Abstract
Objective
The purpose of this study was to identify risk factors for acute symptomatic seizures and post‐stroke epilepsy after acute ischemic stroke and evaluate the effects of reperfusion treatment.
Methods
We assessed the risk factors for post‐stroke seizures using logistic or Cox regression in a multicenter study, including adults from 8 European referral centers with neuroimaging‐confirmed ischemic stroke. We compared the risk of post‐stroke seizures between participants with or without reperfusion treatment following propensity score matching to reduce confounding due to treatment selection.
Results
In the overall cohort of 4,229 participants (mean age 71 years, 57% men), a higher risk of acute symptomatic seizures was observed in those with more severe strokes, infarcts located in the posterior cerebral artery territory, and strokes caused by large‐artery atherosclerosis. Strokes caused by small‐vessel occlusion carried a small risk of acute symptomatic seizures. 6% developed post‐stroke epilepsy. Risk factors for post‐stroke epilepsy were acute symptomatic seizures, more severe strokes, infarcts involving the cerebral cortex, and strokes caused by large‐artery atherosclerosis. Electroencephalography findings within 7 days of stroke onset were not independently associated with the risk of post‐stroke epilepsy. There was no association between reperfusion treatments in general or only intravenous thrombolysis or mechanical thrombectomy with the time to post‐stroke epilepsy or the risk of acute symptomatic seizures.
Interpretation
Post‐stroke seizures are related to stroke severity, etiology, and location, whereas an early electroencephalogram was not predictive of epilepsy. We did not find an association of reperfusion treatment with risks of acute symptomatic seizures or post‐stroke epilepsy. ANN NEUROL 2021;90:808–820
In general, post‐stroke seizures occur in 6% to 8% of adults with ischemic stroke. 1 , 2 They may increase metabolic stress and cell death, leading to an increase in infarct size, mortality rate, and negative functional outcome. 3 , 4 In the long‐term, seizures may cause injuries, impair the ability to work or operate vehicles, and impact the quality of life. 5 Anti‐seizure medications (ASM) may cause side‐effects or lead to drug–drug interactions, which could reduce the effect of secondary post‐stroke preventive treatment. 4 , 6
Post‐stroke seizures are termed as acute symptomatic (occurring within the first week after stroke) or remote symptomatic (late‐onset; occurring after the first week). 7 According to the International League Against Epilepsy (ILAE) definition, a single unprovoked remote symptomatic post‐stroke seizure >7 days after stroke qualifies as structural epilepsy due to the high (>60%) risk of recurrence within the next 10 years. 8 , 9
Several clinical risk factors for the development of acute or remote symptomatic seizures following stroke have been identified. 10 , 11 There is little knowledge on the role of brain imaging or electroencephalography (EEG) findings for the prediction of post‐stroke seizures. Brain imaging showing a large infarct involving the cortex, particularly in the territory of the middle cerebral artery (MCA), may be associated with a higher risk of seizures. 10 , 11 Two studies using EEG identified diffuse background slowing, background asymmetry, and interictal epileptiform discharges as potential predictors of post‐stroke epilepsy. 12 , 13
Thrombolysis (IV or IA) and endovascular thrombectomy are effective and frequently utilized reperfusion treatments for acute ischemic stroke. Recent reports have suggested that reperfusion treatments may increase the risk of post‐stroke seizures. 14 , 15 , 16 , 17 Others, however, have not found a correlation. 16 , 18 , 19 , 20 , 21 , 22 The potential association raised concerns as seizures or post‐stroke epilepsy were not recognized as complications of reperfusion treatments in pivotal trials. 23 , 24 Several of the reports were, however, based on uncontrolled studies with small sample sizes and were confounded by treatment‐selection (eg, thrombolysis vs no thrombolysis). A recent systematic review concluded that there is still uncertainty about the association. 25
Here, we compared the incidence of post‐stroke seizures in a large multicenter registry using procedures to reduce confounds due to treatment‐selection and, thus, replicating some of the characteristics of controlled trials. 26 We also assessed the role of brain imaging and EEG findings as predictors of post‐stroke seizures.
Methods
Study Population
We used data from the SeLECT study of post‐stroke seizures, currently involving 8 international cohorts. 1 In brief, we included consecutive individuals aged 18 years or older with neuroimaging‐confirmed acute ischemic stroke. We excluded those with a medical history of seizures or epilepsy before stroke, primary hemorrhagic stroke, no evidence of stroke on neuroimaging, transient ischemic attack, potentially epileptogenic comorbidities (ie, intracranial tumor, cerebral venous thrombosis, history of severe traumatic brain injury, and history of brain surgery), or re‐infarction during follow‐up. The study flow‐chart is shown in Fig 1. Follow‐up was performed using face‐to‐face interviews (in Austria, Italy, Portugal, and Switzerland [2]), telephone interviews (in Germany [2], Spain, and Switzerland [2]), medical chart review (in Germany [1]), or telephone screening followed by face‐to‐face interview (in Switzerland [1]).
FIGURE 1.
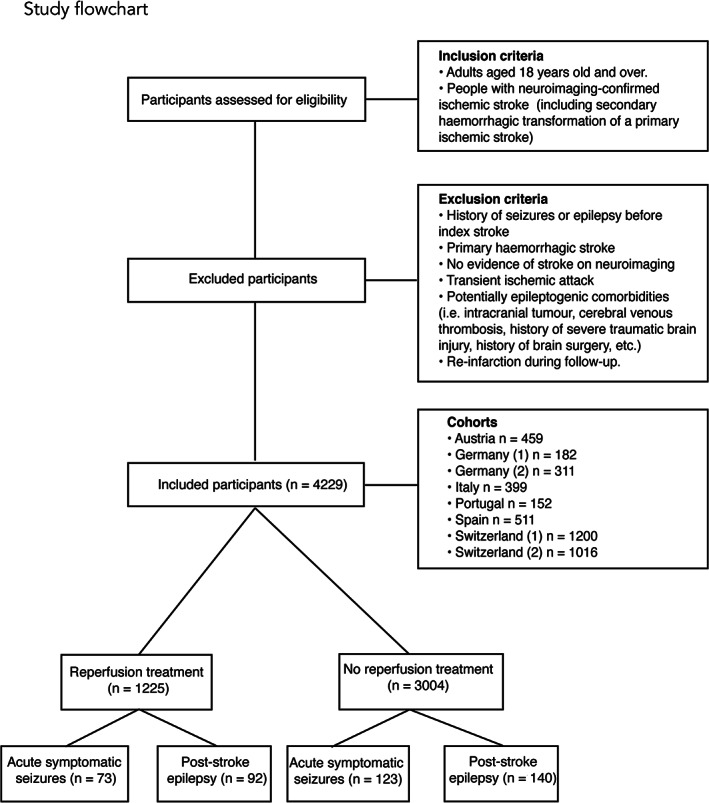
Study flowchart.
Regulatory approval was granted from all local ethical committees. All subjects in the Italian, Spanish, and Portuguese cohort and those having a face‐to‐face interview in the Swiss cohort gave written informed consent. All subjects evaluated by telephone in the Swiss and German (2) cohorts gave verbal informed consent. According to Swiss and German law, the regional ethical committees exempted these cohorts from requiring written informed consent. The Austrian case–control study was classified as a retrospective service evaluation by the regional ethical committee and informed consent was not required.
Definitions
We used the World Health Organization (WHO) definitions for stroke and ILAE definitions for seizures, which were classified as acute symptomatic (≤7 days post‐stroke) or remote symptomatic (spontaneous unprovoked seizures >7 days post‐stroke, congruent with post‐stroke epilepsy). 27 , 28 Acute provoked seizures (eg, due to electrolyte disturbance, induced by medication, etc.) were included as acute symptomatic, however, remote provoked seizures were not considered as post‐stroke epilepsy. Stroke severity was estimated using the National Institutes of Health Stroke Scale (NIHSS). Stroke etiology was categorized according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification. Involvement of the cerebral cortex and/or vascular territory by irreversible infarction was determined by best available neuroimaging (computed tomography [CT] or magnetic resonance imaging [MRI]).
We also evaluated a subset of patients who received a routine EEG using the 10–20 system within the first 7 days after stroke onset. We assessed diffuse background activity slowing, focal slowing, interictal epileptiform discharges, lateralized periodic discharges, electrographic seizures, and status epilepticus in the EEG recordings based on established definitions. 29 , 30 The EEG was classified as abnormal if any of the above factors were present.
Missing values (0.8%) in the 4 original SeLECT cohorts were imputed using multiple imputation methods, as previously described (see online supplement for details on missing values). 1 Data in the 4 newly added cohorts was complete and imputation was not necessary.
Our primary outcome was time to post‐stroke epilepsy. Our secondary outcome was the occurrence of acute symptomatic seizures after starting reperfusion treatment in participants with acute ischemic stroke.
Statistical Analysis
To evaluate variables that are associated with the risk of acute symptomatic seizures, we first used univariable logistic regression and subsequently analyzed all significant (p < 0.05) variables in a multivariable logistic regression model (Table 1). We applied a similar 2‐step approach using Cox regression to assess factors associated with time to post‐stroke epilepsy (Table 2). We also evaluated the predictive role of early (within the first 7 days following stroke) EEG for post‐stroke epilepsy using Cox regression and the two‐step approach (Table 3). For this, we selected individuals with available early EEG data after stroke (n = 673).
TABLE 1.
Logistic Regression Analysis of Predictors Associated with Acute Symptomatic Seizures Following Acute Ischemic Stroke
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | p value | aOR (95% CI) | p value | |
| Age | 1.00 (0.98–1) | 0.532 | ||
| Sex (male) | 1.02 (0.91–1.64) | 0.178 | ||
| NIHSS at admission | ||||
| 0–3 | 0.77 (0.56–1.02) | 0.075 | ||
| 4–10 | 0.86 (0.62–1.15) | 0.315 | ||
| ≥11 | 2.14 (1.58–2.87) | <0.001 | 1.68 (1.22–2.31) | 0.001 |
| Stroke etiology | ||||
| Large‐artery atherosclerosis | 1.82 (1.32–2.51) | <0.001 | 1.43 (1.03–1.98) | 0.025 |
| Small‐vessel occlusion | 0.14 (0.05–0.26) | <0.001 | 0.19 (0.08–0.39) | <0.001 |
| Cardioembolic | 1.09 (0.8–1.48) | 0.553 | ||
| Stroke location | ||||
| Middle cerebral artery | 1.22 (0.89–1.71) | 0.204 | ||
| Anterior cerebral artery | 1.34 (0.71–2.3) | 0.321 | ||
| Posterior cerebral artery | 1.51 (1.1–2.04) | 0.008 | 1.46 (1.07–2.00) | 0.015 |
| Cortical involvement | 0.02 (1.32–2.39) | <0.001 | 1.32 (0.98–1.79) | 0.069 |
| Reperfusion treatment | 1.48 (1.09–1.99) | 0.008 | 1.04 (0.75–1.42) | 0.817 |
OR = odds ratio; aOR = adjusted odds ratio; CI = confidence interval; NIHSS = National Institutes of Health Stroke Scale.
TABLE 2.
Cox Regression Analysis of Factors Associated with Time to Post‐Stroke Epilepsy Following Acute Ischemic Stroke
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p value | aHR (95% CI) | p value | |
| Age | 0.99 (0.99–1.01) | 0.096 | ||
| Sex (male) | 0.86 (0.66–1.11) | 0.266 | ||
| NIHSS at admission | ||||
| 0–3 | 0.41 (0.31–0.55) | <0.001 | 0.75 (0.52–1.09) | 0.136 |
| 4–10 | 0.79 (0.59–1.05) | 0.108 | ||
| ≥11 | 3.62 (2.79–4.69) | <0.001 | 2.33 (1.69–3.21) | <0.001 |
| Stroke etiology | ||||
| Large‐artery atherosclerosis | 2.07 (1.52–2.64) | <0.001 | 1.51 (1.13–2.01) | 0.004 |
| Small‐vessel occlusion | 0.37 (0.24–0.57) | <0.001 | 0.92 (0.57–1.46) | 0.728 |
| Cardioembolic | 0.98 (0.73–1.30) | 0.901 | ||
| Stroke location | ||||
| Middle cerebral artery | 2.26 (1.62–3.15) | <0.001 | 1.35 (0.95–1.92) | 0.087 |
| Anterior cerebral artery | 0.98 (0.50–1.91) | 0.957 | ||
| Posterior cerebral artery | 1.15 (0.86–1.54) | 0.330 | ||
| Cortical involvement | 2.75 (2.07–3.66) | <0.001 | 2.07 (1.54–2.80) | <0.001 |
| ASS | 5.87 (4.11–8.38) | <0.001 | 4.18 (2.90–6.02) | <0.001 |
| Reperfusion treatment | 1.85 (1.41–2.40) | <0.001 | 1.05 (0.78–1.40) | 0.727 |
HR = hazard ratio; aHR = adjusted hazard ratio; ASS = acute symptomatic seizure; CI = confidence interval; NIHSS = National Institutes of Health Stroke Scale.
TABLE 3.
Cox Regression Analysis of Early EEG Parameters Associated with Time to Post‐Stroke Epilepsy Following Acute Ischemic Stroke
| Predictors | A: Univariable | B: Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p value | aHR (95% CI) | p value | |
| Age at stroke | 0.99 (0.97–1.00) | 0.566 | ||
| Sex (male) | 1.14 (0.67–1.93 | 0.625 | ||
| NIHSS at admission | ||||
| 0‐3 | 0.36 (0.2–0.66) | 0.001 | 0.55 (0.25–1.21) | 0.140 |
| 4–10 | 0.93 (0.53–1.63) | 0.807 | ||
| ≥11 | 3.37 (1.99–5.70) | <0.001 | 1.27 (0.65–242) | 0.460 |
| Stroke etiology | ||||
| Large‐artery atherosclerosis | 2.78 (1.62–4.78) | 0.002 | 1.62 (0.90—2.90) | 0.104 |
| Small‐vessel occlusion | 0.55 (0.28–1.10) | 0.093 | ||
| Cardioembolic | 0.63 (0.33–1.23) | 0.182 | ||
| Stroke location | ||||
| Middle cerebral artery | 2.22 (1.05–4.90) | 0.05 | 1.00 (0.39–2.43) | 0.969 |
| Anterior cerebral artery | 0.96 (0.13–7.08) | 0.96 | ||
| Posterior cerebral artery | 1.66 (0.63–4.39) | 0.303 | ||
| Cortical involvement | 2.15 (1.21–3.84) | 0.008 | 2.00 (1.10–3.63) | 0.022 |
| Early ASM treatment | 12.72 (7.55–21.43) | <0.001 | 10.47 (5.63–19.47) | <0.001 |
| Early EEG findings | ||||
| Abnormal | 2.36 (1.18–4.68) | 0.014 | 2.09 (0.90–4.82) | 0.084 |
| Diffuse slow | 2.96 (1.61–5.44) | <0.001 | 1.26 (0.65–2.43) | 0.487 |
| Focal slow | 1.79 (1.05–3.05) | 0.03 | 0.56 (0.30–1.32) | 0.110 |
| Interictal epileptiform discharges | 0.87 (0.21–3.60) | 0.855 | ||
| Lateralized periodic discharges | 1.76 (0.63–4.95) | 0.278 | ||
| Early seizure on EEG | 11.11 (4.42–27.93) | <0.001 | 2.01 (0.72–5.58) | 0.177 |
| Status epilepticus on EEG | 2.62 (0.36–19.00) | 0.34 | ||
N = 673.
aHR = adjusted hazard ratio; ASM = anti‐seizure medication; CI = confidence interval; EEG = electroencephalogram; HR = hazard ratio; NIHSS = National Institutes of Health Stroke Scale.
Comparisons of those who did and did not receive reperfusion treatment are potentially confounded by treatment selection bias. Severe strokes with large vessel occlusion affecting the cerebral hemispheres are more likely to receive reperfusion treatment, and more likely to be associated with subsequent seizures. We used propensity score matching (PSM) to reduce treatment‐selection bias and to mimic the characteristics of a randomized controlled trial in regard to comparing treatment groups without the need for additional adjustment using regression methods. 26 PSM is considered a reliable method to achieve an optimal between‐group balance in covariates. 31 It provides treatment effects estimates that are closer to the true marginal treatment effect, making it a superior alternative to classical regression particularly when the number of covariates is 5 or more. 32 , 33
First, we compared the baseline characteristics between participants who received reperfusion treatments (IV or IA thrombolysis or endovascular thrombectomy) and those who did not using chi‐square tests for normally distributed data and Wilcoxon rank sum tests for those with a non‐normal distribution (Supplementary Table S1). The reported baseline characteristics also included those that were previously identified as independent predictors of seizure risk (severity of stroke, large‐artery atherosclerotic etiology, cortical involvement, and middle cerebral artery territory involvement). 1 We also used multivariable Cox proportional hazards analysis to explore covariates associated with post‐stroke epilepsy and multivariable logistic regression to identify those associated with acute symptomatic seizures before matching. All predictors were assessed for proportional hazards by statistical and visual inspection of log(−log) plots. Multicollinearity was assessed with the variance inflation factor (VIF). Martingale residuals plots were analyzed to assess for nonlinearity. Assumptions related to the study design and data collection (left censoring, noninformative censoring, and secular trends) were fulfilled.
Second, we selected a subgroup matched on the logit of the propensity score using a greedy nearest neighbor algorithm within a caliper width of 0.2 of the standard deviation of the logit of the propensity score. This method results in less biased estimates compared to other matching algorithms. 34 Matching was performed on all covariates (see Supplementary Table S1). Last, to assess for balance of covariates between groups before and after matching, we visually evaluated density function plots and estimated the standardized mean difference (SMD), an SMD greater than 0.15 being indicative of poor balance.
As a secondary analysis, we repeated the same matching procedure to compare participants who received IV thrombolysis using recombinant tissue plasminogen activator (rtPA) versus those who received no reperfusion treatment (Supplementary Table S2). The rationale was to specifically explore the effects of rtPA on post‐stroke seizures rather than the effects of reperfusion, per se. As another secondary analysis, we performed the same procedures for individuals who received mechanical thrombectomy only versus those who received no reperfusion treatment. Data in the German (2) cohort did not distinguish between types of reperfusion treatment, so participants receiving reperfusion treatment in this cohort were not included for these secondary analyses.
We compared time to post‐stroke epilepsy (primary outcome) between participants with or without reperfusion treatment using the log‐rank test and the Kaplan Meier method. The occurrence of acute symptomatic seizures (secondary outcome) was compared using logistic regression, because time to event was <1 week and thus pragmatically classified as binomial. Separate analyses were performed using the initial and the matched samples.
Third, we performed additional analyses:
We used causal mediation analysis to evaluate the relationship between reperfusion treatment and secondary hemorrhage with the occurrence of acute symptomatic seizures or post‐stroke epilepsy. As suggested by other authors, we hypothesized that the epileptogenic effect could be mediated by the occurrence of secondary hemorrhage and not by the reperfusion treatment itself. We performed this analysis in the Swiss (1) and Italian cohorts (n = 737) that had information on secondary hemorrhage. To control for exposure‐outcome confounding, we applied the same matching for treatment propensity as described above and evaluated the average causal mediation effect (ie, ACME) of secondary hemorrhage on the outcome after matching. Confidence intervals (CIs) around mediation effect estimates were generated using bootstrapping with 1,000 simulations.
We evaluated the association between time to IV thrombolysis (door‐to‐needle) or successful recanalization after IA or mechanical recanalization and risks of acute symptomatic seizures or post‐stroke epilepsy using linear and Cox regression analyses. For this, we only included the Swiss (2) cohort, given that data on time to IV thrombolysis (n = 415) or successful recanalization after interventional reperfusion treatment (n = 195) were collected in a standardized manner in this center
We calculated the power to reject the null hypothesis (reperfusion treatment is associated with acute symptomatic seizures or post‐stroke epilepsy) with a 5% type I error rate 35 and using the risks and sample sizes obtained in the above calculations.
We followed established recommendations (ie, STROBE checklist). Analyses were performed using R version 1.1.453, using the packages “survival,” “survminer,” “MatchIt,” “stddiff,” “mediation,” and “ggplot2.”
Results
The overall registry included 4,229 individuals (mean age = 71 years old; 57% men, see Supplementary Table S1) from 8 European cohorts (Austria n = 459, Germany [1] n = 182, Germany [2] n = 311, Italy n = 399, Portugal n = 152, Spain = 511, Switzerland [1] n = 1,200, and Switzerland [2] n = 1,016). Four of these cohorts (Switzerland [1], Austria, Germany [2], and Italy) were part of the original SeLECT study and 2 of the 4 additional cohorts were previously published. 1 , 36 , 37 , 38 , 39 Overall, 1,225 patients (29%) received reperfusion treatment, 196 (5%) experienced acute symptomatic seizures, and 232 (6%) had post‐stroke epilepsy during follow‐up of a median of 1.6 years (interquartile range [IQR] = 1.0–3.3).
The frequency of acute symptomatic seizures and post‐stroke epilepsy among reperfusion groups were as follows: those who received reperfusion treatment, 6% and 8%; IV thrombolysis, 6% and 8%; IA thrombolysis 7% and 5%; and mechanical thrombectomy 8% and 5%, respectively. Median time to post‐stroke epilepsy was 1.87 years (IQR = 1.0–3.2) in participants without reperfusion treatment and 1.1 years (IQR = 1.0–2.2) in participants with reperfusion treatment.
Factors Associated with Acute Symptomatic Seizures or Post‐Stroke Epilepsy
Variables independently associated with a higher risk of acute symptomatic seizures in the overall cohort (n = 4,299; see Table 1) were NIHSS at admission ≥11 points (adjusted odds ratio [aOR] = 1.7, 95% CI = 1.2–2.3, p < 0.001), stroke located in the posterior cerebral artery (PCA) territory (aOR = 1.5, 95% CI = 1.1–2.0, p = 0.02), and stroke caused by large‐artery atherosclerosis (aOR = 1.4, 95% CI = 1.0–2.0, p = 0.03). Stroke caused by small‐vessel occlusion had a lower risk of acute symptomatic seizures (aOR = 0.2, 95% CI = 0.1–0.4, p < 0.001).
Factors independently associated with a shorter time to develop post‐stroke epilepsy (see Table 2) included acute symptomatic seizures (adjusted hazard ratio [aHR] = 4.2, 95% CI = 2.9–6.0, p < 0.001), NIHSS at admission ≥11 points (aHR = 2.3, 95% CI = 1.7–3.2, p < 0.001), stroke involving the cerebral cortex (aHR = 2.1, 95% CI = 1.5–2.8, p < 0.001), and stroke caused by large‐artery atherosclerosis (aHR = 1.5, 95% CI = 1.1–2.0, p = 0.004).
Reperfusion treatment was associated with a higher risk of post‐stroke epilepsy (HR = 1.9, 95% CI = 1.4–2.4, p < 0.001; Fig 2A1) and acute symptomatic seizures (OR = 1.5, 95% CI = 1.1–2.0, p = 0.008; Fig 3A) in univariable analysis. This association was nonsignificant after adjusting for other covariates in multivariable analysis (acute symptomatic seizures: aOR = 1.0, 95% CI = 0.8–1.4, p = 0.82; post‐stroke epilepsy: aHR = 1.1, 95% CI = 0.8–1.4, p = 0.74).
FIGURE 3.
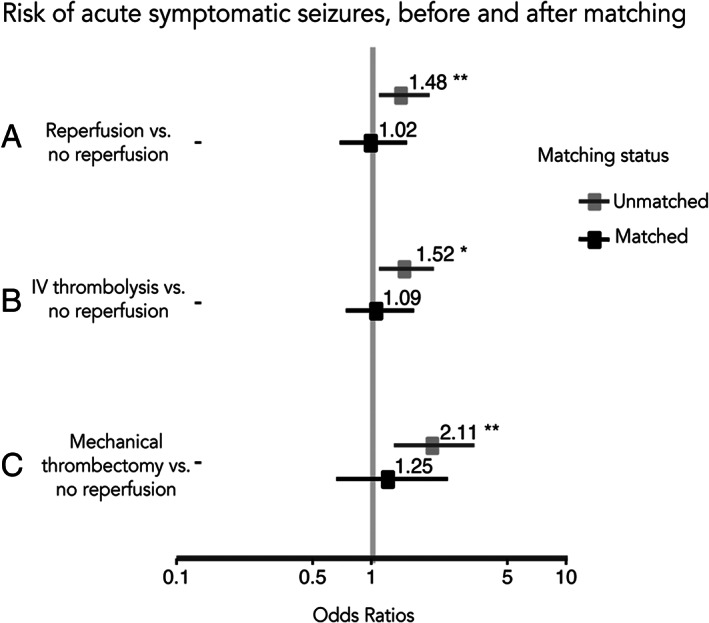
Risk of acute symptomatic seizures, before and after matching. Forest plot showing odd ratios and 95% confidence intervals (horizontal lines) for the risk of acute symptomatic seizures after acute ischemic stroke for each of the scenarios.
Predictive Role of Early EEG Findings for Post‐Stroke Epilepsy
We studied the role of EEG data within the 7 days of stroke onset for prediction of post‐stroke epilepsy in a subset of 673 subjects from 3 cohorts (Portugal and Switzerland [1] and [2]) with available EEG data. Abnormal early EEG (HR = 2.36, 95% CI = 1.18–4.68), diffuse background slowing (HR = 2.96, 95% CI = 1.61–5.44), focal slowing (HR = 1.79, 95% CI = 1.05–3.05), and early seizures on EEG (HR = 11.11, 95% CI = 4.42–27.93) were significant predictors of post‐stroke epilepsy in univariable Cox regression analysis (see Table 3A). None of the studied EEG abnormalities were significant after multivariable correction for covariates (see Table 3B).
Reperfusion (IV, IA, or Mechanical Thrombectomy) Versus No Reperfusion Treatment
We matched 936 patients receiving reperfusion treatment and the same number of controls for reperfusion treatment propensity. No significant differences in baseline characteristics were observed after matching. Standardized differences (see Supplementary Table S1) and density plots (Fig 4A) showed improved balance for all included covariates.
FIGURE 4.
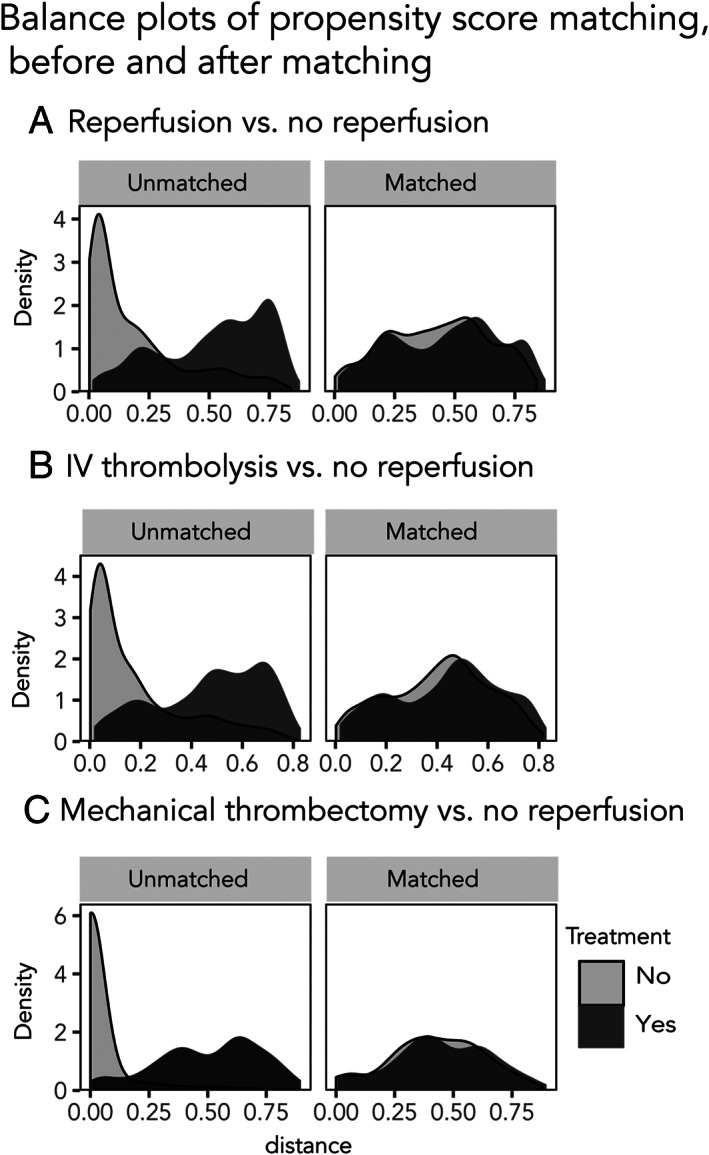
Density plots of propensity score matching. Density plots showing the covariate balance before and after matching, for each of the scenarios.
After matching, we did not observe an association between reperfusion treatment and time to post‐stroke epilepsy (HR = 1.05, 95% CI = 0.75–1.48, p = 0.74; see Fig 2A2) and risk of acute symptomatic seizures (OR = 1.04, 95% CI = 0.70–1.55, p = 0.84; see Fig 3A).
FIGURE 2.
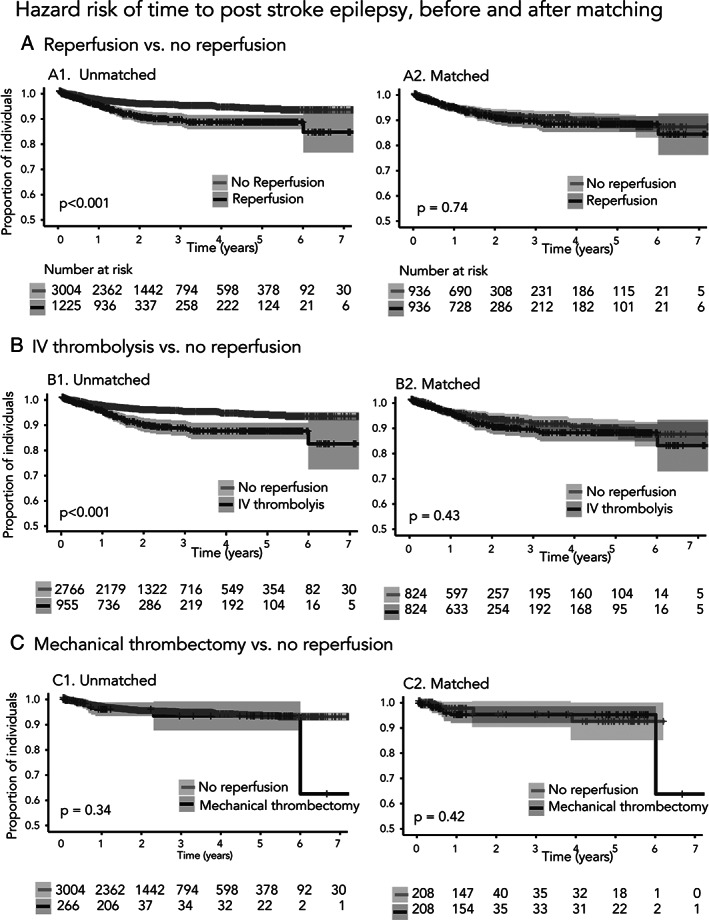
Hazard ratios of time to post‐stroke epilepsy, before and after matching. Kaplan–Meier estimates of time to post‐stroke epilepsy after acute ischemic stroke before and after matching for the propensity to receive or not treatment, for each of the scenarios. Time at baseline was index stroke. Shaded bands represent 95% confidence intervals.
Our study had an estimated power of 80% to reject the null hypothesis that reperfusion treatment is associated with acute symptomatic seizures and a 96% power to reject the null hypothesis that reperfusion treatment is associated with post‐stroke epilepsy.
IV Thrombolysis Versus No Reperfusion Treatment
Before matching, participants who received IV thrombolysis had a shorter time to post‐stroke epilepsy (HR = 1.98, 95% CI = 1.49–2.64, p < 0.001; see Fig 2B1) and higher risk of acute symptomatic seizures (OR = 1.52, 95% CI = 1.09–2.08, p = 0.001; see Fig 3B) compared to those that did not receive reperfusion treatment.
We matched 824 cases and controls for IV thrombolysis treatment propensity. No significant differences in baseline characteristics were observed after matching. Standardized differences (see Supplementary Table S2) and density plots (see Fig 4B) showed improved balance for all included covariates.
After matching, we did not observe any association between IV thrombolysis and time to post‐stroke epilepsy (HR = 1.15, 95% CI = 0.80–1.65, p = 0.43; see Fig 2B2) or risk of acute symptomatic seizures (OR = 1.09, 95% CI = 0.72–1.62, p = 0.68; see Fig 3B).
Mechanical Thrombectomy Only Versus No Reperfusion
The risk of acute symptomatic seizures for individuals that received mechanical thrombectomy after ischemic stroke versus those that received no reperfusion treatment was significant (OR = 2.11, 95% CI = 1.28–3.31, p = 0.001; see Fig 3C), whereas the risk of post‐stroke epilepsy was not (HR = 1.3, 95% CI = 0.74–2.32, p = 0.34; see Fig 2C1). After matching on the propensity to receive mechanical thrombectomy, we found no significant association with acute symptomatic seizures (OR = 1.24, 95% CI = 0.64–2.44, p = 0.51; see Fig 3C) or post‐stroke epilepsy (HR = 1.4, 95% CI = 0.56–3.79, p = 0.42; see Fig 2C2). Standardized differences and density plots (see Fig 4C) showed improved balance for all included covariates.
The Impact of Secondary Hemorrhage
The effect of reperfusion treatments (IV, IA, and/or mechanical thrombectomy) on the likelihood of acute symptomatic seizures and post‐stroke epilepsy was not mediated by secondary hemorrhage after matching for the propensity to receive reperfusion treatment. For acute symptomatic seizures, the unstandardized indirect effect was 0.99 (95% CI = 0.98–1.01, p = 0.91). For post‐stroke epilepsy, the unstandardized indirect effect was 0.98 (95% CI = 0.94–1.01, p = 0.26). The results were comparable when comparing IV thrombolysis with no reperfusion treatment (acute symptomatic seizure: unstandardized indirect effect 1.00, 95% CI = 0.98–1.01, p = 0.90; and post‐stroke epilepsy: 0.98, 95% CI = 0.93–1.01, p = 0.26). Further sensitivity analyses were not performed as the indirect effects were not significant. 40
The Impact of Time to Thrombolysis and Successful Recanalization
Neither time to thrombolysis nor successful recanalization after IA or mechanical thrombolysis were associated with the risk of acute symptomatic seizures (p = 0.18 and p = 0.30, respectively) or post‐stroke epilepsy (p = 0.59 and p = 0.53) in univariable and multivariable analyses in the Swiss (2) cohort.
Discussion
We used data from a large multicenter registry to show that the risk of post‐stroke seizures is associated with stroke severity, etiology, and location. Acute symptomatic seizures were the strongest predictor of post‐stroke epilepsy. Findings on routine EEG within the first week were not independently associated with post‐stroke epilepsy.
Reperfusion treatment, be it IV or IA thrombolysis or mechanical thrombectomy, for ischemic stroke was not associated with an increased risk of post‐stroke seizures after correction for treatment selection bias by PSM. Our analyses suggest that the previously described associations were potentially confounded by treatment selection, with perfusion therapy being more likely to occur in those with more severe cerebral infarcts.
The risk factors for acute symptomatic seizures and post‐stroke epilepsy identified in the current investigation were comparable with previous studies. 1 , 7 Acute symptomatic seizures were associated with more severe strokes (NIHSS at admission ≥11 points) and stroke etiology (higher risk in large‐artery atherosclerosis and lower risk in small‐vessel occlusion). Similarly, the risk of post‐stroke epilepsy was highest after more severe strokes (NIHSS at admission ≥11 points) and strokes caused by large‐artery atherosclerosis. The most relevant predictor of post‐stroke epilepsy were acute symptomatic seizures. Acute symptomatic seizures indicate an increased susceptibility to generate seizures following an insult thus being related to a markedly increased risk of developing epilepsy. Moreover, seizure‐related head injuries, in particular head trauma, may increase the risk of subsequent seizures and post‐traumatic epilepsy. 41
Infarct location was identified as a potential risk factor. Acute symptomatic seizures were associated with PCA territory infarcts. Future studies refining the areas affected with lesion‐symptom mapping could address these aspects and clarify whether there are particular areas that are most likely to be associated with developing seizures (eg, the posterior medial temporal lobe). For post‐stroke epilepsy, however, the most relevant prognostic factor was cortical damage, whereas the arterial location of the infarct did not play a significant role. This confirms the clinical observation that lesions in all supratentorial vascular territories may cause post‐stroke epilepsy, particularly if the cerebral cortex is involved.
In a subset of our cohort with EEG within the first 7 days following stroke, EEG findings did not predict post‐stroke epilepsy. This is in contrast with 2 smaller previous studies. 12 , 42 The differences between studies may be explained by adjustment for covariates. In our study, we rigorously adjusted our models for potential covariates, including the presence of acute symptomatic seizures. The EEG findings that were significant in univariable analysis were not relevant after correction for clinical variables and stroke location. In contrast, one previous study used a prospective assessment of repeated EEG recordings using a 64‐channel system, which may have increased the sensitivity and specificity of the EEG findings. 12 Thus, the chosen variables which were extracted from the visual analysis of early routine EEG may not be reliable independent biomarkers for the development of post‐stroke epilepsy, whereas extended EEG monitoring with a 64‐channel system showed some benefit in a previous study. 12
Acute symptomatic seizures after stroke are uncommon but they have a negative impact on outcome. 43 Seizures and periodic discharges on EEG are associated with increased metabolic stress and the release of extracellular glutamate, 44 which may contribute to neurotoxicity after stroke. Thus, reports that reperfusion treatment after stroke may cause seizures raised concern. 14 , 17 , 45 Proposed potential mechanisms are a pro‐epileptic effect of a maintained penumbra or blood–brain barrier disruption following reperfusion or a direct proconvulsive effect of rtPA. Treatment using rtPA has also been found to increase the likelihood of hemorrhagic transformation, 46 which in turn may increase the risk of post‐stroke seizures. 3 , 14 , 47
The optimal study design to assess the contribution of reperfusion treatment to seizure risk after stroke is a randomized controlled trial, but such a trial would require a large sample size. It would also be unethical to withhold reperfusion treatment due to its proven benefits. 48 , 49 Thus, the best available approach is to analyze prospectively acquired data from nonrandomized cohort studies. Such comparisons are confounded by treatment selection. We used PSM, to reduce treatment selection bias, to mimic important aspects of a randomized controlled trial.
Our results show that the previously reported association of reperfusion treatment with seizures can be attributed to treatment selection bias. Both time to post‐stroke epilepsy and the occurrence of acute symptomatic seizures were associated with higher stroke severity, infarcts involving the cortex and large‐artery atherosclerotic etiology. These factors are also associated with a higher propensity to receive reperfusion treatment (see Supplementary Table S1). This observation highlights the importance of adequately addressing confounders in nonrandomized trials and the need for a critical approach when interpreting data from nonrandomized studies.
We found no differences in the risk of acute or post‐stroke epilepsy after correction for reperfusion treatment propensity, neither in the overall population nor in the subgroup of individuals with stroke affecting the cortex in the MCA territory. As it has been previously hypothesized that rtPA may have a direct pro‐seizure effect, 25 , 50 we compared participants who received IV thrombolysis to those that had no reperfusion treatment. We did not find an effect of IV thrombolysis on post‐stroke seizures in the matched cohorts. It has also been hypothesized that mechanical thrombectomy may have pro‐convulsive effects. In our study, there was no effect of mechanical thrombectomy on post‐stroke seizures in the matched comparison.
Thrombolysis and thrombectomy reduce overall disability after stroke. 48 , 49 These treatments did, however, not appear to reduce the risk of post‐stroke seizures and epilepsy. We speculate that, despite the removal of the thrombus, there may be ongoing excitotoxicity, inflammation, or blood–brain‐barrier disruption leading to the development of an epileptogenic network. This highlights the importance to develop other strategies to prevent seizures and epileptogenesis after stroke. 47
Secondary hemorrhage has been described as a risk factor for seizures after stroke. 2 Our results show that secondary hemorrhage after reperfusion treatment or IV thrombolysis does not mediate the likelihood of having post‐stroke seizures. This result is in line with the lack of association between reperfusion treatment and occurrence of seizures.
Time to thrombolysis and successful recanalization have been shown to be strongly associated with function outcome and reduced mortality. 51 , 52 This is the first study examining the effect of these variables on the risk of post stroke epilepsy. Door‐to‐needle times and recanalization rates were not associated with the risk of acute symptomatic seizures or post‐stroke epilepsy.
Our study has several strengths. First, the assembled cohort is one of the largest studies of ischemic stroke participants with long follow‐up regarding seizures. We had a high power to detect potential differences between patients with or without reperfusion treatment. Second, our matching approach mimics some of the aspects of a randomized controlled trial and reduces confounds. Third, using data from 8 international centers provides support for the generalizability of the findings. Fourth, our results are applicable to a wide range of patients with ischemic stroke and also in those with stroke affecting the cortex in the MCA territory, that may have a larger a priori risk of seizures. Our results were valid for all types of reperfusion treatment and also in the subgroups receiving IV thrombolysis or mechanical thrombectomy.
Our study has limitations. First, we only considered clinically apparent seizures and may have underestimated the incidence of nonconvulsive seizures. Using continuous EEG after stroke might have increased the detection of seizures with subtle or no clinical signs but it would not be feasible in a retrospective study not designed to robustly detect asymptomatic seizures. Second, data on time to IV thrombolysis and recanalization rates after IA or mechanical reperfusion treatment were collected in a standardized manner only in the Swiss (2) cohort, restricting these analyses to a smaller sample. Last, even though we reduced between‐group baseline differences using PSM, we cannot rule out the possibility of residual confounding due to unmeasured or unknown factors.
To conclude, our results describe risk factors for seizures following stroke and show that reperfusion treatment after ischemic stroke is not associated with an increased risk of acute symptomatic seizures or of post‐stroke epilepsy.
Author Contributions
C.F.A. and M.G. contributed to the conception and design of the study. C.F.A., N.D., B.E.C., A.F., P.S., N.S., G.B., J.S., L.S., L.I., M.K., L.A., E.S., J.A.S., M.W., T.J.O., J.N.W., G.L.G., A.S., F.J., G.M., M.V., G.G., J.C., S.E., P.L., F.R., F.B., C.B., A.R.P., T.P.M., B.T., M.R.K., J.S.D., J.W.S., B.T., M.J.K., and M.G. contributed to the acquisition and analysis of the data. C.F.A. and M.G. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
The authors declared no conflict of interest.
Supporting information
Table S1. Baseline characteristics for participants with and without reperfusion treatment, before and after propensity score matching.
Table S2. Baseline characteristics for participants with IV thrombolysis and without reperfusion treatment, before and after propensity score matching.
Acknowledgments
J.S.D., J.W.S., and M.J.K. are based at the NIHR University College London Hospitals Biomedical Research Centre which is sponsored by the UK Department of Health. Open access funding provided by Universitat Zurich.
Contributor Information
Carolina Ferreira‐Atuesta, Email: carolina.ferreira@mssm.edu.
Marian Galovic, Email: marian.galovic@usz.ch.
References
- 1. Galovic M, Dohler N, Erdelyi‐Canavese B, et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol 2018;17:143–152. 10.1016/S1474-4422(17)30404-0. [DOI] [PubMed] [Google Scholar]
- 2. Zou S, Wu X, Zhu B, et al. The pooled incidence of post‐stroke seizure in 102 008 patients. Top Stroke Rehabil 2015;22:460–467. 10.1179/1074935715Z.00000000062. [DOI] [PubMed] [Google Scholar]
- 3. Brondani R, de Almeida AG, Cherubini PA, et al. Risk factors for epilepsy after thrombolysis for ischemic stroke: a cohort study. Front Neurol 2020;10:1256. 10.3389/fneur.2019.01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holtkamp M, Beghi E, Benninger F, et al. European stroke organisation guidelines for the management of post‐stroke seizures and epilepsy. Eur Stroke J 2017;2:103–115. 10.1177/2396987317705536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baranowski CJ. The quality of life of older adults with epilepsy: a systematic review. Seizure 2018;60:190–197. 10.1016/j.seizure.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 6. Stöllberger C, Finsterer J. Interactions between non‐vitamin K oral anticoagulants and antiepileptic drugs. Epilepsy Res 2016;126:98–101. 10.1016/j.eplepsyres.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 7. Beghi E, D'Alessandro R, Beretta S, et al. Incidence and predictors of acute symptomatic seizures after stroke. Neurology 2011;77:1785–1793. 10.1212/WNL.0b013e3182364878. [DOI] [PubMed] [Google Scholar]
- 8. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475–482. 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 9. Hesdorffer DC, Benn EKT, Cascino GD, Hauser WA. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia 2009;50:1102–1108. 10.1111/j.1528-1167.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 10. Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol 2000;57:1617–1622. 10.1001/archneur.57.11.1617. [DOI] [PubMed] [Google Scholar]
- 11. Galovic M, Ferreira‐Atuesta C, Abraira L, et al. Seizures and epilepsy after stroke: epidemiology, biomarkers and management. Drugs Aging 2021;38:285–299. 10.1007/S40266-021-00837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bentes C, Martins H, Peralta AR, et al. Early EEG predicts poststroke epilepsy. Epilepsia Open 2018;3:203–212. 10.1002/epi4.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Reuck J, Goethals M, Claeys I, et al. EEG findings after a cerebral territorial infarct in patients who develop early‐ and late‐onset seizures. Eur Neurol 2006;55:209–213. 10.1159/000093871. [DOI] [PubMed] [Google Scholar]
- 14. Naylor J, Thevathasan A, Churilov L, et al. Association between different acute stroke therapies and development of post stroke seizures. BMC Neurol 2018;18:1–7. 10.1186/s12883-018-1064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teunissen C, Menge T, Altintas A, et al. Consensus definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis. Mult Scler J 2013;19:1802–1809. 10.1177/1352458513488232. [DOI] [PubMed] [Google Scholar]
- 16. Belcastro V, Brigo F, Ferlazzo E, et al. Incidence of early poststroke seizures during reperfusion therapies in patients with acute ischemic stroke: an observational prospective study: (TESI study: “Trombolisi/Trombectomia e crisi Epilettiche precoci nello stroke Ischemico”). Epilepsy Behav 2019;104:106476. 10.1016/j.yebeh.2019.106476. [DOI] [PubMed] [Google Scholar]
- 17. Burneo JG, Antaya TC, Allen BN, et al. The risk of new‐onset epilepsy and refractory epilepsy in older adult stroke survivors. Neurology 2019;93:e568–e577. 10.1212/WNL.0000000000007895. [DOI] [PubMed] [Google Scholar]
- 18. Gasparini S, Ascoli M, Brigo F, et al. Younger age at stroke onset but not thrombolytic treatment predicts poststroke epilepsy: an updated meta‐analysis. Epilepsy Behav 2020;104:106540. 10.1016/j.yebeh.2019.106540. [DOI] [PubMed] [Google Scholar]
- 19. Lekoubou A, Awoumou JJL, Kengne AP, et al. Incidence of seizure in stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta‐analysis. Int J Stroke 2017;12:923–931. 10.1177/1747493017729239. [DOI] [PubMed] [Google Scholar]
- 20. Bentes C, Martins H, Peralta AR, et al. Epileptic manifestations in stroke patients treated with intravenous alteplase. Eur J Neurol 2017;24:755–761. 10.1111/ene.13292. [DOI] [PubMed] [Google Scholar]
- 21. de Reuck J, van Maele G. Acute ischemic stroke treatment and the occurrence of seizures. Clin Neurol Neurosurg 2010;112:328–331. 10.1016/j.clineuro.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 22. Tan ML, Ng A, Pandher PS, et al. Tissue plasminogen activator does not alter development of acquired epilepsy. Epilepsia 2012;53:1998–2004. 10.1111/j.1528-1167.2012.03635.x. [DOI] [PubMed] [Google Scholar]
- 23. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European cooperative acute stroke study (ECASS). J Am Med Assoc 1995;274:1017–1025. 10.1001/jama.1995.03530130023023. [DOI] [PubMed] [Google Scholar]
- 24. Group TNI of ND and S rt‐PSS . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1588. 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 25. Bentes C, Brigo F, Zelano J, Ferro JM. Reperfusion therapies and poststroke seizures. Epilepsy Behav 2020;104:106524. 10.1016/j.yebeh.2019.106524. [DOI] [PubMed] [Google Scholar]
- 26. Austin PC. A critical appraisal of propensity‐score matching in the medical literature between 1996 and 2003. Stat Med 2008;27:2037–2049. 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 27. Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia 2010;51:671–675. 10.1111/j.1528-1167.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 28. Aho K, Harmsen P, Hatano S, et al. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ 1980;58:113–130. Accessed June 26, 2020. [PMC free article] [PubMed] [Google Scholar]
- 29. Hirsch LJ, Fong MWK, Leitinger M, et al. American clinical neurophysiology Society's standardized critical care EEG terminology: 2021 version. J Clin Neurophysiol 2021;38:1–29. 10.1097/WNP.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leitinger M, Beniczky S, Rohracher A, et al. Salzburg consensus criteria for non‐convulsive status epilepticus ‐ approach to clinical application. Epilepsy Behav 2015;49:158–163. 10.1016/j.yebeh.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 31. Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol 2017;69:345–357. 10.1016/j.jacc.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 32. Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol 2003;158:280–287. 10.1093/aje/kwg115. [DOI] [PubMed] [Google Scholar]
- 33. Martens EP, Pestman WR, de Boer A, et al. Systematic differences in treatment effect estimates between propensity score methods and logistic regression. Int J Epidemiol 2008;37:1142–1147. 10.1093/ije/dyn079. [DOI] [PubMed] [Google Scholar]
- 34. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2013;33:1057–1069. 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schlesselman J. Case‐Control Studies: Design, Conduct, Analysis. 1982.
- 36. Abraira L, Giannini N, Santamarina E, et al. Correlation of blood biomarkers with early‐onset seizures after an acute stroke event. Epilepsy Behav 2020;104:106549. 10.1016/j.yebeh.2019.106549. [DOI] [PubMed] [Google Scholar]
- 37. Bentes C, Peralta AR, Martins H, et al. Seizures, electroencephalographic abnormalities, and outcome of ischemic stroke patients. Epilepsia Open 2017;2:441–452. 10.1002/epi4.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Serafini A, Gigli GL, Gregoraci G, et al. Are early seizures predictive of epilepsy after a stroke? Results of a population‐based study. Neuroepidemiology 2015;45:50–58. 10.1159/000382078. [DOI] [PubMed] [Google Scholar]
- 39. Conrad J, Pawlowski M, Dogan M, et al. Seizures after cerebrovascular events: risk factors and clinical features. Seizure 2013;22:275–282. 10.1016/j.seizure.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 40. Garcia MA, Vallejo Seco G, Lozano EA. Classical and causal inference approaches to statistical mediation analysis. Psicothema 2014;26:252–259. http://www.psicothema.com/pdf/4186.pdf Accessed August 3, 2020. [DOI] [PubMed] [Google Scholar]
- 41. Pingue V, Mele C, Nardone A. Post‐traumatic seizures and antiepileptic therapy as predictors of the functional outcome in patients with traumatic brain injury. Sci Rep 2021;11:1–12. 10.1038/s41598-021-84203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campos M, Godoy J, Mesa M, et al. Temporal lobe epilepsy surgery with limited resources: results and economic considerations. Epilepsia 2000;41:S18–S21. 10.1111/j.1528-1157.2000.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 43. Scoppettuolo P, Gaspard N, Depondt C, et al. Epileptic activity in neurological deterioration after ischemic stroke, a continuous EEG study. Clin Neurophysiol 2019;130:2282–2286. 10.1016/j.clinph.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 44. Witsch J, Frey HP, Schmidt JM, et al. Electroencephalographic periodic discharges and frequency‐dependent brain tissue hypoxia in acute brain injury. JAMA Neurol 2017;74:301–309. 10.1001/jamaneurol.2016.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hafeez F, Razzaq MA, Levine RL, Ramirez MAN. Reperfusion seizures: a manifestation of cerebral reperfusion injury after Administration of Recombinant Tissue Plasminogen Activator for acute ischemic stroke. J Stroke Cerebrovasc Dis 2007;16:273–277. 10.1016/j.jstrokecerebrovasdis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 46. Yaghi S, Willey JZ, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017;48:e343–e361. 10.1161/STR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 47. Chen Z, Churilov L, Chen Z, et al. Association between implementation of a code stroke system and poststroke epilepsy. Neurology 2018;90:e1126–e1133. 10.1212/WNL.0000000000005212. [DOI] [PubMed] [Google Scholar]
- 48. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic. Stroke 2008;13:1317–1346. 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 49. Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of Intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 50. Iyer AM, Zurolo E, Boer K, et al. Tissue plasminogen activator and urokinase plasminogen activator in human epileptogenic pathologies. Neuroscience 2010;167:929–945. 10.1016/j.neuroscience.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 51. Man S, Xian Y, Holmes DN, et al. Association between thrombolytic door‐to‐needle time and 1‐year mortality and readmission in patients with acute ischemic stroke. J Am Med Assoc 2020;323:2170–2184. 10.1001/jama.2020.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta‐analysis. Stroke 2007;38:967–973. 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics for participants with and without reperfusion treatment, before and after propensity score matching.
Table S2. Baseline characteristics for participants with IV thrombolysis and without reperfusion treatment, before and after propensity score matching.


