Abstract
We wanted to explore a new method for the determination of monosaccharides in Polygonatum cyrtonema Hua polysaccharide using the ultra‐high‐performance liquid chromatography quadrupole trap tandem mass spectrometry. In this study, hydrochloric acid was used instead of trifluoroacetic acid to hydrolyze polysaccharides, and hydrolysis time was greatly reduced from 5–9 h to 1 h. The 1‐phenyl‐3‐methyl‐5‐pyrazolone was used for pre‐column derivatization of monosaccharides. The parameters of linearity (R 2 > 0.999), stability (1.63–2.52%), intra‐day and inter‐day precision (0.69–0.95%, 1.81–2.77%), repeatability (1.89–2.65%), and recovery (97.63–102.24%) of the method were verified. Satisfactory validation results showed this method could be used to determine the target components. The results indicated the polysaccharide contained glucose, mannose, rhamnose, galactose, ribose, and arabinose. Technique for order preference by similarity to an ideal solution and principal component analysis were used to build an evaluation model based on the monosaccharide composition. The evaluation results showed that the samples from the Qingyang County of Anhui Province were the best when the monosaccharides were used as the evaluation index. Therefore, a new method was established to detect the monosaccharide content of polysaccharides from Polygonatum cyrtonema Hua and comprehensively evaluate the quality of Chinese medicines with polysaccharides as the main active ingredient.
Keywords: component analysis, monosaccharide composition, Polygonatum cyrtonema Hua, precolumn derivatization
Article Related Abbreviations
- PCA
principal component analysis
- PCH
Polygonatum cyrtonema Hua
- PCP
Polygonatum cyrtonema Hua polysaccharide
- TOPSIS
technique for order preference by similarity to an ideal solution
- QTRAP
quadrupole trap
1. INTRODUCTION
Herbal plants play a vital role in traditional medicine worldwide and are the main therapeutic agents for treating diseases [1, 2]. Many herbal plants are used for medicinal purposes and contain a wide range of bioactive compounds. For example, Ganoderma lucidum, one of the most famous herbal plants, has been used for centuries in promoting health and longevity [3]. Therefore, the impact of herbal medicine on human beings is unparalleled, improving the life span and quality of life of human beings.
Polygonatum cyrtonema Hua (PCH) is a medicinal herb of the genus Liliaceae, which is widely used as a tonic remedy in traditional Chinese medicine for the treatment of lung, spleen, and stomach diseases [4]. The rhizome of PCH, one of the original plants of the Chinese herbal medicine “Huang‐jing” recorded in the Chinese Pharmacopoeia, has been widely used in ancient times and the first monograph on pharmacology in China and “Shennong Bencao Jing” has classified this plant as top‐grade herb [5]. Hence, Huang‐jing is an important traditional Chinese medicine and a wild Chinese medicine resource with more than 2000 years of history. Besides being a kind of Chinese herbal medicine with great biological activity, it can also be processed into many healthy foods, such as functional botanical beverages, wines, tea, and even medicinal diets with meat [6].
PCH contains polysaccharides, steroidal saponins, flavonoids, amino acids, and other components [7, 8, 9, 10, 11]. Polysaccharides are the major biologically active components of PCH, comprising up to 17.79% [12]. Modern pharmacological studies have shown that PCH polysaccharide (PCP) has a variety of pharmacological effects, such as anti‐aging, anti‐tumor, lowering blood sugar, preventing atherosclerosis, and improving immunity [13, 14, 15, 16, 17]. However, PCH obtained from different areas and its chemical analysis methods have been rarely compared using polysaccharides as an index. Polysaccharides are polymers condensed from monosaccharides, which are widely distributed in plants and microorganisms in nature. They are important active substances that play an indispensable role in biological activities. Therefore, it is evident that the analysis of polysaccharide composition plays a vital role in explaining the mechanism of action in organisms. Hence, the study of the composition of monosaccharides is indispensable for the structural analysis and structural activity relationship of polysaccharides.
In recent years, the qualitative and quantitative determination methods of monosaccharides mainly include HPCE, GC, HPLC, and UHPLC with quadrupole trap‐MS/MS (QTRAP‐MS/MS) [18, 19, 20, 21]. The low sensitivity and poor reproducibility of the HPCE greatly limit its application [22]. The operation of the GC method is tedious because of the many types of reagents and their long duration [23]. The data measured by the HPLC method is not sufficiently accurate, and the analysis is time‐consuming [24]. The UHPLC‐QTRAP‐MS/MS is commonly used for monosaccharide analysis, which can operate rapidly, efficiently, and simply [25]. Meanwhile, in the study of the structure and composition of the polysaccharide, the polysaccharides must be hydrolyzed to obtain hydrolysates of different molecular weights to obtain the composition information of the corresponding monosaccharides. trifluoroacetic acid is commonly used for polysaccharide hydrolysis, but it takes 5−9 h or more to complete [26, 27, 28]; hence, we used hydrochloric acid which takes only 1 h.
Therefore, a UHPLC‐QTRAP‐MS/MS method was established to detect the composition and content of six main monosaccharides, glucose, mannose, rhamnose, galactose, ribose, and arabinose in PCP [29, 30, 31]. Our method can be applied to determine the monosaccharide content and composition of PCH from different species and producing areas. In particular, an effective measurement and chemical analysis method was established for plants with polysaccharides as the main component, which provided a theoretical basis for their mechanism of action and quality evaluation in vivo.
2. MATERIALS AND METHODS
2.1. Reagents
A total of 14 batches of PCH samples were collected from the wild in the main producing areas of Anhui Province and then dried until the water content did not exceed 18%, as shown in Table 1. The botanical origins of the samples were identified by Professor Nianjun Yu, Anhui University of Traditional Chinese Medicine. The mannose, ribose, rhamnose, glucose, galactose, arabinose, ammonium, acetate, and 1‐phenyl‐3‐methyl‐5‐pyrazolone were purchased from Yuanye Biotechnology (Shanghai, China). The acetonitrile was purchased from Merck Drugs & Biotechnology (Darmstadt, Germany). Ethanol and petroleum ether (boiling range: 60–90°C) were purchased from Sinopharm Chemical Reagent (Beijing, China). The water used in the experiment was ultrapure water produced by a Milli‐Q Purification System (Millipore, USA).
TABLE 1.
The information of samples from different regions
| Sample number | Cultivation region |
|---|---|
| S1 | Yanzihe Town, Jinzhai County, Anhui |
| S2 | Changling Township, Jinzhai County, Anhui |
| S3 | Wuwei City, Anhui |
| S4 | Tongcheng City, Anhui |
| S5 | Shitai County, Anhui |
| S6 | Qimen County, Anhui |
| S7 | Jingde County, Anhui |
| S8 | Baishan village, Jing County, Anhui |
| S9 | Mayuan village, Jing County, Anhui |
| S10 | Huoshan County, Anhui |
| S11 | Kuaiyuan village, Qingyang County, Anhui |
| S12 | Yangchong village, Qingyang County, Anhui |
| S13 | Shexian County, Anhui |
| S14 | Huizhou District, Huangshan City, Anhui |
2.2. Preparation of standard solution
The six monosaccharide standard substances of 1.0 mg were precisely weighed as D‐rhamnose, D‐arabinose, D‐galactose, D‐mannose, D‐ribose, and D‐glucose, respectively. The standard samples were diluted with ultra‐pure water to prepare a standard stock solution of 1.0 g/L. About 0.1 mL of each standard stock solution was taken and then diluted to the target concentration to plot the calibration curve. The solution was transferred to a refrigerator at 4°C for storage after passing through a 0.22 μm microporous filter membrane. Figure 1 shows the chromatogram of the standard solution.
FIGURE 1.
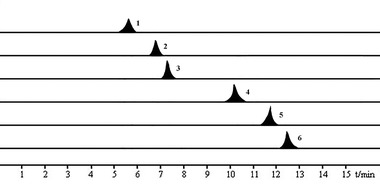
Multiple reaction monitoring (MRM) chromatograms of six monosaccharides were derivatized by 1‐phenyl‐3‐methyl‐5‐pyrazolone (PMP). Note: The six monosaccharides were 1, Mannose; 2, Ribose; 3, Rhamnose; 4, Glucose; 5, Galactose; and 6, Arabinose
2.3. Preparation of sample solution
2.3.1. PCP extraction
The powder of the PCH sample was precisely weighed 5.0 g and added with 50 mL petroleum ether was added and refluxed in a water bath at 75°C for 1.5 h. Then, 50 mL of 75% ethanol was added to the filter residue and refluxed in a water bath at 85°C for 1 h. After filtering the solution, the filtered residue was extracted using 100 mL of boiling water at 100°C for 2 h. The filtrate was concentrated using a rotary evaporator, and methanol (3:1) was added to the concentrate and properly shaken to allow the polysaccharide to precipitate. The solution was placed in a refrigerator at 4°C overnight, centrifuged (centrifugation conditions: 4°C, 4000 rpm, and 10 min), and the lower layer was precipitated and dried to obtain PCP.
2.3.2. Hydrolysis of PCP
The dried PCP 5.0 mg was precisely weighed, and 0.5 mL of 3 mol/L HCl and 1 mL ultra‐pure water were added. After the bottle mouth was sealed, the solution was hydrolyzed at 110°C for 1 h. The solution was then cooled, and the pH was adjusted to neutral by using 3 mol/L HCl and 3 mol/L NaOH. Finally, the volume of the solution was fixed to 3 mL with ultra‐pure water.
2.3.3. Sample derivatization
Each 400 μL of PCP solution, 1‐phenyl‐3‐methyl‐5‐pyrazolone solution, and 0.3 mol/L NaOH were measured and added precisely, and then the reaction solution was shaken and placed in a water bath at 70°C for 100 min to react adequately. After the solution was cooled to room temperature, 500 μL of 0.3 mol/L HCl and 2 mL chloroform solution were added, and the solution was shaken at a uniform speed to allow the reaction to occur for 5−10 min. The upper liquid was repeatedly washed with chloroform 2−3 times to remove the organic phase. The solution was filtered through a 0.22 μm microporous membrane for further analysis.
2.4. Liquid chromatographic and MS conditions
The samples were analyzed using a UHPLC system (Agilent Infinity 1290, USA) and a triple quadrupole‐linear ion trap mass spectrometer (QUAD‐4500; AB Sciex, Framingham, MA, USA). To obtain a better liquid‐phase separation effect, we investigated the conditions of different chromatographic columns, column temperatures, mobile phase systems, and mobile phase gradients. It was found that under the following chromatographic conditions, the separation effect was the best, the number of peaks was large, the peak shape was good, and the baseline was stable. The optimum conditions for HPLC were as follows. The chromatographic column used was an Agilent ZORBAX Eclipse Plus C18 (2.1× 100 mm 1.8‐μm; Agilent, USA). Mobile phase: 0.003% ammonium acetate (A) and acetonitrile (B). Gradient elution was 0−14 min, 80% A; 14−16 min, 80−75% A; 16−20 min, 80% A. The flow rate was 0.2 mL/min, the column temperature was 30°C, and the injection volume was 5 μL.
Under ionization mode, ESI+, multi‐reaction monitoring detection, and quantitative analysis were performed. The gas temperature was 550°C, the Gas‐1 pressure was 379.2 kPa (55 psi), the Gas‐2 pressure was 379.2 kPa (55 psi), the Curtain Gas was 241.3 kPa (35 psi). The ion‐pair detection, de‐cluster voltage (DP), and collision voltage (CE) were optimized. The optimized MS detection condition parameters of the six target components are listed in Table 2.
TABLE 2.
The optimized mass spectrometry condition parameters in the determination of six monosaccharides were derivatized by 1‐phenyl‐3‐methyl‐5‐pyrazolone (PMP)
| MRM parameter | ||||||
|---|---|---|---|---|---|---|
| Component | t R/min | Molecular mass | Precursor ion | Product ion | CE/V | DP/V |
| D‐Galactose | 11.334 | 180.16 | 511.22 | 175.10/217.10 | 35 | 171 |
| D‐Arabinose | 12.534 | 150.13 | 481.21 | 175.10/217.10 | 33 | 163 |
| D‐Mannose | 5.050 | 180.16 | 511.22 | 175.10/217.10 | 35 | 171 |
| D‐Rhamnose | 6.713 | 164.16 | 495.22 | 175.10/217.10 | 33 | 174 |
| D‐Ribose | 6.363 | 150.13 | 481.21 | 175.10/217.11 | 33 | 163 |
| D‐Glucose | 9.956 | 180.16 | 511.22 | 175.10/217.10 | 35 | 171 |
Abbreviations: CE, collision voltage; DP, de‐cluster voltage; MRM, multiple reaction monitoring; t R, retention time
2.5. Quantitative detection method verification
We verified the established quantitative method using methodological experiments. In the validation experiments of quantitative detection methods, the following indicators were accurately verified: linearity, LOQ, LOD, precision, stability, repeatability, and recovery. Each 1 μL mixed reference solution was used for GC‐MS, and the calibration curve was drawn with the peak area integral value of the reference substance and the mass concentration of the reference substance. The LODs and LOQs were determined as the concentration of the standard solution with S/N of 3 and 10, LOD = 3.3 SD/S, LOQ = 10 SD/S, respectively, where S is the slope of the linearity test and SD is five injections of blank of peak area [32].
To calculate the precision, 1 μL of the mixed standard solution at a certain concentration was used as the internal standard solution. Under the condition of chromatography‐MS, the sample was injected continuously six times to measure the peak area of each standard solution. These values were used to calculate the RSDs for the six reference substances to determine their precision. Six replicates of the PCH sample solution were hydrolyzed using hydrochloric acid and prepared in parallel, and the RSD of the six solutions was calculated to investigate the repeatability. To evaluate stability, we analyzed the PCH samples from different locations at different time points (0, 2, 4, 8, 12, and 24 h). The RSDs of the six standard solutions were calculated. Six S1 samples were accurately weighed 1.0 g and added to the standard solution with the same content of each component as the sample solution, and then the RSDs of the six standard solutions were calculated to verify the recovery of the sample.
3. RESULT
3.1. Method validation
The method validation results for the six components are shown in Table 3. The calibration curves of the six monosaccharides in the samples within their suitable ranges showed good linear ranges (R 2) > 0.999. The linear ranges were 0.25−27.49 μg/mL for D‐galactose; 0.29−31.85 μg/mL for D‐arabinose; 0.62−67.79 μg/mL for D‐mannose; 0.26−27.44 μg/mL for D‐rhamnose; 0.23−25.96 μg/mL for D‐ribose; and 0.26−27.97 μg/mL for D‐glucose. The LODs and LOQs of the six monosaccharides ranged from 0.063−0.27 and 0.25−0.72 μg/mL are shown in Table 3. The good sensitivity results showed that this method can be used to detect PCH samples. The RSD of the intra‐day and inter‐day precision of the six components were in the range of 0.69−0.95 and 1.81−2.77%, respectively. The stability of the six components of the specific RSD values were all in the range of 1.63−2.52 and 1.89−2.65%, respectively, indicating the feasibility of the method. In addition, the recovery rates were between 97.63−102.24% and the RSD values ranged between 1.75−2.71%. In summary, these results proved that the established method is accurate, feasible, and sensitive for the determination of monosaccharide content.
TABLE 3.
Method linearity, LOD, LOQ, precision, stability, repeatability, recovery, and repeatability (n = 6) of six monosaccharides
| Linear range | LOD | LOQ | Precision RSD (%) | stability | repeatability | recovery | RSD (n = 3) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Linear equation | (μg/mL) | R 2 | (μg/mL) | (μg/mL) | Intraday (n = 6) | Interday (n = 6) | RSD (%)(n = 6) | RSD (%)(n = 6) | Mean | RSD (%) |
| D‐Galactose | y = 1.6 × 105 + 41.245 | 0.25–27.49 | 0.9998 | 0.079 | 0.28 | 0.82 | 2.27 | 2.37 | 2.48 | 99.57 | 1.75 |
| D‐Arabinose | y = 1.9 × 105 + 69.989 | 0.29–31.85 | 0.9997 | 0.063 | 0.25 | 0.95 | 1.81 | 2.12 | 2.24 | 98.35 | 2.37 |
| D‐Mannose | y = 1.3 × 105 + 47.824 | 0.62–67.79 | 0.9998 | 0.21 | 0.51 | 0.69 | 2.44 | 1.78 | 2.39 | 99.84 | 1.89 |
| D‐Rhamnose | y = 1.7 × 105 – 15.436 | 0.26–27.44 | 0.9996 | 0.27 | 0.69 | 0.71 | 1.89 | 2.52 | 2.35 | 101.47 | 1.96 |
| D‐Ribose | y = 2.0 × 105 + 51.271 | 0.23–25.96 | 0.9999 | 0.23 | 0.66 | 0.77 | 2.77 | 2.43 | 1.89 | 97.63 | 2.71 |
| D‐Glucose | y = 1.6 × 105 + 18.779 | 0.26–27.97 | 0.9999 | 0.21 | 0.72 | 0.86 | 2.14 | 1.63 | 2.65 | 102.24 | 2.15 |
3.2. Quantitative analysis
The composition and content of six kinds of monosaccharides in 14 batches of samples were determined using the UHPLC‐QTRAP‐MS/MS, and the specific results are shown in Table 4. The results showed that S3 and S4 did not contain rhamnose, S7 did not contain ribose and rhamnose, and S13 did not contain ribose. The order of the total monosaccharide content was S12>S8>S1>S11>S2>S9>S13>S14>S5>S6>S7>S4>S10>S3. S12 (Qingyang, 4.2870 mg/g) had the highest monosaccharide content and S3 (Wuwei, 0.8037 mg/g) had the lowest monosaccharide content. In addition, for individual monosaccharide constituents, the content of mannose in all the samples was the most abundant in PCH, with an average proportion content as high as 67.71% (1.6014 mg/g), followed by glucose, galactose, arabinose, rhamnose, and the lowest content of ribose (0.31%, 0.0069 mg/g).
TABLE 4.
Determination and content of six monosaccharides in Polygonatum cyrtonema Hua
| Sample | Content (mg/g) | Content ratio (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D‐Man | D‐Rib | D‐Rha | D‐Glu | D‐Gal | D‐Ara | D‐Man | D‐Rib | D‐Rha | D‐Glu | D‐Gal | D‐Ara | |
| S1 | 2.7867 | 0.0162 | 0.0117 | 0.3238 | 0.2280 | 0.1156 | 80.03 | 0.46 | 0.34 | 9.30 | 6.55 | 3.32 |
| S2 | 2.5861 | 0.0179 | 0.0244 | 0.3451 | 0.2794 | 0.1456 | 76.10 | 0.53 | 0.72 | 10.15 | 8.22 | 4.28 |
| S3 | 0.4808 | 0.0058 | 0 | 0.2777 | 0.0227 | 0.0168 | 59.82 | 0.72 | 0 | 34.56 | 2.82 | 2.09 |
| S4 | 0.5389 | 0.0038 | 0 | 0.3171 | 0.0263 | 0.0186 | 59.57 | 0.42 | 0 | 35.05 | 2.90 | 2.06 |
| S5 | 1.0498 | 0.0041 | 0.0097 | 0.2325 | 0.1297 | 0.0776 | 69.83 | 0.27 | 0.64 | 15.46 | 8.63 | 5.16 |
| S6 | 0.9979 | 0.0036 | 0.0052 | 0.2787 | 0.0861 | 0.0388 | 70.76 | 0.26 | 0.37 | 19.76 | 6.11 | 2.75 |
| S7 | 0.5129 | 0 | 0 | 0.3344 | 0.0603 | 0.0341 | 54.46 | 0 | 0 | 35.51 | 6.40 | 3.63 |
| S8 | 3.3261 | 0.0063 | 0.0164 | 0.2676 | 0.3025 | 0.1339 | 82.07 | 0.16 | 0.40 | 6.60 | 7.46 | 3.30 |
| S9 | 2.2211 | 0.0046 | 0.0143 | 0.3016 | 0.2694 | 0.1469 | 75.09 | 0.16 | 0.48 | 10.20 | 9.11 | 4.97 |
| S10 | 0.4875 | 0.0036 | 0.0024 | 0.2616 | 0.0335 | 0.0213 | 60.19 | 0.45 | 0.29 | 32.30 | 4.14 | 2.63 |
| S11 | 1.9745 | 0.0174 | 0.0475 | 1.1308 | 0.2548 | 0.0339 | 57.09 | 0.50 | 1.37 | 32.69 | 7.37 | 0.98 |
| S12 | 2.4760 | 0.0114 | 0.0310 | 1.5800 | 0.1665 | 0.0221 | 57.76 | 0.27 | 0.72 | 36.86 | 3.88 | 0.52 |
| S13 | 1.6400 | 0 | 0.0094 | 0.3238 | 0.3220 | 0.2310 | 64.92% | 0% | 0.37 | 12.82 | 12.75 | 9.14 |
| S14 | 1.3410 | 0.0023 | 0.0069 | 0.2357 | 0.0593 | 0.0244 | 80.32% | 0.14% | 0.41 | 14.12 | 3.55 | 1.46 |
3.3. Technique for order preference by similarity to an ideal solution analysis
The technique for order preference by similarity to an ideal solution (TOPSIS) method is a statistical method used to determine the optimal scheme and the worst scheme in the ideal condition from the normalized original data matrix. It determines the quality of the evaluation object by calculating the relative proximity between the evaluation object, the optimal scheme, and the worst scheme [33]. In this study, the contents of six kinds of monosaccharides were used as experimental indices, and the TOPSIS method was used to comprehensively evaluate and rank the PCH.
3.3.1. Establishment of the normalized standard decision matrix
According to the content determination results (Table 4), the original test data of six kinds of monosaccharides of the 14 batches of PCH were normalized according to Eq. (1) to establish a standardized decision matrix and calculate the Z value.
| (1) |
Note: The measured value of the ith evaluation object on the j index is denoted by Xij, and the normalized value of the ith evaluation object on the j index is denoted by Zij.
3.3.2. Determination of the optimal and worst schemes
The optimal scheme Z + and the worst schemes Z – were obtained according to the weighted decision matrix. By converting the normalized data, we got the Z + and Z – [34, 35]:
3.3.3. Determination of the distance between the evaluation project and the two schemes
The best and worst schemes are calculated, and the distance between the PCH samples from the optimal scheme (Di +) and the distance from the worst scheme (Di −) can be calculated according to Eqs. (2) and (3).
| (2) |
| (3) |
3.3.4. Calculation of the relative closeness
Ci of the proximity degree between each evaluation object and the optimal scheme was calculated, and the evaluation objects were ranked according to the value of Ci . The value of Ci is in the range of [0, 1]. A value closer to 1 indicates that the evaluation object is closer to the optimal level, and a value closer to 0 indicates that the evaluation object is closer to the worst level. The Eq. (4) is as follows [36, 37]:
| (4) |
3.3.5. Analytical results of the method
The TOPSIS ranking results according to the above equations are shown in Table 5. We used the content of six monosaccharides as an index and sorted the values of Ci . The results were as follows: S11 and S12 had the best quality, ranking 1st and 2nd, respectively; S17 was the worst, ranking 14th.
TABLE 5.
The quality of samples was sorted by technique for order preference by similarity to an ideal solution (TOPSIS) method
| Sample | Di + | Di – | Ci | Ranking |
|---|---|---|---|---|
| S1 | 0.849110960 | 0.717434465 | 0.457972334 | 4 |
| S2 | 0.930204412 | 0.782569992 | 0.456901966 | 5 |
| S3 | 1.271828347 | 0.169707273 | 0.117726729 | 11 |
| S4 | 1.274048736 | 0.116901388 | 0.084044270 | 13 |
| S5 | 1.086773053 | 0.298493603 | 0.215477361 | 8 |
| S6 | 1.216034220 | 0.168087743 | 0.121439980 | 10 |
| S7 | 1.278729447 | 0.083896007 | 0.061569382 | 14 |
| S8 | 0.858530763 | 0.712162700 | 0.453406547 | 6 |
| S9 | 0.807636762 | 0.687112348 | 0.459684066 | 3 |
| S10 | 1.248791537 | 0.126865735 | 0.092221906 | 12 |
| S11 | 0.605983607 | 1.028654198 | 0.629285702 | 1 |
| S12 | 0.680241946 | 0.902884369 | 0.570317327 | 2 |
| S13 | 0.977235632 | 0.740476037 | 0.431082847 | 7 |
| S14 | 1.199897424 | 0.181822426 | 0.131591383 | 9 |
3.3.6. Cluster analysis of the total amount of monosaccharides
Cluster analysis gradually aggregates the samples according to the degree of similarity of quality characteristics, prioritizes the ones with the greatest similarity, and finally aggregates multiple varieties according to the comprehensive properties of the category. By using the cluster analysis program of SPSS 26.0 software, the TOPSIS comprehensive scores of the six monosaccharide components of PCH from each producing area were used as the evaluation index. The samples from 14 producing areas were systematically analyzed using the square Euclidean distance method, and the results are shown in Figure 2. When the classification distance was 10, all the samples could be divided into two categories: category I included S3, S4, S7, and S10 (group 1) and S5, S6, S13, and S14 (group 2); category II included S11, S12 (group 3), and S1, S2, S8, and S9 (group 4). The comprehensive score and the quality of PCH in Qingyang County were the best and could be classified into a group (S11, S12).
FIGURE 2.
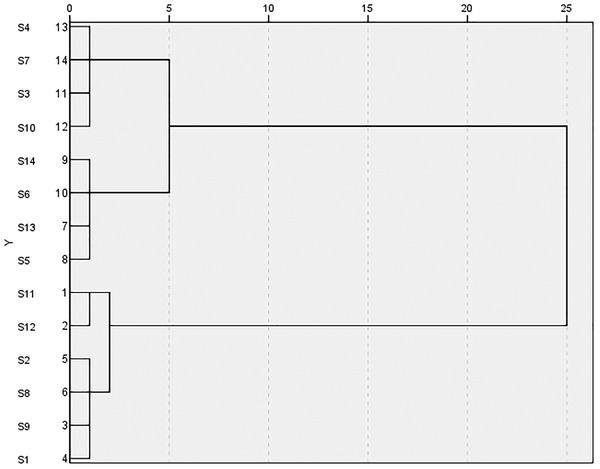
Dendrogram of cluster analysis for comprehensive evaluation of samples from different areas
3.4. Principal component analysis of monosaccharide
The principal component analysis (PCA) is a dimensionality reduction method that converts many original variables into several integrated new variables to reflect the entire dataset as much as possible without losing the principal variable information and shows the difference in data on the premise of not losing principal variable information [38, 39]. The standardized scores of the sample content determination results in Table 4 were taken as variables, and principal component analysis was carried out using the SPSS26.0 software. The characteristic values are listed in Table 6a. The analysis results showed that the characteristic values of the two PC extracted samples were 3.222 and 1.763, respectively, and the cumulative contribution rate of each sample was 53.699 and 83.087%, respectively. The cumulative variance contribution rates of these two principal components were 83.087%, which is very representative. The PCA initial factor loading matrix was extracted and the data are listed in Table 6b.
TABLE 6.
Principal component analysis results of the samples
| (a) Principal component eigenvalues and the variance contribution rate of monosaccharides | ||||
| Principal component | Eigenvalue | Contribution rate (%) | Cumulative contribution rate (%) | |
| F1 | 3.222 | 53.699 | 53.699 | |
| F2 | 1.763 | 29.388 | 83.087 | |
| (b) Principal component factor load matrix | ||||
| Component | F 1 | F 2 | ||
| Mannose | 0.905 | –0.187 | ||
| Ribose | 0.703 | 0.318 | ||
| Rhamnose | 0.744 | 0.467 | ||
| Glucose | 0.531 | 0.756 | ||
| Galactose | 0.892 | –0.421 | ||
| Arabinose | 0.527 | –0.813 | ||
| (c) The comprehensive evaluation results of samples were based on principal component analysis | ||||
| Sample | F 1 | F 2 | F | Ranking |
| S1 | 0.8831 | –0.4062 | 0.3548 | 3 |
| S2 | 0.9050 | ‐0.8410 | 0.2388 | 4 |
| S3 | –1.1032 | 0.3875 | –0.4785 | 12 |
| S4 | –1.1273 | 0.3449 | –0.5040 | 13 |
| S5 | –0.4507 | –0.2359 | –0.3113 | 8 |
| S6 | –0.8190 | –0.0151 | –0.4443 | 10 |
| S7 | –1.1390 | 0.0791 | –0.5884 | 14 |
| S8 | 0.9722 | –1.0077 | 0.2259 | 5 |
| S9 | 0.7226 | –0.7277 | 0.1742 | 6 |
| S10 | –1.0734 | 0.3358 | –0.4777 | 11 |
| S11 | 1.4688 | 1.7517 | 1.3035 | 1 |
| S12 | 1.0784 | 1.9689 | 1.1577 | 2 |
| S13 | 0.4545 | ‐1.7810 | –0.2794 | 7 |
| S14 | –0.7718 | 0.1468 | –0.3713 | 9 |
According to the loading of each monosaccharide component on the first two main PCs, the principal component F1 mainly reflects the information on mannose, and the principal component F2 mainly represents the glucose information.
The comprehensive score F value of PCH from each region was obtained according to the two main components and the weight of the variance contribution rate, as shown in Table 6c. The calculation formula was F = 0.53669 × F1 + 0.29388 × F2 .
The results showed that S11 and S12 had the best quality and S7 had the worst quality of PCH with six kinds of monosaccharides as evaluation indexes.
4. CONCLUDING REMARKS
In this study, six monosaccharides (glucose, mannose, rhamnose, galactose, ribose, and arabinose) in PCH were determined by the UHPLC‐QTRAP‐MS/MS, and the monosaccharide components in the polysaccharides were quantitatively and qualitatively detected by the multi‐reaction monitoring scanning method. Two multivariate statistical methods, TOPSIS and PCA, were combined to comprehensively evaluate the quality of 14 batches of PCH from different producing areas. Under the analysis of these two methods, highly similar results were obtained. The comprehensive evaluation of PCH from different producing areas was used to evaluate the monosaccharide as the index showed that Qingyang County (S11, S12) had the best quality, ranking in the 1st and 2nd places, respectively.
Hydrochloric acid was used instead of trifluoroacetic acid to hydrolyze polysaccharide samples, and the hydrolysis time was shortened from more than 5−9 h to 1 h. The process was simple and easy, and the experimental efficiency was significantly improved. The experimental results showed that PCH from Qingyang had a higher polysaccharide content and better quality. However, the difference in monosaccharide content and proportion in PCP may be related to the differences in the climatic conditions and geographical environments in the different producing areas, because there are obvious differences in precipitation, temperature, and altitude [40, 41]. The emerging potential methods of pretreatment and extraction [42] may provide unexpected insights into the active material bases of herbal medicine. In this study, a comprehensive evaluation model of PCH was established based on the content and composition of monosaccharides in PCP. This method can effectively evaluate the quality of Chinese medicines or natural plants with polysaccharides as the main active ingredient and can provide a theoretical basis for the rational development and utilization of Chinese medicinal resources.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This work was financially funded by the National Key R&D Project (2017YFC1701600, 2017YFC1701602), Major Science and Technology Special Project of Anhui Province (202003a07020011), and the National Census of Chinese medicine resources project of public health service subsidy of traditional Chinese medicine in 2017 (CS [2017] No. 66).
Hu J, Cheng H, Xu J, Liu J, Xing L, Shi S, Wang R, Wu Z, Yu N, Peng D. Determination and analysis of monosaccharides in Polygonatum cyrtonema Hua polysaccharides from different areas by ultra‐high‐performance liquid chromatography quadrupole trap tandem mass spectrometry. J Sep Sci. 2021;44:3506–3515. 10.1002/jssc.202100263
Contributor Information
Nianjun Yu, Email: ynj2005288@sina.com.
Daiyin Peng, Email: pengdy@ahtcm.edu.cn.
REFERENCES
- 1. Cruz Martínez C, Diaz Gómez M, Sook Oh M. Use of traditional herbal medicine as an alternative in dental treatment in Mexican dentistry: a review. Pharm Biol. 2017;55:1992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fu‐Shuang L, Jing‐Ke W. Demystifying traditional herbal medicine with modern approach. Nat Plants. 2017;3:17109. [DOI] [PubMed] [Google Scholar]
- 3. Sanodiya BS, Thakur GS, Baghel RK, Prasad GB, Bisen PS. Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharm Biotechnol. 2009;10:717–42. [DOI] [PubMed] [Google Scholar]
- 4. Editorial Committee of Chinese Flora, Chinese Academy of Sciences . Flora of China. Beijing: Science Press; 1993. [Google Scholar]
- 5. Long JL, Chen SS, Wang HL, Liu HC, Zhao Z, Li JL. Effects of Polygonatum cyrtonema plants and rhizosphere soil water extract on seed germination and seed bud growth. J Chin Med Mater. 2021;2:269–272. 10.13863/j.issn1001-4454.2021.02.001 [DOI] [Google Scholar]
- 6. Mu CL, Sheng YF, Wang Q, Amin A, Li XG, Xie YQ. Potential compound from herbal food of Rhizoma Polygonati for treatment of COVID‐19 analyzed by network pharmacology: Viral and cancer signaling mechanisms. J Funct Foods. 2021;77:104149, 10.1016/J.JFF.2020.104149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi JX, Xu XM, Yu JN. Extraction method, structural identification and pharmacological activity of Polygonatum sibiricum polysaccharide. Chin Wild Plant Resour. 2019;38:36–42. [Google Scholar]
- 8. Wang J, Liang S, Chen YP, Wang ZP, Zhu Y, Wu HH. Research advances in non‐saponain chemical constituents of Polygonatum genus. J Liaoning Univ Tradit Chin Med. 2016;18:74–8. [Google Scholar]
- 9. Ning HH, Yuan MM, Wu QP, Ping YH, Zhou ZQ, Xu Y, Wu Yi, Yin HX. Identification of chemical constituents from Polygonatum cyrtonema. Chin J Exp Tradit Med Formulae. 2018;24:77–82. [Google Scholar]
- 10. Tao AE, Zhang XC, Du ZF, Zhao FY, Xia CL, Duan BZ. Research progress on flavonoids in plants of Polygonatum Mill. and their pharmacological activities. Chin Tradit Herb Drugs. 2018;49:2163–71. [Google Scholar]
- 11. Cheng ZY, Ke ZC, Wu YX. Study on secondary metabolites of endophytic fungus Aspergillus ochraceus from Polygonatum cyrtonema. Chin Tradit Herb Drugs. 2019;50:5424–8. [Google Scholar]
- 12. Chen H, Feng SS, Sun YJ, Hao ZY, Feng WS, Zheng XK. Advances in studies on chemical constituents of three medicinal plants from Polygonatum Mill. and their pharmacological activities. Chin Tradit Herb Drugs. 2015;46:2329–38. [Google Scholar]
- 13. Wang K, Yue YD, Tang F, Xun H, Sun J, Wang J. Sequential extraction and structural analysis of polysaccharides from Polygonatum cyrtonema Hua. Nat Prod Res Dev. 2014;26:364–9. [Google Scholar]
- 14. Jiang CX, Zhang TJ, Chen CQ, Li XK, Liu CX. Research progress in molygonati ohizoma and predictive analysis on Q‐marker. Chin Tradit Herb Drugs. 2017;48:1–16. [Google Scholar]
- 15. Xu JX, Liu L, Yang SX, Kuang Y. Chemical constituents from aerial part of Polygonatum cyrtonema. Chin Tradit Herb Drugs. 2016;47:3569–72. [Google Scholar]
- 16. Jia L, Shi J, Duan ZQ, Dong RP, Wan M, Zheng JY. The influence of polygonatum polysaccharide on function of glucose metabolism of high‐fat‐diet‐fed diabetic mice. China Med Her. 2017;14:24–8. [Google Scholar]
- 17. Ko JH, Kwon HS, Yoon JM, Yoo JS, Jang HS, Kim JY, Yeon SW, Kang JH. Effects of Polygonatum sibiricum rhizome ethanol extract in high‐fat diet‐fed mice. Pharm Biol. 2015;53:563–70. [DOI] [PubMed] [Google Scholar]
- 18. Pei H, Yong H, Hylands P, Legido‐Quigley C. Assessment of Polygonum capitatum Buch.‐Ham. ex D.Don by metabolomics based on gas chromatography with mass spectrometry. J Sep Sci. 2016;39:1979–86 . [DOI] [PubMed] [Google Scholar]
- 19. Hu DJ, Cheong KL, Zhao J, Li SP. Chromatography in characterization of polysaccharides from medicinal plants and fungi. J Sep Sci. 2013;36:1–19. [DOI] [PubMed] [Google Scholar]
- 20. Chen J, Yang F, Guo H, Wu F, Wang X. Optimized hydrolysis and analysis of Radix Asparagi polysaccharide monosaccharide composition by capillary zone electrophoresis. J Sep Sci. 2015;38:2327–31. [DOI] [PubMed] [Google Scholar]
- 21. Xu Z, Dai XX, Su SL, Yan H, Guo S, Qian DW, Duan JA. Investigation of dynamic accumulation and regularity of nine glycosides and saccharides in Rehmannia glutinosa by rapid quantitative analysis technology. J Sep Sci. 2019;42:1489–99. [DOI] [PubMed] [Google Scholar]
- 22. Ma HN, Hua YJ, Tu CY, Yuan LH, Wei P. Analysis of monosaccharides in the saffron corm glycoconjugate by capillary electrophoresis. Chin J Chromatogr. 2012;30:304–8. [DOI] [PubMed] [Google Scholar]
- 23. Wang XR, Liu HH, Luan CF, Mao M, Zhang P. Comparison of two methods for analysis of monosaccharide composition of polysaccharides from pomegranate peel. Guangzhou Chem Ind. 2018;46:105–7. [Google Scholar]
- 24. Akabane M, Yamamoto A, Aizawa SI, Taga A, Kodama S. Simultaneous enantioseparation of monosaccharides derivatized with L‐tryptophan by reversed phase HPLC. Anal Sci. 2014;30:739. [DOI] [PubMed] [Google Scholar]
- 25. Li PP, Yan ZY, Chen Y, He PF, Yang WG. Analysis of monosaccharide composition of water‐soluble polysaccharides from Codium fragile by ultra performance liquid chromatography‐tandem mass spectrometry. J Sep Sci. 2021;44:1452–60. 10.1002/jssc.202001140 [DOI] [PubMed] [Google Scholar]
- 26. Han XY, Xu ZP, Zhou CB, Feng W‐J, Liu JL, Wu HF. Extraction, hydrolysis and component analysis of zymosan. Chin J Biol. 2013;26:1513–6. [Google Scholar]
- 27. Niu F, Gao JM, Duan XW, Zheng F, Tian W. Optimization of hydrolysis process of polysaccharides from Chrysanthemum indicum flowers by response surface methodology. Sci Technol Food Ind. 2019;40:156–61. [Google Scholar]
- 28. Dong MY, Ding J, Ren WJ, Li XL, Jiang HQ, Li J, Liu YH. Comparison of GC and HPLC methods for analysis of monosaccharide composition of polysaccharides from Lonicera japonica. China Food Addit. 2020;31:124–8. [Google Scholar]
- 29. Tang LF, Wang F, Su XJ, Li QM, Guo HY, Sun YZ, Sun ZL. Effects of extracting with deep eutectic solvents on the properties and antioxidant activity of polysaccharides from Polygonatum cyrtonema Hua. Food Ferment Ind. 2021;47:151–157. 10.13995/j.cnki.11-1802/ts.026440 [DOI] [Google Scholar]
- 30. He LJ, Gan YP, Lv WD, Rao JF, Yang JM, Yu JS. Monosaccharide composition analysis on polysaccharides in Polygonatum cyrtonema by high performance anion‐exchange chromatography with pulsed amperometric detection. Chin Tradit Herb Drugs. 2017;48:1671–6. [Google Scholar]
- 31. Tao AE, Du ZF, Zhao FY, Xia CL, Duan BZ. Quality evaluation and discrimination of Polygonati Rhizoma from three origins based. Chin Tradit Herb Drugs. 2019;50:2467–73. [Google Scholar]
- 32. Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29:S49–52. [PMC free article] [PubMed] [Google Scholar]
- 33. Li YM, Shao JJ. TOPSIS analysis in evaluation of medical service quality in a Chinese hospital. Acad J Second Mil Med Univ. 2008;29:1531–3. [Google Scholar]
- 34. Guo YY, Xu CP, Nie X, Xiong GB, Li Y, Wang H. Evaluation of transdermal absorption of diclofenac sodium enhanced by different percutaneous absorption enhancers with TOPSIS method based on entropy weight. Chin J Hosp Pharm. 2016;36:1282–6. [Google Scholar]
- 35. Tian T, Ji YH, Wang SF, Chen DY, Liu YJ, Cheng XR. Study on determination method of plantain extract and quality evaluation of plantain by entropy weight TOPSIS. J Guangdong Pharm Univ. 2021;37:16–22. [Google Scholar]
- 36. Li X, Wang K, Liu L, Xin J, Yang H, Gao C. Application of the entropy weight and topsis method in safety evaluation of coal mines. Procedia Eng. 2011;26:2085–91. [Google Scholar]
- 37. Wu LT, Luo JL, Huang YF, Bai DY, Chen L. Applicability evaluation of TOPSIS attribute stratification method in the assessment of safflower yellow injection. Chin J Hosp Pharm. 2020;40:922–8. [Google Scholar]
- 38. Li BX, Dong ST, Ma Z. Determination of five hazardous elements in Wikstroemia from different areas and principal component analysis and cluster analysis of inorganic elements. Chin J New Drugs. 2019;28:2548–52. [Google Scholar]
- 39. Liu W, Guo HJ, Qin JP, Luo YD, Zheng LZ. Quality control of Vernonia patula Merrbased on multi‐component determination and principal component analysis. Chin J New Drugs. 2021;41:135–8. [Google Scholar]
- 40. Luo XC, Yang B, Zhang YS, Wang CH, Yao YQ. Distribution characteristics of minute rainfall rate in Anhui Province. Chin J Radio. 2020;35:908–13. [Google Scholar]
- 41. Shi XF, Xu GL. Time series analysis of meteorological elements in southern anhui under the background of climate change. J Subtrop Resour Environ. 2019;14:44–50. [Google Scholar]
- 42. Zhao C, Qiao XL, Shao QJ, Hassan M, Ma Z. Evolution of lignin chemical structure during bioethanol production process and its inhibition to enzymatic hydrolysis. Energy Fuels. 2020;34:5938–47. 10.1021/acs.energyfuels.0c00293 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


