐999. [DOI] [PubMed] [Google Scholar]
- 76. Fjorback LO, Arendt M, Ornbol E, Fink P, Walach H. Mindfulness‐based stress reduction and mindfulness‐based cognitive therapy: a systematic review of randomized controlled trials. Acta Psychiatr Scand. 2011;124(2):102‐119. [DOI] [PubMed] [Google Scholar]
- 77. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness‐based stress reduction in mood, breast‐ and endocrine‐related quality of life, and well‐being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335‐1342. [DOI] [PubMed] [Google Scholar]
- 78. Zhang Q, Zhao H, Zheng Y. Effectiveness of mindfulness‐based stress reduction (MBSR) on symptom variables and health‐related quality of life in breast cancer patients‐a systematic review and meta‐analysis. Support Care Cancer. 2019;27(3):771‐781. [DOI] [PubMed] [Google Scholar]
- 79. Kay‐Stacey M, Attarian H. Advances in the management of chronic insomnia. BMJ. 2016;354:i2123. [DOI] [PubMed] [Google Scholar]
- 80. Carlson LE, Speca M, Faris P, Patel KD. One year pre‐post intervention follow‐up of psychological, immune, endocrine and blood pressure outcomes of mindfulness‐based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21(8):1038‐1049. [DOI] [PubMed] [Google Scholar]
- 81. DiMatteo MR. Social support and patient adherence to medical treatment: a meta‐analysis. Health Psychol. 2004;23(2):207‐218. [DOI] [PubMed] [Google Scholar]
- 82. Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psycho Oncol. 2018;27(3):713‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1
FIGURE S2
TABLE S1
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


