Abstract
Background and purpose
Previously it has been shown that patients with painful diabetic neuropathy (PDN) have greater corneal nerve loss compared to patients with painless diabetic neuropathy. This study investigated if the severity of corneal nerve loss was related to the severity of PDN.
Methods
Participants with diabetic neuropathy (n = 118) and healthy controls (n = 38) underwent clinical and neurological evaluation, quantitative sensory testing, nerve conduction testing and corneal confocal microscopy and were categorized into those with no (n = 43), mild (n = 34) and moderate‐to‐severe (n = 41) neuropathic pain.
Results
Corneal nerve fibre density (p = 0.003), corneal nerve fibre length (p < 0.0001) and cold perception threshold (p < 0.0001) were lower and warm perception threshold was higher (p = 0.002) in patients with more severe pain, but there was no significant difference in the neuropathy disability score (p = 0.5), vibration perception threshold (p = 0.5), sural nerve conduction velocity (p = 0.3) and amplitude (p = 0.7), corneal nerve branch density (p = 0.06) and deep breathing heart rate variability (p = 0.08) between patients with differing severity of PDN. The visual analogue scale correlated significantly with corneal nerve fibre density (r = −0.3, p = 0.0002), corneal nerve branch density (r = −0.3, p = 0.001) and corneal nerve fibre length (r = −0.4, p < 0.0001). Receiver operating curve analysis showed that corneal nerve fibre density had an area under the curve of 0.78 with a sensitivity of 0.73 and specificity of 0.72 for the diagnosis of PDN.
Conclusions
Corneal confocal microscopy reveals increasing corneal nerve fibre loss with increasing severity of neuropathic pain and a good diagnostic outcome for identifying patients with PDN.
Keywords: cornea, corneal confocal microscopy, corneal nerve, neuropathic pain, painful diabetic neuropathy
There is evidence that patients with painful diabetic neuropathy (PDN) have greater corneal nerve fibre loss compared to painless diabetic neuropathy. The current study investigated if the severity of corneal nerve loss was related to the severity of PDN. Corneal confocal microscopy shows increasing corneal nerve fibre loss with increasing severity of neuropathic pain and a good diagnostic outcome for identifying patients with PDN.
![]()
INTRODUCTION
Painful diabetic neuropathy (PDN) affects at least one in five adults with diabetes and has a major impact due to associated depression, anxiety and sleep disturbance [1]. Current therapies for PDN show limited efficacy [2] reflecting in part diverse aetiological mechanisms involving the peripheral nociceptor, spinal cord and brain [3]. It has been suggested that phenotyping patients and identifying the primary site and mechanism of their symptoms may allow a more tailored therapeutic approach and improved response to treatment [4]. However, sensory testing, skin punch biopsy and brain imaging currently have insufficient evidence to support their use as biomarkers in clinical trials of PDN [5] and an analysis of seven clinical trials utilizing sensory phenotyping showed a limited predictive impact on the efficacy of drugs used to relieve neuropathic pain [6].
A recent metanalysis has shown increased heat pain thresholds in patients with PDN compared to painless diabetic neuropathy [7]. However, a skin biopsy study showed no difference in intraepidermal nerve fibre density (IENFD) in patients with and without painful neuropathy [8]. In our previous study, whilst IENFD was comparable, there was a reduction in intraepidermal nerve fibre length (IENFL) in patients with PDN compared to painless diabetic neuropathy [9]. A study also found no difference in IENFD or axonal swellings between patients with PDN and painless diabetic neuropathy [10]. Additionally, another study found no difference in IENFD but an increase in the density of dermal nerve fibres containing substance P and calcitonin gene‐related peptide in patients with PDN compared to painless diabetic neuropathy [11]. Bönhof et al. found comparable IENFD and IENFL, but increased dermal nerve GAP‐43 staining, indicative of regenerating nerves in patients with PDN [12].
Corneal confocal microscopy (CCM) is a rapid, non‐invasive technique that images C fibres in the cornea and has demonstrated good diagnostic utility for diabetic neuropathy [13, 14], comparable to IENFD [15, 16]. Initially a significant reduction in corneal nerve fibre length (CNFL) was shown in a small cohort of patients with PDN compared to painless diabetic neuropathy [9]. Subsequently, a lower central and inferior whorl CNFL in patients with PDN was confirmed by ourselves and others [17, 18]. Here it is investigated whether the severity of corneal nerve loss and other measures of neuropathy are related to the severity of PDN.
METHODOLOGY
Study participants
This study included 118 participants with diabetes (type 1, n = 45; type 2, n = 73) and 38 control subjects who underwent detailed assessment of peripheral neuropathy and CCM. Participants were excluded if they had a history of connective tissue or infectious disease, malignancy, deficiency of B12 or folate, chronic renal or liver failure, current or active diabetic foot ulceration, previous ocular trauma, systemic disease other than diabetes that involves the cornea, surgery and a history of or current contact lens wear. Informed consent was obtained from patients prior to study participation. The research adhered to the tenets of the Declaration of Helsinki and was approved by the Greater Manchester East Research Ethics Committee.
Clinical and neuropathy assessment
A medical history was taken and body mass index, blood pressure, glycated haemoglobin (HbA1c) and lipid profile were evaluated. The neuropathy disability score (NDS) was used to assess vibration, pinprick sensation, temperature perception and the presence or absence of ankle reflexes. All patients had confirmed diabetic neuropathy based on an NDS > 2 [19]. All patients were asked not to use pain medications 24 h before the study assessments. The severity of pain at the study visit was recorded using a 0–100 mm visual analogue scale (VAS). Patients were stratified into three groups according to pain severity: no pain (VAS 0–4; n = 43), mild pain (VAS 5–44; n = 34), moderate‐to‐severe pain (VAS 45–100; n = 41) [20].
Deep breathing heart rate variability was assessed using the ANX 3.0 autonomic nervous system monitoring device (ANSAR Medical Technologies).
Vibration perception threshold (VPT) was assessed using a neurothesiometer (Scientific Laboratory Supplies). Cold perception threshold (CPT) and warm perception threshold (WPT) were tested on the dorsolateral aspect of the non‐dominant foot (S1) using a TSA‐II NeuroSensory Analyser (Medoc Ltd). Electrodiagnostic studies were undertaken by a consultant neurophysiologist using a Dantec ‘Keypoint’ system (Dantec Dynamics Ltd). Sural sensory nerve conduction velocity and amplitude were tested.
Ophthalmic assessment
Corneal sensitivity threshold was measured using a non‐contact corneal aesthesiometer (Glasgow Caledonian University) [21]. CCM examination using a laser scanning corneal confocal microscope HRT III (Heidelberg Engineering) was performed for both eyes according to our established protocol [22]. Six images (three per eye) from the central sub‐basal nerve plexus were selected by a single expert in a masked fashion, taking into account the quality, depth and variability according to our established protocol [23] and quantified manually using CCMetrics (University of Manchester). The corneal nerve fibre density (CNFD) (the total number of main nerves per square millimetre, no./mm2), the corneal nerve branch density (CNBD) (the total number of branches per square millimetre, no./mm2) and the CNFL (the total length of main nerves and nerve branches per square millimetre, mm/mm2) were quantified.
Statistical analysis
Analysis was carried out using SPSS (Version 22.0 for Windows, IBM Corporation). The Shapiro–Wilk test was employed to assess whether the data were normally distributed. The non‐parametric Kruskal–Wallis H test was used to compare the differences between groups of patients whilst the Mann–Whitney U test was used for comparison between two groups. Data are expressed as median (range). For CCM parameters, data were adjusted for age and are presented as estimated marginal means ± SEM using ANCOVA (with least significant difference [LSD] correction). The ANCOVA (LSD) test was used to assess for differences between groups of patients. Spearman's rho correlation coefficient was used to assess associations between CCM parameters, other measures of neuropathy and the VAS score. Receiver operating characteristic (ROC) curves were used to define the optimum cut‐off points with highest sensitivity and specificity for the diagnosis of PDN, and Youden's index, where J = sensitivity + specificity – 1, was used to measure the optimum cut‐off points. Graphs were created using Graphpad Prism (Version 7.0c for Windows, Graphpad Software).
RESULTS
Clinical and demographic assessment
Patients and healthy controls were matched for gender (p = 0.07). There was no significant difference in duration of diabetes (H(2) = 5.97, p = 0.051) between groups of patients (Table 1). Age (H(2) = 9.19, p = 0.01), type of diabetes (p < 0.0001), HbA1c (H(2) = 6.03, p = 0.05) and triglycerides (H(2) = 7.6, p = 0.02) differed significantly between groups of patients. The type of diabetes differed significantly between the patient groups with a significantly greater proportion of patients with type 2 diabetes in those with mild pain compared to comparable numbers with type 1 and type 2 diabetes in those with moderate‐to‐severe pain (p = 0.002) and no pain (p = 0.003). HbA1c was significantly higher in patients with no (7.2 [7.4], p < 0.0001), mild (7.4 [4.3], p < 0.0001) and moderate‐to‐severe pain (8.1 [7.3], p < 0.0001) compared to controls (5.6 [1.3]) and in patients with moderate‐to‐severe pain compared to no pain (p = 0.03). Triglycerides were significantly lower in patients with no pain (1.10 [2.4]) compared to mild pain (1.65 [5.0], p = 0.007) and healthy controls (1.60 [4.3], p = 0.008). Low density lipoprotein cholesterol was significantly lower in patients with mild pain (1.97 [3.6], p < 0.0001) compared to controls (2.84 [2.7]). 47.1% of patients with mild pain and 41.5% of patients with moderate‐to‐severe pain were on neuropathic pain relief medication.
TABLE 1.
Demographic and clinical data in controls and diabetic patients with no, mild and moderate‐to‐severe neuropathic pain
| Controls n = 38 | No pain n = 43 | Mild pain n = 34 | Moderate‐to‐severe pain n = 41 | Kruskal–Wallis test | |
|---|---|---|---|---|---|
| Age (years) | 57 (36) | 68 (47) a | 68 (34) a | 61 (50) b | H(2) = 9.19, p = 0.01 |
| Sex (female) | 18 | 11 | 12 | 21 | 0.07 c |
| Type of diabetes (T1DM/T2DM) | 0 | 20/23 | 5/29 | 20/21 | <0.0001 c |
| Duration of diabetes (years) | 0 | 16 (66) | 15 (50) | 24 (66) b | H(2) = 5.97, p = 0.051 |
| BMI (kg/m2) | 26 (24) | 29 (35) | 32 (45) a | 29 (18) | H(2) = 4.46, p = 0.1 |
| HbA1c (%) | 5.6 (1.3) | 7.2 (7.4) a | 7.4 (4.3) a | 8.1 (7.3) a , d | H(2) = 6.03, p = 0.05 |
| IFCC (mmol/mol) | 37.35 (20.4) | 55 (81.0) a | 57 (46.0) a | 65 (79.0) a , d | H(2) = 5.9, p = 0.05 |
| HDL‐C (mmol/l) | 1.53 (1.72) | 1.38 (2.32) | 1.33 (2.10) | 1.44 (2.78) | H(2) = 2.1, p = 0.3 |
| Triglycerides (mmol/l) | 1.60 (4.3) | 1.10 (2.4) a | 1.65 (5.0) d | 1.40 (3.6) | H(2) = 7.6, p = 0.02 |
| LDL‐C (mmol/l) | 2.84 (2.7) | 1.63 (2.62) | 1.97 (3.6) a | 1.86 (2.9) | H(2) = 2.6, p = 0.2 |
Kruskal–Wallis test is performed between groups of patients. All data are presented as median (range).
Abbreviations: BMI, body mass index; HDL‐C, high density lipoprotein cholesterol; IFCC, International Federation of Clinical Chemistry; LDL‐C, low density lipoprotein cholesterol; T1DM, type 1 diabetes; T2DM, type 2 diabetes.
Significant difference compared to controls.
Significant difference compared to mild pain.
p value refers to Fisher's exact test.
Significant difference compared to no pain.
Neuropathy assessment
Neuropathy disability score was significantly higher in diabetic patients with no pain (5 [7], p < 0.0001), mild pain (6 [7], p < 0.0001) and moderate‐to‐severe pain (6 [7], p < 0.0001) compared to controls, but was comparable between the groups of patients with differing severity of neuropathic pain (H(2) = 1.2, p = 0.5) (Table 2). VPT was significantly higher in patients with no (21 [43], p < 0.0001), mild (21 [44.5], p < 0.0001) and moderate‐to‐severe pain (24 [44.5], p < 0.0001) compared to healthy controls (6 [23.75]) but was comparable between groups of patients with differing severity of neuropathic pain (H(2) = 1.2, p = 0.5). Sural sensory nerve conduction velocity was significantly lower in patients with no (40.0 [18.9], p < 0.0001), mild (41.2 [26.6], p < 0.0001) and moderate‐to‐severe pain (40.0 [24.9], p < 0.0001) compared to healthy controls (50.0 [20.9]) but did not differ between groups of patients with differing severity of neuropathic pain (H(2) = 1.98, p = 0.3). Sural nerve action potential was significantly lower in patients with no (4.5 [25], p < 0.0001), mild (5.35 [17], p < 0.0001) and moderate‐to‐severe pain (4.2 [26], p < 0.0001) compared to healthy controls (16.0 [37.4]) but did not differ between groups of patients with differing severity of neuropathic pain (H(2) = 0.6, p = 0.7). CPT was significantly lower in patients with no (26.1 [31.6], p < 0.0001), mild (25.25 [30.3], p = 0.004) and moderate‐to‐severe pain (22.1 [29], p < 0.0001) compared to controls (27.9 [12.9]) and was significantly lower in patients with moderate‐to‐severe pain compared to no (p < 0.0001) and mild (p = 0.009) pain. WPT was significantly higher in patients with no (41.0 [16.6], p = 0.01), mild (42.1 [16.1], p < 0.0001) and moderate‐to‐severe pain (44.8 [15.2], p < 0.0001) compared to controls (38.5 [19.1]) and was higher in patients with moderate‐to‐severe pain compared to no pain (p = 0.002). CPT and WPT differed significantly between groups of patients (H(2) = 15.4, p < 0.0001; and H(2) = 12.17, p = 0.002). Deep breathing heart rate variability was significantly lower only in patients with moderate‐to‐severe pain compared to controls (p = 0.03) and did not differ significantly between groups of patients (H(2) = 4.9, p = 0.08).
TABLE 2.
Neuropathy testing in controls and diabetic patients with no, mild and moderate‐to‐severe neuropathic pain
| Controls n = 38 | No pain n = 43 | Mild pain n = 34 | Moderate‐to‐severe pain n = 41 | Kruskal–Wallis test | |
|---|---|---|---|---|---|
| NDS (0–10) | 0 (6) | 5 (7) a | 6 (7) a | 6 (7) a | H(2) = 1.2, p = 0.5 |
| DB‐HRV (beats per min) | 16 (5) | 15 (41) | 13 (38) | 9 (56) a | H(2) = 4.9, p = 0.08 |
| VPT (V) | 6 (23.75) | 21 (43) a | 21 (44.5) a | 24 (44.5) a | H(2) = 1.2, p = 0.5 |
| CPT foot (°C) | 27.9 (12.9) | 26.1 (31.6) a | 25.25 (30.3) a | 22.1 (29) a , b , c | H(2) = 15.4, p < 0.0001 |
| WPT foot (°C) | 38.5 (19.1) | 41.0 (16.6) a | 42.1 (16.1) a | 44.8 (15.2) a , b | H(2) = 12.17, p = 0.002 |
| SNCV (m/s) | 50.0 (20.9) | 40.0 (18.9) a | 41.2 (26.6) a | 40.0 (24.9) a | H(2) = 1.98, p = 0.3 |
| SNAP (mV) | 16.0 (37.4) | 4.5 (25.0) a | 5.35 (17.0) a | 4.2 (26.0) a | H(2) = 0.6, p = 0.7 |
Data presented as median (range). p value less than 0.01 was considered as significant. Kruskal–Wallis test is performed between groups of patients.
Abbreviations: CPT, cold perception threshold; DB‐HRV, deep breathing heart rate variability; NDS, neuropathy disability score; SNAP, sural nerve action potential; SNCV, sural nerve conduction velocity; VPT, vibration perception threshold; WPT, warm perception threshold.
Significant difference compared to controls.
Significant difference compared to no pain.
Significant difference compared to mild pain.
Corneal assessment
Corneal sensitivity threshold was significantly higher in the group with moderate‐to‐severe pain (1.63 ± 0.24) compared to controls (0.6 ± 0.19, p = 0.001), no pain (0.89 ± 0.20, p = 0.02) and mild pain (0.65 ± 0.24, p = 0.005). The CNFD was significantly lower in patients with no (23.67 ± 1.15, p < 0.0001), mild (20.85 ± 1.29, p < 0.0001) and moderate‐to‐severe (17.27 ± 1.18, p < 0.0001) pain compared to controls (Table 3, Figures 1 and 2) and in patients with moderate‐to‐severe pain compared to no pain (p < 0.0001) and mild pain (p = 0.01). CNBD was significantly lower in patients with no (56.47 ± 4.56, p < 0.0001), mild (45.20 ± 5.14, p < 0.0001) and moderate‐to‐severe (40.60 ± 4.71, p < 0.0001) pain compared to controls and in moderate‐to‐severe pain (p = 0.02) compared to no pain. CNFL was lower in no (22.47 ± 1.03, p = 0.004), mild (20.21 ± 1.17, p < 0.0001) and moderate‐to‐severe pain (15.82 ± 1.07, p < 0.0001) compared to controls and was lower in moderate‐to‐severe pain compared to mild pain (p = 0.007) and no pain (p < 0.0001). There was a significant difference in corneal sensitivity (F(2, 113) = 4.2, p = 0.007), CNFD (F(2, 113) = 6.3, p = 0.003) and CNFL (F(2, 113) = 8.18, p < 0.0001) but no difference in CNBD (F(2, 113) = 2.9, p = 0.06) between groups of patients.
TABLE 3.
Corneal sensitivity and confocal microscopy parameters in controls and diabetic patients with no, mild and moderate‐to‐severe pain
| Controls n = 38 | No pain n = 43 | Mild pain n = 34 | Moderate‐to‐severe pain n = 41 | ANCOVA | |
|---|---|---|---|---|---|
| NCCA (mbar) | 0.6 ± 0.19 | 0.89 ± 0.20 | 0.65 ± 0.24 | 1.63 ± 0.24 b , c | F(2, 113) = 4.2, p = 0.007 |
| CNFD (no./mm2) | 33.56 ± 1.25 | 23.67 ± 1.15 c | 20.85 ± 1.29c | 17.27 ± 1.18 b , c | F(2, 113) = 6.3, p = 0.003 |
| CNBD (no./mm2) | 92.75 ± 5.09 | 56.47 ± 4.56 c | 45.20 ± 5.14 c | 40.60 ± 4.71 c | F(2, 113) = 2.9, p = 0.06 |
| CNFL (mm/mm2) | 27.06 ± 1.13 | 22.47 ± 1.03 c | 20.21 ± 1.17 c | 15.82 ± 1.07 b , c | F(2, 113) = 8.18, p < 0.0001 |
Data are adjusted for age and presented as estimated marginal means ± SEM using ANCOVA (LSD correction). The ANCOVA (LSD correction) test is done between groups of patients.
Abbreviations: CNBD, corneal nerve branch density; CNFD, corneal nerve fibre density; CNFL, corneal nerve fibre length; NCCA, non‐contact corneal aesthesiometer.
Significant compared to no pain.
Significant difference compared to mild pain.
Significant difference compared to controls.
FIGURE 1.
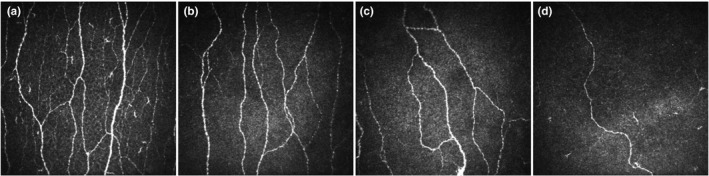
CCM images of a healthy control (a) and age‐matched patients with no (b), mild (c) and moderate‐to‐severe (d) neuropathic pain
FIGURE 2.
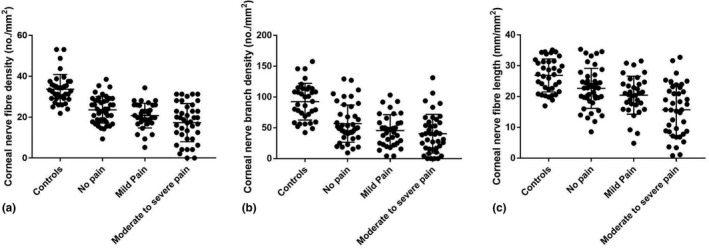
Corneal nerve fibre parameters as dot plots and mean ± SD for controls and patients with no, mild and moderate‐to‐severe pain: (a) corneal nerve fibre density; (b) corneal nerve branch density; (c) corneal nerve fibre length
Association between VAS, CCM and other measures of neuropathy
Visual analogue scale correlated significantly with CNFD (r = −0.3, p = 0.0002), CNBD (r = −0.3, p = 0.001) and CNFL (r = −0.4, p < 0.0001) (Figure 3) and with WPT (r = 0.3, p = 0.0004) and CPT (r = −0.35, p = 0.0001). There was no correlation between VAS and measures of large fibre neuropathy. Corneal sensitivity threshold was significantly associated with CNFD (r = −0.4, p = 0.005).
FIGURE 3.
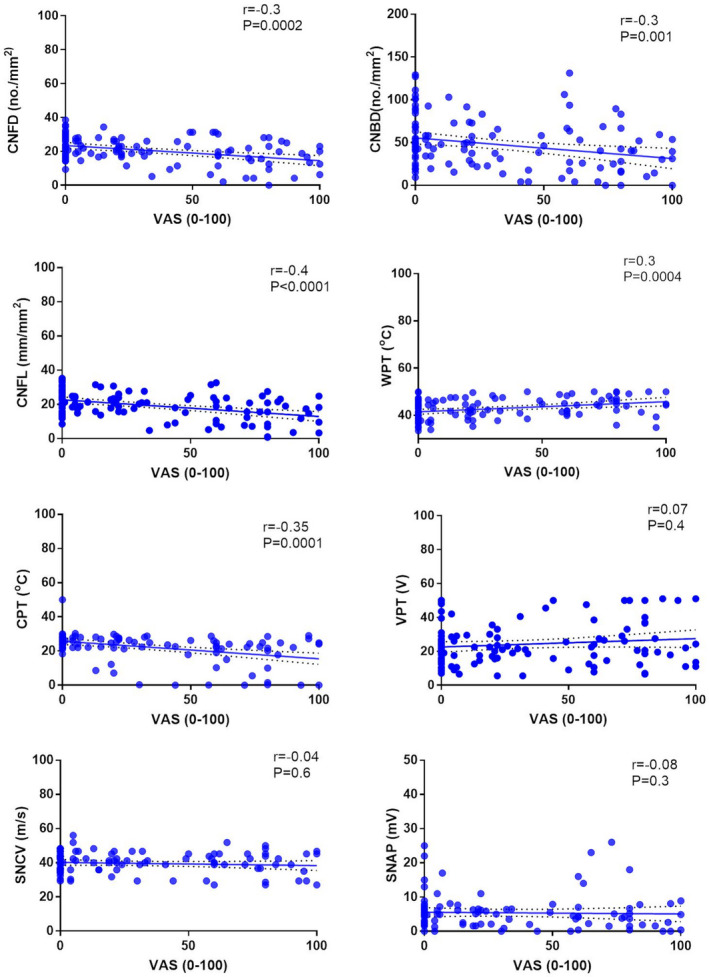
Association plots between VAS and measures of small and large fibre neuropathy
Diagnostic utility of CCM for PDN
For the diagnosis of PDN (VAS > 4 and NDS > 2) ROC curve analysis revealed an area under the curve (AUC) for CNFD of 0.78 with a sensitivity of 0.73, specificity of 0.72 and optimal threshold of 23.69 (no./mm2) (Figure 4, Table 4). CNBD had an AUC of 0.75 with sensitivity of 0.66, specificity of 0.66 and an optimal threshold of 51.04 (no./mm2). CNFL had an AUC of 0.74 with a sensitivity of 0.66 and specificity of 0.65 and an optimal threshold of 21.48 (mm/mm2).
FIGURE 4.
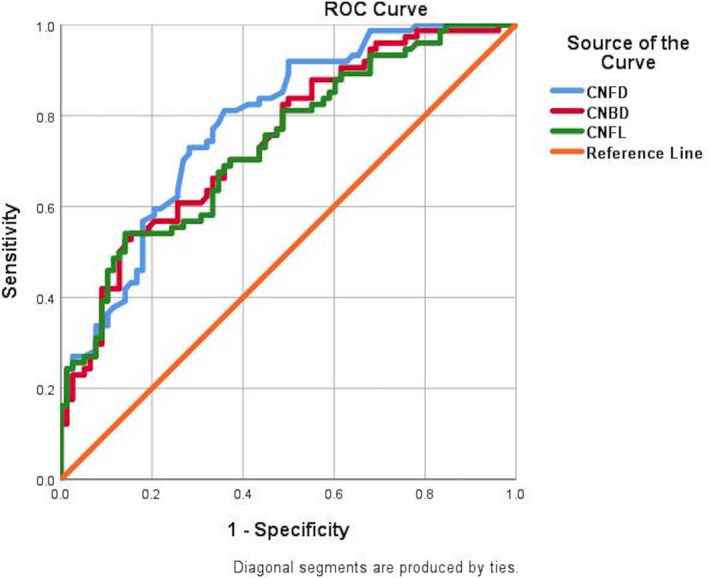
ROC curves for corneal nerve fibre density CNFD (no./mm2), corneal nerve branch density (CNBD, no./mm2) and corneal nerve fibre length (CNFL, mm/mm2) for painful diabetic neuropathy
TABLE 4.
Diagnostic performance of corneal nerve fibre parameters for the diagnosis of painful diabetic neuropathy
| CCM parameters | AUC | p value | Optimal threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|
| CNFD (no./mm2) | 0.78 | <0.0001 | 23.69 | 0.73 | 0.72 |
| CNBD (no./mm2) | 0.75 | <0.0001 | 51.04 | 0.66 | 0.66 |
| CNFL (mm/mm2) | 0.74 | <0.0001 | 21.48 | 0.66 | 0.65 |
Abbreviations: AUC, area under the curve; CCM, corneal confocal microscopy; CNBD, corneal nerve branch density; CNFD, corneal nerve fibre density; CNFL, corneal nerve fibre length.
DISCUSSION
In the current study, greater small nerve fibre dysfunction and damage was evidenced by increased cold and warm thermal thresholds and greater corneal nerve loss, respectively, with increasing severity of PDN. The aetiology of PDN is complex with a contribution of genetics, gender, glycaemic control peripheral nerve, spinal cord and brain pathology [24, 25]. However, there is an increasing body of evidence for the permissive role of peripheral small nerve fibre dysfunction and damage in the development and maintenance of PDN [4, 9, 17, 26].
Benbow et al. (1994) followed 50 patients with PDN over 3.6 years and found no association between the initial or follow‐up pain scores and a range of measures of small fibre function including thermal limen, heat pain threshold and weighted pinprick threshold [27]. However, recently two large cross‐sectional cohort studies have reported an association between thermal sensory loss and neuropathic pain [28, 29]. There is debate regarding the association between alterations in intraepidermal nerve fibre morphology and the severity or characteristics of PDN. Thus, whilst some studies of patients with PDN have shown intraepidermal nerve fibre loss [12, 30], others have not [10, 12, 31, 32]. Furthermore, other recent studies have shown comparable IENFD but increased dermal nerve fibres in PDN compared to painless diabetic neuropathy [11, 12].
Corneal nerve fibre damage has been reported by ourselves and others in adults with diabetic peripheral neuropathy [33] children with type 1 diabetes without neuropathy [34], adults with type 1 diabetes without overt microvascular complications [35], subjects with impaired glucose tolerance [36] and recently diagnosed patients with type 2 diabetes [37]. However, patients with PDN have greater corneal nerve loss compared to patients with painless diabetic neuropathy [9, 17, 26, 38, 39]. Furthermore, greater corneal nerve loss has been shown in patients with more painful symptoms [38]. Further support for the relationship between the severity of corneal nerve loss and neuropathic pain is now provided, by showing a greater reduction in CNFD and length in patients with more severe PDN and a significant correlation between the severity of PDN and corneal nerve fibre loss [38]. CNBD did not differ significantly between patients with differing severity of PDN and may reflect concomitant small fibre degeneration and regeneration. Indeed, Püttgen et al. (2019) also recently reported increased CNBD, indicative of small nerve fibre regeneration in patients with PDN [40]. Significantly lower corneal sensitivity has been shown previously in patients with PDN compared to painless diabetic neuropathy [39]. In the current study, lower corneal sensitivity is also reported in patients with moderate‐to‐severe pain compared to those with mild or no pain.
Deep breathing heart rate variability, a marker of cardiac autonomic dysfunction, was also lower in patients with moderate‐to‐severe PDN, which is consistent with a previous study showing greater autonomic dysfunction in patients with PDN [41]. The relationship between PDN and autonomic dysfunction may indicate an effect on cutaneous blood flow as impaired cutaneous sympathetic vasoconstriction has been shown previously in patients with PDN [42].
It is important to note that the associations observed do not simply reflect more severe neuropathy in those with more severe PDN as there was no difference in the NDS, VPT and sural nerve conduction and amplitude between patients with differing severity of PDN. These findings are consistent with our recent studies where significantly greater small nerve fibre damage was demonstrated but there was no difference in measures of large fibre neuropathy between patients with PDN compared to painless diabetic peripheral neuropathy [17, 38]. Several other studies have also shown no difference in measures of large fibre dysfunction or damage between patients with PDN and painless diabetic neuropathy [40]. Krämer et al. found no difference in nerve conduction studies [NCS] and VPT between patients with PDN and painless diabetic neuropathy; however, in the PDN group there was a correlation between pain intensity ratings and the cold detection threshold [43]. Spallone et al. have demonstrated a significant correlation between the 24‐h average pain score with the Michigan diabetic neuropathy score and Valsalva ratio [44].
Several previous studies have shown that CCM has a good diagnostic accuracy for diabetic peripheral neuropathy [13, 14]. This is the first study to demonstrate good diagnostic accuracy for all three corneal nerve parameters in the diagnosis of PDN.
It is acknowledged that this is a cross‐sectional study and VAS is a subjective tool; however, this is a validated score to measure pain intensity in patients with PDN. Also skin biopsy to quantify IENFD or IENFL, a sensitive marker of small fibre neuropathy, was not performed although as discussed earlier there is considerable debate regarding the association between IENFD and PDN. A significant proportion of patients were on medication for neuropathic pain, 24 h prior to their assessment, which could have impacted on the assessment of the severity of neuropathic pain.
In conclusion, it is shown that CCM has good diagnostic accuracy for PDN and detects progressively greater small nerve fibre loss in patients with increasing severity of PDN. CCM may have clinical utility as a rapid and objective test for the assessment of PDN.
CONFLICT OF INTEREST
There is no conflict of interest related to this work for any of the authors.
AUTHOR CONTRIBUTIONS
Alise Kalteniece: Data curation (equal); investigation (equal); methodology (equal); writing—original draft (lead). Maryam Ferdousi: Data curation (equal); formal analysis (lead); investigation (equal); methodology (equal); writing—original draft (equal). Shazli Azmi: Investigation (equal). Saif Ullah Khan: Investigation (equal). Anne Worthington: Investigation (equal). Andrew Marshall: Investigation (equal). Catharina G. Faber: Funding acquisition (equal); writing—review and editing (equal). Giuseppe Lauria: Funding acquisition (equal); writing—review and editing (equal). Andrew JM Boulton: Writing—review and editing (equal). Handrean Soran: Writing—review and editing (equal). Rayaz Malik: Conceptualization (lead); funding acquisition (lead); resources (lead); supervision (lead); writing—review and editing (lead).
ACKNOWLEDGEMENTS
Supported by the Manchester Biomedical Research Centre, the Greater Manchester Comprehensive Local Research Network and the Wellcome Trust Research Facility. The authors alone are responsible for the content and writing of the paper. Dr Mitra Tavakoli undertook corneal confocal microscopy and Dr Hassan Fadavi undertook clinical assessment and QST in a proportion of patients.
Kalteniece A, Ferdousi M, Azmi S, et al. Corneal nerve loss is related to the severity of painful diabetic neuropathy. Eur J Neurol. 2022;29:286–294. 10.1111/ene.15129
Funding information
The research received the funding from the European Union Seventh Framework Programme FP7/2007‐2013 (no. 602273)
DATA AVAILABILITY STATEMENT
The datasets generated and analysed during the study are available from the corresponding author on a reasonable request.
REFERENCES
- 1. Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community‐based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta‐analysis. Lancet Neurol. 2015;14(2):162‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456‐2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calcutt NA. Diabetic neuropathy and neuropathic pain: a (con)fusion of pathogenic mechanisms? Pain. 2020;161(Suppl 1):S65‐S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Backonja MM, Attal N, Baron R, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154(9):1807‐1819. [DOI] [PubMed] [Google Scholar]
- 6. Holbech JV, Bach FW, Finnerup NB, Jensen TS, Sindrup SH. Pain phenotype as a predictor for drug response in painful polyneuropathy—a retrospective analysis of data from controlled clinical trials. Pain. 2016;157(6):1305‐1313. [DOI] [PubMed] [Google Scholar]
- 7. Sierra‐Silvestre E, Somerville M, Bisset L, Coppieters MW. Altered pain processing in patients with type 1 and 2 diabetes: systematic review and meta‐analysis of pain detection thresholds and pain modulation mechanisms. BMJ Open Diabetes Res Care. 2020;8(1):e001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Truini A, Biasiotta A, Di Stefano G, et al. Does the epidermal nerve fibre density measured by skin biopsy in patients with peripheral neuropathies correlate with neuropathic pain? Pain. 2014;155(4):828‐832. [DOI] [PubMed] [Google Scholar]
- 9. Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148‐2154. [DOI] [PubMed] [Google Scholar]
- 10. Cheung A, Podgorny P, Martinez JA, Chan C, Toth C. Epidermal axonal swellings in painful and painless diabetic peripheral neuropathy. Muscle Nerve. 2015;51(4):505‐513. [DOI] [PubMed] [Google Scholar]
- 11. Karlsson P, Provitera V, Caporaso G, et al. Increased peptidergic fibers as a potential cutaneous marker of pain in diabetic small fiber neuropathy. Pain. 2020;162(3):778‐786. [DOI] [PubMed] [Google Scholar]
- 12. Bönhof GJ, Strom A, Püttgen S, et al. Patterns of cutaneous nerve fibre loss and regeneration in type 2 diabetes with painful and painless polyneuropathy. Diabetologia. 2017;60(12):2495‐2503. [DOI] [PubMed] [Google Scholar]
- 13. Perkins BA, Lovblom LE, Bril V, et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia. 2018;61(8):1856‐1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams BM, Borroni D, Liu R, et al. An artificial intelligence‐based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: a development and validation study. Diabetologia. 2020;63(2):419‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen X, Graham J, Dabbah MA, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. 2015;38(6):1138‐1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alam U, Jeziorska M, Petropoulos IN, et al. Diagnostic utility of corneal confocal microscopy and intra‐epidermal nerve fibre density in diabetic neuropathy. PLoS One. 2017;12(7):e0180175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalteniece A, Ferdousi M, Petropoulos I, et al. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci Rep. 2018;8(1):3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang H, Fan D, Zhang S, Wang X. Early diagnosis of painful diabetic neuropathy by corneal confocal microscopy. Zhonghua Yi Xue Za Zhi. 2014;94(33):2602‐2606. [PubMed] [Google Scholar]
- 19. Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150‐154. [DOI] [PubMed] [Google Scholar]
- 20. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S240‐S252. [DOI] [PubMed] [Google Scholar]
- 21. Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Malik RA. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care. 2007;30(7):1895‐1897. [DOI] [PubMed] [Google Scholar]
- 22. Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non‐invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp. 2011;47:2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalteniece A, Ferdousi M, Adam S, et al. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS One. 2017;12(8):e0183040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr DiabRep. 2019;19(6):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Selvarajah D, Wilkinson ID, Fang F, et al. Structural and functional abnormalities of the primary somatosensory cortex in diabetic peripheral neuropathy: a multimodal MRI study. Diabetes. 2019;68(4):796‐806. [DOI] [PubMed] [Google Scholar]
- 26. Marshall AG, Lee‐Kubli C, Azmi S, et al. Spinal disinhibition in experimental and clinical painful diabetic neuropathy. Diabetes. 2017;66(5):1380‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benbow SJ, Chan AW, Bowsher D, MacFarlane IA, Williams G. A prospective study of painful symptoms, small‐fibre function and peripheral vascular disease in chronic painful diabetic neuropathy. Diabet Med. 1994;11(1):17‐21. [DOI] [PubMed] [Google Scholar]
- 28. Raputova J, Srotova I, Vlckova E, et al. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross‐sectional observational study. Pain. 2017;158(12):2340‐2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Themistocleous AC, Ramirez JD, Shillo PR, et al. The Pain in Neuropathy Study (PiNS): a cross‐sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157(5):1132‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galosi E, La Cesa S, Di Stefano G, et al. A pain in the skin. Regenerating nerve sprouts are distinctly associated with ongoing burning pain in patients with diabetes. Eur J Pain. 2018;22(10):1727‐1734. [DOI] [PubMed] [Google Scholar]
- 31. Ekman L, Thrainsdottir S, Englund E, et al. Evaluation of small nerve fiber dysfunction in type 2 diabetes. Acta Neurol Scand. 2020;141(1):38‐46. [DOI] [PubMed] [Google Scholar]
- 32. Bechakra M, Nieuwenhoff MD, Rosmalen JV, et al. Pain‐related changes in cutaneous innervation of patients suffering from bortezomib‐induced, diabetic or chronic idiopathic axonal polyneuropathy. Brain Res. 2020;1730:146621. [DOI] [PubMed] [Google Scholar]
- 33. Ferdousi M, Kalteniece A, Azmi S, et al. Diagnosis of neuropathy and risk factors for corneal nerve loss in type 1 and type 2 diabetes: a corneal confocal microscopy study. Diabetes Care. 2021;44(1):150‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferdousi M, Romanchuk K, Mah JK, et al. Early corneal nerve fibre damage and increased Langerhans cell density in children with type 1 diabetes mellitus. Sci Rep. 2019;9(1):8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petropoulos IN, Green P, Chan AW, et al. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PLoS One. 2015;10(4):e0123517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Azmi S, Ferdousi M, Petropoulos IN, et al. Corneal confocal microscopy identifies small‐fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care. 2015;38(8):1502‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ziegler D, Papanas N, Zhivov A, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63(7):2454‐2463. [DOI] [PubMed] [Google Scholar]
- 38. Kalteniece A, Ferdousi M, Azmi S, et al. Corneal confocal microscopy detects small nerve fibre damage in patients with painful diabetic neuropathy. Sci Rep. 2020;10(1):3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferdousi M, Azmi S, Kalteniece A, et al. Greater small nerve fibre damage in the skin and cornea of type 1 diabetic patients with painful compared to painless diabetic neuropathy. Eur J Neurol. 2021;28(5):1745‐1751. [DOI] [PubMed] [Google Scholar]
- 40. Püttgen S, Bonhof GJ, Strom A, et al. Augmented corneal nerve fiber branching in painful compared to painless diabetic neuropathy. J Clin Endocrinol Metab. 2019;104:6220‐6228. [DOI] [PubMed] [Google Scholar]
- 41. Gandhi RA, Marques JL, Selvarajah D, Emery CJ, Tesfaye S. Painful diabetic neuropathy is associated with greater autonomic dysfunction than painless diabetic neuropathy. Diabetes Care. 2010;33(7):1585‐1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quattrini C, Harris ND, Malik RA, Tesfaye S. Impaired skin microvascular reactivity in painful diabetic neuropathy. Diabetes Care. 2007;30(3):655‐659. [DOI] [PubMed] [Google Scholar]
- 43. Krämer HH, Rolke R, Bickel A, Birklein F. Thermal thresholds predict painfulness of diabetic neuropathies. Diabetes Care. 2004;27(10):2386‐2391. [DOI] [PubMed] [Google Scholar]
- 44. Spallone V, Morganti R, D'Amato C, et al. Clinical correlates of painful diabetic neuropathy and relationship of neuropathic pain with sensorimotor and autonomic nerve function. Eur J Pain. 2011;15(2):153‐160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the study are available from the corresponding author on a reasonable request.


