Summary
Surgery and general anaesthesia have the potential to disturb the body’s circadian timing system, which may affect postoperative outcomes. Animal studies suggest that anaesthesia could induce diurnal phase shifts, but clinical research is scarce. We hypothesised that surgery and general anaesthesia would result in peri‐operative changes in diurnal sleep–wake patterns in patients. In this single‐centre prospective cohort study, we recruited patients aged ≥18 years scheduled for elective surgery receiving ≥30 min of general anaesthesia. The Munich Chronotype Questionnaire and Pittsburgh Sleep Quality Index were used to determine baseline chronotype, sleep characteristics and sleep quality. Peri‐operative sleeping patterns were logged. Ninety‐four patients with a mean (SD) age of 52 (17) years were included; 56 (60%) were female. The midpoint of sleep (SD) three nights before surgery was 03.33 (55 min) and showed a phase advance of 40 minutes to 02.53 (67 min) the night after surgery (p < 0.001). This correlated with the midpoint of sleep three nights before surgery and was not associated with age, sex, duration of general anaesthesia or intra‐operative dexamethasone use. Peri‐operatively, patients had lower subjective sleep quality and worse sleep efficiency. Disruption started from one night before surgery and did not normalise until 6 days after surgery. We conclude that there is a peri‐operative phase advance in midpoint of sleep, confirming our hypothesis that surgery and general anaesthesia disturb the circadian timing system. Patients had decreased subjective sleep quality, worse sleep efficiency and increased daytime fatigue.
Keywords: anaesthesia, circadian rhythm, circadian timing system, sleep–wake timing, surgery
Introduction
All mammals possess a circadian timing system that generates 24‐h rhythms in many physiological processes. The central clock is situated in the hypothalamic suprachiasmatic nucleus, and this nucleus forwards its timing signal to peripheral ‘clocks’ throughout the body via hormonal and neuronal signals [1]. The suprachiasmatic nucleus co‐ordinates 24‐h rhythms in sleep–wake activity, hormone secretion, metabolism, cognitive functioning and autonomous nervous system activity [2]. Individuals differ in chronotype, reflected by their preferred sleep–wake timing, expressed as the midpoint of sleep (i.e. time at which a person is in mid‐sleep on work‐free days), thus resulting in ‘larks’ (people with an early midpoint of sleep who generally go to bed early and wake up early) and ‘owls’ (people with a later midpoint). Further definitions of these and other terms used in this field are given in online Supporting Information Appendix S1.
Several aspects of anaesthetics have the potential to disturb the circadian timing system. The suprachiasmatic nucleus neurons contain NMDA and GABA receptors, and activation of these receptors affects clock gene expression and entrainment of the internal clock [3]. Most drugs used for general anaesthesia are either NMDA receptor antagonists or GABA agonists [3]. In animal studies, general anaesthesia induced strong diurnal (i.e. daily) phase shifts, depending on the internal time at which hypnotics are given [4]. A phase shift means that the peak and trough of a diurnal rhythm will shift to earlier or later in the day (phase advance vs. phase delay). In the case of sleep–wake rhythms, a phase advance means that the midpoint of sleep shifts to earlier in the night, and a phase delay means that the midpoint of sleep shifts to later in the night. Human observational postoperative studies showed a delay in the endogenous rhythm of plasma melatonin levels and melatonin metabolite excretion. Moreover, disturbances in the core body temperature and in the daily rhythm of serum cortisol secretion were also found [5, 6, 7]. Glucocorticoids, which are frequently administered during surgery, mimic endogenous cortisol and have strong effects on the molecular clock [8].
The potential disruption of the circadian timing system by anaesthesia is relevant, as circadian disturbances and sleep irregularity can worsen human health [9], and disturbance of the circadian timing system might negatively affect an individual’s ability to recover from surgery [10]. Knowledge about the impact of anaesthesia on the internal clock could enable ‘chronotherapy’, where the timing of medical interventions such as surgery and administration of medication are timed to match an individual patient’s endogenous rhythm. This principle is already applied in the administration of chemotherapy [11] and statins [12].
We examined the peri‐operative changes in sleep‐wake rhythm in patients undergoing elective surgery with general anaesthesia by measuring pre‐ and post‐surgery sleep timing and subjective sleep quality. We hypothesised that surgery and general anaesthesia have a phase‐shifting effect on an individual’s rhythm, whereby the effect depends on medication types used and their timing relative to the individual’s normal midpoint of sleep, resulting in a worsened subjective sleep quality after surgery.
Methods
The CLOCKS (could jet lag be caused by operations: improving circadian rhythm knowledge in surgery) study was a single‐centre prospective cohort study. The study was approved by the local medical ethics committee, and informed consent was obtained from all patients before the start of the study.
Adult patients aged ≥ 18 y and scheduled for elective surgery under general anaesthesia lasting ≥30 min were recruited during pre‐operative assessment at the outpatient clinic of the Department of Anaesthesiology, Amsterdam UMC. Patients were screened for eligibility and were analysed during July and August 2020.
To minimise bias from known contributors to disturbed peri‐operative circadian rhythm, patients with an increased risk of postoperative delirium were not eligible to participate in the study (i.e. patients undergoing cardiac surgery; those of ASA physical status 3 or 4; and patients scheduled for postoperative ICU admission). Further exclusion criteria were night‐shift work the week before or after surgery, or general anaesthesia in the month before inclusion. We determined that patients experiencing postoperative delirium, diagnosed using the Delirium Observation Screening scale, would be excluded post hoc.
In order to determine a shift in sleep–wake rhythm, a day‐to‐day sleeping log was used for tracking sleep–wake patterns during the study period. The Munich ChronoType Questionnaire (MCTQ) [13] was used to calculate baseline midpoint sleep on work‐free days and to determine chronotype, which is determined by correcting midpoint sleep on work‐free days for sleep deficit on workdays. The MCTQ contains questions about sleep duration; sleep on‐ and offset time; sleep latency; sleep inertia; alarm clock use; and proportion of patients taking naps. It is a validated questionnaire to determine chronotype and correlates with diurnal patterns in the plasma levels of melatonin and cortisol [14].
To assess subjective sleep quality, the Pittsburgh Sleep Quality Index (PSQI) [15] was used, a useful tool for studying subjective sleep quality in clinical groups [16]. The PSQI has 19 questions measuring seven domains important in impaired sleep quality: subjective sleep quality; sleep duration; sleep disturbances; sleep latency; habitual sleep efficiency; use of sleep medication; and daytime fatigue, making up a total score between 0 and 21, with higher scores indicating a lower sleep quality. The PSQI was filled in twice: 3 days before surgery and again 7 days after surgery. The first questionnaire was used to determine baseline subjective sleep quality in the month before surgery, so patients could act as their own control. The second questionnaire measured subjective sleep quality in the week after surgery. Relevant patient characteristics and peri‐operative data were also obtained.
Our primary outcome was the diurnal phase shift the night after surgery, measured by comparing the midpoint of sleep three nights before surgery (as measured by the sleeping log) with midpoint of sleep the night after surgery.
As secondary outcomes, we analysed the night‐to‐night changes from the three nights before to seven nights after surgery in midpoint of sleep and other sleep variables (i.e. sleep duration; sleep on‐ and offset times; sleep latency and inertia; alarm clock use; and proportion of patients taking naps). To account for possible confounding due to hospitalisation, sleep variables for day surgery were compared with data for inpatient surgery. To determine which factors might affect a possible phase shift the night after surgery, baseline and peri‐operative patient characteristics were correlated with possible change in the midpoint of sleep the night after surgery, Δ‐NAS denoting the deduction of the midpoint of sleep the night after surgery from the midpoint of sleep three nights before surgery. Pre‐operative and 1 week postoperative subjective sleep quality, as measured by the PSQI questionnaire, were compared to assess how surgery and general anaesthesia affect the quality of sleep.
One study, observing changes in circadian rhythm in 35 stroke patients, showed a mean (SD) midpoint sleep on work‐free days corrected for sleep deficit on workdays of 03.00 (48 min) before and 03.18 (48 min) after stroke [17]. To be able to detect a shift of 18 min with the reported SD of 48 min, we needed a sample size of at least 58 patients to obtain this effect size (Cohen’s d) at a significance level of 0.05 (ß = 0.8).
Baseline characteristics such as age, ASA physical status [18], surgical risk stratification [19] and peri‐operative complications, were assessed and compared for all included patients, including those lost to follow‐up. Normality was assessed using the Shapiro–Wilk test. When data were normally distributed, a paired t‐test was performed, and when data were non‐normally distributed, a Mann–Whitney U‐test was used for comparison.
Phase shift on the first night after surgery vs. midpoint of sleep three nights before surgery was analysed using a paired t‐test. Linear regression was used to analyse the effects of midpoint of sleep three nights before surgery on the diurnal phase shift the first night after surgery. Multivariable regression was used to determine whether any of the potential predictors were significant. Sleeping log data (sleep duration; sleep on‐ and offset times; sleep latency and inertia; alarm clock use; and proportion of patients taking naps) were compared and seen using repeated measures ANOVA for continuous dependent variables and Wilcoxon signed‐rank tests for categorical dependent variables. Spearman's correlation was used to correlate patient characteristics, peri‐operative data and circadian variables with Δ‐NAS. Pre‐ and postoperative total PSQI scores and PSQI subscales were compared using paired t‐tests to evaluate if subjective sleep quality deteriorated after surgery and general anaesthesia. Lastly, Spearman's correlation was used to examine if increased Δ‐NAS resulted in a lower subjective sleep quality after surgery.
Results
We included patients over a period of 2 months (July and August 2020). A total of 319 patients were eligible for inclusion, of whom 238 provided informed consent. One hundred participants filled in both sets of questionnaires. After exclusion, 94 participants were analysed (Fig. 1). Patient characteristics of those who were not studied are presented and compared with the included participants in online Supporting Information Appendix S2. The mean age (SD) of included participants was 52 (17) y and 56 (60%) were female. Type of surgery, anaesthetic drugs used and duration of general anaesthesia are listed in Table 1. Surgical characteristics and length of hospital stay are listed in online Supporting Information Appendix S3.
Figure 1.
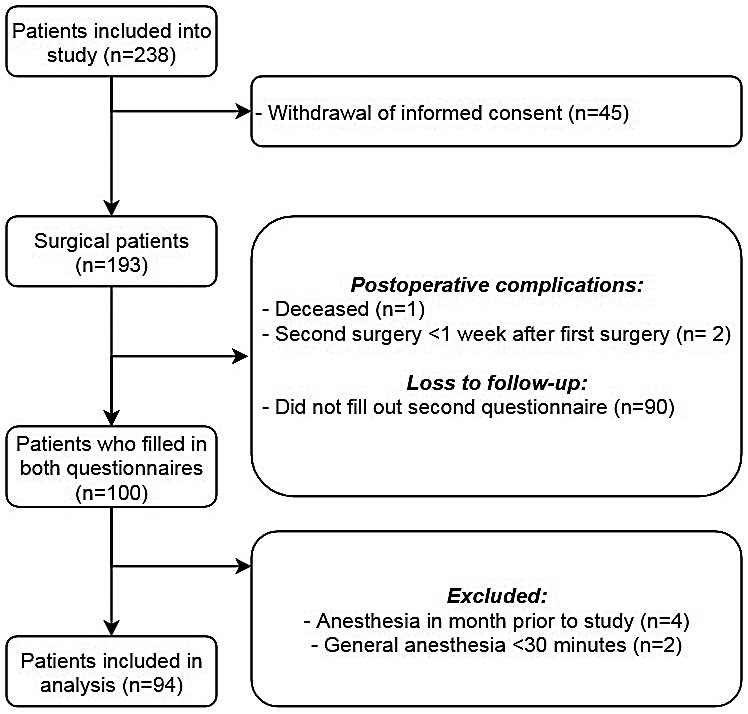
Flow chart denoting recruitment of patients into study.
Table 1.
Participant characteristics (n = 94). Values are mean (SD), number (proportion) or median (IQR [range]).
| Age, y | 52 (16.9) |
| Female sex | 56 (59.6%) |
| ASA physical status | |
| 1 | 33 (35.1%) |
| 2 | 61 (64.9%) |
| Surgical risk stratification | |
| Minor | 49 (52.1%) |
| Moderate | 43 (45.7%) |
| Major | 2 (2.1%) |
| Anaesthetic induction agent | |
| Propofol | 92 (97.8%) |
| Sevoflurane | 1 (1.1%) |
| Thiopentone | 1 (1.1%) |
| Anaesthetic maintenance agent | |
| Propofol | 85 (90.4%) |
| Sevoflurane | 7 (7.4%) |
| Propofol+sevoflurane | 2 (2.1%) |
| Duration of general anaesthesia | |
| 30 min–1 h | 12 (12.8%) |
| 1–2 h | 40 (42.5%) |
| 2–4 h | 27 (28.7%) |
| ≥ 4 h | 15 (16.0%) |
| Sleep parameter characteristics | |
| Number of workdays per week | |
| 0 workdays | 26 (27.7%) |
| 1–3 workdays | 27 (28.7%) |
| 4–6 workdays | 41 (43.6%) |
| Shift work in previous 3 months | 6 (6.4%) |
| Sleep variables, workdays | |
| Sleep onset time | 23.15 (23.00–00.00 [20.45–02.15]) |
| Sleep latency, min | 15 (10–15 [0–120]) |
| Time of awakening | 07.00 (06.11–07.45 [04.45–10.00]) |
| Sleep inertia, min | 15 (5–30 [0–165]) |
| Use of alarm clock | 45 (47.9%) |
| Total duration of sleep, h and min | 7 h 45 min (1 h 13 min) |
| Patients taking a regular nap | 15 (16.0%) |
| Total nap time, min | 45 (30–75 [15–180]) |
| Total time spent outside in daylight, min | 180 (88–300 [0–650]) |
| Sleep variables, work‐free days | |
| Sleep onset time | 23.45 (23.15–00.45 [21.15–02.30]) |
| Sleep latency, min | 15 (15–15 [0–120]) |
| Time of awakening | 08.00 (07.00–08.30 [05.00–11.30]) |
| Sleep inertia, min | 30 (15–49 [0–150]) |
| Use of alarm clock | 10 (10.6%) |
| Total duration of sleep, h and min | 8 h (2 h 4 min) |
| Patients taking a regular nap | 20 (21.3%) |
| Total nap time, min | 60 (34–90 [15–240]) |
| Total time spent outside in daylight, min | 240 (150–350 [10–720]) |
| Chronotype a , hh.mm | 03.35 (55 min) |
midpoint sleep on work‐free days corrected for sleep deficit on workdays.
The midpoint of sleep (SD) three nights before surgery was 03.33 (55 min) and showed a phase advance to 02.53 (67 min) on the night after surgery (p < 0.001). The phase advance correlated with the midpoint of sleep three nights before surgery (p < 0.001) with an R2 of 0.143 and a regression coefficient (95%CI) of 0.38 (0.20–0.67) (Fig. 2), and was not associated with age (p = 0.83), sex (p = 0.81), duration of general anaesthesia (p = 0.32) or intra‐operative dexamethasone use (p = 0.82). This means that the later a patient's midpoint of sleep normally is, the more phase advance is to be expected after surgery and general anaesthesia. In contrast, the earlier a patient's midpoint of sleep normally is, the smaller the phase advance, and in very early chronotypes surgery may induce a phase delay (Fig. 2).
Figure 2.
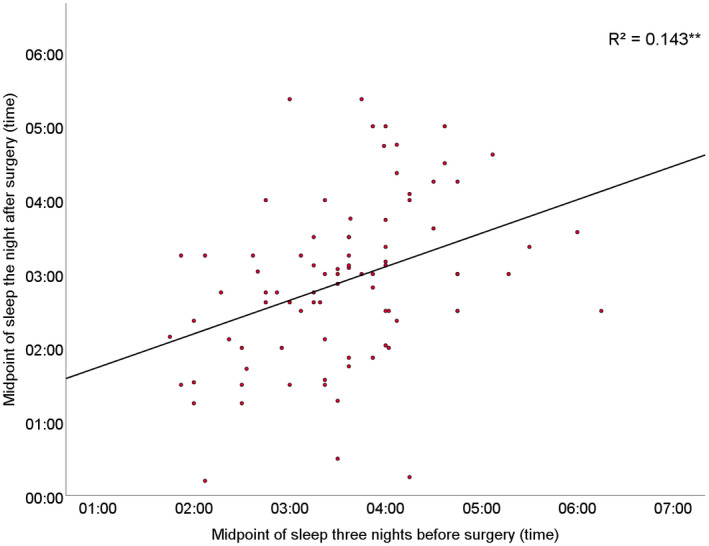
Correlation of midpoint of sleep the night after surgery with midpoint of sleep three nights before surgery. ** p ≤ 0.01.
A phase advance of sleep timing compared with three nights before surgery was observed from the night before surgery, up to one night after surgery (F (7.27, 538.18) = 12.13; p < 0.001) (Fig. 3a). Time of sleep offset and sleep duration also significantly changed from the night before surgery, which persisted until the night after surgery (Figs. 3b and 3d).
Figure 3.
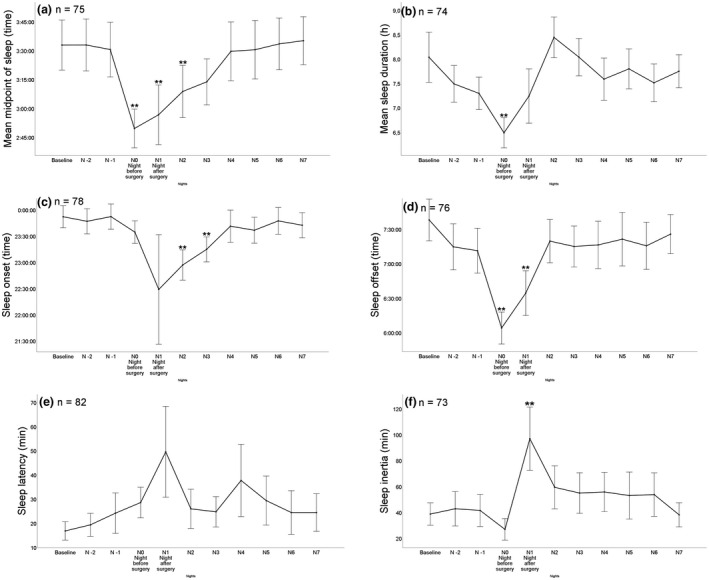
Peri‐operative sleep variables over time (baseline to seven nights after surgery). (a) Midpoint of sleep; (b) Sleep duration; (c) Sleep onset; (d) Sleep offset; (e) Sleep latency; (f) Sleep inertia; and (g) Proportion of patients using an alarm clock. Number of patients completing each item is shown in the upper left corner. Greenhouse–Geisser corrected ** p ≤ 0.01.
An increased use of an alarm clock the days preceding surgery, compared with baseline alarm clock use, was observed (Fig. 4a). Surgery with general anaesthesia elicited an increase in the proportion of participants taking naps on the first day after surgery, which was not associated with the duration of general anaesthesia (p = 0.80), age (p = 0.783), intra‐operative dexamethasone use (p = 0.557) and sex (p = 0.28), and this increase lasted until the sixth day after surgery (Fig. 4b). Time of sleep onset, sleep latency and sleep inertia also significantly changed from baseline after surgery and did not normalise until 2 days postoperatively (Figs. 3c, 3e and 3f). There were no between‐group differences in sleep variables in the nights immediately following surgery when comparing day surgery with inpatient surgery.
Figure 4.
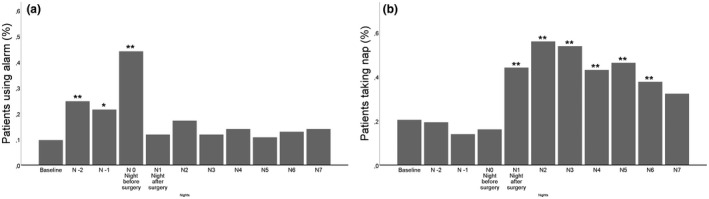
(a) Proportion of patients using an alarm clock; or (b) taking naps over time (baseline to seven nights after surgery). Number of patients completing each item is shown in the upper left corner. * p ≤ 0.05. ** p ≤ 0.01.
In participants undergoing surgery under general anaesthesia, Δ‐NAS increased when participants underwent surgery during the afternoon compared with the morning (rs = 0.29; p = 0.01) or with an increased Δ‐induction (i.e. midpoint of sleep 3 days before surgery subtracted from the time of induction of anaesthesia) (rs = 0.209; p = 0.043). Thus, the later surgery or induction took place, the larger the observed phase advance. Conversely, there was a moderate negative correlation between the Δ‐NAS and patients’ baseline chronotype (rs = −0.43; p < 0.001), meaning that later chronotypes have smaller phase shifts (see online Supporting Information Appendix S4). No significant correlations were found between Δ‐NAS and patient characteristics or peri‐operative data (see online Supporting Information Appendix S5).
Pre‐ and postoperative total PSQI scores were correlated with question 6 of the PSQI (“How would you rate your own sleep quality in the last month?”), showing that a high total score corresponds to poor subjective sleep quality (rs = 0.75; p < 0.001 and rs = 0.77; p < 0.001). Pre‐ and postoperative PSQI scores and multiple subscales were compared. Total PSQI scores (SD) rose from 6.43 (3.98) to 9.06 (4.38) (p < 0.001). Several components of the PSQI were significantly different after surgery and general anaesthesia: participants went to bed earlier; had a shorter total sleep duration; worse sleep efficiency; more sleep disturbance; increased sleep latency; more daytime fatigue; used more sleep medications; and had a lower subjective sleep quality (Table 2). Finally, the data show a more negative Δ‐NAS is associated with worse subjective sleep quality after surgery (rs = −0.326; p = 0.002).
Table 2.
Comparison between pre‐ and postoperative Pittsburgh Sleep Quality Index (PSQI) scores. Values are mean (SD) or median (IQR [range]).
| Pre‐operative | 1‐week postoperative | p value | |
|---|---|---|---|
| Total PSQI score (all seven subscales) | 6.43 (3.98) | 9.06 (4.38) | <0.001 |
| Time patient went to bed | 22.53 (54 min) | 22.40 (52 min) | 0.006 |
| Time patient got out of bed | 07.43 (1 h 32 min) | 07.58 (1 h 5 min) | 0.10 |
| Sleep duration, h and min | 7 h 20 min (1 h 21 min) | 6 h 57 min (1 h 31 min) | 0.013 |
| Time spent in bed, h and min | 9 h 13 min (2 h 26 min) | 9 h 34 min (2 h 1 min) | 0.19 |
| Percentage sleep efficiency | 82.0 (15.4) | 74.4 (17.2) | <0.001 |
| Subscale ‘Sleep Disturbance’ (range 0–3) | 1 (1–2 [0–2]) | 1 (1–2 [1–3]) | 0.007 |
| Subscale ‘Latency’ (range 0–3) | 1 (1–2 [0–3]) | 1 (0–2 [0–3]) | 0.02 |
| Subscale ‘Daytime Fatigue’ (range 0–3) | 1 (0–1 [0–3]) | 1 (1–2 [0–3]) | <0.001 |
| Use of sleep medication (range 0–3) | 0 (0–0 [0–3]) | 0 (0–2 [0–3]) | <0.001 |
| Subjective sleep quality (range 0–3) | 1 (1–1 [0–3]) | 1 (1–2 [0–3]) | <0.001 |
Discussion
In this prospective cohort study, we observed a significant peri‐operative diurnal phase advance in patients undergoing surgery and general anaesthesia. This phase advance correlated with the midpoint of sleep three nights before surgery, Δ‐induction (midpoint of sleep three days before surgery subtracted from the time of induction of anaesthesia) and time of surgery, but not with other baseline and peri‐operative patient characteristics, and was associated with lower subjective sleep quality in the week after surgery.
Interestingly, the midpoint of sleep on the night before surgery was already advanced compared with three nights before surgery. Our data suggest that the phase advance before surgery can be explained by a shorter sleep duration, earlier wake up time and alarm clock use. Other possible factors such as pre‐operative fasting and stress from the impending surgery might also play a role. Conversely, the phase advance in the nights after surgery was associated with higher sleep latency and inertia. Other postoperative sleep alterations were observed, such as increased sleep duration. The changes in sleep variables immediately after surgery were independent of whether a patient had undergone day surgery or had been hospitalised overnight.
Our results support earlier research showing that sleep and sleep–wake rhythms can be negatively affected by surgery. Patients’ pre‐operative diurnal rhythm of physical activity may be associated with an increased risk of developing postoperative delirium [20]. Other studies have described associations between surgery and reduced night‐time melatonin levels [7]; reduced sleep duration and sleep efficiency; reduced daytime physical activity; more frequent and lengthy night‐time awakenings; and increased numbers of naps during the daytime [21]. Reduced postoperative sleep quality has also been linked to worse postoperative recovery [22]. Although daytime napping in non‐surgical populations has been linked to better cognitive function [23], unintentional napping is associated with worse cognitive functioning [24]. In our study, subjective sleep quality deteriorated in the nights after surgery despite increased sleep duration. This reduced subjective sleep quality after surgery may be due to physical recovery from surgery and pain [25].
Two smaller studies have previously revealed a phase delay, rather than an advance, after surgery [5, 6]. However, these studies had very small sample sizes and did not examine sleep–wake timing, but rather assessed core body temperature and melatonin release. Nonetheless, it is possible that rather than phase advancing the whole circadian system, surgery with general anaesthesia creates internal desynchronisation. The increased proportion of patients taking naps after surgery and the deteriorated sleep efficiency at night observed in this study might be an expression of this.
Our findings suggest that peri‐operative sleep interventions to speed up postoperative recovery should not only entail improving sleep quality and duration, but should also focus on circadian realignment. However, means of preventing this peri‐operative phase shift and improving sleep quality need to be examined in clinical intervention studies.
Previous studies have found propofol to cause a phase shift dependent upon the timing of administration, with the largest phase advance observed when administered close to the end of the light period [26]. Most of our patients (98%) received propofol, therefore the observed correlation between phase shift and Δ‐induction might be explained with a time of induction closer to the end of the light period [27]. Peri‐operative stress [28] on the suprachiasmatic nucleus or exposure to bright light [13] may also contribute to diurnal phase shifts. Furthermore, dexamethasone administration has timing‐dependent circadian effects [29], and a large proportion of patients in this study (88%) received intra‐operative dexamethasone. This was not associated with a larger phase advance; however, our sample size was not calculated to detect these changes.
A limitation of our study was the considerable loss to follow‐up, as often experienced with studies using questionnaires sent via email [30]. This may have caused bias, as patients with sleeping difficulties may have been more inclined to fill in the questionnaires. A total of 45 patients withdrew their consent before the first questionnaires were completed, for the most part because patients thought it would take them too long to complete the questionnaires around the time of a surgical procedure. The ongoing COVID‐19 pandemic may also have played a role in this. It cannot be excluded that this may have caused some bias, but the group of patients who withdrew their informed consent did not differ significantly from the other patients (both inclusions and exclusions). Furthermore, only patients undergoing elective surgery with general anaesthesia were included to test the hypothesis. As such, we did not include patients undergoing elective surgery with regional anaesthesia in the cohort, and therefore cannot distinguish the role of surgery from that of general anaesthesia. Nonetheless, this is the first adequately powered study in humans to examine the role of surgery and general anaesthesia on sleep–wake timing.
Due to our sample size and the included low‐risk surgical population, we cannot draw conclusions on the relation between peri‐operative phase shifts and postoperative surgical recovery or postoperative complications. Tracking diurnal phase shifts and sleeping patterns in a control group of patients scheduled for elective surgery under regional anaesthesia may further delineate the specific effects of surgery and general anaesthesia on sleep–wake behaviour. Future research should also focus on the question of whether surgery and/or general anaesthesia cause a phase advance of the entire circadian system or a desynchronisation of the circadian rhythm by simultaneously assessing central and peripheral clock rhythms [26].
This study showed that surgery with general anaesthesia causes a significant phase shift of diurnal sleep‐wake rhythms and worsens sleep quality. Potential mechanisms include timing‐dependent effects of propofol administration or effects of peri‐operative stress on the suprachiasmatic nucleus. It is important that future studies distinguish the role of surgery in diurnal phase shifts from that of general anaesthesia. In the future, we will further investigate the role of peri‐operative phase shifts in surgical recovery and postoperative complications.
Supporting information
Appendix S1. List of abbreviations and glossary of terms.
Appendix S2. Comparison of baseline characteristic inclusions vs. all patients.
Appendix S3. Surgical characteristics and length of stay in hospital.
Appendix S4. Relationship between Δ‐midpoint sleep on night after surgery [Δ‐NAS] and circadian variables.
Appendix S5. Relationship between Δ‐NAS and patient characteristics and peri‐operative data.
Acknowledgements
The study was registered on The Netherlands Trial Register (NL8709). MH served as executive section editor of pharmacology for Anesthesia and Analgesia, section editor of anaesthesiology for the Journal of Clinical Medicine and received speaker’s fees from CSL Behring and Eurocept BV. No external funding or other competing interests declared.
References
- 1. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 2002; 418: 935–41. [DOI] [PubMed] [Google Scholar]
- 2. Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nature Reviews. Endocrinology 2019; 15: 75–89. [DOI] [PubMed] [Google Scholar]
- 3. Poulsen RC, Warman GR, Sleigh J, Ludin NM, Cheeseman JF. How does general anaesthesia affect the circadian clock? Sleep Medicine Reviews 2018; 37: 35–44. [DOI] [PubMed] [Google Scholar]
- 4. Orts‐Sebastian A, Ludin NM, Pawley MDM, Cheeseman JF, Warman GR. Impact of anaesthesia on circadian rhythms and implications for laboratory experiments. Experimental Neurology 2019; 311: 318–22. [DOI] [PubMed] [Google Scholar]
- 5. Gögenur I, Middleton B, Kristiansen VB, Skene DJ, Rosenberg J. Disturbances in melatonin and core body temperature circadian rhythms after minimal invasive surgery. Acta Anaesthesiologica Scandinavica 2007; 51: 1099–106. [DOI] [PubMed] [Google Scholar]
- 6. Gögenur I, Ocak U, Altunpinar O, Middleton B, Skene DJ, Rosenberg J. Disturbances in melatonin, cortisol and core body temperature rhythms after major surgery. World Journal of Surgery 2007; 31: 290–8. [DOI] [PubMed] [Google Scholar]
- 7. Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Melatonin secretion after surgery. Lancet 2000; 356: 1244–5. [DOI] [PubMed] [Google Scholar]
- 8. Oster H, Challet E, Ott V, et al. The functional and clinical significance of the 24‐hour rhythm of circulating glucocorticoids. Endocrine Reviews 2017; 38: 3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allada R, Bass J. Circadian mechanisms in medicine. New England Journal of Medicine 2021; 384: 550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gögenur I. Postoperative circadian disturbances. Danish Medical Bulletin 2010; 57: B4205. [PubMed] [Google Scholar]
- 11. Sancar A, Van Gelder RN. Clocks, cancer, and chronochemotherapy. Science 2021; 371. [DOI] [PubMed] [Google Scholar]
- 12. Awad K, Serban M‐C, Penson P, et al. Effects of morning vs evening statin administration on lipid profile: a systematic review and meta‐analysis. Journal of Clinical Lipidology 2017; 11: 972–85.e9. [DOI] [PubMed] [Google Scholar]
- 13. Burgess HJ, Kikyo F, Valdespino‐Hayden Z, et al. Do the Morningness‐Eveningness questionnaire and Munich ChronoType questionnaire change after morning light treatment? Sleep Science and Practice 2018; 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Facer‐Childs ER, Campos BM, Middleton B, Skene DJ, Bagshaw AP. Circadian phenotype impacts the brain's resting‐state functional connectivity, attentional performance, and sleepiness. Sleep 2019; 42: zsz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 16. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non‐clinical samples: a systematic review and meta‐analysis. Sleep Medicine Reviews 2016; 25: 52–73. [DOI] [PubMed] [Google Scholar]
- 17. Kantermann T, Meisel A, Fitzthum K, Penzel T, Fietze I, Ulm L. Changes in chronotype after stroke: a pilot study. Frontiers in Neurology 2014; 5: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doyle DJ, Goyal A, Bansal P, et al. American Society of Anesthesiologists Classification. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 19. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043–9. [DOI] [PubMed] [Google Scholar]
- 20. Tan C, Saito N, Miyawaki I, Shiotani H. Preoperative circadian physical activity rhythm and postoperative delirium in cardiovascular surgery patients. Chronobiology International 2020; 37: 1059–66. [DOI] [PubMed] [Google Scholar]
- 21. Madsen MT, Rosenberg J, Gögenur I. Actigraphy for measurement of sleep and sleep‐wake rhythms in relation to surgery. Journal of Clinical Sleep Medicine 2013; 9: 387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kjølhede P, Langström P, Nilsson P, Wodlin NB, Nilsson L. The impact of quality of sleep on recovery from fast‐track abdominal hysterectomy. Journal of Clinical Sleep Medicine 2012; 8: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cai H, Su N, Li W, Li X, Xiao S, Sun L. Relationship between afternoon napping and cognitive function in the ageing Chinese population. General Psychiatry 2021; 34: e100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Owusu JT, Wennberg AMV, Holingue CB, Tzuang M, Abeson KD, Spira AP. Napping characteristics and cognitive performance in older adults. International Journal of Geriatric Psychiatry 2019; 34: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asif N, Iqbal R, Nazir CF. Human immune system during sleep. American Journal of Clinical and Experimental Immunology 2017; 6: 92–6. [PMC free article] [PubMed] [Google Scholar]
- 26. Dispersyn G, Pain L, Touitou Y. Circadian disruption of body core temperature and rest‐activity rhythms after general (propofol) anesthesia in rats. Anesthesiology 2009; 110: 1305–15. [DOI] [PubMed] [Google Scholar]
- 27. Challet E. Minireview: entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 2007; 148: 5648–55. [DOI] [PubMed] [Google Scholar]
- 28. Manou‐Stathopoulou V, Korbonits M, Ackland GL. Redefining the perioperative stress response: a narrative review. British Journal of Anaesthesia 2019; 123: 570–83. [DOI] [PubMed] [Google Scholar]
- 29. Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000; 289: 2344–7. [DOI] [PubMed] [Google Scholar]
- 30. Phillips AW, Reddy S, Durning SJ. Improving response rates and evaluating nonresponse bias in surveys: AMEE Guide No. 102. Medical Teacher 2016; 38(3): 217–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. List of abbreviations and glossary of terms.
Appendix S2. Comparison of baseline characteristic inclusions vs. all patients.
Appendix S3. Surgical characteristics and length of stay in hospital.
Appendix S4. Relationship between Δ‐midpoint sleep on night after surgery [Δ‐NAS] and circadian variables.
Appendix S5. Relationship between Δ‐NAS and patient characteristics and peri‐operative data.


