Abstract
The DNA fragment coding for kringle 2 plus serine protease domains (K2S) of tissue plasminogen activator (tPA) was inserted into a phagemid vector, pComb3HSS. In the recombinant vector, pComb3H-K2S, the K2S gene was fused to gpIII of ΦM13 and linked to the OmpA signal sequence. The resulting gene, rK2S-gpIII, was inducibly expressed in Escherichia coli XL-1 Blue. The protein was presented on the phage particle. To stop the expression of gpIII, a stop codon between K2S and the gpIII gene was inserted by site-directed mutagenesis. This mutated vector, MpComb3H-K2S, was transformed in XL-1 Blue. After induction with IPTG (isopropyl-β-d-thiogalactopyranoside), rK2S was found both in the periplasm as an inactive form of approximately 32% and in the culture supernatant as an active form of approximately 68%. The secreted form of rK2S was partially purified by ammonium sulfate (55%) precipitation. The periplasmic form was isolated from whole cells by chloroform extraction. The fibrin binding site of kringle 2 was demonstrated in all expressed versions (phage-bound, periplasmic, and secreted forms) using the monoclonal anti-kringle 2 antibody (16/B). Only the secreted form of rK2S revealed a fibrinogen-dependent amidolytic activity with the specific activity of 236 IU/μg. No amidolytic activity of rK2S was observed in either the periplasmic or the phage-bound form. The secretion of rK2S as an active enzyme offers a novel approach for the production of the active-domain deletion mutant tPA, rK2S, without any requirements for bacterial compartment preparation and in vitro refolding processes. This finding is an important technological advance in the development of large-scale, bacterium-based tPA production systems.
Tissue plasminogen activator (tPA) is a polypeptide containing 527 amino acid residues (27, 33) with a molecular mass of 72 kDa. The molecule is divided into five structural domains. Near the N-terminal region is a looped finger domain followed by a growth factor domain and the two domains kringle 1 and kringle 2. Both finger and kringle 2 domains bind specifically to the fibrin clots, thereby accelerating tPA protein activation of bound plasminogen. Next to the kringle 2 domain is the serine protease domain, which has the catalytic site located at the C terminus. This domain is responsible for converting plasminogen to plasmin, which is important for the homeostasis of fibrin formation and clot dissolution. The correct folding of tPA requires the correct pairing of 17 disulfide bridges in the molecule (1).
Clinically, tPA is a thrombolytic agent of choice for the treatment of acute myocardial infarction. It has the advantage of causing no side effects such as systemic hemorrhaging and fibrinogen depletion (7). Bowes melanoma cells were first used as a source of tPA production for therapeutic purposes (12). Since a consistent process efficiently producing high yields of highly purified protein is required for clinical use, the construction of full-length recombinant tPA (rtPA) progressed to mammalian cells. Chinese hamster ovary cells were transfected with the tPA gene to synthesize rtPA (8, 22). The recombinant product produced by a mammalian fermentation system was harvested from the culture medium. Attracted by simplicity and economy of production, investigators made a number of efforts in producing rtPA from bacteria, especially from Escherichia coli (10, 13, 30). Numerous strategies have been proposed to overcome the problems of low yield and the formation of inclusion bodies, which result in misfolding and in an inactive enzyme. The other major criterion is to synthesize the smallest molecule which is still active, instead of full-length tPA.
Several deletion-mutant variants including kringle 2 plus serine protease (K2S) have been considered. However, the enzymatic activity of the recombinant K2S (rK2S) was obtained only when refolding of the purified inclusion bodies from the cytoplasmic compartment was achieved (16, 29). In order to avoid cumbersome refolding processes and periplasmic protein delivery, special bacterial expression systems were exploited (6, 31). Despite periplasmic expression of tPA, overexpression led to inactive aggregates, even in the relatively high-oxidizing conditions in the periplasm. The other possibility is synthesis of the recombinant protein as an extracellular component. Kipriyanov et al. (17) fused the PelB signal peptide to the N terminus of the single chain (ScFv) and were able to obtain an active recombinant product from E. coli culture supernatant. Recently, the expression of certain recombinant antibody fragments in L forms of Proteus mirabilis was described (28). As the bacterial cells lack periplasmic space, the active recombinant antibody fragment could be directly harvested from the culture supernatant. However, the properties of an extracellular form of the heterologous protein may be distinct from those of the periplasmic accumulated form.
In this study, we describe the production of extracellular and periplasmic forms of K2S in E. coli. In a recent report, the enzymatic properties of phage-displayed kringle 5 with the plasminogen serine protease have been successfully demonstrated (20). Since the cysteine number of this protein is similar to that of K2S, we have evaluated whether the phage-displayed rK2S exerts protease activity. Moreover, we applied a novel strategy to secrete rK2S into the culture supernatant of E. coli. The phage-displayed rK2S and the periplasmic and secreted forms were compared with respect to fibrinogen-dependent serine protease activity.
MATERIALS AND METHODS
Primer design.
In order to amplify a specific part of the tPA gene, a pair of primers, SK2/174 (5′ GAGGAGGAGGTGGCCCAGGCGGCCTCTGAGGGAAACAGTGAC 3′) and ASSP (5′ GAGGAGGAGCTGGCCGGCCTGGCCCGGTCGCATGTTGTCACG 3′), were synthesized (Life Technologies, Grand Island, N.Y.). These primers were designed based on the human tPA gene retrieved from the National Center for Biotechnology Information databases (accession no. g137119). They were synthesized with SfiI end cloning sites (underlined) in such a way that the reading frame from the ATG codon of the gp3 gene in the phagemid vector, pComb3HSS, would be maintained throughout the inserted sequence.
Another primer set for site-directed mutagenesis was designed to anneal at the sequence between the K2S gene and gpIII in pComb3H-K2S. The primers with mutation bases (underlined) for generating a new stop codon were MSTPA (5′ ACATGCGACCGTGACAGGCCGGCCAG 3′) and MASTPA (5′ CTGGCCGGCCTGTCACGGTCGCATGT 3′).
Amplification of the K2S gene by PCR.
One microgram of SK2/174 and ASSP primers together with 50 ng of p51-3 template (obtained from Hiroshi Sasaki, Fujisawa Pharmaceutical, Osaka, Japan) was suspended in 100 μl of PCR mixture. Taq polymerase (2.5 U; Roche Molecular Biochemicals, Indianapolis, Ind.) was finally added to the solution. The titrated amplification condition was initiated with a jump start at 85°C for 4 min and then denaturation at 95°C for 50 s, annealing at 42°C for 50 s, and extension at 72°C for 1.5 min. Thirty-five rounds were performed. The mixture was further incubated at 72°C for 10 min. The 1,110-bp amplified product was subsequently purified with the QIAquick PCR purification kit (Qiagen, Hilden, Germany). The correctness of the purified product was confirmed by restriction enzymes.
Construction of phagemid expressing K2S.
The purified PCR product of K2S and pComb3HSS phagemid (kindly provided by Carlos F. Barbas, Scripps Institute, La Jolla, Calif.) were digested with SfiI (Roche Molecular Biochemicals) to prepare specific cohesive cloning sites. Four micrograms of the purified PCR product was digested with 60 U of SfiI at 50°C for 18 h. For pComb3HSS, 20 μg of phagemid vectors was treated with 100 U of SfiI. Digested products of the purified PCR product of K2S and pComb3HSS (∼3,300 bp) were subsequently gel purified with the QIAquick gel extraction kit (Qiagen). Five units of T4 ligase (Roche Molecular Biochemicals) was introduced to the mixture of 0.7 μg of purified SfiI-digested pComb3HSS and 0.9 μg of purified SfiI-digested PCR product. The ligation reaction mixture was incubated at 30°C for 18 h. The newly constructed phagemid was named pComb3H-K2S.
Transformation of XL-1 Blue.
Two hundred microliters of CaCl2-competent E. coli XL-1 Blue (Stratagene, La Jolla, Calif.) was transformed with 70 ng of ligated or mutated product. The transformed cells were propagated by being spread on Luria-Bertani agar containing 100 μg of ampicillin and 10 μg of tetracycline (Sigma, St. Louis, Mo.) per ml. After cultivation at 37°C for 18 h, several antibiotic-resistant colonies were selected for plasmid minipreparations by the alkaline lysis method. Each purified plasmid was subjected to SfiI restriction site analysis. A transformant-harboring plasmid with the correct SfiI restriction site(s) was subsequently propagated for 18 h at 37°C in 100 ml of Luria-Bertani broth with ampicillin (100 μg/ml) and tetracycline (10 μg/ml). A plasmid maxipreparation was performed using the Qiagen Plasmid Maxi kit (Qiagen). The purified plasmid was reexamined for specific restriction sites by SfiI and sequenced with the AmpliTaq DNA Polymerase Terminator Cycle Sequencing kit (Perkin-Elmer Corporation, Foster City, Calif.).
Site-directed mutagenesis of pComb3H-K2S.
Ten nanograms of pComb3H-K2S template was mixed with 125 ng of MSTPA and MASTPA primers. PfuTurbo DNA polymerase (Stratagene; 2.5 U) was added to the mixture for cycle amplification. The reaction started with one round of 95°C for 30 s followed by 16 rounds consisting of 95°C for 30 s, 55°C for 1 min, and 68°C for 9 min. The reaction tube was subsequently placed on ice for 2 min. In order to destroy the template strands, 10 U of DpnI restriction enzyme (Stratagene) was added to the amplification reaction and incubated for 1 h at 37°C. This synthesized product (MpComb3H-K2S) was further used to transform E. coli XL-1 Blue.
Preparation of phage-displayed rK2S.
After pComb3H-K2S was transformed to XL-1 Blue, the phage display technique was used. A clone of pComb3H-K2S-transformed XL-1 Blue was propagated in 10 ml of super broth (3% [wt/vol] tryptone, 2% [wt/vol] yeast extract, and 1% [wt/vol] morpholinepropanesulfonic acid [MOPS]) containing ampicillin (100 μg/ml) and tetracycline (10 μg/ml) at 37°C until the optical density (OD) at 600 nm of 1.5 was reached. The bacterial culture was subsequently propagated in 100 ml of the same medium and cultured for 2 h. An amount (1012 PFU) of VCSM13 helper phage (Stratagene) was used to infect the transformed XL-1 Blue. After 3 h of incubation, kanamycin at a final concentration of 70 μg/ml was added to the culture. The culture was left shaking (200 rpm) for 18 h at 37°C. Bacteriophages which harbored K2S on gp3 (K2S-φ) were then harvested by adding 4% (wt/vol) polyethylene glycol (PEG) with a molecular weight of 8,000 (Sigma) and 3% (wt/vol) NaCl. Finally, the harvested phage was resuspended in 2 ml of phosphate-buffered saline, pH 7.4. The phage number was determined by infecting XL-1 Blue. The CFU per milliliter was calculated as described previously (21).
Expression of rK2S in shaker flasks.
MpComb3H-K2S-transformed XL-1 Blue was cultivated in 100 ml of super broth at pH 7.0 in the presence of ampicillin (100 μg/ml) at 37°C until an OD at 600 nm of 0.8 was reached. Subsequently, the protein synthesis was induced by 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside; Promega, Madison, Wis.). The bacteria were further cultured by shaking (200 rpm) for 6 h at 30°C. The culture supernatant was collected and precipitated with 55% saturated ammonium sulfate (32). The precipitate was reconstituted with phosphate-buffered saline, pH 7.2, and dialyzed in the same buffer solution at 4°C for 18 h. Periplasmic proteins from bacterial cells were extracted by using a chloroform shock as previously described by Ames et al. (2).
Immunoassay quantification of rK2S.
In order to detect rK2S, solid phase was coated with monoclonal anti-kringle 2 domain (16/B) (generously provided by Ute Zacharias, Central Institute of Molecular Biology, Berlin-Buch, Germany). The standard enzyme-linked immunosorbent assay (ELISA) washing and blocking processes were performed. Fifty microliters of 1011-CFU/ml K2S-φ or secretory rK2S was added into each anti-kringle 2-coated well. Antigen-antibody detection was carried out as follows. Either sheep anti-M13 conjugated with horseradish peroxidase (HRP; Pharmacia Biotech, Uppsala, Sweden) or sheep anti-tPA conjugated with HRP (Cedarlane, Hornby, Ontario, Canada) was added to each reaction well after the washing step. The substrate tetramethylbenzidine was added to every well, and the reaction was finally stopped with H2SO4 solution after 30 min of incubation. The standard melanoma tPA 86/670 (National Institute for Biological Standards and Control, Potters Bar, Hertfordshire, United Kingdom) was used as a positive control.
Amidolytic activity assay.
A test kit for the detection of tPA amidolytic activity was purchased from Chromogenix (Molndal, Sweden). The substrate mixture containing plasminogen and S-2251 was used to determine serine protease activity. The 10−2 dilution of each ammonium-precipitated sample was assayed with and without the stimulant, human fibrinogen fragments. The assay procedure was carried out according to the COASET tPA manual (Chromogenix).
SDS-PAGE and immunoblotting.
The dialyzed precipitate product from the culture supernatant was further concentrated 10-fold with a Centricon 10 concentrator (Amicon, Beverly, Mass.). The concentrated sample was subjected to protein separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), 10% resolving gel, in the reducing buffer followed by electroblotting to nitrocellulose. The nitrocellulose was then blocked with 4% skimmed milk for 2 h. In order to detect rK2S, a proper dilution of monoclonal anti-tPA (107/1) (provided by Stefano Grammatikos, Zellbiologie, Boehringer Ingelheim Pharma KG, Biberach [Riss], Germany) was used. After the washing step, sheep anti-mouse immunoglobulin conjugated with HRP was applied to the nitrocellulose. The chemiluminescence substrate, ECL Western blotting detection reagent (Amersham, Little Chalfont, United Kingdom), was used to locate the reactive band. The immunoreactive band was visualized by exposing the processed nitrocellulose membrane to Hyperfilm ECL (Amersham).
Copolymerized plasminogen PAGE.
An 11% resolving polyacrylamide gel was copolymerized with plasminogen and gelatin as previously described by Heussen and Dowdle (14). The stacking gel was prepared as a 4% concentration without plasminogen and gelatin. Electrophoresis was performed at 4°C in a constant current of 8 mA. The residual SDS in the gel slab was removed after gentle shaking at room temperature for 1 h in 2.5% Triton X-100. Then, the gel slab was incubated in 0.1 M glycine-NaOH, pH 8.3, for 5 h at 37°C. Finally, the gel slab was stained and destained by a standard Coomassie brilliant blue (R-250) dyeing system. The location of the peptide harboring enzymatic activity was not stained by dye, in contrast to the blue-paint background.
RESULTS
Construction of K2S gene carrying vector.
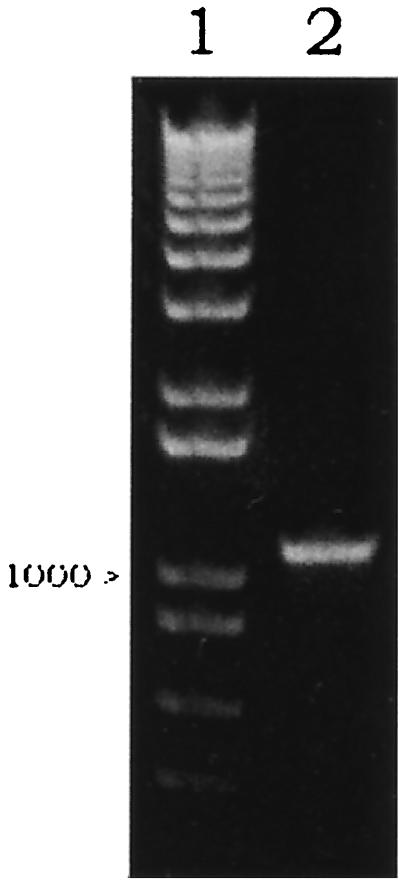
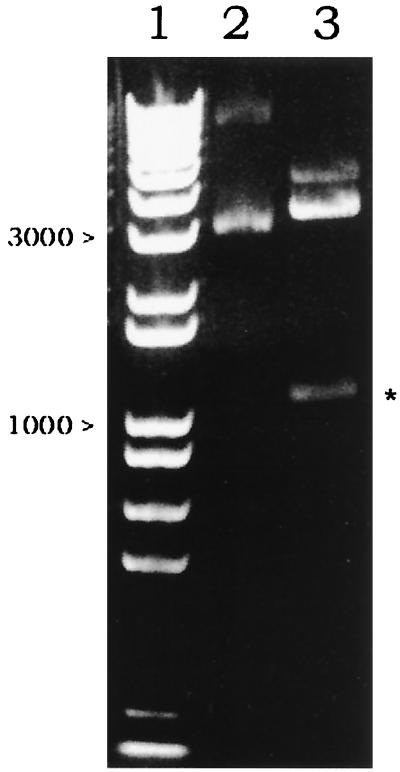
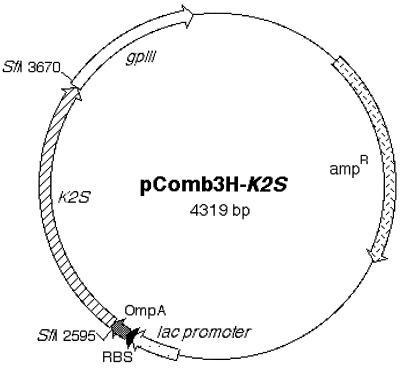
The kringle 2 and the serine protease portion of tPA (Ser174 in kringle 2 domain to Pro527 in the serine protease) in the vector p51-3 were amplified using primers SK2/174 and ASSP. The amplified 1,110-bp product was demonstrated by agarose gel electrophoresis (Fig. 1, lane 2) and was inserted into pComb3HSS phagemid by double SfiI cleavage sites on 5′ and 3′ ends in the correct reading frame. Thus, a new vector harboring the K2S gene, pComb3H-K2S, was generated. In this vector, K2S was flanked upstream by the OmpA signal sequence and downstream by gpIII. The correct insertion of K2S was verified by restriction analysis with SfiI (Fig. 2, lane 3), PCR analysis (demonstration of a single band at 1,110 bp), and DNA sequencing. The map of pComb3H-K2S is shown in Fig. 3.
FIG. 1.
Validation of PCR amplification product of the K2S gene from p51-3 vector using SK2/174 and ASSP primers. Lane 1 shows the 1-kb marker (Roche Molecular Biochemicals). Lane 2 was loaded with 1 μl of amplified product. A single band at 1,110 bp is depicted. The electrophoresis was performed on a 1% agarose gel.
FIG. 2.
Identification of the inserted K2S gene at 1,110 bp (∗) after SfiI-digested pComb3H-K2S was demonstrated (lane 3). Lane 1 shows the 1-kb marker. Lane 2 was loaded with uncut pComb3H-K2S. The electrophoresis was performed on a 1% agarose gel.
FIG. 3.
Map of pComb3H-K2S showing two SfiI cloning sites into which the K2S gene was inserted. The signal sequence (OmpA), the ribosome binding site (RBS), the lac promoter, and the gpIII gene are also depicted.
Phage-displayed rK2S.
VCSM13 filamentous phage was used to infect the pComb3H-K2S transformant of XL-1 Blue, X[K2S]. VCSM13 was propagated and incorporated into the K2S-gpIII fusion protein during the viral packaging processes. The harvested recombinant phage (K2S-φ) gave a concentration of 5.4 × 1011 CFU/ml determined by reinfecting XL-1 Blue with PEG-precipitated phages. These recombinant phage particles were verified for the expression of rK2S by the sandwich ELISA. The phage-bound heterologous K2S protein was recognized by the monoclonal anti-kringle 2 antibody (16/B) using an HRP-conjugated sheep anti-tPA antibody detection system. The absorbance of this assay was 1.12 ± 0.03 (Table 1). The detectable amount of K2S on 1012 phage particles is 336 ng of protein using a standard melanoma tPA as a control. In order to corroborate that K2S-gpIII fusion protein was associated with phage particles, HRP-conjugated sheep anti-tPA antibody was replaced by HRP-conjugated sheep anti-M13 antibody. This immunoreaction exhibited an absorbance of 1.89 ± 0.07 (Table 1). When the capture antibody was sheep anti-M13 antibody, an extremely low level of K2S was observed with HRP-conjugated sheep anti-tPA antibody. The absorbance was only 0.17 ± 0.01 (Table 1). This suggested that there was only a minority of purified phage particles which carried the K2S-gpIII fusion protein. VCSM13 prepared from nontransformed XL-1 Blue was used as a negative control.
TABLE 1.
Detection of rK2S molecule in phage preparation by sandwich ELISA
| Capture antibody | Value for tracer antibody (conjugated with HRP)c
|
|||
|---|---|---|---|---|
| Anti-tPA
|
Anti-M13
|
|||
| K2S-φ | VCSM13a | K2S-φ | VCSM13a | |
| Anti-kringle 2b | 1.12 ± 0.04 | 0.12 ± 0.03 | 1.89 ± 0.02 | 0.16 ± 0.02 |
| Anti-M13 | 0.17 ± 0.01 | 0.14 ± 0.05 | 1.91 ± 0.02 | 1.88 ± 0.03 |
VCSM13 was harvested from XL-1 Blue transformed with pComb3HSS.
Mouse monoclonal anti-kringle 2 (16/B) antibody was used. The other antibodies were prepared from sheep immunoglobulin.
Data are the absorbance values (means ± standard deviations) of samples assayed in triplicate.
Construction of MpComb3H-K2S.
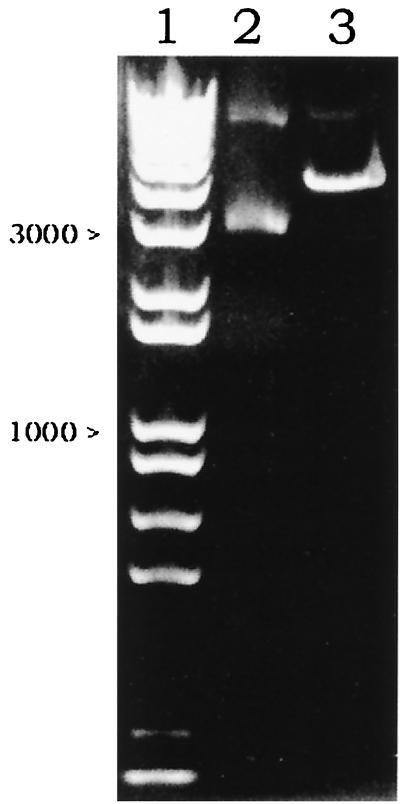
A stop codon between K2S and gpIII in pComb3H-K2S was generated with the aid of mutagenic primers (MSTPA and MASTPA) (Fig. 4). In order to enrich the newly synthesized and mutated MpComb3H-K2S, the cycle amplification mixture was thoroughly digested with DpnI to degrade the old dam-methylated pComb3H-K2S template (DpnI prefers dam-methylated DNA). After transforming XL-1 Blue with MpComb3H-K2S, a transformant, XM[K2S], was selected for further study. As a consequence of base pair substitution, one SfiI cleavage site close to the 3′ end of the K2S gene was lost after site-directed mutagenesis. A linear version of SfiI-cleaved MpComb3H-K2S was observed at 4,319 bp without the appearance of the inserted K2S gene fragment (Fig. 5, lane 3). Thus, the K2S gene encoded by MpComb3H-K2S was expressed in a non-gpIII fusion form in XM[K2S].
FIG. 4.
Diagram of the mutation site at the junction between the K2S and gpIII genes on pComb3H-K2S. The annealing site of pComb3H-K2S was bound with a set of mutation primers (MSTPA and MASTPA) containing modified oligonucleotides (underlined). After the cycle amplification was performed, the SfiI site 1 (in boldface) was modified and lost in the newly synthesized strand.
FIG. 5.
Characterization of newly synthesized MpComb3H-K2S by the SfiI restriction enzyme. A single band at 4,319 bp indicating a single cleavage site of MpComb3H-K2S was observed in lane 3. No inserted K2S band at 1,110 bp is visualized. Lane 1 shows the 1-kb marker. Lane 2 was loaded with the uncut MpComb3H-K2S. The electrophoresis was performed on a 1% agarose gel.
Expression and purification of K2S.
The K2S expression in XM[K2S] was induced by IPTG. The rK2S was detected both in the periplasmic space and in the culture supernatant by an ELISA technique. The amount of the heterologous protein in each preparation was determined by sandwich ELISA using standard tPA for comparison. From 100 ml of the bacterial culture in the shake flask with the OD at 600 nm of 5.0, the periplasmic fraction yielded 1.38 μg of rK2S (approximately 32%) whereas 2.96 μg (approximately 68%) was obtained in the ammonium-precipitated culture supernatant. Sandwich ELISA was used to verify the PEG-precipitated phage from the VCSM13-infected XM[K2S]. No rK2S captured by the monoclonal anti-kringle 2 antibody was detected by an HRP-conjugated anti-M13 antibody, indicating that K2S was not present on the phage particles when gpIII was missing.
Amidolytic activity measurement.
If the serine protease domain is present in the sample, plasminogen will be converted to plasmin. The plasmin produced will further digest the S-2251 substrate to a color product, p-nitroaniline, which has a maximum absorbance at 405 nm. The specific activity of the recombinant product is directly proportional to the absorbance. The fibrinogen-dependent enzymatic activities of K2S-φ, periplasmic rK2S, and the culture supernatant rK2S were evaluated and compared. Both K2S-φ and periplasmic rK2S demonstrated notably low enzymatic activity below the detection limit of the assay (0.25 IU/ml). The culture supernatant rK2S gave fibrinogen-dependent enzymatic activity of 7 IU/ml, i.e., 700 IU from a 100-ml culture. Without fibrinogen, no enzymatic activity of the rK2S purified from culture supernatant was observed, whereas the standard melanoma tPA showed some activity.
Demonstration of recombinant protein by immunoblotting.
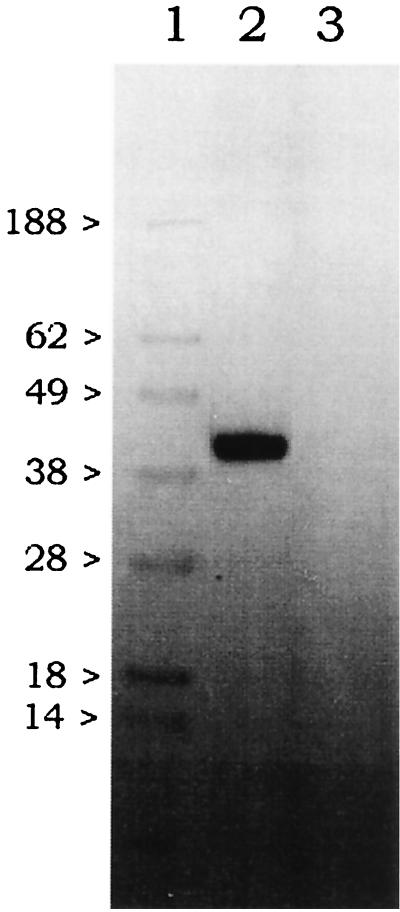
Partially purified K2S from the culture supernatant of XM[K2S] revealed a molecular mass of 39 kDa by using monoclonal anti-tPA (107-30/1) (Fig. 6). For the negative control, the partially purified culture supernatant of nontransformed XL-1 Blue showed no reactive band of a similar size.
FIG. 6.
Identification of an immunologically reactive band of recombinant protein purified from XM[K2S] culture supernatant with monoclonal anti-tPA antibody (107/1). Lane 1 was loaded with SeeBlue prestained standards (NOVEX, Frankfurt am Main, Germany). The partially purified and concentrated culture supernatants from XM[K2S] and nontransformed XL-1 Blue were applied to lanes 2 and 3, respectively. The distinct reactive band was demonstrated in lane 2 at 39 kDa. The visualization of the immunoreactive band was performed by the chemiluminescence system described in Materials and Methods.
Localization of active enzyme by PAGE.
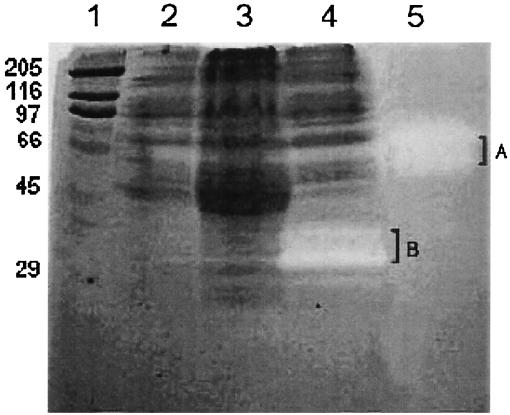
The plasminogen was copolymerized and immobilized with gelatin in the polyacrylamide gel prior to electrophoresis. The ammonium sulfate-precipitated culture supernatants of XL-1 Blue, XL-1 Blue transformed with pComb3HSS, and XM[K2S] were analyzed (Fig. 7). All samples were processed under nonreducing conditions in order to preserve the correct conformation and activity of the serine protease domain. The transparent areas of the serine protease-digested plasminogen were observed only in the ammonium sulfate-precipitated culture supernatants of XM[K2S] at 34- and 37-kDa positions. Other samples gave no clear zones. The positive control lane of the standard melanoma tPA demonstrated enzymatic activities at 66- and 72-kDa positions.
FIG. 7.
Molecular weight determination of extracellular rK2S harboring the active serine protease domain by copolymerized plasminogen PAGE. Lane 1 contains the indicated molecular weight standards (103), SDS-6H (Sigma). Fifty micrograms of the 55% saturated ammonium sulfate-precipitated culture supernatant of XL-1 Blue, XL-1 Blue transformed with pComb3HSS, and XM[K2S] was loaded in lanes 2, 3, and 4, respectively. Lane 5 contains 50 mIU of standard melanoma tPA (86/670). Transparent zones of the digested plasminogen in the polyacrylamide gel are visible only in lane 4 at molecular masses of 34 and 37 kDa (B) and in lane 5 at molecular masses of 66 and 72 kDa (A).
DISCUSSION
In this study, we constructed an expression vector, pComb3H-K2S, encoding kringle 2 plus serine protease domains. Phage display of K2S was successfully performed since the backbone of pComb3H-K2S was phagemid pComb3HSS, a variant of pComb3 (3). The expressed heterologous protein was fused to the essential domain of gpIII. The K2S portion was identified in the phage preparation by a sandwich ELISA technique. By using HRP-conjugated anti-M13, the packed filamentous phages presenting K2S on their surfaces were confirmed. However, only small numbers of phage particles having the K2S-gpIII fusion molecule were present. This was due to the low turnover of substrate in the ELISA used to detect the anchored phages on a solid phase with HRP-conjugated anti-tPA. The low amount of fusion protein was likely due to the fact that K2S-gpIII expression was not induced by IPTG. Overexpression of K2S-gpIII led to an incomplete packaging of the fusion protein on the phage particles (data not shown). The number of harvested phages packaging pComb3H-K2S was independent of foreign protein incorporation. The concentration of the harvested phages was significantly high (5.4 × 1011 CFU/ml). These data were unambiguous because K2S was not detected in the control (VCSM13 phage from the infected XL-1 Blue).
In general, pComb3HSS has been used to clone Fd and light chain genes (4). The gpIII gene plus the first stop codon can be removed by cleaving with NheI 3′ to the first stop codon and SpeI 5′ to gpIII and religated (NheI and SpeI leave complementary cohesive ends). Consequently, the second stop codon is placed behind the Fd gene and the free form of Fd is obtained (please see the PhageAB website at http://www.ams.cmu.ac.th/clinimm/phageab.htm). In our study, K2S was inserted at the two SfiI cloning sites; as a consequence, one SpeI cleavage site is lost. Thus, to synthesize rK2S in a free form, a stop codon was introduced 3′ of the K2S gene by non-PCR site-directed mutagenesis using a set of mutation primers. After transformation, the MX[K2S] carrying the mutated vector (MpComb3H-K2S) which lacked one of the SfiI cleavage sites was established. This strategy facilitated the production of free rK2S and its release to the periplasmic space and the culture supernatant. Free rK2S also lost its competence to assemble phage particles since the gpIII essential domain was absent. This cloning strategy can be applied to other genes whose gene products should be presented on phages or found in the free form. This approach appears to be advantageous, since it uses only a single restriction enzyme in cloning and does not require further restriction enzyme digestion, fragment purification, and ligation steps.
The K2S polypeptide has nine disulfide bridges (5, 29), which are needed for proper folding. For the detection of rK2S either in the free-form rK2S or the K2S-φ, a monoclonal antibody (16/B) recognizing the kringle 2 domain was used. This antibody recognizes only the correctly folded rK2S at the kringle 2 domain (36). In addition, this antibody also binds to the area involved in fibrin binding. Therefore, the recombinant molecule detected by sandwich ELISA retained at least a fibrin binding site. Our data demonstrated that the free-form rK2S and the K2S-φ consisted of an active kringle 2 domain.
The amidolytic activity assay was used to evaluate the functions of the periplasmic rK2S, ammonium-precipitated culture supernatant rK2S, and K2S-φ. The role of the kringle 2-specific region in augmenting the enzymatic activity was verified under the condition of fibrinogen stimulation (25). The digestion of plasminogen to plasmin by every heterologous protein preparation was remarkably low when fibrinogen was not included in the reaction. Interestingly, only ammonium-precipitated culture supernatant rK2S demonstrated specific activity in the fibrinogen stimulation experiment. This implies that partially purified rK2S from the culture supernatant harbors functional kringle 2 and serine protease. The molecular sizes of these active products, which were analyzed by a copolymerized plasminogen PAGE, were 34 and 37 kDa. They were not exactly equal to that demonstrated by immunoblotting (39 kDa) because the samples for copolymerized plasminogen PAGE were prepared under nonreducing conditions. rK2S obtained from the periplasmic fraction gave low enzymatic activity. This may be due to the effect of improper folding and aggregation inside the periplasmic space resulting from overproduction of the heterologous protein. However, this explanation cannot be used for the low enzymatic activity of K2S-φ, since the K2S-gpIII fusion protein was not overexpressed.
Recently, Lasters et al. (20) succeeded in displaying an active enzyme of a plasminogen derivative containing kringle 5 to serine protease (K5S) on phage particles using the Fd-Tet-SN vector. The main difference between that work and our study is that our pComb3HSS contains residues 230 to 406 of gpIII whereas their vector has complete sequences of 406 amino acids of gpIII (26). According to the smaller molecular size, the serine protease active site of rK2S was supposed to be closer to the phage particle than to rK5S. The steric hindrance of the huge phage particle may affect the binding of plasminogen to the catalytic site of the K2S molecule, resulting in the low p-nitroaniline product of plasmin-digested S-2251. Moreover, the activity of K5S-φ was measured by using direct plasmin substrate S-2403. This small substrate molecule eventually can reach the enzyme groove without being obstructed by the phage particle. Therefore, if the ligand-binding site of the recombinant protein is proximal to the C terminus, the length of gpIII encoded on the vector has to be considered.
The specific activity of rK2S from the ammonium-precipitated culture supernatant was 236 IU/μg, which was approximately twofold less than the activity of the standard melanoma tPA. We presume that not all of the rK2S was successfully folded and left the periplasmic space before inactive molecules were formed. The beneficial effect of using L-form cells of P. mirabilis lacking periplasmic space for production of active secretory ScFv supports this notion (28). The enzymatic activity of rK2S from ammonium-precipitated E. coli culture supernatant can be increased by varying the amounts of inducer (IPTG), the incubation conditions, and the harvesting period (17). One recent report suggested the use of glycerol in combination with IPTG to increase the productive yield of insulin-like growth factor in E. coli (23). The amount of active recombinant protein depends on the amount of glycerol used. This finding will be useful in further studies. As has been known, oxidizing compounds such as H2O2 might favor the formation of disulfide bridges. We have also investigated the influence of H2O2 concentrations ranging from 0.005 to 2.0% on extracellular K2S production and folding (data not shown). Not only was the positive effect not observed, but also H2O2 concentrations above 0.005% stopped cell growth and caused cell damage.
So far, at least four reports have described the preparation of active rK2S from E. coli. Obukowicz et al. (25) expressed and purified rK2S from periplasmic space. High enzymatic activity, of 396 IU/μg, was obtained for this heterologous protein since purification of the active form was done with Erythrina inhibitor-Sepharose (15, 19). The obvious disadvantage of this method was an extra periplasmic extraction step, which was not suitable for large-scale production. Therefore, we employed the OmpA instead of the PhoA signal peptide. As the OmpA signal peptide translocates the recombinant proteins to the outer surface, it facilitates the release of the molecule into the culture medium to a greater extent than does the PhoA signal peptide. The OmpA delivery system has been successfully demonstrated in a number of studies, e.g., studies using streptokinase (18) and TolAII (35). In addition, incomplete cleavage of the PhoA leader peptide was used to produce human interleukin-1β (9, 11). This can affect the acquisition of the mature and active form of the heterologous protein. Apart from the release of rK2S into the periplasm, the expressed rK2S found in the cytoplasm is also of interest. Saito et al. (29) introduced in vivo renaturation processes for the expressed rK2S, which was purified from the cytoplasmic space of E. coli as inclusion bodies. In fact, the improvement of refolding processes was established by Boehringer Mannheim for the commercial product Reteplase (24). However, the renaturation procedure is not cost-effective.
In 1991, Waldenström et al. (34) first constructed a vector (pEZZK2P) for the secretion of kringle 2 plus the serine protease domain in E. coli culture supernatant. Hydroxylamine was used to remove the ZZ fusion peptide from the immunoglobulin G-Sepharose-purified fraction. The cleavage sites of the kringle 2 plus serine protease were modified (Asn177 → Ser and Asn184 → Gln) to protect the protein from hydroxylamine digestion. Thus, the synthetic molecule is not suitable for therapeutic purposes since the unusual sequence may activate the human immune system. Moreover, the conformation of the kringle 2 domain that enhances the amidolytic activity was not elucidated.
We describe here an alternative strategy for synthesizing active rK2S and its partial purification from the E. coli culture supernatant. This improved method will also facilitate the study of other complex polypeptides. The selection of a suitable host strain of E. coli and large-scale production of rK2S in an appropriate fermenting system will be established in the near future.
ACKNOWLEDGMENTS
This work was supported by The Thailand Research Fund (TRF) under the Royal Golden Jubilee Ph.D. Scholarship Program (RGJ-TRF) and Institute for Science and Technology Research and Development (IST), Chiang Mai University, Chiang Mai, Thailand.
REFERENCES
- 1.Allen S, Naim H Y, Bulleid N J. Intracellular folding of tissue-type plasminogen activator. Effects of disulfide bond formation on N-linked glycosylation and secretion. J Biol Chem. 1995;270:4797–4804. doi: 10.1074/jbc.270.9.4797. [DOI] [PubMed] [Google Scholar]
- 2.Ames G F, Prody C, Kustu S. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J Bacteriol. 1984;160:1181–1183. doi: 10.1128/jb.160.3.1181-1183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbas C F, III, Kang A S, Lerner R A, Benkovic S J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci USA. 1991;88:7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbas C F, III, Wagner J. Synthetic human antibodies: selecting and evolving functional proteins. Compan Methods Enzymol. 1995;8:94–103. [Google Scholar]
- 5.Bennett W F, Paoni N F, Keyt B A, Botstein D, Jones A J, Presta L, Wurm F M, Zoller M J. High resolution analysis of functional determinants on human tissue-type plasminogen activator. J Biol Chem. 1991;266:5191–5201. [PubMed] [Google Scholar]
- 6.Betton J M, Sassoon N, Hofnung M, Laurent M. Degradation versus aggregation of misfolded maltose-binding protein in the periplasm of Escherichia coli. J Biol Chem. 1998;273:8897–8902. doi: 10.1074/jbc.273.15.8897. [DOI] [PubMed] [Google Scholar]
- 7.Camiolo S M, Thorsen S, Astrup T. Fibrinogenolysis and fibrinolysis with tissue plasminogen activator, urokinase, streptokinase-activated human globulin and plasmin. Proc Soc Exp Biol Med. 1971;38:277–280. doi: 10.3181/00379727-138-35878. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright T. Production of t-PA from animal cell culture. Anim Cell Biotechnol. 1992;5:217–245. [Google Scholar]
- 9.Curry K A, Yem A W, Deibel M R, Jr, Hatzenbuhler N T, Hoogerheide J G, Tomich C S. Escherichia coli expression and processing of human interleukin-1 beta fused to signal peptides. DNA Cell Biol. 1990;9:167–175. doi: 10.1089/dna.1990.9.167. [DOI] [PubMed] [Google Scholar]
- 10.Datar R V, Cartwright T, Rosen C-G. Process economics of animal cell and bacterial fermentations: a case study analysis of tissue plasminogen activator. Bio/Technology. 1993;11:349–357. doi: 10.1038/nbt0393-349. [DOI] [PubMed] [Google Scholar]
- 11.Denefle P, Kovarik S, Ciora T, Gosselet N, Benichou J C, Latta M, Guinet F, Ryter A, Mayaux J F. Heterologous protein export in Escherichia coli: influence of bacterial signal peptides on the export of human interleukin 1 beta. Gene. 1989;85:499–510. doi: 10.1016/0378-1119(89)90444-7. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths J B, Electricwala A. Production of tissue plasminogen activators from animal cells. Adv Biochem Eng Biotechnol. 1987;34:147–166. doi: 10.1007/BFb0000678. [DOI] [PubMed] [Google Scholar]
- 13.Harris T J, Patel T, Marston F A, Little S, Emtage J S, Opdenakker G, Volckaert G, Rombauts W, Billiau A, De Somer P. Cloning of cDNA coding for human tissue-type plasminogen activator and its expression in Escherichia coli. Mol Biol Med. 1986;3:279–292. [PubMed] [Google Scholar]
- 14.Heussen C, Dowdle E B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 15.Heussen C, Joubert F, Dowdle E B. Purification of human tissue plasminogen activator with Erythrina trypsin inhibitor. J Biol Chem. 1984;259:11635–11638. [PubMed] [Google Scholar]
- 16.Hu C K, Kohnert U, Wilhelm O, Fischer S, Llinas M. Tissue-type plasminogen activator domain-deletion mutant BM 06.022: modular stability, inhibitor binding, and activation cleavage. Biochemistry. 1994;33:11760–11766. doi: 10.1021/bi00205a011. [DOI] [PubMed] [Google Scholar]
- 17.Kipriyanov S M, Moldenhauer G, Little M. High level production of soluble single chain antibodies in small-scale Escherichia coli cultures. J Immunol Methods. 1997;200:69–77. doi: 10.1016/s0022-1759(96)00188-3. [DOI] [PubMed] [Google Scholar]
- 18.Ko J H, Park D K, Kim I C, Lee S H, Byun S M. High-level expression and secretion of streptokinase in Escherichia coli. Biotechnol Lett. 1995;17:1019–1024. [Google Scholar]
- 19.Kouzuma Y, Yamasaki N, Kimura M. The tissue-type plasminogen activator inhibitor ETIa from Erythrina variegata: structural basis for the inhibitory activity by cloning, expression, and mutagenesis of the cDNA encoding ETIa. J Biochem (Tokyo) 1997;121:456–463. doi: 10.1093/oxfordjournals.jbchem.a021610. [DOI] [PubMed] [Google Scholar]
- 20.Lasters I, Van Herzeele N, Lijnen H R, Collen D, Jespers L. Enzymatic properties of phage-displayed fragments of human plasminogen. Eur J Biochem. 1997;244:946–952. doi: 10.1111/j.1432-1033.1997.00946.x. [DOI] [PubMed] [Google Scholar]
- 21.Lobel L I, Rausch P, Trakht I, Pollak S, Lustbader J W. Filamentous phage displaying the extracellular domain of the hLH/CG receptor bind hCG specifically. Endocrinology. 1997;138:1232–1239. doi: 10.1210/endo.138.3.5017. [DOI] [PubMed] [Google Scholar]
- 22.Lubiniecki A, Arathoon R, Polastri G, Thomas J, Wiebe M, Garnick R, Jones A, van Reis R, Builder S. Selected strategies for manufacture and control of recombinant tissue plasminogen activator prepared from cell culture. In: Spier R E, Griffiths J B, Stephenne J, Crooy P J, editors. Advances in animal cell biology and technology for bioprocesses. London, United Kingdom: Butterworths; 1990. pp. 442–451. [Google Scholar]
- 23.Lucic M R, Forbes B E, Grosvenor S E, Carr J M, Wallace J C, Forsberg G. Secretion in Escherichia coli and phage-display of recombinant insulin-like growth factor binding protein-2. J Biotechnol. 1998;61:95–108. doi: 10.1016/s0168-1656(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 24.Martin U, Fischer S, Kohnert U, Lill H, Rudolph R, Sponer G, Stern A, Strein K. Properties of a novel plasminogen activator (BM 06.022) produced in Escherichia coli. Z Kardiol. 1990;79:167–170. [PubMed] [Google Scholar]
- 25.Obukowicz M G, Gustafson M E, Junger K D, Leimgruber R M, Wittwer A J, Wun T C, Warren T G, Bishop B F, Mathis K J, McPherson D T, Siegel N R, Jenning M G, Brightwell B B, Diaz-Clier J A, Bell L D, Craik C S, Tacon W C. Secretion of active kringle-2-serine protease in Escherichia coli. Biochemistry. 1990;29:9737–9745. doi: 10.1021/bi00493a033. [DOI] [PubMed] [Google Scholar]
- 26.Parmley S F, Smith G P. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene. 1988;73:305–318. doi: 10.1016/0378-1119(88)90495-7. [DOI] [PubMed] [Google Scholar]
- 27.Pennica D, Holmes W E, Kohr W J, Harkins R N, Vehar G A, Ward C A, Bennett W F, Yelverton E, Seeburg P H, Heyneker H I, Goeddel D V, Collen D. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983;301:214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- 28.Rippmann J F, Klein M, Hoischen C, Brocks B, Rettig W J, Gumpert J, Pfizenmaier K, Mattes R, Moosmayer D. Procaryotic expression of single-chain variable-fragment (scFv) antibodies: secretion in L-form cells of Proteus mirabilis leads to active product and overcomes the limitations of periplasmic expression in Escherichia coli. Appl Environ Microbiol. 1998;64:4862–4869. doi: 10.1128/aem.64.12.4862-4869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito Y, Ishii Y, Sasaki H, Hayashi M, Fujimura T, Imai Y, Nakamura S, Suzuki S, Notani J, Asada T, Horiai H, Masakazu K, Mineo N. Production and characterization of a novel tissue-type plasminogen activator derivative in Escherichia coli. Biotechnol Prog. 1994;10:472–479. doi: 10.1021/bp00029a004. [DOI] [PubMed] [Google Scholar]
- 30.Sarmientos P, Duchesne M, Denèfle P, Boiziau J, Fromage N, Delporte N, Parker F, Lelièvre Y, Mayaux J-F, Cartwright T. Synthesis and purification of active human tissue plasminogen activator from Escherichia coli. Bio/Technology. 1989;7:495–501. [Google Scholar]
- 31.Scherrer S, Robas N, Zouheiry H, Branlant G, Branlant C. Periplasmic aggregation limits the proteolytic maturation of the Escherichia coli penicillin G amidase precursor polypeptide. Appl Microbiol Biotechnol. 1994;42:85–89. doi: 10.1007/BF00170229. [DOI] [PubMed] [Google Scholar]
- 32.Soeda S, Kakiki M, Shimeno H, Nagamatsu A. Rapid and high-yield purification of porcine heart tissue-type plasminogen activator by heparin-sepharose choromatography. Life Sci. 1986;39:1317–1324. doi: 10.1016/0024-3205(86)90329-2. [DOI] [PubMed] [Google Scholar]
- 33.Szarka S J, Sihota E G, Habibi H R, Wong S-L. Staphylokinase as a plasminogen activator component in recombinant fusion proteins. Appl Environ Microbiol. 1999;65:506–513. doi: 10.1128/aem.65.2.506-513.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waldenström M, Holmgren E, Attersand A, Kalderen C, Lowenadler B, Raden B, Hansson L, Pohl G. Synthesis and secretion of a fibrinolytically active tissue-type plasminogen activator variant in Escherichia coli. Gene. 1991;99:243–248. doi: 10.1016/0378-1119(91)90133-v. [DOI] [PubMed] [Google Scholar]
- 35.Wan E W-M, Baneyx F. TolAIII co-overexpression facilitates the recovery of periplasmic recombinant proteins into the growth medium of Escherichia coli. Protein Expr Purif. 1998;14:13–22. doi: 10.1006/prep.1998.0941. [DOI] [PubMed] [Google Scholar]
- 36.Zacharias U, Fischer B, Noll F, Will H. Characterization of human tissue-type plasminogen activator with monoclonal antibodies: mapping of epitopes and binding sites for fibrin and lysine. Thromb Haemostasis. 1992;67:88–94. [PubMed] [Google Scholar]