Abstract
Background and purpose
Although the majority of migraine with aura (MwA) patients experience simple visual aura, a discrete percentage also report somatosensory, dysphasic or motor symptoms (the so‐called complex auras). The wide aura clinical spectrum led to an investigation of whether the heterogeneity of the aura phenomenon could be produced by different neural correlates, suggesting an increased visual cortical excitability in complex MwA. The aim was to explore whether complex MwA patients are characterized by more pronounced connectivity changes of the visual network and whether functional abnormalities may extend beyond the visual network encompassing also the sensorimotor network in complex MwA patients compared to simple visual MwA patients.
Methods
By using a resting‐state functional magnetic resonance imaging approach, the resting‐state functional connectivity (RS‐Fc) of both visual and sensorimotor networks in 20 complex MwA patients was compared with 20 simple visual MwA patients and 20 migraine without aura patients.
Results
Complex MwA patients showed a significantly higher RS‐Fc of the left lingual gyrus, within the visual network, and of the right anterior insula, within the sensorimotor network, compared to both simple visual MwA and migraine without aura patients (p < 0.001). The abnormal right anterior insula RS‐Fc was able to discriminate complex MwA patients from simple aura MwA patients as demonstrated by logistic regression analysis (area under the curve 0.83).
Conclusion
Our findings suggest that higher extrastriate RS‐Fc might promote cortical spreading depression onset representing the neural correlate of simple visual aura that can propagate to sensorimotor regions if an increased insula RS‐Fc coexists, leading to complex aura phenotypes.
Keywords: complex aura, insula, lingual gyrus, migraine with aura, sensorimotor network, visual network
(i) Hyper‐connected cortical areas encompassing visual and somatosensory networks in migraine with aura patients compared to migraine without aura patients may represent the breeding ground for cortical spreading depression wave ignition and propagation.
(ii) Higher resting‐state functional connectivity of the visual network might promote cortical spreading depression initiation representing the neural correlate of simple visual aura that, if an increased insula resting‐state functional connectivity coexists, will be able to propagate to sensorimotor regions leading to complex migraine aura.
(iii) The abnormal resting‐state functional connectivity of visual and sensorimotor networks could represent diagnostic biomarkers able to discriminate complex aura phenotypes.
INTRODUCTION
About one‐third of migraine patients report fully reversible focal neurological symptoms, gradually spreading up to slowly disappear, defining the so‐called migraine aura, a heterogeneous phenomenon with inter‐individual and sometimes intra‐individual variability [1, 2]. Although the majority of migraine with aura (MwA) patients report visual aura, a discrete percentage also experience somatosensory, dysphasic or, more rarely, motor symptoms allowing a clinical sub‐classification as either simple or complex auras [3, 4].
In the last decades, in order to investigate the neural substrates underlying the wide spectrum of aura phenotypes, advanced neuroimaging investigations have identified structural, microstructural and functional connectivity (Fc) abnormalities in the extrastriate cortex, a strategic area of the visual network involved in the genesis of visual aura [5, 6]. Furthermore, neurophysiological and spectroscopic observations have demonstrated an even more increased visual cortical excitability in complex MwA [7, 8]. Nevertheless, no resting‐state functional magnetic resonance imaging (RS‐fMRI) studies have specifically evaluated differences in visual network RS‐Fc between patients experiencing simple aura or complex auras.
The present study aimed to explore visual network RS‐Fc during the interictal period in migraine patients with simple—exclusively visual—aura (simple visual MwA) and patients with migraine with complex aura (complex MwA). It was hypothesized that greater visual network RS‐Fc changes could characterize complex MwA patients compared to simple visual MwA patients. Moreover, it was speculated that RS‐Fc abnormalities in complex MwA patients may extend beyond the visual network, affecting also the sensorimotor network.
Finally, to examine the specificity of any putative RS‐Fc differences between the two groups of MwA patients (e.g., with simple visual MwA and complex MwA), a group of migraine without aura (MwoA) patients and a group of healthy controls (HCs) was further studied.
PATIENTS AND METHODS
Study population and study design
Forty right‐handed patients with exclusively episodic MwA [1.2.1.1] according to the International Headache Society criteria (Headache Classification Subcommittee of the International Headache Society, 2013 and 2018) [9] were recruited between 2015 and 2020 from the migraine population referred to the Headache Centre of the Department of Neurology at the University of Campania ‘Luigi Vanvitelli’. All MwA patients underwent the Migraine Aura Complexity Score (MACS) [3] to be classified, based on aura symptoms, as patients experiencing ‘simple aura’ (MACS score ≤1) or ‘complex aura’ (MACS score >1). The latter includes both moderately complex and complex aura according to MACS. Amongst patients with simple aura, exclusively patients with visual aura were enrolled (e.g., experiencing only visual symptoms during the aura). Demographic data were obtained as well as the following clinical features: age at migraine onset, disease duration, attack frequency (days/month), aura duration, attack pain intensity (assessed using the visual analogue scale) and related disability (using the Migraine Disability Assessment Scale and the Headache Impact Test 6) (Table 1) [10, 11]. Patients with neurological, psychiatric and internal disorders as well as pregnancy, claustrophobia and chronic pain conditions were excluded. Twenty right‐handed episodic MwoA patients [1.1] [9] were recruited. To avoid the confounding interference of migraine attack or pharmacologic intake with the RS‐fMRI investigation, all patients were both migraine‐free and not taking rescue medications at least 3 days before scanning. Patients were interviewed 3 days after scanning to ascertain if they were migraine‐free also during the post‐scan days. All patients were naïve for commonly prescribed migraine preventive medications at the time of the brain scan.
TABLE 1.
Demographic and clinical characteristics of patients with complex MwA, patients with simple visual MwA, patients with MwoA and HCs
| Parameter | Group | p value | |
|---|---|---|---|
| Gender | Complex MwA | 8 M; 12F | – |
| Simple visual MwA | 8 M; 12F | ||
| MwoA | 8 M; 12F | ||
| HCs | 7 M; 13F | ||
| Age (mean years ±SD) | Complex MwA | 30.4 ± 9.41 | 0.875 |
| Simple visual MwA | 31.4 ± 7.81 | ||
| MwoA | 31.25 ± 9.09 | ||
| HCs | 28.45 ± 6.38 | ||
| Disease duration (mean years ±SD) | Complex MwA | 9.95 ± 7.77 | 0.546 |
| Simple visual MwA | 9.65 ± 8.37 | ||
| MwoA | 11.6 ± 8.27 | ||
| Frequency (mean attacks/year ±SD) | Complex MwA | 21.48 ± 25.59 | <0.001 |
| Simple visual MwA | 16.37 ± 26.41 | ||
| MwoA | 63.00 ± 38.71 | ||
| MIDAS (median ±IQR) | Complex MwA | 5 ± 27 | 0.003 |
| Simple visual MwA | 8.5 ± 8 | ||
| MwoA | 18 ± 16.5 | ||
| HIT‐6 (median ±IQR) | Complex MwA | 57 ± 14.25 | <0.001 |
| Simple visual MwA | 58.5 ± 6.5 | ||
| MwoA | 64 ± 7.75 | ||
| VAS (mean score ±SD) | Complex MwA | 8.40 ± 1.02 | 0.655 |
| Simple visual MwA | 7.82 ± 1.33 | ||
| MwoA | 8.14 ± 1.03 | ||
| Aura duration (min) | Complex MwA | 23, 40 ± 12, 34 | 0.16 |
| Simple visual MwA | 20, 03 ± 11, 43 | ||
| MACS (median ±IQR) | Complex MwA | 3 ± 2 | <0.001 |
| Simple visual MwA | 1 ± 0 |
Abbreviations: F, female; HCs, healthy controls; HIT‐6, Headache Impact Test 6; IQR, interquartile range; M, male; MACS, Migraine Aura Complexity Score; MIDAS, Migraine Disability Assessment Scale; MwA, migraine with aura; MwoA, migraine without aura; VAS, visual analogue scale.
Finally, 20 age‐ and sex‐matched, right‐handed subjects with less than a few spontaneous non‐throbbing headaches per year, with no family history of migraine, pregnancy, claustrophobia, hypertension, diabetes mellitus, heart disease, other chronic systemic diseases, stroke, cognitive impairment, substance abuse, chronic pain, as well as other neurological or psychiatric disorders were recruited as HCs. The HC recruitment was conducted via advertisements placed in the hospital (e.g., posters and flyers), word‐of‐mouth referrals, and from a database of research volunteers maintained by the MRI Research Centre of the University of Campania ‘Luigi Vanvitelli’.
Standard protocol approvals, registrations and patient consents
The study was approved by the Ethics Committee of the University of Campania ‘Luigi Vanvitelli’, and written informed consent was obtained from all subjects according to the Declaration of Helsinki.
Imaging parameters
Magnetic resonance images were acquired on a General Electric 3‐T MRI scanner equipped with an eight channel parallel‐head coil (HDxt Signa GE, Milwaukee, WI, USA). The imaging protocol included 3D T1‐weighted sagittal images (gradient‐echo sequence inversion recovery prepared fast spoiled gradient recalled‐echo; repetition time 6988 ms; inversion time 1100 ms; echo time 3.9 ms; flip angle 10; voxel size 1 × 1 × 1.2 mm) [3]. fMRI data consisted of 240 volumes of a repeated gradient‐echo planar imaging T2*‐weighted sequence (repetition time 1508 ms; axial slices 29; matrix 64 × 64; field of view 256 mm; thickness 4 mm; interslice gap 0 mm). During the functional scan, subjects were asked simply to stay motionless, awake and relaxed, and to keep their eyes closed; no visual or auditory stimuli were presented at any time during functional scanning. The criterion used to exclude scans was formulated on the basis of head movements as estimated during the motion correction procedures. To include scans, the estimated translation parameters had to be higher than the dimension of the functional voxel used for the analysis (3 mm isotropic) and the rotation parameters had to be not higher than 3°.
Resting‐state fMRI preprocessing
Image data preprocessing and statistical analysis were performed with BrainVoyager QX (Brain Innovation BV, Maastricht, The Netherlands). Data preprocessing included the correction for slice scan timing acquisition, a 3D rigid‐body motion correction based on a six‐parameter rigid body alignment to correct for minor head movements, and the application of a temporal high‐pass filter with cut‐off set to three cycles per time course. Translational motion parameters were verified to be always less than one functional voxel for all included participants. Structural and functional data were coregistered and spatially normalized to the Talairach standard space using a 12‐parameter affine transformation. During this procedure, the functional images were resampled to an isometric 3‐mm grid covering the entire Talairach box. Single‐subject and group‐level independent component analysis (ICA) was carried out respectively with the fastICA and the self‐organizing group ICA (sogICA) algorithms. For each subject, 40 independent components (corresponding to one‐sixth of the number of time points) were extracted and scaled to spatial z‐scores. All single‐subject component maps were then ‘clustered’ at the group level, resulting in 40 single‐group average maps that were visually inspected to recognize the main functional resting‐state networks and, particularly, to select visual network and sensorimotor network components. The sign‐adjusted ICA components of all subjects were then submitted to a second‐level, multi‐subject random effects two‐way analysis of variance (ANOVA) that treated the individual subject map values as random observations at each voxel, cluster memberships as one within‐subject factor with 40 levels (corresponding to 40 group components) and subject group as one between‐subject factor with four levels (corresponding to complex MwA patients, simple visual MwA patients, MwoA patients and HCs). Starting from the ANOVA, a single‐group one‐sample t test was used to analyse the whole‐brain distribution of the cognitive networks components in each group separately and the resulting t maps were calibrated at p = 0.05 (Bonferroni corrected over the entire brain). An inclusive mask was also created from the HC group maps and was used to define a new search volume within‐network, between‐group comparison. The resulting statistical maps were overlaid on the standard ‘Colin‐27’ brain T1 template. To correct for multiple comparisons, regional effects were only accepted for clusters exceeding a minimum size determined with a non‐parametric randomization approach. Namely, an initial voxel‐level threshold was set to p = 0.001 (uncorrected) and a minimum cluster size was estimated after 1000 Monte Carlo simulations that protected against false positive clusters up to 5%. Cluster‐level correction is a very common and effective way to correct for multiple comparisons in fMRI statistical maps, including random effects maps, obtained from RS‐fMRI studies. Individual ICA z‐scores for all groups were extracted from visual network and sensorimotor network clusters identified in the above analyses and used for linear correlation analyses with clinical parameters of disease severity. ICA z‐scores express the relative modulation of a given voxel by a specific ICA and hence reflect the amplitude of the correlated fluctuations within the corresponding Fc network.
Statistical analysis
For the analysis of differences between groups on demographic and clinical variables non‐parametric tests (Kruskal–Wallis H test to compare four samples and the Mann–Whitney U test to compare two samples) were used to avoid biases because of the small sample size. p < 0.05 was considered statistically significant. Within the sample of MwA patients, the correlation analysis between the imaging and clinical parameters of disease severity was carried out by means of Spearman's rank correlation coefficient. Although a p value <0.05 was considered statistically significant, the Bonferroni correction for multiple comparisons was applied. Finally, a logistic regression analysis was performed to ascertain the effects of RS‐Fc of the left lingual gyrus and anterior insula (the only areas showing statistically significant differences in the groups comparison) on the likelihood that patients belong to each group. More specifically, in a first analytical step, whether the Fcs of the lingual gyrus and insula were able to discriminate MwA patients (as a group) from MwoA patients was evaluated. In a second analytical step, whether the Fcs of the lingual gyrus and insula were able to discriminate complex MwA patients from simple visual MwA patients was determined (Table 2). Finally, receiver operating characteristic curve analysis for each model was conducted. All statistics were performed using the Statistical Package for the Social Sciences version 20 (SPSS, Chicago, IL, USA).
TABLE 2.
Logistic regression analyses assessing whether RS‐Fcs of the lingual gyrus within the visual network and insula within the sensorimotor network are able to discriminate between migraine with aura and migraine without aura patients
| Variable | Coefficient (95% CI) | p value | SE | 95% CIs for odds ratio | ||
|---|---|---|---|---|---|---|
| Lower | Odds | Upper | ||||
| Simple regression | ||||||
| Left lingual gyrus | 0.58 (0.13, 1.04) | 0.01 | 0.23 | 1.13 | 1.79 | 2.84 |
| Right anterior insula | 0.98 (0.21, 1.74) | 0.01 | 0.39 | 1.24 | 2.66 | 5.70 |
Model χ 2(2) =17.42, p value < 0.001, R 2 = 0.35 (Nagelkerke).
Abbreviations: CI, confidence interval; RS‐Fc, resting‐state functional connectivity.
RESULTS
Clinical findings
The experimental groups (e.g., complex MwA, simple visual MwA, MwoA and HCs) did not differ in age and male/female ratio. Similarly, they did not show differences in clinical parameters of migraine severity (disease duration, average of pain intensity of migraine attacks) with an exception concerning attack frequency (attacks/year), Headache Impact Test 6 and Migraine Disability Assessment Scale scores which, as expected based on the migraine attack frequency, were significantly lower in MwA patients (both complex MwA and simple visual MwA) compared to MwoA patients. No statistically significant difference was found between the duration of the aura experience in the two MwA groups (e.g., complex MwA patients, simple visual MwA patients) (Table 1). In complex MwA patients, visual, somatosensory (20 patients) and dysphasic symptoms (six patients) were reported starting with visual symptoms and followed by somatosensory and, finally, dysphasic symptoms.
Resting‐state fMRI
Visual network
Each group exhibited a visual network RS‐Fc pattern consistent with previous reports, encompassing retinotopic occipital cortex and temporo‐occipital regions, including the middle temporal area [12, 13]. The two‐sample t tests revealed significant group differences in the left lingual gyrus. Specifically, MwA patients as a group (including both patients with complex and patients with simple visual aura) showed a higher visual network RS‐Fc centred in the right lingual gyrus (Talairach coordinates x, y, z: 4, –77, 1; t = 5.276) compared to both MwoA patients (p < 0.001) and HCs (Figure 1). Interestingly, complex MwA patients showed a significantly higher component time course related activity of the left lingual gyrus (Talairach coordinates x, y, z: −14, –76, –7; t = 7.842) compared to simple visual MwA patients, MwoA patients and HCs (p < 0.001) (Figures 2 and 3). No differences were found in visual network RS‐Fc between MwoA patients and HCs.
FIGURE 1.
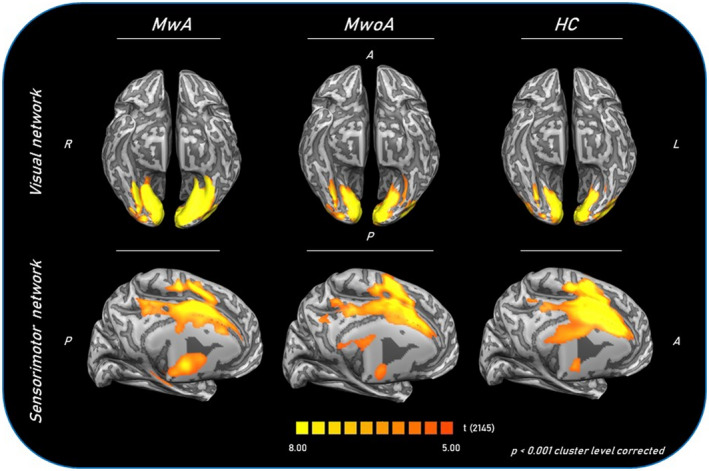
Group‐level (main effects) functional connectivity of visual and sensorimotor networks in MwA patients (as a group), MwoA patients and HCs. Statistical maps were obtained overlaying an inflated 3D brain surface from the ‘Colin 27’ atlas. A, anterior; HC, healthy control; MwA, migraine with aura; MwoA, migraine without aura; P, posterior; R, right; L, left
FIGURE 2.
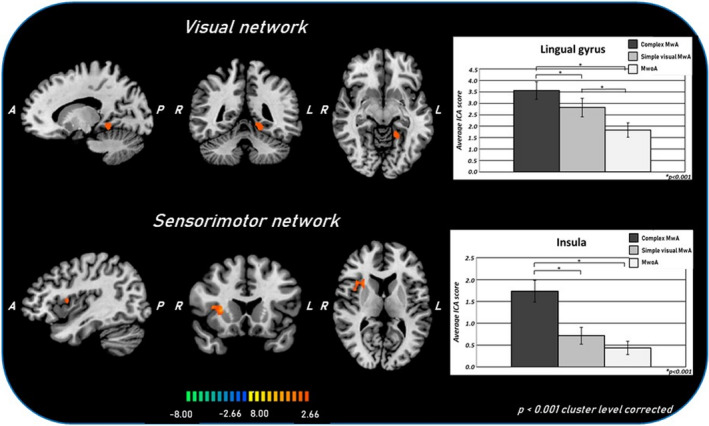
T‐map of statistically significant differences within the visual and sensorimotor networks between complex MwA and simple visual MwA overlaid on the standard ‘Colin‐27’ brain T1 template. Corresponding bar graphs of the averaged ICA z‐scores for complex MwA, simple visual MwA and MwoA groups. A, anterior; ICA, independent component analysis; L, left; complex MwA, migraine with complex aura; simple visual MwA, migraine with simple visual aura; MwoA, migraine without aura; P, posterior; R, right
FIGURE 3.
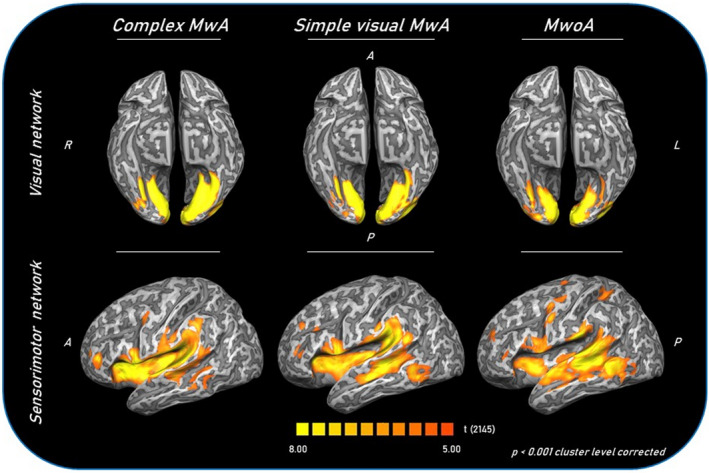
Group‐level (main effects) functional connectivity of visual and sensorimotor networks in complex MwA, simple visual MwA and MwoA patients. Statistical maps were obtained overlaying an inflated 3D brain surface from the ‘Colin 27’ atlas. A, anterior; cMwA, migraine with complex aura; MwoA, migraine without aura; simple visual MwA, migraine with simple visual aura; P, posterior; R, right; L, left
Sensorimotor network
Each group exhibited a sensorimotor network connectivity pattern consistent with previous reports, encompassing somatosensory post‐central gyrus and motor pre‐central gyrus and extending to the supplementary motor areas such as the posterior insula [13]. The two‐sample t tests revealed significant group differences in the RS‐Fc of the sensorimotor network. In particular MwA patients as a group (including patients with both complex and simple visual aura) showed a significantly lower component time course related activity of (left −43, –38, 15, t = −6.641; and right 56, –2, –3, t = −6.728) superior temporal gyri (Talairach coordinates x, y, z: 34, 14, 9; t = 6.288), left pre‐central gyrus (Talairach coordinates x, y, z: −36, –18, 38; t = −6.967) and cingulate gyrus (Talairach coordinates x, y, z: 11, 1, 33; t = −9.507) compared to HCs (Figure 1).
On the other hand, complex MwA patients showed a significantly higher component time course related activity of the right insula compared to both simple visual MwA and MwoA patients (p < 0.001) (Figures 2 and 3) (Talairach coordinates x, y, z: 34, 14, 9; t = 6.288).
Correlation analysis and logistic regression analysis
Post hoc analyses did not reveal any statistically significant correlation between RS‐Fc changes of intrinsic brain networks and clinical parameters of disease severity.
The first logistic regression analysis showed that, based on the sole left lingual gyrus and right anterior insula RS‐Fc, MwA can be discriminated from MwoA patients. Specifically, the logistic regression model was statistically significant, χ 2(4) =17.423, p < 0.001. The model explained 35.0% (Nagelkerke R 2) of the variance and correctly classified 73.3% of patients. Odds ratio analysis demonstrated that an increasing lingual gyrus and insula RS‐Fc was associated with an increased likelihood of exhibiting MwA (b 1 = 0.584 and b 2 = 0.977, p = 0.012) (Table 2). Receiver operating characteristic curves showed an area under the curve (AUC) = 0.80 for the full model.
The second logistic regression analysis showed that the left lingual gyrus and right anterior insula RS‐Fc is able to distinguish complex MwA from simple visual MwA patients. Specifically, the logistic regression model was statistically significant, χ 2(4) = 14.602, p < 0.001. The model explained 41.0% (Nagelkerke R 2) of the variance and correctly classified 77.5% of patients. Odds ratio analysis demonstrated that an increasing insula (but not lingual gyrus) RS‐Fc was associated with an increased likelihood of exhibiting MwA (b = 1.459, p = 0.006) (Table 3). Receiver operating characteristic curves showed AUC = 0.83 for the full model (Figure 4).
TABLE 3.
Logistic regression analyses assessing whether RS‐Fcs of the lingual gyrus within the visual network and insula within the sensorimotor network are able to discriminate between complex migraine with aura and simple visual migraine with aura patients
| Variable | Coefficient (95% CI) | p value | SE | 95% CIs for odds ratio | ||
|---|---|---|---|---|---|---|
| Lower | Odds | Upper | ||||
| Simple regression | ||||||
| Left lingual gyrus | 0.46 (−0.02, 0.94) | 0.058 | 0.24 | 0.98 | 1.59 | 2.56 |
| Right anterior insula | 1.46 (0.42, 2.49) | 0.006 | 0.53 | 1.53 | 4.29 | 12.05 |
Model χ 2(2) =14.60, p value < 0.001, R 2 = 0.41 (Nagelkerke).
Abbreviations: CI, confidence interval; RS‐Fc, resting‐state functional connectivity.
FIGURE 4.
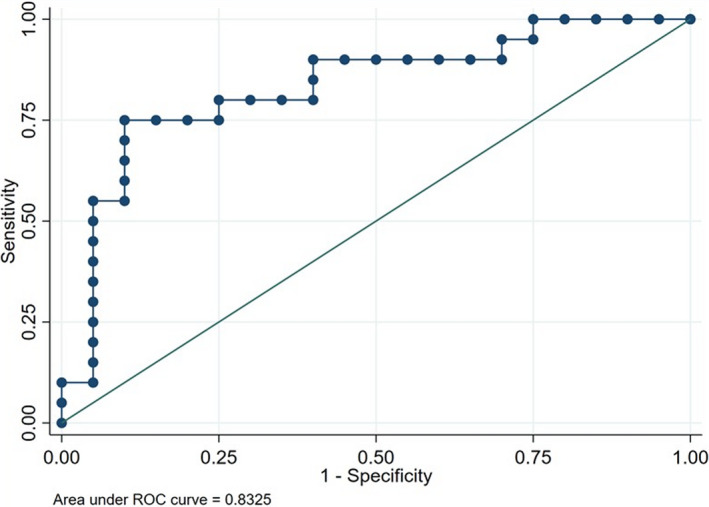
Receiver operating characteristic (ROC) curve of the logistic regression model considering the left lingual gyrus and right anterior insula functional connectivity for the discrimination of complex MwA from simple visual MwA patients. Area under the curve is 0.83. MwA, migraine with aura
DISCUSSION
In the present study, an increased visual network RS‐Fc was found in MwA patients (as a group) compared with MwoA patients and HCs. Furthermore, for the first time, a reduced sensorimotor network RS‐Fc of the sensorimotor network in MwA patients was demonstrated, as previously shown in MwoA patients compared with HCs [15, 16, 17]. However, the main findings of the present study are the significant differences in the intrinsic RS‐Fc of both visual network and sensorimotor network between complex MwA patients and simple visual MwA patients as well as compared with MwoA patients investigated during the interictal period.
About 30% of migraine patients report fully reversible focal neurological symptoms, constituting the so‐called aura, gradually spreading up (in about 5 min) and then slowly disappearing (between 5 and 60 min) [17]. Migraine aura may include a wide array of clinical manifestations [1]. In particular, focusing on the intricacy of aura presentation, whilst the majority of MwA patients exclusively experience visual symptoms, a minority of patients report, along with visual phenomena, also somatosensory or dysphasic symptoms representing the so‐called ‘complex aura’ [3]. In the latter, visual, somatosensory and dysphasic symptoms follow one another, usually beginning with visual, then somatosensory and finally motor or aphasic manifestations [18].
In the last decades, neurophysiological and advanced neuroimaging investigations have provided converging evidence supporting the original idea of ‘cortical spreading depression’ (CSD) as a neurophysiological process underlying migraine aura [5, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28].
In this scenario, the milestone study of Hadjikhani et al. demonstrated, by means of the blood oxygenation level dependent fMRI technique, that a focal signal increase followed by a signal decrease spreads contiguously from the extrastriate cortex towards the anterior cortical brain area, at a rate of 3 mm/min [21]. Interestingly, the spreading extrastriate activity involved the lingual gyrus, from then on considered as the cortical ‘aura generator’. Moreover, the lingual gyrus also showed changes in cortical thickness and cortical surface as well as increased intrinsic RS‐Fc in MwA patients experiencing visual aura, during the interictal period, compared to MwoA patients [12].
Only few studies have been conducted to explore whether the wide spectrum of MwA clinical phenotypes can be produced by different neural correlates. It can be argued that CSD spreading widely into further cortical areas (e.g., somatosensory and the adjacent motor cortices other than the visual cortex) could imply different aura symptoms, resulting overall in a complex aura [29]. The CSD propagation throughout different cortical regions and consequent aura phenotype strongly depend on the balance between predisposing and inhibiting factors, the latter allowing the brain parenchyma to recover by means of an adequate neurovascular coupling [30, 31]. However, due to subtle mitochondrial metabolic abnormalities affecting both the energy reserve and ATP levels, neurovascular coupling seems to be impaired in MwA patients [7, 32]. This concept has been further supported by recent observations showing a transient increase of lactate during visual stimulation in the visual cortex of complex MwA patients compared to simple visual MwA patients, witnessing that an increased metabolic activity may be related to the enhanced cortical excitability [6]. In line with these observations, complex MwA patients showed also a significantly greater amplitude of the visual evoked potentials (e.g., N1‐P1 waves) compared to simple visual MwA patients and HCs [8].
However, the difference between complex MwA and simple visual MwA patients seems not to be exclusively functional in nature. Indeed, although no differences in visual and primary somatosensory cortical thickness and density have been found between complex MwA and simple visual MwA patients, significant changes in temporal sulcal depth have been observed in complex MwA [33, 34].
In the present study, an increased visual network RS‐Fc in MwA patients (as a group) compared with MwoA patients and HCs as well as a reduced sensorimotor network RS‐Fc in migraine patients (both MwA and MwoA patients) compared with HCs have been observed. From the anatomo‐functional point of view, as extensively discussed above, functional and structural abnormalities of the extrastriate cortex, centred in the lingual gyrus, testify to their critical role in the genesis of CSD in MwA patients. Similarly, several studies have consistently reported a reduced RS‐Fc of the sensorimotor network in MwoA patients suggesting these abnormalities as the functional substrate of a migraine‐related impaired pain processing and modulation in these patients [15, 16, 17]. On the other hand, for the first time, a reduced RS‐Fc of the sensorimotor network also in MwA patients was demonstrated, suggesting a superimposable disrupted nociceptive pathway in these patients.
Nevertheless, the main finding of the present study is the higher RS‐Fc of both the visual network and sensorimotor network centred respectively in the lingual gyrus and the anterior insula observed in complex MwA patients compared with both simple visual MwA and MwoA patients. Although the role of the migraine condition rather than the aura phenomenon could be evoked to justify the observed findings, the migraine shared by the three groups of patients make an argument that the RS‐Fc differences in the sensorimotor network may depend strictly on the aura phenomenon.
Amongst the structures encompassing the sensorimotor network, the insula through the integration from somatosensory and visceral sensory modalities may reveal its involvement in the interception and perception of pain [35]. Indeed, as demonstrated by Penfield in the 1950s [36], electrical stimulation of the insula was able to induce somatic sensations such as tingling or numbness in the face, hand, arm and tongue, similar to those experienced by MwA patients in the course of the aura phenomenon. In addition, the anterior insula is strongly connected with the amygdala as demonstrated in MwA patients, interpreted by some authors as the putative ‘missing link’ between the CSD and the trigeminovascular system activation during MwA attacks [37].
From the pathophysiological point of view, ‘hyper‐connected’ cortical networks involved in visual and somatosensory processing, by means of predisposing the synaptic drive from subcortical structures, seem more prone to be overwhelmed by the CSD wave with consequent ignition and propagation of the aura phenomenon [38]. In other terms, our findings suggest that higher extrastriate cortex RS‐Fc might promote CSD initiation (i.e., the underlying neural correlate of simple visual aura) that, if an increased insula RS‐Fc coexists, will be able to propagate to sensorimotor regions, leading to complex aura phenotypes.
Our RS‐fMRI findings showed no correlations with clinical parameters of disease severity in MwA patients. Thus, it is believed that a complex aura per se, probably an innate genetic‐based predisposition, could imply the connectivity changes observed exclusively in complex MwA patients independently from the disease burden.
Logistic regression analysis showed that the full model, considering only both the left lingual gyrus and right anterior insula RS‐Fc, can discriminate MwA patients (as a group) from MwoA (AUC = 0.80). However, surprisingly, the full model considering only both the left lingual gyrus and right anterior insula RS‐Fc can distinguish complex MwA from simple visual MwA patients (AUC = 0.83). Moreover, as demonstrated by odds ratio analysis, an increased RS‐Fc of the insula, more than an increased RS‐Fc of the lingual gyrus, was associated with an increased likelihood of experiencing complex MwA.
The present study is not exempt from limitations. First, it is not known whether the RS‐Fc changes in both the visual network and sensorimotor network are due to hereditary liability, predisposing to simple visual MwA or complex MwA, or due to visual and somatosensory pathway plastic changes (in terms of growth or loss of dendritic spines) as a result of migraine aura experience. In this regard, a complex aura might be a pathophysiological and clinical evolution from a previous simple visual aura, although this hypothesis seems not to be supported by the correlation analyses.
In conclusion, our findings showing the involvement of the visual network further support the prominent role of visual pathways in the whole MwA pathophysiology. On the other hand, our data exhibiting a different pattern of RS‐Fc beyond the visual network and specifically encompassing sensorimotor networks strongly suggest that more widespread Fc changes may represent the fingerprint of complex aura phenotypes.
Conclusions
(i) Cortical areas encompassing the somatosensory network show reduced RS‐Fc in MwA patients compared with HCs, as previously demonstrated in MwoA patients.
(ii) Hyper‐connected cortical areas encompassing visual and somatosensory networks in MwA patients compared to MwoA patients may represent the breeding ground for CSD wave ignition and propagation.
(iii) Higher RS‐Fc of the visual network might promote CSD initiation representing the neural correlate of simple visual aura that, if an increased insula RS‐Fc coexists, will be able to propagate to sensorimotor regions leading to complex migraine aura.
(iv) The abnormal RS‐Fc of visual and sensorimotor networks could represent diagnostic biomarkers able to discriminate complex aura phenotypes.
ACKNOWLEDGEMENTS
Open Access Funding provided by Universita degli Studi della Campania Luigi Vanvitelli within the CRUI‐CARE Agreement. [Correction added on 21 May 2022, after first online publication: CRUI funding statement has been added.]
CONFLICT OF INTEREST
The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article. Dr Silvestro has received speaker honoraria from Novartis, Teva and Lilly. Professor Tessitore has received speaker honoraria from Novartis, Schwarz Pharma/UCB, Lundbeck, Abbvie and Glaxo. Professor Tedeschi has received speaker honoraria from Sanofi‐Aventis, Merck Serono, Bayer Schering Pharma, Novartis, Biogen‐Dompe AG, Teva and Lilly; has received funding for travel from Bayer Schering Pharma, Biogen‐Dompe AG, Merck Serono, Novartis and Sanofi Aventis; and serves as an associate editor of Neurological Sciences. Professor Russo has received speaker honoraria from Allergan, Lilly, Novartis and Teva and serves as an associate editor of Frontiers in Neurology (Headache Medicine and Facial Pain session). The other authors have nothing to declare.
AUTHOR CONTRIBUTIONS
Marcello Silvestro: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); writing—original draft (lead). Alessandro Tessitore: Conceptualization (equal); supervision (equal); validation (equal). Federica Di Nardo: Data curation (lead); formal analysis (lead); methodology (lead). Fabrizio Scotto di Clemente: Formal analysis (equal); methodology (equal). Francesca Trojsi: Supervision (equal); visualization (equal); writing—review and editing (equal). Mario Cirillo: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal). Fabrizio Esposito: Data curation (equal); formal analysis (equal); methodology (equal). Gioacchino Tedeschi: Conceptualization (equal); supervision (equal); validation (equal); writing—review and editing (equal). Antonio Russo: Conceptualization (lead); data curation (lead); investigation (lead); methodology (lead); supervision (lead); validation (lead); writing—original draft (lead).
Silvestro M, Tessitore A, Di Nardo F, et al. Functional connectivity changes in complex migraine aura: beyond the visual network. Eur J Neurol. 2022;29:295–304. 10.1111/ene.15061
Funding information
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Data Availability Statement
All data generated or analysed during this study are available from the corresponding author (dottor.russo@gmail.com).
REFERENCES
- 1. Charles A. The migraine aura. Continuum (Minneap Minn). 2018;24(4, Headache):1009‐1022. [DOI] [PubMed] [Google Scholar]
- 2. Viana M, Sances G, Linde M, et al. Clinical features of migraine aura: results from a prospective diary‐aided study. Cephalalgia. 2017;37(10):979‐989. [DOI] [PubMed] [Google Scholar]
- 3. Petrusic I, Viana M, Dakovic M, Zidverc‐Trajkovic J. Application of the Migraine Aura Complexity Score (MACS): clinical and neuroimaging study. Front Neurol. 2019;18(10):1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petrusic I, Viana M, Zecca C, et al. Dysphasia and other higher cortical dysfunctions during the migraine aura – a systematic review of literature. Curr Pain Headache Rep. 2020;2:3. [DOI] [PubMed] [Google Scholar]
- 5. Russo A, Silvestro M, Tessitore A, et al. Shedding light on migraine with aura: the clarifying role of advanced neuroimaging investigations. Expert Rev Neurother. 2019;19(8):739‐750. [DOI] [PubMed] [Google Scholar]
- 6. Russo A, Silvestro M, Tessitore A, et al. Advances in migraine neuroimaging and clinical utility: from the MRI to the bedside. Expert Rev Neurother. 2018;18(7):533‐544. [DOI] [PubMed] [Google Scholar]
- 7. Sándor PS, Dydak U, Schoenen J, et al. MR‐spectroscopic imaging during visual stimulation in subgroups of migraine with aura. Cephalalgia. 2005;25(7):507‐518. [DOI] [PubMed] [Google Scholar]
- 8. Coppola G, Bracaglia M, Di Lenola D, et al. Visual evoked potentials in subgroups of migraine with aura patients. J Headache Pain. 2015;16:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 10. Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) questionnaire to assess headache‐related disability. Neurology. 2001;56(6 Suppl 1):zS20‐zS28. [DOI] [PubMed] [Google Scholar]
- 11. Yang M, Rendas‐Baum R, Varon SF, et al. Validation of the Headache Impact Test (HIT‐6™) across episodic and chronic migraine. Cephalalgia. 2011;31(3):357‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tedeschi G, Russo A, Conte F, et al. Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia. 2016;36(2):139‐147. [DOI] [PubMed] [Google Scholar]
- 13. Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848‐13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mantini D, Perrucci MG, Del Gratta C, et al. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA. 2007;104:13170‐13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chenji S, Jha S, Lee D, et al. Investigating default mode and sensorimotor network connectivity in amyotrophic lateral sclerosis. PLoS One. 2016;11(6):e0157443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei HL, Chen J, Chen YC, et al. Impaired effective functional connectivity of the sensorimotor network in interictal episodic migraineurs without aura. J Headache Pain. 2020;21(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J, Su J, Wang M, et al. The sensorimotor network dysfunction in migraineurs without aura: a resting‐state fMRI study. J Neurol. 2017;264(4):654‐663. [DOI] [PubMed] [Google Scholar]
- 18. Qin Z, Su J, He XW, et al. Disrupted functional connectivity between sub‐regions in the sensorimotor areas and cortex in migraine without aura. J Headache Pain. 2020;21(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154(Suppl. 1):S44‐S53. [DOI] [PubMed] [Google Scholar]
- 20. Petrusic I, Zidverc‐Trajkovic J. Cortical spreading depression: origins and paths as inferred from the sequence of events during migraine aura. Funct Neurol. 2014;3:207‐212. [PMC free article] [PubMed] [Google Scholar]
- 21. Leão AA. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:359‐390. [DOI] [PubMed] [Google Scholar]
- 22. Leão AA, Morison RS. Propagation of spreading cortical depression. J Neurophysiol. 1945;8:33‐45. [Google Scholar]
- 23. Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arngrim N, Hougaard A, Ahmadi K, et al. Heterogenous migraine aura symptoms correlate with visual cortex functional magnetic resonance imaging responses. Ann Neurol. 2017;82(6):925‐939. [DOI] [PubMed] [Google Scholar]
- 25. Datta R, Aguirre GK, Hu S, et al. Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia. 2013;33(6):365‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sarchielli P, Tarducci R, Presciutti O, et al. Functional 1H‐MRS findings in migraine patients with and without aura assessed interictally. NeuroImage. 2005;24(4):1025–1031. [DOI] [PubMed] [Google Scholar]
- 27. Huang J, Zong X, Wilkins A, et al. fMRI evidence that precision ophthalmic tints reduce cortical hyperactivation in migraine. Cephalalgia. 2011;31:925‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Messina R, Rocca MA, Colombo B, et al. Cortical abnormalities in patients with migraine: a surface‐based analysis. Radiology. 2013;268(1):170‐180. [DOI] [PubMed] [Google Scholar]
- 29. Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol. 2013;9(11):637‐644. [DOI] [PubMed] [Google Scholar]
- 30. Tong CK, Chesler M. Modulation of spreading depression by changes in extracellular pH. J Neurophysiol. 2000;84:2449‐2457. [DOI] [PubMed] [Google Scholar]
- 31. Gorji A. Spreading depression: a review of the clinical relevance. Brain Res Rev. 2001;38:33‐60. [DOI] [PubMed] [Google Scholar]
- 32. Gross EC, Lisicki M, Fischer D, et al. The metabolic face of migraine – from pathophysiology to treatment. Nat Rev Neurol. 2019;15(11):627‐643. [DOI] [PubMed] [Google Scholar]
- 33. Petrusic I, Dakovic M, Kacar K, et al. Migraine with aura: surface‐based analysis of the cerebral cortex with magnetic resonance imaging. Korean J Radiol. 2018;19(4):767‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hougaard A, Amin FM, Arngrim N, et al. Sensory migraine aura is not associated with structural grey matter abnormalities. Neuroimage Clin. 2016;17(11):322‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399(4):440‐468. [DOI] [PubMed] [Google Scholar]
- 36. Penfield W, Faulk M. The insula, further observation on its function. Brain. 1955;78:445‐70. [DOI] [PubMed] [Google Scholar]
- 37. Hadjikhani N, Ward N, Boshyan J, et al. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia. 2013;33(15):1264‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vinogradova LV. Initiation of spreading depression by synaptic and network hyperactivity: insights into trigger mechanisms of migraine aura. Cephalalgia. 2018;38(6):1177‐1187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are available from the corresponding author (dottor.russo@gmail.com).


