Abstract
Baylisascaris procyonis is a common gastrointestinal parasite of raccoons (Procyon lotor) in their native range, and both have been introduced to Europe. Humans may ingest ascarid eggs shed via the racoons’ faeces, and this could lead to severe infections affecting the central nervous system. Here, we report the first occurrence of B. procyonis in Austria. The parasite was detected in a two‐year‐old male raccoon that was road‐killed in November 2019 near Hittisau (Vorarlberg). Genetic profiling provided strong evidence that the raccoon (and its parasite) originated from the nearest German raccoon population. The first finding in Austria highlights the need for monitoring the parasite and information of the public and practitioners.
Keywords: Austria, Baylisascaris procyonis, pica, raccoon, zoonosis
1. INTRODUCTION
The raccoon (Procyon lotor), a North American carnivore (Kaufmann, 1982), has become one of the most successful invasive species in Europe (Stubbe, 1999). Animals were imported to the continent several times for fur production, mainly in the 1930s and 1940s, and spread to the wild after deliberate or accidental release (Fischer et al., 2015, 2017). In some founder populations, a very weak parasite burden was observed, and it is hypothesized that those originated from fur farms with an effective deworming regime (Duscher et al., 2017). In some other populations, Baylisascaris procyonis has become established (Heddergott et al., 2020).
Baylisascaris procyonis is a very common nematode parasite in raccoons in its native range, with prevalences up to 82% (Kazacos, 2016), and might reach similar prevalences (71%) in their new habitats (Gey, 1998). Raccoons harbour the adult worms in their intestines, and the eggs are shed via their faeces. Small mammals or birds infect themselves by ingesting B. procyonis eggs while feeding in raccoon latrines (Kazacos, 2016). These eggs contain third‐stage larvae (Graeff‐Teixeira et al., 2016) that remain infectious for years if situated in conditions with suitable amounts of moisture. Third‐stage larvae continue their growth in the paratenic hosts, and the migration of some larvae into in the central nervous system can lead to debilitation or death of the host (Gavin et al., 2005). This increases the likelihood of predation or scavenging by raccoons, which then, in turn, become infected. Paratenic hosts are not obligatory, however, and raccoons might infect themselves directly via ingestion of eggs, this being the supposed infection path for sub‐adult raccoons (Gavin et al., 2005).
Humans are accidental hosts, who presumably become infected either by taking up eggs from the latrines and their surroundings, or while handling raccoons carrying eggs in their fur. Based on documented cases, the infection via geophagia or pica is the most common course of infection, and therefore, most human cases are infants and children (Gavin et al., 2005; Wise et al., 2005). Depending on the localization of the ingested growing larvae, different syndromes can evolve, such as visceral (VLM), ocular and neural larva migrans syndrome (NLM), the latter being of major concern and displaying the most devastating disease outcome (Gavin et al., 2002; Gavin et al., 2005; Kazacos et al., 1984; Sorvillo et al., 2002). Very often, there is a combination of the syndromes such as NLM being accompanied by VLM. Although documented human cases are rare, all known cases were severe, often fatal, and none of the survivors has recovered neurologically from the infection (Gavin et al., 2002).
While the first reported sighting of the raccoon in Austria dates to 1974, the population density of the carnivore remained low for some time. Few animals have thus been investigated for the presence of parasites. In recent years, however, the hunting bag has increased (Duscher et al., 2017), and the raccoon can be considered as an emerging invasive species in the country. Between 2017 and 2019, 41 individuals were documented in several places in Austria, but none of the investigated raccoons was positive for B. procyonis (Figure 1).
FIGURE 1.
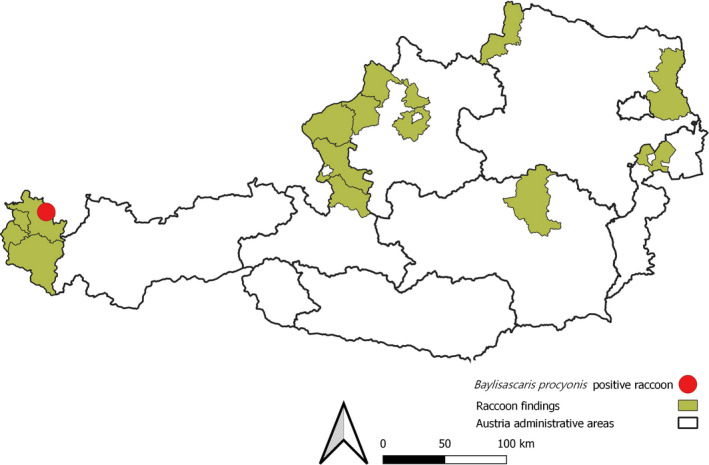
Finding of the B. procyonis‐positive raccoon as well as the districts where raccoons were documented in the past 3 years
2. MATERIALS AND METHODS
Intestinal helminths were collected from a two‐year‐old male raccoon that was road‐killed on 2 November 2019 near Hittisau (Vorarlberg; Figure 1). The age of the raccoon was determined by counting incremental growth lines of a mandibular canine (Heddergott et al., 2017). Worms were determined morphologically and further analysed using molecular tools. We used established protocols (Franssen et al., 2013) and the methods outlined in Osten‐Sacken et al. (2018) to amplify a 441 base pair (bp) fragment of the B. procyonis mitochondrial cytochrome oxidase 1 (CO1) gene in all the recovered worms.
Following the methods described in Osten‐Sacken et al. (2018), we generated a genetic profile of 17 microsatellite loci of the raccoon host. Similarly, we used the methodology to genotype all recovered roundworms using B. procyonis‐specific microsatellite loci. We focussed on the 13 loci that were polymorphic and inherited in a Mendelian fashion (see Osten‐Sacken et al., 2018). We used the genetic profiles to assess whether the raccoon host and the roundworm originated from Germany. Of the five major genetic raccoon populations identified in Germany (Fischer et al., 2015, Figure S1), the ‘Hessen’ population extends into southern Germany and is thus located closest to Austria. The roundworm is present in only two of these populations, including the ‘Hessen’ population (Heddergott et al., 2020). We used GENECLASS 2.0.h (Piry et al. 2004, to first estimate the probability of the raccoon or the parasites not originating from Germany. For each reference population, we estimated the so‐called exclusion probabilities based on the Monte Carlo method of Paetkau et al. (2004). In the case of the raccoon, the population genetic reference data were taken from Heddergott et al. (2020; see also Figure S1) and from Osten‐Sacken et al. (2018) in the case of the nematode. For each reference dataset, we simulated 10,000 multi‐locus genotypes and set the exclusion threshold to 0.01. We then used GENECLASS to assign individuals to their most likely population of origin (assignment test) using the partial Bayesian approach of Rannala and Mountain (1997).
3. RESULTS AND DISCUSSION
We collected seven adult B. procyonis from the intestine of the raccoon that was road‐killed in Vorarlberg. We managed to generate a 381‐bp‐long fragment of the mitochondrial CO1 gene in all seven nematodes from Austria. All seven sequences were identical (GenBank MW255834) and completely matched the (24‐bp shorter) B. procyonis haplotype HT1 previously reported from the vast majority of B. procyonis from Germany (Osten‐Sacken et al., 2018). Only the Saxony and Luxembourg populations could be excluded at the p < .01‐ level as the raccoon host's source population, while in the case of the seven roundworms, neither an origin in the Hessen nor in the Harz populations could be excluded (Table S1). The raccoon and six of the seven roundworms were assigned with high probability (score ≥ 99.1%) to their respective Hesse genetic population (Table S2). The genetic data thus provided strong evidence that the raccoon (and its parasite) originated from Hesse, the nearest German founder population.
Raccoons in Austria were previously supposed to harbour very few parasites (Duscher et al., 2017) and their occurrence was mainly observed in the light of an ecological threat concerning competition with or endangering of indigenous species. This finding of B. procyonis highlights the need for further monitoring and countermeasures to fight against this devastating zoonosis. A potential distribution over the whole of Austria could represent a serious threat to humans.
Surveillance programmes to identify the origin of raccoons as well as the worms and information of the public are urgently needed. Raccoons are known to adapt to the human life style and they can be found in close vicinity to humans, representing a source of infection. Access to pet food and garbage favours the occurrence of raccoons and therefore needs to be restricted. Safety measures while handling dead raccoons or after contact with latrines need to be publicized and implemented. Furthermore, raccoon population control and deworming of the animals, as well as of pets, which also might act as hosts, are of crucial importance, and prevention of infection remains the most important public health strategy (Gavin et al., 2002).
CONFLICT OF INTEREST
None declared.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Ana Paula Lopes Cruz for help with laboratory work.
Duscher GG, Frantz AC, Kuebber‐Heiss A, Fuehrer H‐P, Heddergott M. A potential zoonotic threat: First detection of Baylisascaris procyonis in a wild raccoon from Austria. Transbound Emerg Dis. 2021;68:3034–3037. 10.1111/tbed.13963
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in GenBank, reference number MW255834.
REFERENCES
- Duscher, T. , Hodžić, A. , Glawischnig, W. , & Duscher, G. G. (2017). The raccoon dog (Nyctereutes procyonoides) and the raccoon (Procyon lotor)‐their role and impact of maintaining and transmitting zoonotic diseases in Austria. Central Europe. Parasitology Research, 116(4), 1411–1416. 10.1007/s00436-017-5405-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, M. L. , Hochkirch, A. , Heddergott, M. , Schulze, C. , Anheyer‐Behmenburg, H. E. , Lang, J. , Michler, F. U. , Hohmann, U. , Ansorge, H. , Hoffmann, L. , Klein, R. , & Frantz, A. C. (2015). Historical invasion records can be misleading: genetic evidence for multiple introductions of invasive raccoons (Procyon lotor) in Germany. PLoS One, 10(5), e0125441. 10.1371/journal.pone.0125441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, M. L. , Salgado, I. , Beninde, J. , Klein, R. , Frantz, A. C. , Heddergott, M. , Cullingham, C. I. , Kyle, C. J. , & Hochkirch, A. (2017). Multiple founder effects are followed by range expansion and admixture during the invasion process of the raccoon (Procyon lotor) in Europe. Diversity Distributions, 23, 409–420. 10.1111/ddi.12538 [DOI] [Google Scholar]
- Franssen, F. , Xie, K. , Sprong, H. , & van der Giessen, J. (2013). Molecular analysis of Baylisascaris columnaris revealed mitochondrial and nuclear polymorphisms. Parasites & Vectors, 6, 124. 10.1186/1756-3305-6-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin, P. J. , Kazacos, K. R. , & Shulman, S. T. (2005). Baylisascariasis. Clinical Microbiology Reviews, 18(4), 703–718. 10.1128/CMR.18.4.703-718.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin, P. J. , Kazacos, K. R. , Tan, T. Q. , Brinkman, W. B. , Byrd, S. E. , Davis, A. T. , Mets, M. B. , & Shulman, S. T. (2002). Neural larva migrans caused by the raccoon roundworm Baylisascaris procyonis . The Pediatric Infectious Disease Journal, 21(10), 971–975. 10.1097/00006454-200210000-00017 [DOI] [PubMed] [Google Scholar]
- Gey, A. B. (1998). Synopsis der Parasitenfauna des Waschbären (Procyon lotor) unter Berücksichtigung von Befunden aus Hessen. PhD thesis, Justus Liebig Universität. Fachbereich Veterinärmedizin, Gießen. 1998; 1–203. [Google Scholar]
- Graeff‐Teixeira, C. , Morassutti, A. L. , & Kazacos, K. R. (2016). Update on Baylisascariasis, a highly pathogenic zoonotic infection. Clinical Microbiology Reviews, 29(2), 375–399. 10.1128/CMR.00044-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddergott, M. , Frantz, A. C. , Stubbe, M. , Stubbe, A. , Ansorge, H. , & Osten‐Sacken, N. (2017). Seroprevalence and risk factors of Toxoplasma gondii infection in invasive raccoons (Procyon lotor) in Central Europe. Parasitology Research, 116(8), 2335–2340. 10.1007/s00436-017-5518-7 [DOI] [PubMed] [Google Scholar]
- Heddergott, M. , Steinbach, P. , Schwarz, S. , Anheyer‐Behmenburg, H. E. , Sutor, A. , Schliephake, A. , Jeschke, D. , Striese, M. , Müller, F. , Meyer‐Kayser, E. , Stubbe, M. , Osten‐Sacken, N. , Krüger, S. , Gaede, W. , Runge, M. , Hoffmann, L. , Ansorge, H. , Conraths, F. J. , & Frantz, A. C. (2020). Geographic distribution of Raccoon Roundworm, Baylisascaris procyonis . Germany and Luxembourg. Emerging Infectious Diseases, 26(4), 821–823. 10.3201/eid2604.191670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann, J. H. , Raccoon and allies. In: Chapman, J.A., & Feldhamer, G.A. (1982). Wild mammals of North America (pp. 567–585). John Hopkins University Press. [Google Scholar]
- Kazacos, K. R. (2016). Baylisascaris larva migrans. Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 858. Retrieved from http://digitalcommons.unl.edu/parasitologyfacpubs/858 [Google Scholar]
- Kazacos, K. R. , Vestre, W. A. , & Kazacos, E. A. (1984). Raccoon ascarid larvae (Baylisascaris procyonis) as a cause of ocular larva migrans. Investigative Ophthalmology & Visual Science, 25(10), 1177–1183. [PubMed] [Google Scholar]
- Osten‐Sacken, N. , Heddergott, M. , Schleimer, A. , Anheyer‐Behmenburg, H. E. , Runge, M. , Horsburgh, G. J. , Camp, L. , Nadler, S. A. , & Frantz, A. C. (2018). Similar yet different: Co‐analysis of the genetic diversity and structure of an invasive nematode parasite and its invasive mammalian host. International Journal for Parasitology, 48(3–4), 233–243. 10.1016/j.ijpara.2017.08.013 [DOI] [PubMed] [Google Scholar]
- Paetkau, D. , Slade, R. , Burden, M. , & Estoup, A. (2004). Genetic assignment methods for the direct, real‐time estimation of migration rate: A simulation‐based exploration of accuracy and power. Molecular Ecology, 13(1), 55–65. 10.1046/j.1365-294x.2004.02008.x [DOI] [PubMed] [Google Scholar]
- Piry, S. , Alapetite, A. , Cornuet, J. M. , Paetkau, D. , Baudouin, L. , & Estoup, A. (2004). GENECLASS2: A software for genetic assignment and first‐generation migrant detection. The Journal of Heredity, 95(6), 536–539. 10.1093/jhered/esh074 [DOI] [PubMed] [Google Scholar]
- Rannala, B. , & Mountain, J. L. (1997). Detecting immigration by using multilocus genotypes. Proceedings of the National Academy of Sciences of the United States of. America, 94(17), 9197–9201. 10.1073/pnas.94.17.9197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorvillo, F. , Ash, L. R. , Berlin, O. G. , & Morse, S. A. (2002). Baylisascaris procyonis: An emerging helminthic zoonosis. Emerging Infectious Diseases, 8(4), 355–359. 10.3201/eid0804.010273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe, M. Procyon lotor (Linnaeus, 1758). In: Mitchell‐Jones, A. J., Amori, G., Bogdanowicz, W., Krystufek, B., Reijnders, P. J. H., Spitzenberger, F., Stubbe, M., Thissen, J. B. M., Vohralik, V., & Zima, J. (1999): The atlas of European mammals (pp. 326–327). Academic Press. [Google Scholar]
- Wise, M. E. , Sorvillo, F. J. , Shafir, S. C. , Ash, L. R. , & Berlin, O. G. (2005). Severe and fatal central nervous system disease in humans caused by Baylisascaris procyonis, the common roundworm of raccoons: A review of current literature. Microbes and Infection, 7(2), 317–323. 10.1016/j.micinf.2004.12.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are openly available in GenBank, reference number MW255834.


