Abstract
Purpose
To investigate the efficacy of myopia control defined by axial elongation and safety of orthokeratology lenses (OKL) in a Scandinavian (Danish) population.
Methods
Sixty Danish children aged 6–12 years with myopia ranging from 0.5 to 4.75 dioptres (D) spherical component and refractive astigmatism ≤2.5 D in both eyes were randomly assigned to either OKL or single‐vision spectacles (SVS). Study duration was 18 months. Outcome measures were axial length (AL) measured with Lenstar LS900 (Haag‐Streit, Koeniz, Switzerland) and adverse events graded with Efron Grading Scale for Contact Lens Complications.
Results
Nineteen participants completed the 18‐month follow‐up in the OKL group and 28 in the SVS group. The average AL elongation in the OKL group was 0.24 mm smaller as compared to the SVS group (95% confidence interval 0.12–0.36, mixed model adjusted for baseline sex, age and AL). There were no fast progressors (>0.75 D/year) in the OKL group during the follow‐up period in contrast to 22% in the SVS group. No treatment‐requiring or vision‐threatening adverse events were observed.
Conclusion
Orthokeratology lenses reduced AL elongation in myopic Scandinavian children by 59%, with no treatment‐requiring or vision‐threatening adverse events. The results align with outcomes of previous clinical trials.
Keywords: dropout, myopia, myopia control, orthokeratology lenses, progression rate, randomized, safety
Introduction
The prevalence of myopia is increasing and is expected to affect about 50% of the world’s population in 2050 (Holden et al. 2016). In urbanized areas of East Asia, prevalence rates of over 80% have been reported in young adults (Lin et al. 2004; Lee et al. 2013). The Scandinavian countries are less affected, but increase in myopia prevalence from 13% to 25% has been reported in Danish adolescents (Jacobsen et al. 2007; Hansen et al. 2020). The increased prevalence of myopia is of concern due to sight‐threatening conditions associated with high degrees of myopia, such as retinal detachment, chorioretinal atrophy and myopic choroidal neovascularization (Ikuno 2017). Accordingly, several studies have investigated the effects of different treatment modalities on the reduction in myopia progression, with the most promising treatments being atropine eye drops and orthokeratology lenses (OKL) (Prousali et al. 2019).
Orthokeratology lenses are custom fitted, rigid gas‐permeable contact lenses that are used every night during sleep. They induce a temporary flattening of the central cornea, thus reducing or eliminating the need for myopia correction after removal. In prospective studies of low to moderate myopes, OKL have been shown to effectively reduce axial elongation compared with controls in Asian (Cho et al. 2005; Kakita et al. 2011; Cho & Cheung 2012; Hiraoka et al. 2012; Swarbrick et al. 2015), Spanish (Santodomingo‐Rubido et al. 2012; Paune et al. 2015) and American (Walline et al. 2009) children. However, these studies are heterogeneous in design. Two of the mentioned studies were randomized, hereof one used a contralateral‐eye design, four of them were non‐randomized, and two used matched controls, hereof one with a historic control group. The vision correction of the control groups comprised of three different modalities: single‐vision spectacles (SVS), single‐vision contact lenses and daytime rigid gas‐permeable lenses. The duration of OKL wear was six months to five years. Six of the studies reported two‐year efficacy which varied from 32% to 56%.
The major concern with OKL treatment is the risk of microbial keratitis (MK). Although no MK was reported in a meta‐analysis comprising 667 children from nine OKL studies, the odds ratio for mild adverse events was 8.87 when compared with controls using SVS (95% confidence interval (CI) 3.79 to 20.74; Li et al. 2016).
The variation in myopia prevalence among children worldwide is attributed to both genetic and environmental factors (Lopes et al. 2009; Guggenheim et al. 2012; Williams et al. 2019). Therefore, the efficacy of myopia control treatments may differ between ethnic groups. Thus, we conducted an 18‐month randomized controlled trial including Scandinavian children with low to moderate myopia assigned to receive either OKL or SVS. The objectives of this paper are treatment efficacy, safety and post hoc analysis on dropout rates in the OKL group.
Methods
Study design
This was an 18‐month, 1:1 randomized controlled trial. The intervention was orthokeratology lenses, and the control group used single‐vision spectacles. The primary outcome variable was axial length (AL) which was measured every six months. Additional visits for lens fitting and ocular health evaluation were performed in the OKL group. An overview of visits for both groups is presented in Fig. 1.
Fig. 1.
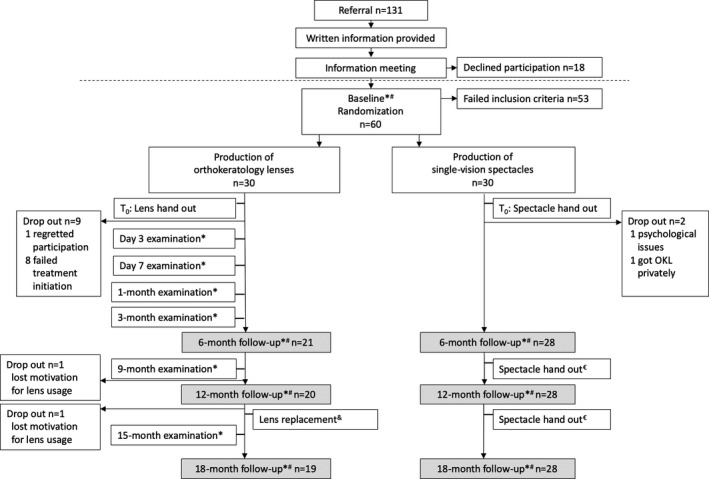
Overview of visits and examinations from baseline to 18‐month follow‐up in a randomized trial on myopic, Danish children using either orthokeratology lenses or single‐vision spectacles. Examinations: *Ocular health by slit‐lamp examination, topography, non‐cycloplegic subjective refraction and visual acuity. #Axial length, cycloplegic autorefraction. €New spectacles were provided for all subjects if a change (≥0.25 D SER) had occurred since prior examination. &Orthokeratology lenses were replaced every 12 months followed by a day 7 lens check with examinations as indicated by *.
Subjects
Sixty Scandinavian (Danish) children were enrolled between April 2017 and September 2018 at the Department of Ophthalmology, Vejle Hospital, University Hospital of Southern Denmark. The subjects were referred from private ophthalmic practitioners. The inclusion criteria were one or both parents being ethnically Scandinavian, age 6–12 years, cycloplegic spherical value of −0.5 to −4.75 D in both eyes, and refractive astigmatism ≤2.5 D in both eyes. Complete inclusion and exclusion criteria are presented in Table 1. When a subject met the inclusion criteria, the author (TMJ) included the subject, who then proceeded to the randomization process.
Table 1.
Inclusion and exclusion criteria in a randomized clinical study on myopic Danish children.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
ETDRS = The Early Treatment Diabetic Retinopathy Study.
Randomization
Random numbers were computer generated (www.randomizer.org) in three blocks throughout the study, packed in envelopes by a secretary and kept in the secretary’s office. Even numbers indicated assignment to the SVS group and odd numbers, to the OKL group. To ensure allocation concealment, the envelope was handed directly to the subjects at randomization and the last envelope from each randomization block was omitted.
Materials and procedures
The OKL used was Dreamlite® (Procornea, LZ Eerbeek, the Netherlands), a four‐zone reverse geometry lens with a 6 mm optic zone diameter and 0.75 D compression factor. The compression factor (also known as the Jessen factor) is additional refractive power added to compensate for the gradual refractive regression throughout the day. The lens material is Boston XO with oxygen permeability (Dk) of 100 × 10−11 (cm2/seconds) (ml O2/ml × mmHg).
The lenses were fitted according to Procornea’s guidelines based on corneal topography analysis (Pentacam HR, Oculus, Wetzlar, Germany). Periphery toric lens designs were made when the height difference between 2 meridians 4 mm from the apex of the cornea was more than 20 μm. Cleadew GP (Ophtecs, Tokyo, Japan), a povidone iodine based cleaner, was used for daily cleaning. Intolerance was suspected in some subjects, which led to the replacement of the product with Ever Clean (Avizor International SL, Madrid, Spain), a hydrogen peroxide solution. Rinsing of the lenses was performed twice within the storage case and thereafter in the subject’s hand before insertion. Regard® (Advanced Eyecare Research, Bucks, United Kingdom), a preservative‐free multipurpose solution, was initially used for this procedure and during the study was gradually replaced by sterile 0.9% sodium chloride ampoules (Fresenius Kabi, Bad Homburg, Germany) to avoid non‐physiological substances. For intensive cleaning, Menicon Progent (Menicon Co., Ltd., Nagoya, Japan) was used when lenses had been in use for 6 and 9 months. OKL, lens solutions and SVS were provided to the subjects free of charge.
Orthokeratology lenses were changed every 12 months or earlier if change in design was required. The indication for change of lens design was evaluated in each individual case. Compromised corneal health suspected to be caused by unsatisfying lens fitting always led to design modification. Other parameters considered were unaided visual acuity, topographical changes, and the subject’s subjective description of daily visual function and lens insertion/removal or comfort complaints. When OKL with design modifications were handed out, additional visits were scheduled to evaluate the lens fitting. No spare lenses were ordered. Accordingly, if lenses were lost or broken, the subject was without lenses for approximately 3 weeks until the new lenses were produced and delivered. SVS with optimal correcting were ordered at each follow‐up visit if change in SER of ≥0.25 D had occurred since prior examination. Furthermore, the subjects were instructed to make an appointment for an additional control in cases of decreasing vision between controls. For both groups, assessment of optimal correction in the OKL or SVS was dependent on the age of the subject. Cycloplegic autorefraction was used for children under the age of 10 years, and manifest subjective refraction according to the ‘push plus’ principle was used for children aged 10 years or older. This was chosen to ensure the best possible visual acuity for all subjects and to minimize the risk of over‐ and under‐correction.
Data collection
Data collected at the specific visits are presented in Fig. 1. If T0 exceeded 5 weeks from the baseline visit, a new measurement of axial length (AL) was performed to replace the baseline measurement. Visits of the subjects in the OKL group were made as early in the morning as possible to permit the evaluation of corneal health shortly after lens removal.
Measurement of variables
Best‐corrected visual acuity was measured with the Early Treatment Diabetic Retinopathy Study charts (Precision Vision, Woodstock, IL, USA) following the guidelines for clinical research (Ferris et al. 1982). Cycloplegic spherical equivalent refractive error (SER) was measured in all subjects at visits specified in Figure 1 with Shin‐Nippon Nvision‐K 5001 autorefractor (Rexxam Co, Kagawa, Japan, also known as Grand Seiko WR‐5100K). Five readings were made with stable fixation, and the optimum value chosen by the autorefractor was used for analysis. Extra measures were made to ensure the precision of the optimum value in cases where the precision of a reading could be doubted. The autorefractor has exhibited good accuracy and repeatability with intrasession variation of the optimum value of ± 0.09 D for the spherical equivalent (Davies et al. 2003). Cycloplegia was obtained by instilling three drops of cyclopentolate 1% (Minims®, Bausch & Lomb, Chauvin Pharmaceuticals Ltd., UK) with an interval of 5 min. Autorefraction was performed more than 30 min after instillation of the last drop and after inspecting pupillary dilation by slit‐lamp examination. Cyclopentolate has been shown to be a strong cycloplegia agent in myopic children (Yazdani et al. 2018). Biometric measurements of AL and central corneal thickness (CCT) were performed before cycloplegia using Lenstar LS900 (Haag‐Streit, Koeniz, Switzerland). Five or six consecutive readings were recorded, and the means of the measurements were used for analysis. Both AL and CCT measurements with Lenstar have good repeatability and yield an intraobserver standard deviation of three consecutive measurements of 0.01 mm for AL and of 0.0015 mm for CCT (Cruysberg et al. 2010).
Orthokeratology lenses induce flattening of the cornea, which reduces AL. Therefore, at follow‐up visits, AL in the OKL group was adjusted for the decrease in CCT: adjusted ALfollow‐up = ALfollow‐up + (CCTBL – CCTfollow‐up). Adverse events were graded using the Efron Grading Scale for Contact Lens Complications (Efron score). To evaluate corneal and conjunctival staining, a fluorescein strip wetted with one drop of sterile 0.9% NaCl was instilled in the inferior conjunctival fornix, and the eye was examined in cobalt blue light in the slit lamp. For consistency, the grading was performed by the same clinician (TMJ) throughout the study. Efron score has been shown to be a valid grading instrument (Efron et al. 2001). Adverse event was defined as any vision‐threatening or treatment‐requiring conditions related to contact lens usage and corneal staining Efron score Grade 2 or more. Efron score Grade 2 or more was handled with paused lens usage for the number of days that was found clinically relevant and the elimination of presumed causation (re‐education for lens handling issues; solution replacement for suspicion of intolerance) or lens design modification.
Progression status at 18 months
Five progression groups were created to evaluate AL progression in a clinical context. Emmetropic axial growth in Danish children has previously been investigated (Fledelius et al. 2014). Using the median progression rates within the age groups representative for the present study (5–7.9 years, 8–10.9 years, 11–13.9 years), emmetropic axial growth was found to be 0.146 mm/year for the group as a whole. To make it comparable with the results in the current study, this was multiplied by 1.5 resulting in 0.22 mm progression in 18 months. Hence, we defined group 1 ‘no progression’: ≤0 mm and group 2 ‘emmetropic progression’: >0 to ≤0.22 mm. Hereafter, we defined low, moderate and fast myopic progression as <0.50 D, 0.50 to <0.75 D and ≥0.75 D annual rate of myopia progression, respectively. Group 3 ‘Low progression’ was defined based on considerations about acceptable annual myopic progression, and group 5 ‘fast progression’ was defined based on previous definitions (Jong et al. 2018). The corresponding axial length changes in 18 months were computed based on the correlation between SER and AL of the SVS group at 12‐month follow‐up in the present study and multiplied by a factor 1.5.
Power calculation
The sample size calculation was based on three studies (Hiraoka et al. 2012; Santodomingo‐Rubido et al. 2012; Paune et al. 2015) that evaluated the effect of OKL on myopia progression. Given a significance level (alpha) of 0.05 and a statistical power of 90%, a sample size of 20 subjects in each group was required, assuming a difference in AL of 0.17 mm between treatment groups at 18 months. To compensate for a 20% dropout rate, 25 patients were required in each group. The dropout rate exceeded 20% in the OKL group. Consequently, the total number of study subjects was increased from 50 to 60 in spring 2018 after approval from the Regional Committee on Health Research Ethics.
Statistics
For comparison of numerical data at baseline, unpaired t‐test was used on parametric data and Mann–Whitney rank sum test on non‐parametric data. For binary data, chi‐square test was used. Normality was tested using the Shapiro–Wilk test. For analysis of the outcome variable AL, a mixed model was chosen due to the longitudinal study design with repeated measures. A random slope was added because of great inter‐individual axial growth. Model improvement was confirmed using Akaike’s information criteria. Additional to group, we used the covariates visit, AL at baseline, age at baseline and SER at baseline. The model contained an interaction between group and visit. The latter three covariates were included in the model to adjust for their possible confounding effect. Because interim analysis showed equally random elongation in the right and left eyes of each subject, the mean AL of right and left eyes at each visit was used in the analysis. For subjects dropping out of the study, data were included in the analysis until dropout occurred. Qq‐plot and best linear unbiased predictors 1 and 2 were used to evaluate model assumptions and were deemed satisfactory. The mixed model showed good predictive value with p < 0.001. For postestimation test, a global test of the effect of group and group‐by‐visit interaction was first used (testparm). Since the global test was statistically significant (p < 0.001), pairwise comparison (pwcompare) was used for significance testing between the two groups at each follow‐up visit. Margins plots were made to visualize the change in AL between 6‐ and 18‐month follow‐up. The progression status at 18‐month follow‐up in the OKL and SVS groups was visualized using cumulative proportions and significance tested using Fisher’s exact test. For post hoc analysis of the dropout rate in the OKL group, multiple logistic regression was used. Statistical analyses were conducted using stata software (version 16.0; StataCorp, College Station, TX, USA) and SigmaPlot (version 14.0, Systat Software Inc., San Jose, California).
Results
Of the 60 subjects enrolled, 30 were allocated to OKL and 30 to SVS. Out of these, 11 dropped out of the OKL group and 2 dropped out of the SVS group. The time and reason for dropping out are presented in Figure 1. Baseline data for both groups are presented in Table 2. There were no statistically significant differences between the two groups at baseline or after removal of dropouts from the analyses. The mean difference in AL progression was statistically significantly lower in the OKL group (adjusted AL) compared to the SVS group. At 6, 12 and 18 months, subjects in the OKL groups had a smaller mean adjusted AL increase of 0.12 mm, 0.19 mm and 0.24 mm, respectively, compared to the SVS group (95% CI 0.06 to 0.18, p < 0.001; 95% CI 0.10 to 0.27, p < 0.001; 95% CI 0.12 to 0.36, p < 0.001, respectively, pairwise comparison, Table 3, Figure 2). Adjusted mean axial progression based on the output from the mixed model is presented in Table 3. Unadjusted (observed) axial progressions were 0.06 ± 0.11 mm, 0.12 ± 0.16 mm and 0.19 ± 0.18 mm for 6‐, 12‐ and 18‐month follow‐up, respectively, in the OKL group and 0.17 ± 0.11 mm, 0.30 ± 0.17 mm and 0.43 ± 0.23 mm for 6‐, 12‐ and 18‐month follow‐up, respectively, in the SVS group. Of the three covariates included in the mixed model, AL at baseline, age at baseline, and SER at baseline, AL at baseline was statistically associated with AL progression (p < 0.001).
Table 2.
Baseline characteristics with in‐between group significance testing for all subjects (left side) and for subjects completing the study (right side).
| Baseline characteristics |
All subjects Mean ± SD Median (range) |
All subjects |
Completing Mean ± SD Median (range) |
Completing | ||
|---|---|---|---|---|---|---|
| OKL (n = 30) | SVS (n = 30) | p‐Value | OKL (n = 19) | SVS (n = 28) | p‐Value | |
| Age, years | 9.77 ± 1.38 | 10.16 ± 1.68 | 0.33* | 10.03 ± 1.60 | 10.06 ± 1.70 | 0.95* |
| Sex, Male (%) | 14 (46.7) | 12 (40.0) | 0.60 ‡ | 7 (36.8) | 10 (35.7) | 0.94 ‡ |
| SER cyclo, D | –2.00 (–0.69 to –4.88) | –2.16 (–0.81 to –4.81) | 0.81 † | –1.88 (–0.69 to –3.63) | –2.16 (–0.81 to –4.81) | 0.59 † |
| Axial length, mm | 24.12 ± 0.70 | 24.33 ± 0.67 | 0.24* | 23.92 ± 0.52 | 24.32 ± 0.69 | 0.08* |
D = dioptre; F = female; M = male; mm = millimetres; n = number; OKL = Orthokeratology lens group; SD = standard deviation; SER cyclo = cycloplegic spherical equivalent refractive error; SVS = single‐vision spectacles group.
For the variables SER cyclo and axial length, the value for right and left eyes for each subject has been averaged.
Unpaired t‐test.
Mann–Whitney rank sum test.
Chi‐square test.
Table 3.
Axial growth between the orthokeratology lens group and single‐vision spectacle groups.
| Pairwise comparison | Contrast, mm | Std. Error | 95% CI | Axial progression | OKL, mm | SVS, mm |
|---|---|---|---|---|---|---|
| (SVS#M06) versus (OKL#M06) | 0.12 | 0.03 | 0.06 to 0.18 | M6 | – | – |
| (SVS#M12) versus (OKL#M12) | 0.19 | 0.05 | 0.10 to 0.27 | M12 | 0.09 | 0.28 |
| (SVS#M18) versus (OKL#M18) | 0.24 | 0.06 | 0.12 to 0.36 | M18 | 0.17 | 0.41 |
CI = confidence interval; M12 = 12‐month follow‐up; M18 = 18‐month follow‐up; M6 = 6‐month follow‐up; OKL#M = interaction of OKL and month; OKL = orthokeratology group; Prog. = progression; Std. Error = standard error; SVS#M = interaction of SVS and month; SVS = single‐vision spectacle group.
Left: Postestimation test for the mixed model with pairwise comparison of groups and interaction between group and follow‐up visits at 6, 12 and 18 months and accounting for the exposure variables baseline axial length, age at time of inclusion, and cycloplegic spherical equivalent refractive error at time of inclusion, and with the allowance of a random slope. Right: actual axial progression calculated based on the output of the mixed model.
Fig. 2.
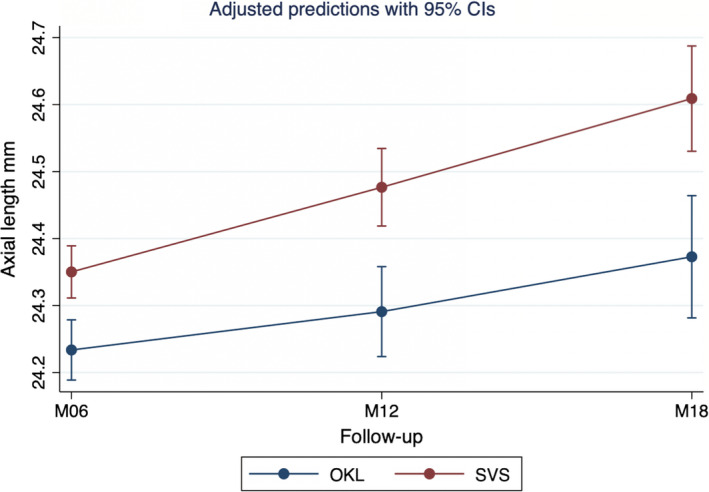
Adjusted predictions with 95% confidence intervals of axial length at follow‐up visits 6 to 18 months. The adjusted predictions are based on a mixed model with axial length at 6‐, 12‐ and 18‐month follow‐up. M06 = 6‐month follow‐up; M12 = 12‐month follow‐up; M18 = 18‐month follow‐up; OKL = orthokeratology group; SVS = single‐vision spectacle group.
The computed correlation between SER and AL in the SVS group showed that a change in SER of 0.5 D/year was equivalent to a change in AL of 0.275 mm/year or 0.41 mm/18 months (linear regression). Hence, change in AL in group 3 ‘low myopic progression’ (<0.5 D/year) was defined as >0.22 to ≤0.41 mm/18 months, in group 4 ‘intermediate myopic progression’ (>0.5 to <0.75D/year) as > 0.41 to 0.62 mm/18 months, and in group 5 ‘fast progression’ (>0.75 D/year) as >0.62 mm/18 months. The progression status at 18‐month follow‐up showed that approximately 50% of subjects in the OKL group were within the categories no or emmetropic progression and over 40% were in the low progression group (Fig. 3). Only about 5% were in the intermediate progression group, and no subjects were in the fast progression group. In the SVS group, only about 10% were in the no or emmetropic progression groups, and 22% were in the fast progression group. The difference in group distribution was statistically significant (p < 0.001, Fisher’s exact test).
Fig. 3.
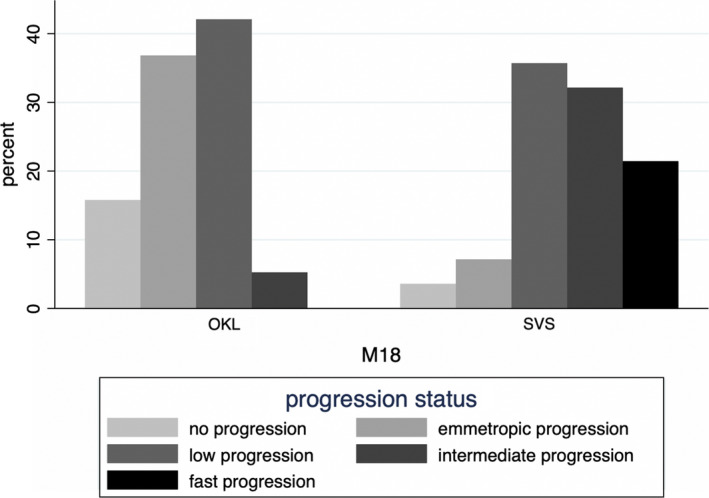
Progression status at 18‐month follow‐up for the orthokeratology and single‐vision spectacle groups. No progression: ≤0 mm; emmetropic progression (0 D/year): >0–0.22 mm; low progression (<0.5 D/year): >0.22‐0.41 mm; intermediate progression (>0.5 < 0.75D/year): >0.41‐0.62 mm; fast progression (>0.75D/year): >0.62 mm. M18 = 18‐month follow‐up; OKL = orthokeratology group; SVS = single‐vision spectacle group.
No vision‐threatening or treatment‐requiring adverse events occurred during the 18‐month follow‐up. An overview of corneal staining at the scheduled follow‐up visits is presented in Table 4. Corneal staining Grade 3 occurred twice during the study. First, it was seen bilaterally in a 9‐year‐old subject at an additional visit after 2 months of treatment when the subject, rather than her parent, started handling the lenses herself. No further corneal issues occurred once the original procedure was re‐established. Second, one subject presented grade 3 staining in the right eye at 18‐month follow‐up. This subject had presented intermittent corneal staining grade 1 or 2 in the right eye throughout the study. Modifications in lens design were effective and resulted in periods of good corneal health up until the 18‐month follow‐up visit.
Table 4.
Efron Grading Scale for Contact Lens Complications for corneal staining at follow‐up visits for right and left eyes.
| Follow‐up | D3 (n 21) | D7 (n 21) | M01 (n 21) | M03 (n 21) | M06 (n 21) | M09 (n 21) | M12 (n 20) | M15 (n 19) | M18 (n 19) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Dxt | Sin | Dxt | Sin | Dxt | Sin | Dxt | Sin | Dxt | Sin | Dxt | Sin | Dxt | Sin | Dxt | Sin | Dxt | Sin |
| 0 | 13 | 12 | 11 | 13 | 11 | 14 | 14 | 13 | 12 | 12 | 10 | 13 | 8 | 12 | 15 | 12 | 16 | 16 |
| 1 | 8 | 8 | 10 | 8 | 9 | 7 | 4 | 7 | 8 | 8 | 8 | 7 | 9 | 8 | 4 | 7 | 1 | 3 |
| 2 | 0 | 1 | 0 | 0 | 1 | 0 | 3 | 1 | 1 | 1 | 3 | 1 | 3 | 0 | 0 | 0 | 1 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
D = day; Dxt = right eye; M = month; Sin = left eye.
In the OKL group, 30% of the subjects dropped out before the treatment was well established. The dropout rate in the OKL group underwent post hoc analysis for two factors that could have influenced motivation and perseverance within the group. The factors were time from referral to baseline less than 2.5 months (75 days) and handout of lenses during the dark season from November to March with the shortest day at winter solstice of only 7 hr from sunrise to sunset. The odds ratio (OR) for dropout was 8.6 if time from referral to baseline was <75 days (p = 0.08, multiple logistic regression) and 10.0 for dropout when lens handout occurred during the dark season (p = 0.03). No statistically significant difference in age was found between dropouts and non‐dropouts in the OKL group (p = 0.25, unpaired t‐test).
Discussion
This is the first study to evaluate the efficacy and safety of OKL in a Scandinavian (Danish) study population. This randomized clinical trial showed that OKL significantly reduces axial growth with no severe adverse events in myopic Scandinavian children aged 6–12 years during 18 months of follow‐up.
The treatment efficacy of OKL is in accordance with previous studies, which were performed, however, on different ethnic populations (Walline et al. 2009; Cho & Cheung 2012; Hiraoka et al. 2012; Santodomingo‐Rubido et al. 2012; Paune et al. 2015). In a recently published systematic review on treatments for myopia, an overall effect of OKL on 524 children was 0.19 mm (95% CI 0.16 to 0.21) decrease in axial growth during one year (Prousali et al. 2019). A meta‐analysis based on nine studies, eight Asian and one European, with a total of 667 children, evaluated the effect of OKL on axial growth (Li et al. 2016). They found contrasts between axial growth in the OKL and the control groups of 0.13 mm (95% CI 0.09 to 0.18), 0.19 mm (95% CI 0.17 to 0.22) and 0.23 mm (95% CI 0.17 to 0.29) for 6‐, 12‐ and 18‐month follow‐up, respectively. In comparison the current study found contrasts of 0.12 mm (95% CI 0.06 to 0.18), 0.19 mm (0.10 to 0.27) and 0.24 mm (95%CI 0.12 to 0.36) at 6‐, 12‐ and 18‐month follow‐up, respectively. This indicates that OKL’s myopia control ability in Scandinavian children is comparable to that in children of other ethnicities.
Orthokeratology lenses treatment efficacy varies between individuals. Surprisingly, we found that about 50% of subjects in the OKL group had an axial growth less or equivalent to emmetropic axial growth (≤0.22 mm/18 months). In addition, no fast progressors (>0.62 mm/18 months) existed in the OKL group. This aligns with the hypothesis that treatment with OKL may reduce axial growth in all subjects and thus limit the number of fast progressors. An Asian study also evaluated the difference in progression rates between OKL and SVS subjects. This study comprised data from two prospective trials and found 6% fast progressors in the OKL group in contrast to 49% fast progressors in the SVS group (Cho & Cheung 2017). Fast progression was defined as >1 D/year equivalent to >0.36 mm/year axial growth. We defined fast progression as >0.75 D/year equivalent to >0.62 mm/18 months or >0.41 mm/year. Accordingly, the two studies show the same overall tendency, that treatment with OKL reduces the number of fast progressors.
No treatment‐requiring or vision‐threatening adverse events were observed during the follow‐up period of this study. Corneal staining occurred in all subjects. Therefore, we recommend to perform regular clinical controls at a maximum interval of 3 months to eliminate agents and courses that affect corneal surface health. According to a meta‐analysis comprising 333 OKL subjects, no severe adverse events were reported in clinical studies (Li et al. 2016). However, MK has been reported in case reports and case series (Kam et al. 2017). One study compared the lifetime risk of MK to the risk of vision impairment associated with myopia in subjects wearing different types of contact lenses. The lifetime likelihood of myopia‐induced vision impairment was 3.9 (95% CI 2.2 to 19.2), 3.8 (95% CI 2.4 to 9.3) and 1.1 (95% CI 0.9 to 1.3) in eyes with AL of 26 to <28 mm, 28 to <30 mm and >30 mm, respectively (Gifford 2019). These findings suggest that treatment with OKL is indicated in myopic subjects at risk of developing high myopia. Growth charts based on three European study populations have been developed to estimate individual risks of high myopia based on age and AL (Tideman et al. 2018). Using the growth charts, clinicians can estimate the risk of high myopia before advising and initiating myopia control.
The motivation among subjects and their parents at the initial stages of treatment seemed essential to successful treatment initiation. Handout during the dark season was significantly associated with a higher number of dropouts. One explanation could be that the subjects were more tired and less motivated for the OKL handling at bedtime during the dark season. Other studies have revealed similar high dropout rates (Walline et al. 2009; Hiraoka et al. 2012; Paune et al. 2015), which highlights the need to make different treatment modalities available for the control of myopia.
The strength of this study is its randomized design. Because the treatment effect was higher than assumed during sample size calculation, smaller than planned sample size provided sufficient statistical power for the pre‐defined level of type I error (e.g. adjusted alpha of 0.002).
Since the dropout rate was higher in the OKL group than in the SVS group, known and unknown prognostics factors may have been unevenly distributed among subjects who completed the study. To correct for this, the statistical analysis was adjusted for baseline AL, baseline SER and baseline age. An assumption of linear progression was made when multiplying the emmetropic one‐year growth rate from a previous study (Fledelius et al. 2014) by 1.5, and hence, the emmetropic growth rate used for the progression groups was an approximation. The duration of the study was relatively short. However, the contrasts in axial progression between intervention and control groups at follow‐ups were comparable to other studies, and hence, it can be speculated that two‐year results would also be similar.
Conclusion
This randomized study showed that OKL effectively reduce axial growth in myopic Danish children without treatment‐requiring or vision‐threatening adverse events. There were no fast progressors in the OKL group. In the OKL group, the dropout rate was significantly associated with lens handout during the dark season.
The authors acknowledge OPEN, Odense Patient Data Explorative Network, Odense University Hospital, Odense, Denmark, for data storage and data management support.
The study was supported by grants from the Region of Southern Denmark; Forskningsrådet at University Hospital of Southern Denmark, Vejle Hospital; Bagenkop Nielsen Myopia Foundation; Fabrikant Einar Willumsen Foundation; Læge Frk. K Rasmussen Foundation; Gangsted Foundation, Fight for Sight, Denmark; Overlæge Jørgen Werner Schous og hustru Else‐Marie Schou, født Wonge Foundation; Henry og Astrid Møller Foundation; Grosserer Chr Andersen og hustru Foundation; The Danish Eye Research Foundation; Helsefonden.
The study was approved by the Regional Committee on Health Research Ethics and adhered to the tenets of the Declaration of Helsinki. The legal guardians of all subjects gave written informed consent after receiving oral and written information about the study from TMJ. The study was registered at ClinicalTrial.gov (NCT03246464).
References
- Cho P & Cheung SW (2012): Retardation of myopia in Orthokeratology (ROMIO) study: a 2‐year randomized clinical trial. Invest Ophthalmol Vis Sci 53: 7077–7085. [DOI] [PubMed] [Google Scholar]
- Cho P & Cheung SW (2017): Protective role of orthokeratology in reducing risk of rapid axial elongation: a reanalysis of data from the ROMIO and TO‐SEE studies. Invest Ophthalmol Vis Sci 58: 1411–1416. [DOI] [PubMed] [Google Scholar]
- Cho P, Cheung SW & Edwards M (2005): The Longitudinal Orthokeratology Research in Children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res 30: 71–80. [DOI] [PubMed] [Google Scholar]
- Cruysberg LP, Doors M, Verbakel F, Berendschot TT, De Brabander J & Nuijts RM (2010): Evaluation of the Lenstar LS 900 non‐contact biometer. Br J Ophthalmol 94: 106–110. [DOI] [PubMed] [Google Scholar]
- Davies LN, Mallen EA, Wolffsohn JS & Gilmartin B (2003): Clinical evaluation of the Shin‐Nippon NVision‐K 5001/Grand Seiko WR‐5100K autorefractor. Optom Vis Sci 80: 320–324. [DOI] [PubMed] [Google Scholar]
- Efron N, Morgan PB & Katsara SS (2001): Validation of grading scales for contact lens complications. Ophthalmic Physiol Opt 21: 17–29. [PubMed] [Google Scholar]
- Ferris FL 3rd, Kassoff A, Bresnick GH & Bailey I (1982): New visual acuity charts for clinical research. Am J Ophthalmol 94: 91–96. [PubMed] [Google Scholar]
- Fledelius HC, Christensen AS & Fledelius C (2014): Juvenile eye growth, when completed? An evaluation based on IOL‐Master axial length data, cross‐sectional and longitudinal. Acta Ophthalmol 92: 259–264. [DOI] [PubMed] [Google Scholar]
- Gifford KL (2019): Childhood and lifetime risk comparison of myopia control with contact lenses. Cont Lens Anterior Eye 43: 26–32. [DOI] [PubMed] [Google Scholar]
- Guggenheim JA, Northstone K, McMahon G, Ness AR, Deere K, Mattocks C, Pourcain BS & Williams C (2012): Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci 53: 2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MH, Laigaard PP, Olsen EM, Skovgaard AM, Larsen M, Kessel L & Munch IC (2020): Low physical activity and higher use of screen devices are associated with myopia at the age of 16–17 years in the CCC2000 Eye Study. Acta Ophthalmol 98: 315–321. [DOI] [PubMed] [Google Scholar]
- Hiraoka T, Kakita T, Okamoto F, Takahashi H & Oshika T (2012): Long‐term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5‐year follow‐up study. Invest Ophthalmol Vis Sci 53: 3913–3919. [DOI] [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA et al. (2016): Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- Ikuno Y (2017): Overview of the complications of high myopia. Retina 37: 2347–2351. [DOI] [PubMed] [Google Scholar]
- Jacobsen N, Jensen H & Goldschmidt E (2007): Prevalence of myopia in Danish conscripts. Acta Ophthalmol Scand 85: 165–170. [DOI] [PubMed] [Google Scholar]
- Jong M, Sankaridurg P, Naduvilath TJ, Li W & He M (2018): The relationship between progression in axial length/corneal radius of curvature ratio and spherical equivalent refractive error in myopia. Optom Vis Sci 95: 921–929. [DOI] [PubMed] [Google Scholar]
- Kakita T, Hiraoka T & Oshika T (2011): Influence of overnight orthokeratology on axial elongation in childhood myopia. Invest Ophthalmol Vis Sci 52: 2170–2174. [DOI] [PubMed] [Google Scholar]
- Kam KW, Yung W, Li GKH, Chen LJ & Young AL (2017): Infectious keratitis and orthokeratology lens use: a systematic review. Infection 45: 727–735. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jee D, Kwon JW & Lee WK (2013): Prevalence and risk factors for myopia in a rural Korean population. Invest Ophthalmol Vis Sci 54: 5466–5471. [DOI] [PubMed] [Google Scholar]
- Li SM, Kang MT, Wu SS, Liu LR, Li H, Chen Z & Wang N (2016): Efficacy, safety and acceptability of orthokeratology on slowing axial elongation in myopic children by meta‐analysis. Curr Eye Res 41: 600–608. [DOI] [PubMed] [Google Scholar]
- Lin LL, Shih YF, Hsiao CK & Chen CJ (2004): Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singapore 33: 27–33. [PubMed] [Google Scholar]
- Lopes MC, Andrew T, Carbonaro F, Spector TD & Hammond CJ (2009): Estimating heritability and shared environmental effects for refractive error in twin and family studies. Invest Ophthalmol Vis Sci 50: 126–131. [DOI] [PubMed] [Google Scholar]
- Paune J, Morales H, Armengol J, Quevedo L, Faria‐Ribeiro M & Gonzalez‐Meijome JM (2015): Myopia control with a novel peripheral gradient soft lens and orthokeratology: a 2‐year clinical trial. Biomed Res Int 2015: 507572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prousali E, Haidich AB, Fontalis A, Ziakas N, Brazitikos P & Mataftsi A (2019): Efficacy and safety of interventions to control myopia progression in children: an overview of systematic reviews and meta‐analyses. BMC Ophthalmol. 19: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santodomingo‐Rubido J, Villa‐Collar C, Gilmartin B & Gutierrez‐Ortega R (2012): Myopia control with orthokeratology contact lenses in Spain: refractive and biometric changes. Invest Ophthalmol Vis Sci 53: 5060–5065. [DOI] [PubMed] [Google Scholar]
- Swarbrick HA, Alharbi A, Watt K, Lum E & Kang P (2015): Myopia control during orthokeratology lens wear in children using a novel study design. Ophthalmology 122: 620–630. [DOI] [PubMed] [Google Scholar]
- Tideman JWL, Polling JR, Vingerling JR, Jaddoe VWV, Williams C, Guggenheim JA & Klaver CCW (2018): Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol 96: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walline JJ, Jones LA & Sinnott LT (2009): Corneal reshaping and myopia progression. Br J Ophthalmol 93: 1181–1185. [DOI] [PubMed] [Google Scholar]
- Williams KM, Kraphol E, Yonova‐Doing E, Hysi PG, Plomin R & Hammond CJ (2019): Early life factors for myopia in the British Twins Early Development Study. Br J Ophthalmol 103: 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani N, Sadeghi R, Momeni‐Moghaddam H, Zarifmahmoudi L & Ehsaei A (2018): Comparison of cyclopentolate versus tropicamide cycloplegia: a systematic review and meta‐analysis. J Optom 11: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]


