Abstract
Lichen Planopilaris (LPP) is a lymphatic disease affecting the scalp that is characterized by a chronic and destructive inflammation process, named as ‘cicatricial alopecia’ in which the hair follicles are targeted and may involve predominantly lymphocytes or neutrophils. Scalp and biopsy layers have never been used to investigate microbial community composition and its relative taxa abundances in LPP. We sought to examine the significant taxa of this chronic relapsing inflammatory skin disease, together with inspect the existing connections with metabolic pathways featuring this microbial community. We used a multilevel analysis based on 16S rRNA marker sequencing in order to detect OTU abundances in pathologic/healthy samples, real time PCR for measuring the levels of IL-23 interleukin expression and urinary metabolomics to find out volatile organic metabolites (VOMs). By using a linear regression model, we described peculiar taxa that significantly differentiated LPP and healthy samples. We inspected taxa abundances and interleukin mRNA levels and the Microbacteriaceae family resulted negatively correlated with the IL-23 expression. Moreover, starting from 16S taxa abundances, we predicted the metabolic pathways featuring this microbial community. By inspecting microbial composition, sample richness, metabolomics profiles and the relative metabolic pathways in a cohort of LPP and healthy samples we deepened the contribution of significant taxa that are connected to inflammation maintenance and microbiota plasticity in LPP pathology.
1. Introduction
The Scalp possesses unique features (high follicular density and sebum production) that make it susceptible to several conditions including the possibility to undergo to an inflammatory status [1]. Psoriasis and seborrheic dermatitis are the most frequent pathologies [2] and their manifestations could be often similar in the initial steps that specifically include erythema, skin scaling or desquamation, and pruritus.
Other less frequent inflammatory conditions of the scalp can mimic the most common forms [3]. Among them, Lichen Planopilaris (LPP) occurs when Lichen Planus, a common skin disease involves the scalp, especially the vertex area [4]. First described in 1895 and also named as “follicular lichen planus of the scalp” it usually manifests in the form of irregular cicatricial alopecia, always irreversible [5].
According to the North American Hair Research Society (NAHRS) LPP is now classified as a primary lymphocytic disease based on lymphocytic, neutrophilic, or mixed infiltrates [6].
Noticeably, few data are nowadays available as regards of its epidemiology. Authors [7] reported higher frequency in Caucasians and Indians whereas lower incidence was found for Asians. Looking at gender ratio, females result more affected than males (1.8:1) with a typical presentation occurring between 40–60 years [8].
Based on differentiated symptoms, three different variants of LPP can be observed. The first one usually involves the vertex and is characterized by a perifollicular violaceous erythema and keratotic plugs that in some cases can be accompanied by inflammation and hyperkeratosis [4]. The second variant is known as Frontal Fibrosing Alopecia (FFA); it is characterized by a distinctive pattern of a progressive recession on frontal hairline and eyebrow, scalp pruritus, and perifollicular erythema [9]. This signs mostly affect middle-aged women. The so-called Graham-Little Piccardi Lassueur Syndrome is reported as the third LPP variant; its common manifestations are patchy cicatricial alopecia on the scalp, non-cicatricial alopecia on the axilla and groin, and the formation of the so called ‘follicular spinous papule’ on the body and/or scalp [10].
More recently, a fourth variant has been included in the list of sub variants: Fibrosing Alopecia in a Pattern Distribution (FAPD). It occurs in patients affected by Androgenetic Alopecia (AGA), that present also the typical lesions of LPP [11].
Regardless of the type of variant, LPP evolves in a very extensive and active way, up to involving the entire scalp and leading to irreversible baldness [12, 13]. In this clinical picture, common clinical manifestations include perifollicular erythema and hyperkeratosis, persisting itching on the scalp, scaling, and enduring painful sensation on the scalp, namely ‘trichodynia’ [14]. Compared to AGA, the hair count per follicular unit is found to be lower, probably as result of the follicular dropout which happens as a consequence of a cicatricial event [15].
Nowadays LPP diagnosis is based on clinical and histopathological findings and on an accurate differential diagnosis that mainly evidences the presence of seborrheic dermatitis, psoriasis and AGA at an initial stage [16]. Due also to poor information on physiopathology and a not fully understood etiology, a complete and accurate diagnosis of LPP remains an open challenge.
Its clinical expression has been reported to be triggered mainly by cell-mediated immunity [17]. The immune response mostly involves the bulge area, a contiguous part of outer root sheath enriched in stem cells, with the engagement of T lymphocytes (CD4 and CD) that are activated by the increasing of Langerhans cells both in the dermis and epidermis. The Th17 (subset of CD4+ T helper) cells has been recently reported to play a crucial role in promoting immune-inflammatory reactions also in autoimmune diseases [18] and some of their secreted cytokines are involved in the defense against pathogenic microorganisms and can correlate with specific bacterial genera in Oral Lichen Planus [19].
A link between LPP and the microbial population inhabiting the scalp has been also recently reported [20]. As reported for the gut [21] and skin microbiome [22], bacteria and fungi are strongly involved in the healthy status, and the host immune system is recognized as a major stress able to modulate the microbial composition [23].
Perturbation in host immune response as well as the alteration of the scalp microbiota observed in our previous study on another model of scalp auto-immune disease [24], may suggest a disequilibrium within the scalp microbiota in LPP patients and a role in its physiopathology.
To test this hypothesis, we studied the scalp bacterial abundance and diversity, as well as distinct scalp metagenomic profiles, cytokine profile, and urine metabolite expression in subjects affected by LPP compared to healthy subjects. We also explored the relationships between clinical manifestations and bacterial microbiota also in terms of predicted microbial biochemical pathways and we inspected the urine metabolomics profiles of LPP patients. Analyses were conducted both on the scalp and the subepidermal scalp compartments.
2. Material and methods
Study design and participants
A cohort of 27 LPP and healthy subjects (people never showing the symptoms related to any dermatologic disease) of both sex (six samples for each one of the three biopsy subepidermal compartments plus eleven lichen swabs), aged between 18–60 years, were enrolled. LPP patients were diagnosed clinically and confirmed as having LPP by biopsy, according to the WHO criteria. Essential clinical data were collected at baseline under dermatological control.
Patients were evaluated for erythema and desquamation (scaling) using a 5-point scale: 0 = none, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe. Besides, the investigator assessed itching/pruritus over the past 24 hours using a visual analogue scale (VAS) from 0 = no pruritus to 10 = severe pruritus.
Patients from both groups were evaluated and enrolled in the study, after signed informed consent, by the RS Dermatologic Clinic, Milan, Italy.
The study was under the approval of the Ethical Independent Committee for Clinical, not pharmacological investigation in Genoa (Italy) and following the ethical standards of the 1964 Declaration of Helsinki. All the volunteers signed an informed consent.
Subjects had also to accept to not receive drug/cosmetic treatments during the study. Exclusion criteria include pregnancy or breastfeeding; any other medical condition or other scalp or hair disorders; last shampoo performed 48 h before sampling; no anti-tumor, immunosuppressant, or radiation therapy in the last 3 months; no antibiotic in the last 30 days before sampling; no presence of underlying infection; no probiotic in the last 15 days; no topical or hormonal therapy on the scalp in the last 3 months.
Subject recruitment and sample collection
Enrolled subjects were sampled through swab procedure according to previously reported methods [24]. eNAT™ kit (1 ml eNAT™ transport and preservation medium and FLOQSwab™) (Copan, Brescia, Italy) was used for sampling a 16 cm2 area. Samples were stored at 4°C until DNA extraction.
A 4-mm biopsy punch was also used to assess the microbial community in the subepidermal compartments of the scalp [24]. Some enrolled healthy subjects were already analyzed in a previously published cohort [24], and here reanalyzed for the metagenomic and metabolite profiles in urinary samples.
DNA extraction and 16S amplicon generation, sequencing, and analysis-Illumina sequencing
Bacterial DNA from collected swabs was extracted by means of QIAamp Dneasy Tissue kit (Qiagen, Milan, Italy) according to manufacturer protocol, with minor modifications [25] following by quantification with QIAexpert system (Qiagen, Milan, Italy) before sequencing.
Following universal prokaryotic primers were used for the V3-V4 variable region: 341 F CTGNCAGCMGCCGCGGTAA [26, 27] and 806bR GGACTACNVGGGTWTCTAAT [28–30] at Personal Genomics (Verona, Italy) following the method of Caporaso et al. [31] and Kozich et al. [32], with minor modifications. The 300PE instrument (Illumina, San Diego, CA) was used for libraries generation. Bioinformatics analyses, from processing of raw fastQ files to alpha index estimates were conducted in QIIME2 [33] () microbiome platform (version 2020.8). QIIME plugin q2-deblur [34] was used for the 16S denoising step. The ad hoc customized classifier “gg-13-8-99-515-806-nb-classifier.qza”, was used to infer taxonomy. QIIME2 plugins were used to compute alpha diversity metrics including Shannon entropy and Faith’s PD.
Predictive functional profiling of microbial LPP communities
16S bacteria rRNA gene sequences were the starting point for the prediction of metabolic functions in the LPP scalp microbiota by using PICRUSt—Phylogenetic Investigation of Communities by Reconstruction of Unobserved States [35]. Specifically, the make.biom command of the Mothur program, based on a Greengenes database [36], was used to obtain a BIOM-formatted OTU table. In order to reflect the true taxa abundance, each OTU was corrected by normalizing the 16S rRNA copy number. By running the Picrust “predict_metagenomes.py” script, KEGG orthology abundances for a given OTU were table-picked using the latest version of Greengenes database.
The gene functions classified by KO were further categorized into KEGG pathways using the “categorize_by_function.py” PICRUSt script, that collapses thousands of predicted functions into higher categories (KEGG pathways). A two-sided corrected (BH) Welch’s t-test (p < 0.05), within the STAMP software [37] was used to assess the enrichment of predicted KEGG pathways.
Cytokine assay
DNA extracted from biopsy samples was used for quantitative real-time PCR (RT-PCR) on interleukin 23 (IL-23) gene. DNA was amplified with a Stratagene Mx3000P Real-Time PCR System (Agilent Technologies Italia S.p.A., Milan, Italy). Following Taqman gene expression assays were used: Hs00372324_m1 (IL-23A) and Hs999999 m1 (GAPDH, human glyceraldehyde-3-phosphate dehydrogenase). Human GAPDH was used as the housekeeping gene. PCR amplifications were carried out in a 20 μl total volume: 10 μl of 2 × Premix Ex Taq (Takara, Japan), 1 μl of 20 × TaqMan gene expression assay, 0.4 μl of RoX Reference Dye II (Takara, Japan), 4.6 μl of water, and 4 μl of DNA at following PCR conditions: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 20 s. PCR reactions were performed in duplicate. The relative abundance of the expression of each gene was calculated by comparing delta cycle thresholds.
Volatile urinary metabolomics
Volatile organic metabolites (VOMs) from urine samples were evaluate as potential biomarkers in healthy and LPP subjects. Two grams of collected urine were supplied in a 20 ml glass vial and spiked with 10 μl of internal standard solution (2-pentanol-4-methyl) at 33 ppm. To obtain the best extraction efficiency, the solid phase microextraction (SPME) was performed by exposing a conditioned 75 μm Carboxen/PDMS fiber (Supelco, Bellefonte, PA, USA) to the headspace of 2 g of acidified (pH 2) urine sample with 1 g of NaCl for 60 min at 60°C after a 35 min incubation [38]. The e following step of extraction was carried out with a CombiPAL system injector autosampler (CTC Analytics). The extracted compounds were desorbed in splitless for 3 min at 280°C [39]. A Clarus 680 (PerkinElmer, Waltham, MA, USA) gas chromatograph equipped with an Elite-624Sil MS Capillary Column (30 m × 0.25 mm i.d., 1.4-μm film thickness; PerkinElmer) was used. The gas chromatography system was coupled to a single quadrupole mass spectrometer Clarus SQ 8C (Perkin Elmer). The source and transfer line temperatures were kept at 250 and 230°C, respectively. Electron ionization masses were recorded at 70 eV, and the mass-to-charge ratio interval was m/z 34 to 350. The obtained chromatogram was analyzed for peak identification using the National Institute of Standard and Technology 2008 (NIST) library. A peak area threshold >1,000,000 and 90% or a greater probability of matches was used for VOMs identification, followed by inspection of the fragment patterns when required. Quantitative data for the compounds identified were obtained by the interpolation of the relative areas vs. the internal standard area.
Statistical analysis
Statistically significant differences in alpha diversity and bacterial communities were obtained by Welch’s t-test corrected by multiple tests (Benjamini-Hochberg). Differences between groups were reported only when significative. Significant taxa at different taxonomic levels were computed by using a regression linear model implemented in MaAsLin2 software (https://huttenhower.sph.harvard.edu/maaslin/).
3. Results
Clinical evaluation and cytokine assay
Twenty-seven healthy or suffering from LPP subjects were enrolled in the study and the groups of lichen and healthy subjects have comparable demographic characteristics (S1 File, sheet 1).
Clinical manifestations typical of LPP were observed in erythema severity score (mean of 2.76±0.7), scaling (mean of 3.25±0.9) and itching/pruritus (mean of 7.15±1.9).
The expression of IL-23 was measured by means of qRT-PCR and resulted higher in the LPP samples than healthy subjects (S1 Fig).
Richness of the bacterial population in LPP
The complexity among the analyzed biopsy layers was estimated by the analysis of community richness. Regardless of the considered sub-epidermal layer, a higher alpha diversity in LPP versus healthy samples was detected both with Shannon entropy and Faith’s PD metrics (S2 Fig).
Taxonomic differences among healthy individuals and LPP
Raw read fastQ files have been demultiplexed and denoised in Qiime2 (S1 File, sheets 2–3).
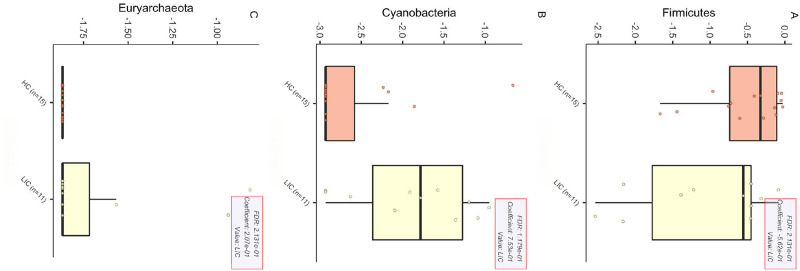
To investigate the existence of a disease-specific microbiota hint on the scalp of LPP patients, we evaluated the bacterial community composition at all different taxonomic levels (S1 File, sheets 4–9) and then we applied a statistical approach based on a regression linear model. As a result, three taxa at the phylum level were found as significantly different among all paired couples if considering the swab (Fig 1).
Fig 1. Statistically significant phyla that differed in the LPP swab.
The three panels report all the statistically different phyla found by applying the regression linear model implemented in MaAsLin2 software. Metadata group information were defined considering the healthy/pathologic status (HC = healthy, LIC = lichen).
At the phylum level all other coupled layers have been tested (DE = Deep Epidermis, D = Dermis HYP = Hypodermis), but no other statistically significant result emerged. Table 1 reports all the significant results found for the other coupled pairs. Noteworthy, only the family of Microbacteriaceae was significantly different in the dermis layer. All the other significative results reported at the family and genus levels were found for the swab microbiota.
Table 1. Statistically different taxa emerged from the application of a multivariable linear regression model.
To test the association between microbial taxa healthy abundances versus Lichen diagnosis, the software MaAsLin2 has been used at different taxonomic level for swab and sub-epidermal layers (swab, deep epidermis, dermis, hypodermis).
| Layer | taxa level | feature | coeff | stderr | N | pval |
|---|---|---|---|---|---|---|
| dermis | family | Microbacteriaceae | -1.03 | 0.2 | 8 | 0.002 |
| swab | phylum | Cyanobacteria | 0.75 | 0.27 | 26 | 0.01 |
| swab | phylum | Euryarchaeota | 0.21 | 0.1 | 26 | 0.053 |
| swab | phylum | Firmicutes | -0.56 | 0.27 | 26 | 0.051 |
| swab | family | Pasteurellaceae | -1.02 | 0.39 | 26 | 0.014 |
| swab | family | Unassigned_Lactobacillales | -0.92 | 0.29 | 26 | 0.004 |
| swab | family | Neisseriaceae | -0.73 | 0.28 | 26 | 0.017 |
| swab | family | Other_Firmicutes | -0.63 | 0.24 | 26 | 0.013 |
| swab | family | Leptotrichiaceae | -0.61 | 0.25 | 26 | 0.021 |
| swab | family | Clostridiaceae | 0.1 | 0.04 | 26 | 0.033 |
| swab | family | Cystobacteraceae | 0.1 | 0.04 | 26 | 0.034 |
| swab | family | Chlamydomonadaceae | 0.1 | 0.04 | 26 | 0.035 |
| swab | family | Unassigned_Rhodospirillales | 0.1 | 0.04 | 26 | 0.037 |
| swab | family | Unassigned_Pseudomonadales | 0.11 | 0.05 | 26 | 0.038 |
| swab | family | Erythrobacteraceae | 0.11 | 0.05 | 26 | 0.039 |
| swab | family | Phormidiaceae | 0.12 | 0.06 | 26 | 0.041 |
| swab | family | Unassigned_Rickettsiales | 0.12 | 0.06 | 26 | 0.044 |
| swab | family | Pseudanabaenaceae | 0.12 | 0.06 | 26 | 0.047 |
| swab | family | Aeromonadaceae | 0.13 | 0.06 | 26 | 0.039 |
| swab | family | Unassigned_Chroococcales | 0.14 | 0.06 | 26 | 0.042 |
| swab | family | Rhodospirillaceae | 0.32 | 0.12 | 26 | 0.018 |
| swab | family | Xanthomonadaceae | 0.38 | 0.16 | 26 | 0.023 |
| swab | family | Oxalobacteraceae | 0.4 | 0.17 | 26 | 0.026 |
| swab | family | Bradyrhizobiaceae | 0.49 | 0.23 | 26 | 0.047 |
| swab | family | Unassigned_Burkholderiales | 0.53 | 0.22 | 26 | 0.023 |
| swab | family | Caulobacteraceae | 0.6 | 0.24 | 26 | 0.018 |
| swab | family | Other_Cyanobacteria | 0.86 | 0.31 | 26 | 0.01 |
| swab | genus | Unclassified_Pasteurellaceae | -1.02 | 0.39 | 26 | 0.014 |
| swab | genus | Unclassified_Neisseriaceae | -0.67 | 0.29 | 26 | 0.031 |
| swab | genus | Unclassified_Leptotrichiaceae | -0.56 | 0.24 | 26 | 0.03 |
| swab | genus | Verrucomicrobium | 0.09 | 0.04 | 26 | 0.033 |
| swab | genus | Haloferula | 0.09 | 0.04 | 26 | 0.033 |
| swab | genus | Clostridium | 0.1 | 0.04 | 26 | 0.033 |
| swab | genus | Unclassified_Cystobacteraceae | 0.1 | 0.04 | 26 | 0.034 |
| swab | genus | Unclassified_Erythrobacteraceae | 0.1 | 0.04 | 26 | 0.034 |
| swab | genus | Novosphingobium | 0.1 | 0.04 | 26 | 0.034 |
| swab | genus | Unclassified_Pseudanabaenaceae | 0.1 | 0.04 | 26 | 0.036 |
| swab | genus | Lysobacter | 0.11 | 0.05 | 26 | 0.036 |
| swab | genus | Unclassified_Hyphomicrobiaceae | 0.11 | 0.05 | 26 | 0.036 |
| swab | genus | Mucilaginibacter | 0.12 | 0.05 | 26 | 0.037 |
| swab | genus | Pedobacter | 0.12 | 0.05 | 26 | 0.038 |
| swab | genus | Pseudoxanthomonas | 0.12 | 0.06 | 26 | 0.04 |
| swab | genus | Unclassified_Phormidiaceae | 0.12 | 0.06 | 26 | 0.041 |
| swab | genus | Frondihabitans | 0.12 | 0.06 | 26 | 0.043 |
| swab | genus | Unclassified_mitochondria | 0.12 | 0.06 | 26 | 0.044 |
| swab | genus | Aeromonas | 0.13 | 0.06 | 26 | 0.039 |
| swab | genus | Unclassified_Chlamydomonadaceae | 0.13 | 0.06 | 26 | 0.041 |
| swab | genus | Caulobacter | 0.15 | 0.06 | 26 | 0.013 |
| swab | genus | Clostridium_2 | 0.2 | 0.07 | 26 | 0.006 |
| swab | genus | Unclassified_Pseudomonadaceae | 0.27 | 0.12 | 26 | 0.036 |
| swab | genus | Unclassified_Xanthomonadaceae | 0.28 | 0.13 | 26 | 0.049 |
| swab | genus | Arthrobacter | 0.35 | 0.16 | 26 | 0.044 |
| swab | genus | Unclassified_Bradyrhizobiaceae | 0.49 | 0.23 | 26 | 0.047 |
| swab | genus | Other_Cyanobacteria | 0.89 | 0.32 | 26 | 0.009 |
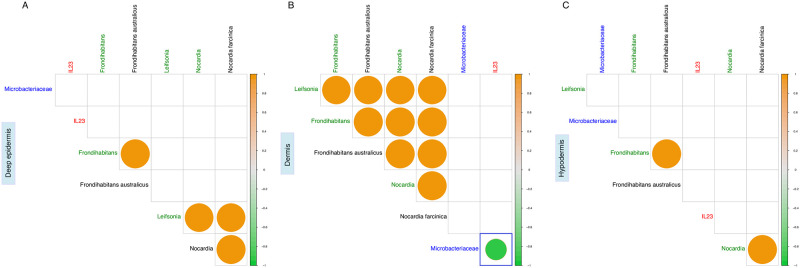
We than investigated about a possible statistical correlation between the genus and species belonging to the Microbacteriaceae family (together with the family abundance itself) against the IL-23 real time ΔCt values in each one of the three biopsy layers. Only the Microbacteriaceae family strongly and negatively correlated (R = -0.92, p<0.05) with the IL-23 mRNA expression (Fig 2). Noteworthy statistical significance dissolved at genus and species levels (Fig 2B).
Fig 2. Significative correlation between taxa (genus and species) belonging to Microbacteriaceae family and IL-23 in the three scalp layers.
Spearman correlations between families (blue), genera (green) and species (black) normalized abundances together with IL-23 ΔCt values. Panel A—deep epidermis, panel B—dermis, panel C—hypodermis. Only statistically significant correlations (p<0.05) have been plotted; the blue delimited square is relative to the only significant correlation between IL-23 and taxonomic data. The color graduated scale ranges from -1 (green—negative correlations) to 1 (orange—positive correlations).
Metabolic function prediction in LPP skin biopsy layers
PiCRUSt software was used to predict microbiota associated biochemical pathways in LPP and healthy samples. Pathways that significantly differed between healthy and LPP samples were selected by applying a Welch’s Test for Unequal Variances corrected by multiple tests.
In the biopsy dermis layers a total of thirty-two metabolic pathways significantly differed between healthy and LPP samples (S3 Fig and S1 Table). Differences in mean proportions statistics evidenced five pathways out of thirty-two that were downregulated in LPP samples and specifically: glycolysis and gluconeogenesis, methane metabolism and three pathways belonging to “genetic information processing” metabolism (i.e. aminoacyl-tRNA biosynthesis, RNA polymerase and ribosome).
GC-MS VOM profiles
Complete profiles of VOMs in six LPP versus six healthy subjects were obtained by mass spectrometry and as a result a total of 73 compounds emerged (S2 Table). Only two VOMs significantly differed between the group of healthy and LPP subjects (correct p < 0.05); specifically, the 4-Terpinenol and the Ionene were detected (Table 2). Both the compounds have a higher relative mean frequency in LPP samples.
Table 2. VOM mean relative frequency assessed in STAMP software using a two-sided Welch’s t-test corrected by using a Benjamini-Hochberg (p < 0.05).
Urine sample metabolites detected in LPP patients were compared to healthy subjects.
| Compound | Healthy: mean rel.freq. (st.dev) | LPP: mean rel.freq. (st.dev) | p value | q value | Diff. between means | 95.0% lower CI* | 95.0% upper CI |
|---|---|---|---|---|---|---|---|
| Ionene | 1.64 (0.3983) | 3.28 (0.8859) | 0.0072 | 0.0411 | -1.6345 | -2.6634 | -0.6056 |
| 4-Terpinenol | 0.63 (0.1030) | 1.15 (0.2603) | 0.0050 | 0.0393 | -0.5197 | -0.8202 | -0.2193 |
*CI Confidence Interval
4. Discussion
Although numerous studies stated the role of bacterial populations in many skin conditions [40–43], the role of the scalp microbiome in LPP has not been investigated yet. In a previously published work, we reported preliminary data on the superficial epidermis of fifteen LPP subjects [20]. In the present study, we showed for the first time, the peculiar features of the core microbiome in cutaneous samples from LPP patients. Also, we contextually studied the differences in microbial populations inhabiting the scalp and its subepidermal compartments in subjects affected by LPP compared to those in a healthy cohort.
Increasing evidence is suggesting the role of the dysbiosis of the complex commensal communities inhabiting the human body, including the skin, on the modulation of the innate and adaptative immune response [44]. This can result in the development of autoimmune diseases with arising inflammation, including Lichen planus (LP). LPP is the form of LP affecting the scalp and hair for which Th17-derived cytokines (IL-17 and IL-23) and it has been reported to be involved in the loss of immune privilege and inflammation [45].
In the current study, we highlighted increased abundances of the phyla Cyanobacteria and Euryarcheota in LPP group compared to the healthy group. Firmicutes were found also less abundant in LPP group. Noteworthy, all these statistically significant phyla (p < 0.05) were found in the swab samples with the only exception of Microbacteriaceae family harboring the dermis layer. These results suggest the involvement of these bacteria in the exacerbation and severity of LPP, with a day by day raising incidence as the result of an improvement of a correct diagnosis by physicians but also linked to an increased impact of etiological factors, especially the environmental ones [13]. In 1994 the Industrial Toxicology Research Center reported for the first time the negative effect of air pollution on the scalp [14].
Nowadays we are witnessing to shifting to metro cities, especially for young people and this produces an increase of scalp discomfort and pains as results from exposure to pollution. As a consequence of pollution, particulate matter and heavy metals may accumulate on the hair [13], inducing oxidative stress by increasing the production of reactive oxygen species [46] (ROS) and leading to clinical conditions related to hair loss, including LPP [47].
Also, in the last decade some members of the Cyanobacteria phylum have been reported to bloom in the atmosphere because of the global climate change [48] but especially of air pollution [49]. This evidence could in some way correlate with the higher abundance of Cyanobacteria we found in LPP cohort. Members of this phylum and the metabolites they produced are reported to be responsible of some negative effects on human health and their extensive blooming leads to several consequences, including negative effects on the skin (irritation, rashes, desquamation, swelling, sores, allergic reactions) [50].
At the same time, it could also be hypothesized that lipopolysaccharide endotoxins which are typical components of the plasma membranes of Gram-negative microorganisms such as Cyanobacteria could play a role in the etiopathology of LPP by the stimulation of the immune system [51]. Therefore, Euryarcheota phylum was also found to be higher in LPP samples.
Even though archaeal colonization of human anatomical sites, including the skin, has long been neglected by researchers, there is a growing number of reports regarding their occurrence at sites of infection [52]. This finding is in line with the hypothesis that infection must be considered as one of the drivers triggering LPP manifestations.
Also, compared to the healthy controls, LPP samples have fewer Firmicutes. This is in line with previous findings on LP affecting the oral mucosa [19].
A statistically significant difference resulted in the Microbacteriaceae abundance (p < 0.05) at dermis layer of LPP group if compared with control samples. A shift in the Microbacteriaceae family abundance has been recently linked to the susceptibility to the otitis media in outer ear skin due to SPINK5 gene variants [53].
We also found a higher microbial diversity in LPP group; this is in line with previous findings [54] where a higher susceptibility of an unhealthy scalp leads the ground to an easier colonization of microorganisms.
Starting with 16S sequencing data, we also explored the differences in predicted KEGG pathways between LPP and healthy samples.
Glycolysis and gluconeogenesis, methane metabolism and three pathways belonging to genetic information processing were found to be downregulated in LPP samples.
Increase in methane metabolism has been reported in atopic dermatitis (AD) [55], another dermatologic disease strongly associated with unbalanced immunological response. It has been hypothesized that dietary metabolites, including methane, produced by gut microbiota, may impact on host immune system leading to an increase in susceptibility to the disease, including LPP.
Hair follicle (HF) possess interesting features related to carbohydrate metabolism including aerobic glycolysis, storage and mobilization of glycogen and the amount of ATP available for follicle activity resulted as greatly influenced by the relative contributions of these metabolic pathways [56]. Because of their alteration, also the hair growth may be altered.
More recently, the role of glycogen metabolism in human HF (HF) has been investigated by Figlak and collaborators [57] and HF outer root sheath (ORS) were found to be the main responsible of glycogen’s synthesis with high levels of glycogen found in the ORS of anagen HF and decreased levels in catagen and absent in telogen phase of the hair cycle. The downregulation of glycolysis and gluconeogenesis metabolism that we found in LPP samples is in line with the above assumptions. Therefore, also peroxisome proliferator-activated receptor γ (PPAR-γ) has been reported to play a role in the pathogenesis of cicatricial alopecia, including LPP [58]. This ligand-activated nuclear receptor is linked to lipid homeostasis but also to inflammatory regulation of sebaceous glands whose function is critical for hair follicle cycling. The main function of sebaceous gland is lipogenesis and glycogen and glycerophosphate represent the main substrates for the synthesis of sebum lipids [57].
Pathways related to aminoacyl-tRNA biosynthesis, RNA polymerase and ribosome were downregulated in LPP samples. Certain aminoacyl-tRNA synthetases (ARSs) are reported to be closely related to different types of immune responses since their involvement in the maturation, transcription, activation and recruitment of immune cells [59]. Interestingly, they can also act as regulators and signaling molecules in various immune diseases including LPP. Indeed, in LPP the immune privilege collapse of the hair follicle’s epithelial stem cell niche is observed [60].
In LPP sample, the downregulation of pathways related to the immune response corresponds to a significant increase in cellular antigens pathway. This is in line with previous evidence on the role of microbial-derived antigens for the susceptibility of the HF under an autoimmune attack [61]. Therefore, associated autoantigen has been recently identified for Lichen Planus [62].
We contextually detected a significant up-regulation in the production of ribosomes and RNA polymerase activity which may be linked to cellular stress, a well know reported marker of LP. Indeed, Wang and collaborators reported a dysregulation of ribosomes biosynthesis as a consequence of cellular stress [48].
Some other pathways, mainly related to metabolism of glycosaminoglycan (GAG) degradation, isoquinoline alkaloid biosynthesis, lipopolysaccharides biosynthesis and antimicrobial resistance, bacterial chemotaxis and flagellar assembly were detected with higher normalized abundance values and resulted predominant in LPP samples.
The findings of the present work are in line with our previous investigation on another form of auto-immune disease affecting the scalp, Alopecia areata [63]. Indeed, the alterations of GAG degradation have been reported as responsible of abnormalities in hair morphology [39] and of the alteration of the morphology of the connective tissue surrounding the HF [64]. Bacteria themselves can use GAGs, as a mask to avoid the recognition by the immune system collaborators [65].
Some recent evidence argued about the possibility that significant differences in OTU abundances may be limited to the epidermal communities, and possibly no effect on the communities inhabiting subepidermal compartments [66]. Accordingly, the great majority of statistically significant taxa at genus and family levels emerged when we compared swab samples.
Some specific upregulated pathways in the LPP sample, such as bacterial chemotaxis, flagellar assembly, and lipopolysaccharide biosynthesis, could be an indication of the presence of specific bacterial community, probably inhabiting the dermal compartment.
Therefore, bacterial chemotaxis is reported to promote activated T lymphocytes in autoimmune disorders [66, 67] and to ensure the access of bacterial species to host niches, where proapoptotic host cell factors were delivered [67].
The pathway relative to isoquinoline alkaloids biosynthesis was predicted to be significantly higher in LPP samples. The synthesis of this type of alkaloids, produced as secondary metabolites by several microorganisms, may be a consequence of the enrichment of microbial population by members of Cyanbacteria.
Skin microbiota, both superficial and the deeper one, comes into contact with epidermal cells; keratinocytes interact also with the cutaneous immune system [68] influencing the immunological response of the scalp and HF [69]. In the present work we noticed in the scalp a strong negative correlation between the Microbacteriaceae family and the IL-23 mRNA levels. The IL-23/IL-17 axis has a pivotal role in the pathogenesis of many chronic inflammatory diseases, including LP [70]. One proposed mechanism from LPP regards the T cell-mediated destruction of the hair follicle bulge and IL-12 and IL-23 are key cytokines involved in this process [71].
By correlating the gene expression of one of the main cytokines involved in the T cell-mediated response in subjects affected by LPP and the microbial population inhabiting the scalp of these subjects, we highlighted the strict connection existing between microbial dysbiosis and clinical manifestations of LPP.
Moreover, by inspecting the urine metabolomic sample profiles we identified two VOMs that resulted significantly higher in LPP than healthy samples. Ionene belongs to a family of polycations known for their antimicrobial activity [72] and probably its higher detected presence in LPP samples may derive from the food or topical medication usage. No scientific association emerged for autoimmune or inflammatory disease and ionene. On the other hand, low concentration of the 4-Terpineol exerts a broad-spectrum antimicrobial and anti-inflammatory effect; this monoterpen is used in different topical formulations based on essential oils [73]. Specifically, the anti-inflammatory effect relies on a selective regulation of monocyte activity, thus toning down immune responses in the skin by inhibiting the production of IL-1β, IL-6 and IL-10 [74]. In another study in vitro tests reveal how only terpinen-4-ol suppressed the production after 40 h of TNFα, IL-1β, IL-8, IL-10 and PGE2 by LPS-activated monocytes [75]. 4-Terpineol and alpha-terpineol have been used in skin topical formulation, but the former has usually a higher percentage than the latter. In our metabolomics results we detected both the compounds but the alpha-terpineol resulted as not statistically significant (S2 Table). As confirmed by our data, the LPP chronic inflammatory disease has an immune response that is triggered by pro inflammatory cytokine IL-23, whose activity together with its downstream effector molecules have been considered as therapeutic targets.
The involvement of the microbiota, both as population and pathways, open to novel therapeutic approaches for the treatment of LPP. Currently available therapeutic options mainly aim to counteract the progression of the lesion with systemic oral corticosteroid, griseofulvin, dapsone, and thalidomide therapy as the first line therapy in case of severe, quickly evolutive LPP [76]. Tosti and collaborators reported the efficacy of clobetasol propionate foam 0.05% [77].
As suggested for atopic dermatitis [78], another inflammatory cutaneous disease, it can be postulated that the use of corticosteroid might suffice to normalize the cutaneous microbial communities on the scalp of LPP subjects. Although the topical treatment with high-potency corticosteroids remains the most effective and safest therapy in the early treatment of LPP, the findings of the present work shed new light of the use of postbiotics [79]. The term “postbiotic” refers to bioactive compounds produced as a result of the metabolic activity of probiotic bacteria. Due to their high specificity of action on resident microbiota and their health-promoting effects in dermatological conditions, postbiotics represent a novel frontier in dermatology.
5. Conclusions
In conclusions, by investigating the bacterial communities inhabiting the superficial epidermis and three biopsy sub-cutaneous layers and reconstructing the biochemical pathways, we shed lights on LPP, a neglected skin pathology. Our results indicated that bacterial community composition and the relative richness are subject to dramatic alterations in the scalp of LPP patients. Thanks to a systematic and multi-level analysis we described the differential abundance of various taxa in the swab and dermis of LPP patients together with their related biochemical pathways. Metagenomics approaches and multi-omics studies are needed to further identify bacterial species and pathways related to LPP immune-related functions.
As the main limitation of the study was the small sample size. Further studies consisting of larger number of patients are needed to better support the provided evidence.
Supporting information
Thirty-two PiCRUSt metabolic pathways from dermis layer belonging to healthy and LPP samples. Statistics are based on Welch’s test for group comparison and Benjamini-Hochberg procedure for multiple test correction. Rel.freq = relative frequency; std.dev. = standard deviation; CI = confidence interval.
(DOCX)
(DOCX)
mRNA levels of IL-23 detected by RT-PCR plus error bars.
(DOCX)
Three different alpha diversity metrics have been computed in Qiime2. PanelA: Observed OTUs; PanelB: Shannon’s diversity index. the dermis layer belonging to healthy and LPP samples PanelC: Faith’s Phylogenetic Diversity (phylogenetic generalization of species richness).
(DOCX)
Pathway normalized abundances from PICRUSt have been analyzed by Welch’s test than corrected by multiple test (Benjamini-Hochberg). Only statistically significant pathways (q value < 0.05) have been reported. Higher mean proportions for healthy and LPP subjects have been plotted as orange or blus dots, respectively. Biochemical pathways which differ significantly in relative abundance between the dermis samples of healthy and LPP samples. The statistical analysis was performed and visualized using the STAMP package. Mean abundance (mean proportion) and difference in mean proportion for pathways showing significant difference in abundance are shown. The 95% confidence intervals and statistical significance (corrected q value) are indicated as well.
(DOCX)
Sheet 1. “lichen metadata”: Sample ID, layer and type of sampled material (biopsy or swab); sheet 2. “demux-filtered-stats”: Qiime2 quality read control demultiplex statistics; sheet 3. “deblur_stats”: Denoising statistics from QIIME2 Deblur plugin; sheet 4. “phylum_assigned_OTU”: Qiime 2 phylum relative frequency table; sheet 5. “class_assigned_OTU”: Qiime 2 class relative frequency table; sheet 6. “order_assigned_OTU”: Qiime 2 order relative frequency table; sheet 7. “family_assigned_OTU”: Qiime 2 family relative frequency table; sheet 8. “genus_assigned_OTU”: Qiime 2 genus relative frequency table; sheet 9. “species_assigned_OTU”: Qiime 2 species relative frequency table.
(XLSX)
Data Availability
16S sequences from both swab and biopsy samples of enrolled subjects obtained with the IlluminaSeq platform were deposited into the National Centre for Biotechnology Information (NCBI) BioProject database under the project number PRJNA510206 and PRJNA692816.
Funding Statement
This research was funded by Giuliani S.p.A.
References
- 1.Wolff H, Fischer TW, Blume-Peytavi U. The Diagnosis and Treatment of Hair and Scalp Diseases. Dtsch Arztebl Int. 2016. May 27;113(21):377–86. doi: 10.3238/arztebl.2016.0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orfanos CE, Frost Ph. Seborrhoeic Dermatitis, Scalp Psoriasis and Hair. in Hair and Hair Diseases (eds. Orfanos C. E. & Happle R.) 641–661 (Springer, 1990). doi: 10.1007/978-3-642-74612-3_25 [DOI] [Google Scholar]
- 3.Suchánková D. [Diseases affecting the scalp]. Cas Lek Cesk; 2017:156, 137–140. [PubMed] [Google Scholar]
- 4.Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009. Mar;28(1):3–10. doi: 10.1016/j.sder.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 5.Lyakhovitsky A, Amichai B, Sizopoulou C, Barzilai A. A case series of 46 patients with lichen planopilaris: Demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015. Jun;26(3):275–9. doi: 10.3109/09546634.2014.933165 Epub 2014 Jul 1. [DOI] [PubMed] [Google Scholar]
- 6.Mehregan DA, Van Hale HM, Muller SA. Lichen planopilaris: clinical and pathologic study of forty-five patients. J Am Acad Dermatol. 1992. Dec;27(6 Pt 1):935–42. doi: 10.1016/0190-9622(92)70290-v [DOI] [PubMed] [Google Scholar]
- 7.Lehman JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009. Jul;48(7):682–94. doi: 10.1111/j.1365-4632.2009.04062.x [DOI] [PubMed] [Google Scholar]
- 8.Soares VC, Mulinari-Brenner F, Souza TE. Lichen planopilaris epidemiology: a retrospective study of 80 cases. An Bras Dermatol. 2015;90(5):666–670. doi: 10.1590/abd1806-4841.20153923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010. Dec;163(6):1296–300. doi: 10.1111/j.1365-2133.2010.09965.x [DOI] [PubMed] [Google Scholar]
- 10.Pai VV, Kikkeri NN, Sori T, Dinesh U. Graham-little piccardi lassueur syndrome: an unusual variant of follicular lichen planus. Int J Trichology. 2011. Jan;3(1):28–30. doi: 10.4103/0974-7753.82129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinkernagel MS, Trüeb RM. Fibrosing alopecia in a pattern distribution: patterned lichen planopilaris or androgenetic alopecia with a lichenoid tissue reaction pattern? Arch Dermatol. 2000. Feb;136(2):205–11. doi: 10.1001/archderm.136.2.205 [DOI] [PubMed] [Google Scholar]
- 12.Cevasco NC, Bergfeld WF, Remzi BK, de Knott HR. A case-series of 29 patients with lichen planopilaris: the Cleveland Clinic Foundation experience on evaluation, diagnosis, and treatment. J Am Acad Dermatol. 2007. Jul;57(1):47–53. doi: 10.1016/j.jaad.2007.01.011 Epub 2007 Apr 30. [DOI] [PubMed] [Google Scholar]
- 13.d’Ovidio R, Sgarra C, Conserva A, Angelotti UF, Erriquez R, Foti C. Alterated integrin expression in lichen planopilaris. Head Face Med. 2007. Feb 8;3:11. doi: 10.1186/1746-160X-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baquerizo Nole KL, Nusbaum B, Pinto GM, Miteva M. Lichen Planopilaris in the Androgenetic Alopecia Area: A Pitfall for Hair Transplantation. Skin Appendage Disord. 2015;1(1):49–53. doi: 10.1159/000381588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horenstein MG, Bacheler CJ. Follicular density and ratios in scarring and nonscarring alopecia. Am J Dermatopathol. 2013. Dec;35(8):818–26. doi: 10.1097/DAD.0b013e3182827fc7 [DOI] [PubMed] [Google Scholar]
- 16.Sorbellini E, Pinto D, Marzani B, Rinaldi F. It is not invisible! A case report of 2 patients with scalp Lichen Planopilaris mimicking Androgenic Alopecia. Ann Dermatol Res; 2017:1, 012–017. [Google Scholar]
- 17.Lepe K, Nassereddin A, Salazar FJ. Lichen Planopilaris. 2021 Jun 25. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. [PubMed]
- 18.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008. Apr;28(4):454–67. doi: 10.1016/j.immuni.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Wang K, Zhang B, Tu Q, Yao Y, Cui B, et al. Salivary mycobiome dysbiosis and its potential impact on bacteriome shifts and host immunity in oral lichen planus. Int. J. Oral Sci; 2019. 11(2), 13 doi: 10.1038/s41368-019-0045-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinaldi F, Pinto D, Marzani B, Rucco M, Sorbellini E, Giuliani G. Human microbiome: What’s new in scalp diseases. J. Transl. Sci; 2018: 4. doi: 10.15761/JTS.1000234 [DOI] [Google Scholar]
- 21.Mukherjee PK, Sendid B, Hoarau G, Colombel JF, Poulain D, Ghannoum MA. Mycobiota in gastrointestinal diseases. Nat Rev Gastroenterol Hepatol. 2015. Feb;12(2):77–87. doi: 10.1038/nrgastro.2014.188 Epub 2014 Nov 11. . [DOI] [PubMed] [Google Scholar]
- 22.Weyrich LS, Dixit S, Farrer AG, Cooper AJ, Cooper AJ. The skin microbiome: Associations between altered microbial communities and disease. Australas J Dermatol. 2015. Nov;56(4):268–74. doi: 10.1111/ajd.12253 Epub 2015 Feb 25. [DOI] [PubMed] [Google Scholar]
- 23.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020. Jun;30(6):492–506. doi: 10.1038/s41422-020-0332-7 Epub 2020 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto D, Sorbellini E, Marzani B, Rucco M, Giuliani G, Rinaldi F. Scalp bacterial shift in Alopecia areata. PLoS One. 2019;14(4):e0215206. Published 2019 Apr 11. doi: 10.1371/journal.pone.0215206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Z, Perez-Perez GI, Chen Y, Blaser MJ. Quantitation of major human cutaneous bacterial and fungal populations. J Clin Microbiol. 2010;48(10):3575–3581. doi: 10.1128/JCM.00597-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi H, Echizen H. Pharmacogenetics of warfarin elimination and its clinical implications. Clin Pharmacokinet. 2001;40(8):587–603. doi: 10.2165/00003088-200140080-00003 [DOI] [PubMed] [Google Scholar]
- 27.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013. Jan 7;41(1):e1. doi: 10.1093/nar/gks808 Epub 2012 Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apprill A, McNally S, Parsons R, Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2019;75,129–137. doi: 10.3354/ame01753 [DOI] [Google Scholar]
- 29.Parada AE, Needham DM, Fuhrman JA. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol. 2016. May;18(5):1403–14. doi: 10.1111/1462-2920.13023 Epub 2015 Oct 14. [DOI] [PubMed] [Google Scholar]
- 30.Walters W, Hyde ER, Berg-Lyons D, Ackermann G, Humphrey G, Parada A, et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems. 2015. Dec 22;1(1):e00009–15. doi: 10.1128/mSystems.00009-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 108 (Suppl 1), 4516–4522. doi: 10.1073/pnas.1000080107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79(17):5112–5120. doi: 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.10.1038/s41587-019-0209-9. [DOI]
- 34.https://github.com/qiime2/q2-deblur.
- 35.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol;2013: 31(9), 814–821. doi: 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May 2013 ver.; http://greengenes.lbl.gov.
- 37.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014. Nov 1;30(21):3123–4. doi: 10.1093/bioinformatics/btu494 Epub 2014 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Živković Semren T, Brčić Karačonji I, Safner T, Brajenović N, Tariba Lovaković B, Pizent A. Gas chromatographic-mass spectrometric analysis of urinary volatile organic metabolites: Optimization of the HS-SPME procedure and sample storage conditions. Talanta. 2018. Jan 1;176:537–543. doi: 10.1016/j.talanta.2017.08.064 Epub 2017 Aug 29. . [DOI] [PubMed] [Google Scholar]
- 39.Pinto D, Calabrese FM, De Angelis M, Celano G, Giuliani G, Gobbetti M, et al. Predictive Metagenomic Profiling, Urine Metabolomics, and Human Marker Gene Expression as an Integrated Approach to Study Alopecia Areata. Front Cell Infect Microbiol. 2020. Apr 29;10:146. doi: 10.3389/fcimb.2020.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008. Mar;158(3):442–55. doi: 10.1111/j.1365-2133.2008.08437.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brogden NK, Mehalick L, Fischer CL, Wertz PW, Brogden KA. The emerging role of peptides and lipids as antimicrobial epidermal barriers and modulators of local inflammation. Skin Pharmacol Physiol. 2012;25(4):167–81. doi: 10.1159/000337927 Epub 2012 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeeuwen PL, Kleerebezem M, Timmerman HM, Schalkwijk J. Microbiome and skin diseases. Curr Opin Allergy Clin Immunol. 2013. Oct;13(5):514–20. doi: 10.1097/ACI.0b013e328364ebeb [DOI] [PubMed] [Google Scholar]
- 43.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014. Jul;16(7):1024–33. doi: 10.1111/cmi.12308 Epub 2014 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci. 2019. Jul 10;20(14):3394. doi: 10.3390/ijms20143394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dlova N, Goh CL, Tosti A. Familial frontal fibrosing alopecia. Br. J. Dermatol;2013; 168: 220–222. doi: 10.1111/j.1365-2133.2012.11101.x [DOI] [PubMed] [Google Scholar]
- 47.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006. Dec;126(12):2565–75. doi: 10.1038/sj.jid.5700340 . [DOI] [PubMed] [Google Scholar]
- 48.Wang W, Nag S, Zhang X, et al. Ribosomal proteins and human diseases: pathogenesis, molecular mechanisms, and therapeutic implications. Med Res Rev. 2015;35(2):225–285. doi: 10.1002/med.21327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paerl HW, Huisman J. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Rep. 2009. Feb;1(1):27–37. doi: 10.1111/j.1758-2229.2008.00004.x [DOI] [PubMed] [Google Scholar]
- 50.Rastogi RP, Madamwar D, Incharoensakdi A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front Microbiol. 2015. Nov 17;6:1254. doi: 10.3389/fmicb.2015.01254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernstein JA, Ghosh D, Levin LS, Zheng S, Carmichael W, Lummus Z, et al. Cyanobacteria: an unrecognized ubiquitous sensitizing allergen? Allergy Asthma Proc. 2011. Mar-Apr;32(2):106–10. doi: 10.2500/aap.2011.32.3434 [DOI] [PubMed] [Google Scholar]
- 52.Drobac Backović D, Tokodi N, Nikolin B, Svirčev Z. Cyanobacteria—insidious foe of the skin? J Water Health. 2020. Jun;18(3):314–330. doi: 10.2166/wh.2020.001 [DOI] [PubMed] [Google Scholar]
- 53.Horz HP, Conrads G. The discussion goes on: What is the role of Euryarchaeota in humans? Archaea. 2010. Dec 30;2010:967271. doi: 10.1155/2010/967271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank DN, Giese APJ, Hafren L, Bootpetch TC, Yarza TKL, Steritz MJ, et al. Otitis media susceptibility and shifts in the head and neck microbiome due to SPINK5 variants. J Med Genet. 2021. Jul;58(7):442–452. doi: 10.1136/jmedgenet-2020-106844 Epub 2020 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiu YH, Wu YW, Hung JI, Chen MC. Epigallocatechin gallate/L-ascorbic acid-loaded poly-γ-glutamate microneedles with antioxidant, anti-inflammatory, and immunomodulatory effects for the treatment of atopic dermatitis. Acta Biomater. 2021;130:223–233. doi: 10.1016/j.actbio.2021.05.032 Epub 2021 Jun 1. . [DOI] [PubMed] [Google Scholar]
- 56.Clavaud C, Jourdain R, Bar-Hen A, Tichit M, Bouchier C, Pouradier F, et al. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One. 2013;8(3):e58203. doi: 10.1371/journal.pone.0058203 Epub 2013 Mar 6. Erratum in: PLoS One. 2013;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Figlak K, Williams G, Bertolini M, Paus R, Philpott MP. Human hair follicles operate an internal Cori cycle and modulate their growth via glycogen phosphorylase. Sci Rep. 2021. Oct 21;11(1):20761. doi: 10.1038/s41598-021-99652-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harries MJ, Paus R. Scarring alopecia and the PPAR-gamma connection. J Invest Dermatol. 2009. May;129(5):1066–70. doi: 10.1038/jid.2008.425 [DOI] [PubMed] [Google Scholar]
- 59.Downie M. M., & Kealey T. (1998). Lipogenesis in the human sebaceous gland: glycogen and glycerophosphate are substrates for the synthesis of sebum lipids. J. invest. Dermatol. 111(2), 199–205. doi: 10.1046/j.1523-1747.1998.00264.x [DOI] [PubMed] [Google Scholar]
- 60.Nie A, Sun B, Fu Z, Yu D. Roles of aminoacyl-tRNA synthetases in immune regulation and immune diseases. Cell Death Dis. 2019. Nov 28;10(12):901. doi: 10.1038/s41419-019-2145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paus R, Bulfone-Paus S, Bertolini M. Hair Follicle Immune Privilege Revisited: The Key to Alopecia Areata Management. J Investig Dermatol Symp Proc. 2018. Jan;19(1):S12–S17. doi: 10.1016/j.jisp.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 62.Roitt IM. The role of autoantigens in the driving of autoimmune diseases. Immunol Ser. 1993;59:119–29. [PubMed] [Google Scholar]
- 63.Schmidt T, Solimani F, Pollmann R, Stein R, Schmidt A, Stulberg I, et al. TH1/TH17 cell recognition of desmoglein 3 and bullous pemphigoid antigen 180 in patients with lichen planus. J Allergy Clin Immunol. 2018. Aug;142(2):669–672.e7. doi: 10.1016/j.jaci.2018.02.044 Epub 2018 Apr 4. [DOI] [PubMed] [Google Scholar]
- 64.Malinowska M, Jakóbkiewicz-Banecka J, Kloska A, Tylki-Szymańska A, Czartoryska B, Piotrowska E, et al. Abnormalities in the hair morphology of patients with some but not all types of mucopolysaccharidoses. Eur J Pediatr. 2008. Feb;167(2):203–9. doi: 10.1007/s00431-007-0462-7 Epub 2007 Mar 15. [DOI] [PubMed] [Google Scholar]
- 65.Maniatopoulou E, Bonovas S, Sitaras N. Isolation and Quantification of Glycosaminoglycans from Human Hair Shaft. Ann Dermatol. 2016. Oct;28(5):533–539. doi: 10.5021/ad.2016.28.5.533 Epub 2016 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cole JN, Barnett TC, Nizet V, Walker MJ. Molecular insight into invasive group A streptococcal disease. Nat Rev Microbiol. 2011. Sep 16;9(10):724–36. doi: 10.1038/nrmicro2648 [DOI] [PubMed] [Google Scholar]
- 67.Bay L, Barnes CJ, Fritz BG, et al. Universal Dermal Microbiome in Human Skin. mBio. 2020; 11. doi: 10.1128/mBio.02945-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rolig AS, Carter JE, Ottemann KM. Bacterial chemotaxis modulates host cell apoptosis to establish a T-helper cell, type 17 (Th17)-dominant immune response in Helicobacter pylori infection. Proc Natl Acad. Sci U. S. A; 2011. 108, 19749–19754. doi: 10.1073/pnas.1104598108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, et al. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006. Oct 9;175(1):179–91. doi: 10.1083/jcb.200603176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scharschmidt TC, Vasquez KS, Pauli ML, Leitner EG, Chu K, Truong HA, et al. Commensal Microbes and Hair Follicle Morphogenesis Coordinately Drive Treg Migration into Neonatal Skin. Cell Host Microbe. 2017. Apr 12;21(4):467–477.e5. doi: 10.1016/j.chom.2017.03.001 Epub 2017 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu R, Zeng X, Han Q, Lin M, Long L, Dan H, et al. Overexpression and selectively regulatory roles of IL-23/IL-17 axis in the lesions of oral lichen planus. Mediators Inflamm. 2014;2014:701094. doi: 10.1155/2014/701094 Epub 2014 Jul 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H, Carnevale G, Polese B, Simard M, Thurairajah B, Khan N, et al. CD109 Restrains Activation of Cutaneous IL-17-Producing γδ T Cells by Commensal Microbiota. Cell Rep. 2019. Oct 8;29(2):391–405.e5. doi: 10.1016/j.celrep.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 73.Mayr J, Bachl J, Schlossmann J, Díaz DD. Antimicrobial and Hemolytic Studies of a Series of Polycations Bearing Quaternary Ammonium Moieties: Structural and Topological Effects. Int J Mol Sci. 2017. Jan 30;18(2):303. doi: 10.3390/ijms18020303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Su CW, Tighe S, Sheha H, Cheng AMS, Tseng SCG. Safety and efficacy of 4-terpineol against microorganisms associated with blepharitis and common ocular diseases. BMJ Open Ophthalmol. 2018. Jun 4;3(1):e000094. doi: 10.1136/bmjophth-2017-000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nogueira MN, Aquino SG, Rossa C Junior, Spolidorio DM. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages. Inflamm Res. 2014. Sep;63(9):769–78. doi: 10.1007/s00011-014-0749-x Epub 2014 Jun 20. [DOI] [PubMed] [Google Scholar]
- 76.de Cássia da Silveira e Sá R, Andrade LN, de Sousa DP. A review on anti-inflammatory activity of monoterpenes. Molecules. 2013;18(1):1227–54. doi: 10.3390/molecules18011227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tosti A, Iorizzo M, Botta GL, Milani M. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006. Nov;20(10):1243–7. doi: 10.1111/j.1468-3083.2006.01781.x [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez ME, Schaffer JV, Orlow SJ, Gao Z, Li H, Alekseyenko AV, et al. Cutaneous microbiome effects of fluticasone propionate cream and adjunctive bleach baths in childhood atopic dermatitis. J Am Acad Dermatol. 2016. Sep;75(3):481–493.e8. doi: 10.1016/j.jaad.2016.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rinaldi F, Pinto D, Giuliani G. Postbiotic Evolution in Dermatology. EC Microbiology; 2020: 16.3, 01–04. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Thirty-two PiCRUSt metabolic pathways from dermis layer belonging to healthy and LPP samples. Statistics are based on Welch’s test for group comparison and Benjamini-Hochberg procedure for multiple test correction. Rel.freq = relative frequency; std.dev. = standard deviation; CI = confidence interval.
(DOCX)
(DOCX)
mRNA levels of IL-23 detected by RT-PCR plus error bars.
(DOCX)
Three different alpha diversity metrics have been computed in Qiime2. PanelA: Observed OTUs; PanelB: Shannon’s diversity index. the dermis layer belonging to healthy and LPP samples PanelC: Faith’s Phylogenetic Diversity (phylogenetic generalization of species richness).
(DOCX)
Pathway normalized abundances from PICRUSt have been analyzed by Welch’s test than corrected by multiple test (Benjamini-Hochberg). Only statistically significant pathways (q value < 0.05) have been reported. Higher mean proportions for healthy and LPP subjects have been plotted as orange or blus dots, respectively. Biochemical pathways which differ significantly in relative abundance between the dermis samples of healthy and LPP samples. The statistical analysis was performed and visualized using the STAMP package. Mean abundance (mean proportion) and difference in mean proportion for pathways showing significant difference in abundance are shown. The 95% confidence intervals and statistical significance (corrected q value) are indicated as well.
(DOCX)
Sheet 1. “lichen metadata”: Sample ID, layer and type of sampled material (biopsy or swab); sheet 2. “demux-filtered-stats”: Qiime2 quality read control demultiplex statistics; sheet 3. “deblur_stats”: Denoising statistics from QIIME2 Deblur plugin; sheet 4. “phylum_assigned_OTU”: Qiime 2 phylum relative frequency table; sheet 5. “class_assigned_OTU”: Qiime 2 class relative frequency table; sheet 6. “order_assigned_OTU”: Qiime 2 order relative frequency table; sheet 7. “family_assigned_OTU”: Qiime 2 family relative frequency table; sheet 8. “genus_assigned_OTU”: Qiime 2 genus relative frequency table; sheet 9. “species_assigned_OTU”: Qiime 2 species relative frequency table.
(XLSX)
Data Availability Statement
16S sequences from both swab and biopsy samples of enrolled subjects obtained with the IlluminaSeq platform were deposited into the National Centre for Biotechnology Information (NCBI) BioProject database under the project number PRJNA510206 and PRJNA692816.