ABSTRACT
Adult stem cells (ASCs) in vertebrates and model invertebrates (e.g. Drosophila melanogaster) are typically long‐lived, lineage‐restricted, clonogenic and quiescent cells with somatic descendants and tissue/organ‐restricted activities. Such ASCs are mostly rare, morphologically undifferentiated, and undergo asymmetric cell division. Characterized by ‘stemness’ gene expression, they can regulate tissue/organ homeostasis, repair and regeneration. By contrast, analysis of other animal phyla shows that ASCs emerge at different life stages, present both differentiated and undifferentiated phenotypes, and may possess amoeboid movement. Usually pluri/totipotent, they may express germ‐cell markers, but often lack germ‐line sequestering, and typically do not reside in discrete niches. ASCs may constitute up to 40% of animal cells, and participate in a range of biological phenomena, from whole‐body regeneration, dormancy, and agametic asexual reproduction, to indeterminate growth. They are considered legitimate units of selection. Conceptualizing this divergence, we present an alternative stemness metaphor to the Waddington landscape: the ‘wobbling Penrose’ landscape. Here, totipotent ASCs adopt ascending/descending courses of an ‘Escherian stairwell’, in a lifelong totipotency pathway. ASCs may also travel along lower stemness echelons to reach fully differentiated states. However, from any starting state, cells can change their stemness status, underscoring their dynamic cellular potencies. Thus, vertebrate ASCs may reflect just one metazoan ASC archetype.
Keywords: adult stem cells, marine invertebrates, niche, gene expression, Waddington landscape, germ cells, totipotency, cell lineages, regeneration, asexual reproduction
I. INTRODUCTION
The prevailing vertebrate‐centric paradigm suggests the existence of idiosyncratic populations of adult stem cells (ASCs) in animals (Raff, 2003; Wagers & Weissman, 2004; Clevers, 2015; Wiggans & Pearson, 2021). In vertebrates, ASCs are defined as lineage‐restricted with tissue or organ‐specific activities, and are capable of regulating homeostasis, repair and regeneration of tissues and organs (Clevers & Watt, 2018). Vertebrate ASCs are located in defined niches, where they normally lie in a quiescent state (Slack, 2018; Marescal & Cheeseman, 2020) until called upon to activate by specific stimuli such as injury or disease (Clevers, 2015; Clevers & Watt, 2018). The literature on mammalian stem cells further defines ASCs as undifferentiated cellular entities that give rise to either daughter stem cells, self‐renewing progenitors, or lineage‐specific differentiated cells (Raff, 2003; Clevers & Watt, 2018). While at early embryogenesis vertebrate stem cells are totipotent, giving rise to both somatic and germline descendants, post‐embryonic stem cells are multipotent at best [e.g. haematopoietic stem cells (Raff, 2003; Wagers & Weissman, 2004)].
Over time, two distinct evolving views of ASCs in vertebrates have been proposed. The first considers ASCs as ‘entities’: discrete units of selection, development and regeneration (Weissman, 2000). The second focuses on their ‘state’ or ‘function’, and posits that the biological state of a cell dictates its status as an ASC or as a differentiated cell (Blau & Baltimore, 1991; Blau, Brazelton & Weismann, 2001). The latter view is supported by the controversial findings that restrictions in cell fates are flexible and that differentiated cells may regain levels of lost stemness.
In vertebrates, ASCs have been categorized by their morphology, tissue of origin, plasticity, and potency. While existing in a quiescent state, they still maintain the power to resume cellular proliferation. They tend to be found in small numbers, but are long‐lived as a population, and often express specific ‘stemness’ genes (Poulsom et al., 2002; Raff, 2003; Wagers & Weissman, 2004; Clevers, 2015; Rumman, Dhawan & Kassem, 2015; Grün et al., 2016; Clevers & Watt, 2018; Marescal & Cheeseman, 2020). Yet other authors have referred to specific ‘conditions’, rather than ‘characters’ or ‘functional potency’ when defining the ASC concept (Loeffler & Roeder, 2002; Zipori, 2004). The above views consider, as a prime defining feature, an ASC's ability to give rise to one or more differentiated cell types as part of regular bodily homeostasis, and in acute states such as those that require repairing damage (Slack, 2018).
While ASCs are inherently defined morphologically (Fig. 1), phenotype alone provides only tantalizing hints for their identification. For instance, it took decades of targeted research to define the population of haematopoietic stem cells (Eaves, 2015), and many years of work before the discovery of intestinal stem cells (van der Flier & Clevers, 2009). Likewise, other ASC identification criteria may conceal the authentic plasticity in their transcriptome profiles (Grün et al., 2016), and the detection of asymmetric cell divisions, often used to identify stem cells ‘unambiguously’, is particularly elusive. Similarly, the criteria of ASC potency and plasticity are a source of confusion (Poulsom et al., 2002; Raff, 2003; Wagers & Weissman, 2004). Notwithstanding such caveats, it is widely accepted that vertebrate ASCs are rare, clonogenic, and undifferentiated (characterized by a high nucleo‐cytoplasmic ratio and small cell size compared to lineage‐differentiated progenies). Moreover, they are multi/oligo/unipotent cells capable of self‐renewal and multilineage differentiation, often interacting with specialized stem cell niches, and are considered slow‐cycling cells that show distinct germ/somatic lineage potential. The somatic ASCs are tissue specific and function in homeostasis and, with constraints, in regeneration of organs/tissues.
Fig 1.
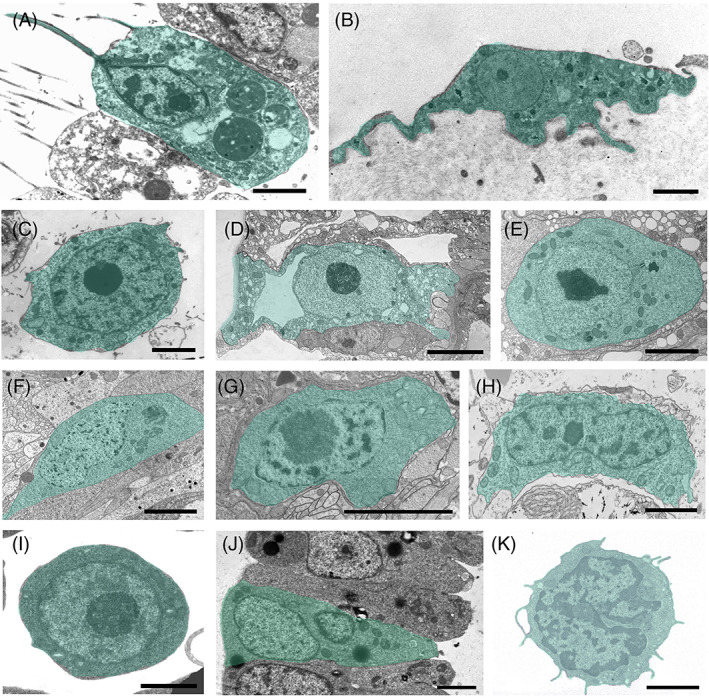
Adult stem cells (ASCs) from selected marine invertebrate phyla and a human haematopoietic stem cell visualized by transmission electron microscopy. (A–C) Porifera: a choanocyte of Leucosolena variabilis (A), a pinacocyte of Oscarella sp. (B), an archaeocyte of Crellomima imparidens (C). (D, E) Cnidaria: epitheliomuscular (D) and interstitial (E) stem cells of Hydra magnipapillata. (F, G) Platyhelminthes: neoblasts of the planarian Schmidtea sp. (F) and the rhabditophoran Macrostomum lignano (G). (H) Acoela: neoblast of Isodiametra pulchra. (I, J) Tunicata: haemoblast (I) and bud primordium cell (J) of Botryllus schlosseri. (K) Mammalia: quiescent haematopoietic stem cell of Mus musculus (modified from Radley et al., 1999). ASCs in invertebrates occur as two basic cell types, either as epithelial cells integrated into organized two‐dimensional tissue layers (A, B, D, J) or as smaller cells located in mesenchymal tissues (C, F–H), in interstitial spaces of epithelia (E), and in the circulating haemolymph/blood (I, K). Epithelial ASCs exhibit the hallmarks of typical epithelial cells including a distinct apical–basal polarity. Mammalian ASCs typically show a high nuclear to cytoplasmic ratio, round interphase nuclei with prominent nucleoli, and a ribosome‐rich cytoplasm. Scale bars: A–C, E–K, 5 μm; D, 5 μm. Photograph credits: A–C, A. Ereskovsky; D–H, B. Hobmayer; I, J, L. Manni).
Does the above vertebrate ASC ‘archetype’ apply to the animal kingdom (Metazoa) as a whole? Comparative approaches may shed light on this important question. A glance at the metazoan phylogenetic tree puts in stark relief the fact that ASCs have only been studied in a limited number of taxa, mainly those capable of asexual reproduction and/or with high competency for regeneration, including some spiralian protostomes (lophotrochozoans, i.e. Platyhelminthes) and deuterostomes (i.e. tunicates, echinoderms), as well as many non‐bilaterian lineages (i.e. cnidarians, poriferans). Somatic ASCs have not been reported in most ecdysozoans (i.e. Nematoidea, Scalidophora and Panarthropoda), except for a few arthropods (Shukalyuk et al., 2007; Alié et al., 2015).
To fill this conceptual lacuna, we evaluate the distribution and the properties of ASCs in non‐vertebrate metazoans in the context of the vertebrate ASC exemplar, excluding invertebrates such as fruit flies and nematodes, which while excellent genetic model systems are by all accounts highly derived ecdysozoans. Using inter/intra‐phyla comparative analyses of ASC properties, their gene expression and the cellular environment, as well as their role in unique biological processes (e.g. whole‐body regeneration), we put forward the hypothesis that vertebrates represent only one particular prototype of ASC, and that ASCs in fact exhibit a wider range of properties and abilities, some non‐existent in vertebrates. In light of this, we propose a unified model to explain ASC diversity in metazoans – ‘the wobbling Penrose landscape’, a modification of the traditional Waddington landscape metaphor.
II. VERTEBRATE VERSUS INVERTEBRATE ASCs AT A GLANCE
Apart from two fundamental properties of stem cells, i.e. self‐renewal and differentiation potential, it appears that many cardinal ASC traits differ between vertebrates and other phyla. Fifteen traits are highlighted in Table 1, together spanning a wide range of characteristics from morphology, differentiation state and somatic/germ lineage characteristics, to some key biological properties. Vertebrate ASCs are constrained to one of the three germ layers (Weissman, Anderson & Gage, 2001) and they give rise to lineage‐restricted progenies that are limited to specific organs/tissues (Tanaka & Reddien, 2011), with the germline being sequestered from the somatic lineages early in ontogeny. ASCs are generally rare in vertebrates (e.g. only 0.001–0.01% of mononuclear cells isolated from a Ficoll density gradient of feline bone marrow aspirate are mesenchymal stem cells; Martin et al., 2002) and pluripotent at best; they are slow cycling and reside in compartmentalized niches, with restricted migration potential (Moore & Lyle, 2011). These vertebrate traits are inconsistent with many of the ASC attributes found in other groups (Table 1). Even the statement that the ‘ability of stem cells to reside within niches is an evolutionarily conserved phenomenon’ (Fuchs, Tumbar & Guasch, 2004, p. 771) is not applicable to all, or even the majority, of metazoan ASCs. Further, ASCs in other lineages may arise de novo by trans‐differentiation from somatic cells (Ferrario et al., 2020), which is not a common phenomenon in the vertebrates (Goodell, Nguyen & Shroyer, 2015; Merrell & Stanger, 2016), and even from germ cells under specific conditions (Table 1). The aforementioned disparate characters have particularly emerged in long‐lived and indeterminately growing animals, where organismal senescence (sensu Rinkevich & Loya, 1986) has not been documented or is delayed (e.g. sponges, corals, and the immortal Hydra).
Table 1.
Central traits distinguishing vertebrate adult stem cell (ASCs) from non‐ecdysozoan invertebrate ASCs [selected citations; aberrant status such as cancer cells in vertebrates or reprogramming approaches such as iPS (induced pluripotent stem) cells are not included]
| ASC trait | Status in vertebrates | Status in marine invertebrates (most cases) |
|---|---|---|
| Abundance | Rare, 0.001–0.01% (e.g. Martin et al., 2002) | Up to 20–30% of all cells in flatworms (Handberg‐Thorsager, Fernandez & Salo, 2008; Gentile, Cebrià & Bartscherer, 2011); 20–30% of all cells in the freshwater hydrozoan Hydra (Bosch et al., 2010; Hobmayer et al., 2012); up to 50–80% (choanocytes) of all cells in Calcarea (Jones, 1961; A. E., unpublished results) and up to 3–14% of all cells in Demospongiae (Diaz, 1979; Custodio, Hajdu & Muricy, 2004). |
| Potency | Primarily uni/oligopotency, some pluripotency | Pluri‐ and totipotency, with differentiation potential towards cell lineages from more than a single germ layer (Müller, Teo & Frank, 2004; Manni et al., 2007; Rinkevich & Matranga, 2009; Rinkevich, Matranga & Rinkevich, 2009; Wagner, Wang & Reddien, 2011; Reyes‐Bermudez, Hidaka & Mikheyev, 2021). |
| Stemness outcomes | Limited to organs and tissues | May develop whole organisms via asexual reproduction (e.g. budding) or via regeneration of minute fragments (Manni et al., 2007, 2019; Rinkevich et al., 2007, 2009, 2011; Voskoboynik et al., 2007; Bely & Nyberg, 2010; Bosch et al., 2010; Lehoczky, Robert & Tabin, 2011; Lavrov & Kosevich, 2016; Lai & Aboobaker, 2018). |
| Amoeboid cell motility | Not recorded under normal conditions | Demosponge archaeocytes (Funayama, 2008), hydrozoan interstitial cells (Bode, 1996), planarian neoblasts (Isaeva, Aleksandrova & Reunov, 2005a ; Abnave et al., 2017) and amoebocytes in stellate echinoderms (Khadra et al., 2018) competent for amoeboid motility and/or active migration. |
| Exhibiting morphologies of differentiated cells | Not recorded under normal conditions | Recorded in various phyla. Examples are the morphologies of choanocytes in sponges and amoebocytes in anthozoans (Gold & Jacobs, 2013; Ereskovsky et al., 2015; Funayama, 2018); epithelial cells in Hydra (Hobmayer et al., 2012); filopodia/extended cell processes in flatworm neoblasts (Baguñà, 2012; Abnave et al., 2017; Ivankovic et al., 2019); also hypothesized for flagellated coelomic epithelial cells in a starfish (Bossche & Jangoux, 1976). |
| Soma/germ stem cell boundaries | The germline is sequestered at early ontogeny | Boundaries between soma/germ stem cells are blurred in many taxa and germ cells can arise from ASCs (Buss, 1982; Blackstone & Jasker, 2003; Rinkevich & Yankelevich, 2004; Seipel, Yanze & Schmid, 2004; Rinkevich et al., 2009; Rosner et al., 2009; Gold & Jacobs, 2013; Dannenberg & Seaver, 2018; DuBuc et al., 2020; Vasquez‐Kuntz et al., 2020). |
| Expression of germ cell markers in ASCs | Not recorded (except in some cancers) | Present in ASCs and various somatic cells (e.g. Vasa, Piwi and POU genes) (Raz, 2000; Mochizuki, Nishimiya‐Fujisawa & Fujisawa, 2001; Seipel et al., 2004; Shukalyuk et al., 2007; Rosner et al., 2009; Rinkevich et al., 2010; Rosner & Rinkevich, 2011; Fierro‐Constaín et al., 2017; Xu & Sun, 2020). |
| Germ stem cell trans‐differentiation to ASCs | Not recorded | Present, recorded in some regenerative scenarios such as in flatworms (Gremigni & Puccinelli, 1977). |
| De novo emergence of ASCs | Not recorded | Present in cnidarians, sponges and tunicates (Müller et al., 2004; Manni et al., 2007, 2019; Schmich et al., 2007; Rinkevich et al., 2010; Rinkevich & Rinkevich, 2013; Borisenko et al., 2015; Ereskovsky et al., 2015; Ferrario et al., 2020; Xu & Sun, 2020). |
| Source cells for regeneration | Tissue resident; mostly lineage‐restricted ASCs | Whole organismal residency; potential mobilization and expansion of ASCs from other sites/tissues; in planarians and some tunicates, a single ASC may regenerate a whole organism (Rinkevich, Shlemberg & Fishelson, 1995; Rinkevich et al., 2010, 2011; Lehoczky et al., 2011; Wagner et al., 2011; Rinkevich & Rinkevich, 2013; Blanchoud, Rinkevich & Wilson, 2018; Fields & Levin, 2018). Presence of dedifferentiation processes (Ferrario et al., 2020; Xu & Sun, 2020). |
| Contribution to dormancy | Inconclusive | Hibernation and aestivation in botryllid ascidians (Hyams et al., 2017). |
| ASC niche | Essential for ASC quiescence and long‐term survival (Marescal & Cheeseman, 2020) | No distinct anatomical stem cell niche has been elucidated for the vast majority of non‐ecdysozoan invertebrates (Rinkevich, 2009; Rinkevich et al., 2009). A few ephemeral ASC niches were identified in botryllid ascidians (Voskoboynik et al., 2008; Rinkevich et al., 2013; Rosner et al., 2013; Rosental et al., 2018). |
| Contribution to indeterminate growth | Indeterminate growth does not exist in birds and mammals | Indeterminate growth exists in various taxa within sponges, cnidarians, annelids, bryozoans, and tunicates (Jackson & Coates, 1986; Hughes, 1987; Vogt, 2012; Gazave et al., 2013); Direct evidence for the role of ASCs found in sponges, flatworms, cnidarians and annelids (e.g. in atokous worms). |
| Contribution to immortal lifespan | Immortality does not exist | Immortality exists in cnidarians (Martínez, 1998; Schmich et al., 2007; Dańko, Kozłowski & Schaible, 2015), planarians [further associated with neoblasts (Saló, 2006; Tan et al., 2012)] and sponges (which may live for thousands of years) (Gatti, 2002; McMurray, Blum & Pawlik, 2008); extended lifespan in bivalves, the longest lived non‐colonial animals (Gruber et al., 2015). |
| ASCs as units of selection | Unspecified; yes, in transmissible tumours | Present, potentially in all marine invertebrates with a somatic embryogenesis type of ontogeny (Buss, 1982; Rinkevich, 2000, 2009, 2011; Weissman, 2000; Laird, De Tomaso & Weissman, 2005; Fields & Levin, 2018). |
III. THE WIDE RANGE OF METAZOAN ASC MORPHOTYPES
Almost no study on ASCs outside vertebrates has been devoted to capturing their degree of potency by using criteria of increased stringency, as has recently been proposed for mammalian systems (Posfai et al., 2021). However, many phyla (e.g. Porifera, Cnidaria, Ctenophora, Annelida, Acoela, Platyhelminthes, Echinodermata, Cephalochordata and Tunicata) possess large pools of bona fide ASCs throughout the lifespan of the organism, most of which are multipotent (in sponges, flatworms, acoels, cnidarians, annelids and tunicates; Fig. 2; see online Supporting Information, Tables S1 and S2) and some of which (e.g. cnidarians, flatworms and tunicates) have been suggested to be totipotent (Müller et al., 2004; Manni et al., 2007; Rinkevich & Matranga, 2009; Rinkevich, Matranga & Rinkevich, 2009; Wagner et al., 2011; Kassmer, Langenbacher & De Tomaso, 2020). In many groups, ASCs give rise not only to somatic lineages, but also to germ cell lineages, with no signature of germ‐cell sequestration (Gschwentner et al., 2001; Takamura, Fujimura & Yamaguchi, 2002; Rinkevich, 2009; Juliano, Swartz & Wessel, 2010; Juliano & Wessel, 2010; Gold & Jacobs, 2013; Solana, 2013; Yoshida et al., 2017; Adamska, 2018; Dannenberg & Seaver, 2018; DuBuc et al., 2020; Vasquez‐Kuntz et al., 2020), and in some animals, ASCs are the only proliferative cells (Bely & Sikes, 2010a ).
Fig 2.

Plasticity, self‐renewal, and differentiation dynamics in selected invertebrate and vertebrate adult stem cell (ASC) lineages. ASCs are highlighted in colour, differentiation products shown as black and white schemes. Conversion of one ASC type into another occurs in pre‐bilaterian sponges and hydrozoans, and within the flatworm neoblast lineage (A–D). Differentiation of gametes as descendants of ASCs is a common feature in the pre‐bilaterian sponges and hydrozoans (A, B). The dashed arrows in sponge ASC lineages represent capacities for self‐renewal, phenotypic conversion and differentiation based on observations of cellular behaviour during growth, tissue renewal and regeneration, which have not yet been validated by stringent experimental analysis. In C stippled arrows represent the formation of hydrozoan epithelial cells from interstitial stem cells as described in Hydractinia spp., which does not occur in Hydra spp. In D red arrows in the planarian neoblast system are based on the lineage‐restricted expression of gene sets, which require further validation using precise lineage tracing and functional interference assays. The self‐renewal capacity of zeta‐, gamma‐, and nu‐neoblasts is under discussion. Species sources: (A) Amphimedon queenslandica, Ephydatia fluviatilis; (B) Oscarella lobularis; (C) Hydractinia spp., Hydra vulgaris; (D) Schmidtea mediterranea; (E) Botryllus schlosseri, Ciona robusta; (F) Homo sapiens. Schemes in C and D are modified from Gold & Jacobs (2013) and Zhu & Pearson (2016), respectively.
ASCs in invertebrates represent a wide range of phylum‐specific and characteristic cell types, morphologies and behaviours (Figs 1 and 2; Table S1), which range from sponge archaeocytes and choanocytes (Simpson, 1984; Ereskovsky, 2010), hydrozoan interstitial cells (i‐cells) (Bosch, 2009; Plickert, Frank & Müller, 2012) and platyhelminth or acoel neoblasts (Wagner et al., 2011; Baguñà, 2012) to tunicate haemoblasts (Freeman, 1964; Voskoboynik et al., 2008; Kawamura & Sunanaga, 2010; Rinkevich et al., 2013; Kassmer et al., 2020). Comparisons within phyla reveal a considerable degree of additional variation, where ASC properties are possessed only by particular taxa within a phylum (e.g. demosponge archaeocytes, hydrozoan i‐cells). Similarly, ASC lineages and progenitors may show intra‐phylum modifications (e.g. Müller et al., 2004; Borisenko et al., 2015; Funayama, 2018; Lavrov et al., 2018; Fig. 2; Table S1).
Outside the vertebrates, ASCs are often highly abundant (primarily choanocytes in sponges, ecto/endodermal epitheliomuscular cells in cnidarian polyps and neoblasts in flatworms; Simpson, 1984; Handberg‐Thorsager et al., 2008; Bosch et al., 2010; Gentile et al., 2011; Hobmayer et al., 2012; Table 1) and the literature reveals cases of putative totipotency, as high differentiation potential contributes to more than a single germ layer (Fig. 2; Rinkevich et al., 2009; Wagner et al., 2011). Emblematic structures in many of these ASCs are the so‐called chromatoid bodies [reported in neoblasts, i‐cells and archaeocytes, as well as most recently in a small pool of notochord cells in cephalochordates (Rossi et al., 2008; Isaeva et al., 2009; Isaeva & Akhmadiev, 2011; Holland & Somorjai, 2020)] – electron‐dense aggregates often adjacent to the nuclear envelope that resemble the germline granules of vertebrates and insects.
Many invertebrate ASCs consist of epithelial tissues, exhibiting epithelial cell hallmarks (lacking the characteristic large nucleus/cytoplasmic ratio of other ASCs), with distinct apical–basal and planar cell polarities, apical cell–cell junctions, and basal cell–extracellular matrix interactions, all of which are features of differentiated cells. The most prominent example is found in sponges, where cells of the inner and outer epithelia – choanocytes and pinacocytes, respectively (Fig. 1A, B) – function as true epithelial cells, while potentially acting as stem cells during tissue renewal and regeneration (Ereskovsky et al., 2015; Lavrov et al., 2018). The same applies to the hydrozoan cnidarian ectodermal and endodermal epitheliomuscular cells (Fig. 1D; Bosch et al., 2010; Hobmayer et al., 2012) and the colonial tunicate bud primordium (Fig. 1J; Manni et al., 2007, 2019). Further, the literature reveals cases where these ASCs not only express genes associated with germline stem cells, but are also able to differentiate into somatic cells or gametes, indicating the lack of strict boundaries between somatic/germline lineages. Examples include sponge archaeocytes and choanocytes (Fierro‐Constaín et al., 2017; Funayama, 2018), hydrozoan i‐cells (Bode, 1996), flatworm and acoel neoblasts (Shibata, Rouhana & Agata, 2010; Chiodin et al., 2013; Lai & Aboobaker, 2018), the posterior stem cells of the annelid growth zone (Giani et al., 2011; Gazave et al., 2013; Kozin & Kostyuchenko, 2015) and tunicate haemoblasts (Magor et al., 1999; Stoner, Rinkevich & Weissman, 1999; Laird et al., 2005; Voskoboynik et al., 2007; Rosner et al., 2009; Brown et al., 2009a ; Rinkevich, 2017; Rosner, Kravchenko & Rinkevich, 2019; Kassmer et al., 2020).
IV. GENE EXPRESSION IN INVERTEBRATE ASCs
Invertebrate ASCs express orthologues of many vertebrate ‘stemness’ genes, as well as genes that contribute to cancer cell ‘stem cell potential’ (Conte et al., 2009; Mashanov et al., 2010; Yun et al., 2017; Ben‐Hamo et al., 2018). A list of selected genes and gene families is provided in Fig. 3 and Table S2. However, it is challenging to identify or compare stemness gene signatures across diverse taxa separated by wide evolutionary distances (Alié et al., 2015; Wiggans & Pearson, 2021). Also, the molecular mechanisms by which invertebrates maintain viable ASC stocks, with long‐term stability and constant proliferation during their lifespan, remain elusive (Conte et al., 2009). This is true for Myc, one of the major vertebrate stem cell maintenance factors, and which has been associated with ASC self‐renewal in hydrozoan i‐cells (Hartl et al., 2010, 2019; Plickert et al., 2012). In‐depth single‐cell transcriptome analysis of hydrozoan i‐cell and flatworm neoblast lineages failed to identify common sets of stemness factors (Fincher et al., 2018; Plass et al., 2018; Siebert et al., 2019).
Fig 3.

The expression of ‘stemness’ genes in somatic cells of invertebrates. Five functional gene categories are depicted, each represented by 3–9 specific genes (in grey boxes). Bilaterian phyla are grouped by colour, with pink for Deuterostomia (Chordata and Ambulacraria) and blue (Spiralia) and yellow (Ecsysozoa) for Protostomia. Ticks indicate that expression of stemness genes in ASCs in at least one species for the phylum has been reported. Note that for most metazoan phyla and many gene categories, no data are available. Only taxa for which sufficient information on ASCs is available are included. The red skull and crossbones indicate the absence/loss of the gene(s) in the phylum. RRM, RNA‐recognition motif. Data from model ecdysozoans are excluded (Drosophila, nematodes; see text for details). See Table S2 for the original data on which this figure is based.
As in the vertebrates (Lander, 2009), the essence of ASC stemness cannot be distilled down to a single shared molecular fingerprint, further highlighted by the co‐expression of somatic/germ stem cell signatures in invertebrate ASCs (Table 1), wherever these ASCs have been studied. This includes the expression of genes such as POU, SOX, Piwi, Bruno, Vasa and Pl10 orthologues in a number of metazoan ASCs, including sponge archaeocytes and choanocytes (Funayama, 2008, 2018; Fierro‐Constaín et al., 2017), hydrozoan i‐cells (Seipel et al., 2004; Rebscher et al., 2008; Leclère et al., 2012), neoblasts of acoels and planarians (Guo, Peters & Newmark, 2006; Pfister et al., 2008; De Mulder et al., 2009b ; Önal et al., 2012), tunicate ASCs (Sunanaga, Watanabe & Kawamura, 2007; Rosner et al., 2009, 2019; Rinkevich et al., 2010), putative stem cells from annelid growth zones (Rebscher et al., 2007; Giani et al., 2011; Gazave et al., 2013), and presumably in regenerating nemertean tissues (Xu & Sun, 2020). This toti/pluripotency in non‐vertebrate phyla maintains functions such as gametogenesis, embryogenesis, homeostasis, asexual reproduction and regeneration (Fierro‐Constaín et al., 2017), supporting the idea of global conservation in pluripotency‐associated genes for day‐to‐day needs (Fig. 3; Table S2), as reported for cell adhesion receptors and nuclear receptors (Gamulin et al., 1994).
In contrast to the vertebrates, somatic and germline stemness markers (e.g. Vasa, Pl10, Piwi, Nanos, Bruno, Pumilio, Tudor, etc.; Fig. 3), as well as alkaline phosphatase (Isaeva, 2011), are co‐expressed in differentiated somatic cells/tissues in many invertebrate phyla (Table 1). This character has been recorded in sponges (Funayama, 2018), cnidarians (Mochizuki et al., 2001), ctenophores (Alié et al., 2011), annelids (Rebscher et al., 2007; Dill & Seaver, 2008; Gazave et al., 2013), parasitic crustaceans (Shukalyuk et al., 2007; Shukayuk & Isaeva, 2012), molluscs and echinoderms (Lai & Aboobaker, 2018) and colonial tunicates (Rabinowitz, Alphasi & Rinkevich, 2009; Rosner et al., 2009; Brown et al., 2009a ; Rinkevich et al., 2010; Rabinowitz & Rinkevich, 2011). These observations may either imply distinct functions or pleiotropy for the genes in differentiated somatic cells (Juliano et al., 2010). Alternatively, this may suggest that the conventional view of distinct ‘stem cell genes’ should be reconsidered.
By tracing shared transcriptomic signatures for demosponge archaeocytes, flatworm neoblasts and Hydra i‐cells, Alié et al. (2015) revealed 180 orthology groups, considered as a relevant proxy for the core set for ancestral stem cells. Most of these genes pre‐dated animal origins, with only a few representing true metazoan innovations. These findings reinforce the idea of a conserved ancestral multipotency program associated with pluri/totipotency (Önal et al., 2012; Fierro‐Constaín et al., 2017; Fig. 3), although the putative gene regulatory networks have been rewired throughout evolution to generate clade‐specific morphologies/physiologies. These observations are in line with the hypothesis of the existence of primordial stem cells (Solana, 2013). Interestingly, the ancestral stem cell transcriptomic landscape (Alié et al., 2015) is noticeably poor in transcription factors, yet it is rich in RNA regulatory players, including many RNA‐binding proteins, which are typical regulators of mammalian embryonic stem cells.
V. THE ENVIRONMENT – ASC NICHES IN INVERTEBRATES
The term ‘stem cell niche’, originally conceptualized by Schofield (1978), refers to a discrete anatomical microenvironment within which stem cells reside, as well as their milieu, which together play critical roles in maintaining/regulating ‘stemness’ properties (Spradling, Drummond‐Barbosa & Kai, 2001; Fuchs et al., 2004; Li & Xie, 2005; Saez, Yusuf & Scadden, 2017). Morphologically, all ‘niches’ consist of homing stem cells and their progeny, heterologous cell types and the surrounding niche‐specific extracellular matrix (Chacón‐Martínez, Koester & Wickström, 2018; Christodoulou et al., 2020). Studies in vertebrate models have elucidated a wide range of core elements associated with stem cell niche environments, encompassing networks of cell–cell and cell–extracellular matrix interactions and soluble signalling factors (autocrine, paracrine, systemic), which act as biochemical cues to determine ASC fates and behaviours (Scadden, 2006; Chacón‐Martínez et al., 2018; Singh et al., 2019). Thus, the maintenance of a niche is associated with, and based on, active crosstalk between ASCs and their niche components (Saez et al., 2017; Durand, Charbord & Jaffredo, 2018). The niche architecture in model organisms (e.g. mice, Caenorhabditis elegans, Drosophila melanogaster) constitutes one of the basic consensus feature central to the definition of ASCs (Slack, 2018).
Fuchs et al. (2004) argued that ASC competence to reside within discrete niches is an evolutionarily conserved feature between Drosophila and vertebrates, and that ASC niches are armed with shared properties, such as three‐dimensional spaces, basement membranes, extracellular matrices and paracrine signalling (Spradling et al., 2001; Scadden, 2006). ASC niches further generate extrinsic factors, such as BMP (bone morphogenetic protein) and Wnt (wingless‐related integration site) signals, that have emerged as common pathways for controlling stem cell self‐renewal and lineage fate from Drosophila to mammals (Li & Xie, 2005). Yet no such distinct anatomical stem cell niche has thus far been convincingly elucidated in non‐ecdysozoan invertebrates (Rinkevich, 2009; Rinkevich et al., 2009), and few putative stem cell niches have been identified (Table S3) that satisfy the strict criteria set for the vertebrate/insect ASC niches.
While knowledge gained from mammalian, D. melanogaster and C. elegans models provides guidelines for defining comparable niches in other metazoans, studies on sponge archaeocytes and choanocytes, hydrozoan i‐cells and platyhelminth and acoel neoblasts have failed to define either discrete anatomical microenvironments where stem cells reside, or a niche‐specific extracellular matrix to which ASCs home. Nevertheless, by employing the niche concept more loosely (Morrison & Spradling, 2008), the existence of ‘permissive’ stem cell niches for i‐cells in Hydra (e.g. Khalturin et al., 2007; Table S3) and for planarians neoblasts (Pellettieri & Sanchez Alvarado, 2007; Dingwall & King, 2016; Table S3) has been proposed. These claims were later adjusted by viewing the whole animal or tissue as a single functional stem cell niche. In Hydra, it was first suggested that the body column of the polyp could be considered a stem cell niche (Bosch et al., 2010). In planarians, a ‘global niche’ (macro‐environment) tenet was postulated, implying that the potential niche is ‘extended to the entire planarian body, in which long‐range signals, released by various differentiated tissues, regulate stem cell behaviour in response to environmental variations’ (Rossi & Salvetti, 2019, p. 33).
Botryllid ascidians reveal a different scenario relative to other taxa, with putative ASCs homing to discrete, yet ephemeral, microenvironments (Table S3). The first presumed niche, considered a somatic stem cell niche, was identified in the endostyle area (Voskoboynik et al., 2008), to which haemoblasts and proliferating cells migrate. Whole‐blood transcriptomes revealed a shared expression of >300 genes with human neural precursors and haematopoietic bone marrow, suggesting that the endostyle represents the haematopoietic stem cell niche (Rosental et al., 2018). Rinkevich et al. (2013) revealed the transient presence of ASC niches around zooid endostyles, termed ‘cell islands’. They host cycling putative stem cells that migrate weekly via the blood vasculature, from degenerating cell islands to newly formed ones in developing buds, which are also regarded as ‘ephemeral soma’ (Qarri et al., 2020). Cells within cell islands express a wide range of markers, including somatic stem cell markers [including PKC (protein kinase C), STAT (signal transducer and activator of transcription)], germ cell markers (Nanos, Vasa, alkaline phosphatase, Piwi) and signalling components of the BMP, FGF (fibroblast growth factor) and Slit/Robo (secreted SLIT glycoproteins and their roundabout receptors) pathways. Trafficking of germ stem cells between other putative transient niches was suggested to occur during the weekly blastogenic cycles in botryllid ascidians (Kawamura, Tachibana & Sunanaga, 2008b ; Rosner et al., 2013).
VI. IDIOSYNCRATIC FEATURES ASSOCIATED WITH ASCs IN INVERTEBRATES
Many of the characters used to identify vertebrate ASCs are associated with their functions, primarily with the perpetuation of lineages, replacement of cells due to wear‐and‐tear and the supply of differentiated cells for maintenance (Raff, 2003; Wagers & Weissman, 2004; Morrison & Spradling, 2008; Rumman et al., 2015; Clevers & Watt, 2018). By contrast, beyond their functions in supporting homeostasis, ASCs in many metazoans (Fig. 4; Table S4) also play major roles in supporting key biological features such as regeneration in adults, including whole‐body regeneration, and agametic asexual reproduction such as budding and fission (Weissman, 2000; Raff, 2003; Rinkevich et al., 2007, 2009, 2011; De Mulder et al., 2009b ; Isaeva et al., 2009; Bely & Nyberg, 2010; Funayama, 2018; Lai & Aboobaker, 2018; Ivankovic et al., 2019; Rossi & Salvetti, 2019; Tables S5 and S6; Figs S1 and S2), as well as regulation of dormancy or torpor‐like states (Hyams et al., 2017; Table S7).
Fig 4.

Adult stem cells (ASCs) are involved in four major biological processes in Metazoa: homeostasis, adult regeneration, dormancy and agametic asexual reproduction. The presence of the biological process, involvement of undifferentiated/differentiated putative ASCs or progenitors and their level of potency, as well as the specific classes of stemness gene families they express are mapped for all phyla, when present in at least a single member of the group considered. In the metazoan phylogeny, Deuterostomia are in pink, Ecdysozoa are in yellow, and Spiralia are in green (Gnathifera) and blue (Lophotrochozoa). The position of the Acoelomorpha is debated (dotted line). Circles: empty circle – documented presence of the biological process; filled circle – cases where putative ASCs or progenitors are involved; dotted line circle – inconclusive evidence for the presence of the biological process. A red cross signifies the absence of the biological process in the clade as currently documented. As homeostasis is a property of life, all phyla are shown with an empty circle. For adult regeneration, an asterisk within a circle documents the presence of whole‐body regeneration. Dormancy refers to any documented type of dormant stage or torpor‐like process and has likely evolved independently in each lineage. For dormancy, the dotted line circle indicates potential involvement in non‐adults. A – quiescence, diapause, growth/degrowth; D – diapause; G – growth/degrowth; O – ontogeny reversal; Q – quiescence. For agametic asexual reproduction, B – any form of budding; F – any form of fission/fragmentation. Triangles indicate the level of documented potency for ASCs (filled) and progenitors (empty). Red = lineage restricted/unipotent; cyan = totipotent; blue = multi/pluripotent; gradient triangle = documented cases of several ASCs or progenitors with different potency. Selected stemness gene families whose members are expressed in ASCs or progenitors during the biological process are listed in a box for each process and phylum. The relative contribution of undifferentiated (U) versus differentiated (D) ASCs or progenitors within each phylum is mapped onto the phylogeny if known; levels of confidence are represented by solid (higher) and dotted (lower) diamonds, while the sizes of D and U reflect their presumed level of contribution. See Tables [Link], [Link] and Figs. S1 and S2 for the original data used to generate this figure.
A comprehensive survey across 26 metazoan phyla identifies ASCs and progenitors with putative roles in homeostasis (10 out of 26 phyla), regeneration (9 out of 23 phyla able to regenerate, of which 14 exhibit the capacity for whole‐body‐regeneration), asexual reproduction (5 out of 15 phyla), and in regulating dormant states (6 out of 20 phyla; Fig. 4; Tables [Link], [Link]). Regeneration patterns, type of dormancy and asexual modes of reproduction differ among phyla (Fig. 4) as well as within specific taxonomic groups (Tables [Link], [Link]; Figs S1 and S2), and are further tuned by the contributions of dedifferentiation processes (Ferrario et al., 2020). While proper identification of stem cells or lineage‐committed progenitor cells is still lacking for many lineages, the literature already indicates major differences between ASCs in various species in terms of general and specific markers for ASCs (the current terminology is based on the vertebrate ASC literature). Many metazoan phyla show ASC‐associated phenomena not recorded in vertebrates, both under normal physiological and hostile environmental conditions, including whole‐body regeneration, budding, fission and fusion of body fragments, and cycles of growth/decay. When studied in detail, the involvement of multi/pluri/totipotent ASCs is often revealed (Fig. 4; Tables [Link], [Link]; Figs S1 and S2). Thus, at least some ASCs in invertebrates can produce differentiated lineages and can impart stemness at the totipotent level.
An additional biological feature of ASCs is their roles in organisms with indeterminate growth (where growth does not cease at adulthood), reflecting an unfolding ontogenic trait from birth to death (Vogt, 2012). This rarely studied phenomenon is characteristic of particular lineages (e.g. bivalve molluscs, echinoderms, solitary ascidians, annelids) as well as colonial/modular marine invertebrates (e.g. corals, sponges, bryozoans, ascidians).
VII. DISCUSSION
This review describes ASC states across the breadth of non‐vertebrate metazoans, fuelling the argument that ASCs in many taxa possess modified and diversified repertoires relative to the status and properties of vertebrate ASCs. Indeed, current ACS concepts were constructed from studies on vertebrates and select canonical ecdysozoan models (fruit flies, nematodes). It is evident that the ASC attributes detailed here are not shared by all animal phyla. However, cumulatively this review emphasizes that vertebrate ASCs represent a ‘unique’ case that could be considered distinct from most other animals. Additional work is needed to reach a better understanding of ASC diversity and properties in other lineages in order to obtain a comprehensive view of the similarities and differences across the Metazoa.
ASCs in many aquatic invertebrates are the engine for agametic asexual reproduction and whole‐body regeneration; they can be far from rare (up to 40% of the animal's cells), and encompass entities with unorthodox cellular shapes and behaviours (e.g. amoeboid movement). These ASCs drive whole‐organismal functions (dormancy, fission, fragmentation, budding); co‐express repertoires of germ and somatic lineage markers, refuting the rule of germ cell sequestration; and may emerge de novo according to need, without the requirement for a stem cell niche. Additionally, as the shared stemness capacity of all ASCs ‘cannot be reduced to the molecular properties of individual cells’ (Lander, 2009, p. 5), we suggest that other ASCs exhibiting extensive lineage‐specific adaptations or distant evolutionary affinities of ‘stemness’ may go unnoticed.
The traditional powerful metaphor of Waddington's landscape (e.g. Waddington, 1957; Noble, 2015; Moris, Pina & Arias, 2016; Rajagopal & Stanger, 2016), is an iconic illustration that describes how sequential developmental fate decisions allow an ASC to transform along alternative descending cell lineages. Discussed extensively, this metaphor reveals the conceptual framework for ASC stemness, hitherto through the vertebrate perspective. However, Waddington's metaphor does not cover many ASC phenomena, such as regeneration in non‐vertebrate deuterostomes (echinoderms, hemichordates and cephalochordates), which is largely based on local dedifferentiation rather than on undifferentiated ASCs (Ferrario et al., 2020) or transdifferentiation in regenerating medusae (Schmid & Reber‐Muller, 1995). These disparities lead us to propose an alternative metaphor, termed the ‘wobbling Penrose landscape’, which illustrates metazoan stemness better (Fig. 5). It defines the continuously acquired totipotency through ontogeny and astogeny observed in many phyla, and thus differs fundamentally from the unidirectional trajectory of differentiation in the Vertebrata, typified by gradually diminished cellular potency through ontogeny.
Fig 5.

A graphical visualization of the ‘wobbling Penrose landscape’ metaphor. In the Penrose Staircase of stemness (the dark‐blue stairs), totipotent adult stem cells (ASCs) make turns in ascending or descending courses, forming a continuous loop, so that the stemness course of a totipotent stem cell could extend throughout ontogeny (presenting endless totipotency; with no niche involvement) and never acquires any upper or lower values. At any step during this journey (represented by funnels), an ASC may start a labyrinthine journey down stemness echelons (the grey downhill walls), descending from one tier (where they can stay, or continue onwards) to a lower one, downhill to a fully differentiated state (with multipotency to unipotency levels of stemness correspondingly coloured in paler blues, see key). The Penrose landscape carries the property of Escherian movement, allowing continuous passage of stem cells at any stemness status either up (towards totipotency, even from fully differentiated states; shown by the ladders) or sideways to change their stemness status (through transdifferentiation/dedifferentiation; shown by the ropes). In the Penrose landscape, as opposed to the hilly Waddingtonian landscape metaphor (see insert), there is no automatic downhill route (symbolized by valleys) in potency and no determinant bifurcated choices, but stemness is portrayed by a flexible, multi‐choice status without a decisive fate. Depending on the internal and external cues experienced, the Penrose landscape can ‘wobble’, representing a dynamic landscape of stemness. Not all ASCs from every lineage display the full range of movements possible within the wobbling Penrose landscape, but the cumulative data suggest its existence.
In the classical Waddington's landscape metaphor (Waddington, 1957; see inset to Fig. 5), a stem cell begins its journey at the top of a hill (representing the highest stemness level, or totipotency) and slides down to bi‐ or multifurcated paths within inescapable valleys (signifying determined fates) in a landscape driven by a metaphorical gravitational force, which guides the cell into one of several possible decisions or fates (each leads to a different cell type and altered level of specification). The kernel of ASC stemness in invertebrates, on the other hand, relies on the logic of the Penrose staircase (https://en.wikipedia.org/wiki/Penrose_stairs), an ‘Escherian stairwell’ of stemness. Here the stairs make turns in ascending or descending courses, yet form continuous loops, from birth to death, where the totipotent stemness course of a stem cell lasts for the duration of the animal's lifespan (Fig. 5). At any point in the Penrose staircase, an ASC may start a journey down stemness echelons to initiate cascades of cellular phenotypes and lineage segregations that recapitulate hierarchies of potency and differentiated cell types. This cascading landscape further allows cells at any point in the slope to turn back into an ascending trajectory towards higher levels of stem cell potency. Cells may thus travel all the way up to the Penrosian loop of totipotency, or move to different statuses (dedifferentiation, transdifferentiation; Fig. 5), depicting a dynamic (wobbling) landscape that does not inevitably entail progressive loss of stemness. Thus, when a cell ‘makes a decision’, the subsequent journey is not bound by this decision. Importantly, in this model, there is no need for the existence of any ASC niches.
The wobbling Penrose landscape diverges conceptually from the Waddingtonian landscape in three key ways: (i) there is no bifurcation ‘choice’, or travelling along symbolic valleys, as in the Waddingtonian landscape, which is subject to a gravity force. The Penrose landscape is a gravity‐independent construct, allowing continual gradients of cellular potency, without any predetermined decision. (ii) The likelihood of backward/sideways trajectories in the Waddingtonian landscape has rarely been raised in the literature (e.g. Pesaresi, Sebastian‐Perez & Cosma, 2019) as, conceptually, such processes necessitate invested energy. In the wobbling Penrose landscape, stem cells, progenitors and even fully differentiated cells at any level of stemness status can move up or change stemness position (Fig. 5). (iii) There is no single downward route in the potency slope but, instead, multiple trajectories of cellular potency can emerge.
The vertebrate literature also reveals cases more in keeping with the wobbling Penrose than the Waddingtonian unidirectional landscape (e.g. Furusawa & Kaneko, 2012; Clevers, 2015; Sieweke, 2015; Kholodenko & Yarygin, 2017; Buczacki, 2019), including ASCs that are not endowed with a determined fate while subjected to stochastic events (Clevers, 2015; Post & Clevers, 2019) or cases of ‘stem cell plasticity’ (Loeffler & Roeder, 2002; Poulsom et al., 2002; Raff, 2003; Wagers & Weissman, 2004; Chacón‐Martínez et al., 2018) where committed stem cells differentiate or transdifferentiate into different cell lineages. While we do not review the vertebrate literature extensively here, such putative cases fitting a wobbling landscape may commonly exist.
As in the vertebrates, ASCs in invertebrates maintain lineages, replace cell losses caused by wear‐and‐tear, and regulate between quiescence and proliferation. Yet, in many invertebrate taxa, stemness is further associated with (i) sets of responses to environmental assaults (e.g. whole‐body regeneration, dormancy), or ecotoxicological impacts (Rosner et al., 2021). (ii) novel biological traits expressed irrespective of environmental cues (e.g. budding, fission, fragmentation), (iii) innate immunity (Ballarin et al., 2021), and (iv) indeterminate growth [e.g. sponges, cnidarians, annelids (e.g. atokous worm stage), tunicates (Jackson & Coates, 1986; Hughes, 1987; Gazave et al., 2013)], a largely neglected trait as the conventional models in stem cell research follow determinate growth plans (Vogt, 2012). Along this line of acquired traits, aquatic invertebrate ASCs not only demonstrate a higher fidelity of stem cell renewal, even when compared with tumorigenesis (Robert, 2010; Vogt, 2012; Tascedda & Ottaviani, 2014), but in some cases, are also elevated to the level of legitimate units of selection (Buss, 1982; Rinkevich, 2000, 2009, 2011; Weissman, 2000; Fields & Levin, 2018).
VIII. CONCLUSIONS
(1) The current paradigm suggests the lifelong existence of adult stem cells (ASCs) in Metazoa. In vertebrates, ASCs are defined as lineage‐restricted cells, limited to tissue or organ‐specific activities, that are capable of regulating homeostasis, repair and regeneration of tissues and organs. While during early embryogenesis stem cells in vertebrates are totipotent and then pluripotent, post‐embryonic ASCs are multipotent at best. It is widely accepted that vertebrate ASCs are rare, clonogenic, undifferentiated, and often express specific ‘stemness’ genes. They are capable of self‐renewal and multilineage differentiation, often interacting with specialized stem cell niches, and are considered slow‐cycling cells that show distinct germ/somatic lineage potential. They function in homeostasis and, with constraints, in the regeneration of organs/tissues.
(2) Numerous key ASC traits in invertebrates differ from those assigned to ASCs of vertebrates. Fifteen such traits are highlighted herein, revealing a wide range of disparate characteristics from morphology, differentiation states and somatic/germ lineage characteristics, to some essential biological properties and roles. Numerous predominantly marine phyla (e.g. Porifera, Cnidaria, Ctenophora, Annelida, Acoela, Platyhelminthes, Echinodermata, Cephalochordata and Tunicata) possess large pools of bona fide ASCs throughout the lifespan of the organism (sometimes consisting of up to 40% of all animals' cells), most of which are multipotent, pluripotent and even totipotent, with high differentiation potential that contribute to more than a single germ layer. They may arise de novo by transdifferentiation from somatic cells and even from germ cells, with no signature of germ‐cell sequestration, and are key players in phenomena such as whole‐body regeneration, asexual budding and dormancy. Many invertebrate ASCs consist of epithelial tissues, exhibiting epithelial cell hallmarks with distinct apical–basal and planar cell polarities, apical cell–cell junctions, and basal cell–extracellular matrix interactions, all of which are features of differentiated cells.
(3) ASCs in invertebrates represent a wide range of phylum‐specific and characteristic cell types, morphologies and behaviours, ranging from sponge archaeocytes and choanocytes, hydrozoan i‐cells, platyhelminth or acoel neoblasts to tunicate haemoblasts. Even within phyla, comparisons reveal a considerable degree of additional variation, where ASC properties are possessed by only particular taxa within a phylum. In the same way, ASC lineages and progenitors may show intra‐phylum specializations.
(4) Invertebrate ASCs express orthologues of many vertebrate ‘stemness’ genes, as well as genes that contribute to cancer cell ‘stem cell potential’. However, it is challenging to identify let alone compare stemness gene signatures across diverse invertebrate taxa spanning wide evolutionary distances. The molecular mechanisms by which invertebrates hold viable ASC stocks, with long‐term stability and constant proliferation during their lifespan, remain elusive. In addition, the essence of ASC stemness in marine invertebrates cannot be distilled down to a single shared molecular fingerprint. Also, in contrast to the vertebrates, somatic and germline stemness markers (e.g. Vasa, Pl10, Piwi, Nanos, Bruno, Pumilio, Tudor, etc.) are co‐expressed in differentiated somatic cells/tissues in many invertebrate phyla.
(5) While knowledge gained from mammalian, D. melanogaster and C. elegans models provide guidelines for defining comparable niches in other metazoans, studies on sponge archaeocytes and choanocytes, hydrozoan i‐cells and platyhelminth and acoel neoblasts have failed to define either discrete anatomical microenvironments where stem cells reside, or a niche‐specific extracellular matrix to which ASCs home. In hydrozoans and planarians, studies further view the whole animal or tissue as a single functional stem cell niche. Botryllid ascidians, by contrast, reveal a different scenario relative to other taxa, with putative ASCs homing to discrete, yet ephemeral, microenvironments.
(6) Beyond their functions in supporting homeostasis, ASCs in many metazoans also play major roles in supporting key biological processes such as regeneration in adults, including whole‐body regeneration, agametic asexual reproduction such as budding and fission, indeterminate growth, postponed ageing and dormancy phenomena.
(7) Conceptualizing the above disparities, we present an alternative stemness metaphor to the Waddington landscape, termed the ‘wobbling Penrose’ landscape. In this metaphor, totipotent ASCs adopt ascending/descending courses of an ‘Escherian stairwell’, in a lifelong totipotency pathway. ASCs may also travel along lower stemness echelons to reach fully differentiated states. However, from any starting state, cells can change their stemness status, underscoring their dynamic cellular potencies. Thus, vertebrate ASCs may reflect just one metazoan ASC archetype.
IX. ACKNOWLEDGEMENTS
This study is based upon work from COST Action 16203 ‘Stem cells of marine/aquatic invertebrates: from basic research to innovative applications’ (MARISTEM), supported by COST (European Cooperation in Science and Technology). The idea for this review was initially discussed by the Action core group (B. R., B. H., L. B., P. M., I. S.) and developed during a specific workshop entitled ‘Adult stem cells (ASC) from marine/aquatic invertebrates’ held at the Obergurgl Center of the University of Innsbruck from March 29 to 31, 2019. B. R. is supported by a grant from the United States‐Israel Binational Science Foundation (BSF no. 2015012), Jerusalem, Israel. E. R. is supported by funding from the French Government (National Research Agency, ANR) through the ‘Investments for the Future’ programs IDEX UCAJedi (ANR‐15‐IDEX‐01) as well as the grant RENEW (ANR‐19‐PRC). B. H. is supported by the Marie Skłodowska‐Curie COFUND program ARDRE ‘Ageing, Regeneration and Drug Research’ (research grant nr. 847681).
X. AUTHOR CONTRIBUTIONS
B. R. conceived the idea and wrote the first version of the manuscript. B. H., L. B., P. M. and I. S. further developed the idea and coordinated analyses. E. G., O. P., M. S., I. S., P. M., L. B., B. H., A. E. and D. K. actively collected and analysed the literature. B. R., B. H., L. B., P. M., I. S. and O. B.‐H. discussed and created Fig. 5. All co‐authors read and commented on drafts, and approved the final version.
Supporting information
Fig. S1. Diversity of adult stem cell (ASC) contributions to four major biological processes in the Cnidaria: homeostasis, dormancy, regeneration and agametic asexual reproduction. The presence of the biological process, involvement of undifferentiated/differentiated putative ASCs or progenitors and their level of potency, as well as the specific classes of stemness gene families they express are mapped for major cnidarian lineages. Circles: empty circle – documented presence of the biological process; filled circle – cases where putative ASCs or progenitors are involved. A red cross signifies the absence of the biological process in the lineage as currently documented. As homeostasis is a property of life, all groups are shown with an empty circle. For adult regeneration, the asterisk documents the presence of whole‐body regeneration. Dormancy refers to any documented type of dormant stage or torpor‐like process and has likely evolved independently in each lineage. For dormancy, A – quiescence, diapause, growth/degrowth; G – growth/degrowth; O – ontogeny reversal; Q – quiescence. For agametic asexual reproduction, B – any form of budding, F – any form of fission/fragmentation. Triangles indicate the level of documented potency for ASCs: red = lineage restricted/unipotent; cyan = totipotent; blue = multi/pluripotent; gradient triangle = documented cases of several ASCs or progenitors with different potency. Selected stemness gene families whose members are expressed in ASCs during the biological process are listed in a box for each process and group where known. The relative contribution of undifferentiated (U) versus differentiated (D) ASCs or progenitors within each subclass is mapped onto the phylogeny where known; levels of confidence are represented by solid (higher) and dotted (lower) diamonds, while the sizes of D and U reflect their presumed level of contribution. A hypothetical ancestral state for this character is proposed at the corresponding node of the simplified cnidarian phylogenetic tree. A general consensus for all features across Cnidaria is proposed at the top of the figure. Key species for which data exist in each subclass are named. Data are derived from Tables [Link], [Link].
Fig. S2. Diversity of adult stem cell (ASC) contributions to four major biological processes in the Echinodermata: homeostasis, dormancy, regeneration and agametic asexual reproduction. The presence of the biological process, involvement of undifferentiated/differentiated putative ASCs or progenitors and their level of potency, as well as the specific classes of stemness gene families they express are mapped for major echinoderm lineages. Circles: empty circle – documented presence of the biological process; filled circle – cases where putative ASCs or progenitors are involved. A red cross signifies the absence of the biological process in the lineage as currently documented. As homeostasis is a property of life, all groups are shown with an empty circle. For adult regeneration, the asterisk documents the presence of whole‐body regeneration. Dormancy refers to any documented type of dormant stage or torpor‐like process and has likely evolved independently in each lineage. For dormancy, the dotted line circle indicates potential involvement in the respective biological feature in non‐adults. Q – quiescence. For agametic asexual reproduction, F – any form of fission/fragmentation. Triangles indicate the level of documented potency for ASCs (filled) and progenitors (empty). Red = lineage restricted/unipotent; blue = multi/pluripotent; gradient triangle = documented cases of several ASCs or progenitors with different potency. Selected stemness gene families whose members are expressed in ASCs or progenitors during the biological process are listed in a box for each process and group where known. The relative contribution of undifferentiated (U) versus differentiated (D) ASCs or progenitors within each class is mapped onto the phylogeny; levels of confidence are represented by solid (higher) and dotted (lower) diamonds, while the sizes of D and U reflect their presumed level of contribution. A hypothetical ancestral state for this character is proposed at the corresponding node of the simplified echinoderm phylogenetic tree. A general consensus for all features across Echinodermata is proposed at the top of the figure. Key species for which data exist in each class are named. Data are derived from Tables [Link], [Link].
Table S1. Properties of selected, well‐studied adult stem cell (ASC) lineages in invertebrates.
Table S2. Genes expressed in invertebrate adult stem cell (ASCs) and progenitor cells during potency state changes.
Table S3. Suggested stem cell niches (SCNs) present in invertebrates.
Table S4. Overview of the involvement of adult stem cell (ASCs) and progenitors during homeostasis in metazoans.
Table S5. Overview of the involvement of adult stem cell (ASCs) and progenitors in regeneration processes in metazoans.
Table S6. Overview of the involvement of adult stem cell (ASCs) and progenitors in agametic asexual reproduction (budding, fission/fragmentation) in metazoans.
Table S7. Overview of the involvement of adult stem cell (ASCs) and progenitors in dormancy in metazoans.
REFERENCES
References used in the main text are marked with asterisks.
- * Abnave, P. , Aboukhatwa, E. , Kosaka, N. , Thompson, J. , Hill, M. A. & Aboobaker, A. A. (2017). Epithelial‐mesenchymal transition transcription factors control pluripotent adult stem cell migration in vivo in planarians. Development 144, 3440–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams, M. J. , Basinger, T. , Yuan, W. , Guo, C. L. & Goentoro, L. (2015). Self‐repairing symmetry in jellyfish through mechanically driven reorganization. Proceedings of the National Academy of Sciences of the United States of America 112, E3365–E3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achatz, J. G. , Chiodin, M. , Salvenmoser, W. , Tyler, S. & Martinez, P. (2013). The Acoela: on their kind and kinships, especially with nemertodermatids and xenoturbellids (Bilateria incertae sedis). Organisms Diversity and Evolution 13, 267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamska, M. (2016). Sponges as models to study emergence of complex animals. Current Opinion in Genetics and Development 39, 21–28. [DOI] [PubMed] [Google Scholar]
- * Adamska, M. (2018). Differentiation and transdifferentiation of sponge cells. In Marine Organisms as Model Systems in Biology and Medicine (eds Kloc M. and Kubiak J. Z.), pp. 229–253. Springer, Cham. [DOI] [PubMed] [Google Scholar]
- Adamska, M. , Degnan, B. M. , Green, K. & Zwafink, C. (2011). What sponges can tell us about the evolution of developmental processes. Zoology 114, 1–10. [DOI] [PubMed] [Google Scholar]
- Adamson, K. J. , Wang, T. , Rotgans, B. A. , Kruangkum, T. , Kuballa, A. V. , Storey, K. B. & Cummins, S. F. (2017). Genes and associated peptides involved with aestivation in a land snail. General and Comparative Endocrinology 246, 88–98. [DOI] [PubMed] [Google Scholar]
- Åkesson, B. , Gschwentner, R. , Hendelberg, J. , Ladurner, P. , Müller, J. & Rieger, R. (2001). Fission in Convolutriloba longifissura: asexual reproduction in acoelous turbellarians revisited. Acta Zoologica 82, 231–239. [Google Scholar]
- Alexander, B. E. , Liebrand, K. , Osinga, R. , van der Geest, H. G. , Admiraal, W. , Cleutjens, J. P. , Schutte, B. , Verheyen, F. , Ribes, M. , van Loon, E. & de Goeij, J. M. (2014). Cell turnover and detritus production in marine sponges from tropical and temperate benthic ecosystems. PLoS One 9(10), e109486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Alié, A. , Hayashi, T. , Sugimura, I. , Manuel, M. , Sugano, W. , Mano, A. , Satoh, N. , Agata, K. & Funayama, N. (2015). The ancestral gene repertoire of animal stem cells. Proceedings of the National Academy of Sciences of the United States of America 112, E7093–E7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alié, A. , Hiebert, L. S. , Simion, P. , Scelzo, M. , Prünster, M. M. , Lotito, S. , Delsuc, F. , Douzery, E. J. P. , Dantec, C. , Lemaire, P. & Darras, S. (2018). Convergent acquisition of nonembryonic development in styelid ascidians. Molecular Biology and Evolution 35, 1728–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Alié, A. , Leclère, L. , Jager, M. , Dayraud, C. , Chang, P. , Le Guyader, H. , Quéinnec, E. & Manuel, M. (2011). Somatic stem cells express Piwi and Vasa genes in an adult ctenophore: ancient association of “germline genes” with stemness. Developmental Biology 350(1), 183–197. [DOI] [PubMed] [Google Scholar]
- Alvarino, A. (1983). Chaetognatha. In Reproductive Biology of Invertebrates (eds Adiyodi K. G. and Adiyodi R. G.), pp. 585–610. John Wiley & Sons. Vol. 1, Oogenesis, Oviposition, and Oosorption, New York. [Google Scholar]
- Alves, L. S. S. , Pereira, A. & Ventura, C. (2002). Sexual and asexual reproduction of Coscinasterias tenuispina (Echinodermata: Asteroidea) from Rio de Janeiro, Brazil. Marine Biology 140, 95–101. [Google Scholar]
- Alwes, F. , Enjolras, C. & Averof, M. (2016). Live imaging reveals the progenitors and cell dynamics of limb regeneration. eLife 5, e19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano, S. & Hori, I. (1993). Metamorphosis of calcareous sponges. II. Cell rearrangement and differentiation in metamorphosis. Invertebrate Reproduction and Development 24(1), 13–26. [Google Scholar]
- Amiel, A. R. , Johnston, H. T. , Nedoncelle, K. , Warner, J. F. , Ferreira, S. & Röttinger, E. (2015). Characterization of morphological and cellular events underlying oral regeneration in the sea anemone, Nematostella vectensis . International Journal of Molecular Sciences 16(12), 28449–28471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, E. A. (1893). An Undescribed Acraniate, Asymmetron lucayanum. Studies. Biological Laboratory (Volume 5), pp. Baltimore, Maryland: Johns Hopkins University, 213–247. [Google Scholar]
- Anlauf, A. (1990). Cyst formation of Tubifex tubifex (Müller)–an adaptation to survive food deficiency and drought. Hydrobiologia 190(1), 79–82. [Google Scholar]
- Arboleda, E. , Hartenstein, V. , Martinez, P. , Reichert, H. , Sen, S. , Sprecher, S. & Bailly, X. (2018). An emerging system to study photosymbiosis, brain regeneration, chronobiology, and behavior: the marine acoel Symsagittifera roscoffensis . BioEssays 40, e1800107. [DOI] [PubMed] [Google Scholar]
- Arimoto, A. & Tagawa, K. (2018). Regeneration in the enteropneust hemichordate, Ptychodera flava, and its evolutionary implications. Development Growth & Differentiation 60, 400–408. [DOI] [PubMed] [Google Scholar]
- * Baguñà, J. (2012). The planarian neoblast: the rambling history of its origin and some current black boxes. International Journal of Developmental Biology 56, 19–37. [DOI] [PubMed] [Google Scholar]
- Bai, L. , Liu, B. , Ji, C. , Zhao, S. , Liu, S. , Wang, R. , Wang, W. , Yao, P. , Li, X. , Fu, X. , Yu, H. , Liu, M. , Han, F. , Guan, N. , Liu, H. , et al. (2019). Hypoxic and cold adaptation insights from the Himalayan marmot genome. iScience 11, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Ballarin, L. , Karahan, A. , Salvetti, A. , Rossi, L. , Manni, L. , Rinkevich, B. , Rosner, A. , Voskoboynik, A. , Rosental, B. , Canesi, L. , Anselmi, C. , Pinsino, A. , Tohumcu, B. E. , Jemec Kokalj, A. , Dolar, A. , et al. (2021). Stem cells and innate immunity in aquatic invertebrates: bridging two seemingly disparate disciplines for new discoveries in biology. Frontiers in Immunology 12, 688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa, J. S. , Sanchez‐Gonzalez, R. , Di Giaimo, R. , Baumgart, E. V. , Theis, F. J. , Gotz, M. & Ninkovic, J. (2015). Neurodevelopment. Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science 348, 789–793. [DOI] [PubMed] [Google Scholar]
- Bavestrello, G. , Puce, S. , Cerrano, C. & Senes, L. (2000). Strobilation in a species of Bougainvillioidea (Cnidaria: Hydrozoa). Scientia Marina 64(S1), 147–150. [Google Scholar]
- Bavestrello, G. , Sommer, C. & Sarà, M. (1992). Bidirectional conversion in Turritopsis nutricula (Hydrozoa). Scientia Marina 56(2‐3), 137–140. [Google Scholar]
- * Bely, A. E. & Nyberg, K. G. (2010). Evolution of animal regeneration: re‐emergence of a field. Trends in Ecology & Evolution 25, 161–170. [DOI] [PubMed] [Google Scholar]
- * Bely, A. E. & Sikes, J. M. (2010a). Acoel and platyhelminth models for stem‐cell research. Journal of Biology 9, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bely, A. E. & Sikes, J. M. (2010b). Latent regeneration abilities persist following recent evolutionary loss in asexual annelids. Proceedings of the National Academy of Sciences of the United States of America 107(4), 1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bely, A. E. & Wray, G. A. (2001). Evolution of regeneration and fission in annelids: insights from engrailed‐ and orthodenticle‐class gene expression. Development 128, 2781–2279. [DOI] [PubMed] [Google Scholar]
- Bely, A. E. , Zattara, E. E. & Sikes, J. M. (2014). Regeneration in spiralians: evolutionary patterns and developmental processes. The International Journal of Developmental Biology 58, 623–634. [DOI] [PubMed] [Google Scholar]
- Ben Khadra, Y. , Sugni, M. , Ferrario, C. , Bonasoro, F. , Oliveri, P. , Martinez, P. & Candia Carnevali, M. D. (2018). Regeneration in stellate echinoderms: Crinoidea, Asteroidea, and Ophiuroidea. Results and Problems in Cell Differentiation 65, 285–320. [DOI] [PubMed] [Google Scholar]
- Ben Khadra, Y. , Sugni, M. , Ferrario, C. , Bonasoro, F. , Varela Coelho, A. , Martinez, P. & Candia Carnevali, M. D. (2017). An integrated view of asteroid regeneration: tissues, cells and molecules. Cell and Tissue Research 370, 13–28. [DOI] [PubMed] [Google Scholar]
- * Ben‐Hamo, O. , Rosner, A. , Rabinowitz, C. , Oren, M. & Rinkevich, B. (2018). Coupling astogenic aging in the colonial tunicate Botryllus schlosseri with the stress protein mortalin. Developmental Biology 433, 33–46. [DOI] [PubMed] [Google Scholar]
- Bennet, A. F. (1994). Exercise performance of reptiles. Advances in Veterinary Science and Comparative Medicine 38B, 113–138. [PubMed] [Google Scholar]
- Berrill, N. J. (1941). The development of the bud in Botryllus . Biological Bulletin 80(2), 169–184. [Google Scholar]
- Berrill, N. J. (1951). Regeneration and budding in tunicates. Biological Reviews 26, 456–475. [Google Scholar]
- Betti, F. , Bo, M. , Di Camillo, C. G. & Bavestrello, G. (2012). Life history of Cornularia cornucopiae (Anthozoa: Octocorallia) on the Conero promontory (north Adriatic Sea). Marine Ecology 33, 49–55. [Google Scholar]
- Bhambri, A. , Dhaunta, N. , Patel, S. S. , Hardikar, M. , Bhatt, A. , Srikakulam, N. , Shridhar, S. , Vellarikkal, S. , Pandey, R. , Jayarajan, R. & Verma, A. (2018). Large scale changes in the transcriptome of Eisenia fetida during regeneration. PLoS One 13(9), e0204234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, K. N. , Chaki, K. K. , Sarkar, A. K. & Misra, K. K. (2012). Ultrastructure of the salivary gland cells in active and aestivated mollusk, Pila globosa (Gastropoda: Orthogastropoda: Ampularidae). Proceedings of the Zoological Society 65, 64–69. [Google Scholar]
- Bird, A. , von Dassow, G. & Maslakova, S. (2014). How the pilidium larva grows. EvoDevo 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biressi, A. , Zou, T. , Dupont, S. , Dahlberg, C. , Di Benedetto, C. , Bonasoro, F. , Thorndyke, M. & Candia Carnevali, M. D. (2010). Wound‐healing and arm regeneration in Ophioderma longicaudum and Amphiura filiformis (Ophiuroidea, Echinodermata): comparative morphogenesis and histogenesis. Zoomorphology 129, 1–19. [Google Scholar]
- Bisbee, J. W. , Francis, J. C. & Harrison, F. W. (1989). Cytological examination of freshwater sponge regeneration from reduction bodies. Transactions of the American Microscopical Society 108(3), 299–303. [Google Scholar]
- * Blackstone, N. W. & Jasker, B. D. (2003). Phylogenetic considerations of clonality, coloniality, and mode of germline development in animals. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 297, 35–47. [DOI] [PubMed] [Google Scholar]
- * Blanchoud, S. , Rinkevich, B. & Wilson, M. J. (2018). Whole‐body regeneration in the colonial tunicate Botrylloides leachii . In Marine Organisms as Model Systems in Biology and Medicine (eds Kloc M. and Kubiak J. Z.), pp. 337–355. Switzerland: Springer. [DOI] [PubMed] [Google Scholar]
- * Blau, H. M. & Baltimore, D. (1991). Differentiation requires continuous regulation. Journal of Cell Biology 112(5), 781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Blau, H. M. , Brazelton, T. R. & Weismann, J. M. (2001). The evolving concept of a stem cell: entity or function? Cell 105(7), 829–841. [DOI] [PubMed] [Google Scholar]
- * Bode, H. R. (1996). The interstitial cell lineage of hydra: a stem cell system that arose early in evolution. Journal of Cell Science 109, 1155–1164. [DOI] [PubMed] [Google Scholar]
- Bode, H. R. & David, C. N. (1978). Regulation of a multipotent stem cell, the interstitial cell of Hydra. Progress in Biophysics & Molecular Biology 33, 189–206. [DOI] [PubMed] [Google Scholar]
- Bode, H. R. , Flick, K. M. & Smith, G. S. (1976). Regulation of interstitial cell differentiation in Hydra attenuata. I. Homeostatic control of interstitial cell population size. Journal of Cell Science 20, 29–46. [DOI] [PubMed] [Google Scholar]
- Boehm, A. M. , Khalturin, K. , Anton‐Erxleben, F. , Hemmrich, G. , Klostermeier, U. C. , Lopez‐Quintero, J. A. , Oberg, H. H. , Puchert, M. , Rosenstiel, P. , Wittlieb, J. & Bosch, T. C. (2012). FoxO is a critical regulator of stem cell maintenance in immortal Hydra . Proceedings of the National Academy of Sciences of the United States of America 109, 19697–19702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boero, F. , Bouillon, J. , Piraino, S. & Schmid, V. (2002). Asexual reproduction in the Hydrozoa (Cnidaria). In Reproductive Biology of Invertebrates. XI Progress in Asexual Reproduction (ed. Hughes R. N.), pp. 141–158. Oxford & IBH Publishing Co, New Delhi & Kolkata. [Google Scholar]
- Bonuccelli, L. , Rossi, L. , Lena, A. , Scarcelli, V. , Rainaldi, G. , Evangelista, M. , Iacopetti, P. , Gremigni, V. & Salvetti, A. (2010). An RbAp48‐like gene regulates adult stem cells in planarians. Journal of Cell Science 123, 690–698. [DOI] [PubMed] [Google Scholar]
- * Borisenko, I. E. , Adamska, M. , Tokina, D. B. & Ereskovsky, A. V. (2015). Transdifferentiation is a driving force of regeneration in Halisarca dujardini (Demospongiae, Porifera). PeerJ 3, e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Bosch, T. C. G. (2009). Hydra and the evolution of stem cells. BioEssays 31, 478–486. [DOI] [PubMed] [Google Scholar]
- * Bosch, T. C. G. , Anton‐Erxleben, F. , Hemmrich, G. & Khalturin, K. (2010). The Hydra polyp: nothing but an active stem cell community. Development, Growth & Differentiation 52, 15–25. [DOI] [PubMed] [Google Scholar]
- Bosch, T. C. G. & David, C. N. (1984). Growth regulation in Hydra: relationship between epithelial cell cycle length and growth rate. Developmental Biology 104(1), 161–171. [DOI] [PubMed] [Google Scholar]
- Bosch, T. C. G. & David, C. N. (1987). Stem cells of Hydra magnipapillata can differentiate into somatic cells and germ line cells. Developmental Biology 121(1), 182–191. [Google Scholar]
- * Bossche, J. P. V. & Jangoux, M. (1976). Epithelial origin of starfish coelomocytes. Nature 261, 227–228. [DOI] [PubMed] [Google Scholar]
- Braden, B. P. , Taketa, D. A. , Pierce, J. D. , Kassmer, S. , Lewis, D. D. & De Tomaso, A. W. (2014). Vascular regeneration in a basal chordate is due to the presence of immobile, bi‐functional cells. PLoS One 9, e95460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw, B. , Thompson, K. & Frank, U. (2015). Distinct mechanisms underlie oral vs aboral regeneration in the cnidarian Hydractinia echinata . eLife 4, e05506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneis, G. & Scholtz, G. (2014). The ‘ventral organs’ of Pycnogonida (Arthropoda) are neurogenic niches of late embryonic and post‐embryonic nervous system development. PLoS One 9(4), e95435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington, S. (2001). The seasonal energetics of the Antarctic bivalve Laternula elliptica (King and Broderip) at Rothera point, Adelaide Island. Polar Biology 24(7), 523–530. [Google Scholar]
- Brooks, N. E. , Myburgh, K. H. & Storey, K. B. (2015). Muscle satellite cells increase during hibernation in ground squirrels. Comparative Biochemistry and Physiology ‐ Part B: Biochemistry and Molecular Biology 189, 55–61. [DOI] [PubMed] [Google Scholar]
- Brown, F. D. & Swalla, B. J. (2007). Vasa expression in a colonial ascidian, Botrylloides violaceus . Developmental Biology 9, 165–177. [DOI] [PubMed] [Google Scholar]
- Brown, F. D. & Swalla, B. J. (2012). Evolution and development of budding by stem cells: ascidian coloniality as a case study. Developmental Biology 369, 151–162. [DOI] [PubMed] [Google Scholar]
- * Brown, F. D. , Keeling, E. L. , Le, A. D. & Swalla, B. J. (2009a). Whole body regeneration in a colonial ascidian, Botrylloides violaceus . Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 312, 885–900. [DOI] [PubMed] [Google Scholar]
- Brown, F. D. , Tiozzo, S. , Roux, M. M. , Ishizuka, K. , Swalla, B. J. & De Tomaso, A. W. (2009b). Early lineage specification of long‐lived germline precursors in the colonial ascidian Botryllus schlosseri . Development 136(20), 3485–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczacki, S. (2019). Fate plasticity in the intestine: the devil is in the detail. World Journal of Gastroenterology 25, 3116–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burighel, P. , Brunetti, R. & Zaniolo, G. (1976). Hibernation of the colonial ascidian Botrylloides leachi (Savigny): histological observations. Italian Journal of Zoology 43(3), 293–301. [Google Scholar]
- Burns, G. , Ortega‐Martinez, O. , Thorndyke, M. C. & Peck, L. S. (2012). Dynamic gene expression profiles during arm regeneration in the brittle star Amphiura filiformis . Journal of Experimental Marine Biology and Ecology 407(2), 315–322. [Google Scholar]
- Burton, P. M. & Finnerty, J. R. (2009). Conserved and novel gene expression between regeneration and asexual fission in Nematostella vectensis . Development Genes and Evolution 219, 79–87. [DOI] [PubMed] [Google Scholar]
- Buscema, M. , De Sutter, D. & Van de Vyver, G. (1980). Ultrastructural study of differentiation processes during aggregation of purified sponge archaeocytes. Roux's Archives of Developmental Biology 188, 45–53. [DOI] [PubMed] [Google Scholar]
- * Buss, L. W. (1982). Somatic cell parasitism and the evolution of somatic tissue compatibility. Proceedings of the National Academy of Sciences of the United States of America 79, 5337–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzgariu, W. , Al Haddad, S. , Tomczyk, S. , Wenger, Y. & Galliot, B. (2015). Multifunctionality and plasticity characterize epithelial cells in Hydra . Tissue Barriers 3, 1068908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzgariu, W. , Crescenzi, M. & Galliot, B. (2014). Robust G2 pausing of adult stem cells in Hydra . Differentiation 87, 83–99. [DOI] [PubMed] [Google Scholar]
- Càceres, C. E. (1997). Dormancy in invertebrates. Invertebrate Biology 116, 371–383. [Google Scholar]
- Candia Carnevali, M. D. , Bonasoro, F. & Biale, A. (1997). Pattern of bromodeoxyuridine incorporation in the advanced stages of arm regeneration in the feather star Antedon mediterranea . Cell and Tissue Research 289, 363–374. [DOI] [PubMed] [Google Scholar]
- Candia Carnevali, M. D. , Bonasoro, F. , Lucca, E. & Thorndyke, M. C. (1995). Pattern of cell proliferation in the early stages of arm regeneration in the feather star Antedon mediterranea . Journal of Experimental Zoology 272(6), 464–474. [Google Scholar]
- Cao, P. L. , Kumagai, N. , Inoue, T. , Agata, K. & Makino, T. (2019). JmjC domain‐encoding genes are conserved in highly regenerative metazoans and are associated with planarian whole‐body regeneration. Genome Biology and Evolution 11, 552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carla, E. C. , Pagliara, P. , Piraino, S. , Boero, F. & Dini, L. (2003). Morphological and ultrastructural analysis of Turritopsis nutricula during life cycle reversal. Tissue and Cell 35, 213–222. [DOI] [PubMed] [Google Scholar]
- * Chacón‐Martínez, C. A. , Koester, J. & Wickström, S. A. (2018). Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development 145, dev165399. [DOI] [PubMed] [Google Scholar]
- Chen, C. P. , Fok, S. K. W. , Hsieh, Y. W. , Chen, C. Y. , Hsu, F. P. , Chang, Y. H. & Chen, J.‐H. (2020). General characterization of regeneration in Aeolosoma viride (Annelida, Aeolosomatidae). Invertebrate Biology 139, 12277. [Google Scholar]
- Cherif‐Feildel, M. , Kellner, K. , Goux, D. , Elie, N. , Adeline, B. , Lelong, C. & Heude Berthelin, C. (2019). Morphological and molecular criteria allow the identification of putative germ stem cells in a lophotrochozoan, the Pacific oyster Crassostrea gigas . Histochemistry and Cell Biology 151(5), 419–433. [DOI] [PubMed] [Google Scholar]
- * Chiodin, M. , Børve, A. , Berezikov, E. , Ladurner, P. , Martinez, P. & Hejnol, A. (2013). Mesodermal gene expression in the acoel Isodiametra pulchra indicates a low number of mesodermal cell types and the endomesodermal origin of the gonads. PLoS One 8, e55499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen, B. , Robles, V. , Raya, M. , Paramonov, I. & Izpisua Belmone, J. C. (2010). Regeneration and reprogramming compared. BMC Biology 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Christodoulou, C. , Spencer, J. A. , Yeh, S. C. A. , Turcotte, R. , Kokkaliaris, K. D. , Panero, R. , Ramos, A. , Guo, G. , Seyedhassantehrani, N. , Esipova, T. V. & Vinogradov, S. A. (2020). Live‐animal imaging of native haematopoietic stem and progenitor cells. Nature 578, 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima, F. , Perin, A. , Burighel, P. & Ballarin, L. (2001). Morpho‐functional characterization of haemocytes of the compound ascidian Botrylloides leachi (Tunicata, Ascidiacea). Acta Zoologica 82(4), 261–274. [Google Scholar]
- * Clevers, H. (2015). What is an adult stem cell? Science 350, 1319–1320. [DOI] [PubMed] [Google Scholar]
- * Clevers, H. & Watt, F. M. (2018). Defining adult stem cells by function, not by phenotype. Annual Review of Biochemistry 87, 1015–1027. [DOI] [PubMed] [Google Scholar]
- Coe, W. R. (1929). Regeneration in nemerteans. Journal of Experimental Zoology 54(3), 411–459. [Google Scholar]
- Coe, W. R. (1930). Asexual reproduction in nemerteans. Physiological Zoology 3, 297–308. [Google Scholar]
- Collins, J. J. , Wang, B. , Lambrus, B. G. , Tharp, M. E. , Iyer, H. & Newmark, P. A. (2013). Adult somatic stem cells in the human parasite Schistosoma mansoni . Nature 494, 476–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conand, C. (1995). Asexual reproduction by fission in Holothuria atra: variability of some parameters in populations from the tropical lndo‐Pacific. Oceanologica Acta 19, 3–4. [Google Scholar]
- * Conte, M. , Deri, P. , Isolani, M. E. , Mannini, L. & Batistoni, R. (2009). A mortalin‐like gene is crucial for planarian stem cell viability. Developmental Biology 334, 109–118. [DOI] [PubMed] [Google Scholar]
- Cortés Rivera, Y. , Hernández, R. I. , San Martin del Angel, P. , Zarza Meza, E. & Cuervo, G. (2016). Regenerative potential of the sea star Linckia guildinguii . Hidrobiológica 26, 103–108. [Google Scholar]
- Coutinho, C. C. , Rosa, I.d. A. , Teixeira, J. D.d. O. , Andrade, L. R. , Costa, M. L. & Mermelstein, C. (2017). Cellular migration, transition and interaction during regeneration of the sponge Hymeniacidon heliophila . PLoS One 12(5), e0178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Custodio, M. R. , Hajdu, E. & Muricy, G. (2004). Cellular dynamics of in vitro allogeneic reactions of Hymeniacidon heliophila (Demospongiae: Halichondrida). Marine Biology 144(5), 999–1010. [Google Scholar]
- Czarkwiani, A. , Ferrario, C. , Dylus, D. V. , Sugni, M. & Oliveri, P. (2016). Skeletal regeneration in the brittle star Amphiura filiformis . Frontiers in Zoology 13, e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerneková, M. , Janelt, K. , Student, S. , Jönsson, K. I. & Poprawa, I. (2018). A comparative ultrastructure study of storage cells in the eutardigrade Richtersius coronifer in the hydrated state and after desiccation and heating stress. PLoS One 13(8), e0201430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Dańko, M. J. , Kozłowski, J. & Schaible, R. (2015). Unraveling the non‐senescence phenomenon in Hydra . Journal of Theoretical Biology 382, 137–149. [DOI] [PubMed] [Google Scholar]
- * Dannenberg, L. C. & Seaver, E. C. (2018). Regeneration of the germline in the annelid Capitella teleta . Developmental Biology 440, 74–87. [DOI] [PubMed] [Google Scholar]
- David, C. N. (2012). Interstitial stem cells in Hydra: multipotency and decision‐making. The International Journal of Developmental Biology 56, 489–497. [DOI] [PubMed] [Google Scholar]
- David, C. N. & Murphy, S. (1977). Characterization of interstitial stem cells in Hydra by cloning. Developmental Biology 58, 372–383. [DOI] [PubMed] [Google Scholar]
- David, C. N. & Plotnick, I. (1980). Distribution of interstitial stem cells in Hydra . Developmental Biology 76, 175–184. [DOI] [PubMed] [Google Scholar]
- Davies, E. L. , Lei, K. , Seidel, C. W. , Kroesen, A. E. , McKinney, S. A. , Guo, L. , Mc Robb, S. , Ross, E. J. , Gotting, K. & Sánchez Alvarado, A. (2017). Embryonic origin of adult stem cells required for tissue homeostasis and regeneration. eLife 6, e21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawydoff, M. C. (1928). Sur la réversibilité des processus du développement. Les phases extrêmes de la réduction des Némertes. Comptes rendus hebdomadaires des séances de l'Académie des sciences 186, 911–913. [Google Scholar]
- De Goeij, J. M. , De Kluijver, A. , Van Duyl, F. C. , Vacelet, J. , Wijffels, R. H. , De Goeij, A. F. P. M. , Cleutjens, J. P. M. & Schutte, B. (2009). Cell kinetics of the marine sponge Halisarca caerulea reveal rapid cell turnover and shedding. Journal of Experimental Zoology 212, 3892–3900. [DOI] [PubMed] [Google Scholar]
- de Jong, D. M. & Seaver, E. C. (2018). Investigation into the cellular origins of posterior regeneration in the annelid Capitella teleta . Regeneration 5(1), 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mulder, K. , Kuales, G. , Pfister, D. , Willems, M. , Egger, B. , Salvenmoser, W. , Thaler, M. , Gorny, A. K. , Hrouda, M. , Borgonie, G. & Ladurner, P. (2009a). Characterization of the stem cell system of the acoel Isodiametra pulchra . BMC Developmental Biology 9, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * De Mulder, K. , Pfister, D. , Kuales, G. , Egger, B. , Salvenmoser, W. , Willems, M. , Steger, J. , Fauster, K. , Micura, R. , Borgonie, G. & Ladurner, P. (2009b). Stem cells are differentially regulated during development, regeneration and homeostasis in flatworms. Developmental Biology 334(1), 198–212. [DOI] [PubMed] [Google Scholar]
- De Sutter, D. & Van de Vyver, G. (1977). Aggregative properties of different cell types of fresh‐water sponge Ephydatia fluviatilis isolated on ficoll gradients. Roux's Archives of Developmental Biology 181, 151–161. [DOI] [PubMed] [Google Scholar]
- De Vito, D. , Piraino, S. , Schmich, J. , Bouillon, J. & Boero, F. (2006). Evidence of reverse development in Leptomedusae (Cnidaria, Hydrozoa): the case of Laodicea undulata (Forbes and Goodsir 1851). Marine Biology 149, 339–346. [Google Scholar]
- Denker, E. , Manuel, M. , Leclère, L. , Le Guyader, H. & Rabet, N. (2008). Ordered progression of nematogenesis from stem cells through differentiation stages in the tentacle bulb of Clytia hemisphaerica (Hydrozoa, Cnidaria). Developmental Biology 315, 99–113. [DOI] [PubMed] [Google Scholar]
- Di Benedetto, C. , Parma, L. , Barbaglio, A. , Sugni, M. , Bonasoro, F. & Carnevali, M. D. (2014). Echinoderm regeneration: an in vitro approach using the crinoid Antedon mediterranea . Cell and Tissue Research 358, 189–201. [DOI] [PubMed] [Google Scholar]
- Di Maio, A. , Setar, L. , Tiozzo, S. & De Tomaso, A. W. (2015). Wnt affects symmetry and morphogenesis during post‐embryonic development in colonial chordates. EvoDevo 6, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Diaz, J. P. (1979). Variations, differentiations et functions des categories cellulaires de la demosponge d'eaux saumatres, Suberites massa, Nardo, au cours du cycle biologique annuel et dans des conditions experimentales. PhD thesis, University of Montpellier, Languedoc.
- * Dill, K. K. & Seaver, E. C. (2008). Vasa and nanos are coexpressed in somatic and germ line tissue from early embryonic cleavage stages through adulthood in the polychaete Capitella sp. I. Development Genes and Evolution 218, 453–463. [DOI] [PubMed] [Google Scholar]
- Dingwall, C. B. & King, R. S. (2016). Muscle‐derived matrix metalloproteinase regulates stem cell proliferation in planarians. Developmental Dynamics 245, 963–970. [DOI] [PubMed] [Google Scholar]
- Dolmatov, I. Y. (2014). Asexual reproduction in holothurians. Scientific World Journal 2014, 527234–527213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray, N. , Bedu, S. , Vuillemin, N. , Alunni, A. , Coolen, M. , Krecsmarik, M. , Supatto, W. , Beaurepaire, E. & Bally‐Cuif, L. (2015). Large‐scale live imaging of adult neural stem cells in their endogenous niche. Development 142, 3592–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * DuBuc, T. Q. , Schnitzler, C. E. , Chrysostomou, E. , McMahon, E. T. , Gahan, J. M. , Buggie, T. , Gornik, S. G. , Hanley, S. , Barreira, S. N. , Gonzalez, P. & Baxevanis, A. D. (2020). Transcription factor AP2 controls cnidarian germ cell induction. Science 367, 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducati, C. C. , Carnevali, M. C. & Barker, M. F. (2004). Regenerative potential and fissiparity in the forcipulate starfish Coscinasterias muricata . In Echinoderms (eds Heinzeller T. and Nebelsick J. H.), pp. 113–118. Taylor & Francis, Munich. [Google Scholar]
- Duffy, D. J. , Plickert, G. , Kuenzel, T. , Tilmann, W. & Frank, U. (2010). Wnt signalling promotes oral but suppresses aboral structures in Hydractinia metamorphosis and regeneration. Development 137(18), 3057–3066. [DOI] [PubMed] [Google Scholar]
- * Durand, C. , Charbord, P. & Jaffredo, T. (2018). The crosstalk between hematopoietic stem cells and their niches. Current Opinion in Hematology 25, 285–289. [DOI] [PubMed] [Google Scholar]
- * Eaves, C. J. (2015). Hematopoietic stem cells: concepts, definitions, and the new reality. Blood 125, 2605–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling, F. J. P. & Lewis, J. E. (2018). Tanycytes and hypothalamic control of energy metabolism. Glia 66, 1176–1184. [DOI] [PubMed] [Google Scholar]
- Edmonson, C. H. (1935). Autotomy and regeneration in Hawaiian starfishes. Bernice P. Bishop Museum Occasional Papers 11, 3–20. [Google Scholar]
- Egger, B. , Gschwentner, R. & Rieger, R. (2007). Free‐living flatworms under the knife: past and present. Development Genes and Evolution 217, 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger, B. , Gschwentner, R. , Hess, M. W. , Nimeth, K. T. , Adamski, Z. , Willems, M. , Rieger, R. & Salvenmose, W. (2009a). The caudal regeneration blastema is an accumulation of rapidly proliferating stem cells in the flatworm Macrostomum lignano . BMC Developmental Biology 9, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger, B. , Steinke, D. , Tarui, H. , De Mulder, K. , Arendt, D. , Borgonie, G. , Funayama, N. , Gschwentner, R. , Hartenstein, V. , Hobmayer, B. , Hooge, M. , Hrouda, M. , Ishida, S. , Kobayashi, C. , Kuales, G. , et al. (2009b). To be or not to be a flatworm: the acoel controversy. PLoS One 4, 5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhoffer, G. T. , Kang, H. & Sánchez Alvarado, A. (2008). Molecular analysis of stem cells and their descendents during cell turnover and regeneration in the planarian Schmidtea mediterranea . Cell Stem Cell 3, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliseikina, M. G. , Magarlamov, T. Y. & Dolmatov, I. Y. (2010). Stem cells of holothuroid coelomocytes. In Echinoderms: Durham (eds Harris L. G., Bottger S. A., Walker C. W. and Lesser M. P.), pp. 163–166. CRC Press, Boca Raton. [Google Scholar]
- Emig, C. C. (1972). Régénération de la région antérieure chez Phoronis psammophila Cori (Phoronida). Zeitschrift für Morphologie der Tiere 73(2), 117–144. [Google Scholar]
- Emson, R. H. & Wilkie, I. C. (1980). Fission and autotomy in echinoderms. Oceanography and Marine Biology 18, 155–250. [Google Scholar]
- Ereskovsky, A. V. (2003). Problems of coloniality, modularity, and individuality in sponges and special features of their morphogeneses during growth and asexual reproduction. Russian Journal of Marine Biology 29, s46–s56. [Google Scholar]
- * Ereskovsky, A. V. (2010). The Comparative Embryology of Sponges. Springer Netherlands, Dordrecht. 329 pp. [Google Scholar]
- * Ereskovsky, A. V. , Borisenko, I. E. , Lapébie, P. , Gazave, E. , Tokina, D. B. & Borchiellini, C. (2015). Oscarella lobularis (Homoscleromorpha, Porifera) regeneration: epithelial morphogenesis and metaplasia. PLoS One 10, e0134566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereskovsky, A. V. , Geronimo, A. & Pérez, T. (2017a). Asexual and puzzling sexual reproduction of the Mediterranean sponge Haliclona fulva (Demospongiae): life cycle and cytological structures. Invertebrate Biology 136(4), 403–421. [Google Scholar]
- Ereskovsky, A. V. , Konjukov, P. & Willenz, P. (2007a). Experimental metamorphosis of Halisarca dujardini larvae (Demospongiae, Halisarcida): evidence of flagellated cell totipotentiality. Journal of Morphology 268(6), 529–536. [DOI] [PubMed] [Google Scholar]
- Ereskovsky, A. , Lavrov, A. , Bolshakov, F. & Tokina, D. (2017b). Regeneration in White Sea sponge Leucosolenia complicata (Porifera, Calcarea). Invertebrate Zoology 14(2), 108–113. [Google Scholar]
- Ereskovsky, A. V. & Tokina, D. B. (2007). Asexual reproduction of homoscleromorph sponges (Porifera; Homoscleromorpha). Marine Biology 151, 425–434. [Google Scholar]
- Ereskovsky, A. V. , Tokina, D. B. , Bezac, C. & Boury‐Esnault, N. (2007b). Metamorphosis of cinctoblastula larvae (Homoscleromorpha, Porifera). Journal of Morphology 268(6), 518–528. [DOI] [PubMed] [Google Scholar]
- Ereskovsky, A. V. , Tokina, D. B. , Saidov, D. M. , Baghdiguian, S. , Le Goff, E. & Lavrov, A. I. (2020). Transdifferentiation and mesenchymal‐to‐epithelial transition during regeneration in Demospongiae (Porifera). Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 334, 37–58. [DOI] [PubMed] [Google Scholar]
- Ewert, M. (1991). Cold torpor, diapause, delayed hatching and aestivation in reptiles and birds. In Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles (eds Deeming D. C. and Ferguson M. W. J.), pp. 173–191. Cambridge University Press, Cambridge. [Google Scholar]
- Fang, Z. , Feng, Q. , Chi, Y. , Xie, L. & Zhang, R. (2008). Investigation of cell proliferation and differentiation in the mantle of Pinctada fucata (Bivalve, Mollusca). Marine Biology 153, 745–754. [Google Scholar]
- Fassini, D. , Parma, L. , Wilkie, I. C. , Bavestrello, G. , Bonasoro, F. & Candia Carnevali, M. D. (2012). Ecophysiology of mesohyl creep in the demosponge Chondrosia reniformis (Porifera: Chondrosida). Journal of Experimental Marine Biology and Ecology 428, 24–31. [Google Scholar]
- Fautin, D. G. (2002). Reproduction of Cnidaria. Canadian Journal of Zoology 80, 1735–1754. [Google Scholar]
- Felix, D. A. , Gutiérrez‐Gutiérrez, Ó. , Espada, L. , Thems, A. & González‐Estévez, C. (2019). It is not all about regeneration: planarians striking power to stand starvation. Seminars in Cell & Developmental Biology 87, 169–181. [DOI] [PubMed] [Google Scholar]
- Fernandéz‐Taboada, E. , Moritz, S. , Zeuschner, D. , Stehling, M. , Schöler, H. R. , Saló, E. & Gentile, L. (2010). Smed‐SmB, a member of the LSm protein superfamily, is essential for chromatoid body organization and planarian stem cell proliferation. Development 137, 1055–1065. [DOI] [PubMed] [Google Scholar]
- * Ferrario, C. , Sugni, M. , Somorjai, I. M. L. & Ballarin, L. (2020). Beyond adult stem cells: dedifferentiation as a unifying mechanism underlying regeneration in invertebrate deuterostomes. Frontiers in Cell and Developmental Biology 8, 587320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Fields, C. & Levin, M. (2018). Are planaria individuals? What regenerative biology is telling us about the nature of multicellularity. Evolutionary Biology 45, 237–247. [Google Scholar]
- * Fierro‐Constaín, L. , Schenkelaars, Q. , Gazave, E. , Haguenauer, A. , Rocher, C. , Ereskovsky, A. , Borchiellini, C. & Renard, E. (2017). The conservation of the germline multipotency program, from sponges to vertebrates: a stepping stone to understanding the somatic and germline origins. Genome Biology and Evolution 9, 474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Fincher, C. T. , Wurtzel, O. , de Hoog, T. , Kravarik, K. M. & Reddien, P. W. (2018). Cell type transcriptome atlas for the planarian Schmidtea mediterranea . Science 360, eaaq1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink, T. , Rasmussen, J. G. , Emmersen, J. , Pilgaard, L. , Fahlman, Å. , Brunberg, S. , Josefsson, J. , Arnemo, J. M. , Zachar, V. , Swenson, J. E. & Fröbert, O. (2011). Adipose‐derived stem cells from the brown bear (Ursus arctos) spontaneously undergo chondrogenic and osteogenic differentiation in vitro. Stem Cell Research 7, 89–95. [DOI] [PubMed] [Google Scholar]
- Fischer, A. B. & Hofmann, D. K. (2004). Budding, bud morphogenesis, and regeneration in Carybdea marsupialis Linnaeus, 1758 (Cnidaria:Cubozoa). Hydrobiologia 530(1), 331–337. [Google Scholar]
- Forsthoefel, D. J. , James, N. P. , Escobar, D. J. , Stary, J. M. , Vieira, A. P. , Waters, F. A. & Newmark, P. A. (2012). An RNAi screen reveals intestinal regulators of branching morphogenesis, differentiation, and stem cell proliferation in planarians. Developmental Cell 23, 691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato, S. A. V. , Vervoort, M. , Adamski, M. & Adamska, M. (2016). Conservation and divergence of bHLH genes in the calcisponge Sycon ciliatum . EvoDevo 7, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraguas, S. , Cárcel, S. , Vivancos, C. , Molina, M. D. , Ginés, J. , Mazariegos, J. , Sekaran, T. , Bartscherer, K. , Romero, R. & Cebrià, F. (2021). Planarian CREB‐binding protein (CBP) gene family regulates stem cell maintenance and differentiation. Developmental Biology 476, 53–67. [DOI] [PubMed] [Google Scholar]
- Frank, U. , Plickert, G. & Müller, W. A. (2009). Cnidarian interstitial cells: the dawn of stem cell research. In Stem Cells in Marine Organisms (eds Rinkevich B. and Matranga V.), pp. 33–59. Springer, Dordrecht. [Google Scholar]
- * Freeman, G. (1964). The role of blood cells in the process of asexual reproduction in the tunicate Perophora viridis . Journal of Experimental Zoology 156(2), 157–183. [DOI] [PubMed] [Google Scholar]
- Freeman, G. (1967). Studies on regeneration in the creeping ctenophore, Vallicul multiformis . Journal of Morphology 123(1), 71–83. [DOI] [PubMed] [Google Scholar]
- Freitas, P. , Lovely, A. & Monaghan, J. (2019). Investigating Nrg1 signaling in the regenerating axolotl spinal cord using multiplexed FISH. Developmental Neurobiology 79, 453–467. [DOI] [PubMed] [Google Scholar]
- Friedländer, M. R. , Adamidi, C. , Han, T. , Lebedeva, S. , Isenbarger, T. A. , Hirst, M. , Marra, M. , Nusbaum, C. , Lee, W. L. , Jenkin, J. C. , Sánchez Alvarado, A. , Kim, J. K. & Rajewsky, N. (2009). High‐resolution profiling and discovery of planarian small RNAs. Proceedings of the National Academy of Sciences of the United States of America 106, 11546–11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Z. , Shibata, M. , Makabe, R. , Ikeda, H. & Uye, S. I. (2014). Body size reduction under starvation, and the point of no return, in ephyrae of the moon jellyfish Aurelia aurita . Marine Ecology Progress Series 510, 255–263. [Google Scholar]
- Fuchs, B. , Wang, W. , Graspeuntner, S. , Li, Y. , Insua, S. , Herbst, E. M. , Dirksen, P. , Böhm, A. M. , Hemmrich, G. , Sommer, F. & Domazet‐Lošo, T. (2014). Regulation of polyp‐to‐jellyfish transition in Aurelia aurita . Current Biology 24, 263–273. [DOI] [PubMed] [Google Scholar]
- * Fuchs, E. , Tumbar, T. & Guasch, G. (2004). Socializing with the neighbors: stem cells and their niche. Cell 116(6), 769–778. [DOI] [PubMed] [Google Scholar]
- Fuchs, J. , Martindale, M. & Hejnol, A. (2011). Gene expression in bryozoan larvae suggest a fundamental importance of pre‐patterned blastemic cells in the bryozoan life‐cycle. EvoDevo 2, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara, S. , Isozaki, T. , Mori, K. & Kawamura, K. (2011). Expression and function of myc during asexual reproduction of the budding ascidian Polyandrocarpa misakiensis . Development Growth & Differentiation 53, 1004–1014. [DOI] [PubMed] [Google Scholar]
- Fukuyama, M. , Kontani, K. , Katada, T. & Rougvie, A. E. (2015). The C. elegans hypodermis couples progenitor cell quiescence to the dietary state. Current Biology 25, 1241–1248. [DOI] [PubMed] [Google Scholar]
- * Funayama, N. (2008). Stem cell system of sponge. In Stem Cells (ed. Bosch T. C. G.), pp. 17–35. Springer, Dordrecht. [Google Scholar]
- Funayama, N. (2013). The stem cell system in demosponges: suggested involvement of two types of cells: archeocytes (active stem cells) and choanocytes (food‐entrapping flagellated cells). Development Genes and Evolution 223, 23–38. [DOI] [PubMed] [Google Scholar]
- * Funayama, N. (2018). The cellular and molecular bases of the sponge stem cell systems underlying reproduction, homeostasis and regeneration. International Journal of Developmental Biology 62, 513–525. [DOI] [PubMed] [Google Scholar]
- Funayama, N. , Nakatsukasa, M. , Mohri, K. , Masuda, Y. & Agata, K. (2010). Piwi expression in archeocytes and choanocytes in demosponges: insights into the stem cell system in demosponges. Evolution and Development 12, 275–287. [DOI] [PubMed] [Google Scholar]
- * Furusawa, C. & Kaneko, K. (2012). A dynamical‐systems view of stem cell biology. Science 338, 215–217. [DOI] [PubMed] [Google Scholar]
- Gahan, J. M. , Bradshaw, B. , Flici, H. & Frank, U. (2016). The interstitial stem cells in Hydractinia and their role in regeneration. Current Opinion in Genetics and Development 40, 65–73. [DOI] [PubMed] [Google Scholar]
- Gaino, E. , Manconi, R. & Pronzato, R. (1995). Organizational plasticity as a successful conservative tactics in sponges. Animal Biology 4, 31–43. [Google Scholar]
- Gaino, E. , Liaci, L. S , Sciscioli, M. & Corriero, G. (2006). Investigation of the budding process in Tethya citrina and Tethya aurantium (Porifera, Demospongiae). Zoomorphology 125, 87–97. [Google Scholar]
- * Gamulin, V. , Rinkevich, B. , Schaecke, H. , Kruse, M. , Mueller, I. M. & Mueller, W. E. G. (1994). Cell adhesion receptors and nuclear receptors are highly conserved from the lowest metazoa (marine sponges) to vertebrates. Biological Chemistry Hoppe‐Seyler 375, 583–588. [DOI] [PubMed] [Google Scholar]
- García‐Arrarás, J. E. , Lázaro‐Peña, M. I. , & Díaz‐Balzac, C. A. (2018) Holothurians as a Model System to Study Regeneration. In: Kloc M., Kubiak J. (eds) Marine Organisms as Model Systems in Biology and Medicine. Results and Problems in Cell Differentiation, vol 65. Springer, Cham. 10.1007/978-3-319-92486-1_13 [DOI] [PubMed] [Google Scholar]
- Garcia‐Cisneros, A. , Perez‐Portela, R. , Almroth, B. C. , Degerman, S. , Palacin, C. & Nilsson Sköld, H. (2015). Long telomeres are associated with clonality in wild populations of the fissiparous starfish Coscinasterias tenuispina . Heredity 115(5), 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Roger, E. M. , Lubzens, E. , Fontaneto, D. & Serra, M. (2019). Facing adversity: dormant embryos in rotifers. Biological Bulletin 237, 119–144. [DOI] [PubMed] [Google Scholar]
- Gasparini, F. , Shimeld, S. M. , Ruffoni, E. , Burighel, P. & Manni, L. (2011). Expression of a Musashi‐like gene in sexual and asexual development of the colonial chordate Botryllus schlosseri and phylogenetic analysis of the protein group. Journal of Experimental Zoology 316B, 562–573. [DOI] [PubMed] [Google Scholar]
- * Gatti, S. (2002). The role of sponges in high‐Antarctic carbon and silicon cycling‐a modelling approach. Reports on Polar and Marine Research 434, 126pp. ISSN 1618 – 3193. [Google Scholar]
- * Gazave, E. , Béhague, J. , Laplane, L. , Guillou, A. , Préau, L. , Demilly, A. , Balavoine, G. & Vervoort, M. (2013). Posterior elongation in the annelid Platynereis dumerilii involves stem cells molecularly related to primordial germ cells. Developmental Biology 382, 246–267. [DOI] [PubMed] [Google Scholar]
- Gazave, E. , Guillou, A. & Balavoine, G. (2014). History of a prolific family: the Hes/Hey‐related genes of the annelid Platynereis . EvoDevo 5, e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazave, E. , Lemaître, Q. & Balavoine, G. (2017). The Notch pathway in the annelid Platynereis: insights into chaetogenesis and neurogenesis processes. Open Biology 7, 160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke, A. R. , Neverett, E. , Luo, Y. J. , Brandt, A. , Ricci, L. , Hulett, R. E. , Gompers, A. , Ruby, J. G. , Rokhsar, D. S. , Reddien, P. W. & Srivastava, M. (2019). Acoel genome reveals the regulatory landscape of whole‐body regeneration. Science 363, eaau6173. [DOI] [PubMed] [Google Scholar]
- * Gentile, L. , Cebrià, F. & Bartscherer, K. (2011). The planarian flatworm: an in vivo model for stem cell biology and nervous system regeneration. Disease Models & Mechanisms 4, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber, T. , Murawala, P. , Knapp, D. , Masselink, W. , Schuez, M. , Herman, S. , Gac‐Santel, M. , Nowoshilow, S. , Kageyama, J. , Khattak, S. , Currie, J. D. , Camp, J. G. , Tanaka, E. M. & Treutlein, B. (2018). Single‐cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science 362(6413), eaaq0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Giani, V. C. , Yamaguchi, E. , Boyle, M. J. & Seaver, E. C. (2011). Somatic and germline expression of piwi during development and regeneration in the marine polychaete annelid Capitella teleta . EvoDevo 2, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, G. D. & Harvey, J. M. L. (2000). Morphogenesis during asexual reproduction in Pygospio elegans Claparede (Annelida, Polychaeta). Biological Bulletin 199, 41–49. [DOI] [PubMed] [Google Scholar]
- Gierer, A. , Berking, S. , Bode, H. , David, C. N. , Flick, K. , Mhansmann, G. , Schaller, H. & Trenkner, E. (1972). Regeneration of Hydra from reaggregated cells. Nature: New Biology 239, 98–101. [DOI] [PubMed] [Google Scholar]
- Giora, J. , Tarasconi, H. M. & Fialho, C. B. (2012). Reproduction and feeding habits of the highly seasonal Brachyhypopomus bombilla (Gymnotiformes: Hypopomidae) from southern Brazil with evidence for a dormancy period. Environmental Biology of Fishes 94, 649–662. [Google Scholar]
- Glynn, P. W. , Coffman, B. , Primov, K. , Renegar, D. A. , Gross, J. , Blackwelder, P. , Martinez, N. , Dominguez, J. , Vanderwoude, J. & Riegl, B. M. (2019). Benthic ctenophore (Order Platyctenida) reproduction, recruitment, and seasonality in south Florida. Invertebrate Biology 138, e12256. [Google Scholar]
- * Gold, D. A. & Jacobs, D. K. (2013). Stem cell dynamics in Cnidaria: are there unifying principles? Development Genes and Evolution 223, 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, D. A. , Lau, C. L. F. , Fuong, H. , Kao, G. , Hartenstein, V. & Jacobs, D. K. (2019). Mechanisms of cnidocyte development in the moon jellyfish Aurelia . Evolution and Development 21, 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontcharoff, M. (1951). Biologie de la régénération et de la reproduction chez quelques Lineidae de France. Annales des Sciences Naturelles. Zoologie et Biologie Animale Série 11(13), 149–235. [Google Scholar]
- González‐Estévez, C. , Felix, D. A. , Rodríguez‐Esteban, G. & Aboobaker, A. A. (2012). Decreased neoblast progeny and increased cell death during starvation‐induced planarian degrowth. International Journal of Developmental Biology 56, 83–91. [DOI] [PubMed] [Google Scholar]
- * Goodell, M. A. , Nguyen, H. & Shroyer, N. (2015). Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nature Reviews Molecular Cell Biology 16, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbushin, A. M. , Levakin, I. A. , Panchina, N. A. & Panchin, Y. V. (2001). Hydrobia ulvae (Gastropoda: Prosobranchia): a new model for regeneration studies. Journal of Experimental Zoology 204, 283–289. [DOI] [PubMed] [Google Scholar]
- Gorbushin, A. M. & Yakovleva, N. V. (2006). Haemogram of Littorina littorea . Journal of the Marine Biological Association of the UK 86(5), 1175–1181. [Google Scholar]
- * Gremigni, V. & Puccinelli, I. (1977). A contribution to the problem of the origin of the blastema cells in planarians: a karyological and ultrastructural investigation. Journal of Experimental Zoology 199, 57–71. [Google Scholar]
- Gross, V. , Bährle, R. & Mayer, G. (2018). Detection of cell proliferation in adults of the water bear Hypsibius dujardini (Tardigrada) via incorporation of a thymidine analog. Tissue and Cell 51, 77–83. [DOI] [PubMed] [Google Scholar]
- * Gruber, H. , Wessels, W. , Boynton, P. , Xu, J. , Wohlgemuth, S. , Leeuwenburgh, C. , Qi, W. , Austad, S. N. , Schaible, R. & Philipp, E. E. (2015). Age‐related cellular changes in the long‐lived bivalve A. islandica . Age 37, e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudniewska, M. , Mouton, S. , Simanov, D. , Beltman, F. , Grelling, M. , De Mulder, K. , Arindrarto, W. , Weissert, P. M. , Van Der Elst, S. & Berezikov, E. (2016). Transcriptional signatures of somatic neoblasts and germline cells in Macrostomum lignano . eLife 5, e20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Grün, D. , Muraro, M. J. , Boisset, J. C. , Wiebrands, K. , Lyubimova, A. , Dharmadhikari, G. , van den Born, M. , van Es, J. , Jansen, E. , Clevers, H. & de Koning, E. J. (2016). De novo prediction of stem cell identity using single‐cell transcriptome data. Cell Stem Cell 19, 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Gschwentner, R. , Ladurner, P. , Nimeth, K. & Rieger, R. (2001). Stem cells in a basal bilaterian. S‐phase and mitotic cells in Convolutriloba longifissura (Acoela, Platyhelminthes). Cell and Tissue Research 304, 401–408. [DOI] [PubMed] [Google Scholar]
- Guidetti, R. , Altiero, T. & Rebecchi, L. (2011). On dormancy strategies in tardigrades. Journal of Insect Physiology 57(5), 567–576. [DOI] [PubMed] [Google Scholar]
- Guidi, L. , Eitel, M. , Cesarini, E. , Schierwater, B. & Balsamo, M. (2011). Ultrastructural analyses support different morphological lineages in the phylum Placozoa Grell, 1971. Journal of Morphology 272, 371–378. [DOI] [PubMed] [Google Scholar]
- * Guo, T. , Peters, A. H. & Newmark, P. A. (2006). A Bruno‐like gene is required for stem cell maintenance in planarians. Developmental Cell 11, 159–169. [DOI] [PubMed] [Google Scholar]
- Gurska, D. & Garm, A. (2014). Cell proliferation in cubozoan jellyfish Tripedalia cystophora and Alatina moseri . PLoS One 9, e102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, S. & Brown, F. D. (2017). Vascular budding in Symplegma brakenhielmi and the evolution of coloniality in styelid ascidians. Developmental Biology 423, 152–169. [DOI] [PubMed] [Google Scholar]
- Hammond, L. S. (1983). Experimental studies of salinity tolerance, burrowing behavior and pedicle regeneration in Lingula anatina (Brachiopoda, Inarticulata). Journal of Paleontology 57, 1311–1316. [Google Scholar]
- Hand, S. C. , Denlinger, D. L. , Podrabsky, J. E. & Roy, R. (2016). Mechanisms of animal diapause: recent developments from nematodes, crustaceans, insects, and fish. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology 310, R1193–R1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Handberg‐Thorsager, M. , Fernandez, E. & Salo, E. (2008). Stem cells and regeneration in planarians. Frontiers in Bioscience 13, 6374–6394. [DOI] [PubMed] [Google Scholar]
- Handberg‐Thorsager, M. & Saló, E. (2007). The planarian nanos‐like gene Smednos is expressed in germline and eye precursor cells during development and regeneration. Development Genes and Evolution 217, 403–411. [DOI] [PubMed] [Google Scholar]
- Harrison, F. W. & Davis, D. A. (1982). Morphological and cytochemical patterns during early stages of reduction body formation in Spongilla lacustris (Porifera: Spongillidae). Transactions of the American Microscopical Society 101(4), 317–324. [Google Scholar]
- Harrison, F. W. , Dunkelberger, D. & Watabe, N. (1975). Cytological examination of reduction bodies of Corvomeyenia carolinensis harrison (Porifera: Spongillidae). Journal of Morphology 145(4), 483–491. [DOI] [PubMed] [Google Scholar]
- * Hartl, M. , Glasauer, S. , Gufler, S. , Raffeiner, A. , Puglisi, K. , Breuker, K. , Bister, K. & Hobmayer, B. (2019). Differential regulation of myc homologs by Wnt/beta‐Catenin signaling in the early metazoan Hydra . FEBS Journal 286, 2295–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl, M. , Glasauer, S. , Valovka, T. , Breuker, K. , Hobmayer, B. & Bister, K. (2014). Hydra myc2, a unique pre‐bilaterian member of the myc gene family, is activated in cell proliferation and gametogenesis. Biology Open 3, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hartl, M. , Mitterstiller, A. M. , Valovka, T. , Breuker, K. , Hobmayer, B. & Bister, K. (2010). Stem‐cell specific activation of an ancestral myc protooncogene with conserved basic functions in the early metazoan Hydra . Proceedings of the National Academy of Sciences of the United States of America 107, 4051–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauenschild, C. (1966). Der hormonale Einfluss des Gehirns auf die sexuelle Entwicklung bei dem Polychaeten Platynereis dumerilii . General and Comparative Endocrinology 6, 26–73. [Google Scholar]
- He, J. , Zheng, L. , Zhang, W. & Lin, Y. (2015). Life cycle reversal in Aurelia sp.1 (Cnidaria, Scyphozoa). PLoS One 10(12), e0145314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm, R. R. (2018). Evolution and development of scyphozoan jellyfish. Biological Reviews 93, 1228–1250. [DOI] [PubMed] [Google Scholar]
- Hemmrich, G. , Khalturin, K. , Boehm, A.‐M. , Puchert, M. , Anton‐Erxleben, F. , Wittlieb, J. , Klostermeier, U. C. , Rosenstiel, P. , Oberg, H.‐H. , Domazet‐Loso, T. , Sugimoto, T. , Niwa, H. & Bosch, T. C. G. (2012). Molecular signatures of the three stem cell lineages in Hydra and the emergence of stem cell function at the base of multicellularity. Molecular Biology and Evolution 29, 3267–3280. [DOI] [PubMed] [Google Scholar]
- Hendelberg, J. & Åkesson, B. (1988). Convolutriloba retrogemma gen. et sp.n., a turbellarian (Acoela, Platyhelminthes) with reversed polarity of reproductive buds. Fortschritte der Zoologie 36, 321–327. [Google Scholar]
- Hengherr, S. & Schill, R. O. (2011). Dormant stages in freshwater bryozoans–an adaptation to transcend environmental constraints. Journal of Insect Physiology 57(5), 595–601. [DOI] [PubMed] [Google Scholar]
- Henry, J. Q. & Martindale, M. Q. (2000). Regulation and regeneration in the ctenophore Mnemiopsis leidyi . Developmental Biology 227, 720–733. [DOI] [PubMed] [Google Scholar]
- Henry, L. A. , Kenchington, E. L. R. & Silvaggio, A. (2003). Effects of mechanical experimental disturbance on aspects of colony responses, reproduction and regeneration in the cold water octocoral Gersemia rubiformis . Canadian Journal of Zoology 81(10), 1691–1701. [Google Scholar]
- Highsmith, R. C. (1982). Reproduction by fragmentation in corals. Marine Ecology Progress Series 7, 207–226. [Google Scholar]
- Higuchi, S. , Hayashi, T. , Tarui, H. , Nishimura, O. , Nishimura, K. , Shibata, N. , Sakamoto, H. & Agata, K. (2008). Expression and functional analysis of musashi‐like genes in planarian CNS regeneration. Mechanisms of Development 125, 631–645. [DOI] [PubMed] [Google Scholar]
- * Hobmayer, B. , Jenewein, M. , Eder, D. , Eder, M.‐K. , Glasauer, S. , Gufler, S. , Hartl, M. & Salvenmoser, W. (2012). Stemness in Hydra – a current perspective. International Journal of Developmental Biology 56(6‐7‐8), 509–517. [DOI] [PubMed] [Google Scholar]
- Höhr, D. (1977). Differenzierungsvorgänge in der keimenden Gemmula von Ephydatia fluviatilis . Wilhelm Roux' Archiv 182, 329–346. [DOI] [PubMed] [Google Scholar]
- * Holland, N. & Somorjai, I. (2020). Serial blockface SEM suggests that stem cells may participate in adult notochord growth in an invertebrate chordate, the Bahamas lancelet. EvoDevo 11, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm, K. , Dupont, S. , Skold, H. , Stenius, A. , Thorndyke, M. & Hernroth, B. (2008). Induced cell proliferation in putative haematopoietic tissues of the sea star, Asterias rubens (L.). Journal of Experimental Zoology 211, 2551–2558. [DOI] [PubMed] [Google Scholar]
- Holstein, T. W. , Hobmayer, E. & David, C. N. (1991). Pattern of epithelial cell cycling in Hydra . Developmental Biology 148, 602–611. [DOI] [PubMed] [Google Scholar]
- Hori, I. & Kishida, Y. (1998). A fine structural study of regeneration after fission in the planarian Dugesia japonica . Hydrobiologia 383, 131–136. [Google Scholar]
- Hori, I. & Kishida, Y. (2001). Further observation on the early regenerates after fission in the planarian Dugesia japonica . Belgian Journal of Zoology 131(Suppl. 1), 117–121. [Google Scholar]
- Hu, C. K. , Wang, W. , Brind'Amour, J. , Singh, P. P. , Reeves, G. A. , Lorincz, M. C. , Alvarado, A. S. & Brunet, A. (2020). Vertebrate diapause preserves organisms long term through Polycomb complex members. Science 367(6480), 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hughes, R. N. (1987). The functional ecology of clonal animals. Functional Ecology 1(1), 63–69. [Google Scholar]
- Humphreys, T. , Sasaki, A. , Uenishi, G. , Tamparra, K. , Arimoto, A. & Tagawa, K. (2010). Regeneration in the hemichordate Ptychodera flava . Zoological Science 27(2), 91–95. [DOI] [PubMed] [Google Scholar]
- * Hyams, Y. , Paz, G. , Rabinowitz, C. & Rinkevich, B. (2017). Insights into the unique torpor of Botrylloides leachi, a colonial urochordate. Developmental Biology 428, 101–117. [DOI] [PubMed] [Google Scholar]
- Hygum, T. L. , Clausen, L. K. B. , Halberg, K. A. , Jørgensen, A. & Møbjerg, N. (2016). Tun formation is not a prerequisite for desiccation tolerance in the marine tidal tardigrade Echiniscoides sigismundi . Zoological Journal of the Linnean Society 178(4), 907–911. [Google Scholar]
- Hyman, L. H. (1955). The Invertebrates. IV. Echinodermata, Asteroidea. McGraw Hill, New York. [Google Scholar]
- Iguchi, N. & Kidokoro, H. (2006). Horizontal distribution of Thetys vagina Tilesius (Tunicata, Thaliacea) in the Japan Sea during spring 2004. Journal of Plankton Research 28, 537–541. [Google Scholar]
- Imperadore, P. & Fiorito, G. (2018). Cephalopod tissue regeneration: consolidating over a century of knowledge. Frontiers in Physiology 9, e593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperadore, P. , Uckermann, O. , Galli, R. , Steiner, G. , Kirsch, M. & Fiorito, G. (2018). Nerve regeneration in the cephalopod mollusc Octopus vulgaris: label‐free multiphoton microscopy as a tool for investigation. Journal of the Royal Society Interface 15, 20170889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Isaeva, V. V. (2011). Pluripotent gametogenic stem cells of asexually reproducing invertebrates. In Embryonic Stem Cells‐Basic Biology to Bioengineering (ed. Kallos M. S.), pp. 449–478. Rijeka, Croatia: IntechOpen. 10.5772/23740. [DOI] [Google Scholar]
- * Isaeva, V. V. & Akhmadiev, A. V. (2011). Germinal granules in archaeocytes of the sponge Oscarella malakhovi Ereskovsky, 2006. Russian Journal of Marine Biology 37, 209–216. [Google Scholar]
- * Isaeva, V. V. , Akhmadieva, A. V. , Aleksandrova, Y. N. & Shukalyuk, A. I. (2009). Morphofunctional organization of reserve stem cells providing for asexual and sexual reproduction of invertebrates. Russian Journal of Developmental Biology 40, 57–68. [PubMed] [Google Scholar]
- * Isaeva, V. V. , Aleksandrova, Y. & Reunov, A. (2005a). Interaction between chromatoid bodies and mitochondria in neoblasts and gonial cells of the asexual and spontaneously sexualized planarian, Girardia (Dugesia) tigrina . Invertebrate Reproduction & Development 48, 119–128. [Google Scholar]
- Isaeva, V. V. , Dolganov, S. M. & Shukalyuk, A. I. (2005b). Rhizocephalan barnacles‐ parasites of commercially important crabs and other decapods. Russian Journal of Marine Biology 31(4), 215–220. [Google Scholar]
- Israelsson, O. (2006). Observations on some unusual cell types in the enigmatic worm Xenoturbella (phylum uncertain). Tissue and Cell 38, 233–242. [DOI] [PubMed] [Google Scholar]
- * Ivankovic, M. , Haneckova, R. , Thommen, A. , Grohme, M. A. , Vila‐Farré, M. , Werner, S. & Rink, J. C. (2019). Model systems for regeneration: planarians. Development 146, dev167684. [DOI] [PubMed] [Google Scholar]
- * Jackson, J. B. C. & Coates, A. G. (1986). Life cycles and evolution of clonal (modular) animals. Philosophical Transactions of the Royal Society of London. B, Biological Sciences 313(1159), 7–22. [Google Scholar]
- Jager, M. , Quéinnec, E. , Le Guyader, H. & Manuel, M. (2011). Multiple Sox genes are expressed in stem cells or in differentiating neuro‐sensory cells in the hydrozoan Clytia hemisphaerica . EvoDevo 2, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob, W. , Sagasser, S. , Dellaporta, S. , Holland, P. , Kuhn, K. & Schierwater, B. (2004). The Trox‐2 Hox/ParaHox gene of Trichoplax (Placozoa) marks an epithelial boundary. Development Genes and Evolution 214, 170–175. [DOI] [PubMed] [Google Scholar]
- Janelt, K. & Poprawa, I. (2020). Analysis of encystment, excystment, and cyst structure in freshwater Eutardigrade Thulinius ruffoi (Tardigrada, Isohypsibioidea: Doryphoribiidae). Diversity 12, e62. [Google Scholar]
- Jeffery, W. R. (2014). Distal regeneration involves the age dependent activity of branchial sac stem cells in the ascidian Ciona intestinalis . Regeneration 2(1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery, W. R. (2015). Regeneration, stem cells, and aging in the tunicate Ciona: insights from the oral siphon. International Review of Cell and Molecular Biology 319, 255–282. [DOI] [PubMed] [Google Scholar]
- Jeffery, W. R. (2019). Progenitor targeting by adult stem cells in Ciona homeostasis, injury, and regeneration. Developmental Biology 448, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn, J. , Gebert, D. , Pipilescu, F. , Stern, S. , Kiefer, J. S. T. , Hewel, C. & Rosenkranz, D. (2018). PIWI genes and piRNAs are ubiquitously expressed in mollusks and show patterns of lineage‐specific adaptation. Communications Biology 1, e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez‐Merino, J. , Santos de Abreu, I. , Hiebert, L. S. , Allodi, S. , Tiozzo, S. , De Barros, C. & Brown, F. D. (2019). Putative stem cells in the hemolymph and the intestinal submucosa of the solitary ascidian Styela plicata . EvoDevo 10, e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Jones, W. C. (1961). Properties of the wall of Leucosolenia variabilis: I. The skeletal layer. Journal of Cell Science 3, 531–542. [Google Scholar]
- Joven, A. , Elewa, A. & Simon, A. (2019). Model systems for regeneration: salamanders. Development 146(14), dev167700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Juliano, C. & Wessel, G. (2010). Versatile germline genes. Science 329, 640–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano, C. E. , Reich, A. , Liu, N. , Götzfried, J. , Zhong, M. , Uman, S. , Reenan, R. A. , Wessel, G. M. , Steele, R. E. & Lin, H. (2014). PIWI proteins and PIWI‐interacting RNAs function in Hydra somatic stem cells. Proceedings of the National Academy of Sciences of the United States of America 111, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Juliano, C. E. , Swartz, S. Z. & Wessel, G. M. (2010). A conserved germline multipotency program. Development 137, 4113–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajihara, H. & Hookabe, N. (2019). Anterior regeneration in Baseodiscus hemprichii (Nemertea: Heteronemertea). Tropical Natural History 19(1), 39–42. [Google Scholar]
- Kalacheva, N. V. , Eliseikina, M. G. , Frolova, L. T. & Dolmatov, I. Y. (2017). Regeneration of the digestive system in the crinoid Himerometra robustipinna occurs by transdifferentiation of neurosecretory‐like cells. PLoS One 12(7), e0182001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenev, Y. O. & Dolmatov, I. Y. (2015). Posterior regeneration following fission in the holothurian Cladolabes schmeltzii (Dendrochirotida: Holothuroidea). Microscopy Research and Technique 78, 540–552. [DOI] [PubMed] [Google Scholar]
- Kamenev, Y. O. & Dolmatov, I. Y. (2017). Anterior regeneration after fission in the holothurian Cladolabes schmeltzii (Dendrochirotida: Holothuroidea). Microscopy Research and Technique 80, 183–194. [DOI] [PubMed] [Google Scholar]
- Kaneko, N. , Katsuyama, Y. , Kawamura, K. & Fujiwara, S. (2010). Regeneration of the gut requires retinoic acid in the budding ascidian Polyandrocarpa misakiensis . Development, Growth & Differentiation 52, 457–468. [DOI] [PubMed] [Google Scholar]
- Kaneto, S. & Wada, H. (2011). Regeneration of amphioxus oral cirri and its skeletal rods: implications for the origin of the vertebrate skeleton. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 316, 409–417. [DOI] [PubMed] [Google Scholar]
- Kanska, J. & Frank, U. (2013). New roles for Nanos in neural cell fate determination revealed by studies in a cnidarian. Journal of Cell Science 126, 3192–3203. [DOI] [PubMed] [Google Scholar]
- * Kassmer, S. H. , Langenbacher, A. D. & De Tomaso, A. W. (2020). Integrin‐alpha‐6+ candidate stem cells are responsible for whole body regeneration in the invertebrate chordate Botrylloides diegensis . Nature Communications 11, e4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassmer, S. H. , Nourizadeh, S. & De Tomaso, A. W. (2019). Cellular and molecular mechanisms of regeneration in colonial and solitary ascidians. Developmental Biology 448, 271–278. [DOI] [PubMed] [Google Scholar]
- Kassmer, S. H. , Rodriguez, D. & De Tomaso, A. W. (2016). Colonial ascidians as model organisms for the study of germ cells, fertility, whole body regeneration, vascular biology and aging. Current Opinion in Genetics and Development 39, 101–106. [DOI] [PubMed] [Google Scholar]
- Kawamura, K. & Fujiwara, S. (1995a). Cellular and molecular characterization of transdifferentiation in the process of morphallaxis of budding tunicates. Seminars in Cell and Developmental Biology 6(3), 117–126. [DOI] [PubMed] [Google Scholar]
- Kawamura, K. & Fujiwara, S. (1995b). Establishment of cell lines from multipotent epithelial sheet in the budding tunicate, Polyandrocarpa misakiensis . Cell Structure and Function 20(1), 97–106. [DOI] [PubMed] [Google Scholar]
- Kawamura, K. , Fujiwara, S. & Sugino, Y. M. (1991). Budding‐specific lectin induced in epithelial cells is an extracellular matrix component for stem cell aggregation in tunicates. Development 113, 995–1005. [DOI] [PubMed] [Google Scholar]
- Kawamura, K. , Kitamura, S. , Sekida, S. , Tsuda, M. & Sunanaga, T. (2012). Molecular anatomy of tunicate senescence: reversible function of mitochondrial and nuclear genes associated with budding cycles. Development 139, 4083–4093. [DOI] [PubMed] [Google Scholar]
- Kawamura, K. & Nakauchi, M. (1986). Mitosis and body patterning during morphallactic development of palleal buds in ascidians. Developmental Biology 116, 39–50. [Google Scholar]
- Kawamura, K. , Sugino, Y. , Sunanaga, T. & Fujiwara, S. (2008a). Multipotent epithelial cells in the process of regeneration and asexual reproduction in colonial tunicates. Development Growth & Differentiation 50, 1–11. [DOI] [PubMed] [Google Scholar]
- * Kawamura, K. & Sunanaga, T. (2010). Hemoblasts in colonial tunicates: are they stem cells or tissue‐restricted progenitor cells? Development, Growth & Differentiation 52, 69–76. [DOI] [PubMed] [Google Scholar]
- Kawamura, K. & Sunanaga, T. (2011). Role of Vasa, Piwi, and Myc‐expressing coelomic cells in gonad regeneration of the colonial tunicate, Botryllus primigenus . Mechanisms of Development 128, 457–470. [DOI] [PubMed] [Google Scholar]
- * Kawamura, K. , Tachibana, M. & Sunanaga, T. (2008b). Cell proliferation dynamics of somatic and germline tissues during zooidal life span in the colonial tunicate Botryllus primigenus . Developmental Dynamics 237(7), 1812–1825. [DOI] [PubMed] [Google Scholar]
- Kawamura, K. , Yoshida, T. & Sekida, S. (2018). Autophagic dedifferentiation induced by cooperation between TOR inhibitor and retinoic acid signals in budding tunicates. Developmental Biology 433, 384–393. [DOI] [PubMed] [Google Scholar]
- Kenny, N. J. , de Goeij, J. M. , de Bakker, D. M. , Whalen, C. G. , Berezikov, E. & Riesgo, A. (2018). Towards the identification of ancestrally shared regenerative mechanisms across the Metazoa: a transcriptomic case study in the demosponge Halisarca caerulea . Marine Genomics 37, 135–147. [DOI] [PubMed] [Google Scholar]
- * Khadra, Y. B. , Sugni, M. , Ferrario, C. , Bonasoro, F. , Oliveri, P. , Martinez, P. & Carnevali, M. D. C. (2018). Regeneration in stellate echinoderms: Crinoidea, Asteroidea and Ophiuroidea. In Marine Organisms as Model Systems in Biology and Medicine (ed. Kubiak K. M.), pp. 285–320. Springer, Cham. [DOI] [PubMed] [Google Scholar]
- * Khalturin, K. , Anton‐Erxleben, F. , Milde, S. , Plötz, C. , Wittlieb, J. , Hemmrich, G. & Bosch, T. C. (2007). Transgenic stem cells in Hydra reveal an early evolutionary origin for key elements controlling self‐renewal and differentiation. Developmental Biology 309, 32–44. [DOI] [PubMed] [Google Scholar]
- Khan, S. J. , Schuster, K. J. & Smith‐Bolton, R. K. (2016). Regeneration in crustaceans and insects. eLS, 14 pp. Chichester: John Wiley & Sons. 10.1002/9780470015902.a0001098.pub2. [DOI] [Google Scholar]
- Kharin, A. , Zagainova, I. & Kostyuchenko, R. (2006). Formation of the paratomic fission zone in freshwater oligochaetes. Russian Journal of Developmental Biology 37, 354–365. [Google Scholar]
- * Kholodenko, I. V. & Yarygin, K. N. (2017). Cellular mechanisms of liver regeneration and cell‐based therapies of liver diseases. BioMed Research International 2017, 8910821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchin, I. M. (1994). The Biology of Tardigrades. Portland Press, London. pp. 186. [Google Scholar]
- King, G. M. (1998). Reproduction in the Hemichordata. In Encyclopedia of Reproduction (Volume 2, eds Knobil E. and Neill J. D.), pp. 599–603. Academic Press, San Diego. [Google Scholar]
- Kipreos, E. T. & van den Heuvel, S. (2019). Developmental control of the cell cycle: insights from Caenorhabditis elegans . Genetics 211, 797–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, M. & Akasaka, K. (2010). Regeneration in crinoids. Development Growth & Differentiation 52, 57–68. [DOI] [PubMed] [Google Scholar]
- Konstantinides, N. & Averof, M. (2014). A common cellular basis for muscle regeneration in arthropods and vertebrates. Science 343(6172), 788–791. [DOI] [PubMed] [Google Scholar]
- Korotkova, G. P. (1972). Regeneration of the calcareous sponge Sycon lingua . Transactions of the Leningrad Society of Naturalists 78, 155–171 (in Russian). [Google Scholar]
- Kostyuchenko, R. P. , Kozin, V. V. & Kupriashova, E. E. (2016). Regeneration and asexual reproduction in annelids: cells, genes, and evolution. Biological Bulletin 43(3), 185–194. [Google Scholar]
- Kozin, V. V. , Filippova, N. A. & Kostyuchenko, R. P. (2017). Regeneration of the nervous and muscular system after caudal amputation in the polychaete Alitta virens (Annelida: nereididae). Russian Journal of Developmental Biology 48, 198–210. [Google Scholar]
- * Kozin, V. V. & Kostyuchenko, R. P. (2015). Vasa, PL10, and Piwi gene expression during caudal regeneration of the polychaete annelid Alitta virens . Development Genes and Evolution 225, 129–138. [DOI] [PubMed] [Google Scholar]
- Krichinskaya, E. B. & Martynova, M. G. (1975). Distribution of neoblasts and mitoses during the asexual reproduction of the planarian Dugesia tigrina (Girard). Soviet Journal of Developmental Biology 5, 309–314. [PubMed] [Google Scholar]
- Kubota, S. (2006). Life cycle reversion of Laodicea undulata (Hydrozoa, Leptomedusae) from Japan. Bulletin of the Biogeographical Society of Japan 61, 85–88. [Google Scholar]
- Kuenzel, T. , Heiermann, R. , Frank, U. , Müller, W. A. , Tilmann, W. , Bause, M. , Nonn, A. , Helling, M. , Schwarz, R. S. & Plickert, G. (2010). Migration and differentiation potential of stem cells in the cnidarian Hydractinia analysed in eGFP‐transgenic animals and chimeras. Developmental Biology 348, 120–129. [DOI] [PubMed] [Google Scholar]
- Kürn, U. , Rendulic, S. , Tiozzo, S. & Lauzon, R. J. (2011). Asexual propagation and regeneration in colonial ascidians. Biological Bulletin 221(1), 43–61. [DOI] [PubMed] [Google Scholar]
- Kusserow, A. , Pang, K. , Sturm, C. , Hrouda, M. , Lentfer, J. , Schmidt, H. A. , Technau, U. , von Haeseler, A. , Hobmayer, B. , Martindale, M. Q. & Holstein, T. W. (2005). Unexpected complexity of the Wnt gene family in a sea anemone. Nature 433, 156–160. [DOI] [PubMed] [Google Scholar]
- * Lai, A. G. & Aboobaker, A. A. (2018). EvoRegen in animals: time to uncover deep conservation or convergence of adult stem cell evolution and regenerative processes. Developmental Biology 433, 118–131. [DOI] [PubMed] [Google Scholar]
- Lai, A. G. , Kosaka, N. , Abnave, P. , Sahu, S. & Aboobaker, A. A. (2018). The abrogation of condensin function provides independent evidence for defining the self‐renewing population of pluripotent stem cells. Developmental Biology 433, 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Laird, D. J. , De Tomaso, A. W. & Weissman, I. L. (2005). Stem cells are units of natural selection in a colonial ascidian. Cell 123, 1351–1360. [DOI] [PubMed] [Google Scholar]
- Laird, D. J. & Weissman, I. L. (2004). Telomerase maintained in self‐renewing tissues during serial regeneration of the urochordate Botryllus schlosseri . Developmental Biology 273, 185–194. [DOI] [PubMed] [Google Scholar]
- * Lander, A. D. (2009). The 'stem cell' concept: is it holding us back? Journal of Biology 8, e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanna, E. & Klautau, M. (2019). The choanoderm of Sycettusa hastifera (Calcarea, Porifera) is able to generate new individuals. Invertebrate Biology 138, 12262. [Google Scholar]
- Lasker, H. (1984). Asexual reproduction, fragmentation, and skeletal morphology of a plexaurid gorgonian. Marine Ecology Progress Series 19, 261–268. [Google Scholar]
- * Lavrov, A. I. , Bolshakov, F. V. , Tokina, D. B. & Ereskovsky, A. V. (2018). Sewing up the wounds: the epithelial morphogenesis as a central mechanism of calcaronean sponge regeneration. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 330, 351–371. [DOI] [PubMed] [Google Scholar]
- * Lavrov, A. I. & Kosevich, I. A. (2016). Sponge cell reaggregation: cellular structure and morphogenetic potencies of multicellular aggregates. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 325, 158–177. [DOI] [PubMed] [Google Scholar]
- Laxminarayana, A. (2006). Asexual reproduction by induced transverse fission in the sea cucumbers Bohadschia marmorata and Holothuria atra . SPC Beche‐de‐Mer Information Bulletin 23, 35–37. [Google Scholar]
- Leclère, L. , Copley, R. R. , Momose, T. & Houliston, E. (2016). Hydrozoan insights in animal development and evolution. Current Opinion in Genetics and Development 39, 157–167. [DOI] [PubMed] [Google Scholar]
- * Leclère, L. , Jager, M. , Barreau, C. , Chang, P. , Le Guyader, H. , Manuel, M. & Houliston, E. (2012). Maternally localized germ plasm mRNAs and germ cell/stem cell formation in the cnidarian Clytia . Developmental Biology 364, 236–248. [DOI] [PubMed] [Google Scholar]
- Lee, Y. J. , Bernstock, J. D. , Klimanis, D. & Hallenbeck, J. M. (2018). Akt Protein Kinase, miR‐200/miR‐182 expression and epithelial‐mesenchymal transition proteins in hibernating ground squirrels. Frontiers in Molecular Neuroscience 11, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Lehoczky, J. A. , Robert, B. & Tabin, C. J. (2011). Mouse digit tip regeneration is mediated by fate‐restricted progenitor cells. Proceedings of the National Academy of Sciences of the United States of America 108, 20609–20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, K. , Thi‐Kim Vu, H. , Mohan, R. D. , McKinney, S. A. , Seidel, C. W. , Alexander, R. , Gotting, K. , Workman, J. L. & Sánchez Alvarado, A. (2016). Egf signaling directs neoblast repopulation by regulating asymmetric cell division in planarians. Developmental Cell 38, 413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leininger, S. , Adamski, M. , Bergum, B. , Guder, C. , Liu, J. , Laplante, M. , Bråte, J. , Hoffmann, F. , Fortunato, S. , Jordal, S. , Rapp, H. T. & Adamska, M. (2014). Developmental gene expression provides clues to relationships between sponge and eumetazoan body plans. Nature Communications 5, e3905. [DOI] [PubMed] [Google Scholar]
- Lengfeld, T. , Watanabe, H. , Simakov, O. , Lindgens, D. , Gee, L. , Law, L. , Schmidt, H. A. , Ozbek, S. , Bode, H. & Holstein, T. W. (2009). Multiple Wnts are involved in Hydra organizer formation and regeneration. Developmental Biology 330, 186–199. [DOI] [PubMed] [Google Scholar]
- León‐Espinosa, G. , Regalado‐Reyes, M. , DeFelipe, J. & Muñoz, A. (2018). Changes in neocortical and hippocampal microglial cells during hibernation. Brain Structure and Function 223, 1881–1895. [DOI] [PubMed] [Google Scholar]
- Lewis, J. E. & Ebling, F. J. (2017). Tanycytes as regulators of seasonal cycles in neuroendocrine function. Frontiers in Neurology 8, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S. C. , Dyal, L. A. , Hilburn, C. F. , Weitz, S. , Liau, W. S. , LaMunyon, C. W. & Denver, D. R. (2009). Molecular evolution in Panagrolaimus nematodes: origins of parthenogenesis, hermaphroditism and the Antarctic species P. davidi . BMC Ecology and Evolution 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys, S. P. , Mackie, G. O. & Reiswig, H. M. (2007). The biology of glass sponges. Advances in Marine Biology 52, 1–145. [DOI] [PubMed] [Google Scholar]
- * Li, L. & Xie, T. (2005). Stem cell niche: structure and function. Annual Review of Cell and Developmental Biology 21, 605–631. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Wang, Y. L. , Xie, J. , Sun, W. J. , Zhu, M. , He, L. & Wang, Q. (2015). Characterization and expression of DDX6 during gametogenesis in the Chinese mitten crab Eriocheir sinensis . Genetics and Molecular Research 14, 4420–4437. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Wang, R. , Xun, X. , Wang, J. , Bao, L. , Thimmappa, R. , Ding, J. , Jiang, J. , Zhang, L. , Li, T. , Lv, J. , Mu, C. , Hu, X. , Zhang, L. , Liu, J. , et al. (2018). Sea cucumber genome provides insights into saponin biosynthesis and aestivation regulation. Cell Discovery 4, e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Y. , Rathnayake, D. , Huang, S. , Pathirana, A. , Xu, Q. & Zhang, S. (2019). BMP signaling is required for amphioxus tail regeneration. Development 146(4), dev166017. [DOI] [PubMed] [Google Scholar]
- Lim, R. S. , Anand, A. , Nishimiya‐Fujisawa, C. , Kobayashi, S. & Kai, T. (2014). Analysis of Hydra PIWI proteins and piRNAs uncover early evolutionary origins of the piRNA pathway. Developmental Biology 386, 237–251. [DOI] [PubMed] [Google Scholar]
- Lin, X. & Söderhäll, I. (2011). Crustacean hematopoiesis and the astakine cytokines. Blood 117, 6417–6424. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Bai, Z. , Li, Q. , Zhao, Y. & Li, J. (2013). Healing and regeneration of the freshwater pearl mussel Hyriopsis cumingii Lea after donating mantle saibos. Aquaculture 392–395, 34–43. [Google Scholar]
- * Loeffler, M. & Roeder, I. (2002). Tissue stem cells: definition, plasticity, heterogeneity, self‐organization and models–a conceptual approach. Cells, Tissues, Organs 171, 8–26. [DOI] [PubMed] [Google Scholar]
- Lombardi, C. , Taylor, P. D. , Cocito, S. , Bertolini, C. & Calosi, P. (2017). Low pH conditions impair module capacity to regenerate in a calcified colonial invertebrate, the bryozoan Cryptosula pallasiana . Marine Environmental Research 125, 110–117. [DOI] [PubMed] [Google Scholar]
- Loomis, S. H. (2010). Diapause and estivation in sponges. Progress in Molecular and Subcellular Biology 49, 231–243. [DOI] [PubMed] [Google Scholar]
- Lu, Y. C. , Smielewska, M. , Palakodeti, D. , Lovci, M. T. , Aigner, S. , Yeo, G. W. & Graveley, B. R. (2009). Deep sequencing identifies new and regulated microRNAs in Schmidtea mediterranea . RNA 15, 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz, B. L. P. , Capel, K. C. C. , Zilberberg, C. , Flores, A. A. V. , Migotto, A. E. & Kitahara, M. V. (2018). A polyp from nothing: the extreme regeneration capacity of the Atlantic invasive sun corals Tubastraea coccinea and T. tagusensis (Anthozoa, Scleractinia). Journal of Experimental Marine Biology and Ecology 503, 60–65. [Google Scholar]
- Magor, B. G. , De Tomaso, A. W. , Rinkevich, B. & Weissman, I. L. (1999). Allorecognition in colonial tunicates: protection against predatory cell lineages? Immunological Reviews 167, 69–79. [DOI] [PubMed] [Google Scholar]
- Malinowski, P. T. , Cochet‐Escartin, O. , Kaj, K. J. , Ronan, E. , Groisman, A. , Diamond, P. H. & Collins, E. S. (2017). Mechanics dictate where and how freshwater planarians fission. Proceedings of the National Academy of Sciences of the United States of America 114, 10888–10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Manni, L. , Anselmi, C. , Cima, F. , Gasparini, F. , Voskoboynik, A. , Martini, M. , Peronato, A. , Burighel, P. , Zaniolo, G. & Ballarin, L. (2019). Sixty years of experimental studies on the blastogenesis of the colonial tunicate Botryllus schlosseri . Developmental Biology 448, 293–308. [DOI] [PubMed] [Google Scholar]
- * Manni, L. , Zaniolo, G. , Cima, F. , Burighel, P. & Ballarin, L. (2007). Botryllus schlosseri: a model ascidian for the study of asexual reproduction. Developmental Dynamics 236, 335–352. [DOI] [PubMed] [Google Scholar]
- Manylov, O. G. (1995). Regeneration in Gastrotricha‐ I. Light microscopical observations on the regeneration in Turbanella sp. Acta Zoologica 76(1), 1–6. [Google Scholar]
- * Marescal, O. & Cheeseman, I. M. (2020). Cellular mechanisms and regulation of quiescence. Developmental Cell 55, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow, H. Q. , Srivastava, M. , Matus, D. Q. , Rokhsar, D. & Martindale, M. Q. (2009). Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Developmental Neurobiology 69, 235–254. [DOI] [PubMed] [Google Scholar]
- Marques, I. , Lupi, E. & Mercader, N. (2019). Model systems for regeneration: zebrafish. Development 146, dev167692. [DOI] [PubMed] [Google Scholar]
- Marsden, J. R. (1957). Regeneration in Phoronis vancouverensis . Journal of Morphology 101, 307–323. [Google Scholar]
- * Martin, D. R. , Cox, N. R. , Hathcock, T. L. , Niemeyer, G. P. & Baker, H. J. (2002). Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Experimental Hematology 30, 879–886. [DOI] [PubMed] [Google Scholar]
- Martinand‐Mari, C. , Vacelet, J. , Nickel, M. , Wörheide, G. , Mangeat, P. & Baghdiguian, S. (2012). Cell death and renewal during prey capture and digestion in the carnivorous sponge Asbestopluma hypogea (Porifera: Poecilosclerida). Journal of Experimental Zoology 215, 3937–3943. [DOI] [PubMed] [Google Scholar]
- Martindale, M. Q. (2016). The onset of regenerative properties in ctenophores. Current Opinion in Genetics and Development 40, 113–119. [DOI] [PubMed] [Google Scholar]
- * Martínez, D. E. (1998). Mortality patterns suggest lack of senescence in hydra. Experimental Gerontology 33, 217–225. [DOI] [PubMed] [Google Scholar]
- Martynova, M. G. (1993). Satellite cells in the crayfish heart muscle functions as stem cells and are characterized by molt dependent behaviour. Zoologischer Anzeiger 230, 181–190. [Google Scholar]
- Martynova, M. G. (2004). Proliferation and differentiation processes in the heart muscle elements in different phylogenetic groups. International Review of Cytology 235, 215–250. [DOI] [PubMed] [Google Scholar]
- Maruzzo, D. , Egredzija, M. , Minelli, A. & Fusco, G. (2008). Segmental pattern formation following amputation in the flagellum of the second antennae of Asellus aquaticus (Crustacea, Isopoda). Italian Journal of Zoology 75, 225–231. [Google Scholar]
- Mashanov, V. S. & García‐Arrarás, J. E. (2011). Gut regeneration in holothurians: a snapshot of recent developments. Biological Bulletin 221, 93–109. [DOI] [PubMed] [Google Scholar]
- Mashanov, V. S. , Frolova, L. & Dolmatov, I. (2004). Structure of the digestive tube in the holothurian Eupentacta fraudatrix (Holothuroidea: Dendrochirota). Russian Journal of Marine Biology 30, 314–322. [Google Scholar]
- Mashanov, V. S. & Zueva, O. (2019). Radial glia in Echinoderms. Developmental Neurobiology 79(5), 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov, V. S. , Zueva, O. R. & García‐Arrarás J. E. (2014a). Postembryonic organogenesis of the digestive tube: why does it occur in worms and sea cucumbers but fail in humans? Current Topics in Developmental Biology 108, 185–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov, V. S. , Zueva, O. & Garcia‐Arraras, J. E. (2014b). Transcriptomic changes during regeneration of the central nervous system in an echinoderm. BMC Genomics 15, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov, V. S. , Zueva, O. R. & Garcia‐Arrarás, J. E. (2015a). Expression of pluripotency factors in echinoderm regeneration. Cell and Tissue Research 359(2), 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov, V. S. , Zueva, O. & García‐Arrarás, J. E. (2015b). Myc regulates programmed cell death and radial glia dedifferentiation after neural injury in an echinoderm. BMC Developmental Biology 15, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov, V. S. , Zueva, O. R. & García‐Arrarás J. E. (2015c). Heterogeneous generation of new cells in the adult echinoderm nervous system. Frontiers in Neuroanatomy 9, e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov, V. S. , Zueva, O. , Mashanova, D. & García‐Arrarás, J. E. (2017). Expression of stem cell factors in the adult sea cucumber digestive tube. Cell and Tissue Research 370, 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Mashanov, V. S. , Zueva, O. R. , Rojas‐Catagena, C. & Garcia‐Arraras, J. E. (2010). Visceral regeneration in a sea cucumber involves extensive expression of survivin and mortalin homologs in the mesothelium. BMC Developmental Biology 10, e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozzo, V. , Marin, M. G. , Cima, F. & Ballarin, L. (2008). First evidence of cell division in circulating haemocytes from the Manila clam Tapes philippinarum . Cell Biology International 32, 865–868. [DOI] [PubMed] [Google Scholar]
- Matsumoto, J. , Nakamoto, C. , Fujiwara, S. , Yubisui, T. & Kawamura, K. (2001). A novel C‐type lectin regulating cell growth, cell adhesion and cell differentiation of the multipotent epithelium in budding tunicates. Development 128, 3339–3347. [DOI] [PubMed] [Google Scholar]
- Matsumoto, Y. , Piraino, S. & Miglietta, M. P. (2019). Transcriptome characterization of reverse development in Turritopsis dohrnii (Hydrozoa, Cnidaria). G3: Genes, Genomes, Genetics 9, 4127–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo, R. , Kobayashi, S. , Tanaka, Y. & Ito, E. (2010). Effects of tentacle amputation and regeneration on the morphology and activity of the olfactory center of the terrestrial slug Limax valentianus . Journal of Experimental Zoology 213, 3144–3149. [DOI] [PubMed] [Google Scholar]
- McAvoy, J. W. & Dixon, K. E. (1977). Cell proliferation and renewal in the small intestinal epithelium of metamorphosing and adult Xenopus laevis . Journal of Experimental Zoology 202, 129–138. [Google Scholar]
- McGovern, T. M. (2002). Patterns of sexual and asexual reproduction in the brittle star Ophiactis savignyiin the Florida Keys. Marine Ecology Progress Series 230, 119–126. [Google Scholar]
- * McMurray, S. E. , Blum, J. E. & Pawlik, J. R. (2008). Redwood of the reef: growth and age of the giant barrel sponge Xestospongia muta in the Florida Keys. Marine Biology 155, 159–171. [Google Scholar]
- Merkel, J. , Wanninger, A. & Lieb, B. (2018). Novel and conserved features of the Hox cluster of Entoprocta (Kamptozoa) . Journal of Phylogenetics & Evolutionary Biology 6, e1. [Google Scholar]
- * Merrell, A. J. & Stanger, B. Z. (2016). Adult cell plasticity in vivo: de‐differentiation and transdifferentiation are back in style. Nature Reviews Molecular Cell Biology 17, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. (1865). Über die Reproduktionskraft der Lucernarien. Amtl Ber 40 Verslag Deutsch Naturf Ärzte. Hannover 1865, e217. [Google Scholar]
- Millane, R. C. , Kanska, J. , Duffy, D. J. , Seoighe, C. , Cunningham, S. , Plickert, G. & Frank, U. (2011). Induced stem cell neoplasia in a cnidarian by ectopic expression of a POU domain transcription factor. Development 138, 2429–2439. [DOI] [PubMed] [Google Scholar]
- Miller, C. M. & Newmark, P. A. (2012). An insulin‐like peptide regulates size and adult stem cells in planarians. International Journal of Developmental Biology 56, 75–82. [DOI] [PubMed] [Google Scholar]
- Miranda, L. S. , Collins, A. G. & Marques, A. C. (2010). Molecules clarify a cnidarian life cycle ‐ the “hydrozoan” Microhydrula limopsicola is an early life stage of the staurozoan Haliclystus antarcticus . PLoS One 5(4), e10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda, L. S. , Morandini, A. C. & Marques, A. C. (2012). Do Staurozoa bloom? A review of stauromedusan population biology. Hydrobiologia 690, 57–67. [Google Scholar]
- Miyamoto, N. & Saito, Y. (2010). Morphological characterization of the asexual reproduction in the acorn worm Balanoglossus simodensis . Development Growth & Differentiation 52, 615–627. [DOI] [PubMed] [Google Scholar]
- Mladenov, P. V. , Emson, R. H. , Colpit, L. V. & Wilkie, I. C. (1983). Asexual reproduction in the West Indian brittle star Ophiocomella ophiactoides (H. L. Clark) (Echinodermata: Ophiuroidea). Journal of Experimental Marine Biology and Ecology 72(1), 1–23. [Google Scholar]
- * Mochizuki, K. , Nishimiya‐Fujisawa, C. & Fujisawa, T. (2001). Universal occurrence of the vasa‐related genes among metazoans and their germline expression in Hydra . Development Genes and Evolution 211, 299–308. [DOI] [PubMed] [Google Scholar]
- Mochizuki, K. , Sano, H. , Kobayashi, S. , Nishimiya‐Fujisawa, C. & Fujisawa, T. (2000). Expression and evolutionary conservation of nanos‐related genes in Hydra . Development Genes and Evolution 210, 591–602. [DOI] [PubMed] [Google Scholar]
- Moffett, S. & Austin, D. R. (1982). Generation of new cerebral ganglion neurons in the snail Melampus: an ultrastructural study. Journal of Comparative Neurology 207, 177–182. [DOI] [PubMed] [Google Scholar]
- Moffett, S. B. & Ridgway, R. L. (1988). Structural repair and functional recovery following cerebral ganglion removal in the pulmonate snail Melampus . American Zoologist 28(4), 1109–1122. [Google Scholar]
- Moffett, S. B. (1992). Mating behavior in the pulmonate snail Melampus: can regeneration restore function? Acta Biologica Hungarica 43, 367–374. [PubMed] [Google Scholar]
- Moffett, S. B. (1996). Nervous System Regeneration in the Invertebrates. Springer, New York. [Google Scholar]
- Monge‐Nájera, J. (1994). Ecological biogeography in the phylum Onycophora. Biogeographica 70, 111–123. [Google Scholar]
- * Moore, N. & Lyle, S. (2011). Quiescent, slow‐cycling stem cell populations in cancer: a review of the evidence and discussion of significance. Journal of Oncology 2011, 396076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraczewski, J. (1977). Asexual reproduction and regeneration of Catenula (Turbellaria, Archoophora). Zoomorphology 88, 65–80. [Google Scholar]
- Moraes, G. , Altran, A. E. , Avilez, I. M. , Barbosa, C. C. & Bidinotto, P. M. (2005). Metabolic adjustments during semi‐aestivation of the marble swamp eel (Synbranchus marmoratus, Bloch 1795)–a facultative air breathing fish. Brazilian Journal of Biology 65, 305–312. [DOI] [PubMed] [Google Scholar]
- * Moris, N. , Pina, C. & Arias, A. M. (2016). Transition states and cell fate decisions in epigenetic landscapes. Nature Reviews Genetics 17, 693–703. [DOI] [PubMed] [Google Scholar]
- * Morrison, S. J. & Spradling, A. C. (2008). Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton, S. , Ustyantsev, K. , Beltman, F. , Glazenburg, L. & Berezikov, E. (2021). Tim29 is required for stem cell activity during regeneration in the flatworm Macrostomum lignano . Scientific Reports 11, e1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton, S. , Wudarski, J. , Grudniewska, M. & Berezikov, E. (2018). The regenerative flatworm Macrostomum lignano, a model organism with high experimental potential. International Journal of Developmental Biology 62, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai, H. & Makioka, T. (1978). Studies on the regeneration of an entoproct, Barentsia discreta . Journal of Experimental Zoology 205(2), 261–275. [Google Scholar]
- Müller, W. A. , Frank, U. , Teo, R. , Mokady, O. , Guette, C. & Plickert, G. (2007). Wnt signaling in hydroid development: ectopic heads and giant buds induced by GSK‐3beta inhibitors. International Journal of Developmental Biology 51, 211–220. [DOI] [PubMed] [Google Scholar]
- * Müller, W. A. , Teo, R. & Frank, U. (2004). Totipotent migratory stem cells in a hydroid. Developmental Biology 275, 215–224. [DOI] [PubMed] [Google Scholar]
- Müller, W. E. G. (2006). The stem cell concept in sponges (Porifera): metazoan traits. Seminars in Cell & Developmental Biology 17, 481–491. [DOI] [PubMed] [Google Scholar]
- Myohara, M. (2012). What role do annelid neoblasts play? A comparison of the regeneration patterns in a neoblast‐bearing and a neoblast‐lacking enchytraeid oligochaete. PLoS One 7(5), e37319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi, N. & Jacobs, D. K. (2019). The early evolution of cellular reprogramming in animals. In Deferring development (eds Bishop C. D. and Hall B. K.), pp. 67–86. CRC press, New York. [Google Scholar]
- Nakano, H. (2015). What is Xenoturbella? Zoological Letters 1, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham, A. E. (1945). Peripheral nerve and regeneration in Crustacea. Journal of Experimental Biology 21, 144–146. [DOI] [PubMed] [Google Scholar]
- Nengwen, X. , Ge, F. & Edwards, C. A. (2011). The regeneration capacity of an earthworm, Eisenia fetida, in relation to the site of amputation along the body. Acta Ecologica Sinica 31, 197–204. [Google Scholar]
- Nentwig, M. R. (1978). Comparative morphological studies of head development after decapitation and after fission in the planarian Dugesia dorotocephala . Transactions of the American Microscopical Society 97, 297–310. [PubMed] [Google Scholar]
- Newmark, P. A. & Sánchez Alvarado, A. (2000). Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Developmental Biology 220, 142–153. [DOI] [PubMed] [Google Scholar]
- Newmark, P. A. & Sanchez Alvarado, A. (2002). Not your father's planarian: a classic model enters the era of functional genomics. Nature Reviews Genetics 3, 210–319. [DOI] [PubMed] [Google Scholar]
- Nimeth, K. , Ladurner, P. , Gschwentner, R. , Salvenmoser, W. & Rieger, R. (2002). Cell renewal and apoptosis in Macrostomum sp. [Lignano]. Cell Biology International 26(9), 801–815. [DOI] [PubMed] [Google Scholar]
- Nimeth, K. T. , Mahlknecht, M. , Mezzanato, A. , Peter, R. , Rieger, R. & Ladurner, P. (2004). Stem cell dynamics during growth, feeding, and starvation in the basal flatworm Macrostomum sp. (Platyhelminthes). Developmental Dynamics 230, 91–99. [DOI] [PubMed] [Google Scholar]
- * Noble, D. (2015). Conrad Waddington and the origin of epigenetics. Journal of Experimental Biology 218, 816–818. [DOI] [PubMed] [Google Scholar]
- Nobuyasu, M. , Suetsugu‐Maki, R. , Agata, K. , Rio‐Tsonis, K. & Tsonis, P. (2009). Expression of stem cell pluripotency factors during regeneration in newts. Developmental Dynamics 238, 1613–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez, J. D. , Ocampo, E. H. , Chiaradia, N. M. , Morsan, E. & Cledon, M. (2013). The effect of temperature on the inhalant siphon regeneration of Amiantis purpurata (Lamarck, 1818) (Bivalvia; Veneridae). Marine Biology Research 9, 189–197. [Google Scholar]
- O'Dea, A. (2006). Asexual propagation in the marine bryozoan Cupuladria exfragminis . Journal of Experimental Marine Biology and Ecology 335(2), 312–322. [Google Scholar]
- Ohtaka, A. (2018). Aquatic oligochaete fauna (Annelida, Clitellata) in Lake Tonle Sap and adjacent waters in Cambodia. Limnology 19, 367–373. [Google Scholar]
- Oka, H. & Watanabe, H. (1957). Vascular budding, a new type of budding in Botryllus . Biological Bulletin 112, 225–240. [Google Scholar]
- Okamoto, K. , Nakatsukasa, M. , Alié, A. , Masuda, Y. , Agata, K. & Funayama, N. (2012). The active stem cell specific expression of sponge Musashi homolog EflMsiA suggests its involvement in maintaining the stem cell state. Mechanisms of Development 129, 24–37. [DOI] [PubMed] [Google Scholar]
- * Önal, P. , Grën, D. , Adamidi, C. , Rybak, A. , Solana, J. , Mastobuoni, G. , Wang, Y. , Rahn, H.‐P. , Chen, W. , Kempa, S. , Ziebold, U. & Rajewsky, N. (2012). Gene expression of pluripotency determinants is conserved between mammalian and planarian stem cells. EMBO Journal 31, 2755–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, J. L. , Chng, Y. R. , Ching, B. , Chen, X. L. , Hiong, K. C. , Wong, W. P. , Chew, S. F. & Ip, Y. K. (2017). Molecular characterization of myostatin from the skeletal muscle of the African lungfish, Protopterus annectens, and changes in its mRNA and protein expression levels during three phases of aestivation. Journal of Comparative Physiology B 187, 575–589. [DOI] [PubMed] [Google Scholar]
- Orii, H. , Sakurai, T. & Watanabe, K. (2005). Distribution of the stem cells (neoblasts) in the planarian Dugesia japonica . Development Genes and Evolution 215, 143–157. [DOI] [PubMed] [Google Scholar]
- Otto, J. J. & Campbell, R. D. (1977). Tissue economics of Hydra: regulation of cell cycle, animal size and development by controlled feeding rates. Journal of Cell Science 28, 117–132. [DOI] [PubMed] [Google Scholar]
- Özpolat, B. D. & Bely, A. E. (2015). Gonad establishment during asexual reproduction in the annelid Pristina leidyi . Developmental Biology 405, 123–136. [DOI] [PubMed] [Google Scholar]
- Özpolat, B. D. & Bely, A. E. (2016). Developmental and molecular biology of annelid regeneration: a comparative review of recent studies. Current Opinion in Genetics and Development 40, 144–153. [DOI] [PubMed] [Google Scholar]
- Özpolat, B. D. , Sloane, E. S. , Zattara, E. E. & Bely, A. E. (2016). Plasticity and regeneration of gonads in the annelid Pristina leidyi . EvoDevo 7, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard, A. (1968). Asexual reproduction in Balanoglossus (Stomochordata). Proceedings of the Royal Society B: Biological Sciences 171(1023), 261–272. [Google Scholar]
- Padilla, P. A. & Ladage, M. L. (2012). Suspended animation, diapause and quiescence. Cell Cycle 11, 1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua, A. , Leocorny, P. , Custódio, M. R. & Klautau, M. J. (2016). Fragmentation, fusion, and genetic homogeneity in a calcareous sponge (Porifera, Calcarea). Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 325, 294–303. [DOI] [PubMed] [Google Scholar]
- Palakodeti, D. , Smielewska, M. , Lu, Y. C. , Yeo, G. W. & Graveley, B. R. (2008). The PIWI proteins SMEDWI‐2 and SMEDWI‐3 are required for stem cell function and piRNA expression in planarians. RNA 14, 1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmberg, I. (1986). Cell migration and differentiation during wound healing and regeneration in Microstomum lineare (Turbellaria). Hydrobiologia 132, 181–188. [Google Scholar]
- Palmberg, I. (1990). Stem cells in microturbellarians. Protoplasma 158, 109–120. [Google Scholar]
- Paraskevopoulou, S. , Dennis, A. B. , Weithoff, G. , Hartmann, S. & Tiedemann, R. (2019). Within species expressed genetic variability and gene expression response to different temperatures in the rotifer Brachionus calyciflorus sensu stricto. PLoS One 14(9), e0223134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus, T. & Müller, M. C. M. (2006). Cell proliferation dynamics and morphological differentiation during regeneration in Dorvillea bermudensis (Polychaeta, Dorvilleidae). Journal of Morphology 267, 393–403. [DOI] [PubMed] [Google Scholar]
- Pearse, V. B. (1999). Placozoa. In Encyclopedia of Reproduction (Volume 3, eds Knobil E. and Neill J. D.), pp. 898–901. Academic Press, San Diego. [Google Scholar]
- Peiris, T. H. , Weckerle, F. , Ozamoto, E. , Ramirez, D. , Davidian, D. , García‐Ojeda, M. E. & Oviedo, N. J. (2012). TOR signaling regulates planarian stem cells and controls localized and organismal growth. Journal of Cell Science 125, 1657–1665. [DOI] [PubMed] [Google Scholar]
- * Pellettieri, J. & Sanchez Alvarado, A. (2007). Cell turnover and adult tissue homeostasis: from humans to planarians. Annual Review of Genetics 41, 83–105. [DOI] [PubMed] [Google Scholar]
- Perea‐Atienza, E. , Botta, M. , Salvenmoser, W. , Gschwentner, R. , Egger, B. , Kristof, A. , Martinez, P. & Achatz, J. G. (2013). Posterior regeneration in Isodiametra pulchra (Acoela, Acoelomorpha). Frontiers in Zoology 10, e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez, Y. , Rieger, V. , Martin, E. , Müller, C. H. G. & Harzsch, S. (2013). Neurogenesis in an early protostome relative: progenitor cells in the ventral nerve center of chaetognath hatchlings are arranged in a highly organized geometrical pattern. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 320, 179–193. [DOI] [PubMed] [Google Scholar]
- Persinina, M. & Chaga, O. (1995). Renewal and differentiation of coelomic fluid cells in polychaeta Arenicola marina. III. Autoradiographic analysis. Cytology (Russ) 37, 101–105. [Google Scholar]
- * Pesaresi, M. , Sebastian‐Perez, R. & Cosma, M. P. (2019). Dedifferentiation, transdifferentiation and cell fusion: in vivo reprogramming strategies for regenerative medicine. FEBS Journal 286, 1074–1093. [DOI] [PubMed] [Google Scholar]
- Peter, R. , Ladurner, P. & Rieger, R. M. (2001). The role of stem cell strategies in coping with environmental stress and choosing between alternative reproductive modes: Turbellaria rely on a single cell type to maintain individual life and propagate species. Marine Ecology 22(1‐2), 35–51. [Google Scholar]
- Petersen, H. O. , Hoger, S. K. , Looso, M. , Lengfeld, T. , Kuhn, A. , Warnken, U. , Nishimiya‐Fujisawa, C. , Schnolzer, M. , Kruger, M. , Ozbek, S. , Simakov, O. & Holstein, T. W. (2015). A comprehensive transcriptomic and proteomic analysis of Hydra head regeneration. Molecular Biology and Evolution 32(8), 1928–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, J. A. & Ditadi, A. S. F. (1971). Asexual reproduction in Glossobalanus crozieri (Ptychoderidae, Enteropneusta, Hemichordata). Marine Biology 9(1), 78–85. [Google Scholar]
- Pfeifer, K. , Dorresteijn, A. W. C. & Fröbius, A. C. (2012). Activation of Hox genes during caudal regeneration of the polychaete annelid Platynereis dumerilii . Development Genes and Evolution 222, 165–179. [DOI] [PubMed] [Google Scholar]
- * Pfister, D. , De Mulder, K. , Hartenstein, V. , Kuales, G. , Borgonie, G. , Marx, F. , Morris, J. & Ladurner, P. (2008). Flatworm stem cells and the germ line: developmental and evolutionary implications of macvasa expression in Macrostomum lignano . Developmental Biology 319, 146–159. [DOI] [PubMed] [Google Scholar]
- Pfister, D. , De Mulder, K. , Philipp, I. , Kuales, G. , Hrouda, M. , Eichberger, P. , Borgonie, G. , Hartenstein, V. & Ladurner, P. (2007). The exceptional stem cell system of Macrostomum lignano: screening for gene expression and studying cell proliferation by hydroxyurea treatment and irradiation. Frontiers in Zoology 4, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp, E. E. , Wessels, W. , Gruber, H. , Strahl, J. , Wagner, A. E. , Ernst, I. M. , Rimbach, G. , Kraemer, L. , Schreiber, S. , Abele, D. & Rosenstiel, P. (2012). Gene expression and physiological changes of different populations of the long‐lived bivalve Arctica islandica under low oxygen conditions. PLoS One 7(9), e44621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pila, E. A. , Sullivan, J. T. , Wu, X. Z. , Fang, J. , Rudko, S. P. , Gordy, M. A. & Hanington, P. C. (2016). Haematopoiesis in molluscs: a review of haemocyte development and function in gastropods, cephalopods and bivalves. Developmental & Comparative Immunology 58, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piraino, S. , Boero, F. , Aeschbach, B. & Schmid, V. (1996). Reversing the life cycle: Medusae transforming into polyps and cell transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa). Biological Bulletin 190, 302–312. [DOI] [PubMed] [Google Scholar]
- Piraino, S. , De Vito, D. , Schmich, J. , Bouillon, J. & Boero, F. (2004). Reverse development in Cnidaria. Canadian Journal of Zoology 82, 1748–1754. [Google Scholar]
- Pirger, Z. , Lubics, A. , Reglodi, D. , Laszlo, Z. , Mark, L. & Kiss, T. (2010). Mass spectrometric analysis of activity‐dependent changes of neuropeptide profile in the snail, Helix pomatia . Neuropeptides 44, 475–483. [DOI] [PubMed] [Google Scholar]
- Planques, A. , Malem, J. , Parapar, J. , Vervoort, M. & Gazave, E. (2019). Morphological, cellular and molecular characterization of posterior regeneration in the marine annelid Platynereis dumerilii . Developmental Biology 445, 189–210. [DOI] [PubMed] [Google Scholar]
- * Plass, M. , Solana, J. , Wolf, F. A. , Ayoub, S. , Misios, A. , Glazar, P. , Obermayer, B. , Theis, F. J. , Kocks, C. & Rajewsky, N. (2018). Cell type atlas lineage tree of a whole complex animal by single‐cell transcriptomics. Science 360, eaaq1723. [DOI] [PubMed] [Google Scholar]
- * Plickert, G. , Frank, U. & Müller, W. A. (2012). Hydractinia, a pioneering model for stem cell biology and reprogramming somatic cells to pluripotency. International Journal of Developmental Biology 56, 519–534. [DOI] [PubMed] [Google Scholar]
- * Posfai, E. , Schell, J. P. , Janiszewski, A. , Rovic, I. , Murray, A. , Bradshaw, B. , Yamakawa, T. , Pardon, T. , El Bakkali, M. , Talon, I. , De Geest, N. , Kumar, P. , To, S. K. , Petropoulos, S. , Jurisicova, A. , et al. (2021). Evaluating totipotency using criteria of increasing stringency. Nature Cell Biology 23, 49–60. [DOI] [PubMed] [Google Scholar]
- * Post, Y. & Clevers, H. (2019). Defining adult stem cell function at its simplest: the ability to replace lost cells through mitosis. Cell Stem Cell 25, 174–183. [DOI] [PubMed] [Google Scholar]
- * Poulsom, R. , Alison, M. R. , Forbes, S. J. & Wright, N. A. (2002). Adult stem cell plasticity. The Journal of Pathology 197, 441–456. [DOI] [PubMed] [Google Scholar]
- Presnell, J. S. & Browne, W. E. (2019). Krüppel‐like factor gene function in the ctenophore Mnemiopsis suggests an ancient role in promoting cell proliferation in metazoan stem cell niches. BioRxiv, 527002. 10.1101/527002. [DOI] [Google Scholar]
- * Qarri, A. , Rosner, A. , Rabinowitz, C. & Rinkevich, B. (2020). UV‐B radiation bearings on ephemeral soma in the shallow water tunicate Botryllus schlosseri . Ecotoxicology and Environmental Safety 196, e110489. [DOI] [PubMed] [Google Scholar]
- * Rabinowitz, C. , Alphasi, G. & Rinkevich, B. (2009). Further portrayal of epithelial monolayers, emergent de novo from extirpated ascidians' palleal buds. In Vitro Cellular & Developmental Biology – Animal 45, 334–342. [DOI] [PubMed] [Google Scholar]
- * Rabinowitz, C. & Rinkevich, B. (2011). De novo emerged stemness signatures in epithelial monolayers developed from extirpated palleal buds. In Vitro Cellular & Developmental Biology – Animal 47, 26–31. [DOI] [PubMed] [Google Scholar]
- * Radley, J. M. , Ellis, S. , Palatsides, M. , Williams, B. & Bertoncello, I. (1999). Ultrastructure of primitive hematopoietic stem cells isolated using probes of functional status. Experimental Hematology 27, 365–369. [DOI] [PubMed] [Google Scholar]
- * Raff, M. (2003). Adult stem cell plasticity: fact or artifact? Annual Review of Cell and Developmental Biology 19, 1–22. [DOI] [PubMed] [Google Scholar]
- * Rajagopal, J. & Stanger, B. Z. (2016). Plasticity in the adult: how should the Waddington diagram be applied to regenerating tissues? Developmental Cell 36, 133–137. [DOI] [PubMed] [Google Scholar]
- Rajasethupathy, P. , Antonov, I. , Sheridan, R. , Frey, S. , Sander, C. , Tuschl, T. & Kandel, E. R. (2012). A role for neuronal piRNAs in the epigenetic control of memory‐related synaptic plasticity. Cell 149, 693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajulu, G. & Krishnan, N. (1969). Occurrence of asexual reproduction by budding in sipunculida. Nature 223, 186–187. [Google Scholar]
- Ramon‐Mateu, J. , Tori Ellison, S. , Angelini, T. E. & Martindale, M. Q. (2019). Regeneration in the ctenophore Mnemiopsis leidyi occurs in the absence of blastema, requires cell division, and is temporally separable from wound healing. BMC Biology 17, e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Raz, E. (2000). The function and regulation of vasa‐like genes in germ‐cell development. Genome Biology 1, reviews1017‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi, L. , Altiero, T. & Guidetti, R. (2007). Anhydrobiosis: the extreme limit of dessication tolerance. Invertebrate Survival Journal 4, 65–81. [Google Scholar]
- * Rebscher, N. , Volk, C. , Teo, R. & Plickert, G. (2008). The germ plasm component Vasa allows tracing of the interstitial stem cells in the cnidarian Hydractinia echinata . Developmental Dynamics 237, 1736–1745. [DOI] [PubMed] [Google Scholar]
- * Rebscher, N. , Zelada‐González, F. , Banisch, T. U. , Raible, F. & Arendt, D. (2007). Vasa unveils a common origin of germ cells and of somatic stem cells from the posterior growth zone in the polychaete Platynereis dumerilii . Developmental Biology 306, 599–611. [DOI] [PubMed] [Google Scholar]
- Reddien, P. W. (2018). The cellular and molecular basis for planarian regeneration. Cell 175, 327–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien, P. W. , Oviedo, N. J. , Jennings, J. R. , Jenkin, J. C. & Sánchez Alvarado, A. (2005). SMEDWI‐2 is a PIWI‐like protein that regulates planarian stem cells. Science 310, 1327–1330. [DOI] [PubMed] [Google Scholar]
- Reddien, P. W. & Sanchez Alvarado, A. (2004). Fundamentals of planarian regeneration. Annual Review of Cell and Developmental Biology 20, 725–757. [DOI] [PubMed] [Google Scholar]
- Reilly, B. D. , Schlipalius, D. I. , Cramp, R. L. , Ebert, P. R. & Franklin, C. E. (2013). Frogs and estivation: transcriptional insights into metabolism and cell survival in a natural model of extended muscle disuse. Physiological Genomics 45, 377–388. [DOI] [PubMed] [Google Scholar]
- Reinardy, H. C. , Emerson, C. E. , Manley, J. M. & Bodnar, A. G. (2015). Tissue regeneration and biomineralization in sea urchins: role of Notch signaling and presence of stem cell markers. PLoS One 10(8), e0133860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzel, A. M. , Burton, P. M. , Krone, C. & Finnerty, J. R. (2007). Comparison of developmental trajectories in the starlet sea anemone Nematostella vectensis: embryogenesis, regeneration, and two forms of asexual fission. Invertebrate Biology 126, 99–112. [Google Scholar]
- Renfree, M. B. & Fenelon, J. C. (2017). The enigma of embryonic diapause. Development 144, 3199–3210. [DOI] [PubMed] [Google Scholar]
- Rentzsch, F. , Layden, M. & Manuel, M. (2017). The cellular and molecular basis of cnidarian neurogenesis. Wiley Interdisciplinary Reviews: Developmental Biology 6, e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch, A. M. , Palakodeti, D. , Lu, Y. C. , Horowitz, M. & Graveley, B. R. (2012). Transcriptome analysis reveals strain‐specific and conserved stemness genes in Schmidtea mediterranea . PLoS One 7, e34447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, M. & Kreshchenko, N. (2004). Flatworm asexual multiplication implicates stem cells and regeneration. Canadian Journal of Zoology 82, 334–356. [Google Scholar]
- * Reyes‐Bermudez, A. , Hidaka, M. & Mikheyev, A. (2021). Transcription profiling of cultured Acropora digitifera adult cells reveals the existence of ancestral genome regulatory modules underlying pluripotency and cell differentiation in cnidaria. Genome Biology and Evolution 13, evab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, R. P. , Bleidorn, C. & Aguado, M. T. (2018a). Regeneration mechanisms in Syllidae (Annelida). Regeneration 5(1), 26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, R. P. , Ponz‐Segrelles, G. , Bleidorn, C. & Aguado, M. T. (2018b). Comparative transcriptomics in Syllidae (Annelida) indicates that posterior regeneration and regular growth are comparable, while anterior regeneration is a distinctive process. BMC Genomics 20, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci, L. , Chaurasia, A. , Lapébie, P. , Dru, P. , Helm, R. R. , Copley, R. R. & Tiozzo, S. (2016). Identification of differentially expressed genes from multipotent epithelia at the onset of an asexual development. Scientific Reports 6, 27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rinkevich, B. (2000). A critical approach to the definition of Darwinian units of selection. The Biological Bulletin 199, 231–240. [DOI] [PubMed] [Google Scholar]
- * Rinkevich, B. (2009). Stem cells: autonomy interactors that emerge as causal agents and legitimate units of selection. In Stem Cells in Marine Organisms (eds Rinkevich B. and Matranga V.), pp. 1–20. Springer, Dordrecht, Heidelberg, London, New York. [Google Scholar]
- * Rinkevich, B. (2011). Quo vadis chimerism? Chimerism 2, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rinkevich, B. (2017). Senescence in modular animals‐ botryllid ascidians as a unique aging system. In The Evolution of Senescence in the Tree of Life (eds Salguero‐Gomez R., Shefferson R. and Jones O.), pp. 220–237. Cambridge: Cambridge University Press. [Google Scholar]
- * Rinkevich, B. & Loya, Y. (1986). Senescence and dying signals in a reef building coral. Experientia 42, 320–322. [Google Scholar]
- * Rinkevich, B. & Matranga, V. (2009). Stem Cells in Marine Organisms. London: Springer. 369 pp. [Google Scholar]
- * Rinkevich, B. & Rinkevich, Y. (2013). The “stars and stripes” metaphor for animal regeneration– elucidating two fundamental strategies along a continuum. Cells 2, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rinkevich, B. , Shlemberg, Z. & Fishelson, L. (1995). Whole body protochordate regeneration from totipotent blood cells. Proceedings of the National Academy of Sciences of the United States of America 92, 7695–7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rinkevich, B. & Yankelevich, I. (2004). Environmental split between germ cell parasitism and somatic cell synergism in chimeras of a colonial urochordate. The Journal of Experimental Biology 207, 3531–3536. [DOI] [PubMed] [Google Scholar]
- * Rinkevich, Y. , Lindau, P. , Ueno, H. , Longaker, M. T. & Weissman, I. L. (2011). Germ‐layer and lineage‐restricted stem/progenitors regenerate the mouse digit tip. Nature 476, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rinkevich, Y. , Matranga, V. & Rinkevich, B. (2009). Stem cells in aquatic invertebrates: common premises and emerging unique themes. In Stem Cells in Marine Organisms (eds Rinkevich B. and Matranga V.), pp. 60–103. Springer, Dordrecht, Heidelberg, London, New York. [Google Scholar]
- * Rinkevich, Y. , Paz, G. , Rinkevich, B. & Reshef, R. (2007). Systemic bud induction and retinoic acid signaling underlie whole body regeneration in urochordate Botrylloides leachi . PLoS Biology 5, 900–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich, Y. , Rinkevich, B. & Reshef, R. (2008). Cell signaling and transcription factor genes expressed during whole body regeneration in a colonial chordate. BMC Developmental Biology 8, e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rinkevich, Y. , Rosner, A. , Rabinowitz, C. , Lapidot, Z. , Moiseeva, E. & Rinkevich, B. (2010). Piwi positive cells that line the vasculature epithelium, underlie whole body regeneration in a basal chordate. Developmental Biology 345, 94–104. [DOI] [PubMed] [Google Scholar]
- * Rinkevich, Y. , Voskoboynik, A. , Rosner, A. , Rabinowitz, C. , Oren, M. , Paz, G. , Alfasi, G. , Moiseeva, E. , Ishizuka, K. J. , Palmeri, K. J. , Weissman, I. L. & Rinkevich, B. (2013). Repeated, long‐term cycling of putative stem cells between niches in a basal chordate. Developmental Cell 24, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Robert, J. (2010). Comparative study of tumorigenesis and tumor immunity in invertebrates and nonmammalian vertebrates. Developmental & Comparative Immunology 34, 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogick, M. D. (1941). The resistance of fresh‐water Bryozoa to desiccation. Biodynamica 3, 369–378. [Google Scholar]
- Roncalli, V. , Sommer, S. A. , Cieslak, M. C. , Clarke, C. , Hopcroft, R. R. & Lenz, P. H. (2018). Physiological characterization of the emergence from diapause: a transcriptomics approach. Scientific Reports 8, e12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rosental, B. , Kowarsky, M. , Seita, J. , Corey, D. M. , Ishizuka, K. J. , Palmeri, K. J. , Chen, S. Y. , Sinha, R. , Okamoto, J. , Mantalas, G. & Manni, L. (2018). Complex mammalian‐like haematopoietic system found in a colonial chordate. Nature 564, 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosental, B. , Raveh, T. , Voskoboynik, A. & Weissman, I. L. (2020). Evolutionary perspective on the hematopoietic system through a colonial chordate: allogeneic immunity and hematopoiesis. Current Opinion in Immunology 62, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner, A. , Alfassi, G. , Moiseeva, E. , Paz, G. , Rabinowitz, C. , Lapidot, Z. , Douek, J. , Haim, A. & Rinkevich, B. (2014). The involvement of three signal transduction pathways in botryllid ascidian astogeny, as revealed by expression patterns of representative genes. International Journal of Developmental Biology 58, 677–692. [DOI] [PubMed] [Google Scholar]
- * Rosner, A. , Armengaud, J. , Ballarin, L. , Barnay‐Verdier, S. , Cima, F. , Coelho, A. V. , Domart‐Coulon, I. , Drobne, D. , Genevière, A.‐M. , Jemec, K. A. , Kotlarska, E. , Lyons, D. M. , Mass, T. , Paz, G. , Pazdro, K. , et al. (2021). Stem cells of aquatic invertebrates as an advanced tool for assessing ecotoxicological impacts. Science of the Total Environment 771, e144565. [DOI] [PubMed] [Google Scholar]
- * Rosner, A. , Kravchenko, O. & Rinkevich, B. (2019). IAP genes partake weighty roles in the astogeny and whole body regeneration in the colonial urochordate Botryllus schlosseri . Developmental Biology 448, 320–341. [DOI] [PubMed] [Google Scholar]
- * Rosner, A. , Moiseeva, E. , Rabinowitz, C. & Rinkevich, B. (2013). Germ lineage properties in the urochordate Botryllus schlosseri – from markers to temporal niches. Developmental Biology 384, 356–374. [DOI] [PubMed] [Google Scholar]
- * Rosner, A. , Moiseeva, E. , Rinkevich, Y. , Lapidot, Z. & Rinkevich, B. (2009). Vasa and the germ line lineage in colonial urochordate. Developmental Biology 331, 113–128. [DOI] [PubMed] [Google Scholar]
- Rosner, A. , Paz, G. & Rinkevich, B. (2006). Divergent roles of the DEAD‐box protein BS‐PL10, the urochordate homologue of human DDX3 and DDX3Y proteins, in colony astogeny and ontogeny. Developmental Dynamics 235, 1508–1521. [DOI] [PubMed] [Google Scholar]
- Rosner, A. , Rabinowitz, C. , Moiseeva, E. , Voskoboynik, A. & Rinkevich, B. (2007). BS‐cadherin in the colonial urochordate Botryllus schlosseri: one protein, many functions. Developmental Biology 304, 687–700. [DOI] [PubMed] [Google Scholar]
- * Rosner, A. & Rinkevich, B. (2011). VASA as a specific marker for germ cells lineage: in light of evolution. Trends in Comparative Biochemistry & Physiology 15, 1–15. [Google Scholar]
- * Rossi, L. & Salvetti, A. (2019). Planarian stem cell niche, the challenge for understanding tissue regeneration. Seminars in Cell & Developmental Biology 87, 30–36. [DOI] [PubMed] [Google Scholar]
- * Rossi, L. , Salvetti, A. , Batistoni, R. , Deri, P. & Gremigni, V. (2008). Planarians, a tale of stem cells. Cellular and Molecular Life Sciences 65, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, L. , Salvetti, A. , Lena, A. , Batistoni, R. , Deri, P. , Pugliesi, C. , Loreti, E. & Gremigni, V. (2006). DjPiwi‐1, a member of the PAZ‐Piwi gene family, defines a subpopulation of planarian stem cells. Development Genes and Evolution 216, 335–346. [DOI] [PubMed] [Google Scholar]
- Rossi, L. , Salvetti, A. , Marincola, F. M. , Lena, A. , Deri, P. , Mannini, L. , Batistoni, R. , Wang, E. & Gremigni, V. (2007). Deciphering the molecular machinery of stem cells: a look at the neoblast gene expression profile. Genome Biology 8, R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhana, L. , Shibata, N. , Nishimura, O. & Agata, K. (2010). Different requirements for conserved post‐transcriptional regulators in planarian regeneration and stem cell maintenance. Developmental Biology 341, 429–443. [DOI] [PubMed] [Google Scholar]
- Rubilar, T. , Pastor de Ward, C. T. & Diaz de Vivar, M. (2005). Sexual and asexual reproduction of Allostichaster capensis (Echinodermata: Asteroidea) in Golfo Nuevo. Marine Biology 146, 1083–1090. [Google Scholar]
- Rubliar, T. , Merett, P. E. & Cledon, M. (2015). Regeneration rate after fission in the fissiparous sea star Allostichaster capensis (Asteroidea). Revista de Biología Tropical 63(2), 321–328. [Google Scholar]
- * Rumman, M. , Dhawan, J. & Kassem, M. (2015). Concise review: quiescence in adult stem cells: biological significance and relevance to tissue regeneration. Stem Cells 33, 2903–2912. [DOI] [PubMed] [Google Scholar]
- Rychel, A. L. & Swalla, B. J. (2008). Anterior regeneration in the hemichordate Ptychodera flava . Developmental Dynamics 237, 3222–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychel, A. & Swalla, B. (2009). Regeneration in hemichordates and echinoderms. In Stem Cells in Marine Organisms (eds Rinkevich B. and Matranga V.), pp. 245–265. Springer, Dordrecht. [Google Scholar]
- * Saez, B. , Yusuf, R. Z. & Scadden, D. T. (2017). Harnessing the biology of stem cells' niche. In Biology and Engineering of Stem Cell Niches (eds Vishwakarms A. and Karp J. F.), pp. 15–31. London, UK: Academic Press. [Google Scholar]
- Sakurai, T. , Lee, H. , Kashima, M. , Saito, Y. , Hayashi, T. , Kudome‐Takamatsu, T. , Nishimura, O. , Agata, K. & Shibata, N. (2012). The planarian P2X homolog in the regulation of asexual reproduction. International Journal of Developmental Biology 56, 173–182. [DOI] [PubMed] [Google Scholar]
- * Saló, E. (2006). The power of regeneration and the stem‐cell kingdom: freshwater planarians (Platyhelminthes). BioEssays 28, 546–559. [DOI] [PubMed] [Google Scholar]
- Salvetti, A. , Rossi, L. , Deri, P. & Batistoni, R. (2000). An MCM2‐related gene is expressed in proliferating cells of intact and regenerating planarians. Developmental Dynamics 218, 603–614. [DOI] [PubMed] [Google Scholar]
- Salvetti, A. , Rossi, L. , Lena, A. , Batistoni, R. , Deri, P. , Rainaldi, G. , Locci, M. T. , Evangelista, M. & Gremigni, V. (2005). DjPum, a homologue of Drosophila Pumilio, is essential to planarian stem cell maintenance. Development 132, 1863–1874. [DOI] [PubMed] [Google Scholar]
- Sammarco, P. W. (1982). Polyp bail‐out: an escape response to environmental stress and a new means of reproduction in corals. Marine Ecology Progress Series 10(1), 57–65. [Google Scholar]
- San Miguel‐Ruiz, J. & García‐Arrarás, J. (2007). Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima . BMC Developmental Biology 7, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sànchez, J. A. , Li, Y. & Kirk, M. D. (2000). Regeneration of cerebral‐buccal interneurons and recovery of ingestion buccal motor programs in Aplysia after CNS lesions. Journal of Neurophysiology 84, 2961–2974. [DOI] [PubMed] [Google Scholar]
- Sandoval‐Guzman, T. , Wang, H. , Khattak, S. , Schuez, M. , Roensch, K. , Nacu, E. , Tazaki, A. , Joven, A. , Tanaka, E. M. & Simon, A. (2014). Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell 14, 174–187. [DOI] [PubMed] [Google Scholar]
- Sato, K. , Shibata, N. , Orii, H. , Amikura, R. , Sakurai, T. , Agata, K. , Kobayashi, S. & Watanabe, K. (2006). Identification and origin of the germline stem cells as revealed by the expression of nanos‐related gene in planarians. Development Growth & Differentiation 48, 615–628. [DOI] [PubMed] [Google Scholar]
- * Scadden, D. T. (2006). The stem‐cell niche as an entity of action. Nature 441, 1075–1079. [DOI] [PubMed] [Google Scholar]
- Scelzo, M. , Alié, A. , Pagnotta, S. , Lejeune, C. , Henry, P. , Gilletta, L. , Hiebert, L. S. , Mastrototaro, F. & Tiozzo, S. (2019). Novel budding mode in Polyandrocarpa zorritensis: a model for comparative studies on asexual development and whole body regeneration. EvoDevo 10, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk, S. , Krauditsch, C. , Frühauf, P. , Gerner, C. & Raible, F. (2016). Discovery of methylfarnesoate as the annelid brain hormone reveals an ancient role of sesquiterpenoids in reproduction. eLife 5, e17126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierwater, B. & Hauenschild, C. (1990). A photoperiod determined life‐cycle in an oligochaete worm. Biological Bulletin 178, 111–117. [DOI] [PubMed] [Google Scholar]
- Schiesari, L. & O'Connor, M. B. (2013). Diapause: delaying the developmental clock in response to a changing environment. Current Topics in Developmental Biology 105, 213–246. [DOI] [PubMed] [Google Scholar]
- * Schmich, J. , Kraus, Y. , De Vito, D. , Graziussi, D. , Boero, F. & Piraino, S. (2007). Induction of reverse development in two marine Hydrozoans. International Journal of Developmental Biology 51, 45–56. [DOI] [PubMed] [Google Scholar]
- * Schmid, V. & Reber‐Muller, S. (1995). Transdifferentiation of isolated striated muscle of jellyfish in vitro: the initiation process. Seminars in Cell Biology 6, 109–116. [DOI] [PubMed] [Google Scholar]
- Schmid, V. , Wydler, M. & Alder, H. (1982). Transdifferentiation and regeneration in vitro. Developmental Biology 92, 476–488. [DOI] [PubMed] [Google Scholar]
- Schmid, V. , Alder, H. , Plickert, G. & Weber, C. (1988). Transdifferentiation from striated muscle of medusae in vitro. Cell Differentiation and Development 25, 137–146. [DOI] [PubMed] [Google Scholar]
- Schmidt, T. & David, C. N. (1986). Gland cells in Hydra: cell cycle kinetics and development. Journal of Cell Science 85, 197–215. [DOI] [PubMed] [Google Scholar]
- Schnitzler, C. , Simmons, D. , Pang, K. , Martindale, M. & Baxevanis, A. (2014). Expression of multiple Sox genes through embryonic development in the ctenophore Mnemiopsis leidyi is spatially restricted to zones of cell proliferation. EvoDevo 5, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Schofield, R. (1978). The relationship between the spleen colony‐forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25. [PubMed] [Google Scholar]
- Schwaha, T. , Handschuh, S. , Redl, E. & Walzl, M. G. (2011). Organogenesis in the budding process of the freshwater bryozoan Cristatella mucedo Cuvier, 1798 (Bryozoa, Phylactolaemata). Journal of Morphology 272, 320–341. [DOI] [PubMed] [Google Scholar]
- Schwaha, T. & Wood, T. S. (2011). Organogenesis during budding and lophophoral morphology of Hislopia malayensis Annandale,1916 (Bryozoa, Ctenostomata). BMC Developmental Biology 11, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimone, M. L. , Meisel, J. & Reddien, P. W. (2010). The Mi‐2‐like Smed‐CHD4 gene is required for stem cell differentiation in the planarian Schmidtea mediterranea . Development 137, 1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scognamiglio, R. , Cabezas‐Wallscheid, N. , Their, M. C. , Altamura, S. , Reyes, A. , Prendergast, Á. M. , Baumgärtner, D. , Carnevalli, L. S. , Atzberger, A. , Haas, S. , von Paleske, L. , Boroviak, T. , Wörsdörfer, P. , Essers, M. A. , Kloz, U. , et al. (2016). Myc depletion induces a pluripotent dormant state mimicking diapause. Cell 164, 668–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secor, S. M. & Lignot, J. H. (2010). Morphological plasticity of vertebrate aestivation. In Aestivation. Progress in Molecular and Subcellular Biology (Volume 49, eds Arturo Navas C. and Carvalho J.), pp. 183–208. Springer, Berlin, Heidelberg. [DOI] [PubMed] [Google Scholar]
- * Seipel, K. , Yanze, N. & Schmid, V. (2004). The germ line and somatic stem cell gene Cniwi in the jellyfish Podocoryne carnea . International Journal of Developmental Biology 48, 1–7. [DOI] [PubMed] [Google Scholar]
- Shafir, S. J. , Van Rijn, J. & Rinkevich, B. (2001). Nubbing of coral colonies: a novel approach for the development of island broodstocks. Aquarium Science and Conservation 3, 183–190. [Google Scholar]
- Sharlaimova, N. , Pinaev, G. & Petukhova, O. (2010). Comparative analysis of behavior and proliferative activity in culture of cells of coelomic fluid and of cells of various tissues of the sea star Asterias rubens L. isolated from normal and injured animals. Cell and Tissue Biology 4, 280–288. [Google Scholar]
- Sharlaimova, N. , Shabelnikov, S. & Petukhova, O. (2014). Small coelomic epithelial cells of the starfish Asterias rubens L. that are able to proliferate in vivo and in vitro. Cell and Tissue Research 356, 83–64. [DOI] [PubMed] [Google Scholar]
- Sharlaimova, N. S. & Petukhova, O. A. (2012). Characteristics of populations of the coelomic fluid and coelomic epithelium cells from the starfish Asterias rubens L. able to attach to and spread on various substrates. Cell and Tissue Biology 6(2), 176–188. [Google Scholar]
- Sharlaimova, N. S. & Petukhova, O. A. (2016). The small cells of coelomic fluid and coelomic epithelium isolated from starfish Asterias rubens and Asterias amurensis (Echinodermata: Asteroidea): comparative analysis of cell morphology and proliferative activity in vivo and in vitro. Russian Journal of Marine Biology 42, 199–203. [Google Scholar]
- Shenkar, N. , Koplovitz, G. , Dray, L. , Gissi, C. & Huchon, D. (2016). Back to solitude: solving the phylogenetic position of the Diazonidae using molecular and developmental characters. Molecular Phylogenetics and Evolution 100, 51–56. [DOI] [PubMed] [Google Scholar]
- Shibata, D. , Hirano, Y. & Komatsu, M. (2011). Life cycle of the multiarmed sea star Coscinasterias acutispina (Stimpson, 1862) in laboratory culture: sexual and asexual reproductive pathways. Zoological Science 28, 313–317. [DOI] [PubMed] [Google Scholar]
- Shibata, N. , Hayashi, T. , Fukumura, R. , Fujii, J. , Kudome‐Takamatsu, T. , Nishimura, O. , Sano, S. , Son, F. , Suzuki, N. , Araki, R. , Abe, M. & Agata, K. (2012). Comprehensive gene expression analyses in pluripotent stem cells of a planarian, Dugesia japonica . International Journal of Developmental Biology 56, 93–102. [DOI] [PubMed] [Google Scholar]
- Shibata, N. , Kashima, M. , Ishiko, T. , Nishimura, O. , Rouhana, L. , Misaki, K. , Yonemura, S. , Saito, K. , Siomi, H. , Siomi, M. C. & Agata, K. (2016). Inheritance of a nuclear PIWI from pluripotent stem cells by somatic descendants ensures differentiation by silencing transposons in planarian. Developmental Cell 7, 226–237. [DOI] [PubMed] [Google Scholar]
- * Shibata, N. , Rouhana, L. & Agata, K. (2010). Cellular and molecular dissection of pluripotent adult somatic stem cells in planarians. Development, Growth & Differentiation 52, 27–41. [DOI] [PubMed] [Google Scholar]
- Shibata, N. , Umesono, Y. , Orii, H. , Sakurai, T. , Watanabe, K. & Agata, K. (1999). Expression of vasa(vas)‐related genes in germline cells and totipotent somatic stem cells of planarians. Developmental Biology 206, 73–87. [DOI] [PubMed] [Google Scholar]
- Shinji, J. , Miyanishi, H. , Gotoh, H. & Kaneko, T. (2016). Appendage regeneration after autotomy is mediated by baboon in the crayfish Procambarus Fallax F. Virginalis Martin, Dorn, Kawai, Heiden and Scholtz, (2010) (Decapoda: Astacoidea: Cambaridae). Journal of Crustacean Biology 36(5), 649–657. [Google Scholar]
- * Shukalyuk, A. I. , Golovnina, K. A. , Baiborodin, S. I. , Gunbin, K. V. , Blinov, A. G. & Isaeva, V. V. (2007). Vasa‐related genes and their expression in stem cells of colonial parasitic rhizocephalan barnacle Polyascus polygenea (Arthropoda: Crustacea: Cirripedia: Rhizocephala). Cell Biology International 31, 97–108. [DOI] [PubMed] [Google Scholar]
- * Shukayuk, A. L. & Isaeva, W. (2012). Molecular and sub‐cellular gametogenic machinery of stem and germline cells across Metazoa. In Current Frontiers and Perspectives in Cell Biology (ed. Najman S.), pp. 279–314. Rijeka, Croatia: IntechOpen. [Google Scholar]
- Siebert, S. , Anton‐Erxleben, F. & Bosch, T. C. G. (2008). Cell type complexity in the basal metazoan Hydra is maintained by both stem cell based mechanisms and transdifferentiation. Developmental Biology 313, 13–24. [DOI] [PubMed] [Google Scholar]
- * Siebert, S. , Farrell, J. A. , Cazet, J. , Abeykoon, Y. , Primack, A. S. , Schnitzler, C. & Juliano, C. E. (2019). Stem cell differentiation trajectories in Hydra resolved at single‐cell resolution. Science 365, eaav9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert, S. , Goetz, F. E. , Church, S. H. , Bhattacharyya, P. , Zapata, F. , Haddock, S. H. & Dunn, C. W. (2015). Stem cells in Nanomia bijuga (Siphonophora), a colonial animal with localized growth zones. EvoDevo 6, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Sieweke, M. H. (2015). Waddington's valleys and Captain Cook's islands. Cell Stem Cell 16, 7–8. [DOI] [PubMed] [Google Scholar]
- Sikes, J. M. & Bely, A. E. (2010). Making heads from tails: development of a reversed anterior–posterior axis during budding in an acoel. Developmental Biology 338, 86–97. [DOI] [PubMed] [Google Scholar]
- Silva, J. R. , Mendes, E. G. & Mariano, M. (1995). Wound repair in the Amphioxus (Branchiostoma platae), an animal deprived of inflammatory phagocytes. Journal of Invertebrate Pathology 65, 147–151. [DOI] [PubMed] [Google Scholar]
- Silva, J. R. M. C. , Mendes, E. G. & Mariano, M. (1998). Regeneration in the amphioxus (Branchiostoma platae). Zoologischer Anzeiger 237(2), 107–112. [Google Scholar]
- * Simpson, T. L. (1984). The Cell Biology of Sponges. Springer‐Verlag, New York. [Google Scholar]
- Singh, A. , Pinto, L. , Martin, C. , Rutherford, N. , Ragunathan, A. , Upadhyay, U. , Kapoor, P. , McRae, M. , Siddiqui, A. , Cantelmi, D. & Heyland, A. (2018). Rudiment resorption as a response to starvation during larval development in the sea urchin Strongylocentrotus droebachiensis . Canadian Journal of Zoology 96, 1178–1185. [Google Scholar]
- * Singh, A. , Yadav, C. B. , Tabassum, N. , Bajpeyee, A. K. & Verma, V. (2019). Stem cell niche, dynamic neighbor of stem cells. European Journal of Cell Biology 98, 65–73. [DOI] [PubMed] [Google Scholar]
- Skogh, C. , Garm, A. , Nilsson, D. E. & Ekström, P. (2006). Bilaterally symmetrical rhopalial nervous system of the box jellyfish Tripedalia cystophora . Journal of Morphology 267, 1391–1405. [DOI] [PubMed] [Google Scholar]
- * Slack, J. M. (2018). What is a stem cell? Wiley Interdisciplinary Reviews: Developmental Biology 7, e323. [DOI] [PubMed] [Google Scholar]
- Smirnova, N. P. & Kostyuchenko, R. P. (2007). Cellular sources of paratomy zone in oligochaetes Pristina longiseta (Naididae): cloning and analysis of expression of genes markers of stem and undifferentiated cells. Tsitologiya 49, 794. [Google Scholar]
- Söderhäll, I. , Bangyeekhun, E. , Mayo, S. & Söderhäll, K. (2003). Hemocyte production and maturation in an invertebrate animal; proliferation and gene expression in hematopoietic stem cells of Pacifastacus leniusculus . Developmental & Comparative Immunology 27, 661–672. [DOI] [PubMed] [Google Scholar]
- Sogabe, S. , Hatleberg, W. L. , Kocot, K. M. , Say, T. E. , Stoupin, D. , Roper, K. E. , Fernandez‐Valverde, S. L. , Degnan, S. M. & Degnan, B. M. (2019). Pluripotency and the origin of animal multicellularity. Nature 570, 519–522. [DOI] [PubMed] [Google Scholar]
- * Solana, J. (2013). Closing the circle of germline and stem cells: the primordial stem cell hypothesis. EvoDevo 4, 2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana, J. , Irimia, M. , Ayoub, S. , Rodriguez Orejuela, M. , Zywitza, V. , Jens, M. , Tapial, J. , Ray, D. , Morris, Q. , Hughes, T. R. , Blencowe, B. J. & Rajewsky, N. (2016). Conserved functional antagonism of CELF and MBNL proteins controls stem cell‐specific alternative splicing in planarians. eLife 5, e16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana, J. , Lasko, P. & Romero, R. (2009). Spoltud‐1 is a chromatoid body component required for planarian long‐term stem cell self‐renewal. Developmental Biology 328, 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer, A. M. & Pörtner, H. O. (2004). Mitochondrial function in seasonal acclimatization versus latitudinal adaptation to cold in the lugworm Arenicola marina (L.). Physiological and Biochemical Zoology 77, 174–186. [DOI] [PubMed] [Google Scholar]
- Somorjai, I. M. (2017). Amphioxus regeneration: evolutionary and biomedical implications. International Journal of Developmental Biology 61, 689–696. [DOI] [PubMed] [Google Scholar]
- Somorjai, I. M. , Escrivà, H. & Garcia‐Fernàndez, J. (2012a). Amphioxus makes the cut‐again. Communicative & Integrative Biology 5, 499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somorjai, I. M. , Somorjai, R. L. , Garcia‐Fernàndez, J. & Escrivà, H. (2012b). Vertebrate‐like regeneration in the invertebrate chordate amphioxus. Proceedings of the National Academy of Sciences of the United States of America 109, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Spradling, A. , Drummond‐Barbosa, D. & Kai, T. (2001). Stem cells find their niche. Nature 414, 98–104. [DOI] [PubMed] [Google Scholar]
- Sprecher, S. G. , Bernardo‐Garcia, F. J. , van Giesen, L. , Hartenstein, V. , Reichert, H. , Neves, R. , Bailly, X. , Martinez, P. & Brauchle, M. (2015). Functional brain regeneration in the acoel worm Symsagittifera roscoffensis . Biology Open 4, 1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, M. , Mazza‐Curll, K. L. , van Wolfswinkel, J. C. & Reddien, P. W. (2014). Whole‐body acoel regeneration is controlled by Wnt and Bmp‐Admp signaling. Current Biology 24(10), 1107–1113. [DOI] [PubMed] [Google Scholar]
- Stamatakis, S. A. , Worsaae, K. & Garm, A. (2018). Regeneration of the rhopalium and the rhopalial nervous system in the box jellyfish Tripedalia cystophora . Biological Bulletin 234(1), 22–36. [DOI] [PubMed] [Google Scholar]
- Steele, M. I. (1907). Regeneration in compound eyes of Crustacea. Journal of Experimental Zoology 5, 163–243. [Google Scholar]
- Stockard, C. R. (1908). Studies of tissue growth. I. On experimental study of the rate of regeneration in Cassiopea xamachana . Papers from the Tortugas Laboratory of the Carnegie Institution of Washington 2, 61–102. [Google Scholar]
- Stolyarova, M. & Valkovich, E. (2016). The mechanism of physiological regeneration in the skin and intestinal epithelia of Saccoglossus mereschkowskii (Enteropneusta, Hemichordata). Journal of Evolutionary Biochemistry and Physiology 52, 84–86. [PubMed] [Google Scholar]
- * Stoner, D. S. , Rinkevich, B. & Weissman, I. L. (1999). Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proceedings of the National Academy of Sciences of the United States of America 96, 9148–9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan, S. R. , Chester, E. T. & Robson, B. J. (2015). Freshwater invertebrate life history strategies for surviving desiccation. Springer Science Reviews 3, 57–75. [Google Scholar]
- Sugio, M. , Takeuchi, K. , Kutsuna, J. , Tadokoro, R. , Takahashi, Y. , Yoshida‐Noro, C. & Tochinai, S. (2008). Exploration of embryonic origins of germline stem cells and neoblasts in Enchytraeus japonensis (Oligochaeta, Annelida). Gene Expression Patterns 8, 227–236. [DOI] [PubMed] [Google Scholar]
- Sugio, M. , Yoshida‐Noro, C. , Ozawa, K. & Tochinai, S. (2012). Stem cells in asexual reproduction of Enchytraeus japonensis (Oligochaeta, Annelid): proliferation and migration of neoblasts. Development Growth & Differentiation 54, 439–450. [DOI] [PubMed] [Google Scholar]
- Sugiyama, T. & Fujisawa, T. (1978). Genetic analysis of developmental mechanisms in Hydra . II. Isolation and characterization of an interstitial cell‐deficient strain. Journal of Cell Science 29, 35–52. [DOI] [PubMed] [Google Scholar]
- Sun, L. , Song, Y. , Qu, Y. , Yu, X. & Zhang, W. (2007). Purification and in vitro cultivation of archaeocytes (stem cells) of the marine sponge Hymeniacidon perleve (Demospongiae). Cell and Tissue Research 328, 223–237. [DOI] [PubMed] [Google Scholar]
- Sunanaga, T. , Inubushi, H. & Kawamura, K. (2010). Piwi‐expressing hemoblasts serve as germline stem cells during postembryonic germ cell specification in colonial ascidian, Botryllus primigenus . Development Growth & Differentiation 52, 603–614. [DOI] [PubMed] [Google Scholar]
- Sunanaga, T. , Satoh, M. & Kawamura, K. (2008). The role of Nanos homologue in gametogenesis and blastogenesis with special reference to male germ cell formation in the colonial ascidian, Botryllus primigenus . Developmental Biology 324, 31–40. [DOI] [PubMed] [Google Scholar]
- * Sunanaga, T. , Watanabe, A. & Kawamura, K. (2007). Involvement of vasa homolog in germline recruitment from coelomic stem cells in budding tunicates. Development Genes and Evolution 217, 1–11. [DOI] [PubMed] [Google Scholar]
- Syed, T. & Schierwater, B. (2002). Trichoplax adhaerens: discovered as a missing link, forgotten as a hydrozoan, re‐discovered as a key to metazoan evolution. Vie et Milieu 52, 177–187. [Google Scholar]
- Szabó, R. & Ferrier, D. E. K. (2014). The dynamics of alkaline phosphatase activity during operculum regeneration in the polychaete Pomatoceros lamarckii . International Journal of Developmental Biology 58, 635–642. [DOI] [PubMed] [Google Scholar]
- Tadokoro, R. , Sugio, M. , Kutsuna, J. , Tochinai, S. & Takahashi, Y. (2006). Early segregation of germ and somatic lineages during gonadal regeneration in the annelid Enchytraeus japonensis . Current Biology 16, 1012–1017. [DOI] [PubMed] [Google Scholar]
- Takahashi, T. , Koizumi, O. , Ariura, Y. , Romanovitch, A. , Bosch, T. C. , Kobayakawa, Y. , Mohri, S. , Bode, H. R. , Yum, S. , Hatta, M. & Fujisawa, T. (2000). A novel neuropeptide, Hym‐355, positively regulates neuron differentiation in Hydra . Development 127(5), 997–1005. [DOI] [PubMed] [Google Scholar]
- * Takamura, K. , Fujimura, M. & Yamaguchi, Y. (2002). Primordial germ cells originate from the endodermal strand cells in the ascidian Ciona intestinalis . Development Genes and Evolution 212, 11–18. [DOI] [PubMed] [Google Scholar]
- Tamm, S. L. (2012). Regeneration of ciliary comb plates in the ctenophore Mnemiopsis leidyi. i. morphology. Journal of Morphology 273(1), 109–120. [DOI] [PubMed] [Google Scholar]
- * Tan, T. C. J. , Rahman, R. , Jaber‐Hijazi, F. , Felix, D. A. , Chen, C. , Louis, E. J. & Aboobaker, A. (2012). Telomere maintenance and telomerase activity are differentially regulated in asexual and sexual worms. Proceedings of the National Academy of Sciences of the United States of America 109, 4209–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Tanaka, E. M. & Reddien, P. W. (2011). The cellular basis for animal regeneration. Developmental Cell 21(1), 172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardent, P. (1963). Regeneration in the Hydrozoa. Biological Reviews of the Cambridge Philosophical Society 38, 293–333. [Google Scholar]
- * Tascedda, F. & Ottaviani, E. (2014). Tumors in invertebrates. Invertebrate Survival Journal 11, 197–203. [Google Scholar]
- Tatzuke, Y. , Sunanaga, T. , Fujiwara, S. & Kawamura, K. (2012). RACK1 regulates mesenchymal cell recruitment during sexual and asexual reproduction of budding tunicates. Developmental Biology 368, 393–403. [DOI] [PubMed] [Google Scholar]
- Tavares, M. R. , Costa, P. A. S. & Ventura, C. R. R. (2019). Population size structure, asexual reproduction, and somatic growth estimates of the non‐indigenous brittle star Ophiothela mirabilis (Echinodermata: Ophiuroidea) on the southeastern coast of Brazil. Marine Biodiversity 49, 1713–1725. [Google Scholar]
- Thein, H. , Ikeda, H. & Uye, S. I. (2013). Ecophysiological characteristics of podocysts in Chrysaora pacifica (Goette) and Cyanea nozakii Kishinouye (Cnidaria: Scyphozoa: Semaeostomeae): effects of environmental factors on their production, dormancy and excystment. Journal of Experimental Marine Biology and Ecology 446, 151–158. [Google Scholar]
- Tiozzo, S. , Christiaen, L. , Deyts, C. , Manni, L. , Joly, J. S. & Burighel, P. (2005). Embryonic versus blastogenetic development in the compound ascidian Botryllus schlosseri: insights from Pitx expression patterns. Developmental Dynamics 232, 468–478. [DOI] [PubMed] [Google Scholar]
- Tiozzo, S. & De Tomaso, A. W. (2009). Functional analysis of Pitx during asexual regeneration in a basal chordate. Evolution and Development 11, 152–162. [DOI] [PubMed] [Google Scholar]
- Torre, C. , Abnave, P. , Tsoumtsa, L. L. , Mottola, G. , Lepolard, C. , Trouplin, V. , Gimenez, G. , Desrousseaux, J. , Gempp, S. , Levasseur, A. , Padovani, L. , Lemichez, E. & Ghigo, E. (2017). Staphylococcus aureus promotes Smed‐PGRP‐2/Smed‐setd8‐1 methyltransferase signalling in planarian neoblasts to sensitize anti‐bacterial gene responses during re‐infection. eBioMedicine 20, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traylor‐Knowles, N. (2016). Distinctive wound‐healing characteristics in the corals Pocillopora damicornis and Acropora hyacinthus found in two different temperature regimes. Marine Biology 163(11), e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treffkorn, S. , Hernández‐Lagos, O. & Mayer, G. (2019). Evidence for cell turnover as the mechanism responsible for the transport of embryos towards the vagina in viviparous onychophorans (velvet worms). Frontiers in Zoology 16, e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost, T. , Haines, J. , Dillon, A. , Mersman, B. , Robbins, M. , Thomas, P. & Hubert, A. (2018). Characterizing the role of SWI/SNF‐related chromatin remodeling complexes in planarian regeneration and stem cell function. Stem Cell Research 32, 91–103. [DOI] [PubMed] [Google Scholar]
- Tuchina, O. & Meyer‐Rochow, V. B. (2010). Regeneration of the visual system in gastropods (Mollusca). Invertebrate Biology 129, 27–38. [Google Scholar]
- Turon, X. (2005). A new mode of colony multiplication by modified budding in the ascidian Clavelina gemmae n. sp. (Clavelinidae). Invertebrate Biology 124, 273–283. [Google Scholar]
- Tweeten, K. A. & Anderson, A. (2008). Analysis of cell proliferation and migration during regeneration in Lumbriculus variegatus (Clitellata: Lumbriculidae). BIOS 79, 183–190. [Google Scholar]
- Tweeten, K. A. & Reiner, A. (2012). Characterization of serine proteases of Lumbriculus variegatus and their role in regeneration. Invertebrate Biology 131(4), 322–332. [Google Scholar]
- Vacelet, J. (1990). Storage cells of calcified relict sponges. In New Perspectives in Sponge Biology (ed. Ruetzler K.), pp. 144–152. Smithsonian Institution Press, Washington. [Google Scholar]
- * van der Flier, L. G. & Clevers, H. (2009). Stem cells, self‐renewal, and differentiation in the intestinal epithelium. Annual Review of Physiology 71, 241–260. [DOI] [PubMed] [Google Scholar]
- Van Wolfswinkel, J. C. , Wagner, D. E. & Reddien, P. W. (2014). Single‐cell analysis reveals functionally distinct classes within the planarian stem cell compartment. Cell Stem Cell 15, 326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Vasquez‐Kuntz, K. L. , Kitchen, S. A. , Conn, T. L. , Vohsen, S. A. , Chan, A. N. , Vermeij, M. J. , Page, C. , Marhaver, K. L. & Baums, I. B. (2020). Juvenile corals inherit mutations acquired during the parents´ lifespan. BioRxiv 23pp. 10.1101/2020.10.19.345538. [DOI] [Google Scholar]
- Vogg, M. C. , Galliot, B. & Tsiairis, C. D. (2019). Model systems for regeneration: Hydra . Development 146, dev177212. [DOI] [PubMed] [Google Scholar]
- * Vogt, G. (2012). Hidden treasures in stem cells of indeterminately growing bilaterian invertebrates. Stem Cell Reviews and Reports 8, 305–317. [DOI] [PubMed] [Google Scholar]
- Vorontsova, M. A. & Liosner, L. D. (1960). Asexual Propagation and Regeneration. meryl rose, S., Translator, p. 488. Pergamon Press, Chicago. [Google Scholar]
- * Voskoboynik, A. , Simon‐Blecher, N. , Soen, Y. , Rinkevich, B. , De Tomaso, A. W. , Ishizuka, K. J. & Weissman, I. L. (2007). Striving for normality: whole body regeneration through a series of abnormal generations. FASEB Journal 21, 1335–1344. [DOI] [PubMed] [Google Scholar]
- * Voskoboynik, A. , Soen, Y. , Rinkevich, Y. , Rosner, A. , Ueno, H. , Reshef, R. , Ishizuka, K. J. , Palmeri, K. J. , Moiseeva, E. , Rinkevich, B. & Weissman, I. L. (2008). Identification of the endostyle as a stem cell niche in a colonial chordate. Cell Stem Cell 3, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Waddington, C. H. (1957). The Strategy of the Genes. Allen & Unwin, London. [Google Scholar]
- * Wagers, A. J. & Weissman, I. L. (2004). Plasticity of adult stem cells. Cell 116, 639–648. [DOI] [PubMed] [Google Scholar]
- Wagner, D. E. , Ho, J. J. & Reddien, P. W. (2012). Genetic regulators of a pluripotent adult stem cell system in planarians identified by RNAi and clonal analysis. Cell Stem Cell 10, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wagner, D. E. , Wang, I. E. & Reddien, P. W. (2011). Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332, 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, J. T. & Podrabsky, J. E. (2015). Gene expression patterns that support novel developmental stress buffering in embryos of the annual killifish Austrofundulus limnaeus . EvoDevo 6, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltrick, D. , Awruch, C. & Simpfendorfer, C. (2012). Embryonic diapause in the elasmobranchs. Reviews in Fish Biology and Fisheries 22(4), 849–859. [Google Scholar]
- Watanabe, H. , Hoang, V. T. , Mättner, R. & Holstein, T. W. (2009). Immortality and the base of multicellular life: lessons from cnidarian stem cells. Seminars in Cell & Developmental Biology 20, 1114–1125. [DOI] [PubMed] [Google Scholar]
- Weiler‐Stolt, B. (1960). Über die Bedeutung der interstitiellen Zellen für die Entwicklung und Fortpflanzung mariner Hydrozoen. Roux's Archives of Developmental Biology 152, 398–455. [DOI] [PubMed] [Google Scholar]
- * Weissman, I. L. (2000). Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157–168. [DOI] [PubMed] [Google Scholar]
- * Weissman, I. L. , Anderson, D. J. & Gage, F. (2001). Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annual Review of Cell and Developmental Biology 17, 387–403. [DOI] [PubMed] [Google Scholar]
- Wenemoser, D. & Reddien, P. W. (2010). Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Developmental Biology 344, 979–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielsputz, C. & Saller, U. (1990). The metamorphosis of the parenchymula‐larva of Ephydatia fluviatilis (Porifera, Spongillidae). Zoomorphology 109, 173–177. [Google Scholar]
- Wiens, M. , Belikov, S. I. , Kaluzhnaya, O. V. , Krasko, A. , Schröder, H. C. , Perovic‐Ottstadt, S. & Müller, W. E. G. (2006). Molecular control of serial module formation along the apical–basal axis in the sponge Lubomirskia baicalensis: silicateins, mannose‐binding lectin and mago nashi. Development Genes and Evolution 216(5), 229–242. [DOI] [PubMed] [Google Scholar]
- * Wiggans, M. & Pearson, B. J. (2021). One stem cell program to rule them all? The FEBS Journal 288, 3394–3406. 10.1111/febs.15598. [DOI] [PubMed] [Google Scholar]
- Windsor Reid, P. J. , Matveev, E. , McClymont, A. , Posfai, D. , Hill, A. L. & Leys, S. P. (2018). Wnt signaling and polarity in freshwater sponges. BMC Evolutionary Biology 18, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witchley, J. N. , Mayer, M. , Wagner, D. E. , Owen, J. H. & Reddien, P. W. (2013). Muscle cells provide instructions for planarian regeneration. Cell Reports 4(4), 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittlieb, J. , Khalturin, K. , Lohmann, J. U. , Anton‐Erxleben, F. & Bosch, T. C. G. (2006). Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proceedings of the National Academy of Sciences of the United States of America 103, 6208–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudarski, J. , Simanov, D. , Ustyantsev, K. , de Mulder, K. , Grelling, M. , Grudniewska, M. , Beltman, F. , Glazenburg, L. , Demircan, T. , Wunderer, J. & Qi, W. (2017). Efficient transgenesis and annotated genome sequence of the regenerative flatworm model Macrostomum lignano . Nature Communications 8, e2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Xu, C. M. & Sun, S. C. (2020). Expression of piwi genes during the regeneration of Lineus sanguineus (Nemertea, Pilidiophora, Heteronemertea). Genes 11, e1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, S. , Huang, Y. , Zhangfang, Y. , Zhong, X. , Li, P. , Huang, J. , Liu, D. & Songyang, Z. (2016). SmedOB1 is required for planarian homeostasis and regeneration. Scientific Reports 6, 34013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yomogida, S. & Wani, R. (2013). Higher risk of fatality by predatory attacks in earlier ontogenetic stages of modern Nautilus pompilius in the Philippines: evidence from the ontogenetic analyses of shell repairs. Lethaia 46(3), 317–330. [Google Scholar]
- * Yoshida, K. , Hozumi, A. , Treen, N. , Sakuma, T. & Yamamoto, T. (2017). Germ cell regeneration‐mediated, enhanced mutagenesis in the ascidian Ciona intestinalis reveals flexible germ cell formation from different somatic cells. Developmental Biology 423, 111–125. [DOI] [PubMed] [Google Scholar]
- Yoshida‐Noro, C. & Tochinai, S. (2010). Stem cell system in asexual and sexual reproduction of Enchytraeus japonensis (Oligochaeta, Annelida). Development Growth & Differentiation 52, 43–55. [DOI] [PubMed] [Google Scholar]
- Yoshimura, K. , Morino, Y. & Wada, H. (2019). Regeneration of the acorn worm pygochord with the implication for its convergent evolution with the notochord. Development Growth & Differentiation 61, 158–165. [DOI] [PubMed] [Google Scholar]
- Yousefi, M. , Nakauka‐Ddamba, A. , Berry, C. T. , Li, N. , Schoenberger, J. , Simeonov, K. P. , Cedeno, R. J. , Yu, Z. & Lengner, C. J. (2018). Calorie restriction governs intestinal epithelial regeneration through cell‐autonomous regulation of mTORC1 in reserve stem cells. Stem Cell Reports 10, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Yun, C. O. , Bhargava, P. , Na, Y. , Lee, J. S. , Ryu, J. , Kaul, S. C. & Wadhwa, R. (2017). Relevance of mortalin to cancer cell stemness and cancer therapy. Scientific Reports 7, 42016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaldibar, B. , Cancio, I. & Marigómez, I. (2004). Circatidal variation in epithelial cell proliferation in the mussel digestive gland and stomach. Cell and Tissue Research 318, 395–402. [DOI] [PubMed] [Google Scholar]
- Zattara, E. E. , Fernández‐Álvarez, F. Á. , Hiebert, T. C. , Bely, A. E. & Norenburg, J. L. (2019). A phylum‐wide survey reveals multiple independent gains of head regeneration in Nemertea. Proceedings of the Royal Society B: Biological Sciences 286, 20182524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zattara, E. E. , Turlington, K. W. & Bely, A. E. (2016). Long‐term time‐lapse live imaging reveals extensive cell migration during annelid regeneration. BMC Developmental Biology 16, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, H. , Ye, H. , Li, S. , Wang, G. & Huang, J. (2010). Hepatopancreas cell cultures from mud crab, Scylla paramamosain . In Vitro Cellular & Developmental Biology – Animal 46, 431–437. [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Li, G. , Sun, Y. & Wang, Y. (2009a). Chromosome preparation and preliminary observation of two amphioxus species in Xiamen. Zoological Research 30(2), 131–136. [Google Scholar]
- Zhang, Y. , Allodi, S. , Sandeman, D. C. & Beltz, B. S. (2009b). Adult neurogenesis in the crayfish brain: proliferation, migration and possible origin of precursor cells. Developmental Neurobiology 69, 415–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X. , Battistoni, G. , El Demerdash, O. , Gurtowski, J. , Wunderer, J. , Falciatori, I. , Ladurner, P. , Schatz, M. C. , Hannon, G. J. & Wasik, K. A. (2015). Dual functions of Macpiwi1 in transposon silencing and stem cell maintenance in the flatworm Macrostomum lignano . RNA 21, 1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Zhu, S. J. & Pearson, B. J. (2016). (Neo)blast from the past: new insights into planarian stem cell lineages. Current Opinion in Genetics & Development 40, 74–80. [DOI] [PubMed] [Google Scholar]
- Zhu, W. , Pao, G. , Satoh, A. , Cummings, G. , Monaghan, J. , Harkins, T. , Bryant, S. , Voss, S. , Gardiner, D. & Hunter, T. (2012). Activation of germline‐specific genes is required for limb regeneration in the Mexican axolotl. Developmental Biology 370, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer, R. (1999). Phoronida. In Encyclopedia of Reproduction (eds Knobil E. and Neill J. D.). Academic Press, San Diego. [Google Scholar]
- * Zipori, D. (2004). The nature of stem cells: state rather than entity. Nature Reviews Genetics 5, 873–878. [DOI] [PubMed] [Google Scholar]
- Zuccolotto‐Arellano, J. & Cuervo‐González, R. (2020). Binary fission in Trichoplax is orthogonal to the subsequent division plane. Mechanisms of Development 162, 103608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Diversity of adult stem cell (ASC) contributions to four major biological processes in the Cnidaria: homeostasis, dormancy, regeneration and agametic asexual reproduction. The presence of the biological process, involvement of undifferentiated/differentiated putative ASCs or progenitors and their level of potency, as well as the specific classes of stemness gene families they express are mapped for major cnidarian lineages. Circles: empty circle – documented presence of the biological process; filled circle – cases where putative ASCs or progenitors are involved. A red cross signifies the absence of the biological process in the lineage as currently documented. As homeostasis is a property of life, all groups are shown with an empty circle. For adult regeneration, the asterisk documents the presence of whole‐body regeneration. Dormancy refers to any documented type of dormant stage or torpor‐like process and has likely evolved independently in each lineage. For dormancy, A – quiescence, diapause, growth/degrowth; G – growth/degrowth; O – ontogeny reversal; Q – quiescence. For agametic asexual reproduction, B – any form of budding, F – any form of fission/fragmentation. Triangles indicate the level of documented potency for ASCs: red = lineage restricted/unipotent; cyan = totipotent; blue = multi/pluripotent; gradient triangle = documented cases of several ASCs or progenitors with different potency. Selected stemness gene families whose members are expressed in ASCs during the biological process are listed in a box for each process and group where known. The relative contribution of undifferentiated (U) versus differentiated (D) ASCs or progenitors within each subclass is mapped onto the phylogeny where known; levels of confidence are represented by solid (higher) and dotted (lower) diamonds, while the sizes of D and U reflect their presumed level of contribution. A hypothetical ancestral state for this character is proposed at the corresponding node of the simplified cnidarian phylogenetic tree. A general consensus for all features across Cnidaria is proposed at the top of the figure. Key species for which data exist in each subclass are named. Data are derived from Tables [Link], [Link].
Fig. S2. Diversity of adult stem cell (ASC) contributions to four major biological processes in the Echinodermata: homeostasis, dormancy, regeneration and agametic asexual reproduction. The presence of the biological process, involvement of undifferentiated/differentiated putative ASCs or progenitors and their level of potency, as well as the specific classes of stemness gene families they express are mapped for major echinoderm lineages. Circles: empty circle – documented presence of the biological process; filled circle – cases where putative ASCs or progenitors are involved. A red cross signifies the absence of the biological process in the lineage as currently documented. As homeostasis is a property of life, all groups are shown with an empty circle. For adult regeneration, the asterisk documents the presence of whole‐body regeneration. Dormancy refers to any documented type of dormant stage or torpor‐like process and has likely evolved independently in each lineage. For dormancy, the dotted line circle indicates potential involvement in the respective biological feature in non‐adults. Q – quiescence. For agametic asexual reproduction, F – any form of fission/fragmentation. Triangles indicate the level of documented potency for ASCs (filled) and progenitors (empty). Red = lineage restricted/unipotent; blue = multi/pluripotent; gradient triangle = documented cases of several ASCs or progenitors with different potency. Selected stemness gene families whose members are expressed in ASCs or progenitors during the biological process are listed in a box for each process and group where known. The relative contribution of undifferentiated (U) versus differentiated (D) ASCs or progenitors within each class is mapped onto the phylogeny; levels of confidence are represented by solid (higher) and dotted (lower) diamonds, while the sizes of D and U reflect their presumed level of contribution. A hypothetical ancestral state for this character is proposed at the corresponding node of the simplified echinoderm phylogenetic tree. A general consensus for all features across Echinodermata is proposed at the top of the figure. Key species for which data exist in each class are named. Data are derived from Tables [Link], [Link].
Table S1. Properties of selected, well‐studied adult stem cell (ASC) lineages in invertebrates.
Table S2. Genes expressed in invertebrate adult stem cell (ASCs) and progenitor cells during potency state changes.
Table S3. Suggested stem cell niches (SCNs) present in invertebrates.
Table S4. Overview of the involvement of adult stem cell (ASCs) and progenitors during homeostasis in metazoans.
Table S5. Overview of the involvement of adult stem cell (ASCs) and progenitors in regeneration processes in metazoans.
Table S6. Overview of the involvement of adult stem cell (ASCs) and progenitors in agametic asexual reproduction (budding, fission/fragmentation) in metazoans.
Table S7. Overview of the involvement of adult stem cell (ASCs) and progenitors in dormancy in metazoans.


