Abstract
Aim
To evaluate 26 weeks of liraglutide treatment in type 1 diabetes (T1D) by subgroups in the ADJUNCT ONE and ADJUNCT TWO trials.
Materials and Methods
ADJUNCT ONE and ADJUNCT TWO were randomized controlled phase 3 trials in 1398 and 835 participants with T1D treated with liraglutide (1.8, 1.2, or 0.6 mg) or placebo (adjuncts to insulin). This post hoc analysis evaluated treatment effects by subgroups: HbA1c (< or ≥8.5%), body mass index (BMI; < or ≥27 kg/m2), and insulin regimen (basal bolus or continuous subcutaneous insulin infusion).
Results
In both trials at week 26, reductions in HbA1c, body weight, and daily insulin dose did not differ significantly (P > .05) by baseline HbA1c or BMI. Risk of clinically significant hypoglycaemia or hyperglycaemia with ketosis did not differ significantly (P > .05) by baseline HbA1c, BMI, or insulin regimen. At week 26 in ADJUNCT ONE, these risks did not differ (P > .05) between treatment groups. Placebo‐adjusted reductions in HbA1c, body weight, and insulin dose (−0.30%‐points, −5.0 kg, and −12%, respectively, with liraglutide 1.8 mg), were significant (P < .05), greater than at week 52, and similar to those in ADJUNCT TWO (−0.35%, −4.8 kg, and −10%, respectively, with liraglutide 1.8 mg).
Conclusions
In ADJUNCT ONE and ADJUNCT TWO, the efficacy and glycaemic safety of liraglutide did not depend on subgroups, leaving residual beta‐cell function as the only identified variable impacting the effect of glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) in T1D. These findings support a role for GLP‐1 RAs as adjuncts to insulin in T1D, warranting further study.
Keywords: clinical trial, incretin therapy, liraglutide, type 1 diabetes
1. INTRODUCTION
Despite the introduction of new insulin analogues and glucose monitoring systems, achieving and maintaining strict glycaemic control are difficult for most people with type 1 diabetes. 1 Use of insulin is associated with risk of hypoglycaemia and weight gain. 2 In industrialized parts of the world, adults with type 1 diabetes are often overweight or obese, further complicating obtaining adequate glycaemic control and increasing the risk of cardiovascular complications, which is markedly elevated in type 1 diabetes. 3 , 4 , 5 , 6 In addition, evidence suggests that a high body mass index (BMI) may promote the hallmark pathophysiological feature of type 1 diabetes, that is, immune‐mediated destruction of the beta cell. 7
Safe and efficacious medicines for type 1 diabetes beyond insulin are still urgently needed. While sodium‐glucose co‐transporter‐2 inhibitors have shown clear potential, they have not been approved for use in type 1 diabetes in the United States and their use in Europe is unclear. This epitomizes the lack of major therapeutical advances in type 1 diabetes. In parallel, management of type 2 diabetes has undergone a paradigm shift over the past decade with the introduction of, for example, glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs). GLP‐1 RAs markedly reduce HbA1c, need for exogenous insulin, risk of hypoglycaemia, and body weight, accompanied by other proven benefits such as those on cardiovascular risk. 8
The injectable GLP‐1 RA liraglutide in type 1 diabetes has been evaluated in small‐scale, open‐label clinical studies and in two phase 3 trials: ADJUNCT ONE 9 and ADJUNCT TWO. 10 The ADJUNCT trials confirmed that liraglutide as an adjunct to insulin improved glycaemic control, and reduced body weight and exogenous insulin requirements. However, the benefits were accompanied by an increase in the risk of hypoglycaemia and, very seldomly, by hyperglycaemia with ketosis but without diabetic ketoacidosis (DKA). These findings have been confirmed and expanded on by multiple investigations. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 In the ADJUNCT trials, a more pronounced placebo‐adjusted effect of liraglutide on HbA1c and a lower risk of hypoglycaemia were found for participants with residual beta‐cell function (as measured by C‐peptide levels) compared with participants without. Residual beta‐cell function, a clinically fundamental variable in the disease, remains the hitherto only identified anthropometric with significant impact on the effect of liraglutide in type 1 diabetes.
In this post hoc analysis, we investigated whether key efficacy (glycaemic control, body weight, and insulin requirements) and safety (hypoglycaemia and ketosis‐associated hyperglycaemia) outcomes differed between subgroups of ADJUNCT ONE and ADJUNCT TWO participants as defined by the level of glycaemic control, the type of insulin regimen used, and presence of overweight/obesity.
2. MATERIALS AND METHODS
2.1. Trial designs
The ADJUNCT trials were randomized placebo‐controlled, double‐blind, parallel‐group, phase 3 trials. In the 52‐week ADJUNCT ONE trial (177 centres in 17 countries; 1398 participants; NCT01836523), once‐daily subcutaneous injections of liraglutide were added to insulin, which was continued and titrated on a treat‐to‐target basis. In the 26‐week ADJUNCT TWO trial (59 centres in 12 countries; 835 participants; NCT02098395), subcutaneous liraglutide was used as an adjunct to insulin, which was capped for each participant as the prerandomization 7‐day average total daily dose. Results for the full trial populations have previously been reported for week 52 in ADJUNCT ONE 9 and for week 26 in ADJUNCT TWO. 10 In both trials, liraglutide was introduced at 0.6 mg then escalated as applicable every other week until the target dose (0.6, 1.2, or 1.8 mg once daily) was reached; thereafter, the dose could not be reduced. Volume‐matched placebo injections followed the same regimen. At randomization (week 0), the pretrial total daily insulin dose was decreased by 25%, and further 10% reductions were implemented for a minimum of 24 hours on each dose‐escalation day. Further details on the trial designs, including a list of the ethics committees that approved the study, are available in the primary publications. 9 , 10 Both trials were conducted in accordance with the Declaration of Helsinki.
2.2. Trial populations
The key inclusion criteria were: type 1 diabetes diagnosed 12 months or longer prior to enrolment; BMI of 20 kg/m2 or higher; and HbA1c range of 7.0%‐10% (53‐86 mmol/mol). Further, pretrial treatment for 6 months or longer (stable for ≥3 months) with continuous subcutaneous insulin infusion (CSII) or basal bolus insulin in ADJUNCT ONE and basal bolus insulin in ADJUNCT TWO, was required. Individuals with a history of severe hypoglycaemia or hypoglycaemia unawareness were not excluded, nor were those with a history of ketoacidosis. Key exclusion criteria were: prior use of GLP‐1 RAs or dipeptidyl peptidase‐4 inhibitors; use of medication that interfered with glycaemic control (except insulins), including all antihyperglycaemic agents or steroids; a history of acute or chronic pancreatitis; severely decreased renal function (estimated glomerular filtration rate <30 mL/min/1.73m2); calcitonin level more than 50 ng/L at screening; personal/family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2; and severe neuropathy. All participants gave their informed consent before participating in either of the trials.
2.3. Outcomes measures
In the trials, the primary endpoint was the change from baseline in HbA1c to end‐of‐treatment, which was week 52 in ADJUNCT ONE and week 26 in ADJUNCT TWO. Change from baseline in body weight was a secondary endpoint. Safety endpoints evaluated the occurrence of various categories of hypoglycaemic episodes, including clinically significant episodes (symptomatic hypoglycaemic episodes as defined by Novo Nordisk as severe, according to the American Diabetes Association, and a plasma glucose value <3.1 mmol/L [<56 mg/dL] with symptoms consistent with hypoglycaemia 20 ), as well as the occurrence of episodes of hyperglycaemia with ketosis (plasma glucose >16.7 mmol/L [>301 mg/dL] and plasma ketone >1.5 mmol/L). In the current report, for each of the two ADJUNCT trials separately, we present results for these selected endpoints analysed post hoc by the following subgroups (censored at baseline [week 0]): HbA1c (< or ≥8.5% [69 mmol/mol]); BMI (< or ≥27 kg/m2); and, for hypoglycaemia and hyperglycaemia, also the type of insulin regimen used (basal bolus or CSII). Results for additional efficacy and safety endpoints were reported previously. 9 , 10
Of importance, ADJUNCT ONE and ADJUNCT TWO were powered to document non‐inferiority and superiority, respectively, on the primary endpoint.
2.4. Statistical analyses
The statistical subgroup analyses were performed post hoc and were not controlled for multiplicity. For continuous endpoints (HbA1c, body weight, and insulin dose), estimates were derived from a mixed model for repeated measurements with treatment, subgroup, stratification, visit, and country as fixed factors, and baseline value as a fixed covariate; the model also included the interaction between subgroup and treatment group, and the interactions between each model term and visit, and group mean estimates were adjusted according to the observed baseline distribution in each subgroup. Postbaseline on‐treatment data (data collected on or after the first day on treatment and no later than the day after the last day on treatment) were included in the analysis. Statistically significant differences within a subgroup were determined on the P value of the test of interaction with a cut‐off value of .05; for transparency, we describe only such statistically significant differences.
Rate estimates for treatment‐emergent hypoglycaemic and hyperglycaemic episodes (onset on or after the first day of treatment and no later than the last day on treatment) were derived from a negative binomial regression model with a log‐link, and with treatment, subgroup, country, stratification factor, the interaction between treatment and subgroup as factors, baseline HbA1c as a covariate, and the logarithm of the exposure time as offset.
3. RESULTS
In both ADJUNCT ONE and ADJUNCT TWO and across treatment groups, baseline demographics were in general balanced within subgroups with no consistent patterns of difference (Table 1). In the subgroup of participants who at baseline were on CSII compared with the subgroup who used basal bolus insulin, the mean duration of diabetes was longer and the mean baseline fasting plasma glucose was lower; however, HbA1c did not differ.
TABLE 1.
Baseline characteristics
| Subgroup | ADJUNCT ONE | ADJUNCT TWO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Liraglutide | Liraglutide | ||||||||
| 1.8 mg | 1.2 mg | 0.6 mg | Placebo | 1.8 mg | 1.2 mg | 0.6 mg | Placebo | ||
| Full analysis set, N | HbA1c <8.5% (69 mmol/mol) | 235 | 230 | 236 | 234 | 146 | 141 | 147 | 144 |
| HbA1c ≥8.5% (69 mmol/mol) | 111 | 116 | 114 | 113 | 59 | 68 | 64 | 62 | |
| BMI <27 kg/m2 | 128 | 121 | 122 | 130 | 82 | 87 | 77 | 84 | |
| BMI ≥27 kg/m2 | 218 | 225 | 228 | 217 | 123 | 122 | 134 | 122 | |
| Insulin: Basal bolus | 231 | 246 | 279 | 251 | 153 | 153 | 158 | 154 | |
| Insulin: CSII | 115 | 100 | 71 | 96 | 52 | 56 | 53 | 52 | |
| Female, N (%) | HbA1c <8.5% (69 mmol/mol) | 124 (52.8) | 116 (50.4) | 118 (50.0) | 127 (54.3) | 88 (60.3) | 69 (48.9) | 82 (55.8) | 71 (49.3) |
| HbA1c ≥8.5% (69 mmol/mol) | 57 (51.4) | 63 (54.3) | 68 (59.6) | 53 (46.9) | 25 (42.4) | 37 (54.4) | 36 (56.3) | 41 (66.1) | |
| BMI <27 kg/m2 | 65 (50.8) | 76 (62.8) | 72 (59.0) | 71 (54.6) | 47 (57.3) | 50 (57.5) | 42 (54.5) | 46 (54.8) | |
| BMI ≥27 kg/m2 | 116 (53.2) | 103 (45.8) | 114 (50.0) | 109 (50.2) | 66 (53.7) | 56 (45.9) | 76 (56.7) | 66 (54.1) | |
| Insulin: Basal bolus | 113 (48.9) | 115 (46.7) | 139 (49.8) | 119 (47.4) | 77 (50.3) | 72 (47.1) | 80 (50.6) | 76 (49.4) | |
| Insulin: CSII | 68 (59.1) | 64 (64.0) | 47 (66.2) | 61 (63.5) | 36 (69.2) | 34 (60.7) | 38 (71.7) | 36 (69.2) | |
| Age, mean y | HbA1c <8.5% (69 mmol/mol) | 44.5 (13.1) | 44.1 (12.7) | 44.3 (12.8) | 44.0 (12.7) | 43.6 (13.0) | 43.0 (13.7) | 44.0 (13.5) | 43.5 (13.0) |
| HbA1c ≥8.5% (69 mmol/mol) | 42.0 (13.8) | 43.7 (13.8) | 42.1 (12.6) | 42.3 (12.2) | 42.2 (12.8) | 42.4 (12.6) | 43.5 (11.5) | 40.8 (13.0) | |
| BMI <27 kg/m2 | 42.9 (14.0) | 41.4 (14.5) | 42.2 (13.5) | 40.5 (14.2) | 43.0 (13.9) | 42.2 (14.0) | 41.1 (13.0) | 42.5 (12.6) | |
| BMI ≥27 kg/m2 | 44.1 (12.9) | 45.3 (12.0) | 44.3 (12.3) | 45.2 (11.2) | 43.4 (12.3) | 43.3 (12.8) | 45.5 (12.6) | 42.8 (13.3) | |
| Insulin: Basal bolus | 42.9 (13.5) | 44.4 (12.9) | 44.2 (12.8) | 43.7 (12.3) | 42.6 (12.6) | 42.8 (13.0) | 43.8 (12.4) | 43.0 (13.1) | |
| Insulin: CSII | 45.3 (13.0) | 42.9 (13.5) | 41.3 (12.7) | 42.8 (13.2) | 44.9 (13.7) | 42.8 (14.2) | 44.2 (14.3) | 41.8 (12.7) | |
| Body weight, kg | HbA1c <8.5% (69 mmol/mol) | 85.4 (17.5) | 85.5 (17.1) | 86.7 (16.7) | 85.8 (17.2) | 83.0 (18.1) | 85.7 (18.8) | 83.4 (15.9) | 84.7 (16.5) |
| HbA1c ≥8.5% (69 mmol/mol) | 88.1 (16.9) | 85.1 (17.5) | 86.2 (18.7) | 87.7 (18.9) | 85.4 (16.4) | 82.6 (16.7) | 82.5 (16.9) | 83.1 (16.6) | |
| BMI <27 kg/m2 | 72.2 (10.2) | 69.2 (9.1) | 70.8 (8.7) | 70.8 (9.0) | 70.7 (9.3) | 70.1 (9.3) | 69.7 (9.2) | 71.0 (10.1) | |
| BMI ≥27 kg/m2 | 94.5 (15.2) | 94.1 (13.9) | 95.0 (14.7) | 95.8 (14.9) | 92.3 (16.6) | 95.1 (15.6) | 90.8 (14.1) | 93.3 (13.7) | |
| Insulin: Basal bolus | 87.1 (18.6) | 84.3 (17.3) | 85.6 (17.2) | 86.6 (18.3) | 82.6 (17.3) | 84.7 (17.0) | 82.9 (16.2) | 83.9 (16.1) | |
| Insulin: CSII | 84.7 (14.4) | 88.0 (16.7) | 90.4 (17.4) | 85.9 (16.4) | 86.6 (18.3) | 84.6 (21.1) | 83.6 (16.0) | 85.1 (18.0) | |
| Body mass index, kg/m2 | HbA1c <8.5% (69 mmol/mol) | 29.3 (5.3) | 29.3 (5.1) | 29.5 (5.2) | 29.6 (5.3) | 28.9 (5.7) | 29.0 (5.2) | 29.0 (5.1) | 28.9 (4.9) |
| HbA1c ≥8.5% (69 mmol/mol) | 29.9 (4.9) | 29.3 (5.0) | 29.5 (5.6) | 30.0 (6.1) | 29.1 (5.2) | 28.5 (4.7) | 28.8 (4.8) | 29.0 (4.8) | |
| Insulin: Basal bolus | 29.4 (5.5) | 28.6 (4.8) | 29.1 (5.2) | 29.8 (5.7) | 28.5 (5.6) | 28.8 (4.9) | 28.6 (5.0) | 28.8 (4.6) | |
| Insulin: CSII | 29.7 (4.6) | 31.0 (5.4) | 31.0 (5.4) | 29.6 (5.3) | 30.2 (5.0) | 29.0 (5.3) | 29.8 (4.7) | 29.4 (5.5) | |
| HbA1c, mean % | HbA1c <8.5% (69 mmol/mol) | 7.7 (0.4) | 7.7 (0.5) | 7.8 (0.4) | 7.7 (0.4) | 7.7 (0.4) | 7.7 (0.4) | 7.7 (0.4) | 7.7 (0.4) |
| HbA1c ≥8.5% (69 mmol/mol) | 9.0 (0.4) | 9.1 (0.5) | 9.0 (0.5) | 9.0 (0.4) | 9.0 (0.4) | 8.9 (0.4) | 9.0 (0.4) | 9.0 (0.4) | |
| BMI <27 kg/m2 | 8.1 (0.8) | 8.1 (0.8) | 8.1 (0.8) | 8.2 (0.8) | 8.1 (0.8) | 8.1 (0.8) | 8.0 (0.7) | 8.1 (0.7) | |
| BMI ≥27 kg/m2 | 8.2 (0.7) | 8.2 (0.8) | 8.2 (0.7) | 8.1 (0.7) | 8.0 (0.7) | 8.1 (0.7) | 8.1 (0.8) | 8.1 (0.7) | |
| Insulin: Basal bolus | 8.0 (0.7) | 8.1 (0.8) | 8.2 (0.7) | 8.2 (0.8) | 8.1 (0.8) | 8.1 (0.8) | 8.2 (0.7) | 8.2 (0.7) | |
| Insulin: CSII | 8.2 (0.7) | 8.2 (0.8) | 8.1 (0.7) | 7.9 (0.6) | 7.8 (0.7) | 8.0 (0.6) | 7.8 (0.6) | 8.0 (0.8) | |
| HbA1c, mean mmol/mol | HbA1c <8.5% (69 mmol/mol) | 61 (4.4) | 61 (5.5) | 62 (4.4) | 61 (4.4) | 61 (4.4) | 61 (4.4) | 61 (4.4) | 61 (4.4) |
| HbA1c ≥8.5% (69 mmol/mol) | 75 (4.4) | 76 (5.5) | 75 (5.5) | 75 (4.4) | 75 (4.4) | 74 (4.4) | 75 (4.4) | 75 (4.4) | |
| BMI <27 kg/m2 | 65 (8.7) | 65 (8.7) | 65 (8.7) | 66 (8.7) | 65 (8.7) | 65 (8.7) | 64 (7.7) | 65 (7.7) | |
| BMI ≥27 kg/m2 | 66 (7.7) | 66 (8.7) | 66 (7.7) | 65 (7.7) | 64 (7.7) | 65 (7.7) | 65 (8.7) | 65 (8.7) | |
| Insulin: Basal bolus | 64 (7.7) | 65 (8.7) | 66 (7.7) | 66 (8.7) | 65 (8.7) | 65 (8.7) | 66 (8.7) | 66 (8.7) | |
| Insulin: CSII | 66 (7.7) | 66 (8.7) | 65 (7.7) | 63 (6.6) | 62 (7.7) | 64 (6.6) | 62 (6.6) | 64 (6.6) | |
| Duration of diabetes, y |
HbA1c <8.5% (69 mmol/mol) |
21.9 (12.8) | 22.1 (12.1) | 21.7 (12.7) | 22.0 (12.0) | 22.7 (12.1) | 21.1 (11.5) | 21.7 (13.1) | 21.3 (11.6) |
| HbA1c ≥8.5% (69 mmol/mol) | 20.6 (12.1) | 20.6 (12.4) | 19.1 (10.7) | 20.9 (11.5) | 18.2 (9.7) | 21.1 (11.4) | 19.5 (11.2) | 19.3 (11.4) | |
| BMI <27 kg/m2 | 20.9 (12.6) | 19.9 (11.6) | 20.1 (12.5) | 19.5 (11.6) | 20.7 (11.9) | 20.4 (11.8) | 18.1 (11.2) | 20.7 (11.6) | |
| BMI ≥27 kg/m2 | 21.8 (12.6) | 22.5 (12.5) | 21.3 (12.0) | 22.9 (11.8) | 21.9 (11.4) | 21.6 (11.2) | 22.7 (13.0) | 20.8 (11.6) | |
| Insulin: Basal bolus | 20.3 (12.2) | 20.8 (11.9) | 20.4 (12.4) | 20.6 (11.7) | 19.7 (10.8) | 20.5 (11.0) | 19.8 (12.4) | 20.2 (11.6) | |
| Insulin: CSII | 23.7 (13.2) | 23.6 (12.7) | 22.6 (11.3) | 24.4 (11.8) | 26.5 (12.3) | 22.9 (12.4) | 24.5 (12.5) | 22.3 (11.4) | |
| Fasting plasma glucose, mmol/L |
HbA1c <8.5% (69 mmol/mol) |
9.4 (4.0) | 10.2 (4.0) | 9.6 (4.1) | 9.6 (4.2) | 9.7 (3.9) | 9.8 (4.2) | 9.4 (3.9) | 9.8 (4.1) |
| HbA1c ≥8.5% (69 mmol/mol) | 11.4 (4.7) | 11.5 (4.5) | 11.5 (4.2) | 10.8 (4.1) | 11.8 (4.2) | 11.5 (4.9) | 10.9 (3.9) | 10.9 (4.8) | |
| BMI <27 kg/m2 | 9.9 (4.4) | 10.6 (4.4) | 10.6 (4.2) | 10.0 (4.6) | 10.3 (4.4) | 9.7 (4.5) | 8.9 (3.9) | 9.7 (4.2) | |
| BMI ≥27 kg/m2 | 10.1 (4.3) | 10.7 (4.1) | 10.0 (4.2) | 10.0 (3.9) | 10.3 (3.9) | 10.8 (4.5) | 10.4 (3.9) | 10.4 (4.5) | |
| Insulin: Basal bolus | 10.6 (4.5) | 11.0 (4.3) | 10.3 (4.3) | 10.3 (4.3) | 10.9 (4.3) | 10.6 (4.6) | 10.1 (4.2) | 10.4 (4.6) | |
| Insulin: CSII | 8.8 (3.7) | 9.9 (3.9) | 9.8 (4.0) | 9.2 (3.7) | 8.5 (3.0) | 9.7 (4.4) | 9.0 (3.2) | 9.2 (3.6) | |
Note: Data are observed means (standard deviation) or number of participants (proportion of subgroup) at baseline for participants in the full analysis set.
Abbreviations: BMI, body mass index; CSII, continuous subcutaneous insulin infusion; N, number of participants.
Across subgroups in both ADJUNCT ONE and ADJUNCT TWO, HbA1c (Figure 1A‐D), total daily insulin dose (Figure 1E‐H), and body weight (Figure 2A‐D) had all decreased by week 26 with liraglutide 1.8, 1.2, and 0.6 mg. The one exception was that in ADJUNCT ONE, the total daily insulin dose slightly increased for liraglutide 1.2 and 0.6 mg in participants with a baseline HbA1c level of 8.5% or higher (≥69 mmol/mol). With placebo, smaller decreases or even increases were seen for all three outcomes. The placebo‐adjusted estimated treatment effects at week 26 did not appear to differ systematically between subgroups; the one exception was that with liraglutide 0.6 mg in ADJUNCT TWO, the placebo‐adjusted decrease in HbA1c was statistically significantly greater in participants with a baseline HbA1c level of 8.5% or higher (≥69 mmol/mol) compared with those with an HbA1c level of less than 8.5% (P = .0443). At week 52 in ADJUNCT ONE, the treatment effects on all three outcomes were in general lower than at week 26 across subgroups (Figure S1).
FIGURE 1.
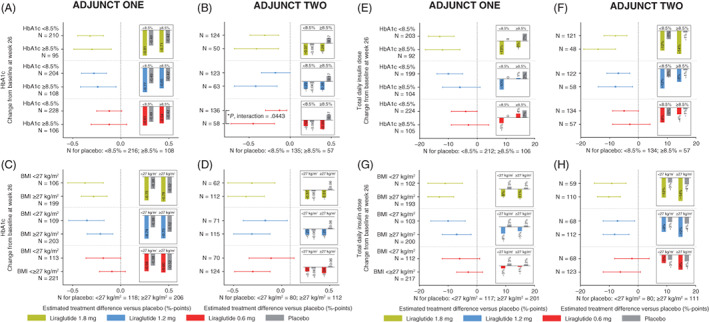
HbA1c and insulin use: week 26. Data are estimated mean changes from baseline (bars) and estimated treatment differences versus placebo with 95% confidence intervals at week 26 in ADJUNCT ONE (left panels) and in ADJUNCT TWO (right panels). Estimates are shown for change in HbA1c (panels A‐D) and total daily insulin dose (panels E‐H) by subgroups by baseline HbA1c level (HbA1c < or ≥8.5% [69 mmol/mol]) and by baseline BMI (< or ≥27 kg/m2). Estimates were derived from a mixed model for repeated measurements with treatment, subgroup, stratification, visit, and country as fixed factors, and baseline value as a fixed covariate; the model also included the interaction between subgroup and treatment group, and the interactions between each model term and visit, and group mean estimates were adjusted according to the observed baseline distribution in each subgroup. Postbaseline on‐treatment data (data collected on or after the first day on treatment and no later than the day after the last day on treatment) were included in the analysis. Unless indicated (*), the estimated placebo‐adjusted treatment effect did not differ statistically significantly within subgroups for all liraglutide dose levels (all tests for interaction between treatment and group were not statistically significant). *Indicates a statistically significant test for interaction between treatment and group. BMI, body mass index; N, number of participants
FIGURE 2.
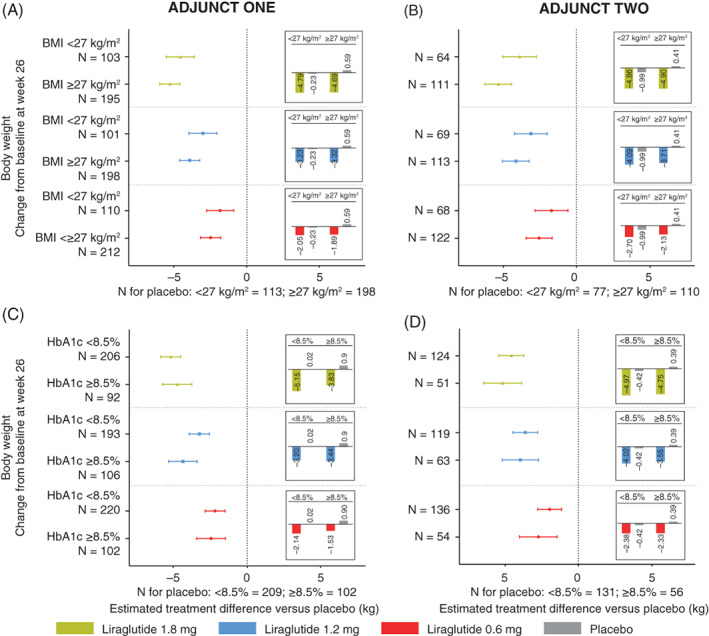
Body weight: week 26. Data are estimated mean changes from baseline (bars) and estimated treatment differences versus placebo with 95% confidence intervals at week 26 in ADJUNCT ONE (left panels) and in ADJUNCT TWO (right panels). Panels A‐D, estimates are shown for change in body weight, by subgroups, by baseline HbA1c level (< or ≥8.5% [69 mmol/mol]), and by baseline BMI (< or ≥27 kg/m2). Estimates were derived from a mixed model for repeated measurements with treatment, subgroup, stratification, visit, and country as fixed factors, and baseline value as a fixed covariate; the model also included the interaction between subgroup and treatment group, and the interactions between each model term and visit, and group mean estimates were adjusted according to the observed baseline distribution in each subgroup. Postbaseline on‐treatment data (data collected on or after the first day on treatment and no later than the day after the last day on treatment) were included in the analysis. The estimated placebo‐adjusted treatment effect did not differ statistically significantly within subgroups for all liraglutide dose levels (all tests for interaction between treatment group and subgroup were not statistically significant). BMI, body mass index; N, number of participants
For the full trial population in ADJUNCT ONE at week 26, HbA1c, body weight, and total daily insulin dose decreased statistically significantly more with liraglutide (change from baseline of −0.78%‐points to −0.59%‐points [−8.5 to −6.4 mmol/mol] in HbA1c, −4.7 to −2 kg, and −10% to −2% in insulin dose) than with placebo (−0.48%‐points [−5.2 mmol/mol], +0.3 kg, and +2%, respectively) (Table S1). The estimated placebo‐adjusted treatment effects at week 26 for liraglutide 1.8, 1.2, and 0.6 mg, respectively, were all statistically significant (P < .0001 unless noted): −0.30%‐points (−3.3 mmol/mol), −0.24%‐points (−2.6 mmol/mol), −0.11%‐points (−1.2 mmol/mol) (95% CI: −0.22% to −0.01%‐points [−2.4 to −0.1 mmol/mol]; P = .0380) for HbA1c; −12%, −8%, and −4% for total daily insulin dose (95% CI: −12% to 0%; P = .0470), and −5.0, −3.6, and −2.2 kg for body weight. As previously reported, 10 in ADJUNCT TWO, the corresponding estimated placebo‐adjusted treatment effects with liraglutide 1.8 mg at week 26 were −0.35%‐points (−3.8 mmol/mol) in HbA1c, −4.8 kg in body weight, and −10% in insulin dose (all P < .0001).
In both ADJUNCT ONE and ADJUNCT TWO at week 26, the estimated differences between liraglutide (all doses) and placebo in the incidences of hyperglycaemia with ketosis (Figure 3A‐F) and clinically significant hypoglycaemia (Figure 3G‐L) did, in general, not appear to differ between subgroups. There were two exceptions: the placebo‐adjusted incidence of hyperglycaemia with ketosis in ADJUNCT ONE appeared to differ (P = .0350) between participants in the liraglutide 1.2 mg group according to the type of insulin used at baseline (basal bolus insulin or on CSII); further, the placebo‐adjusted incidence of clinically significant hypoglycaemia appeared to differ in ADJUNCT TWO according to the baseline level of HbA1c (< or ≥8.5% [69 mmol/mol]) in participants in the liraglutide 1.8 and 0.6 mg groups (P = .0494 and P = .0187, respectively). At week 52 in ADJUNCT ONE, the placebo‐adjusted incidence of either type of episode did not appear to differ between subgroups (Figure S2).
FIGURE 3.
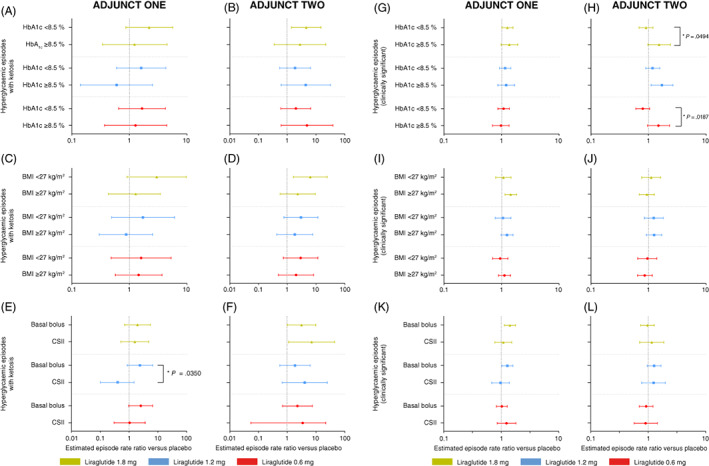
Hyperglycaemia with ketosis and hypoglycaemia (clinically significant): week 26. Data are estimated mean rate ratios versus placebo with 95% confidence intervals at week 26 in ADJUNCT ONE (left panels) and in ADJUNCT TWO (right panels). Estimates are shown for hyperglycaemic episodes with ketosis (episodes with plasma glucose >16.7 mmol/L and plasma ketone >1.5 mmol/L) (panels A‐F), and clinically significant hypoglycaemic episodes (symptomatic hypoglycaemic episodes as defined by Novo Nordisk as severe according to the American Diabetes Association and a plasma glucose value <3.1 mmol/L [<56 mg/dL] with symptoms consistent with hypoglycaemia 20 ) (panels G‐L), by subgroups, by baseline HbA1c level (< or ≥8.5% [69 mmol/mol]), by baseline BMI (< or ≥27 kg/m2), and by type of insulin treatment used at baseline (basal bolus or CSII). Episodes are treatment‐emergent (onset on or after the first day on treatment and no later than the day after the last day on treatment). Estimates were derived from a negative binomial regression model with a log‐link and with treatment, subgroup, country, stratification factor, and the interaction between treatment and subgroup as factors, baseline HbA1c as a covariate, and the logarithm of the exposure time as offset. Unless indicated (*), the episode rate ratio did not differ statistically significantly within subgroups for all liraglutide dose levels. *Indicates a statistically significant test for interaction between treatment and group. BMI, body mass index; CSII, continuous subcutaneous insulin infusion
For the full trial population in ADJUNCT ONE at week 26 (Table S2), the number of episodes of hyperglycaemia with ketosis was higher with liraglutide 1.8 and 0.6 mg than with placebo; however, the incidence did not differ statistically significantly between any of the individual liraglutide doses and placebo (estimated episode rate ratio vs. placebo [95% CI] for liraglutide 1.8, 1.2, and 0.6 mg, respectively: 1.84 [0.86; 3.92], P = .1156; 1.15 [0.51; 2.60], P = .7287; and 1.51 [0.72; 3.20], P = .2776). The number of episodes of clinically significant hypoglycaemia was also higher with liraglutide (all doses) than with placebo; the incidence difference was statistically significant for liraglutide 1.8 and 1.2 mg but not for 0.6 mg (estimated rate ratio vs. placebo [95% CI]): 1.34 (1.10; 1.63), P = .0033; 1.29 (1.06; 1.57), P = .0125; and 1.18 (0.97; 1.43), P = .0995. In ADJUNCT TWO, the estimated incidence of clinically significant hypoglycaemia was greater with liraglutide 1.2 mg than with placebo (rate ratio: 1.3; P = .03), as previously reported. 10
4. DISCUSSION
In the current post hoc analysis, we show that, regardless of the baseline level of glycaemic control, BMI, and pre‐existing insulin regimen, the GLP‐1 analogue liraglutide at dose levels of 1.8, 1.2, and 0.6 mg appears equally efficacious and safe in populations that were generally representative of individuals with type 1 diabetes.
In both trials, the placebo‐adjusted reductions in HbA1c, body weight, and total daily insulin dose with liraglutide 1.8, 1.2, and 0.6 mg did not appear to depend on baseline HbA1c (< or ≥8.5% [69 mmol/mol]) or BMI (< or ≥27 kg/m2). The greatest placebo‐adjusted effect of liraglutide was observed after 26 weeks with the 1.8 mg dose level, which reduced HbA1c by 0.3% and 0.35%‐points (3.3 and 3.8 mmol/mol), body weight by 5.0 and 4.8 kg, and the total daily insulin dose by 10% and 12% in ADJUNCT ONE and ADJUNCT TWO, respectively.
The current report expands on the conclusions of ADJUNCT ONE and ADJUNCT TWO. 9 , 10 The results from the two placebo‐controlled, randomized phase 3 trials confirmed that liraglutide as an adjunct to insulin offers clinically relevant benefits in type 1 diabetes by improving glycaemic control and reducing body weight and insulin requirements. These findings were associated with an increased incidence of clinically relevant hypoglycaemia with liraglutide 1.8 mg (ADJUNCT ONE only) and 1.2 mg. Notably, however, the incidence of adjudication‐confirmed severe hypoglycaemia was lower with liraglutide (all doses in ADJUNCT ONE and 1.8 mg in ADJUNCT TWO) compared with placebo, although the differences between groups were not statistically significant in either trial. Moreover, despite the finding that the incidence of hyperglycaemia with ketosis observed in both trials was numerically higher with liraglutide than with placebo, the risk of DKA was very low with all doses of liraglutide in both ADJUNCT ONE and ADJUNCT TWO. Still, based on the findings related to hyperglycaemia and hypoglycaemia in comparison with the seemingly modest magnitude of the detected glycaemic improvements (e.g. placebo‐adjusted HbA1c reduction of −0.2%‐points [−2.2 mmol/mol] with liraglutide 1.8 mg at week 52 in ADJUNCT ONE), a favourable benefits/risks profile of liraglutide in type 1 diabetes was not immediately obvious.
In ADJUNCT ONE and ADJUNCT TWO, the eligibility criteria ensured a broad trial population, representing a wide range of type 1 diabetes disease stages. As previously noted, 9 , 10 subgroups of participants representing certain disease states may differ in terms of efficacy and safety. In ADJUNCT ONE, for example, better glycaemic efficacy with liraglutide (vs. placebo) and a lower risk of hypoglycaemia was found for participants with residual beta‐cell function (as measured by C‐peptide secretion) compared with those without. With the results of the current investigation, residual beta‐cell function remains the only clinically relevant variable associated with significant impact on the effects of liraglutide in type 1 diabetes.
ADJUNCT ONE was designed to confirm non‐inferiority on change from baseline to week 52 in HbA1c with a non‐inferiority margin of −0.3%‐points (3.3 mmol/mol) (placebo‐adjusted treatment effect of liraglutide 1.8 mg). It may be argued that this design precluded a robust conclusion regarding the full glycaemic efficacy of liraglutide in type 1 diabetes, for example, considering that the trial population was characterized by long‐standing disease. In such patients, and in general, trial fatigue (i.e. in terms of adjustment of insulin doses in relation to carbohydrate intake and premeal glucose levels) may be especially pronounced in a longer term trial, suggesting that the treatment effects observed after a full year of treatment may not represent the full or optimal treatment effects. Accordingly, the confirmatory analysis in the evaluation of sodium‐glucose co‐transporter‐2 inhibitors, 21 , 22 , 23 , 24 , 25 , 26 another drug class of potential benefit in type 1 diabetes, as well as in ADJUNCT TWO, was in general performed for week 26, in line with regulatory recommendations. Indeed, the placebo‐adjusted HbA1c reduction at week 26 with liraglutide 1.8 mg in ADJUNCT ONE, as reported in the current report, was −0.3%‐points (−3.3 mmol/mol) and statistically significant (non‐confirmatory testing, not controlled for multiplicity), whereas the reduction had diminished at week 52. In ADJUNCT TWO, the placebo‐adjusted reduction in HbA1c at week 26 was −0.35%‐points (−3.8 mmol/mol) (P < .0001), as previously reported. 10
As delineated above, it should be noted that several smaller trials have added to the evidence of the effects of liraglutide in people with type 1 diabetes. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 The investigations have unanimously found no major safety concerns, including no increased risk of hypoglycaemia, and a meta‐analysis has reported a lower risk of hypoglycaemia with liraglutide than with placebo. 11 Furthermore, no evidence has been reported to indicate increased risks of ketosis‐accompanied hyperglycaemia or DKA with liraglutide, corroborating the results from the ADJUNCT trials.
It is becoming increasingly recognized that type 1 diabetes is associated with increased body weight; thus, an agent that meaningfully reduces body weight is highly desirable in the management of type 1 diabetes and its associated complications. Excess body weight is associated with insulin resistance, 27 increasing the risk of cardiovascular disease as well as increased insulin secretion requirements, which further stress the remaining beta cells. Compelling evidence is available to suggest that liraglutide may preserve functional beta cells, for example, by relieving them of the metabolic stresses that are believed to unmask the cell to the immune system, leading to its destruction (unpublished data; Matthias von Herrath). Another benefit of liraglutide is its effect on the islet alpha cell, resulting in suppressed glucagon secretion postprandially and attenuated postprandial glucose excursions. 28 Finally, liraglutide and certain other GLP‐1 RAs have recently been approved for the reduction of cardiovascular risk in type 2 diabetes. 29 , 30 , 31 , 32 An agent with cardiovascular benefits is urgently needed in type 1 diabetes, which also is associated with a very high risk of cardiovascular outcomes. 3 , 4 , 5 , 6
It should also be recognized that, while liraglutide is considered a first‐generation GLP‐1 analogue with proven benefits, second‐generation analogues such as semaglutide are even more potent and offer substantially stronger effects on glycaemic control and body weight in type 2 diabetes 28 , 33 compared with the GLP‐1 RAs tested in type 1 diabetes to date (i.e. liraglutide as well as both once‐daily and once‐weekly exenatide). In addition, semaglutide markedly delays gastric emptying and suppresses glucagon secretion, 34 and the once‐weekly (subcutaneous) and oral regimens available for semaglutide may also be beneficial in terms of improving dosing and adherence. Whether these pronounced benefits of semaglutide apply to type 1 diabetes as well deserves to be studied. Considering the scarcity of therapeutical medicines beyond insulin for type 1 diabetes, such investigations are, in general, urgently needed and may also explore the possibility of combining agents such as efficacious GLP‐1 RAs with novel monitoring technologies like real‐time or intermittently scanned continuous glucose monitoring. These glucose monitoring modalities, since the conduct of the ADJUNCT trials, have been developed to a mature stage and may be relevant to include in future studies of GLP‐1 RAs in type 1 diabetes.
The limitations of the current evaluation are related to the statistical analyses and are inherent to the analyses used in the original ADJUNCT trials. These trials were conducted and analysed according to the prevailing standard at the time of planning of the trials, including data collection and handling. No estimands were defined in the ADJUNCT trials; however, the analyses resemble analyses relevant for an estimand applying a “hypothetical” strategy, where data are included in the analyses only if the data pertain to the period when participants were on treatment with the trial drug regimen. The current analyses adhere to those principles and should be interpreted in this context, as well as recognizing the fact that the analyses were conducted post hoc and not controlled for multiplicity. Moreover, the mixed model for repeated measurements applied in the statistical analyses assumes that missing data were missing‐at‐random (MAR). Considering that we used a hypothetical‐like analysis strategy, this assumption is considered reasonable; however, it remains a limitation of the evaluation that the MAR assumption cannot be verified. Overall, these limitations imply that the findings of our evaluation are not confirmatory and that the results should be validated in future studies using an estimand applying a “treatment policy” strategy (intention‐to‐treat principle) in line with regulatory guidelines.
In conclusion, the current post hoc analysis further clarifies the place for GLP‐1 RAs as adjuncts to insulin in the management of type 1 diabetes by showing that the efficacy and safety of liraglutide do not differ across clinically relevant subgroups of participants in ADJUNCT ONE and ADJUNCT TWO. The ADJUNCT programme suggests that residual beta‐cell function remains the only clinically relevant variable associated with significant impact on the effects of liraglutide on HbA1c and risk of hypoglycaemia in type 1 diabetes. These findings, together with the developments since the completion of the two trials, indicate that the benefit/risk profile of liraglutide in type 1 diabetes may need to be reconsidered, warranting further studies.
CONFLICT OF INTEREST
Five of the authors of this report are employees of the sponsor and, as such, were involved in the preparation, review, and approval of the manuscript. TFD: Advisory board for Novo Nordisk; lecture fees from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Sanofi; research grant from Novo Nordisk and AstraZeneca. BJvS: Employee and shareholder of Novo Nordisk A/S. EC: Employee and shareholder of Novo Nordisk A/S. FFK: Employee and shareholder of Novo Nordisk A/S. LB: Employee and shareholder of Novo Nordisk A/S. MvH: Employee and shareholder of Novo Nordisk A/S. CM: serves or has served on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Insulet, and Zealand Pharma. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for CM from Medtronic, Novo Nordisk, Sanofi, and ActoBio Therapeutics; CM serves or has served on the speakers bureau for Novo Nordisk, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, AstraZeneca, and Novartis. Financial compensation for these activities has been received by KU Leuven. SM: Advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Sanofi, and Bayer; lecture fees from AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; research grant from Novo Nordisk and Boehringer Ingelheim.
AUTHOR CONTRIBUTIONS
TFD, BJvS, EC, LB, MvH, CM, and SM were involved in planning the analyses. All authors were involved in interpreting the analyses and writing the manuscript, and all authors approved the final version of the manuscript. TFD is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author, SM, had final responsibility for the decision to submit for publication.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14532.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGEMENTS
The authors thank all subjects and ADJUNCT ONE and ADJUNCT TWO investigators involved in the study. The list of the ADJUNCT ONE and ADJUNCT TWO investigators can be accessed in the Supplementary Materials of ADJUNCT ONE (Mathieu et al. Diabetes Care. 2016;39:1702‐1710) and ADJUNCT TWO (Ahrén et al. Diabetes Care. 2016;39:1693‐1701). Submission support was provided by Ashfield MedComms, an Ashfield Health company, and was funded by Novo Nordisk A/S. The study was sponsored by Novo Nordisk A/S, and ADJUNCT ONE and ADJUNCT TWO are registered with ClinicalTrials.gov (NCT01836523 and NCT02098395, respectively). The sponsor of the study had a role in study design, medical oversight during trial conduct, data cleaning and analysis, and data interpretation.
Dejgaard TF, von Scholten BJ, Christiansen E, et al. Efficacy and safety of liraglutide in type 1 diabetes by baseline characteristics in the ADJUNCT ONE and ADJUNCT TWO randomized controlled trials. Diabetes Obes Metab. 2021;23(12):2752‐2762. doi: 10.1111/dom.14532
Prior presentation/publication: Parts of these data have been accepted as an abstract and were presented as a poster at the American Diabetes Association (ADA) ‐ 81st Annual Scientific Session, 25‐29 June 2021, and the corresponding abstract has been published by ADA (Dejgaard et al. Diabetes 2021; 70(Suppl 1): doi: 10.2337/db21‐675‐P).
Funding information Novo Nordisk, Grant/Award Numbers: NCT02098395, NCT01836523
DATA AVAILABILITY STATEMENT
Individual participant data will be shared in datasets in a deidentified or anonymized format. The redacted clinical study reports will be available in accordance with Novo Nordisk data sharing commitments. Data will be shared with bona fide researchers submitting a research proposal and requesting access to data. Data will be made available for analyses as approved by the independent review board (IRB) in accordance with the IRB charter. The access request proposal form and the access criteria can be found on the Novo Nordisk Trials website. The data will be made available on a specialized SAS data platform.
REFERENCES
- 1. Weinstock RS, Schütz‐Fuhrmann I, Connor CG, et al. Type 1 diabetes in older adults: comparing treatments and chronic complications in the United States T1D exchange and the German/Austrian DPV registries. Diabetes Res Clin Pract. 2016;122:28‐37. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2020. Diabetes Care. 2020;43:S98‐S110. [DOI] [PubMed] [Google Scholar]
- 3. Bebu I, Braffett BH, Pop‐Busui R, Orchard TJ, Nathan DM, Lachin JM. The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: the DCCT/EDIC study. Diabetologia. 2017;60:2084‐2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petrie JR, Sattar N. Excess cardiovascular risk in type 1 diabetes mellitus. Circulation. 2019;139:744‐747. [DOI] [PubMed] [Google Scholar]
- 5. Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407‐1418. [DOI] [PubMed] [Google Scholar]
- 6. Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register‐based cohort study. Lancet. 2018;392:477‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferrara‐Cook C, Geyer SM, Evans‐Molina C, et al. Excess BMI accelerates islet autoimmunity in older children and adolescents. Diabetes Care. 2020;43:580‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aroda VR. A review of GLP‐1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20(Suppl 1):22‐33. [DOI] [PubMed] [Google Scholar]
- 9. Mathieu C, Zinman B, Hemmingsson JU, et al. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat‐to‐target randomized trial. Diabetes Care. 2016;39:1702‐1710. [DOI] [PubMed] [Google Scholar]
- 10. Ahrén B, Hirsch IB, Pieber TR, et al. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care. 2016;39:1693‐1701. [DOI] [PubMed] [Google Scholar]
- 11. Dimitrios P, Michael D, Vasilios K, et al. Liraglutide as adjunct to insulin treatment in patients with type 1 diabetes: a systematic review and meta‐analysis. Curr Diabetes Rev. 2020;16:313‐326. [DOI] [PubMed] [Google Scholar]
- 12. Ghanim H, Batra M, Green K, et al. Liraglutide treatment in overweight and obese patients with type 1 diabetes: a 26‐week randomized controlled trial; mechanisms of weight loss. Diabetes Obes Metab. 2020;22:1742‐1752. [DOI] [PubMed] [Google Scholar]
- 13. Goyal I, Sattar A, Johnson M, Dandona P. Adjunct therapies in treatment of type 1 diabetes. J Diabetes. 2020;12:742‐753. [DOI] [PubMed] [Google Scholar]
- 14. Kuhadiya ND, Prohaska B, Ghanim H, Dandona P. Addition of glucagon‐like peptide‐1 receptor agonist therapy to insulin in C‐peptide‐positive patients with type 1 diabetes. Diabetes Obes Metab. 2019;21:1054‐1057. [DOI] [PubMed] [Google Scholar]
- 15. Wang W, Liu H, Xiao S, Liu S, Li X, Yu P. Effects of insulin plus glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) in treating type 1 diabetes mellitus: a systematic review and meta‐analysis. Diabetes Ther. 2017;8:727‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dejgaard TF, Schmidt S, Frandsen CS, et al. Liraglutide reduces hyperglycaemia and body weight in overweight, dysregulated insulin‐pump‐treated patients with type 1 diabetes: the lira pump trial—a randomized, double‐blinded, placebo‐controlled trial. Diabetes Obes Metab. 2020;22:492‐500. [DOI] [PubMed] [Google Scholar]
- 17. Dejgaard TF, Frandsen CS, Hansen TS, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira‐1): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2016;4:221‐232. [DOI] [PubMed] [Google Scholar]
- 18. Frandsen CS, Dejgaard TF, Holst JJ, Andersen HU, Thorsteinsson B, Madsbad S. Twelve‐week treatment with liraglutide as add‐on to insulin in normal‐weight patients with poorly controlled type 1 diabetes: a randomized, placebo‐controlled, double‐blind parallel study. Diabetes Care. 2015;38:2250‐2257. [DOI] [PubMed] [Google Scholar]
- 19. Johansen NJ, Dejgaard TF, Lund A, et al. Efficacy and safety of meal‐time administration of short‐acting exenatide for glycaemic control in type 1 diabetes (MAG1C): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2020;8:313‐324. [DOI] [PubMed] [Google Scholar]
- 20. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buse JB, Garg SK, Rosenstock J, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American in Tandem1 study. Diabetes Care. 2018;41:1970‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT‐1 52‐week study. Diabetes Care. 2018;41:2552‐2559. [DOI] [PubMed] [Google Scholar]
- 23. Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT‐2 study): 24‐week results from a randomized controlled trial. Diabetes Care. 2018;41:1938‐1946. [DOI] [PubMed] [Google Scholar]
- 24. Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. 2018;41:2560‐2569. [DOI] [PubMed] [Google Scholar]
- 25. Garg SK, Henry RR, Banks P, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 2017;377:2337‐2348. [DOI] [PubMed] [Google Scholar]
- 26. Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38:2258‐2265. [DOI] [PubMed] [Google Scholar]
- 27. Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is 'double diabetes' and what are the risks? Diabetologia. 2013;56:1462‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP‐1 receptor agonists in the treatment of type 2 diabetes ‐ state‐of‐the‐art. Mol Metab. 2020;46:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mann JFE, Ørsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839‐848. [DOI] [PubMed] [Google Scholar]
- 30. Leiter LA, Bain SC, Bhatt DL, et al. The effect of glucagon‐like peptide‐1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across baseline blood pressure categories: analysis of the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab. 2020;22:1690‐1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121‐130. [DOI] [PubMed] [Google Scholar]
- 32. Tuttle KR, Rayner BL, Lakshmanan M, et al. 233‐OR: chronic kidney disease (CKD) outcomes with dulaglutide (DU) vs. insulin glargine (IG) in type 2 diabetes (T2D) and moderate‐to‐severe CKD by albuminuria status: AWARD‐7. Diabetes. 2019;68:233 ‐OR (Abstract). https://diabetes.diabetesjournals.org/content/68/Supplement_1/233-OR [Google Scholar]
- 33. Thethi TK, Pratley R, Meier JJ. Efficacy, safety and cardiovascular outcomes of once‐daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab. 2020;22:1263‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, Blundell J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first‐hour gastric emptying in subjects with obesity. Diabetes Obes Metab. 2018;20:610‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
Individual participant data will be shared in datasets in a deidentified or anonymized format. The redacted clinical study reports will be available in accordance with Novo Nordisk data sharing commitments. Data will be shared with bona fide researchers submitting a research proposal and requesting access to data. Data will be made available for analyses as approved by the independent review board (IRB) in accordance with the IRB charter. The access request proposal form and the access criteria can be found on the Novo Nordisk Trials website. The data will be made available on a specialized SAS data platform.


