Abstract
Active biological containment (ABC) systems have been designed to control at will the survival or death of a bacterial population. These systems are based on the use of a killing gene, e.g., a porin-inducing protein such as the one encoded by the Escherichia coli gef gene, and a regulatory circuit that controls expression of the killing gene in response to the presence or absence of environmental signals. An ABC system for recombinant microorganisms that degrade a model pollutant was designed on the basis of the Pseudomonas putida TOL plasmid meta-cleavage regulatory circuit. The system consists of a fusion of the Pm promoter to lacI, whose expression is controlled by XylS with 3-methylbenzoate, and a fusion of a synthetic Plac promoter to gef. In the presence of the model pollutant, bacterial cells survived and degraded the target compound, whereas in the absence of the aromatic carboxylic acid cell death was induced. The system had two main drawbacks: (i) the slow death of the bacterial cells in soil versus the fast killing rate in liquid cultures in laboratory assays, and (ii) the appearance of mutants, at a rate of about 10−8 per cell and generation, that did not die after the pollutant had been exhausted. We reinforced the ABC system by including it in a Δasd P. putida background. A P. putida Δasd mutant is viable only in complex medium supplemented with diaminopimelic acid, methionine, lysine, and threonine. We constructed a P. putida Δasd strain, called MCR7, with a Pm::asd fusion in the host chromosome. This strain was viable in the presence of 3-methylbenzoate because synthesis of the essential metabolites was achieved through XylS-dependent induction. In the P. putida MCR7 strain, an ABC system (Pm::lacI, xylS, Plac::gef) was incorporated into the host chromosome to yield strain MCR8. The number of MCR8 mutants that escaped killing was below our detection limit (<10−9 mutants per cell and generation). The MCR8 strain survived and colonized rhizosphere soil with 3-methylbenzoate at a level similar to that of the wild-type strain. However, it disappeared in less than 20 to 25 days in soils without the pollutant, whereas an asd+, biologically contained counterpart such as P. putida CMC4 was still detectable in soils after 100 days.
Soil bacteria belonging to the species Pseudomonas putida show high metabolic versatility and a variety of characteristics that make them attractive for environmental applications and agricultural uses (18, 27). They can colonize the surface of plant roots and the rhizosphere, which is the part of the soil in which microorganism activity is influenced by the plant root and where nutrients are obtained from root exudates (7, 18, 22). In turn, some bacterial strains can promote plant growth and are potentially useful in the biocontrol of certain pathogens (18, 34). Many P. putida strains have the ability to degrade toxic organic compounds, which are frequently present as contaminants in the environment. P. putida KT2440 is a soil bacterium whose genome is being sequenced (www.tigr.org/KT2440). This strain was recognized by the National Institutes of Health as a nonpathogenic microorganism and as a suitable host for DNA manipulation (2). P. putida KT2440 and its derivatives have been used widely in biodegradation studies and have been the host for the construction of recombinant pathways to remove recalcitrant xenobiotics (1, 9, 24). This strain can also colonize the rhizosphere of plants at high population densities, making it a candidate suitable for use in rhizoremediation (21, 22) and biological control through the expression of insecticidal proteins (4). The strain is also being used for the synthesis of pharmaceutical products and the biosynthesis of added-value chemicals (5, 16, 25).
Recombinant derivatives of P. putida KT2440 used for bioproduction of added-value pharmaceuticals can be physically contained in reactors; however, recombinant derivatives designed for the treatment of polluted sites will eventually be used in open environments. The consequences of the introduction and persistence of genetically engineered microorganisms (GEMs) in polluted sites, and their effects on recolonization of these sites by indigenous microbiota once the pollutants have been removed, are unknown. These issues raise serious concerns.
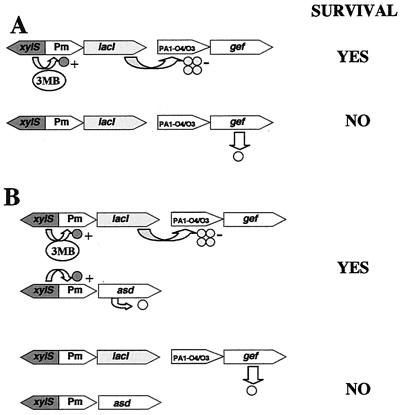
One way to decrease the persistence of GEMs in the environment is to provide them with active biological containment (ABC) systems. These systems are based on the control of a lethal function (e.g., porin-like protein, nuclease) via sensory systems that recognize physical or chemical signals in the surrounding environment (19, 20, 33). We developed a circuit for the biological containment of bacteria that uses the regulatory circuit of the TOL plasmid meta-cleavage pathway, namely, the xylS gene and its cognate Pm promoter, and the porin-like-protein-encoding gef gene of Escherichia coli or the gene E product of the phage φX174 (3, 21, 29, 30). The model (shown in Fig. 1A) predicts survival of the biologically contained strain in the presence of XylS effectors (i.e., a wide variety of alkyl- and halo-substituted benzoates [26]) because expression of the lacI gene gives rise to the production of the LacI repressor, which in turn prevents the expression of the killer gene. Instead, when the pollutant is exhausted, the lack of induction of Pm results in the lack of the repressor protein, thereby permitting the expression of the lethal gene and subsequent cell death. This ABC system has been refined since it was conceived by Contreras et al. (3) to create efficient suicide strains, whose rate of escape from killing was in the range of 10−8 per cell and generation (30). The biologically contained bacteria were shown to be able to colonize the rhizosphere of plants in outdoor experiments in soil with 3-methylbenzoate (3MB) but not in unpolluted soils. In contrast, the parental strain colonized the rhizosphere of plants grown in both polluted and nonpolluted soils (21). However, the rate of cell killing in soil was relatively slow with respect to that observed in the laboratory, and disappearance of the biologically contained strains from the soil took almost 100 days.
FIG. 1.
Scheme of an ABC model system (A) and the dual containment system (B) to control survival of bacteria by varying the availability of 3MB. Pm, promoter for the meta pathway; lacI, repressor for the lac operon; xylS, positive regulator of Pm; asd, aspartate-β-semialdehyde dehydrogenase gene; PA1-O4/O3, modified promoter for the lac operon; gef, killing gene from E. coli.
A way to improve the performance of the ABC system is to increase the rate of disappearance of the contained strains in soils. We hypothesized that this would be possible in a genetically engineered background in which the expression of a gene that gives rise to essential metabolites is under the control of the promoter used for the expression of the repressor that prevents the synthesis of the killing protein. This would guarantee at the same time both the synthesis of the essential metabolites and the expression of the repressor of the killing system (Fig. 1B). In turn, in the absence of the trigger, the expression of the killing protein and the debilitation of the strain should lead to a faster disappearance rate.
The asd gene product is involved in the biosynthesis of aspartate-β-semialdehyde, a key intermediate in the biosynthesis of diaminopimelic acid (DAP) and of amino acids such as lysine, methionine, and threonine. Strains of Salmonella enterica serovar Typhimurium (23) and Pseudomonas aeruginosa (12) that lack the asd gene are unable to grow on minimal medium unless the culture medium is supplied with a complex mixture of nutrients and undergo rapid lysis in the absence of DAP. Inspection of the genome sequence of P. putida KT2440 allowed us to identify the asd gene in this microorganism, and we decided to exploit it to reinforce the biological containment of P. putida strains designed for bioremediation.
In this study we show that the ABC system can be reinforced by using a host strain in which the natural asd gene has been replaced by a fusion of the Pm promoter to the asd gene. The new biologically contained strain dies in soils faster than previous constructs, and the rate of mutations leading to escape from killing in the strain with the reinforcement system is reduced by at least one order of magnitude in comparison with previously assayed GEMs.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study and their relevant characteristics are shown in Table 1. Bacterial strains were grown aerobically in liquid Luria-Bertani (LB) medium. P. putida strains were also grown in M9 minimal medium supplemented with 28 mM glucose and with 5 to 15 mM benzoate or 10 mM 3MB. P. putida was usually incubated at 30°C, and E. coli strains were incubated at 37°C. For growth of the P. putida Δasd mutant constructed in this study, the culture media were supplemented with 5 mM DAP and 40 μg (each) of lysine, methionine, and threonine ml−1. When required, antibiotics were used at the following final concentrations (in micrograms milliter−1): ampicillin, 100; chloramphenicol, 30; kanamycin, 50; streptomycin, 100; rifampin, 20. Isopropyl-β-d-thiogalactopyranoside was used at 2 mM.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| E. coli | ||
| JM109 | F′(traD36 proAB+lacZΔM15 lacIq) recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi-1 Δ(lac-proAB) | 36 |
| CC118λpir | Δ(ara-leu)7697 araD139 ΔlacX74 galK galE phoA20 thi-1 rpsE rpoB argE(Am) recA1 1pir | 11 |
| P. putida | ||
| KT2440 | Prototroph | 8 |
| UWC1 | Rifr derivative of KT2440 | Pseudomonas stock center at Estación Experimental del Zaidín |
| Δasd(pWW0) | KT2440 Δasd::xylE | This study |
| MCR7 | 3MB+, Δasd mini-Tn5/Sm::(Pm::asd)(pWW0) | This study |
| MCR8 | MCR7::mini-Tn5/Km::(xylS Pm::lacI PA1-O4/O3::gef) | This study |
| CMC4(pWW0) | 3MB+, mini-Tn5/Km::(xylS, Pm::lacI PA1-O4/O3::gef) | 21 |
| Δasd(pWW0, pBASD2) | Δasd(pWW0) bearing pBASD2 | This study |
| Plasmids | ||
| pASD1 | Tcr pLAFR3 carrying a 25-kb chromosomal fragment from P. putida KT2440 with the asd gene | This study |
| pBASD2 | pBBR1MCS-5 carrying a 3.6-kb EcoRI fragment from pMCR5 | This study |
| pBBR1MCS-5 | Gmr RK2oriT, α-lacZ | 15 |
| pΔasdXylE | pMCR5Δasd carrying a xylE cassette replacing the asd gene | This study |
| pKNG101 | Smr gene replacement vector, R6KoriV, RK2oriT, sacB | 13 |
| pKNGΔasdXylE | pKNG101 carrying, at the SmaI site, a 3.6-kb EcoRI fragment from pΔasdXylE | This study |
| pMCR5 | pUC18Not carrying a 3.6-kb EcoRI fragment from the cosmid pASD1, Apr | This study |
| pMCR5Δasd | pMCR5 with a deletion of the asd gene | This study |
| pRK600 | Cmr ColE1oriV RK2mob+tra+ | 11 |
| pSM1350 | Kmr AprxylS Pm::lacI PA1-O4/O3::gef | 21 |
| pSPm::asd | Smr Apr Pm::asd fusion cloned into the NotI site of pUT/mini-Tn5/Sm | This study |
| pUC18Not | Apr cloning vector oriColE1, α-lacZ | 11 |
| pUPm::asd | pUC18Not derivative carrying a Pm::asd fusion | This study |
| pUT/mini-Tn5/Sm | Apr Smr R6KroriV RP4oriT | 11 |
| pxylE10 | Kmr, source of the promoterless xylE cassette | 32 |
| pWW0 | IncP9 mob+tra+ 3MB+ | 35 |
Apr, Cmr, Gmr, Kmr, Smr, and Tcr stand for resistance to ampicillin, chloramphenicol, gentamicin, kanamycin, streptomycin, and tetracycline, respectively. PA1-O4/O3 is a synthetic lac promoter (17).
Recombinant DNA techniques.
DNA was manipulated according to standard procedures (31). For PCR amplification of the chromosomal asd gene of P. putida KT2440, appropriate primers designed on the basis of the available genome sequence were used to prime synthesis in a 50-μl reaction mixture containing 10 ng of bacterial DNA, each deoxynucleotide triphosphate at 0.1 mM, each primer at 0.5 μM, 5% (vol/vol) dimethyl sulfoxide, and 0.025 U of Taq polymerase (Pharmacia, Uppsala, Sweden) in the buffer supplied by the manufacturer. The PCR conditions were as follows: 1 cycle at 94°C for 5 min; 25 cycles at 94°C for 1 min, 68°C for 1 min, and 72°C for 2 min; and a final extension at 72°C for 10 min.
In vitro construction of an asd deletion.
The amplified P. putida KT2440 asd gene was labeled with digoxigenin and used to screen a genomic library of the wild-type strain made in the cosmid vector pLAFR1. The cosmid from the clones that showed a positive hybridization signal was isolated, digested with EcoRI, and analyzed by Southern blotting against the same probe. This allowed us to locate the asd gene in a 3.7-kb EcoRI fragment. This fragment was isolated and cloned into pUC18Not to obtain pMCR5. The fragment was sequenced to confirm the presence of the asd gene and to determine the P. putida DNA flanking the asd gene, which was about 1.9 kb in 5′ and 0.6 kb in 3′ with respect to the asd gene. The whole asd gene in pMCR5 was removed as follows. Two oligonucleotides, asd5X (5′-CTAGATCTGTACTCGAGCGGCACCGGGAATTTTGGGGGG-3′) and asd3X (5′-CCGCTCGAGTACAGATCTAGCACCTGAAAAATACCGCAC-3′), were designed outward from the asd gene to carry complementary ends and a XhoI site (underlined in the sequence). In this case, plasmid DNA was treated as previously described (6), and Expand High Fidelity PCR was used under the following conditions: 1 cycle at 94°C for 5 min; 30 cycles at 94°C for 1 min, 50°C for 1 min, and 72°C for 4 min; and a final extension at 72°C for 10 min. The long PCR product was treated with XhoI and was ligated to obtain pMCR5Δasd. Then a 1.1-kb XhoI fragment containing the xylE gene was cloned into the XhoI site of this plasmid. The resulting plasmid was called pΔasdXylE. Finally, the 3.6-kb EcoRI fragment of this plasmid, which contained the xylE gene flanked by P. putida DNA, was blunted and subcloned at the SmaI site of pKNG101 to yield pKNGΔasdXylE.
Estimation of the mutation rate of the suicide system in biologically contained P. putida strains.
Fluctuation tests were done as previously described (14) to estimate the mutation rate in bacteria that escaped from the biological containment system.
Seed coating and soil inoculation.
A natural soil consisting of 6% (wt/wt) CaCO3 and 0.5% (wt/wt) organic matter was mixed with vermiculite (3:1, wt/wt), and the water content of the soil was adjusted to 50% of the field capacity. The pots were kept in a greenhouse at 18 to 22°C with natural light-dark cycles. For these assays, cells were grown on 50 ml of M9 minimal medium with 10 mM 3MB. When they had reached the mid-log growth phase they were harvested from 500 ml of culture medium by centrifugation, washed twice in 50 mM phosphate–1% (wt/vol) NaCl, and resuspended in the same buffer to about 108 CFU ml−1. Sixty corn seeds (Zea mays) were soaked in 25 ml of the cell suspension for 30 min with gentle shaking at 30°C. The number of bacteria attached per seed was estimated as follows. Three seeds coated with P. putida were air-dried and then transferred to a glass tube with 5 ml of M9 minimal medium without a C source. They were vortexed for 1 min to remove cells weakly adhered to the seed. The procedure was repeated two additional times, and serial dilutions were spread on plates. Each seed was found to be coated with about (6 ± 1) × 105 CFU of the target strain. Three seeds were sown per pot at a depth of 2 cm in pots that were 10 cm in diameter and 20 cm deep and that contained 600 g of the soil mixture. Two pots were used per sample, and all samples were analyzed in triplicate.
Monitoring bacteria in soil and in the rhizosphere.
After germination, individual plants were sampled after the appearance of the first true leaf (i.e., 7 days after sowing) and at subsequent time points. Whole plants were gently removed from the soil, and bacteria were counted in the soil attached to the roots (rhizosphere soil). The roots were placed in a 250-ml Erlenmeyer flask with 50 to 100 ml of M9 minimal medium without a C source and shaken for 30 min on a Heidolph bench shaker at 200 strokes per min at 30°C. Soil suspensions were then serially diluted (10-fold), and 0.1-ml aliquots were spread in triplicate on selective medium. The strains were counted on selective minimal medium with 5 mM 3MB as the sole C source and the appropriate antibiotics. The number of CFU per gram of bulk soil was determined as described above except that soil not attached to the root was used.
RESULTS AND DISCUSSION
Construction of a P. putida asd mutant strain.
We constructed a P. putida strain in which the asd gene was deleted by gene replacement. Plasmid pKNGΔasdXylE was used to deliver the Δasd::xylE mutation to the host P. putida chromosome via homologous recombination in two steps (10, 13). The suicide pKNGΔasdXylE plasmid was mobilized from E. coli CC118λpir, also bearing pRK600, into P. putida KT2440 by conjugation (11). P. putida transconjugants bearing a cointegrant of the plasmid in the host chromosome were selected on M9 minimal medium with benzoic acid as the sole C source and streptomycin. Streptomycin-resistant (Smr) transconjugants appeared at a frequency of about 10−7 per recipient, and all turned bright yellow after being sprayed with a 0.5 M solution of catechol. This confirmed the incorporation of xylE. (Catechol 2,3-dioxygenase, encoded by xylE, converts catechol into the yellow 2-hydroxymuconic acid semialdehyde.) The transconjugants were unable to grow in the presence of 7% (wt/vol) sucrose in LB medium with DAP, lysine, methionine, and threonine at 30°C because of the synthesis of levans, products formed by the sacB gene product. One of the transconjugants was selected at random and grown overnight at 30°C in M9 minimal medium with glucose as a sole C source (supplemented with DAP and the three amino acids named above) in the absence of any antibiotics to select for the second recombination event. This resulted in the loss of the wild-type gene, the Smr marker, and the sacB gene. Sucrose-resistant (Sucr) colonies appeared at a frequency of about 10−7. Among the Sucr colonies we looked for catechol 2,3-dioxygenase-positive, Sms clones, and one of them was chosen at random for further characterization. Successful allelic exchange in the selected clone was checked by PCR and by Southern blot hybridization (data not shown). This strain was called P. putida Δasd, and as expected, it grew in M9 minimal medium with benzoate if and only if the medium was supplemented with DAP, methionine, threonine, and lysine.
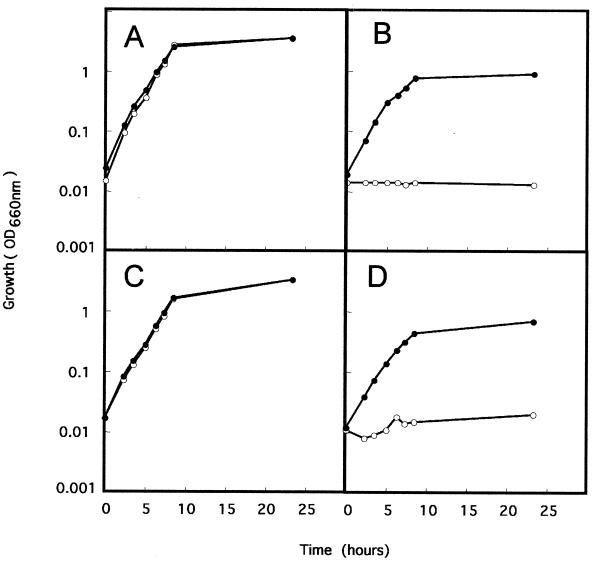
To confirm the nature of the asd mutation, we electroporated the mutant strain P. putida Δasd with pBBR1MCS-5 and its derivative, pBASD2, which bears the asd gene. P. putida KT2440, P. putida KT2440Δasd, P. putida KT2440Δasd(pBASD2), and P. putida KT2440Δasd(pBBR1MCS-5) were grown in liquid minimal medium with glucose, DAP, lysine, methionine, and threonine, and then the cultures were diluted to a turbidity of 0.2 units at 660 nm in M9 minimal medium with glucose as the C source supplemented with DAP, lysine, methionine, and threonine (or not supplemented). As expected, the wild-type strain, P. putida KT2440, was able to grow under both conditions (Fig. 2A), whereas the mutant strain was able to grow only in the medium supplemented with DAP and all three amino acids (Fig. 2B). The complemented mutant strain was able to grow at a rate similar to that of the wild type in the presence and in the absence of DAP and the amino acids (Fig. 2C), whereas the mutant strain bearing the control plasmid pBBR1MCS-5 was unable to thrive in the absence of DAP and the amino acids (Fig. 2D). Therefore, pBASD2 was able to complement the mutation in P. putida Δasd, thus confirming the mutation in this strain.
FIG. 2.
Cell viability of different P. putida strains in M9 culture medium with different supplements. The P. putida strains used were KT2440 (A), KT2440 Δasd (B), KT2440 Δasd(pBASD2) (C), and KT2440 Δasd(pBBR1MCS-5) (D). Cells were inoculated in M9 minimal medium supplemented (●) or not supplemented (○) with DAP, methionine, threonine, and lysine.
Reinforcement of the biological conditional lethal system based on the TOL plasmid meta-cleavage pathway regulatory circuit.
To reinforce the ABC system described in the introduction, we decided to take advantage of the severe growth inhibition imposed by the lack of the asd gene, which can be overcome by providing the asd gene in trans. To incorporate the asd gene into the containment system we decided to express the asd gene from the Pm promoter. We first constructed pUPm::asd, which is a pUC18Not derivative that carries a fusion of the asd gene to the Pm promoter. The 1.5-kb NotI fragment carrying Pm::asd was cloned into the NotI site of pUT/mini-Tn5/Sm and then transferred into the chromosome of P. putida Δasd bearing pWW0 by conjugation. (Note that pWW0 was used as the source of the xylS gene.) Transconjugants appeared at a frequency of about 2 × 10−6 per recipient cell. All transconjugant cells grew on M9 minimal medium containing 3MB as the sole C source but not with glucose as the sole C source. This behavior confirmed that the XylS-3MB complex efficiently induced expression of the Pm:asd fusion to achieve the biosynthesis of metabolites essential for growth and also induced the meta operon for 3MB catabolism.
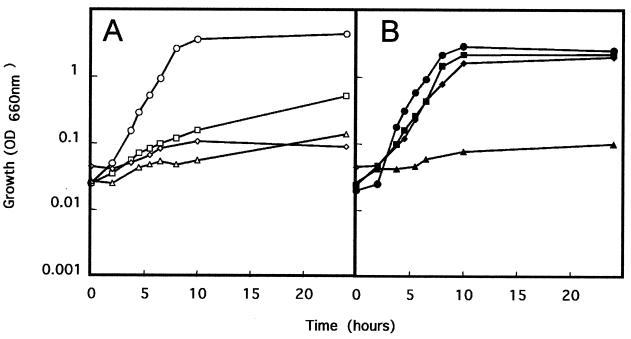
Ideally, the elements of the containment system should be located in chromosomal sites with a low mobilization rate. Ramos-González et al. (28) have shown that the TOL plasmid is able to mobilize the host chromosome and that the rate of mobilization is influenced by the physical location of the marker. To compare the mobilization frequency of the insert of 10 independent clones bearing the Pm::asd fusion, we set up conjugation experiments in which the donor strains were the clones with the mini-Tn5/Sm and the TOL plasmid (Rifs Smr 3MB+) and P. putida UWC1 (Rifr Sms 3MB−) was the recipient. We obtained Rifr 3MB+ transconjugants of P. putida UWC1 cells at a frequency of 10−1 to 10−2 per recipient, whereas the frequency of derivatives that had received the Smr marker from the chromosome varied between 10−6 and fewer than 10−8 per recipient. One clone whose Smr marker was mobilized at a rate lower than 10−8 transconjugants per recipient was selected and named P. putida MCR7. P. putida KT2440(pWW0), P. putida KT2440Δasd(pWW0), and P. putida MCR7 were grown in liquid minimal medium with 3MB or glucose as the sole carbon source. The wild-type strain grew in both media, P. putida MCR7 bearing a Pm::asd fusion grew in the medium with 3MB as a consequence of the expression of the asd gene, and P. putida Δasd(pWW0) did not grow in these culture media, as expected (Fig. 3). (No mutants of this latter strain were found even after prolonged [96 h] incubation of the cultures.) To reinforce the containment system we decided to bring together the Pm:asd fusion and the ABC system which carries a fusion of the lacI gene to the Pm promoter plus the xylS gene and a fusion of the gef gene from E. coli to the PA1-O4/O3 promoter (17) (Fig. 1B). The rationale for the reinforced biological containment system was as follows. In the presence of 3MB, synthesis of LacI protein prevents the expression of the killing gene; on the other hand, synthesis of the Asd protein made from the Pm promoter allows synthesis of essential metabolites, and the cells survive (Fig. 1B). In the absence of effectors of the XylS protein, the killing gene is expressed and the host cells die as a consequence of debilitation due to the lack of DAP bridges between peptidoglycan chains in the periplasmic space and the lack of essential amino acids.
FIG. 3.
Complementation of the Δasd deletion in P. putida by the cloned asd gene. The P. putida strains used were KT2440(pWW0) (circles), MCR7 (squares), Δasd(pWW0) (triangles), and MCR8 (diamonds). Cells were pregrown on M9 minimal medium with 3MB as the sole C source and supplemented with DAP, lysine, methionine, and threonine. Then cultures were diluted 50-fold in unsupplemented M9 minimal medium with glucose (A) (open symbols) or 3MB (B) (closed symbols) as the sole carbon source.
Transfer of the containment system in pSM1350 into the chromosome of P. putida MCR7 was achieved by conjugation as described before (29). The transconjugants appeared at a frequency of about 5 × 10−7 per recipient cell. As described above, we tested the rate of mobilization of the marker in different clones. Five clones whose Kmr marker was mobilized at a rate equal to or lower than 10−8 per recipient were kept. Fluctuation tests of all five clones were done to determine the rate of mutant escape from cell killing. It was found that the rate of mutation was below 10−9 per cell and per generation in all cases. One of the transconjugants carrying the miniTn5/Km (xylS, Pm::lacI, PA1-O4/O3::gef) was selected as a strain in which the containment system had been reinforced and was named P. putida MCR8. This strain grew in liquid medium with 3MB without any other supplement (Fig. 3). In this culture medium, with 3MB as the sole C source, the growth rate (62 ± 4 min) was similar to that of P. putida KT2440(pWW0), which exhibited a growth rate of 58 ± 3 min. These results suggest that the contained strain was as efficient as the parental one in the degradation of the target pollutant.
Survival of the reinforced biologically contained MCR8 strain and other P. putida strains in the rhizosphere of corn plants.
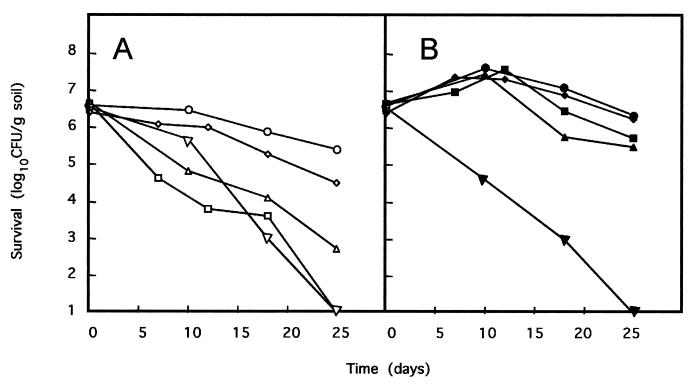
The behavior of P. putida KT2440 Δasd(pWW0), P. putida KT2440 Δasd(pWW0, pBASD2 [Pm::asd]), P. putida MCR7(pWW0), and P. putida MCR8(pWW0 [Pm::asd, Pm::lacI, Plac::gef]) (Δasd, Pm::asd) was assayed in pots planted with corn and kept under greenhouse conditions. In this series of assays we also included P. putida CMC4(pWW0), a strain bearing the same ABC system as MCR8 but in a wild-type asd+ background. This strain had been constructed before (30). The assay conditions are given in Materials and Methods, and the results obtained are shown in Fig. 4. Both in the absence and in the presence of 3MB, the control strain [P. putida Δasd(pWW0, pBASD2)] tended to become established at a density of about 106 to 107 CFU per g of rhizosphere soil. The number of CFU per gram of soil of the mutant P. putida Δasd(pWW0) strain decreased steadily with time and reached levels below our detection limit (i.e., <50 CFU/g of rhizosphere soil) 25 days after the start of the assay regardless of the presence of 3MB in the soil. The behavior of the contained strain, P. putida CMC4(pWW0), was as previously reported (21, 29); namely, the number of cells tended to decrease with time, particularly in the absence of 3MB, although 100 days after the start of the assay we were still able to recover 5 × 102 to 103 CFU per gram of soil (data not shown). P. putida strains MCR7 and MCR8 behaved similarly in soils containing 3MB: we found that they became established at about 106 CFU/g of soil; however, in the absence of 3MB the number of CFU of MCR8 per gram of rhizosphere soil decreased faster than that of MCR7 and fell below our detection limits after about 25 days.
FIG. 4.
Survival of different P. putida strains under greenhouse conditions. Zea mays seeds were coated with P. putida MCR8 (squares), P. putida CMC4 (diamonds), P. putida KT2440 Δasd(pBASD2, pWW0) (circles), P. putida MCR7 (triangles), or P. putida Δasd(pWW0) (inverted triangles). Coated seeds were sown in pots in which the soil was supplemented (B) (closed symbols) or not supplemented (A) (open symbols) with 3MB. The number of CFU per gram of rhizosphere soil was determined at the times indicated. Data are the averages of values from three independent counts, and the standard deviations were between 5 and 17% of the given values.
The concept of ABC was conceived by Molin et al. (19), who showed that cloning of the killer hok gene under the tryptophan promoter of E. coli induced cell killing when cells were transferred to a culture medium without tryptophan. Contreras et al. (3) extended this concept to the control of recombinant bacteria that degrade xenobiotics through the TOL meta-cleavage pathway. These investigators showed that survival or death of a population of bacteria could be controlled with an ABC system, as shown in Fig. 1A.
In all genetic systems mutations appear at a certain frequency, and in the case of containment systems mutations that lead to escape from killing have been reported. In the original system, in which the control element and the killing genes of the containment system were on a plasmid, the rate of gene escape was as high as 10−5 to 10−6 mutants per cell and generation (3, 19). A series of improvements, including the use of minitransposons to incorporate killing genes or the entire biological containment system on the host chromosome, led to the construction of strain P. putida CMC4(pWW0), a strain in which the rate of escape from killing was in the range of 10−8 per cell and generation. This rate of escape was considered satisfactory, and field-release assays were carried out with this contained strain and a control uncontained strain (29). It was found that when the control strain colonized the rhizosphere of plants in unpolluted soils and in soils polluted with 3MB, the survival of the contained strain was compromised by the absence of the model pollutant so that the number of viable cells decreased with time in soils without the aromatic carboxylic acid. However, the contained strain disappeared from the soil much more slowly than was expected from the results obtained in the laboratory. The reasons for this slower killing rate were unknown. Effective killing mediated by the gef gene product results from the collapse of the cell membrane potential upon insertion of the killing protein in the cytoplasmic membrane (20). We reasoned that the killing system could be reinforced by a gene whose product is needed to synthesize essential metabolites and that this gene should be expressed from the same promoter as the killing gene. Behind this idea was also our intention to debilitate the contained strain and increase its rate of suicide. To this end we chose the asd gene, which encodes the Asd protein needed for biosynthesis of DAP, and three amino acids. Because DAP is involved in cross-linking of the peptidoglycan chains of gram-negative bacteria, the lack of synthesis of this compound debilitates cells and prevents growth, effects that eventually lead to cell death. We have shown here that P. putida lacking the asd gene has complex growth requirements but that expression of the asd gene from the XylS positively regulated Pm promoter in the presence of XylS effectors allowed cell growth without the need to add exogenous DAP and amino acids. We then envisaged a way to reinforce the containment of P. putida by combining the killing system and the expression of the asd gene from Pm in a Δasd strain. We have shown that the Δasd strain bearing the ABC system and a Pm::asd fusion is viable in culture medium with 3MB and that mutations leading to escape from killing in the reinforced strain occur at a rate below our detection limits. In addition, the induction of cell death in the rhizosphere of corn plants in the absence of the model pollutant is significantly faster than in asd+ counterparts such as P. putida CMC4(pWW0). Therefore, our results confirm that the ABC system based on the porin-like-protein-encoding killing genes can be reinforced with regard to killing efficiency and rate of killing by incorporation into an asd mutant background. The contained strain, P. putida MCR8, was unable to colonize bulk soil, in contrast with the control (uncontained) strain. This indicates that Δasd-contained strains may be severely limited in their ability to colonize some environments. One of the drawbacks that the dual system still presents is that the two lethal systems are not independent since they are ultimately controlled by the XylS protein. Constitutive XylS mutants, induced by powerful mutagenic compounds such as ethyl methanesulfonate and nitrosoguanidine, have been described before (37), and therefore, if such mutants appear no killing can take place because of the continuous synthesis of LacI and aspartate β-semialdehide dehydrogenase. Other issues that deserve consideration in remediation assays are the viability of contained cells when the target pollutant in the soil is not bioavailable and the number of microorganisms that need to be introduced to achieve an efficient removal of the pollutants.
Although a number of questions still need further research, our present results in this study show that the survival or death of a bacterial population can be accurately controlled. Other applications of ABC systems can be envisaged in fields where biological containment may be desirable, such as crop protection and live vaccines for animals.
ACKNOWLEDGMENTS
This work was supported by grants from the European Commission (BIO4-CT97–2313 and BIO4-CT98–0283).
We thank M. Mar Fandila and Carmen Lorente for secretarial assistance and K. Shashok for checking the use of English in the manuscript.
REFERENCES
- 1.Abril M A, Michán C, Timmis K N, Ramos J L. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J Bacteriol. 1989;171:6782–6790. doi: 10.1128/jb.171.12.6782-6790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagdasarian M, Timmis K N. Host: vector systems for gene cloning in Pseudomonas. Curr Top Microbiol Immunol. 1982;96:47–67. doi: 10.1007/978-3-642-68315-2_4. [DOI] [PubMed] [Google Scholar]
- 3.Contreras A, Molin S, Ramos J L. Conditional-suicide containment system for bacteria which mineralize aromatics. Appl Environ Microbiol. 1991;57:1504–1508. doi: 10.1128/aem.57.5.1504-1508.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook R J, Bruckart W L, Coulson J R, Goettel M S, Humber R A, Lumsden R D, Maddox J V, McManus M L, Moore L, Meyer S F, Quimby P C, Jr, Stack J P, Vaughn J L. Safety of microorganisms intended for pest and plant disease control: a framework for scientific evaluation. Biol Control. 1996;7:333–351. [Google Scholar]
- 5.Delgado A, Wubbolts M G, Abril M A, Ramos J L. Nitroaromatics are substrates for the TOL plasmid upper-pathway enzymes. Appl Environ Microbiol. 1992;58:415–417. doi: 10.1128/aem.58.1.415-417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorrell N, Gyselman V C, Foynes S, Li S-R, Wren B W. Improved efficiency of inverse PCR mutagenesis. BioTechniques. 1996;21:604–608. doi: 10.2144/96214bm07. [DOI] [PubMed] [Google Scholar]
- 7.Espinosa-Urgel M, Salido A, Ramos J L. Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J Bacteriol. 2000;182:2363–2369. doi: 10.1128/jb.182.9.2363-2369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin F C H, Bagdasarian M, Bagdasarian M M, Timmis K N. Molecular and functional analysis of the TOL plasmid pWW0 from Pseudomonas putida and cloning of the genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci USA. 1981;78:7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallardo M E, Fernández A, de Lorenzo V, García J L, Díaz E. Designing recombinant Pseudomonas strains to enhance biodesulfurization. J Bacteriol. 1997;179:7156–7160. doi: 10.1128/jb.179.22.7156-7160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gay P, Le Coq D, Steinmetz M, Berkelman T, Kado C I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 1985;164:918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoang T T, Williams S, Schweizer H P, Lam J S. Molecular genetic analysis of the region containing the essential Pseudomonas aeruginosa asd gene encoding aspartate-β-semialdehyde dehydrogenase. Microbiology. 1997;143:899–907. doi: 10.1099/00221287-143-3-899. [DOI] [PubMed] [Google Scholar]
- 13.Kaniga K, Delor I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 14.Knudsen S M, Karlström O H. Development of efficient suicide mechanisms for biological containment of bacteria. Appl Environ Microbiol. 1991;57:85–92. doi: 10.1128/aem.57.1.85-92.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop II R M, Peterson K M. Four new derivatives of the broad-host-range cloning vector pBBR1MC5, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 16.Kraak M N, Smits T H, Kessler B, Witholt B. Polymerase C1 levels and poly(R-3-hydroxyalkanoate) synthesis in wild-type and recombinant Pseudomonas strains. J Bacteriol. 1997;16:4985–4991. doi: 10.1128/jb.179.16.4985-4991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanzer M, Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci USA. 1988;85:8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lugtenberg B J J, Dekkers L C. What makes Pseudomonas bacteria rhizosphere competent? Environ Microbiol. 1999;1:9–13. doi: 10.1046/j.1462-2920.1999.00005.x. [DOI] [PubMed] [Google Scholar]
- 19.Molin S, Klemm P, Poulsen L K, Biehl H, Gerdes K, Andersson P. Conditional suicide system for containment of bacteria and plasmids. Bio/Technology. 1987;5:1315–1318. [Google Scholar]
- 20.Molin S, Boe L, Jensen L B, Kristensen C S, Giskov M, Ramos J L, Bej A K. Suicidal genetic elements and their use in biological containment of bacteria. Annu Rev Microbiol. 1993;47:139–166. doi: 10.1146/annurev.mi.47.100193.001035. [DOI] [PubMed] [Google Scholar]
- 21.Molina L, Ramos C, Ronchel M C, Molin S, Ramos J L. Construction of an efficient biologically contained Pseudomonas putida strain and its survival in outdoor assays. Appl Environ Microbiol. 1998;64:2072–2078. doi: 10.1128/aem.64.6.2072-2078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molina L, Ramos C, Duque E, Ronchel M C, García J M, Wyke L, Ramos J L. Survival of Pseudomonas putida KT2440 in soil and in the rhizosphere of plants under greenhouse and environmental conditions. Soil Biol Biochem. 2000;32:315–321. [Google Scholar]
- 23.Nakayama K, Kelly S M, Curtiss R. Construction of an Asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Bio/Technology. 1988;6:693–697. [Google Scholar]
- 24.Panke S, Sánchez-Romero J M, de Lorenzo V. Engineering of quasi-natural Pseudomonas putida strains for toluene metabolism through an ortho-cleavage degradation pathway. Appl Environ Microbiol. 1998;64:748–751. doi: 10.1128/aem.64.2.748-751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panke S, de Lorenzo V, Kaiser A, Witholt B, Wubbolts M G. Engineering of a stable whole-cell biocatalyst capable of (S)-styrene oxide formation for continuous two-liquid-phase applications. Appl Environ Microbiol. 1999;65:5619–5623. doi: 10.1128/aem.65.12.5619-5623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos J L, Stolz A, Reineke W, Timmis K N. Altered effector specificities in regulators of gene expression: TOL plasmid xylS mutants and their use to engineer expansion of the range of aromatics degraded by bacteria. Proc Natl Acad Sci USA. 1986;83:8467–8471. doi: 10.1073/pnas.83.22.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos J L, Díaz E, Dowling D, de Lorenzo V, Molin S, O'Gara F, Ramos C, Timmis K N. The behavior of bacteria designed for biodegradation. Bio/Technology. 1994;12:1349–1356. doi: 10.1038/nbt1294-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos-González M I, Ramos-Díaz M A, Ramos J L. Chromosomal gene capture mediated by the Pseudomonas putida TOL catabolic plasmid. J Bacteriol. 1994;176:4635–4641. doi: 10.1128/jb.176.15.4635-4641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronchel M C, Ramos C, Jensen L B, Molin S, Ramos J L. Construction and behaviour of biologically contained bacteria for environmental applications in bioremediation. Appl Environ Microbiol. 1995;61:2990–2994. doi: 10.1128/aem.61.8.2990-2994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronchel M C, Molina L, Witte A, Lutbiz W, Molin S, Ramos J L, Ramos C. Characterization of cell lysis in Pseudomonas putida induced upon expression of heterologous killing genes. Appl Environ Microbiol. 1998;64:4904–4911. doi: 10.1128/aem.64.12.4904-4911.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Stein D C. Plasmids with easily excisable xylE cassettes. Gene. 1992;117:157–158. doi: 10.1016/0378-1119(92)90506-k. [DOI] [PubMed] [Google Scholar]
- 33.Torres B, Jaenecke S, Timmis K N, García J L, Díaz E. A gene containment strategy based on a restriction-modification system. Environ Microbiol. 2000;2:555–563. doi: 10.1046/j.1462-2920.2000.00138.x. [DOI] [PubMed] [Google Scholar]
- 34.Weller D M. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol. 1998;26:379–407. [Google Scholar]
- 35.Worsey M J, Williams P A. Metabolism of toluene and xylenes by Pseudomonas putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975;124:7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, Timmis K N, Ramos J L. Mutations leading to constitutive expression from the TOL plasmid meta-cleavage pathway operon are located at the C-terminal end of the positive regulator protein XylS. J Bacteriol. 1990;172:3707–3710. doi: 10.1128/jb.172.7.3707-3710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]