Abstract
Oral corticosteroids (OCS) are frequently used for asthma treatment. This medication is highly effective for both acute and chronic diseases, but evidence indicates that indiscriminate OCS use is common, posing a risk of serious side effects and irreversible harm. There is now an urgent need to introduce OCS stewardship approaches, akin to successful initiatives that optimized appropriate antibiotic usage. The aim of this TSANZ (Thoracic Society of Australia and New Zealand) position paper is to review current knowledge pertaining to OCS use in asthma and then delineate principles of OCS stewardship. Recent evidence indicates overuse and over‐reliance on OCS for asthma and that doses >1000 mg prednisolone‐equivalent cumulatively are likely to have serious side effects and adverse outcomes. Patient perspectives emphasize the detrimental impacts of OCS‐related side effects such as weight gain, insomnia, mood disturbances and skin changes. Improvements in asthma control and prevention of exacerbations can be achieved by improved inhaler technique, adherence to therapy, asthma education, smoking cessation, multidisciplinary review, optimized medications and other strategies. Recently, add‐on therapies including novel biological agents and macrolide antibiotics have demonstrated reductions in OCS requirements. Harm reduction may also be achieved through identification and mitigation of predictable adverse effects. OCS stewardship should entail greater awareness of appropriate indications for OCS prescription, risk–benefits of OCS medications, side effects, effective add‐on therapies and multidisciplinary review. If implemented, OCS stewardship can ensure that clinicians and patients with asthma are aware that OCS should not be used lightly, while providing reassurance that asthma can be controlled in most people without frequent use of OCS.
Keywords: corticosteroids, position paper, side effects, severe asthma, stewardship
Short abstract
See related Editorial
Contents
Introduction
How did we get here?
The need for OCS stewardship
What is stewardship?
Methods
Writing group membership
Outline of key aspects for inclusion in the position paper
Information gathering and review process
Application of evidence to elicit principles of OCS stewardship in asthma
Rationale for use of OCS
Acute indications and use
Chronic indications and use
Current use of oral steroids
Epidemiology of use
Evidence of overuse or over‐reliance on OCS
Current Australian guidelines
Nature and prevalence of harm arising from oral steroids
Risks associated with long‐term/maintenance use
Risk associated with short‐term use
Adrenal insufficiency
Cumulative risk
Estimating the harm from OCS in asthma
OCS use in practice: perspectives from people with asthma
Principles of acute OCS use and prevention of overuse
Primary prevention of OCS use
Clarification of acute indications
Dose and duration
Secondary prevention
Special groups
Rescue packs for home administration
Principles of chronic OCS use and prevention of overuse
Primary prevention of OCS use
Harm reduction strategies
Monitoring adverse effects
Summary of core principles of OCS stewardship in asthma
Clinical resources and dissemination
Summary
Conflict of interest
References
INTRODUCTION
Asthma is a highly prevalent chronic inflammatory airway disease, affecting approximately 300 million people worldwide 1 and the disease has a considerable personal impact and public health footprint. For example, in Australia and New Zealand, more than 11% of the overall population are reported to take asthma treatment. 2
Inhaled corticosteroid (ICS) medications can achieve adequate symptom control with acceptable disease stability for most people with asthma. However, up to 10% of people have difficult‐to‐treat asthma with ongoing troublesome symptoms and recurrent exacerbations, despite optimized inhaler technique with good adherence. This situation often leads to use of recurrent bursts of oral corticosteroid (OCS) therapy and/or long‐term OCS treatments. 3
How did we get here?
From the early 1900s, it was common for asthma to be treated with anti‐cholinergic agents, often as ‘asthma cigarettes’, 4 with subcutaneous administration of adrenaline employed for acute attacks. 5 It was not until the 1950s that limited observational studies of the effectiveness of corticosteroids in asthma began to appear. 6 At this stage, the common adverse effects of ‘anti‐rheumatic substance X’ were apparent, including peptic ulceration, osteoporosis and psychological disturbance. 7 In 1956, a multicentre controlled trial of cortisone acetate for acute asthma demonstrated markedly better short‐term outcomes in the intervention group 8 (Figure 1).
FIGURE 1.

For every 10 people with acute asthma, two will improve with bronchodilators alone, six will improve if they were also given oral corticosteroids and two will fail to improve. Outcomes at day 4, data from the 1956 MRC trial. 8 This historic study predated introduction of inhaled corticosteroids
A landmark publication by Morrow‐Brown in 1958 demonstrated striking benefits of chronic prednisolone use in individuals with sputum eosinophilia who had failed to achieve a satisfactory response to bronchodilator drugs, but little benefit in those without raised eosinophils. 9 With the subsequent introduction of ICS, prednisolone remained the cornerstone of severe asthma treatment and this approach was enshrined in initial iterations of asthma guidelines, although the need to demonstrate sputum eosinophilia was not considered. 10 , 11 , 12 It is therefore not surprising that after such a long history of use, OCS prescribing and use have remained embedded in the care of patients with asthma. Alternative approaches are often not considered despite several decades of asthma guidelines and health professional education, awareness of side effects and the availability of effective alternative treatments.
The need for OCS stewardship
Despite recent therapeutic advances, OCS use remains very common, particularly in severe asthma. 13 For example, the National Asthma Registry Data collected from the United Kingdom have shown that more than half of the patients with difficult‐to‐control severe asthma referred to a specialist asthma centre were prescribed daily OCS. 14 Concerningly, a great deal of inappropriate OCS use occurs in mild–moderate asthma that is poorly controlled due to underuse of ICS and/or poor adherence. 13
Frequent bursts as well as maintenance OCS therapy are associated with a multitude of acute and chronic adverse effects, which are outlined in subsequent sections. These include short‐term negative outcomes such as dyspepsia, insomnia, fluid retention and mood changes 13 , 15 and long‐term complications including weight gain, osteoporosis, hypertension, glucose intolerance and increased risk of infections. 16 , 17 , 18 , 19 , 20 There is now an increasing body of evidence indicating that serious adverse consequences of OCS arise at lifetime cumulative doses as low as 500 mg. 21 , 22
The proven benefits and clinical efficacy of OCS in patients considered to have severe asthma have given rise to widespread OCS prescription. A recent report from Australia using healthcare prescription data illustrates this reality showing that amongst people with asthma who were prescribed regular inhaled therapy (many with poor adherence), more than a quarter used OCS doses >1000 mg of prednisolone (or equivalent) over a 5‐year period. 23 This and other evidence 13 , 24 , 25 , 26 suggest suboptimal decision‐making regarding the use of OCS in asthma. This situation is in some ways analogous to over‐prescribing of antibiotics for viral infections, a leading cause of bacterial resistance. Promulgation of antibiotic and antimicrobial stewardship principles represents a key initiative that reduced arbitrary antibiotic use 27 , 28 and an analogous strategy has been proposed in asthma. 29
What is stewardship?
The Merriam‐Webster Dictionary defines stewardship as: ‘the conducting, supervising or managing of something; especially the careful and responsible management of something entrusted to one's care’. Stewardship is therefore a behaviour that embodies responsible planning and management of resources applicable not only to health but also to the environment, economics, property and cultural resources. It is abundantly clear that OCS carry serious health risks and their use in individual patients has become an indicator that asthma management requires specialist review. However, collaborative and systematic initiatives as well as implementation strategies are needed to highlight the risks of OCS and to curb widespread use in asthma. Publicizing and implementing OCS stewardship principles and ensuring their dissemination to all stakeholders (including patients) can promote investigation of reversible complicating factors in patients taking OCS, encourage consideration of alternative treatments and prompt reduction of inappropriate OCS use.
This position paper aims to delineate OCS stewardship principles in adults and adolescents with asthma. It is intended for health professionals as well as other persons interested in asthma and optimal disease management. We highlight current use of OCS in asthma, review the nature and prevalence of harm arising from OCS and provide patient perspectives. Pathways for implementation and dissemination of an OCS stewardship initiative are outlined.
METHODS
Writing group membership
The writing group was convened by the Thoracic Society of Australia and New Zealand (TSANZ) following an open call for participants. Applications were reviewed through the standardized TSANZ process and multidisciplinary members and consumer representatives were selected on the basis of their experience and complementary skills. The position paper development group incorporated a range of professions and disciplines that are relevant to the scope of the position paper as well as two consumer representatives with asthma: John Blakey—Consultant in Respiratory and Sleep Medicine; Li Ping Chung—Respiratory Physician, Clinical Lead for Airways Disease, Fiona Stanley Hospital; Vanessa McDonald—Professor of Nursing; Laurence Ruane—community representative and Respiratory Scientist in Lung & Sleep Monash Medical Centre; John Gornall—community representative with long‐term history of severe asthma; Chris Barton—Senior Lecturer, Department of General Practice, Monash University; Sinthia Bosnic‐Anticevich—Professor, Sydney Pharmacy School, University of Sydney and Research Leader, Quality Use of Respiratory Medicines Group, The Woolcock Institute of Medical Research; John Harrington—Clinical Nurse Consultant Airway Disease, John Hunter Hospital; Mark Hew—Professor, Head of Allergy, Asthma & Clinical Immunology, The Alfred; Anne E. Holland—Professor of Physiotherapy, Monash University and Alfred Health; Trudy Hopkins—Clinical Nurse Consultant; Lata Jayaram—Respiratory and Sleep Disorders Physician, Department of Respiratory Medicine, Western Health and University of Melbourne; Helen Reddel—Research Leader and Director, Australian Centre for Airways disease Monitoring (ACAM); John W. Upham—Professor of Respiratory Medicine, The University of Queensland and Respiratory Physician, Princess Alexandra Hospital; Peter G. Gibson—Senior Staff Specialist, Department of Respiratory and Sleep Medicine, John Hunter Hospital; Philip Bardin—Professor and Director, Respiratory Physician, Monash Lung Sleep Allergy & Immunology, Monash University and Medical Centre. Conflict of interest declarations were made by all members and reviewed by TSANZ. The group was chaired by Professors Philip Bardin and Peter G. Gibson, with support from Professor Anne E. Holland as a non‐prescribing group member.
Outline of key aspects for inclusion in the position paper
A broad outline of the position paper was drafted by a core writing group and circulated. Input was received, changes were implemented by consensus and working subgroups were established. After the key components of the document were agreed by consensus amongst the overall group following broad discussion, subgroup members worked in teams to gather information on their allocated topics and draft sections of the document.
Information gathering and review process
Literature searches were conducted by subgroups to identify English language articles published using search terms that included: asthma AND ([corticosteroid OR corticosteroids OR glucocorticoid OR glucocorticoids OR prednisone OR prednisolone OR dexamethasone OR methylprednisolone OR hydrocortisone]). We excluded reviews, letters and information presented at scientific conferences. All studies that reported information on frequency of systemic corticosteroids (SCS) use and treatment patterns, as well as clinical complications and economic impacts were included. Information was summarized qualitatively, and studies were separated according to whether they reported long‐ or short‐term use.
Application of evidence to elicit principles of OCS stewardship in asthma
All authors and consumer representatives had the opportunity to comment on, or revise, all drafted sections through the use of a shared drive. The final draft was crafted by the core writing group, circulated and then edited before being finally approved by the overall group. Throughout the process, the group held regular videoconferences to discuss evidence and produce a position paper outlining a proposed OCS stewardship programme in asthma. A dissemination plan for the position paper was discussed and included in the paper. This position paper is to be reviewed within 24–36 months, given the developing nature of OCS stewardship and its evolving future role in management.
Peer review was conducted jointly by TSANZ and Respirology and the final position paper was approved by the TSANZ Board and Editor‐in‐chief of Respirology.
RATIONALE FOR USE OF OCS
Acute indications and use
Mechanisms driving asthma exacerbations are complex but are underpinned by inflammation. OCS and ICS therapy suppresses inflammation, especially type 2 responses, and has been demonstrated to reduce bronchial mucosal oedema, mucus accumulation and late bronchoconstrictive response to allergens. 30 , 31 Clinical trials have therefore been undertaken examining the role of OCS in the acute treatment of asthma. However, these have been almost exclusively undertaken in individuals presenting to emergency departments (EDs). This raises questions over their generalizability and the application of this finding to practice, as most people with asthma are treated in primary care, where most OCS prescribing also occurs.
Administration of SCS within the ED or on discharge reduces the risk of relapse compared with placebo by more than 50%. 32 Early use of SCS (within the hour) following presentation to the ED has been shown to be superior to beta2‐agonists alone, reducing the risk of hospitalization by 60%. 33
Oral, intravenous or intramuscular administration in asthma exacerbations?
Oral and intramuscular corticosteroids appear equally effective at reducing hospitalization and relapse rate at 7–10 days. 32 Intravenous hydrocortisone, methylprednisolone and dexamethasone at equivalent dosage are equally effective at improving forced expiratory volume in 1 s in acute asthma, 34 but not superior to OCS. 35 Intramuscular triamcinolone appears less effective than intravenous or oral steroids. 36 , 37 The improvement in lung function from SCS is only observed several hours after administration, hence the importance of bronchodilator therapy in the interim when treating an asthma exacerbation. 38 There is no significant difference in length of stay between those patients treated with intravenous or OCS, or in adverse effects. 36 , 39 In the light of these findings, there is no clear rationale for giving intravenous steroids to those individuals who are capable of taking OCS.
What dose of OCS?
Various trials have explored the optimal dose and duration of OCS use for acute exacerbations. Examples include a trial comparing prednisolone (0.5 mg/kg) for 1 or 2 weeks following initial treatment with methylprednisolone, 40 , 41 and a study comparing 40 mg prednisolone for 5 or 10 days. 40 , 41 Neither of these found a difference in peak expiratory flow, rate of readmission or unscheduled hospital visits during follow‐up. Despite the heterogeneity of the trials, subsequent systematic reviews have concurred that there is no convincing difference in clinical outcomes between higher or lower doses of prednisolone, nor duration of use of prednisolone, nor whether a tapering of dose was used. 42 , 43 , 44 A study comparing 2 days of dexamethasone with 5 days of prednisone showed similar rates of relapse and time to return to normal activity suggesting the former strategy may be an option. 45 Caution is required in anyone taking dexamethasone for more than 2 days given its potent metabolic (diabetogenic) effects.
Until further evidence is available, short‐course (5 days) non‐tapered OCS remains the first choice, while intravenous use is reserved for patients unable to tolerate oral medications. 44 , 46
Minimizing acute OCS exposure
Based on the need to minimize the lifetime expose to OCS, strategies to minimize exposure are critical. The aim of asthma management is to minimize exacerbations and reduce symptom burden with minimal medication. This is also the key to minimizing OCS bursts by optimizing asthma management in order to prevent exacerbations. Although limiting exposure to triggers (e.g., environmental irritants, allergens and occupational sensitisers) can be useful in some circumstances, the cornerstone of current therapeutic approaches is the prescription and appropriate usage of ICS. Regular use of ICS reduces the risk of death due to asthma by more than half, 47 and severe exacerbations by almost half, even in patients with very mild asthma. 48 , 49 , 50 Increasing ICS exposure during times of increased symptoms by employing a maintenance and reliever therapy (MART) with ICS‐formoterol is associated with around a third fewer severe exacerbations compared with the same dose of ICS/long‐acting beta‐agonist (LABA) plus as‐needed short‐acting beta‐agonist (SABA) 51 in eligible groups. In contrast, simply doubling a regular ICS dose in response to worsening symptoms is ineffective in preventing the need for OCS, 52 although quadrupling the dose may be helpful in those on the lowest regular adult ICS doses. 53 The addition of regular LABAs to ICS further reduces the incidence of severe exacerbations, 54 with other add‐on therapies such as long‐acting anti‐muscarinic agents (LAMAs) having a modest effect in addition to ICS–LABA. 55
For patients with more severe asthma, early referral for specialist or multidimensional asthma assessment reduces OCS bursts by reducing exacerbations and improving asthma symptom control. 56 , 57 , 58 , 59 The key is to determine whether a patient is likely to benefit from additional treatment which may reduce the need for OCS. A specialist and/or multidimensional approach to asthma diagnosis and management is vital in identifying those individuals most likely to benefit from biological treatments. In this context, recent studies have confirmed the benefits of using blood eosinophils, IgE and fractional exhaled nitric oxide (FeNO) as biomarkers for type 2 asthma and use of monoclonal therapies targeting the relevant pathways that effectively reduce the need for OCS bursts. 60 , 61 , 62 , 63 , 64 , 65 , 66 The reduction in OCS and lower exacerbation rates seen in large randomized trials are substantiated by data from real‐world registries. 67 , 68 , 69 Use of long‐term azithromycin also shows promise in lowering the exacerbation rate in a broader population of adults with persistent severe asthma. 70 , 71 , 72
Current Australian guideline recommendations
The Australian asthma guidelines provide recommendations on strategies to reduce the risk of severe exacerbations requiring OCS use (Australian Asthma Handbook update August 2020). For treatment of severe exacerbations, guidelines currently recommend the use of oral prednisolone at 37.5–50 mg per day for 5–10 days and do not routinely recommend tapering doses except if treatment has continued for more than 2 weeks. Similar, but not entirely consistent, recommendations are seen in other guidelines. For example, the 2019 BTS guidelines recommend 40–50 mg of prednisolone for all acute asthma attacks, and continuation ‘until recovery (minimum 5 days)’. 73
Chronic indications and use
Prior to the availability of ICS, patients with repeated attacks of severe asthma or those with disabling symptoms affecting normal daily function were treated with daily SCS. A number of case series published in the 1960s showed clinical benefits in asthma‐symptom free days, reduced bronchodilator use and improved daily function, usually measured as ability to return to normal work duties. 74 , 75 OCS can effectively suppress eosinophilic inflammation, the key driver of exacerbations in most people with asthma, and relationships between OCS dose and eosinophilic inflammation and the lag between increases in sputum markers and clinical features have been demonstrated in longitudinal observational studies. 76
ICS were subsequently shown to be as effective as low‐dose oral prednisolone for maintenance treatment in the majority of patients with asthma, and with fewer adverse effects. 77 Currently, ICS are the cornerstone for management of chronic asthma across all severity levels, with the majority of benefit seen with low ICS doses, and with medium‐dose (400–800 μg budesonide or equivalent) to high‐dose ICS (>800 μg budesonide or equivalent) providing an approximately equal clinical benefit to a daily dose of oral prednisolone of 7.5–10 mg, 78 with greatly reduced risk of adverse events.
Maintenance OCS are prescribed for complex asthma variants such as allergic bronchopulmonary aspergillosis (ABPA) and eosinophilic granulomatosis with polyangiitis (EGPA). However, recent evidence suggests that monoclonal antibody treatment may be effective for these conditions, so it is likely that the need for maintenance OCS in ABPA and EGPA will decline further as new evidence emerges. 79 , 80
Glucocorticoid resistance
Despite the broadly beneficial anti‐inflammatory and immunomodulatory effects of corticosteroids, relative glucocorticoid resistance is also recognized in asthma and other inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease. Current evidence supports a structured multidimensional approach to confirm the diagnosis, then identify and treat comorbidities and risk factors contributing to difficult‐to‐treat asthma (Figure 2). This effectively reduces asthma symptom burden and exacerbations 59 and minimizes the need for trials of chronic OCS use. In Britain, patients assessed by multidisciplinary asthma services had lower OCS burden with fewer OCS courses and reduction in mean daily steroid dose at follow‐up compared with the time of referral. 58 Importantly, after careful evaluation, a minority of individuals with difficult‐to‐treat asthma were discovered to have a significant degree of OCS resistance. 81 , 82 , 83
FIGURE 2.
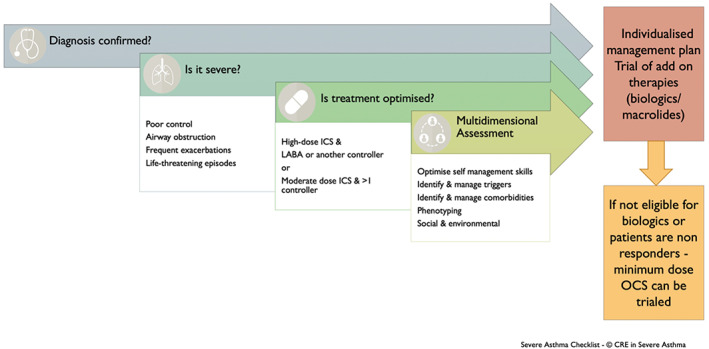
Stepwise multidimensional assessment of individuals who are potentially being considered for treatment with oral corticosteroid. Content has been reproduced with permission from the Centre of Excellence in Severe Asthma, originally developed as part of the Centre of Research Excellence in Severe Asthma (https://toolkit.severeasthma.org.au)
Glucocorticoid resistance, characterized by a lack of clinical response and cushingoid features, was initially described in the 1960s. 84 Subsequent larger studies further illustrated that some individuals with chronic asthma and reversible airflow limitation may not benefit from oral prednisolone. 77 , 85 Paradoxically, these particular individuals may have higher eosinophil and lymphocyte counts in bronchial lavages than steroid responders 86 as well as different patterns of airway remodelling‐associated proteins. 87 ‘Glucocorticoid resistance’ has been defined as no clinical improvement after treatment with prednisolone 40 mg daily for at least 2 weeks. 88 However, this concept is best considered more broadly as a spectrum of ‘corticosteroid insensitivity’. This wider definition includes individuals whose disease cannot be effectively controlled despite adherence to high‐dose ICS treatment. 67 It should also be emphasized that the improvements seen in clinical parameters with high‐dose corticosteroids in this group are often modest 81 and, even in those individuals with high levels of eosinophilic inflammation, corticosteroid treatment may only be partially effective (Figure 3).
Minimizing OCS exposure
It is firmly established that improvements in asthma control, prevention of exacerbations and reductions in the need for OCS therapy can be achieved by improved inhaler technique, adherence to therapy, asthma education, smoking cessation, specialist and/or multidisciplinary review and optimized medications. Recently, add‐on therapies including novel biological agents have also demonstrated reductions in OCS requirements. A full consideration of the available published evidence around monoclonal antibody (biologic) therapies targeting eosinophilic or allergic pathways is beyond the scope of this position paper. However, it is important to recognize that there are robust data showing a substantial reduction in exacerbations, unscheduled healthcare use and minimal adverse effects in the majority of people with asthma who meet criteria for treatment with these agents.
Mepolizumab, benralizumab and dupilumab are currently available in Australia for the treatment of severe eosinophilic asthma, and all three monoclonal antibody therapies facilitate reduction in the dose of maintenance OCS. Randomized controlled trials (RCTs) show a median OCS dose reduction of 50% and complete steroid withdrawal in 14% of mepolizumab‐treated patients, compared with 8% of patients in the placebo arm. 84 In benralizumab trials, median maintenance OCS dose was reduced by 75% in those on active drug compared with 25% in placebo‐treated patients. 89 More than half of the patients treated with benralizumab were no longer using initial maintenance OCS. The reduction in maintenance OCS use has been sustained in subsequent open‐label extension studies. 85 , 86 In general, these outcomes are supported by findings reported from international and national registries, including the Australian Mepolizumab Registry. 68 , 87
Omalizumab also reduces exacerbations 88 but evidence for a reduction in OCS for those patients on maintenance therapy is less robust. In the Australian Omalizumab Registry, 150 of 180 patients with severe allergic asthma demonstrated effective responses defined by improvement in Asthma Control Questionnaire >0.5, and the proportion of these patients who required maintenance OCS was reduced from 54% to 47% at 6 months. 67 A Cochrane analysis reported no difference in median OCS dose or the proportion of patients successfully withdrawn from maintenance OCS in patients treated with omalizumab compared with placebo, in the one study available at the time. 90
Bronchial thermoplasty is a novel treatment modality by which chronic OCS can potentially be reduced. However, further research is essential as reductions in chronic OCS found in initial clinical trials were not confirmed by ‘real‐world’ observational data. 91
CURRENT USE OF OCS IN ASTHMA
Epidemiology of use
The chronic use of OCS amongst people with difficult‐to‐treat asthma is high, particularly when balanced against the harms of treatment. 21 , 92 Data from the Australian Severe Asthma Web‐Based Database, a multicentre study enrolling people with severe asthma from Australia, New Zealand and Singapore, report the prevalence of maintenance OCS treatment at 24%. 93 Those patients using maintenance OCS were prescribed a median dose of 10 mg/day, but the dose range was wide (2–50 mg daily). These data are consistent with regular OCS use (defined as >90 days of OCS use in a year) across five countries reported by the International Severe Asthma Registry (ISAR). The mean proportion of patients with uncontrolled asthma at GINA step 4 and above using regular OCS was 30% (95% CI 24.5, 35.7). 3
A systematic review examined patterns of OCS in 64 published observational and registry studies. Overall, long‐term OCS use was reported in 1%–31% of people with asthma. In people with severe or uncontrolled asthma, use of long‐term OCS (defined as frequent intermittent or daily maintenance treatment) was between 20% and 60%. 13 The dosages of long‐term OCS were reported in 23 of the included studies, and the mean daily prednisolone‐equivalent dose ranged between 4 and 21 mg per day with over half of the studies reporting a mean dose of 10 mg/day or more. In the 58 studies reporting short‐burst OCS use, the number of prescriptions per year increased with increasing asthma severity and was reported to be between 3% and 62% of patients managed in a variety of healthcare settings. 13 These findings on acute asthma and OCS prescriptions are consistent with large‐scale real‐world studies. 94 , 95
Evidence of overuse or over‐reliance on OCS
Recent evidence from Australia supports the occurrence of overuse and over‐reliance on OCS for asthma. A study published in 2020 by Hew et al. 23 used analysis of a 10% sample of Pharmaceutical Benefits Scheme dispensing data from 2014 to 2018 to assess the proportion of people dispensed OCS for asthma management, and the proportion who were dispensed a potentially toxic cumulative dose of ≥1000 mg of prednisolone within that 5 years. Overall, more than half of the individuals were prescribed OCS in the study period, and more than a quarter (28%) were dispensed a cumulative dose ≥1000 mg of prednisolone, consistent with primary care data from the UK. 96 A secondary outcome of this study was to determine the number of people dispensed ≥1000 mg of prednisolone‐equivalent during 2018, stratified by the dose of inhaled preventer. There were 4633 people with asthma using high‐dose ICS who were dispensed ≥1000 mg prednisolone‐equivalent; of these, 2316 (50%) had poor adherence with treatment based on evidence of infrequent dispensing of preventer medications.
These data suggest that OCS use is excessive in relation to asthma severity, and that OCS are being dispensed to people with asthma who currently have suboptimal adherence with ICS treatment. This situation may be more pronounced in rural communities as, globally, regions with limited access to specialist care demonstrate low use of effective preventative therapy in those individuals with recurrent exacerbations. 97
Current Australian guidelines
The Australian Asthma Management Handbook recommends long‐term OCS use for adults and adolescents, but only as an option for those individuals with severe asthma in whom the disease remains uncontrolled despite the use of high‐dose ICS in combination with LABAs. These recommendations include trials of add‐on therapies and referral to specialist care, avoiding chronic OCS if at all possible. Other international guidelines make similar recommendations 98 but it is apparent that these proposals are often not followed, and many patients are prescribed chronic OCS without specialist review or appropriate assessment. 99
FIGURE 3.
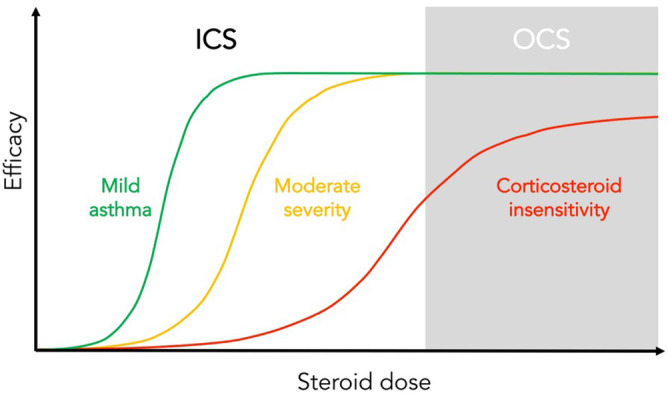
Depiction of inhaled or oral glucocorticoid efficacy and responsiveness in the treatment of asthma. In mild asthma, benefit can be achieved at low doses, efficacy is reduced in moderate severity disease whereas in patients with corticosteroid insensitivity limited benefit is gained, even at high doses
NATURE AND PREVALENCE OF HARM ARISING FROM ORAL STEROIDS
Both long‐term and short‐term OCS use in patients with asthma are associated with steroid‐related adverse effects. 13 Risks of OCS‐related complications including ED presentations, hospitalizations and mortality escalate with increasing OCS exposure. 20 , 100 , 101 This is true both for the cumulative dose derived from repeated acute courses and also for chronic therapy as individuals taking at least 10 mg per day of prednisolone‐equivalent are at substantially higher risk of developing musculoskeletal, metabolic and psychiatric issues. 102 Those who experience steroid‐related adverse effects also have a higher disease burden and exacerbation risk, 103 compounding the detrimental effects on their health.
Risks associated with long‐term/maintenance use
Steroid‐related comorbidities are highly prevalent in people with severe asthma, with over half of this group having three or more steroid‐related comorbidities. 92 The most common steroid‐related effects are shown in Figure 4. 92 , 103
FIGURE 4.
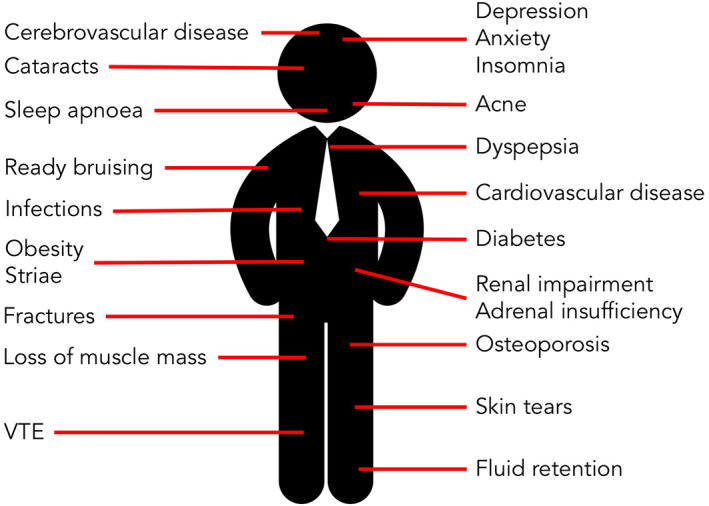
Harmful effects of oral corticosteroids in asthma are common and widespread and affect all organ systems. VTE, venous thrombo‐embolism
Multiple studies report the association between maintenance OCS use and risk of hypertension, hypercholesterolaemia and risk of cardiovascular complications. 13 , 21 For example, a retrospective study comparing individuals whose asthma was treated with OCS against those on any other therapy found an increased risk of all‐cause mortality, coronary heart disease, cerebrovascular disease and heart failure. Effect sizes were substantial, with hazard ratios (HRs) for coronary heart disease and heart failure in patients using OCS being 2.6 and 3.5, respectively. 17 It should be noted that other confounders may have played a role including the possibility that both severe asthma per se and OCS contributed to increases in risk.
The risk of osteoporosis and fractures is also dose‐dependent. 15 , 21 Compared with patients without SCS exposure, the risk of musculoskeletal complications is 1.36‐, 2.28‐ and 2.42‐fold higher in those on lower (<5 mg/day), medium (5‐10 mg) and higher dose (>10 mg) prednisolone‐equivalent, respectively. 102 Furthermore, fractures are more likely to occur in steroid‐dependent asthma patients with bone mineral density above the fracture threshold value than in those with non‐steroid‐related involutional osteoporosis. 104
Depression and anxiety are three times more likely in patients with asthma requiring maintenance OCS than in those with severe asthma who do not take oral steroids. 105 Metabolic complications such as weight gain, fluid retention and electrolyte abnormality have been documented for over 50 years. 106 The risk of type 2 diabetes and obesity is higher in asthma patients with greater or longer OCS use compared with patients with milder disease. 15 , 19 , 21
As a consequence of these complications, healthcare costs of managing patients with severe asthma on maintenance OCS are at least 40% higher than for those not using maintenance OCS. 107 In a primary care study of over 15,000 people with asthma, age‐adjusted annual healthcare costs were much greater in regular OCS users than in non‐OCS users and intermittent OCS users. 108 Furthermore, the annualized incremental costs of managing OCS‐related complications increase with higher maintenance OCS dosages. 101
Risk associated with short‐term use
The risk associated with short‐term OCS use appears often underestimated. In a study of 1.5 million adults, those individuals treated with short‐term OCS for respiratory indications, mostly asthma, were three times more likely to have sepsis or venous thromboembolism within the first 30 days of initiation; the risk of fracture was also increased by two‐fold. Risk persisted for at least 3 months despite a relatively brief duration of treatment (median 6 days) and a modest median dose of 20 mg prednisolone‐equivalent. 109 Other adverse effects such a mood disorders and hyperglycaemia were not assessed.
Adrenal insufficiency
An important consideration for people with asthma is the potential impact of corticosteroid use on normal hypothalamic and/or anterior pituitary function. Exogenous corticosteroids can suppress secretion of steroid hormones from the adrenal cortex (adrenal suppression). Consequently, cessation, decrease in dose or changing the type of exogenous corticosteroid administered may trigger features of secondary adrenal insufficiency (AI). While exogenous corticosteroid therapy is the most common cause of AI, it is under‐recognized in adults with asthma. 110 , 111
A meta‐analysis of studies in adults with asthma identified cortisol suppression in 18%, 27% and 36% of patients prescribed low‐, medium‐ or high‐dose ICS, respectively. 112 A recent systematic review of patients with severe asthma determined that up to 25% of people with asthma prescribed high‐dose, long‐term ICS and 68%–100% of those prescribed high‐dose, long‐term ICS and OCS experienced AI. 113 Even though higher doses and longer duration of use are associated with higher risk of AI, this cannot be excluded with certainty in any patient taking corticosteroids. Ideally, screening for AI should be undertaken in selected patients with asthma, especially people using high‐dose ICS, OCS or both. 111 , 114
The clinical presentation of AI can vary widely, but often includes non‐specific features. 115 , 116 , 117 Signs and symptoms include weakness, fatigue, malaise, gastrointestinal upset (nausea, vomiting, diarrhoea, abdominal pain), anorexia/weight loss, headache, fever, myalgia, arthralgia and psychiatric symptoms. 117 , 118 It is not surprising, therefore, that AI often goes unnoticed until a physiological stress (surgery, trauma, intercurrent illness) precipitates an acute adrenal crisis. 119
The National Patient and Safety Alert (National Health Service UK) has developed a ‘Steroid Emergency Card’ aimed at supporting early identification and treatment of adrenal crisis in adults. 120 The NSW Steroid Emergency Card can be issued by community pharmacies, general practitioners (GPs) or hospital physicians and is intended to be carried by high‐risk patients at all times, leading to increased awareness of the need to promptly commence corticosteroid treatment in this patient population. 121
Cumulative risk
Repeated short‐term OCS use increases the risk of complications. For example, a study from the United States compared over 70,000 people with asthma who had received OCS and twice as many matched individuals with asthma who had not been treated. Individuals prescribed four or more courses of OCS were at significantly higher risk (OR 1.29) of adverse events in the first year including a new diagnosis of osteoporosis, hypertension, obesity, type 2 diabetes, gastrointestinal ulcers or bleeds, fractures and cataracts. This risk appeared additive, with those who had three successive years of ≥4 OCS prescriptions having an OR of 1.73 for adverse events by the third year compared with patients not exposed to OCS. 15 Similar findings arose from a primary care study in the United Kingdom where over 24,000 individuals receiving OCS (and matched controls) were followed up for as long as records were available or until an adverse event occurred. 21 Compared with matched controls without any OCS exposure, those prescribed OCS had significantly higher incidence of developing a variety of adverse outcomes including osteoporosis/fracture (HR 3.11), pneumonia (HR 2.68), cardio/cerebrovascular disease (HR 1.53), cataracts (HR 1.50), sleep apnoea, (HR 1.40) and renal impairment (HR 1.36). Importantly, there was a clear dose–response relationship between adverse events and cumulative OCS exposure, with substantial increases in risk seen above a cumulative dose of 1000 mg.
Despite diverse and individual susceptibilities, the concerning clinical implication from several studies is that a potentially toxic threshold dose (approximately 1000 mg prednisolone or equivalent) is reached with lifetime exposure to just four OCS scripts at dosages typically used for management of asthma exacerbations. The repercussions associated with cumulative use of OCS are supported by data from large retrospective cohort studies and are of concern particularly in Australia and other countries where an initial prescription for a short burst of OCS often results in the dispensing of far more tablets than are needed for treatment of the current event.
Estimating the harm from OCS in asthma
Concordant with studies from the United States and Europe, recent Australian research indicates that OCS use is widespread and leads to considerable harm. As described above, a 10% random sample of the Australian Pharmaceutical Benefits Scheme was used to identify people with asthma aged over 12 years based on inhaled controller dispensing. 23 In the primary analysis of 124,011 people with asthma, 52% were dispensed OCS at some point over a 5‐year period. More than a quarter were prescribed cumulative doses of over 1000 mg prednisolone‐equivalent. As detailed above, a secondary analysis of data from 2018 found that approximately half of these individuals had evidence of suboptimal adherence with preventer treatment. Importantly, patients who had been dispensed >1000 mg prednisolone‐equivalent were significantly more likely to require medication for diabetes mellitus and osteoporosis (Figure 5).
FIGURE 5.
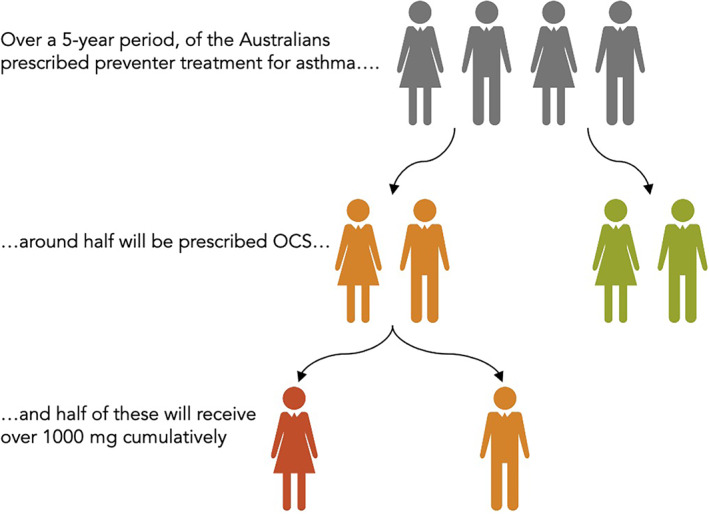
Natural frequency diagram highlighting the prevalence of high cumulative dose oral corticosteroid exposure in asthma. Based on data from studies by Hew et al. 23
OCS USE IN PRACTICE: PERSPECTIVES FROM PEOPLE WITH ASTHMA
I just came off the prednisone and the pictures are not flattering. I'm embarrassed [about the weight gain] ‘cos I can see that I'm so puffy. (female, 45 years). 122
The benefits and risks from the use of OCS in inflammatory diseases have been increasingly and thoroughly documented over the last 70 years. Despite having the potential for both marked positive and negative impacts on the lives of people with asthma, until recently there has been little academic examination of patient perspectives in this area.
Over the last decade, there have been a number of studies that have examined the patient experience of OCS in a severe asthma population. A systematic review of the experience of living with severe asthma emphasized the emotional as well as the physical burden of OCS treatment. 123 Hyland et al. conducted a qualitative study in people with severe asthma to compare the burden of disease and treatment with items in eight asthma‐specific quality of life scales. 124 The authors reported significant negative impacts of OCS in people with severe asthma and patients specifically discussed the major impact of OCS on depression, irritability, sleep, hunger, weight, skin, gastric, pain and anxiety. They concluded that the burden of OCS in this population is neglected in both policy and practice. 124 These findings are supported by those of Foster et al., 122 who conducted a qualitative study in an Australian severe asthma population to explore the experiences of living with severe asthma. The burden of treatment emerged as a theme in this study, with particular reference to the concerns of OCS side effects. Clark et al. also examined the experiences of people with severe asthma who were prescribed either a monoclonal antibody therapy or a macrolide antibiotic. 125 In this study, 20 patients who had been using these treatments for at least 4 months were interviewed. From these interviews, one of the four emergent themes was ‘Prednisone—a necessary evil’. Patients related that, despite the perceived effectiveness of biological therapies and macrolides, prednisone use was an ongoing concern. They shared their frustration of being ‘still stuck on prednisone’, and of their concerns for the ‘damaging side effects’. Despite these concerns, there was an acceptance of OCS treatment and they stated, ‘but it keeps me breathing’. 125
The importance of OCS reduction was highlighted in a cross‐sectional study by the same group, 126 who sought to understand the outcomes that patients wanted to improve following initiation of add‐on asthma treatments for severe asthma. When participants were asked to select their top five outcomes, the most important were improving quality of life, followed by a reduction in acute exacerbations, increased participation in physical activity and less OCS treatment. These were the four most highly ranked priorities from a selection of 17 options. 126 There also appears to be a significant gap between clinicians and people with severe asthma in terms of their perspectives and goals. 123 Costello et al. (2017) reviewed the perspective of individuals with chronic diseases towards various adverse effects of OCS in an online health community survey. Only 9% of the study population had respiratory disease, despite this being the most common indication for OCS. 127 The 604 respondents consistently placed a high importance on the side effects of weight gain, insomnia and facial changes. Categories of adverse effects that might be perceived by clinicians to be more significant (such as cardiovascular disease and diabetes) were rated as less important by patients.
In addition to potentially misjudging what side effects matter most to patients, clinicians are also poor at appraising the chance of adverse outcomes. For example, a UK survey found healthcare professionals appeared to consistently underestimate the risk of commonly reported side effects such as excessive bruising (Figure 6). 128 Concerns regarding overuse of OCS by patients were also associated with lower adherence with therapy. The perception of chronic OCS use was studied specifically in airways disease prior to the availability of biological therapy for asthma. 128 In that era, people with asthma acknowledged the severity of their condition and generally felt OCS were a necessary evil. However, this study also highlighted that patients want more information about OCS side effects than they are often given, and that this information should be provided when therapy is commenced. Subsequent studies have also found that people with asthma perceive OCS to be effective, but they are concerned regarding side effects.
FIGURE 6.
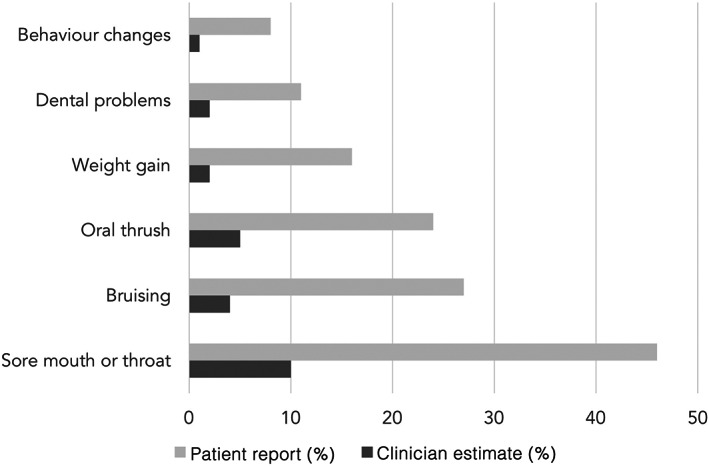
Proportion of people with asthma who reported experiencing specific symptoms attributable to oral corticosteroid side effects and the median proportion of patients estimated by clinicians to experience these side effects. Redrawn from Cooper et al. 128
The adverse effects experienced or anticipated from OCS use have been identified as a ‘push factor’ that motivates people with asthma to use complementary therapies such as homeopathy and supplements not meticulously examined in clinical trials. 129 There is also evidence that patients may have considerable apprehension about using ICS. A Canadian study found that approximately 50% of patients were hesitant to take ICS treatment for reasons that included possible side effects, decreased bone density and reduced efficacy over time. 130 Two‐thirds had not discussed their concerns with the physician. This may mean that patients avoid the very treatment that has least adverse effects and that represents their best chance to avoid OCS medication.
Personal stories relating to OCS use for severe asthma can be found on the Health Talks website (https://healthtalkaustralia.org/severe-asthma/overview/). Individuals often highlight the apparently casual attitude of some prescribers to starting OCS, but also the great symptomatic benefit they can experience. We recommend listening to descriptions of patients' experiences detailing side effects that include weight gain, AI, increased risk of infection and impaired wound healing.
PRINCIPLES OF ACUTE OCS USE AND PREVENTION OF OVERUSE
Primary prevention of OCS use
Guideline‐recommended asthma care reduces the risk of acute asthma exacerbations requiring OCS. Given the high rates of asthma misdiagnosis in the community, every effort should be made to confirm or exclude asthma objectively to reduce the risk of erroneous use of OCS. Guidelines recommend attention to inhaler technique, adherence to therapy and regular medical review. Self‐management education, including the provision of an action plan, can reduce exacerbations requiring emergency presentations and hospital admissions. All these measures should reduce the requirement for OCS prescription for acute exacerbations.
In selected patients with mild asthma, use of combination ICS and formoterol as needed for symptoms can reduce exacerbations requiring OCS. Patients with moderate asthma also experience reduced exacerbations with a combination of maintenance and as‐needed (reliever) therapy. An alternative strategy of quadrupling the dose of fixed‐dose ICS at the first sign of instability has been shown to reduce the likelihood of progression to exacerbations in some adults, 53 but quintupling the dose was not effective in children with good adherence to treatment. 131
Clarification of acute indications
It can be challenging to identify an exacerbation requiring OCS. Guidelines define exacerbations as ‘a change in symptoms or lung function from baseline’. Increasing the dose of ICS in response to a fall in peak flow of 20%–40% may reduce the likelihood of requiring OCS. In patients with mild asthma, a single day of asthma symptoms leading to use of two or more inhalations of as‐needed ICS‐formoterol was associated with a 75% reduction in need for OCS in the subsequent 3 weeks compared with SABA alone 132 ; similar but smaller reductions in short‐term need for OCS were seen in patients prescribed MART, after a day with >6 reliever inhalations. 133
Dose and duration
Doses of between 37.5 and 50 mg of prednisolone‐equivalent for approximately 5–10 days are recommended. To date, there are few data to support a specific dose or duration of treatment.
Secondary prevention
Patients with frequent exacerbations, despite the use of high‐dose ICS, may have multiple contributors to their disease instability. Such patients benefit from medical review of all aspects of their asthma management, ideally by a multidisciplinary team. Observational data demonstrate a reduction in the frequency of exacerbations following such assessment, associated with a reduction in OCS requirements.
In patients with previous exacerbations on ICS and LABA combinations, therapeutic options to reduce the risk of future exacerbations include use of maintenance plus reliever ICS‐formoterol, azithromycin three times per week and/or the addition of tiotropium.
Among patients with previous exacerbations, despite the use high‐dose ICS and LABAs, randomized trials of omalizumab, mepolizumab, benralizumab, reslizumab and dupilumab all demonstrate reductions in exacerbations requiring OCS. Bronchial thermoplasty reduces exacerbations in moderate to severe asthma (if complications in the peri‐procedural period are excluded), but there is a need to obtain further evidence, so guidelines do not currently recommend routine use of thermoplasty for severe asthma.
Special groups
In pregnancy, the occurrence of exacerbations requiring OCS is associated with an increased incidence of pre‐term and low birth weight infants. Balanced against this is an increased risk of adverse foetal outcomes in untreated status asthmaticus. Therefore, OCS should be prescribed whenever clinically indicated. Non‐adherence to inhaler medication is strongly associated with increased risk of exacerbations during pregnancy 134 and this aspect should be carefully reviewed in pregnant patients with unstable asthma.
Rescue packs for home administration
To commence OCS as part of an action plan, patients are often provided with prescriptions that can be filled urgently. This prevents delays in implementing the agreed action plan. However self‐management requires that patients are able to monitor symptoms and/or peak flows, detect deterioration appropriately and decide when to institute OCS therapy. This is most likely to succeed when self‐management is regularly taught and may involve many hours of coaching, over repeated sessions. It is possible that provision of OCS rescue packs without appropriate education may have unwanted effects including inappropriate increases in OCS use, a potential problem that requires further study.
PRINCIPLES OF CHRONIC OCS USE AND PREVENTION OF OVERUSE
Primary prevention of OCS use
Maintenance OCS are required in some patients who have severe asthma. Chronic use should be restricted to those who have had their diagnosis of asthma confirmed and have been unable to achieve adequate asthma control despite the use of high‐dose ICS and a controller. This treatment pathway should only be considered when patients have trialled alternate add‐on therapies, or they are either not eligible or have not responded to biological treatments. 135
Patients who are being considered for maintenance OCS should undergo specialist or multidimensional assessment that involves review of asthma management skills and adherence, comorbidities and assessment of inflammatory phenotype. 59 , 136 , 137 Should asthma management skills and adherence be suboptimal, an attempt to address these should be performed. 138
In the era of biological treatment for severe asthma, there are now distinct opportunities to reduce overuse of maintenance OCS whilst avoiding toxicity of these agents. The advent of biologics will substantially change OCS use in many patients who qualify for biological therapy and maintenance OCS should not be used as a ‘holding position’ prior to initiation of biological therapy. Importantly, patients who do not qualify for biological therapy (high symptom burden, low exacerbation rate, absence of type 2 asthma biomarkers) may be given a therapeutic trial of OCS, but it is critical that this should be ceased if there is no objective evidence of benefit.
When OCS are considered for maintenance asthma treatment, they should be prescribed at the minimum dose required to achieve adequate asthma control and treatment should be regularly reviewed. Doses of OCS can be minimized through the use of biomarkers and by prescribing treatment on alternate days. A schema for this process is outlined in Figure 2 (see also Box 1).
BOX 1.
Recommended principles for weaning and stopping of maintenance OCS in asthma
| I. Assess and manage disease control |
| a. Maintenance OCS can be weaned (‘back‐titrated’) whilst monitoring asthma disease control using symptoms of asthma (e.g., Asthma Control Questionnaire scores) and exacerbation history |
| b. These parameters should be supplemented by objective markers including lung function and blood eosinophil counts |
| c. The interval for dose reduction needs to allow time for a change in disease activity to be reflected in the outcome measure—this is usually for a period of 1–4 weeks |
| d. The quantum dose reduction needs to be a meaningful change to allow for detection of any increase in disease activity. It can be expressed as a percentage of total daily dose, typically 25% or 50% reduction; or a dose amount, such as 5 or 10 mg of prednisone (or equivalent) |
| e. Patients should be provided with clear printed/written information regarding the steroid weaning plan in terms of the dosing schedule and monitoring |
| II. Assess and manage AI |
| a. Once the maintenance OCS dose is approximately 10 mg prednisone (or equivalent) daily, consider the possibility of AI. This can be identified by clinical history and optimally by laboratory testing |
| b. The tests used differ widely but a minimum benchmark would be a morning serum cortisol measurement |
| c. For test interpretation, cortisol values of <100 nmol/L are considered to be indicative of AI until further testing can confirm or refute the diagnosis |
| d. In the absence of confounding factors, measurements of morning cortisol >400 nmol/L reasonably exclude hypocortisolism (some centres use thresholds of 350 nmol/L or lower) |
| e. Dose reduction of OCS in patients with or considered at risk of AI should be done at a slower rate with periodic assessment of the emergence of symptoms of AI |
Abbreviations: AI, adrenal insufficiency; OCS, oral corticosteroid.
Harm reduction strategies
Biomarker‐driven treatment decisions such as those used to choose therapies may also possibly be deployed to reduce exposure to OCS. An Australian proof‐of‐concept study showed that OCS for severe asthma could potentially be reduced by more than 50% using a blood eosinophil biomarker algorithm. 139 In contrast, an RCT in patients with severe asthma failed to show a significant reduction in OCS use employing a strategy based on a composite type 2 biomarker endpoint (FeNO, periostin and blood eosinophils) compared to usual care. 140 However, in this study, many participants did not follow treatment advice. When the findings were analysed on a per‐protocol basis, a greater proportion of those in the intervention group were on a lower dose of OCS (31%) at 48 weeks compared with control (5%, p = 0.026), but the difference in daily OCS dose (−2 mg) was not significant. This study highlights the need for good patient–clinician partnerships and shared decision‐making when implementing novel strategies.
A further challenge to minimizing chronic OCS exposure is the lack of asthma‐specific guidance for reducing the dose or ceasing OCS in clinical practice. An international consensus paper has suggested a tapering strategy for this purpose, and the results of the subsequent evaluation study are awaited with interest. 141 Harm reduction may also be attempted by early identification and mitigation of predictable adverse effects. A checklist based on expert consensus has also been developed for identifying OCS‐related problems. 141 Practical strategies to taper and stop long‐term OCS maintenance therapy are outlined in Box 1.
Effective OCS‐sparing strategies are now available which will lead to reduced OCS requirements and therefore adverse effects of treatment. Add‐on therapies including novel biologicals such as mepolizumab, 84 benralizumab 142 and dupilumab 143 are also effective in reducing OCS requirements. 144 Long‐term macrolide antibiotics reduce exacerbations 71 , 72 , 144 significantly in severe asthma which in turn may lead to reduced OCS requirements, but the risk of microbial resistance must be considered.
Finally, when OCS are prescribed as maintenance treatment, bisphosphonates are recommended for the prevention of fractures in patients with glucocorticoid‐induced osteoporosis or in the presence of osteopenia. This is particularly important if the dose of OCS is ≥7.5 mg prednisolone/day (or equivalent) for 3 months or more. 135
Monitoring adverse effects
In the absence of an evidence‐based guideline that makes recommendations for standard monitoring of adverse effects of OCS in asthma, a recent international study has promulgated a checklist based on expert consensus. 145 The checklist recommends at minimum, glycaemic control, bone mineral density, blood pressure, cataracts and glaucoma, weight change and fracture risk score. These measures should also be performed routinely on patients using regular OCS. The National Asthma Council's Asthma Management Handbook suggest that an annual dual‐energy x‐ray absorptiometry scan be obtained at baseline and repeated every 1–5 years (depending on age, sex and initial result). 135
The Glucocorticoid Toxicity Index (GTI) was developed to assess corticosteroid‐related morbidity and corticosteroid‐sparing ability of therapies. 146 GTI was then used to examine individual glucocorticoid toxicity in patients with steroid‐dependent severe asthma 147 in a study of patients from a UK Regional Severe Asthma Specialist Clinic. Measurements using GTI were found to be strongly correlated with age and asthma quality of life (rather than with corticosteroid exposure in the preceding year) and was reported to be a useful tool to identify and quantify toxicity in individual patients. The authors recommended its use in facilitating assessment of patient response to corticosteroid‐sparing asthma therapy, both in clinical trials and in routine practice. 147
SUMMARY OF CORE PRINCIPLES OF OCS STEWARDSHIP IN ASTHMA
OCS have proven benefit in both acute exacerbations and in chronic severe asthma.
Prescription of OCS for asthma is common.
OCS have potentially severe adverse effects, both acutely and long term.
Strong evidence indicates that there is overuse of OCS to treat asthma and a harmful dose (lifetime dose >1000 mg prednisolone‐equivalent) may be exceeded in up to 25% of patients.
OCS have a significant impact on patients' quality of life.
Optimal dose and duration of OCS in acute and chronic asthma have not been established.
Reductions in OCS use are essential and primary strategies should focus on optimizing inhaler technique, improving treatment adherence, smoking cessation, use of add‐on treatments such as LABA and LAMA as appropriate and ensuring adequate doses of ICS are prescribed.
Comorbidities should be identified and managed, if possible, using multidimensional assessments and other initiatives such as online resources (e.g., Severe Asthma Toolkit: https://toolkit.severeasthma.org.au/resources/infographics/), written action plans and OCS treatment report cards.
Novel biological agents (e.g., targeting IL‐5, IgE and IL‐4/13 activities) mitigate OCS use in patients with type 2 asthma. As they are expensive and require appropriate application and specialist prescription, biological agents are chiefly prescribed in specialist centres. In this context, referral of people with asthma controlled only by frequent OCS courses or maintenance OCS prescription is a priority for primary care.
Restrictions on OCS prescription may be considered in future. For example, regulatory approaches have potential to address OCS stewardship effectively and minimize inappropriate prescribing. Examples include creating an OCS rescue pack with ten 25 mg tablets only (instead of providing 30 tablets), rescheduling maintenance OCS use to authority prescription only and allowing prescribers better access to data on ICS adherence to facilitate detection of and counselling for non‐adherence.
Mechanisms for review of OCS prescriptions that can be conducted within hospital and/or national jurisdictions can permit long‐term monitoring of OCS use (Box 2).
BOX 2.
Summary of core principles of OCS stewardship in asthma
|
|
|
|
|
|
|
|
|
Abbreviations: ICS, inhaled corticosteroid; OCS, oral corticosteroid.
CLINICAL RESOURCES AND DISSEMINATION
Clinical resources are available to assist both clinicians and patients with prescription and use of OCS. The Severe Asthma Toolkit (see below) contain useful resources including:
OCS report cards,
Infographics for chronic use of OCS in asthma (not including ABPA),
Infographics relating to OCS use and harms,
Written asthma action plan and
A patient charter.
Most resources can be downloaded from the Severe Asthma Toolkit (https://toolkit.severeasthma.org.au/resources/infographics/), an online resource for clinicians caring for people with severe asthma. 148
SUMMARY
OCS are frequently used to treat asthma during acute flares (burst therapy) and for chronic disease (maintenance treatment). Indiscriminate use of burst and maintenance OCS therapy is common, posing a risk of irreversible harm affecting multiple organ systems. Patient perspectives have also emphasized detrimental impacts of OCS‐related side effects such a weight gain, insomnia, mood disturbances and skin changes. Careful and thorough assessment of poorly controlled asthma is key to improved asthma control, preventing exacerbations and reducing OCS use. This can be achieved by improved inhaler technique, adherence to therapy, asthma education, smoking cessation, specialist/multidisciplinary review, optimized medications and other strategies. Recently, add‐on therapies including novel biological agents have demonstrated reductions in OCS requirements. Harm reduction may also be gained by early identification and mitigation of predictable adverse effects.
Introduction and application of OCS stewardship programmes have become imperative and this TSANZ position paper outlines principles applicable to this vital initiative. We indicate how steps can be taken to minimize use of OCS, minimize exposure when they are indicated and reduce harm through vigilance for emerging adverse effects. Following the strategies outlined in this paper, OCS use can be minimized and harm mitigated if OCS stewardship practises are established and implemented.
CONFLICT OF INTEREST
John Blakey has received a contract from Astra Zeneca (AZ) to deliver research (phase 2 RCT), grant funding from Novartis for an investigator‐initiated study and a contract from GSK to deliver research (multicentre phase 3 RCT); consulting fees from GSK, Chiesi and Boehringer Ingelheim (BI); honoraria for speaking at educational meetings, podcasts and vodcasts from AZ, GSK and Chiesi; travel support to attending meetings from GSK, BI and AZ; medical writing support from GSK and Teva; payment for advisory work from Asthma Australia; and has served on the advisory board of Asthma WA and the steering committee of Optimum Patient Care Australia. Li Ping Chung has received consulting fees from GSK, AZ and Chiesi for advisory board meetings over the past 5 years on topics related to asthma; and honoraria from GSK, AZ, BI, Novartis and Chiesi for speaking/organizing educational events. Vanessa M. McDonald has received honoraria from AZ for lectures at the TSANZ and the APSR meetings on OCS use in severe asthma. Sinthia Bosnic‐Anticevich has received research grants from Teva, GSK and Mylan in the area of inhaler device use, severe asthma, primary care management of asthma and chronic obstructive pulmonary disease and managing allergic rhinitis in the community pharmacy; consulting fees from Teva, Sanofi, Mylan and GSK for the development of educational materials for GPs and pharmacists; honoraria from Teva, Sanofi, Mylan, GSK, AZ and BI for lectures on medicines use, inhaler devices, patient perspectives to respiratory medicines use and allergic rhinitis management; and has participated on advisory boards of Teva, AZ and BI related to inhaler devices. She is President of the Respiratory Effectiveness Group, Chair of Allergic Rhinitis in Asthma (ARIA) in Pharmacy and Chair of the TSANZ's Primary Care Special Interest group. Mark Hew has received grants and consulting fees from Sanofi, GSK, AZ and Novartis paid to his institution; and honoraria for presentations from Teva, Sanofi, GSK and AZ paid to his institution. Anne E. Holland has received fees and travel support from AZ for a non‐promotional speaking engagement (unrelated to the present work). Helen Reddel has received research grants from AZ for investigator‐sponsored research; research grants GSK and Novartis for investigator‐sponsored research and registries; and honoraria from AZ, GSK, Teva, BI, Sanofi and Chiesi for independent medical education. She's been on advisory boards of AZ, GSK, Novartis, Chiesi and Sanofi; is Chair of GINA Scientific Committee; and a member of the Australian Asthma Guidelines Committee for the National Asthma Council. John W. Upham has received speaker fees from AZ, GSK and Sanofi and served on advisory boards of AZ, GSK and Sanofi. He was the TSANZ President in 2021–2022. Peter G. Gibson received grants from GSK and NHMRC paid to his institution; honoraria from GSK, Novartis and AZ for lectures/presentations; and advisory board payments from Sanofi and Chiesi. Philip Bardin has received honoraria from GSK, Novartis, Sanofi, BI and AZ for lectures/presentations, and from GSK, AZ, Novartis and Sanofi for participation on an Advisory Board, donated to the Departmental Research Institute. Laurence Ruane, John Gornall, Chris Barton, John Harrington, Trudy Hopkins and Lata Jayaram have nothing to disclose.
Blakey J, Chung LP, McDonald VM, Ruane L, Gornall J, Barton C, et al. Oral corticosteroids stewardship for asthma in adults and adolescents: A position paper from the Thoracic Society of Australia and New Zealand. Respirology. 2021;26:1112–1130. 10.1111/resp.14147
Laurence Ruane and John Gornall are consumer representatives.
Handling Editor: Paul Reynolds
See related Editorial
REFERENCES
- 1. Marciniuk D, Nana A, Rabe K, Zar H, Ferkol T & MontesdeOca M et al. Respiratory diseases in the world: realities of today, opportunities for tomorrow. Forum of International Respiratory Societies. 2014, Afr J Resp Dis; 6; 4‐13.
- 2.Australian Institute of Health and Welfare 2020. Asthma. Cat. no. ACM 33. Canberra: AIHW. Viewed 16 September 2021, https://www.aihw.gov.au/reports/chronic-respiratory-conditions/asthma
- 3. Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies‐Gow AN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest. 2020;157(4):790–804. [DOI] [PubMed] [Google Scholar]
- 4. Osler W, McCrae T. Bronchial asthma. The principles and practice of medicine. New York: D. Appleton and Co; 1914. [Google Scholar]
- 5. Melland B. The treatment of spasmodic asthma by the hypodermic injection of adrenalin. Lancet. 1910;1:1407–11. [Google Scholar]
- 6. McCombs RP. Serial courses of corticotrophin or cortisone in chronic bronchial asthma. N Engl J Med. 1952;247(1):1–6. [DOI] [PubMed] [Google Scholar]
- 7. Gray SJ, Benson JA Jr, Spiro HM, Reifenstein RW. Effects of ACTH and cortisone upon the stomach: its significance in the normal and in peptic ulcer. Gastroenterology. 1951;19(4):658–73. [PubMed] [Google Scholar]
- 8. CONTROLLED trial of effects of cortisone acetate in status asthmaticus; report to the Medical Research Council by the subcommittee on clinical trials in asthma. Lancet. 1956;271(6947):803–6. [PubMed] [Google Scholar]
- 9. Brown HM. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet. 1958;2(7059):1245–7. [DOI] [PubMed] [Google Scholar]
- 10. Guidelines for management of asthma in adults: I – Chronic persistent asthma. Statement by the British Thoracic Society, Research Unit of the Royal College of Physicians of London, King's Fund Centre, National Asthma Campaign. BMJ. 1990;301(6753):651–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. GINA Global Strategy for Asthma Management and Prevention. Contract No.: Publication Number 95‐3659. NIH National Heart, Lung, and Blood Institute; Bethesda, MD, USA, 1995.
- 12. Woolcock A, Rubinfeld AR, Seale JP, Landau LL, Antic R, Mitchell C, et al. Thoracic society of Australia and New Zealand. Asthma management plan, 1989. Med J Aust. 1989;151(11–12):650–3. [PubMed] [Google Scholar]
- 13. Bleecker ER, Menzies‐Gow AN, Price DB, Bourdin A, Sweet S, Martin AL, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201(3):276–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sweeney J, Brightling CE, Menzies‐Gow A, Niven R, Patterson CC, Heaney LG, et al. Clinical management and outcome of refractory asthma in the UK from the British Thoracic Society Difficult Asthma Registry. Thorax. 2012;67(8):754–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):110–6.e7. [DOI] [PubMed] [Google Scholar]
- 16. Barry LE, Sweeney J, O'Neill C, Price D, Heaney LG. The cost of systemic corticosteroid‐induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017;18(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iribarren C, Tolstykh IV, Miller MK, Sobel E, Eisner MD. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol. 2012;176(11):1014–24. [DOI] [PubMed] [Google Scholar]
- 18. Lujan M, Gallardo X, Amengual MJ, Bosque M, Mirapeix RM, Domingo C. Prevalence of bronchiectasis in asthma according to oral steroid requirement: influence of immunoglobulin levels. Biomed Res Int. 2013;2013:109219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zazzali JL, Broder MS, Omachi TA, Chang E, Sun GH, Raimundo K. Risk of corticosteroid‐related adverse events in asthma patients with high oral corticosteroid use. Allergy Asthma Proc. 2015;36(4):268–74. [DOI] [PubMed] [Google Scholar]
- 20. Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Tran TN. Burden of chronic oral corticosteroid use by adults with persistent asthma. J Allergy Clin Immunol Pract. 2017;5(4):1050–60.e9. [DOI] [PubMed] [Google Scholar]
- 21. Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long‐term observational study. J Asthma Allergy. 2018;11:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voorham J, Xu X, Price DB, Golam S, Davis J, Zhi Jie Ling J, et al. Healthcare resource utilization and costs associated with incremental systemic corticosteroid exposure in asthma. Allergy. 2019;74(2):273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hew M, McDonald VM, Bardin PG, Chung LP, Farah CS, Barnard A, et al. Cumulative dispensing of high oral corticosteroid doses for treating asthma in Australia. Med J Aust. 2020. Sep 9;213(7):316–20. 10.5694/mja2.50758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McBrien CN, Menzies‐Gow A. Time to FOCUS on oral corticosteroid stewardship in asthma management. Respirology. 2019;24(4):304–5. [DOI] [PubMed] [Google Scholar]
- 25. Menzies‐Gow A, McBrien CN, Baker JR, Donnelly LE, Cohen RT. Update in asthma and airway inflammation 2018. Am J Respir Crit Care Med. 2019;200(1):14–9. [DOI] [PubMed] [Google Scholar]
- 26. Price D, Castro M, Bourdin A, Fucile S, Altman P. Short‐course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev. 2020. Apr 3;29(155):190151. 10.1183/16000617.0151-2019;29(155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buising KL, Thursky KA, Robertson MB, Black JF, Street AC, Richards MJ, et al. Electronic antibiotic stewardship – reduced consumption of broad‐spectrum antibiotics using a computerized antimicrobial approval system in a hospital setting. J Antimicrob Chemother. 2008;62(3):608–16. [DOI] [PubMed] [Google Scholar]
- 28. Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta‐analysis. BMJ. 2010;340:c2096. [DOI] [PubMed] [Google Scholar]
- 29. Asthma and Allergy Foundation of America . Oral Corticosteroid Stewardship Statement. 2018. Available from: https://www.aafa.org/media/2244/oral-corticosteroid-stewardship-statement-november-2018.pdf
- 30. Greenberger PA. Corticosteroids in asthma. Chest. 1992;101(6):418s–21s. [PubMed] [Google Scholar]
- 31. Ramsahai JM, Hansbro PM, Wark PAB. Mechanisms and management of asthma exacerbations. Am J Respir Crit Care Med. 2019;199(4):423–32. [DOI] [PubMed] [Google Scholar]
- 32. Kirkland SW, Cross E, Campbell S, Villa‐Roel C, Rowe BH. Intramuscular versus oral corticosteroids to reduce relapses following discharge from the emergency department for acute asthma. Cochrane Database Syst Rev. 2018;6:CD012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001;(1):CD002178. [DOI] [PubMed] [Google Scholar]
- 34. Sue M, Kwong F, Klaustermeyer W. A comparison of intravenous hydrocortisone, methylprednisolone, and dexamethasone in acute bronchial asthma. Ann Allergy. 1986;56(5):406–9. [PubMed] [Google Scholar]
- 35. Dembla G, Mundle R, Salkar H, Doifoide D. Oral versus intravenous steroids in acute exacerbation of asthma – randomized controlled study. J Assoc Physicians India. 2011;59:621–3. [PubMed] [Google Scholar]
- 36. Rodrigo G, Rodrigo C. Corticosteroids in the emergency department therapy of acute adult asthma: an evidence‐based evaluation. Chest. 1999;116(2):285–95. [DOI] [PubMed] [Google Scholar]
- 37. Rodrigo G, Neffen H. Systematic review on the use of omalizumab for the treatment of asthmatic children and adolescents. Pediatr Allergy Immunol. 2015;26(6):551–6. [DOI] [PubMed] [Google Scholar]
- 38. Rodrigo G, Rodrigo C, Hall J. Acute asthma in adults: a review. Chest. 2004;125(3):1081–102. [DOI] [PubMed] [Google Scholar]
- 39. Rowe BH, Edmonds ML, Spooner CH, Diner B, Camargo CA Jr. Corticosteroid therapy for acute asthma. Respir Med. 2004;98(4):275–84. [DOI] [PubMed] [Google Scholar]
- 40. Hasegawa T, Ishihara K, Takakura S, Fujii H, Nishimura T, Okazaki M, et al. Duration of systemic corticosteroids in the treatment of asthma exacerbation; a randomized study. Intern Med. 2000;39(10):794–7. [DOI] [PubMed] [Google Scholar]
- 41. Jones A, Munavvar M, Vail R, Aldridge R, Hopkinson L, Rayner C, et al. Prospective, placebo‐controlled trial 5 vs 10 days of oral prednisolone in acute adult asthma. Respir Med. 2002;96(11):950–4. [DOI] [PubMed] [Google Scholar]
- 42. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;(5):CD011801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rowe BH, Kirkland SW, Vandermeer B, Campbell S, Newton A, Ducharme FM, et al. Prioritizing systemic corticosteroid treatments to mitigate relapse in adults with acute asthma: a systematic review and network meta‐analysis. Acad Emerg Med. 2017;24(3):371–81. [DOI] [PubMed] [Google Scholar]
- 44. Krishnan JA, Davis SQ, Naureckas ET, Gibson P, Rowe BH. An umbrella review: corticosteroid therapy for adults with acute asthma. Am J Med. 2009;122(11):977–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rowe BH. Similar rate of relapse and time to return to normal activity within 2 to 5 days of systemic corticosteroids after asthma exacerbation but study confirmation of equivalence would require further study. Evid Based Med. 2012;17(1):23–4. [DOI] [PubMed] [Google Scholar]
- 46. O'Driscoll BR, Kalra S, Wilson M, Pickering CA, Carroll KB, Woodcock AA. Double‐blind trial of steroid tapering in acute asthma. Lancet. 1993;341(8841):324–7. [DOI] [PubMed] [Google Scholar]
- 47. Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–6. [DOI] [PubMed] [Google Scholar]
- 48. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reddel HK, Busse WW, Pedersen S, Tan WC, Chen Y‐Z, Jorup C, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post‐hoc efficacy analysis of the START study. Lancet. 2017;389(10065):157–66. [DOI] [PubMed] [Google Scholar]
- 50. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen Y‐Z, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double‐blind trial. Lancet. 2003;361(9363):1071–6. [DOI] [PubMed] [Google Scholar]
- 51. Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long‐acting β‐agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta‐analysis. JAMA. 2018;319(14):1485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harrison TW, Oborne J, Newton S, Tattersfield AE. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet. 2004;363(9405):271–5. [DOI] [PubMed] [Google Scholar]
- 53. McKeever T, Mortimer K, Wilson A, Walker S, Brightling C, Skeggs A, et al. Quadrupling inhaled glucocorticoid dose to abort asthma exacerbations. N Engl J Med. 2018;378(10):902–10. [DOI] [PubMed] [Google Scholar]
- 54. Busse WW, Bateman ED, Caplan AL, Kelly HW, O'Byrne PM, Rabe KF, et al. Combined analysis of asthma safety trials of long‐acting beta2‐agonists. N Engl J Med. 2018;378(26):2497–505. [DOI] [PubMed] [Google Scholar]
- 55. Sobieraj DM, Baker WL, Nguyen E, Weeda ER, Coleman CI, White CM, et al. Association of inhaled corticosteroids and long‐acting muscarinic antagonists with asthma control in patients with uncontrolled, persistent asthma: a systematic review and meta‐analysis. JAMA. 2018;319(14):1473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van der Meer A‐N, Pasma H, Kempenaar‐Okkema W, Pelinck J‐A, Schutten M, Storm H, et al. A 1‐day visit in a severe asthma centre: effect on asthma control, quality of life and healthcare use. Eur Respir J. 2016;48(3):726–33. [DOI] [PubMed] [Google Scholar]
- 57. Tay TR, Lee J, Radhakrishna N, Hore‐Lacy F, Stirling R, Hoy R, et al. A structured approach to specialist‐referred difficult asthma patients improves control of comorbidities and enhances asthma outcomes. J Allergy Clin Immunol Pract. 2017;5(4):956–64. [DOI] [PubMed] [Google Scholar]
- 58. Gibeon D, Heaney LG, Brightling CE, Niven R, Mansur AH, Chaudhuri R, et al. Dedicated severe asthma services improve health‐care use and quality of life. Chest. 2015;148(4):870–6. [DOI] [PubMed] [Google Scholar]
- 59. Clark VL, Gibson PG, Genn G, Hiles SA, Pavord ID, McDonald VM. Multidimensional assessment of severe asthma: a systematic review and meta‐analysis. Respirology. 2017;22(7):1262–75. [DOI] [PubMed] [Google Scholar]
- 60. Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add‐on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–16. [DOI] [PubMed] [Google Scholar]
- 61. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012;380(9842):651–9. [DOI] [PubMed] [Google Scholar]
- 62. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–207. [DOI] [PubMed] [Google Scholar]
- 63. FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti‐interleukin‐5 receptor α monoclonal antibody, as add‐on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2016;388(10056):2128–41. [DOI] [PubMed] [Google Scholar]
- 64. Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–66. [DOI] [PubMed] [Google Scholar]
- 65. Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–96. [DOI] [PubMed] [Google Scholar]
- 66. Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high‐dosage inhaled corticosteroids and long‐acting β2‐agonists (SIROCCO): a randomised, multicentre, placebo‐controlled phase 3 trial. Lancet. 2016;388(10056):2115–27. [DOI] [PubMed] [Google Scholar]
- 67. Gibson PG, Reddel H, McDonald VM, Marks G, Jenkins C, Gillman A, et al. Effectiveness and response predictors of omalizumab in a severe allergic asthma population with a high prevalence of comorbidities: the Australian Xolair Registry. Intern Med J. 2016;46(9):1054–62. [DOI] [PubMed] [Google Scholar]
- 68. Harvey ES, Langton D, Katelaris C, Stevens S, Farah CS, Gillman A, et al. Mepolizumab effectiveness and identification of super‐responders in severe asthma. Eur Respir J. 2020;55(5):1902420. [DOI] [PubMed] [Google Scholar]
- 69. Harrison T, Canonica GW, Chupp G, Lee J, Schleich F, Welte T, et al. Real‐world mepolizumab in the prospective severe asthma REALITI‐A study – initial analysis. Eur Respir J. 2020;56:2000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double‐blind placebo‐controlled trial. Thorax. 2013;68(4):322–9. [DOI] [PubMed] [Google Scholar]
- 71. Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2017;390(10095):659–68. [DOI] [PubMed] [Google Scholar]
- 72. Hiles SA, McDonald VM, Guilhermino M, Brusselle GG, Gibson PG. Does maintenance azithromycin reduce asthma exacerbations? An individual participant data meta‐analysis. Eur Respir J. 2019;54:1901381. [DOI] [PubMed] [Google Scholar]
- 73. Paton J, White J, Annandale J, Boyter A, Brown J, Cant B, et al. British guideline on the management of asthma. London: British Thoracic Society and Scottish Intercollegiate Guideline Network; 2019. [Google Scholar]
- 74. Corticosteroid therapy in asthma. Br Med J. 1961;2(5264):1413–4. [PMC free article] [PubMed] [Google Scholar]
- 75. Phear D, Ball K, Page F. Prolonged treatment with steroids in severe chronic asthma. Lancet. 1960;1(7116):139–41. [DOI] [PubMed] [Google Scholar]
- 76. Pizzichini MM, Pizzichini E, Clelland L, Efthimiadis A, Pavord I, Dolovich J, et al. Prednisone‐dependent asthma: inflammatory indices in induced sputum. Eur Respir J. 1999;13(1):15–21. [DOI] [PubMed] [Google Scholar]
- 77. Inhaled corticosteroids compared with oral prednisone in patients starting long‐term corticosteroid therapy for asthma. A controlled trial by the British Thoracic and Tuberculosis Association. Lancet. 1975;2(7933):469–73. [PubMed] [Google Scholar]
- 78. Mash B, Bheekie A, Jones PW. Inhaled vs oral steroids for adults with chronic asthma. Cochrane Database Syst Rev. 2001;(1):CD002160. [DOI] [PubMed] [Google Scholar]
- 79. Voskamp AL, Gillman A, Symons K, Sandrini A, Rolland JM, O'Hehir RE, et al. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2015;3(2):192–9. [DOI] [PubMed] [Google Scholar]
- 80. Steinfeld J, Bradford ES, Brown J, Mallett S, Yancey SW, Akuthota P, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol. 2019;143(6):2170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Effects of age and disease severity on systemic corticosteroid responses in asthma. Am J Respir Crit Care Med. 2017;195(11):1439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. The ENFUMOSA cross‐sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J. 2003;22(3):470–7. [DOI] [PubMed] [Google Scholar]
- 83. Moore WC, Bleecker ER, Curran‐Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid‐sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–97. [DOI] [PubMed] [Google Scholar]
- 85. Bourdin A, Shaw D, Menzies‐Gow A, FitzGerald JM, Bleecker ER, Busse WW, et al. Two‐year integrated steroid‐sparing analysis and safety of benralizumab for severe asthma. J Asthma. 2019;58:1–9. [DOI] [PubMed] [Google Scholar]
- 86. Khurana S, Brusselle GG, Bel EH, FitzGerald JM, Masoli M, Korn S, et al. Long‐term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther. 2019;41(10):2041–56.e5. [DOI] [PubMed] [Google Scholar]
- 87. Sposato B, Camiciottoli G, Bacci E, Scalese M, Carpagnano GE, Pelaia C, et al. Mepolizumab effectiveness on small airway obstruction, corticosteroid sparing and maintenance therapy step‐down in real life. Pulm Pharmacol Ther. 2020;61:101899. [DOI] [PubMed] [Google Scholar]
- 88. Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes‐Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–82. [DOI] [PubMed] [Google Scholar]
- 89. Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, Barker P, Sproule S, Ponnarambil S, Goldman M. Oral Glucocorticoid–Sparing Effect of Benralizumab in Severe Asthma. New England Journal of Medicine. 2017;376(25):2448–2458. 10.1056/nejmoa1703501 [DOI] [PubMed] [Google Scholar]
- 90. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chupp G, Laviolette M, Cohn L, McEvoy C, Bansal S, Shifren A, et al. Long‐term outcomes of bronchial thermoplasty in subjects with severe asthma: a comparison of 3‐year follow‐up results from two prospective multicentre studies. Eur Respir J. 2017;50(2):1700017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sweeney J, Patterson CC, Menzies‐Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross‐sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71(4):339–46. [DOI] [PubMed] [Google Scholar]
- 93. Chung LP, Upham JW, Bardin PG, Hew M. Rational oral corticosteroid use in adult severe asthma: a narrative review. Respirology. 2020;25(2):161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Woodcock A, Vestbo J, Bakerly ND, New J, Gibson JM, McCorkindale S, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open‐label, parallel group, randomised controlled trial. Lancet. 2017;390(10109):2247–55. [DOI] [PubMed] [Google Scholar]
- 95. Bloom CI, Nissen F, Douglas IJ, Smeeth L, Cullinan P, Quint JK. Exacerbation risk and characterisation of the UK's asthma population from infants to old age. Thorax. 2018;73(4):313–20. [DOI] [PubMed] [Google Scholar]
- 96. Blakey JD, Price DB, Pizzichini E, Popov TA, Dimitrov BD, Postma DS, et al. Identifying risk of future asthma attacks using UK medical record data: a Respiratory Effectiveness Group Initiative. J Allergy Clin Immunol Pract. 2017;5(4):1015–24.e8. [DOI] [PubMed] [Google Scholar]
- 97. Ardura‐Garcia C, Arias E, Hurtado P, Bonnett LJ, Sandoval C, Maldonado A, et al. Predictors of severe asthma attack re‐attendance in Ecuadorian children: a cohort study. Eur Respir J. 2019;54(5):1802419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Global Strategy for Asthma Management and Prevention Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2021. Available at: http://www.ginasthma.org.
- 99. Blakey JD, Gayle A, Slater MG, Jones GH, Baldwin M. Observational cohort study to investigate the unmet need and time waiting for referral for specialist opinion in adult asthma in England (UNTWIST asthma). BMJ Open. 2019;9(11):e031740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bourdin A, Molinari N, Vachier I, Pahus L, Suehs C, Chanez P. Mortality: a neglected outcome in OCS‐treated severe asthma. Eur Respir J. 2017;50(5):1701486. [DOI] [PubMed] [Google Scholar]
- 101. Lefebvre P, Duh MS, Lafeuille MH, Gozalo L, Desai U, Robitaille MN, et al. Acute and chronic systemic corticosteroid‐related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–95. [DOI] [PubMed] [Google Scholar]
- 102. Dalal A, Duh M, Gozalo L, Robitaille MN, Albers F, Yancey S, et al. Dose‐response relationship between long‐term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm. 2016;22(7):833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. McDonald VM, Hiles SA, Godbout K, Harvey ES, Marks GB, Hew M, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology. 2019;24(1):37–47. [DOI] [PubMed] [Google Scholar]
- 104. Luengo M, Picado C, Del Rio L, Gaunabens N, Montserrat J. Vertebral fractures in steroid dependent asthma and involutional osteoporosis: a comparative study. Thorax. 1991;46:803–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Amelink M, Hashimoto S, Spinhoven P, Pasma HR, Sterk PJ, Bel EH, et al. Anxiety, depression and personality traits in severe, prednisone‐dependent asthma. Respir Med. 2014;108(3):438–44. [DOI] [PubMed] [Google Scholar]
- 106. Smyllie H, Connolly C. Incidence of serious complications of corticosteroid therapy in respiratory disease. Thorax. 1968;23:571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. O'Neill S, Sweeney J, Patterson CC, Menzies‐Gow A, Niven R, Mansur AH, et al. The cost of treating severe refractory asthma in the UK: an economic analysis from the British Thoracic Society Difficult Asthma Registry. Thorax. 2015;70(4):376–8. [DOI] [PubMed] [Google Scholar]
- 108. Janson C, Lisspers K, Stallberg B, Johansson G, Telg G, Thuresson M, et al. Health care resource utilization and cost for asthma patients regularly treated with oral corticosteroids – a Swedish observational cohort study (PACEHR). Respir Res. 2018;19(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Waljee AK, Rogers MA, Lin P, Singal AG, Stein JD, Marks RM, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Broersen LH, Pereira AM, Jorgensen JO, Dekkers OM. Adrenal insufficiency in corticosteroids use: systematic review and meta‐analysis. J Clin Endocrinol Metab. 2015;100(6):2171–80. [DOI] [PubMed] [Google Scholar]
- 111. Heffler E, Madeira LNG, Ferrando M, Puggioni F, Racca F, Malvezzi L, et al. Inhaled corticosteroids safety and adverse effects in patients with asthma. J Allergy Clin Immunol Pract. 2018;6(3):776–81. [DOI] [PubMed] [Google Scholar]
- 112. Wlodarczyk JH, Gibson PG, Caeser M. Impact of inhaled corticosteroids on cortisol suppression in adults with asthma: a quantitative review. Ann Allergy Asthma Immunol. 2008;100(1):23–30. [DOI] [PubMed] [Google Scholar]
- 113. Aspin PKY, Allen D, Niven R, Fowler S, Tavernier G. Adrenal insufficiency in adult severe asthma patients on long‐term inhaler, oral or intramuscular corticosteroids: a systematic review. Thorax. 2021;76:A21. [Google Scholar]
- 114. Sannarangappa V, Jalleh R. Inhaled corticosteroids and secondary adrenal insufficiency. Open Respir Med J. 2014;8:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kelly HW, Nelson HS. Potential adverse effects of the inhaled corticosteroids. J Allergy Clin Immunol. 2003;112(3):469–78. quiz 479. [PubMed] [Google Scholar]
- 116. Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360(22):2328–39. [DOI] [PubMed] [Google Scholar]
- 117. Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003;361(9372):1881–93. [DOI] [PubMed] [Google Scholar]
- 118. Schwartz RH, Neacsu O, Ascher DP, Alpan O. Moderate dose inhaled corticosteroid‐induced symptomatic adrenal suppression: case report and review of the literature. Clin Pediatr. 2012;51(12):1184–90. [DOI] [PubMed] [Google Scholar]
- 119. Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383(9935):2152–67. [DOI] [PubMed] [Google Scholar]
- 120. NHS . Steroid Emergency Card to Support Early Recognition and Treatment of Adrenal Crisis in Adults. London; 2020. Available from https://www.england.nhs.uk/publication/national‐patient‐safety‐alert‐steroid‐emergency‐card‐to‐support‐early‐recognition‐and‐treatment‐of‐adrenal‐crisis‐in‐adults/ [Cited on September 16, 2021]
- 121. Simpson H, Tomlinson J, Wass J, Dean J, Arlt W. Guidance for the prevention and emergency management of adult patients with adrenal insufficiency. Clin Med (Lond). 2020;20(4):371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Foster JM, McDonald VM, Guo M, Reddel HK. "I have lost in every facet of my life": the hidden burden of severe asthma. Eur Respir J. 2017;50(3):1700765. [DOI] [PubMed] [Google Scholar]
- 123. Eassey D, Reddel HK, Foster JM, Kirkpatrick S, Locock L, Ryan K, et al. "…I've said I wish I was dead, you'd be better off without me": a systematic review of people's experiences of living with severe asthma. J Asthma. 2019;56(3):311–22. [DOI] [PubMed] [Google Scholar]
- 124. Hyland ME, Whalley B, Jones RC, Masoli M. A qualitative study of the impact of severe asthma and its treatment showing that treatment burden is neglected in existing asthma assessment scales. Qual Life Res. 2015;24(3):631–9. [DOI] [PubMed] [Google Scholar]
- 125. Clark VL, Gibson PG, McDonald VM. The patients' experience of severe asthma add‐on pharmacotherapies: a qualitative descriptive study. J Asthma Allergy. 2021;14:245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Clark VL, Gibson P, McDonald VM. What matters to people with severe asthma? Exploring add‐on asthma medication and outcomes of importance. ERJ Open Res. 2021;7:00497‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Costello R, Patel R, Humphreys J, McBeth J, Dixon WG. Patient perceptions of glucocorticoid side effects: a cross‐sectional survey of users in an online health community. BMJ Open. 2017;7(4):e014603. 10.1136/bmjopen-2016-014603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cooper V, Metcalf L, Versnel J, Upton J, Walker S, Horne R. Patient‐reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side‐effect prevalence: a UK‐wide, cross‐sectional study. NPJ Prim Care Respir Med. 2015;25:15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Shaw A, Thompson EA, Sharp D. Complementary therapy use by patients and parents of children with asthma and the implications for NHS care: a qualitative study. BMC Health Serv Res. 2006;6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Boulet LP. Perception of the role and potential side effects of inhaled corticosteroids among asthmatic patients. Chest. 1998;113(3):587–92. [DOI] [PubMed] [Google Scholar]
- 131. Jackson DJ, Bacharier LB, Mauger DT, Boehmer S, Beigelman A, Chmiel JF, et al. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. N Engl J Med. 2018;378(10):891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. O'Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zheng J, Gustafson P, et al. Effect of a single day of increased as‐needed budesonide‐formoterol use on short‐term risk of severe exacerbations in patients with mild asthma: a post‐hoc analysis of the SYGMA 1 study. Lancet Respir Med. 2021;9(2):149–58. [DOI] [PubMed] [Google Scholar]
- 133. Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez‐Aguilar NE, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high‐dose salmeterol/fluticasone. Respir Med. 2007;101(12):2437–46. [DOI] [PubMed] [Google Scholar]
- 134. Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax. 2006;61(2):169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. National Asthma Council . Asthma Management Handbook. 2020. Available from https://www.asthmahandbook.org.au [Cited on September 16, 2021]
- 136. Hew M, Menzies‐Gow A, Hull JH, Fleming L, Porsbjerg C, Brinke AT, et al. Systematic assessment of difficult‐to‐treat asthma: principles and perspectives. J Allergy Clin Immunol Pract. 2020;8(7):2222–33. [DOI] [PubMed] [Google Scholar]
- 137. Gibson PG, McDonald VM. Management of severe asthma: targeting the airways, comorbidities and risk factors. Intern Med J. 2017;47(6):623–31. [DOI] [PubMed] [Google Scholar]
- 138. Grainge CL, Maltby S, Gibson PG, Wark PA, McDonald VM. Targeted therapeutics for severe refractory asthma: monoclonal antibodies. Expert Rev Clin Pharmacol. 2016;9(7):927–41. [DOI] [PubMed] [Google Scholar]
- 139. Wark PA, McDonald VM, Gibson PG. Adjusting prednisone using blood eosinophils reduces exacerbations and improves asthma control in difficult patients with asthma. Respirology. 2015;20(8):1282–4. [DOI] [PubMed] [Google Scholar]
- 140. Heaney LG, Busby J, Hanratty CE, Djukanovic R, Woodcock A, Walker SM, et al. Composite type‐2 biomarker strategy versus a symptom‐risk‐based algorithm to adjust corticosteroid dose in patients with severe asthma: a multicentre, single‐blind, parallel group, randomised controlled trial. Lancet Respir Med. 2021;9(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Menzies‐Gow A, Corren J, Bel EH, Maspero J, Heaney LG, Gurnell M, et al. Corticosteroid tapering with benralizumab treatment for eosinophilic asthma: PONENTE Trial. ERJ Open Res. 2019;5(3):00009‐2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid‐sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–58. [DOI] [PubMed] [Google Scholar]
- 143. Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med. 2018;378(26):2475–85. [DOI] [PubMed] [Google Scholar]
- 144. Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society Guideline. Eur Respir J. 2019;55:1900588. [DOI] [PubMed] [Google Scholar]
- 145. Suehs CM, Menzies‐Gow A, Price D, Bleecker ER, Canonica GW, Gurnell M, et al. Expert consensus on the tapering of oral corticosteroids for the treatment of asthma: a Delphi study. Am J Respir Crit Care Med. 2021;203(7):871–81. [DOI] [PubMed] [Google Scholar]
- 146. Miloslavsky EM, Naden RP, Bijlsma JW, Brogan PA, Brown ES, Brunetta P, et al. Development of a Glucocorticoid Toxicity Index (GTI) using multicriteria decision analysis. Ann Rheum Dis. 2017;76(3):543–6. [DOI] [PubMed] [Google Scholar]
- 147. McDowell PJ, Stone JH, Zhang Y, Honeyford K, Dunn L, Logan RJ, et al. Quantification of glucocorticoid‐associated morbidity in severe asthma using the Glucocorticoid Toxicity Index. J Allergy Clin Immunol Pract. 2021;9(1):365–72.e5. [DOI] [PubMed] [Google Scholar]
- 148. Maltby S, Gibson PG, Reddel HK, Smith L, Wark PAB, King GG, et al. Severe Asthma Toolkit: an online resource for multidisciplinary health professionals‐needs assessment, development process and user analytics with survey feedback. BMJ Open. 2020;10(3):e032877. [DOI] [PMC free article] [PubMed] [Google Scholar]


