Abstract
Background
Information on large groups of patients with acrodermatitis chronica atrophicans (ACA) is limited.
Methods
We assessed clinical and microbiological characteristics of patients with ACA diagnosed at a single medical centre and compared findings in periods 1991–2004 vs. 2005–2018. The cohort is representative of Slovenian ACA patients.
Results
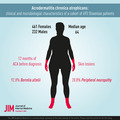
We assessed 693 patients: 461 females and 232 males, with median age of 64 years. Median duration of ACA before diagnosis was 12 months. In all but 2 patients, the skin lesions were located on extremities, more often on the lower (70.0%) than the upper (45.2%), bilaterally in 42.4%. Reddish‐blue discoloration, swelling, thinning and wrinkling of skin were present in 95.2%, 28.1%, 46.4% and 20.5% of patients, respectively. Overall, 64.4% of patients reported constitutional symptoms, 23.1% had local symptoms, and 20.8% had symptoms/signs of peripheral neuropathy. Nodules, arthritis, joint deformity, muscle atrophy and paresis were rare (<3%). Borreliae were isolated from 200/664 (30.1%) skin samples; 92.8% were Borrelia afzelii. B. garinii and B. burgdorferi s.s. were more often isolated from the skin of male patients (OR = 4.17) and from those with arthropathy (OR = 11.74). Patients included in the more recent period were older, complained less often of constitutional symptoms but more often of local symptoms, and more often had local swelling but less often skin atrophy and bilateral involvement, probably as a consequence of earlier diagnosis.
Conclusions
ACA, typically caused by B. afzelii, usually affects older women. Clinical presentation depends on the duration of illness and probably on the Borrelia species causing the disease.
Keywords: acrodermatitis chronica atrophicans, Borrelia afzelii, Borrelia burgdorferi sensu lato, late Lyme borreliosis
Introduction
Acrodermatitis chronica atrophicans (ACA) is a late cutaneous manifestation of European Lyme borreliosis (LB). It starts with a very slowly enlarging reddish‐blue discoloration and swelling of the skin of the distal, extensor parts of the extremities (an inflammatory phase), and if untreated is followed by atrophy. Peripheral neuropathy and/or arthropathy can evolve, typically in the area of impaired skin. In some patients, ACA follows an earlier manifestation of LB, such as erythema migrans (EM). ACA was first reported in 1883, when Buchwald described a diffuse idiopathic skin atrophy [1], named ACA in 1902 [2]. Thus, ACA was known in Europe 100 years before the recognition of LB in the United States in 1983 [3, 4] and the first report that ACA is a manifestation of this disease [5]. However, in recent decades articles on ACA have included only small groups of patients.
The aim of this study was to obtain comprehensive clinical and microbiological data on a large group of patients with ACA.
Patients and methods
The present study is a retrospective cohort study, encompassing patients diagnosed with ACA at LB Outpatient Clinic of University Medical Centre Ljubljana, Slovenia, in the period 1991–2018. Whilst the information on the patients has been systematically collected since 1991, the database has been created only recently. Consequently, the data for individual patient were considerably complete; however, for initial years (1991–1994) documentation was missing for several patients (Fig. S1). Since a large majority of patients with suspected ACA from central part of Slovenia are referred to our LB Outpatient Clinic, the present case series is most probably well representative of Slovenian ACA patients. The age and sex of 590 patients included in the present study have been reported previously [6].
The study was approved by the Medical Ethics Committee of the Ministry of Health of the Republic of Slovenia (No. 0120‐520/2017/5).
Patients
We reviewed the medical records of patients ≥ 15 years of age, diagnosed with ACA at our LB Outpatient Clinic during the 28‐year period, 1991–2018, and for whom the medical documentation was available. Diagnosis of ACA was based on 3 criteria: suggestive clinical presentation, demonstration of borrelial serum IgG antibodies and histological findings compatible with ACA. We also included some patients with typical clinical presentation and established borrelial infection but for whom skin histology was not available. We analysed the epidemiological, clinical and microbiological characteristics of all these patients. To test the assumption that knowledge of ACA has improved over the years and that diagnosis is therefore earlier, we compared findings in subgroups of patients assessed in the periods 1991–2004 and 2005–2018.
Clinical evaluation
Basic approaches remained similar during the overall study period. We obtained the demographic, epidemiological and clinical data, paying particular attention to constitutional and local symptoms, tick bites, past manifestations of LB, previous antibiotic treatment, skin changes, and neurological and/or joint involvement. The data were collected prospectively. For the purpose of this study, only information obtained at the initial presentation (before treatment) was used.
Serological evaluation
Up to 2010, we measured serum antibodies to B. burgdorferi s.l. in an indirect immunofluorescence assay (IFA) with a local isolate of B. afzelii as antigen. Serum dilutions ≥ 1:256 were interpreted as positive, based on results in a control group from the same geographic region [7]. For antibody detection from 2010 onwards, we used an indirect chemiluminescence immunoassay (LIAISON®, DiaSorin, Italy) with recombinant antigens OspC and VlsE for IgM and VlsE for IgG; results were graded according to the manufacturer’s instructions.
Cultivation and typing of B. burgdorferi s.l
Skin biopsy specimens (2.5 × 2 × 2 mm) obtained from ACA lesions were inoculated directly into tubes containing 7 mL of modified Kelly–Pettenkofer medium (MKP). Samples of citrated blood (5 mL until 2000, 9 mL from 2001 onwards) were centrifuged (500 rpm for 10 minutes), and 1 mL samples of plasma were inoculated into tubes containing 7 mL MKP. All samples were incubated at 33ºC and examined weekly by dark‐field microscopy for the presence of spirochetes (up to 9 weeks for skin, 12 weeks for blood specimens). Isolates were identified to species/strain level using pulsed‐field gel electrophoresis after MLuI restriction of genomic DNA [8], or by PCR‐based restriction fragment length polymorphism of the intergenic region [9].
Histopathological evaluation
Skin samples obtained at sites of ACA were placed directly into formalin‐containing tubes and subsequently examined after standard haematoxylin and eosin staining. Dermis atrophy and/or lymphocyte and plasma cell inflammatory infiltrates were regarded as indicative of ACA.
Statistical methods
Categorical variables were summarized with frequencies and percentages and 95% confidence intervals (CI), numerical variables with medians and interquartile ranges (IQRs). The characteristics of the patients diagnosed in the 1991‒2004 period were compared to those diagnosed in the 2005–2018 period using the Mann–Whitney test, Fisher's exact test or chi‐squared test, as appropriate. Several covariates, selected using expert opinion (KO, FS) independently from the observed outcomes, were used for testing associations with three outcomes: the presence of serum borrelial IgM antibodies; positive borrelial skin culture; and B. garinii/B. burgdorferi s.s. versus B. afzelii isolated from the ACA skin lesion (Table S1). For the analyses, univariate and multivariable logistic regression models were employed. The R software was used. The missing values for the duration of ACA and skin histology indicative of ACA were imputed using the observed means; for the other covariates included in the analyses, the information was complete. Sensitivity analysis comparing the results obtained with imputation of means and the results obtained with multiply imputed values for covariates with missing values was performed using mice R package. Since the outcomes obtained with the two imputation methods were very consistent (data not shown), only the results using imputation of the observed means are shown.
Results
A total of 693 patients diagnosed with ACA during the 28‐year period qualified for the study: 461 females (66.5%) and 232 males, with median age of 64 (IQR: 55−71) years. The lower number of patients in the early 1990s was mainly due to incomplete medical documentation (Fig. S1). Basic demographic, epidemiological and clinical data were assessed for the periods 1991–2004 and 2005–2018 (Table 1).
Table 1.
Demographic, epidemiological and clinical characteristics of 693 patients with acrodermatitis chronica atrophicans
|
1991–2004 N = 295 |
2005–2018 N = 398 |
P |
1991–2018 N = 693 |
|
|---|---|---|---|---|
| Female sex | 187 (63.4; 57.6–68.9) | 274 (68.8; 64.0–73.4) | 0.155 | 461 (66.5; 62.9–70.0) |
| Age (years) | 62 (53–69) | 65 (57–74) | < 0.001 | 64 (55–71) |
| Female | 64 (57–70) | 67 (59–74) | 0.002 | 65 (58–72) |
| Male | 60 (48–67) | 61 (53–73) | 0.037 | 61 (50–69) |
| Annual number of tick bites | 1 (0–4) a | 1 (0–4) b | 0.761 | 1 (0–4) |
| Tick bite in 2‐year period before ACA | 191/254 (75.2; 69.4–80.4) | 231/336 (68.8; 63.5–73.7) | 0.104 | 422/590 (71.5; 67.7–75.1) |
| Time interval from tick bite to ACA (months) c | 5 (4–12) d | 6 (2–14) e | 0.883 | 6 (2–14) |
| Past EM | 57 (19.3; 15.0–24.3) | 90 (22.6; 18.6–27.0) | 0.340 | 147 f (21.2; 18.2–24.4) |
| Time from EM to ACA (months) | 19 (6–56) g | 96 (36–178) h | < 0.001 | 55 (18–150) |
| Matching location of ACA and preceding EM | 19/48 (39.6; 25.8–54.7) | 17/55 (30.9; 19.1–44.8) | 0.475 | 36/103 (35.0; 25.8–45.0) |
| Time from EM to ACA in patients with matching locations (months) | 9 (3–22) i | 54 (6–177) j | 0.024 | 19 (3–55) |
| Past LNB k | 1 (0.3; 0–1.9) | 6 (1.5; 0.6–3.3) | 0.126 | 7 l (1.0; 0.4–2.1) |
| Antibiotic therapy with anti‐borrelial activity in the 6 months prior to presentation | 23/290 (7.9; 5.1–11.7) | 33 (8.3; 5.8–11.4) | 0.976 | 56/688 (8.1; 6.2–10.4) |
| Duration of ACA (months) | 12 (6–24) | 8 (4–18) | < 0.001 | 12 (5–24) |
| Constitutional symptoms m | 237 (80.3; 75.3–84.7) | 209 (52.5; 47.5–57.5) | < 0.001 | 446 (64.4; 60.7–67.9) |
| Arthralgia | 180 (61.0; 55.2–66.6) | 118 (29.6; 25.2–34.4) | < 0.001 | 298 (43.0; 39.3–46.8) |
| Headache | 95 (32.2; 26.9–37.9) | 79 (19.8; 16.0–24.1) | < 0.001 | 174 (25.1; 21.9–28.5) |
| Myalgia | 97 (32.9; 27.5–38.6) | 48 (12.1; 9.0–15.7) | < 0.001 | 145 (20.9; 18.0–24.1) |
| Fatigue | 68 (23.1; 18.4–28.3) | 69 (17.3; 13.7–21.4) | 0.076 | 137 (19.8; 16.9–22.9) |
| Vertigo | 42 (14.2; 10.5–18.8) | 32 (8.0; 5.6–11.2) | 0.013 | 74 (10.7; 8.5–13.2) |
| Memory/concentration disorder | 21 (7.1; 4.5–10.7) | 15 (3.8; 2.1–6.1) | 0.073 | 36 (5.2; 3.7–7.1) |
| Duration of constitutional symptoms (months) | 10 (5–24) | 6 (3–12) | 0.007 | 9 (4–24) |
| Local symptoms | 56 (19.0; 14.7–23.9) | 104 (26.1; 21.9–30.7) | 0.034 | 160 (23.1; 20.0–26.4) |
| Pain | 27 (9.2; 6.1–13.0) | 37 (9.3; 6.6–12.6) | 0.946 | 64 (9.2; 7.2–11.6) |
| Burning | 15 (5.1; 2.9–8.2) | 42 (10.6; 7.7–14.0) | 0.014 | 57 (8.2; 6.3–10.5) |
| Paresthesia | 20 (6.8; 4.2–10.3) | 25 (6.3; 4.1–9.1) | 0.914 | 45 (6.5; 4.8–8.6) |
| Itching | 10 (3.4; 1.6–6.1) | 26 (6.5; 4.3–9.4) | 0.095 | 36 (5.2; 3.7–7.1) |
| Hypoesthesia | 2 (0.7; 0.1–2.4) | 5 (1.3; 0.4–2.9) | 0.364 | 7 (1.0; 0.4–2.1) |
| Localization of ACA n | ||||
| Lower extremity | 203 (68.8; 63.2–74.1) | 282 (70.9; 66.1–75.3) | 0.620 | 485 (70.0; 66.4–73.4) |
| Foot | 88 (43.3; 36.4–50.5) | 157 (55.7; 49.7–61.6) | 0.010 | 245 (50.5; 46.0–55.1) |
| Ankle | 84 (41.4; 34.5–48.5) | 167 (59.2; 53.2–65.0) | < 0.001 | 251 (51.7; 47.2–56.3) |
| Shin | 149 (73.4; 66.8–79.3) | 177 (62.8; 56.8–68.4) | 0.018 | 326 (67.2; 62.8–71.4) |
| Knee | 51 (25.1; 19.3–31.7) | 75 (26.6; 21.5–32.2) | 0.795 | 126 (26.0; 22.1–30.1) |
| Thigh | 49 (24.1; 18.4–30.6) | 100 (35.5; 29.9–41.4) | 0.010 | 149 (30.7; 26.6–35.0) |
| Buttocks | 3 (1.5; 0.3–4.3) | 8 (2.8; 1.2–5.5) | 0.251 | 11 (2.3; 1.1–4.0) |
| Lower extremity – bilateral involvement | 111 (54.7; 47.6–61.7) | 75 (26.6; 21.5–32.2) | < 0.001 | 186 (38.4; 34.0–42.8) |
| Upper extremity | 157 (53.2; 47.3–59.0) | 156 (39.2; 34.4–44.2) | < 0.001 | 313 (45.2; 41.4–49.0) |
| Hand | 146 (93.0; 87.8–96.5) | 150 (96.2; 91.8–98.6) | 0.325 | 296 (94.6; 91.4–96.8) |
| Forearm | 32 (20.4; 14.4–27.5) | 49 (31.4; 24.2–39.3) | 0.036 | 81 (25.9; 21.1–31.1) |
| Elbow | 26 (16.6; 11.1–23.3) | 27 (17.3; 11.7–24.2) | 0.980 | 53 (16.9; 12.9–21.6) |
| Upper arm | 15 (9.6; 5.4–15.3) | 19 (12.2; 7.5–18.4) | 0.572 | 34 (10.9; 7.6–14.8) |
| Upper extremity – bilateral involvement | 102 (65.0; 57.0–72.4) | 58 (37.2; 29.6–45.3) | < 0.001 | 160 (51.1; 45.4–56.8) |
| Trunk | 9 (3.1; 1.4–5.7) | 10 (2.5; 1.2–4.6) | 0.846 | 19 (2.7; 1.7–4.2) |
| Face | 1 (0.3; 0–1.9) | 1 (0.3; 0–1.4) | 0.615 | 2 (0.3; 0–1.0) |
| Description of skin lesion n | ||||
| Redness | 199 (67.5; 61.8–72.8) | 290 (72.9; 68.2–77.2) | 0.144 | 489 (70.6; 67.0–73.9) |
| Bluish discoloration | 175 (59.3; 53.5–65.0) | 207 (52.0; 47.0–57.0) | 0.066 | 382 (55.1; 51.3–58.9) |
| No colour change | 26 (8.8; 5.8–12.6) | 7 (1.8; 0.7–3.6) | < 0.001 | 33 (4.8; 3.3–6.6) |
| Swelling | 56 (19.0; 14.7–23.9) | 139 (34.9; 30.2–39.8) | < 0.001 | 195 (28.1; 24.8–31.6) |
| Shining | 47 (15.9; 11.9–20.6) | 44 (11.1; 8.1–14.6) | 0.077 | 91 (13.1; 10.7–15.9) |
| Thin / atrophic | 176 (59.7; 53.8–65.3) | 144 (36.2; 31.5–41.1) | < 0.001 | 320 (46.4; 42.4–50.0) |
| Wrinkled | 95 (32.2; 26.9 –37.9) | 47 (11.8; 8.8–15.4) | < 0.001 | 142 (20.5; 17.5–23.7) |
| Venous prominence | 43 (14.6; 10.8–19.1) | 53 (13.3; 10.1–17.1) | 0.716 | 96 (13.8; 11.4–16.7) |
| At least 1 clinical sign of skin atrophy o | 223 (75.6; 70.3–80.4) | 183 (46.0; 41.0–51.0) | < 0.001 | 406 (58.6; 54.8–62.3) |
| Nodule | 5 (1.7; 0.6–3.9) | 10 (2.5; 1.2–4.6) | 0.640 | 15 (2.2; 1.2–3.5) |
| Peeling | 7 (2.4; 1.0–4.8) | 11 (2.8; 1.4–4.9) | 0.937 | 18 (2.6; 1.5–4.1) |
| Arthritis n | 7 (2.4; 1.0–5.3) | 11 (2.8; 0.8–4.2) | 0.937 | 18 (2.6; 1.3–3.8) |
| Joint deformity n | 8 (2.7; 1.2–5.3) | 3 (0.8; 0.2–2.2) | 0.042 | 11 (1.6; 0.8–2.8) |
| Muscle atrophy n | 3 (1.0; 0.2–2.9) | 0 (0–0.9) | 0.077 | 3 (0.4; 0.1–1.3) |
| Muscle paresis n | 1 (0.3; 0–1.9) | 0 (0–0.9) | 0.426 | 1 (0.1; 0–0.8) |
Data are medians (interquartile range) or frequencies (percentage; 95% confidence interval). P values were obtained with the Mann–Whitney test for numerical variables and with Yates’s corrected chi‐squared test or two‐tailed Fisher’s exact test for categorical variables.
ACA, acrodermatitis chronica atrophicans; EM, erythema migrans; LNB, Lyme neuroborreliosis.
Data available for 93 patients.
Data available for 260 patients.
Patients who attributed ACA to specific tick bite.
Data available for 4 patients.
Data available for 33 patients.
83 patients treated according to recommendations (azithromycin in 27 patients, doxycycline in 9, ceftriaxone in 6, cefuroxime in 3, amoxicillin in 2, penicillin in 1 and unknown antibiotic in 35); 61 patients not treated, data on treatment not available for 3 patients.
Data available for 38 patients.
Data available for 64 patients.
Data available for 18 patients.
Data available for 15 patients.
4 months to 20 years prior to ACA.
All patients treated according to recommendations.
Mostly of low intensity.
Findings at presentation.
Thin/atrophic and/or wrinkled and/or shining skin and/or venous prominence.
Information on tick bites was available for 590/693 patients, most of whom (422/590; 71.5%) recalled a tick bite within a 2‐year period before the onset of ACA, but only 37/590 (6.3%) attributed the skin lesion to a particular tick bite, with a median latency period of 6 months.
Amongst the 693 patients, 147 (21.2%) reported having had EM (Fig. 1) and 7 (1.0%) having had Lyme neuroborreliosis before the ACA. In 36/103 (35.0%) patients with available information on the location of EM, the location matched the later ACA; in these patients, the time interval from EM to onset of ACA was shorter than in patients with nonmatching locations (19 vs. 63 months; P = 0.005).
Fig. 1.
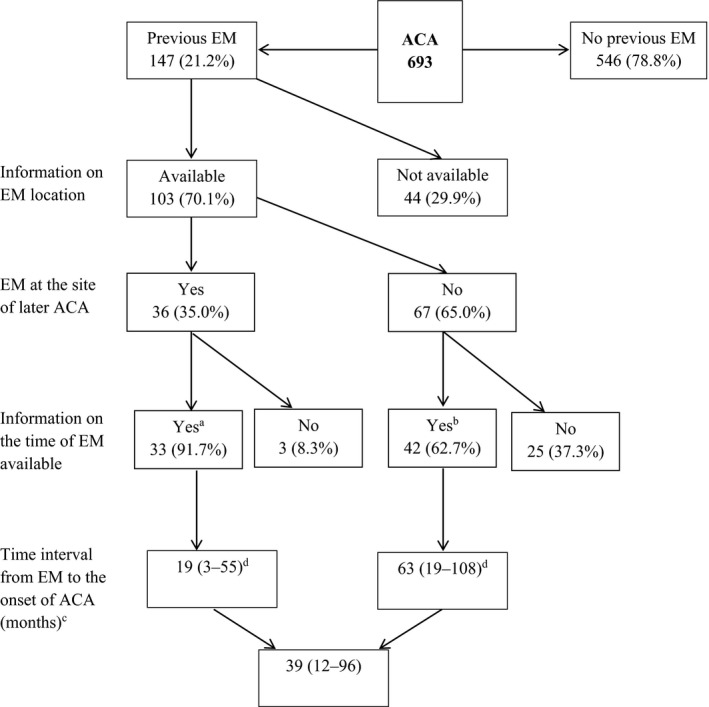
Erythema migrans in patients with acrodermatitis chronica atrophicans. Abbreviations: EM, erythema migrans; ACA, acrodermatitis chronica atrophicans. a11/33 (33.3%) patients treated with antibiotic for EM. b27/42 (64.3%) patients treated with antibiotic for EM. cData are medians (interquartile range). dSignificant difference in the time interval from EM to onset of ACA between the two groups (P = 0.005).
The median duration of ACA before diagnosis was 12 months (Fig. 2). In all but 2 patients, ACA was located on the limbs: on 1, 2, 3 and all 4 extremities in 55%, 31.3%, 5.6% and 7.8% of patients, respectively. The lower extremities were involved in 70%, and the upper extremities were involved in 45%. Bilateral involvement occurred in 42.1%, more often on the upper extremities than the lower (51.1% vs. 38.4%; P < 0.001).
Fig. 2.
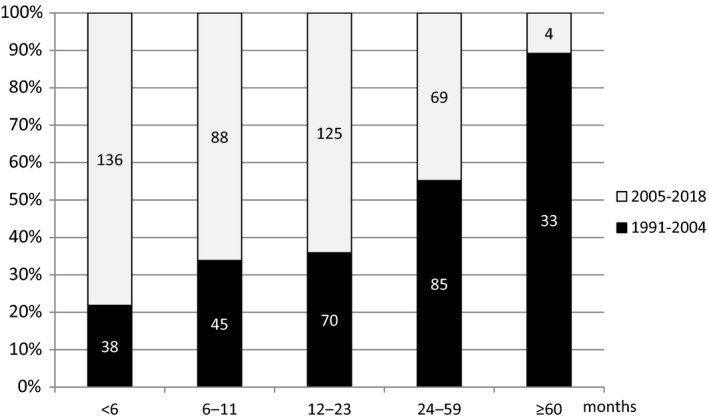
Duration of ACA prior to enrolment in the study. Numbers in the columns represent number of patients according to duration of ACA prior to diagnosis in the two time periods.
The most frequent skin signs (in descending order) were as follows: redness, bluish discoloration, thinning, swelling and wrinkling. In 4.8% of patients, only swelling or atrophic changes were noted, but no colour change. Rare findings included nodules (2.2%, most often located on the extensor side of the elbow), arthritis (2.6%), joint deformity (0.4%), muscle atrophy (0.4%) and paresis (0.1%).
Constitutional symptoms were reported by 64.4% of patients and local symptoms by 23.1%; in the majority, the symptoms were mild. At least 1 symptom (pain, burning, paresthesia, hypesthesia) and/or sign (muscle atrophy, paresis) suggesting ACA‐associated peripheral neuropathy was present in 144 (20.8%) patients; these symptoms and signs were located exclusively at the site of ACA skin lesions.
Borrelial IgG antibody levels were usually very high. Specific IgM antibodies were also present in 32.4% of patients (Table 2). Univariate and multivariable models found no significant associations between predefined covariates and the presence of borrelial serum IgM antibodies (Table 3). Histological findings in skin lesions were available for 567/693 (81.8%) patients and were indicative of ACA in 498/567 (87.8%) (Table 2).
Table 2.
Serology, Borrelia burgdorferi s.l. Culture Results and Histological Findings in 693 Patients with Acrodermatitis Chronica Atrophicans
|
1991–2004 N = 295 |
2005–2018 N = 398 |
P |
1991–2018 N = 693 |
|
|---|---|---|---|---|
| Borrelial serology (IFA or LIAISON®) a | ||||
| Positive serum IgM | 80/294 (27.2; 22.2–32.7) | 144/398 (36.2; 31.5–41.1) | 0.016 | 224/692 (32.4; 28.9–36.0) |
| Positive serum IgG | 295/295 (100; 98.8–100) | 398/398 (100; 99.1–100) | ‐ | 693 b /693 (100; 99.5–100) |
| Borrelia culture results | ||||
| Positive blood culture | 3/123 (2.4; 0.5–7.0) | 1/285 (0.3; 0–1.9) | 0.084 | 4 c /408 (1.0; 0.3–2.5) |
| Positive skin culture | 95/291 (32.6; 27.3–38.4) | 105/373 (28.2; 23.6–33.0) | 0.243 | 200 d /664 (30.1; 26.7–33.8) |
| Positive skin culture without antibiotics in previous 6 months | 91/261 (34.9; 29.1–41.0) | 105/338 (31.1; 26.2–36.3) | 0.371 | 196/599 (32.7; 29.0–36.6) |
| Histological findings indicative of ACA e | 168/205 (81.9; 76.0–87.0) | 330/362 (91.2; 87.7–93.9) | 0.002 | 498/567 (87.8; 84.9–90.4) |
Data are frequencies (percentage; 95% confidence interval). P values were obtained with Yates’s corrected chi‐squared test or two‐tailed Fisher’s exact test.
ACA, acrodermatitis chronica atrophicans; IFA, immunofluorescence assay.
Serological tests: IFA in 423 patients, LIAISON in 184 patients, both assays in 86.
IFA median titre 1:1024 (IQR: 1:512–1:1024), maximum 1:65.536; LIAISON® median IgG antibody level 1204 (IQR: 396–2400) U/mL, maximum value 25.276 U/mL.
3 of 4 blood isolates identified as B. garinii and 1 as B. afzelii.
Typing of 193/200 skin isolates: 179 isolates (92.8%) were B. afzelii, 8 (4.1%) B. garinii and 6 (3.1%) B. burgdorferi sensu stricto.
Atrophy of dermis and/or lymphocyte and plasma cell inflammatory infiltrates were regarded as indicative of ACA. Skin histology results were available for 567 patients but not for 126 patients (no biopsy in 21 patients, original results not obtainable for 78 and samples not representative in 27).
Table 3.
Covariates associated with the presence of serum Borrelial IgM antibodies, positive Borrelia skin culture result, and B. garinii or B. burgdorferi s.s. vs. B. afzelii Isolated from the Skin in Patients with Acrodermatitis Chronica Atrophicans
| Serum borrelial IgM antibodies | ||
|---|---|---|
| Covariate | Univariate analysis OR (95% CI), P | Multivariable analysis a OR (95% CI), P |
| Female sex | 1.00 (0.72–1.41), 0.987 | 1.01 (0.72–1.43), 0.949 |
| Age | 0.99 (0.98–1.01), 0.428 | 1.00 (0.98–1.01), 0.510 |
| Duration of ACA b | 1.00 (1.00–1.01), 0.106 | 1.00 (1.00–1.01), 0.092 |
| Previous EM | 0.98 (0.66–1.44), 0.908 | 0.95 (0.64–1.41), 0.785 |
| Antibiotic therapy in previous 6 months | 0.92 (0.53–1.60), 0.772 | 0.94 (0.54–1.65), 0.841 |
| Constitutional symptoms c | 1.19 (0.85–1.66), 0.313 | 1.20 (0.85–1.68), 0.294 |
| Local symptoms d | 1.13 (0.77–1.64), 0.537 | 1.13 (0.77–1.65), 0.523 |
| Local swelling | 1.13 (0.80–1.61), 0.484 | 1.17 (0.81–1.68), 0.401 |
| Positive Borrelia skin culture result (results available for 664 patients) | ||
|---|---|---|
| Covariate | Univariate analysis OR (95% CI), P |
Multivariable analysis e OR (95% CI), P |
| Female sex | 1.32 (0.92–1.89), 0.130 | 1.36 (0.94–1.99), 0.106 |
| Age | 0.99 (0.97–1.00), 0.059 | 0.99 (0.97–1.00), 0.102 |
| Duration of ACA b | 1.00 (0.99–1.01), 0.914 | 1.00 (0.99–1.01), 0.801 |
| Antibiotic therapy during previous 6 months | 0.13 (0.05–0.38), < 0.001 | 0.13 (0.05–0.37), < 0.001 |
| Local symptoms d | 1.38 (0.94–2.02), 0.097 | 1.28 (0.86–1.91), 0.222 |
| Local swelling | 1.46 (1.02–2.09), 0.038 | 1.33 (0.90–1.95), 0.148 |
| Signs of skin atrophy f | 1.12 (0.80–1.57), 0.511 | 1.36 (0.94–1.97), 0.099 |
| Upper extremity involvement | 0.68 (0.48–0.95), 0.025 | 0.75 (0.45–1.24), 0.259 |
| Lower extremity involvement | 1.49 (1.02–2.17), 0.039 | 1.13 (0.65–1.96), 0.659 |
| B. garinii or B. burgdorferi s.s. versus B. afzelii isolated from skin (B. afzelii isolated from 179 patients, B. garinii or B. burgdorferi s.s. from 14) | ||
|---|---|---|
| Covariate | Univariate analysis OR (95% CI), P |
Multivariable analysis g OR (95% CI), P |
| Female sex | 0.28 (0.09–0.86), 0.025 | 0.24 (0.07–0.85), 0.027 |
| Age | 0.97 (0.94–1.01), 0.164 | 0.97 (0.93–1.02), 0.279 |
| Duration of ACA b | 0.93 (0.86–1.01), 0.067 | 0.93 (0.86–1.01), 0.072 |
| Previous EM | 2.06 (0.65–6.51), 0.218 | 1.92 (0.47–7.79), 0.361 |
| Constitutional symptoms c | 1.21 (0.39–3.76), 0.740 | 0.95 (0.26–3.51), 0.935 |
| Local symptoms d | 0.70 (0.19–2.63), 0.601 | 0.45 (0.01–21.97), 0.690 |
| Symptoms and/or signs of peripheral neuropathy h | 0.77 (0.20–2.87), 0.692 | 1.35 (0.03–65.13), 0.880 |
| Local swelling | 3.76 (1.20–11.71), 0.023 | 3.80 (0.99–14.53), 0.051 |
| Signs of skin atrophy f | 0.88 (0.29–2.63), 0.814 | 1.24 (0.33–4.62), 0.751 |
| Signs of arthropathy i | 3.56 (0.68–18.67), 0.133 | 11.74 (1.48–93.07), 0.020 |
| Skin histology indicative of ACA b | 0.51 (0.10–2.50), 0.406 | 0.30 (0.05–1.91), 0.204 |
OR, odds ratio, CI, confidence interval, ACA, acrodermatitis chronica atrophicans, EM, erythema migrans.
Intercept: estimated coefficient 0.48 (95% CI: 0.20–1.15), P = 0.099.
For duration of ACA (no information available for 84/693 patients) and skin histology indicative of ACA (information not accessible for 126/693), the mean of missing values was imputed. The information was complete for the other covariates included in the analyses.
Arthralgia and/or headache and/or myalgia and/or fatigue and/or vertigo and/or memory/concentration disorder.
Pain and/or burning and/or itching and/or paresthesia and/or hypesthesia.
Intercept: estimated coefficient 0.60 (95% CI: 0.21–1.69), P = 0.335.
Thin/atrophic and/or wrinkled and/or shining skin and/or venous prominence.
Intercept: estimated coefficient 1.85 (95% CI: 0.05–67.24), P = 0.736.
Pain and/or burning and/or paresthesia and/or hypesthesia and/or muscle paresis and/or muscle atrophy at the site of ACA skin lesion.
Arthritis and/or joint deformity.
Borreliae were successfully isolated from 200/664 (30.1%) skin samples and 4/408 (1.0%) blood samples: B. afzelii predominated (92.8%) in skin, whereas 3 of 4 blood isolates were B. garinii (Table 2). In univariate analyses, isolation of borreliae from skin was positively associated with the presence of oedema and location of ACA on the lower extremities, whilst the association was negative for antibiotic treatment within 6 months before skin biopsy and location of ACA on the upper extremities. The multivariable model showed significant association only for antibiotic treatment within 6 months before skin biopsy (odds ratio (OR) 0.13; 95% CI, 0.05–0.37; P < 0.001). In a skin culture‐positive subgroup, multivariable analysis found that isolation of B. garinii or B. burgdorferi s.s. was associated with patient sex (OR for male sex 4.17; 95% CI, 1.18–14.29, P = 0.027) and arthropathy (OR = 11.74; 95% CI, 1.48–93.07; P = 0.020). Further information is given in Table 3.
Comparison of demographic, epidemiological, clinical and laboratory characteristics in the 2 time periods (1991–2004 vs. 2005–2018) showed that patients treated in the more recent period were older, had shorter duration of ACA (Fig. 2), reported constitutional symptoms less often but local symptoms more often, presented more frequently with swelling and less frequently with skin atrophy and deformation of joints, and had bilateral ACA less frequently. Also in the later period, patients more frequently had borrelial IgM antibodies in serum and histological findings suggestive of ACA (Tables 1 and 2).
Discussion
Several articles on ACA have appeared in recent decades, but the reported series are relatively small and most relate to specific clinical or microbiological aspects of ACA. In North America, only sporadic cases imported from Europe have been described [10, 11, 12, 13].
Our study encompasses 693 patients ≥ 15 years old who presented with ACA at our LB Outpatient Clinic in a 28‐year period, 1991–2018. In that same period, EM was diagnosed in 17,654 patients, indicating > 25 cases of EM per 1 case of ACA, and corroborating the appraisal that amongst adult patients with LB, ACA represents < 4% of cases [14, 15]. It is of interest that each year we diagnose more cases of ACA than proven Lyme neuroborreliosis [16].
Amongst our ACA patients, the age and sex distribution, localization on dorsal distal parts of extremities and proportion of patients recalling EM before the onset of ACA were similar to those reported elsewhere [16, 17, 18, 19, 20]; however, several other findings differed (Table 4). For some distinctions in the present study (such as the lower proportion of patients with symptoms/signs of peripheral neuropathy or joint involvement), the shorter duration of ACA (12 months) in comparison with previous reports (≥2 years) might be a reliable explanation, but for the more frequent bilateral involvement and some other distinctions we do not have convincing explanations. For example, peripheral neuropathy and/or arthropathy are reported to occur primarily in the area of impaired skin but in some patients may also occur at other skin sites [21, 24, 28]; in the present study, these complications were found exclusively in the areas of affected skin. We also observed differences within our patient group: those diagnosed more recently (2005–2018) were older, had shorter duration of ACA that was less frequently bilateral, reported constitutional symptoms less often but local symptoms more often, and more frequently had swelling but less frequently had atrophy compared with patients treated earlier (1991–2004) (Table 1). Most of these differences probably relate to the earlier recognition of the disease in more recent years.
Table 4.
Comparison of our results with previous reports on ACA
| Our findings | Previous reports | References a | |
|---|---|---|---|
| Number of patients | 693 | 50 (15–111) b | |
| Sex and age |
Female 66% Median age 64 years |
~ |
Asbrink [18] Brehmer‐Andersson et al. [19] Hulshof et al. [20] Strle et al. [16] |
| Duration | 12 months | ≥ 24 months |
Asbrink et al. [17] Kindstrand et al. [21] Lenormand et al. [22] Picken et al. [23] |
| Location | Distal parts of extremities | ~ |
Asbrink et al. [17] Kindstrand et al. [21] Kristoferitsch et al. [24] |
| Bilateral involvement | 42% | 20% | Tazelaar et al. [25] |
| Previous EM | 21% | 18–55% c |
Asbrink et al. [17] Lenormand et al. [22] Moniuszko‐Malinowska et al. [26] Picken et al. [23] |
| ACA‒EM location matching | 35% | 18–24% c |
Asbrink [27] Asbrink et al. [17] Picken et al. [23] |
| Symptoms of peripheral neuropathy | 20% | 33–64% c |
Hopf [28] Kindstrand et al. [21] Kristoferitsch et al. [24] Tazelaar et al. [25] |
| Signs of peripheral neuropathy (muscle atrophy or paresis) | 0.4% | 9–11% c |
Hopf [28] Kindstrand et al. [21] |
| Signs of arthropathy | 4% | 26% | Hovmark et al. [29] |
| Positive skin culture |
30% 33% (without previous antibiotic) |
22–40% c |
Asbrink et al. [30] Lenormand et al. [22] Picken et al. [31] Picken et al. [23] |
| Borrelia species isolated from skin | B. afzelii>>>B. garinii, B. burgdorferi s.s. | B. afzelii predominated |
Picken et al. [23] Rijpkema et al. [32] Ružić‐Sabljić et al. [33] |
| Positive blood culture | 1% | 3 blood isolates (2 B. afzelii, B. garinii) | Maraspin et al. [34] |
~similar results.
Most studies reported specific clinical or microbiological aspects of ACA.
Median (interquartile range, IQR).
Range.
The pathogenesis of ACA is not well elucidated. It is postulated that ACA does not heal spontaneously, in contrast to the majority of other manifestations of LB [35, 36]. ACA is frequently the only manifestation of LB. Its incubation period is uncertain. In tick‐transmitted infection, incubation signifies the time from a tick bite to the onset of disease. In the current study, >70% of patients reported tick bites in the 2 years before onset of ACA, but only 6% of patients associated a specific bite with subsequent ACA developing 6 (2–14) months after the bite. However, tick bites are numerous, not all bites are noticed and/or remembered, and only some ticks are infected. Moreover, just a small proportion of ticks harbouring borreliae successfully transmit the causative agent to humans, resulting in clinical illness. It is very difficult, therefore, to establish a bite causally associated with ACA. Data on EM, which develops within a month after a bite, enable more reliable assessment. Nevertheless, the assumption that prior EM is related to ACA may not be (always) valid. The chances are probably higher if ACA occurs at the site of previous EM, especially if the EM was not adequately treated. Amongst our patients, 147/693 (21%) reported having EM prior to ACA. In 36/103 (35%) patients with information on EM location, the locations of ACA and EM matched; in these patients, the time interval from EM to the onset of ACA was shorter than in patients with nonmatching locations (median 19 vs. 63 months; P = 0.005), implying nonuniform association between EM and subsequent ACA. These findings suggest that the incubation time for ACA is long, ranging from a few months to a few years.
Our study shows that, as for EM, ACA is more common on the lower extremity than on the upper extremity. The fact that EM develops at the site of a tick bite, and that tick bites in adults are more common on the lower than on the upper part of the body, implies localized illness at the site of tick bite not only for EM but also for ACA.
It is not known why patients with ACA are mostly older, why women are more commonly affected than men, and why the distal parts of the limbs are mainly involved. However, female predominance is not a complete surprise; as in several European countries, including Slovenia, LB is more common in women than in men. Closer insight shows that female predominance is valid only for cutaneous manifestations of LB (EM and ACA, which are by far the most common clinical signs and account for ≥ 90% of all LB cases in Slovenia), but not for Lyme neuroborreliosis and Lyme arthritis, which are more common in males [16]. A recent hypothesis suggests that ACA occurs in older individuals because they are likely to have age‐related anatomic/physiological skin changes in the distal extremities that may predispose to the development of ACA in those particular body parts [16]. However, if this were the case, why do later similar lesions also appear on the distal part of some other limb and why does this happen contralaterally more often than ipsilaterally, resulting in bilateral, more or less symmetrical lesions that are more often present on hands than on feet? A possible theoretical explanation is that the initial localized infection disseminates early on, before the humoral immune response. Consequently, borreliae are present in other parts of the body as early as during the initial infection, but manifest as clinically visible primary ACA after a longer delay because the skin in these areas is less damaged than at the primary location. Alternative hypotheses are that borreliae spread slowly and continuously from the site of primary infection through the skin or along nerves to other parts of the body, or that despite a pronounced humoral immune response intermittent haematogenous dissemination of borreliae occurs in the course of ACA; in both cases, the infection becomes clinically evident on locations with the most ‘favourable’ conditions for ACA. A further possibility would be reinfection, that is new localized infection at the ‘vulnerable’ site with a long delay until clinical manifestation. However, all these hypotheses are only partial explanations and have several weaknesses.
Several other simple questions remain unanswered, such as why do ACA lesions progress from the distal part of the extremity to more proximal sites, why is spreading so slow, and why is bilateral involvement more often on hands than on feet?
Diagnosis of ACA is based on the suggestive clinical presentation, demonstration of borrelial serum IgG antibodies and histological findings compatible with ACA. Although the histopathological pattern of ACA is not diagnostic per se, it is sufficiently characteristic to alert the experienced pathologist [15, 37]; in our patient group, histological findings were indicative of ACA in nearly 90%, whilst in the remainder it was suggestive (Table 2). We found at least one clinical sign of skin atrophy in 59% of our patient group overall. As expected, atrophy was associated with duration of the lesions: signs of atrophy were present in 51% of patients with ACA duration up to a year and in as many as 70% of patients with longer duration (P < 0.001). However, we also found signs of skin atrophy in some patients with relatively short ACA duration, suggesting that in such patients the process of atrophy may begin within the first few months after onset of ACA. Nevertheless, as the onset is gradual, appreciation of the presence of the lesion may be delayed and assessment of its duration underestimated.
All our patients had borrelial IgG antibodies in serum, which was 1 of the inclusion criteria, and in concordance with previous reports [21, 38, 39, 40], the levels of IgG antibodies were very high. Specific IgM antibodies were also present in 32% of our patients. The higher proportion of IgM seropositivity in the more recent time period could relate to the use of different serological tests in the last 9 years of the study.
Borreliae were cultured from the skin of 30% of patients and from blood in 1% (Table 2). The isolation rate from skin was comparable with older reports (22%–40%) [22, 23, 30, 31]. Skin culture was more likely to be positive in patients not treated with antibiotics in the previous 6 months. Amongst the skin isolates, B. afzelii predominated even more strongly (93%) than reported previously [23, 32, 33]. The absence of autochthonous ACA in North America, where B. burgdorferi s.s. is almost the exclusive agent of LB, and the isolation of B. burgdorferi s.s. from the skin of some European patients with ACA, appears contradictory. However, even though North American and European B. burgdorferi s.s. are genetically alike, they vary in inflammatory potential and clinical presentation of the disease [41]. Interestingly, in the present study B. garinii and B. burgdorferi s.s. were more frequently isolated from male patients and from patients with signs of arthropathy. Concerning blood isolates, 3 out of 4 were B. garinii, which is hard to explain, but the total number of blood isolates was small. Until now, only 3 blood isolates (2 B. afzelii, 1 B. garinii) have been reported from patients with ACA [34].
Our study is descriptive, but we hope our insights will encourage analysis of the clinically relevant mechanisms behind the findings. Our results are applicable to European regions with similar ratios of borrelial genospecies causing LB in humans but may not entirely apply to regions where the proportion of non‐B. afzelii borreliae causing skin manifestations of LB (EM) is higher [23, 32].
Conclusions
ACA is a late manifestation of European LB that usually affects older women. B. afzelii is by far the most common, but not exclusive, causative agent. Clinical presentation depends on the duration of the ACA skin lesions and probably also on the Borrelia species causing the disease.
Funding
This research was funded by the Slovenian Research Agency, grant number P3‐0296 (Javna agencija za raziskovalno dejavnost Republike Slovenije; ARRS; www.arrs.si). The funding source had no role in study design, data collection and analysis, interpretation of data, decision to publish or preparation of the manuscript.
Conflicts of interest
F.S. served on the scientific advisory board for Roche on Lyme disease serological diagnostics, received research support from the Slovenian Research Agency (grant numbers P3‐0296, J3‐1744 and J3‐8195) and is an unpaid member of the steering committee of the ESCMID Study Group on Lyme Borreliosis/ ESGBOR. All other authors (K.O., V.M., L.L., T.C.K. and E.R.S.) have declared no conflicts of interest.
Supporting information
Figure S1. Number of patients.
Table S1. Covariates used for testing associations with different outcomes.
Ogrinc K, Maraspin V, Lusa L, Cerar Kišek T, Ružić‐Sabljić E, Strle F (University Medical Centre Ljubljana, Ljubljana; University of Primorska, Koper; and University of Ljubljana, Ljubljana, Slovenia). Acrodermatitis chronica atrophicans: clinical and microbiological characteristics of a cohort of 693 Slovenian patients. J Intern Med 2021; 290: 335–348. 10.1111/joim.13266
References
- 1. Buchwald A. Ein Fall von diffuser idiopathischer Haut Atrophie. Vierteljahresschrift Dermatol Syph.2021;10:553–6. 10.1007/BF01833474. [DOI] [Google Scholar]
- 2. Herxheimer K, Hartmann K. Über Acrodermatitis chronica atrophicans. Arch Dermatol Syph. 1902;61:255–300. [Google Scholar]
- 3. Burgdorfer W, Barbour AG, Hayes SF, Péter O, Aeschlimann A. Erythema chronicum migrans – a tickborne spirochetosis. Acta Trop. 1983;40:79–83.PMID: 6134457. [PubMed] [Google Scholar]
- 4. Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–40. 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 5. Asbrink E, Hovmark A, Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Derm Venereol. 1984;64:506–12.PMID: 6084922. [PubMed] [Google Scholar]
- 6. Ogrinc K, Wormser GP, Visintainer P, Maraspin V, Lotrič‐Furlan S, Cimperman J, et al. Pathogenetic implications of the age at time of diagnosis and skin location for acrodermatitis chronica atrophicans. Ticks Tick Borne Dis. 2017;8:266–9. 10.1016/j.ttbdis.2016.11.011. Epub 2016 Nov 24. [DOI] [PubMed] [Google Scholar]
- 7. Ružić‐Sabljić E, Maraspin V, Cimperman J, Lotrič‐Furlan S, Strle F. Evaluation of immunofluorescence test (IFT) and immuno (western) blot (WB) test in patients with erythema migrans. Wien Klin Wochenschr. 2002;114:586–90.PMID: 12422606. [PubMed] [Google Scholar]
- 8. Ružić‐Sabljić E, Zore A, Strle F. Characterization of Borrelia burgdorferi sensu lato isolates by pulsed‐field gel electrophoresis after MLuI restriction of genomic DNA. Res Microbiol. 2008;159:441–8. 10.1016/j.resmic.2008.05.005. Epub 2008 Jun 6. [DOI] [PubMed] [Google Scholar]
- 9. Postic D, Assous MV, Grimont PA, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)–rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–52. 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 10. Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–73. 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 11. DiCaudo DJ, Su WP, Marshall WF, Malawista SE, Barthold S, Persing DH. Acrodermatitis chronica atrophicans in the United States: clinical and histopathologic features of six cases. Cutis. 1994;54:81–4.PMID: 7956339. [PubMed] [Google Scholar]
- 12. Lavoie PE, Wilson AJ, Tuffanelli DL. Acrodermatitis chronica atrophicans with antecedent Lyme disease in a California. Case report. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;263:262–5. 10.1016/s0176-6724(86)80129-8. [DOI] [PubMed] [Google Scholar]
- 13. Correa‐Selm LM, Bronsnick T, Rao BK, Kirkorian AY, Marcus A, Cha J. A souvenir from France: Acrodermatitis chronica atrophicans presenting in the United States. Skinmed 2016; 14: 217–9.eCollection 2016. [PubMed] [Google Scholar]
- 14. Berglund J, Eitrem R, Ornstein K, Lindberg A, Ringnér Å, Elmrud H, et al. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995;333:1319–24. 10.1056/NEJM199511163332004. [DOI] [PubMed] [Google Scholar]
- 15. Strle F, Stanek G. Clinical manifestations and diagnosis of Lyme borreliosis. Curr Probl Dermatol. 2009;37:51–110. 10.1159/000213070. Epub 2009 Apr 8. [DOI] [PubMed] [Google Scholar]
- 16. Strle F, Wormser GP, Mead P, Dhaduvai K, Longo MV, Adenikinju O, et al. Gender disparity between cutaneous and non‐cutaneous manifestations of lyme borreliosis. PLoS One. 2013;8(5):e64110. 10.1371/journal.pone.0064110. Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asbrink E, Hovmark A, Olsson I. Clinical manifestations of acrodermatitis chronica atrophicans in 50 Swedish patients. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;263:253–61. 10.1016/s0176-6724(86)80128-6. [DOI] [PubMed] [Google Scholar]
- 18. Asbrink E. Acrodermatitis chronica atrophicans. Clin Dermatol. 1993;11:369–75. 10.1016/0738-081x(93)90092-q. [DOI] [PubMed] [Google Scholar]
- 19. Brehmer‐Andersson E, Hovmark A, Asbrink E. Acrodermatitis chronica atrophicans: histopathologic findings and clinical correlations in 111 cases. Acta Derm Venereol. 1998;78:207–13. 10.1080/000155598441558. [DOI] [PubMed] [Google Scholar]
- 20. Hulshof MM, Vandenbroucke JP, Nohlmans LMKE, Spanjaard L, Bavinck JN, Dijkmans BA. Long‐term prognosis in patients treated for erythema chronicum migrans and acrodermatitis chronica atrophicans. Arch Dermatol. 1997;133:33–7.PMID: 9006370. [PubMed] [Google Scholar]
- 21. Kindstrand E, Nilsson BY, Hovmark A, Pirskanen R, Asbrink E. Peripheral neuropathy in acrodermatitis chronica atrophicans ‐ a late Borrelia manifestation. Acta Neurol Scand. 1997;95:338–45. 10.1111/j.1600-0404.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 22. Lenormand C, Jaulhac B, Debarbieux S, Dupin N, Granel‐Brocard F, Adamski H, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans [ACA]): A prospective study of 20 culture‐ and/or polymerase chain reaction (PCR)‐documented cases. J AM Acad Dermatol. 2016;74:685–92. 10.1016/j.jaad.2015.10.046. Epub 2016 Jan 9. [DOI] [PubMed] [Google Scholar]
- 23. Picken RN, Strle F, Picken MM, Ruzic‐Sabljic E, Maraspin V, Lotric‐Furlan S, et al. Identification of three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B. garinii, and B. afzelii) among isolates from acrodermatitis chronica atrophicans lesions. J Invest Dermatol. 1998;110:211–4. 10.1046/j.1523-1747.1998.00130.x. [DOI] [PubMed] [Google Scholar]
- 24. Kristoferitsch W, Sluga E, Graf M, Partsch H, Neumann R, Stanek G, et al. Neuropathy associated with acrodermatitis chronica atrophicans. Clinical and morphological features. Ann N Y Acad Sci. 1988;539:35–45. 10.1111/j.1749-6632.1988.tb31836.x. [DOI] [PubMed] [Google Scholar]
- 25. Tazelaar DJ, Velders AJ, de Koning J, Hoogkamp‐Korstanje JA. Chronic atrophic acrodermatitis; a deceptive form of Lyme borreliosis. Ned Tijdschr Geneeskd. 1991;135:1358–63.PMID: 1865945. [PubMed] [Google Scholar]
- 26. Moniuszko‐Malinowska A, Czupryna P, Dunaj J, Pancewicz S, Garkowski A, Kondrusik M, et al. Acrodermatitis chronica atrophicans: various faces of the late form of Lyme borreliosis. Adv Dermatol Allergol. 2018;35:490–4. 10.5114/ada.2018.77240. Epub 2018 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asbrink E. Erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans: early and late manifestations of Ixodes ricinus‐borne Borrelia spirochetes. Acta Derm Venereol Suppl (Stockh). 1985;118:1–63.PMID: 3901647. [PubMed] [Google Scholar]
- 28. Hopf HC. Peripheral neuropathy in acrodermatitis chronica atrophicans (Herxheimer). J Neurol Neurosurg Psychiatry. 1975;38:452–8. 10.1136/jnnp.38.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hovmark A, Asbrink E, Olsson I. Joint and bone involvement in Swedish patients with Ixodes ricinus‐borne Borrelia infection. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;263:275–84. 10.1016/s0176-6724(86)80132-8. [DOI] [PubMed] [Google Scholar]
- 30. Asbrink E, Hovmark A. Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathol Microbiol Immunol Scand B. 1985;93:161–3. 10.1111/j.1699-0463.1985.tb02870.x. [DOI] [PubMed] [Google Scholar]
- 31. Picken MM, Picken RN, Han D, Cheng Y, Ruzic‐Sabljic E, Cimperman J, et al. A two year prospective study to compare culture and polymerase chain reaction amplification for the detection and diagnosis of Lyme borreliosis. Mol Pathol. 1997;50:186–93. 10.1136/mp.50.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rijpkema SGT, Tazelaar DJ, Molkenboer MJCH, Noordhoek GT, Plantinga G, Schouls LM, et al. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin Microbiol Infect. 1997;3:109–16. 10.1111/j.1469-0691.1997.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 33. Ružić‐Sabljić E, Maraspin V, Lotrič‐Furlan S, Jurca T, Logar M, Pikelj‐Pecnik A, et al. Characterization of Borrelia burgdorferi sensu lato strains isolated from human material in Slovenia. Wien Klin Wochenschr. 2002;114:544–50.PMID: 12422599. [PubMed] [Google Scholar]
- 34. Maraspin V, Ogrinc K, Ružić‐Sabljić E, Lotrič‐Furlan S, Strle F. Isolation of Borrelia burgdorferi sensu lato from blood of adult patients with borrelial lymphocytoma, Lyme neuroborreliosis, Lyme arthritis and acrodermatitis chronica atrophicans. Infection. 2011;39:35–40. 10.1007/s15010-010-0062-8. Epub 2010 Dec 10. [DOI] [PubMed] [Google Scholar]
- 35. Asbrink E, Hovmark A. Early and late cutaneous manifestations in Ixodes‐borne borreliosis (erythema migrans borreliosis, Lyme borreliosis). Ann N Y Acad Sci. 1988;539:4–15. 10.1111/j.1749-6632.1988.tb31833.x. [DOI] [PubMed] [Google Scholar]
- 36. Steere AC. Lyme disease. N Engl J Med. 1989;321:586–96. 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 37. Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, Kaiser R, Krause A, et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 2011;17:69–79. 10.1111/j.1469-0691.2010.03175.x. [DOI] [PubMed] [Google Scholar]
- 38. Asbrink E, Brehmer‐Andersson E, Hovmark A. Acrodermatitis chronica atrophicans – a spirochetosis: clinical and histopathological picture based on 32 patients; course and relationship to erythema chronicum migrans Afzelius. Am J Dermatopathol. 1986;8:209–19. 10.1097/00000372-198606000-00005. [DOI] [PubMed] [Google Scholar]
- 39. Moter SE, Hofmann H, Wallich R, Simon MM, Kramer MD. Detection of Borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by ospA‐specific PCR. J Clin Microbiol. 1994;32:2980–8. 10.1128/JCM.32.12.2980-2988.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flisiak I, Schwartz RA, Chodynicka B. Clinical features and specific immunological response to Borrelia afzelii in patients with acrodermatitis chronica atrophicans. J Med. 1999;30:267–78.PMID: 17312680. [PubMed] [Google Scholar]
- 41. Cerar T, Strle F, Stupica D, Ruzic‐Sabljic E, McHugh G, Steere AC, et al. Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto strains from Europe and the United States. Emerg Infect Dis. 2016;22:818–27. 10.3201/eid2205.151806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Number of patients.
Table S1. Covariates used for testing associations with different outcomes.


