Abstract
Background
Methylphenidate may improve executive functioning in children with attention‐deficit/hyperactivity disorder (ADHD). However, it is unclear if there are still acute effects of methylphenidate on executive functioning after long‐term use.
Methods
In a randomized double‐blind, placebo‐controlled discontinuation study, 94 children and adolescents (ages 8–18 years) who used methylphenidate beyond two years were either assigned to seven weeks of continued treatment with 36 or 54 mg of extended‐release methylphenidate or to gradual withdrawal over three weeks to placebo for four weeks. Performance on neuropsychological tasks, measuring working memory, response inhibition, attentional flexibility and psychomotor speed was compared between both groups using mixed models for repeated measures. Additionally, we investigated within the discontinuation group if a deterioration on the investigator‐rated Clinical Global Impressions Improvement scale after withdrawing to placebo was related to a worse performance on the neuropsychological tasks. This study was registered in the Netherlands Trial Register (www. Trialregister.nl) with identifier 5252.
Results
After withdrawal of methylphenidate, the discontinuation group made more errors on working memory (β = −1.62, SD = 0.56, t = −2.88, p = .01, Cohen’s f2 = .14), independent from reaction time compared to baseline, in contrast to the continuation group. We did not find differences in changes in response inhibition, attentional flexibility and psychomotor speed between the two groups. Also, there were no significant differences in task measures between the participants who deteriorated clinically and those who did not.
Conclusions
Our study shows that methylphenidate has a beneficial effect on working memory after two years of use. Future studies should explore whether cognitive outcomes may aid clinical decision‐making on the continued use of methylphenidate, given dissociation between cognitive and behavioural effects of stimulant medication.
Keywords: attention‐deficit/hyperactivity disorder, executive functioning, long‐term, methylphenidate, working memory
Introduction
Methylphenidate is widely prescribed for children with attention‐deficit/hyperactivity disorder (ADHD), often for many years (Beau‐Lejdstrom, Douglas, Evans, & Smeeth, 2016), despite doubts about its effectiveness after prolonged use. Observational studies have shown that benefits of methylphenidate on ADHD symptom severity may be maintained to a period of two years (Abikoff et al., 2004; Hechtman & Greenfield, 2003; Molina et al., 2009; Swanson et al., 2008, 2017). A recent randomized controlled discontinuation trial study from our group confirmed that on average, methylphenidate was still superior to placebo after treatment for two or more years, albeit with effect sizes that were smaller than those seen in short‐term trials. However, at an individual level 60% of children did not deteriorate after withdrawing to placebo, suggesting that in most children, methylphenidate might no longer be beneficial after two or more years (Matthijssen et al., 2019). Thus, there are indications that the magnitude of the effects of methylphenidate on ADHD symptom severity may, at least in a portion of individuals, diminish after prolonged methylphenidate use. Less is known about the effects of methylphenidate on executive functioning after prolonged use, even though ADHD has often been associated with several executive functioning impairments, such as response inhibition (i.e. the ability to withhold a prepotent response), working memory (i.e. the capacity to temporarily maintain and process information) or attentional flexibility (i.e. the ability to switch between task demands; Pievsky & McGrath, 2018). While it has been currently recognized that ADHD symptomatology and cognitive impairments do not always correlate, likely with clear neuropsychological impairments only in a small number of patients, the importance of monitoring cognitive functioning in parallel to ADHD symptoms has been increasingly stressed (Coghill, Hayward, Rhodes, Grimmer, & Matthews, 2014).
Acute (short‐term) effects of methylphenidate on executive functioning in children and adolescents with ADHD are well‐established; meta‐analyses and reviews conclude that most studies found improvements in working memory, response inhibition and reaction time (Coghill, Seth, et al., 2014; Pietrzak, Mollica, Maruff, & Snyder, 2006), and improvements have also been found in attentional flexibility (Bolfer et al., 2017). Functional magnetic resonance imaging studies have shown that acute administration of methylphenidate normalizes brain dysfunction in treatment‐naïve children with ADHD in the typically affected frontal regions, possibly explaining the effects of methylphenidate on executive functioning (Rubia, Halari, Cubillo, et al., 2011; Rubia, Halari, Mohammad, Taylor, & Brammer, 2011).
Opposed to the studies that focussed on the immediate effects of methylphenidate associated with short‐term use, the acute effects of methylphenidate on executive functioning after prolonged use have, to our knowledge, not yet been studied in humans. So far, a number of studies have investigated whether there would be lasting improvement on executive functioning associated with a history of methylphenidate use; however, little evidence for improvement of long‐term methylphenidate use on executive functions was found when comparing pre‐ and posttreatment functioning off methylphenidate (Huang, Wang, & Chen, 2012; Schweren et al., 2018). Obviously, these observational studies are limited by not assessing the acute effects of methylphenidate in a controlled situation. Thus, to date it remains unknown if ongoing methylphenidate use has an effect on executive functioning after prolonged use in children and adolescents.
In the present study, as part of a double‐blind, placebo‐controlled methylphenidate discontinuation trial in 94 children and adolescents aged 8–18 years treated in regular clinical practice (Matthijssen et al., 2019), we aimed to investigate the acute effects of methylphenidate after long‐term use (>2 years) on a test battery of neuropsychological tasks, measuring executive functioning (i.e. response inhibition, working memory and attentional flexibility), and psychomotor speed (Cepeda, Blackwell, & Munakata, 2013; Kibby, Vadnais, & Jagger‐Rickels, 2019), in comparison with children and adolescents who withdrew to placebo over a seven‐week period. Specifically, we wanted to know whether children and adolescents who gradually withdraw to placebo would deteriorate in their performance on the cognitive measures compared to those who continued to use methylphenidate. Additionally, to find out whether there would be a relation between changes in global clinical improvement and neuropsychological task measures, we investigated whether children who deteriorated clinically, based on ratings on the Clinical Global Impression Scale of Improvement (CGI‐I; Guy, 1976), after withdrawal from methylphenidate performed worse on the task measures compared to children who did not deteriorate.
Method
Participants
A total of 94 children aged 8–18 years participated in this study, taking part in the baseline and seven‐week follow‐up assessment of a randomized placebo‐controlled discontinuation study on the continued benefits of methylphenidate in ADHD after two or more years in clinical practice (Matthijssen et al., 2019). Inclusion criteria for all participants included an IQ over 70 and the use of methylphenidate as prescribed in clinical practice in any dose or form for two years or longer. Participants who originally used immediate‐release methylphenidate could be included if they had switched to methylphenidate extended release of either 36 or 54 mg per day during the last four weeks before the trial. All participants were allowed to use any kind of co‐medication or receive any kind of psychosocial interventions if already ongoing before the trial and if remained stable during the trial. Parents/legal representatives and children ≥12 years provided written informed consent; younger children gave oral assent. The study was approved by the regional ethics board (Medical Ethics Review Board University Medical Center Groningen). This study was registered in the Netherlands Trial Register (www. Trialregister.nl) with identifier 5252, and the reporting of this study is compliant with CONSORT guidelines.
Procedures and measures
Participants were randomly assigned in a 1:1 ratio to either continued use of methylphenidate (n = 47), or to the discontinuation group (n = 47), in which methylphenidate was gradually reduced to placebo over a three‐week period followed by four weeks of complete placebo. The reduction schedule was identical for all children in the discontinuation group, independent of the dose at the beginning of the study, 36 mg or 54 mg: week one: 36 mg, week two: 27 mg, week three: 18 mg and weeks four through seven: placebo. The study medication consisted of an over‐encapsulation (capsules manufactured by Capsugel) of methylphenidate Osmotic Release Oral System (Concerta®; 18, 27, 36, and 54 mg). For a more detailed description of the study design, we refer to Matthijssen et al., (2019). We used the clinician‐rated CGI‐I after seven weeks to rate improvement or worsening in global clinical functioning compared to baseline (i.e. before the start of the seven‐week discontinuation trial) on a seven‐point Likert scale. The outcomes were dichotomized, creating two groups: ‘deteriorated’ (6 = much worse and 7 = very much worse; combining the scores for clinically relevant worsening according to the scoring suggestions for clinical use by Busner & Targum, [2007]) and ‘not deteriorated’ (all else). To keep blinding optimal, side effects were reported by the parents via an online questionnaire which was not available to the clinician. CGI‐I ratings were based on all available information from parents including the ADHD Rating Scale, and Swanson, Nolan and Pelham Questionnaire Oppositional Defiant Disorder subscale.
Neuropsychological assessment
Prior to the start of the discontinuation trial and at the end (after seven weeks), the child completed a set of computerized neuropsychological tasks (+/‐ 45 minutes) from the well‐validated Amsterdam Neuropsychological Tasks (ANT; De Sonneville, 1999; de Sonneville, Geeraets, & Woestenburg, 1993; Hanisch, Konrad, Günther, & Herpertz‐Dahlmann, 2004), assessing a range of executive functions (i.e. working memory by the Memory Search Letters task (Figure S1); response inhibition and attentional flexibility by the Attentional Set Shifting‐Visual task (Figure S2), and simple psychomotor speed from the Baseline Speed task mainly as a control variable (Figure S3; Cepeda et al., 2013). We used parallel tests to prevent possible practice effects. Assessments were all conducted within 12 hr after taking methylphenidate (Concerta) or placebo and we aimed to assess the participants at the same timepoint of day at the baseline and follow‐up assessment to control for possible pharmacokinetic variability within the 12‐hour window. A higher mean reaction time (slower response) and more errors corresponded to a poorer performance in working memory, response inhibition and attentional flexibility. Psychomotor speed was the mean reaction time in milliseconds averaged across both hands. For a detailed description of all neuropsychological tasks, we refer to the Supporting Information (Appendix S1).
Statistical analyses
Statistical analyses were performed with R version 3.6.0 (R Core Team, 2004). All variables were checked for normal distribution and log transformed where appropriate (i.e. working memory error rate and attentional flexibility reaction time). The mean values reported are without log transformation. Outlier values (z‐scores ≥ |3.0|) were removed from further analyses (up to 3.6%).
Differences between the discontinuation and continuation group regarding age, age of onset of methylphenidate use, duration of methylphenidate use and ADHD severity at the baseline visit were analysed with a Mann–Whitney test. Between‐group differences in sex were analysed with a Pearson’s chi‐square test.
We used mixed‐effects models from the ‘lme4’ package for R to assess the changes after 7 weeks in executive functioning (e.g. working memory, response inhibition and attentional flexibility) and psychomotor speed between children with ongoing long‐term methylphenidate use (continuation group) compared to those who gradually stopped with long‐term methylphenidate use (discontinuation group). Mixed models can properly account for correlation between repeated measurements and can handle missing data (14.1% of individual data points in our study; Gueorguieva & Krystal, 2004); therefore, no participants had to be excluded for the analyses. According to a stepwise model comparison procedure, we started with the simplest model for each outcome measure: a model with group (continuation group versus discontinuation group) and time (baseline versus follow‐up after seven weeks) as fixed effects, and age, sex and IQ as confounders, as these are related to executive functioning (Mous et al., 2017). Additionally, we added variables that correlated significantly with the dependent variable to the model, starting with the variable with the strongest correlation to the outcome measure (see Table S1 for the correlations between variables), to adjust for the effect of these correlated variables on the dependent variable. After each addition, the best model was chosen via model comparison on the basis of the Akaike Information Criterium (AIC; Akaike, 1973); that is, models with lower AIC were preferred over models with higher AIC (Cavanaugh & Neath, 2019), until we ended up with an optimal model for each separate outcome measure. Parameter estimates were determined with the restricted maximum likelihood (REML) approach. No assumptions were violated, and the residuals were normally distributed. The Bonferroni correction (Bonferroni, 1936) was used to control for multiple testing, resulting in an alpha level of .00714 (.05 divided by seven cognitive measures). Cohen’s f2 was used to calculate local effect sizes, with f2 ≥ .02, f2 ≥ .15 and f2 ≥ .35, representing small, medium and large effect sizes, respectively (Cohen, 1988).
The same stepwise model comparison procedure was performed in individuals from the methylphenidate discontinuation group, to explore differences in change scores of task measures (between baseline and seven‐week follow‐up) between the children who deteriorated (i.e. CGI‐I ≥ 6) compared to children who did not deteriorate after withdrawal of methylphenidate, with age, sex and IQ included as covariates. We also used here an alpha level of .00714.
Finally, we additionally included reaction time variability (i.e. the standard deviation) of working memory, response inhibition, attentional flexibility and baseline speed to our models. We considered this as exploratory given the scattered and preliminary evidence of methylphenidate reducing reaction time variability across various cognitive measures (Coghill, Seth, et al., 2014; Epstein et al., 2011).
Sensitivity analyses
To check whether a large difference in the timepoint of assessment of the baseline and follow‐up visit (median = 28 min; SD = 112; range = 0–449 min) may have impacted the results, we repeated the analyses including only the participants with a difference smaller than 60 min (n = 66) and again including only participants with a difference smaller than 120 min (n = 75) between the timepoint of the baseline and follow‐up assessment. We also repeated the analyses within the discontinuation group with the definition of deteriorated of CGI ≥ 5. To investigate whether a possible dose effect (35 or 54 mg) had an influence on the withdrawal effects, we also repeated the analyses including dosage as a covariable.
Results
Sample characteristics
See Table 1 for group characteristics at baseline. The methylphenidate discontinuation and continuation groups did not differ regarding age, sex, IQ, and start, duration, and dosage of methylphenidate use, ADHD severity, co‐medication use and use of psychosocial treatments. Table 2 presents the neuropsychological task measures for both groups at baseline and seven weeks. Figure 1 summarizes the flow of participants throughout the study.
Table 1.
Group characteristics of children and adolescents in a randomized, placebo‐controlled discontinuation trial of methylphenidate at baseline: comparing a discontinuation group (withdrawal to placebo) and continuation group (continued methylphenidate use)
|
Discontinuation group n = 47 |
Continuation group n = 47 |
|
|---|---|---|
| Age in years, M (SD)[range] a | 13.65 (2.17) [8.5–17.9] | 13.77 (2.05) [10.3–17.9] |
| Methylphenidate start age, M (SD)[range] a | 9.2 (2.3) [3.6–14.1] | 9.3 (2.2) [5.3–14.1] |
| Duration of methylphenidate use, M (SD)[range] a | 4.5 (1.7) [2.0–8.5] | 4.5 (1.4) [2.0–7.2] |
| Methylphenidate dosage, mg/kg per day (SD) a | 0.91 (0.29) | 0.93 (0.31) |
| Study methylphenidate dosage, n (%)b | ||
| 36 mg/day | 23 (48.9%) | 26 (55.3%) |
| 54 mg/day | 24 (51.1%) | 21 (44.7%) |
| Male sex, n (%)b | 34 (72.3%) | 39 (83.0%) |
| IQ, M (SD) a | 94.9 (10.7) | 93.1 (13.0) |
| ADHD‐RS, M (SD) | ||
| Total score | 19.6 (8.9) | 21.4 (9.7) |
| Inattention score | 12.0 (5.7) | 13.8 (6.2) |
| Hyperactivity–impulsivity score | 7.6 (5.0) | 7.6 (5.2) |
| Comorbidities, n (%) b | ||
| ODDb | 0 (0) | 2 (4.3) |
| ASDb | 7 (14.9) | 8 (17) |
| Otherb | 4 (8.5) | 1 (2.1) |
| Co‐medication, n (%) | 22 (46.8%) | 13 (27.7%) |
| Antipsychotic medicationb | 1 (2.1%) | 0 (0.0%) |
| Psychosocial treatment n (%) | ||
| For externalizing problemsb | 4 (8.5%) | 5 (10.6%) |
| For internalizing problemsb | 1 (2.1%) | 3 (6.4%) |
ADHD‐RS, Attention‐Deficit/Hyperactivity Disorder‐Rating Scale; ASD, Autism Spectrum Disorder; ODD, Oppositional Defiant Disorder.
Between‐group differences were tested by a aMann–Whitney U test, or bPearson’s chi‐squared test. There were no significant group differences (p < .05).
Table 2.
Task measures at baseline and 7 weeks in children and adolescents treated with methylphenidate for >2 years, comparing a discontinuation group (withdrawal to placebo) and a continuation group (continued methylphenidate use)
| Measure |
Discontinuation group n = 47 |
delta a |
Continuation group n = 47 |
delta a | Δ Change between groups b | 95% CI | Cohen’s f2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 7 weeks | Baseline | 7 weeks | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| Working memory | |||||||||||||
| RT | 386 | 272 | 422 | 287 | 35.8 | 378 | 267 | 535 | 319.83 | 157 | 121 | 38.9, 203 | |
| ER | 1.02 | 2.06 | 2.28 | 2.07 | 1.26 | 1.81 | 1.79 | 1.44 | 1.65 | −0.37 | 1.63* | 1.09, 2.17 | .14 |
| Response inhibition | |||||||||||||
| RT | 195 | 121 | 127 | 103 | −68.2 | 186 | 132 | 154 | 124.26 | −32.3 | 35.9 | −66.7, 2.15 | |
| ER | 1.50 | 2.10 | 1.12 | 1.54 | −0.38 | 0.67 | 1.98 | 0.70 | 1.22 | 0.03 | 0.41 | −0.09, 0.91 | |
| Attentional flexibility | |||||||||||||
| RT | 542 | 249 | 395 | 237 | −146 | 542 | 255 | 383 | 162.28 | −159 | 13.0 | −52.4, 78.4 | |
| ER | 3.26 | 3.15 | 2.81 | 3.10 | −0.45 | 2.70 | 2.96 | 2.31 | 2.81 | −0.39 | 0.06 | −0.80, 0.92 | |
| Psychomotor speed | |||||||||||||
| RT | 320 | 49.7 | 328 | 41.2 | 8.10 | 322 | 41.0 | 332 | 40.35 | 10.4 | 2.27 | −10.1, 14.6 | |
RT, reaction time in milliseconds; ER, error rate; working memory by the Memory Search Letters task; response inhibition and attentional flexibility by the Shifting Attentional Set—Visual (SSV) task, and psychomotor speed by the Baseline Speed task (De Sonneville, 1999).
The difference between seven weeks and baseline.
The difference in mean change from baseline to seven weeks between the discontinuation and continuation group, by mixed models analyses.
p < .0074 using Bonferroni correction for multiple testing.
Figure 1.
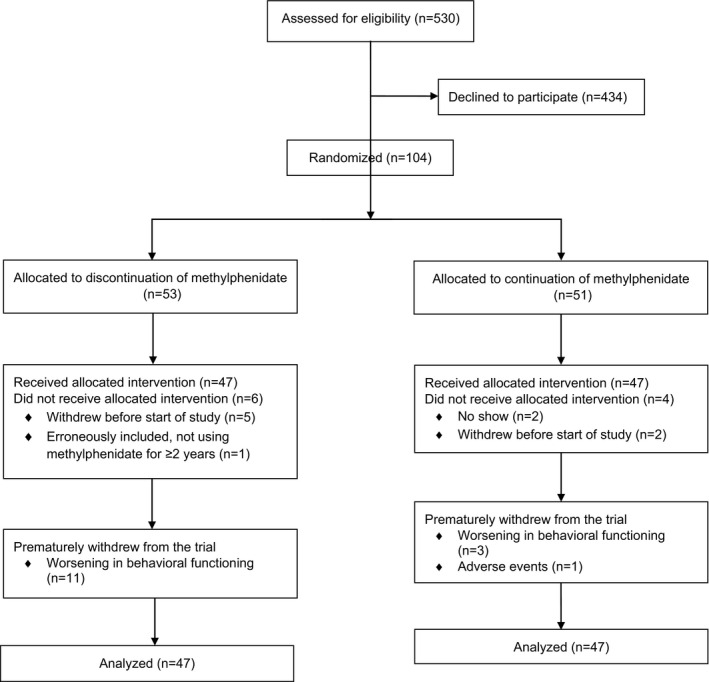
CONSORT flow diagram of participants in the randomized, placebo‐controlled discontinuation study of methylphenidate
Task results between the methylphenidate discontinuation and continuation groups
Working memory
We found an interaction effect between group and time with a near‐medium effect size (β = −1.62, SD = 0.56, t = −2.88, p = .01, Cohen’s f2 = .14), indicating that the continuation group using methylphenidate made fewer errors at the follow‐up visit compared to the baseline visit, whereas the discontinuation group made more errors at the follow‐up visit compared to the baseline visit. Thus, withdrawal of methylphenidate led to making more errors regarding working memory compared to continuing with methylphenidate use, independent from working memory reaction time. No significant differences were observed in changes in working memory reaction time between both groups (β = 58.16, SD = 70.78, t = .82, p = .42).
Response inhibition
We did not find significant differences in changes in response inhibition reaction time (β = 50.12, SD = 31.35, t = 1.60, p = .12) or error rate (β = −.13, SD = 0.67, t = −.20, p = .84) between the two groups from baseline to seven weeks.
Attentional flexibility
There were also no significant differences in changes in attentional flexibility reaction time (β = −26.94, SD = 38.76, t = −.07, p = .49) and error rate (β = −.49, SD = 0.81, t = .61, p = .55) between the two groups from baseline to seven weeks.
Psychomotor speed
We did not find a significant difference in changes in mean reaction time between the two groups from baseline to seven weeks (β = −4.29, SD = 7.94, t = −.54, p = .59).
Reaction time variability
None of the variability measures were significant (all p’s > .10).
Task results in relation to clinical deterioration within the discontinuation group
We did not find significant differences in changes in cognitive measures between children and adolescents who worsened in clinical functioning after discontinuation of methylphenidate (n = 19) versus those who did not deteriorate (n = 28) regarding working memory reaction time (β = 137.41, SD = 105.89, t = 1.30, p = .21) and error rate (β = −1.40, SD = 0.97, t = −1.44, p = .16), response inhibition reaction time (β = −7.16, SD = 47.31, t = −0.15, p = .88) and error rate (β = 1.82, SD = 1.02, t = 1.79, p = .08), attentional flexibility reaction time (β = −.15, SD = 0.16, t = −0.92, p = .36) and error rate (β = −.42, SD = 0.96, t = −0.44, p = .67), psychomotor speed reaction time (β = 18.89, SD = 10.80, t = 1.75, p = .09) and reaction time variability measures (all p’s > .36), see Table 3.
Table 3.
Task measures within the discontinuation group at baseline and seven weeks of youth who deteriorated clinically versus those who did not
| Measure |
Not deteriorated a n = 28 |
delta b |
Deteriorated a n = 19 |
delta b | Δ Change between groups c | Δ 95% CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 7 weeks | Baseline | 7 weeks | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Working memory | ||||||||||||
| RT | 380 | 280 | 390 | 297 | 9.67 | 396 | 267 | 496 | 262 | 100 | 90.8 | 9.50, 172 |
| ER | 1.07 | 1.96 | 2.73 | 2.21 | 1.66 | 0.94 | 2.26 | 1.30 | 1.34 | 0.36 | 1.30 | 0.73, 1.87 |
| Response inhibition | ||||||||||||
| RT | 186 | 124 | 112 | 111 | −74.0 | 189 | 79.5 | 160 | 78.3 | −28.4 | 45.6 | 17.0, 74.2 |
| ER | 1.61 | 2.15 | 0.91 | 1.44 | −0.70 | 1.33 | 2.09 | 1.60 | 1.71 | 0.38 | 1.2 | 0.67, 1.73 |
| Attentional flexibility | ||||||||||||
| RT | 519 | 276 | 398 | 258 | −121 | 576 | 203 | 390 | 199 | −186 | 64.7 | −2.89, 132 |
| ER | 3.31 | 3.20 | 2.50 | 2.26 | −0.81 | 2.20 | 1.66 | 1.92 | 2.43 | −0.28 | 0.53 | −0.17, 1.23 |
| Psychomotor speed | ||||||||||||
| RT | 317 | 52.5 | 309 | 34.5 | −7.86 | 326 | 45.6 | 364 | 26.3 | 37.7 | 45.6 | 33.9, 57.3 |
RT, reaction time in milliseconds; ER, error rate; tasks see Table 2. p < .0074 using Bonferroni correction for multiple testing: none of the Δ changes between groups were significant.
‘Deteriorated’ = CGI‐I ≥ 6 (‘much worse’ and ‘very much worse’) and ‘not deteriorated’ all else.
The difference between seven weeks and baseline.
The difference in mean change from baseline to 7 weeks between the ‘deterioration’ and ‘nondeterioration’ group, by mixed models analyses.
Sensitivity analyses
The results were similar when including only participants with a difference in timepoint of the baseline and follow‐up assessment smaller than 60 min or 120 min. The results were also similar when using CGI ≥ 5 as a definition of deterioration. Furthermore, we found no effect of dosage.
Discussion
This study investigated the acute effects of methylphenidate on executive functioning (i.e. working memory, response inhibition and attentional flexibility) and psychomotor speed in children and adolescents after ongoing long‐term treatment with methylphenidate in regular clinical practice for at least two years, using a randomized, placebo‐controlled discontinuation design. We found that withdrawal of methylphenidate resulted in a worse performance on the working memory task, compared to continued methylphenidate treatment.
The benefit of methylphenidate on working memory in long‐term users is in line with studies in treatment‐naïve children and adolescents assessing acute effects (Coghill, Seth, et al., 2014; Pietrzak et al., 2006). The discontinuation group performed less accurate, making more errors on the working memory task after methylphenidate withdrawal, whereas the continuation group actually improved in accuracy, making fewer errors at the follow‐up visit compared to baseline. According to previous findings, children with ADHD have difficulties prioritizing a correct response over a fast response (Mulder et al., 2010). Even though our results did not show an effect on working memory reaction time, our findings may imply that methylphenidate helps to overcome this. These results may suggest that even after prolonged use, methylphenidate could be of value for better educational and/or occupational outcomes, as working memory has been associated with better school achievements (Gathercole, Pickering, Knight, & Stegmann, 2004; St Clair‐Thompson & Gathercole, 2006).
Our null findings in response inhibition, attentional flexibility and psychomotor speed contrast with our working memory results. It should be noted that working memory underlies the central executive component of executive functioning (Engle, Laughlin, Tuholski, & Conway, 1999) and is the one executive function task that is most consistently impaired in children with ADHD (Kofler et al., 2019). However, previous studies did report positive effects of methylphenidate on response inhibition, attentional flexibility and psychomotor speed in treatment‐naïve children with ADHD (Bolfer et al., 2017; Coghill, Seth, et al., 2014; Konrad, Neufang, Fink, & Herpertz‐Dahlmann, 2007; Pietrzak et al., 2006). Our null findings could result from a diminishing effect after long‐term use due to developing tolerance for methylphenidate (Wang et al., 2013), although an assessment prior to starting methylphenidate treatment would be necessary to confirm this. Also, differences in executive functioning tasks may explain the discrepant findings. For example, the widely used Stop‐Signal task to measure response inhibition requires to withhold an already initiated response (Lipszyc & Schachar, 2010), whereas in our paradigm, children had to execute a response on every trial. Additionally, although unlikely, we cannot rule out that our null findings were due to a prolonged effect of methylphenidate after withdrawing to placebo.
We also looked into possible differences in change in neuropsychological task measures from baseline to 7 weeks within the discontinuation group, between children who deteriorated after methylphenidate withdrawal versus those who did not deteriorate (based on the CGI‐I). However, we did not find significant group differences in changes in executive functioning or psychomotor speed, suggesting that global clinical impressions of symptom improvement or worsening do not necessarily translate to changes in executive functioning, which is in line with earlier findings (Bedard et al., 2015; Coghill, Hayward, et al., 2014). However, findings should be seen in light of the small sample size and should be regarded as preliminary.
This study was the first double‐blind, placebo‐controlled methylphenidate discontinuation study after prolonged use beyond two years. A number of limitations need to be acknowledged. First, we lacked a baseline assessment of participants prior to the discontinuation trial; we were therefore unable to compare the initial acute effects of methylphenidate at the start of the treatment to the ongoing effects during the discontinuation trial. Second, we cannot be sure that participants were on their optimal dosage of methylphenidate. However, our results imply that even unoptimized dosages as prescribed in clinical practice may have a beneficial effect on working memory after long‐term use. Future studies may include the full range of possible dosages, which will allow for a proper assessment of the role of dosage on withdrawal effects. It is possible that higher dosages may result in effects on the other neuropsychological outcome measures. Third, our sample may have been overrepresented by participants in whom methylphenidate was less effective (Matthijssen et al., 2019); still, with that in mind, we found a beneficial effect of methylphenidate. Fourth, although we specifically chose tasks that are insensitive to practice effects, we cannot rule these out. Fifth, although in some participants the baseline and follow‐up neuropsychological assessment were not assessed at the same time of day, this did not affect the results. Finally, the modest sample size has to be mentioned and the fact that more participants in the discontinuation group (n = 11) dropped out compared to the continuation group (n = 4), which may have led to underestimation of effects.
To conclude, we found support for beneficial effects of methylphenidate on working memory after prolonged use, beyond two years of treatment. This effect was independent from global clinical impressions of ADHD improvement or worsening, highlighting the importance of assessing neuropsychological performance as an additional dimension, which may also directly impact various domains of daily life often compromised in individuals with ADHD (such as academic achievements, high‐risk behaviours or social functioning; McQuade, Murray‐Close, Shoulberg, & Hoza, 2013; Romer et al., 2011). Future research should aim at replicating this novel finding in larger samples incorporating a baseline assessment to investigate long‐term effects of methylphenidate use on executive functioning, assess children and adolescents on their optimal dosage, include a range of (neuro)cognitive measurements and investigate possible age‐related differences. Further research should also focus on further understanding the relationship between executive functioning and clinical improvements, including measures to test the ecological validity of executive function deficiencies versus indices of impairment (e.g. school functioning, social relations) related to ADHD. A more profound understanding of this relationship could possibly aid the clinical decision‐making on the continued use of methylphenidate and individual treatment plans. The dissociation between cognitive and behavioural effects of stimulant medication may suggest added value of also considering cognitive outcome measures in clinical practice.
Supporting information
Appendix S1. Detailed description of all the neuropsychological tasks used.
Figure S1. Memory search letters.
Figure S2. Shifting set task.
Figure S3. Baseline speed.
Table S1. Correlations for the outcome measures of the ANT tasks.
Acknowledgements
The authors are extremely grateful to all the children and their parents who participated to make this study possible. They furthermore thank all colleagues who contributed to the data collection. This project has received funding from the Netherlands Organization for Health Research and Development (ZonMw, grant 836011014). This study is registered in the Netherlands Trial Register (www.Trialregister.nl) with identifier 5252. G.H.H.v.d.L‐N. has been a speaker for Takeda without getting any fee or any financial support, is not an employee of any company, nor a stock shareholder. She has no other financial or material support, including expert testimony, patents and royalties. J.K.B. has been consultant to/member of advisory board of and/or speaker for Janssen Cilag BV, Eli Lilly, Takeda/Shire, Roche, Medice, Angelini, Novartis and Servier. He is not an employee of any of these companies, nor a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents and royalties. P.J.H. has been member of an advisory board meeting for Takeda/Shire. The remaining authors have declared that they have no competing or potential conflicts of interest.
Key points.
Methylphenidate can improve executive functioning; however, it is unknown whether there are acute effects of methylphenidate on executive functioning after long‐term use.
In this double‐blind, placebo‐controlled discontinuation trial, methylphenidate had a beneficial effect on working memory beyond two years of treatment; no evidence was found for a beneficial effect of methylphenidate treatment beyond two years on response inhibition, attentional flexibility and psychomotor speed.
There was no relationship between clinical improvement and improvements in working memory.
The effect of long‐term methylphenidate use on executive functioning should be further explored, incorporating baseline assessments and testing children and adolescents on optimized dosages.
Conflict of interest statement: See Acknowledgements for full disclosures.
References
- Abikoff, H. , Hechtman, L. , Klein, R.G. , Gallagher, R. , Fleiss, K. , Etcovitch, J. , … & Pollack, S. (2004). Social functioning in children with ADHD treated with long‐term methylphenidate and multimodal psychosocial treatment. Journal of the American Academy of Child & Adolescent Psychiatry, 43, 820–829. [DOI] [PubMed] [Google Scholar]
- Akaike, H. (1973). Information theory and an extension of the maximum likelihood principle. Second International Symposium on Information Theory (Tsahkadsor, 1971). Available from http://search.ebscohost.com.proxy‐ub.rug.nl/login.aspx?direct=true&db=msn&AN=MR0483125&site=ehost‐live&scope=site [Google Scholar]
- Beau‐Lejdstrom, R. , Douglas, I. , Evans, S.J.W. , & Smeeth, L. (2016). Latest trends in ADHD drug prescribing patterns in children in the UK: Prevalence, incidence and persistence. British Medical Journal Open, 6, e010508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard, A.C. , Stein, M.A. , Halperin, J.M. , Krone, B. , Rajwan, E. , & Newcorn, J.H. (2015). Differential impact of methylphenidate and atomoxetine on sustained attention in youth with attention‐deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry, 56, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolfer, C. , Carreira, W.S. , Tsunemi, M.H. , Pacheco, S.P. , Casella, B.B. , & Casella, E.B. (2017). Attention‐deficit/hyperactivity disorder: The impact of methylphenidate on working memory, inhibition capacity and mental flexibility. Arquivos de Neuro‐Psiquiatria, 75, 204–208. [DOI] [PubMed] [Google Scholar]
- Bonferroni, C.E. (1936). Teoria statistica delle classi e calcolo delle probilitit ’a. Pubblicazioni Del R Instituto Superiore Di Scienze Economiche e Commercialdi Di Firenze, 8, 3–62. [Google Scholar]
- Busner, J. , & Targum, S.D. (2007). Global impressions scale: Applying a research tool in Clinical Practice. Psychiatry, 4, 28–37. [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh, J.E. , & Neath, A.A. (2019). The Akaike information criterion: Background, derivation, properties, application, interpretation, and refinements. Wiley Interdisciplinary Reviews: Computational Statistics, 11, 1–11. [Google Scholar]
- Cepeda, N. , Blackwell, K. , & Munakata, Y. (2013). Speed isn’t everything: Complex processing speed measures mask individual differences and developmental changes in executive control. Applied Attention Theory, 16, 269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill, D.R. , Hayward, D. , Rhodes, S.M. , Grimmer, C. , & Matthews, K. (2014). A longitudinal examination of neuropsychological and clinical functioning in boys with attention deficit hyperactivity disorder (ADHD): Improvements in executive functioning do not explain clinical improvement. Psychological Medicine, 44(5), 1087–1099. 10.1017/S0033291713001761. [DOI] [PubMed] [Google Scholar]
- Coghill, D.R. , Seth, S. , Pedroso, S. , Usala, T. , Currie, J. , & Gagliano, A. (2014). Effects of methylphenidate on cognitive functions in children and adolescents with attention‐deficit/hyperactivity disorder: Evidence from a systematic review and a meta‐analysis. Biological Psychiatry, 76, 603–615. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. London: Routledge. [Google Scholar]
- De Sonneville, L.M.J. (1999). Amsterdam Neuropsychological Task: A computer‐aided assessment program. In den Brinker B.P.L.M. Beek P.J. Brand A.N. Maarse S.J. & Mulder L.J.M. (Eds.), Cognitive ergonomics, clinical assessment and computer‐ assisted learning: Computers in psychology (vol. 6, pp. 204–217). Lisse, The Netherlands: Swets & Zeitlinger. [Google Scholar]
- de Sonneville, L. M. J. , Geeraets, M. H. W. , & Woestenburg, J. C. (1993). Information processing in children with minor neurological dysfunction: Behavioural and neurophysiological indices. Early Human Development, 34(1‐2), 69–78. 10.1016/0378-3782(93)90042-S. [DOI] [PubMed] [Google Scholar]
- Engle, R.W. , Laughlin, J.E. , Tuholski, S.W. , & Conway, A.R.A. (1999). Working memory, short‐term memory, and general fluid intelligence: A latent‐variable approach. Journal of Experimental Psychology: General, 128, 309–331. [DOI] [PubMed] [Google Scholar]
- Epstein, J.N. , Brinkman, W.B. , Froehlich, T. , Langberg, J.M. , Narad, M.E. , Antonini, T.N. , … & Altaye, M. (2011). Effects of stimulant medication, incentives, and event rate on reaction time variability in children with ADHD. Neuropsychopharmacology, 36, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole, S.E. , Pickering, S.J. , Knight, C. , & Stegmann, Z. (2004). Working memory skills and educational attainment: Evidence from national curriculum assessments at 7 and 14 years of age. Applied Cognitive Psychology, 18, 1–16. [Google Scholar]
- Gueorguieva, R. , & Krystal, J.H. (2004). Move over ANOVA: Progress in analyzing repeated‐measures data and its reflection in papers published in the Archives of General Psychiatry. Archives of General Psychiatry, 61, 310–317. [DOI] [PubMed] [Google Scholar]
- Guy, W. (1976). ECDEU Assessment Manual for Psychopharmacology, Revised. US Department of Health, Education, and Welfare Publication (ADM). Rockville, MD: National Institute of Mental Health, 76–338. [Google Scholar]
- Hanisch, C. , Konrad, K. , Günther, T. , & Herpertz‐Dahlmann, B. (2004). Age‐dependent neuropsychological deficits and effects of methylphenidate in children with attention‐deficit/hyperactivity disorder: A comparison of pre‐ and grade‐school children. Journal of Neural Transmission, 111(7), 865–881. 10.1007/s00702-003-0056-0. [DOI] [PubMed] [Google Scholar]
- Hechtman, L. , & Greenfield, B. (2003). Long‐term use of stimulants in children with attention deficit hyperactivity disorder. Paediatric Drugs, 5, 787–794. [DOI] [PubMed] [Google Scholar]
- Huang, Y.S. , Wang, L.J. , & Chen, C.K. (2012). Long‐term neurocognitive effects of methylphenidate in patients with attention deficit hyperactivity disorder, even at drug‐free status. BMC Psychiatry, 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby, M.Y. , Vadnais, S.A. , & Jagger‐Rickels, A.C. (2019). Which components of processing speed are affected in ADHD subtypes? Child Neuropsychology, 25, 964–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler, M.J. , Irwin, L.N. , Soto, E.F. , Groves, N.B. , Harmon, S.L. , & Sarver, D.E. (2019). Executive functioning heterogeneity in Pediatric ADHD. Journal of Abnormal Child Psychology, 47, 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad, K. , Neufang, S. , Fink, G.R. , & Herpertz‐Dahlmann, B. (2007). Long‐term effects of methylphenidate on neural networks associated with executive attention in children with ADHD: Results from a longitudinal functional MRI study. Journal of the American Academy of Child and Adolescent Psychiatry, 46, 1633–1641. [DOI] [PubMed] [Google Scholar]
- Lipszyc, J. , & Schachar, R. (2010). Inhibitory control and psychopathology: A meta‐analysis of studies using the stop signal task. Journal of the International Neuropsychological Society, 16, 1064–1076. [DOI] [PubMed] [Google Scholar]
- Matthijssen, A.‐F.‐M. , Dietrich, A. , Bierens, M. , Kleine Deters, R. , van de Loo‐Neus, G.H.H. , van den Hoofdakker, B.J. , & Hoekstra, P.J. (2019). Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: A Randomized Placebo‐Controlled Discontinuation Study. American Journal of Psychiatry, 176, 754–762. [DOI] [PubMed] [Google Scholar]
- McQuade, J.D. , Murray‐Close, D. , Shoulberg, E.K. , & Hoza, B. (2013). Working memory and social functioning in children. Journal of Experimental Child Psychology, 115, 422–435. [DOI] [PubMed] [Google Scholar]
- Molina, B.S.G. , Hinshaw, S.P. , Swanson, J.M. , Arnold, L.E. , Vitiello, B. , Jensen, P.S. , … & Houck, P.R. (2009). The MTA at 8 Years: Prospective follow‐up of children treated for combined‐type ADHD in a Multisite Study. Journal of the American Academy of Child & Adolescent Psychiatry, 48, 484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mous, S.E. , Schoemaker, N.K. , Blanken, L.M.E. , Thijssen, S. , van der Ende, J. , Polderman, T.J.C. , … & White, T. (2017). The association of gender, age, and intelligence with neuropsychological functioning in young typically developing children: The Generation R study. Applied Neuropsychology: Child, 6, 22–40. [DOI] [PubMed] [Google Scholar]
- Mulder, M.J. , Bos, D. , Weusten, J.M.H. , van Belle, J. , van Dijk, S.C. , Simen, P. , … & Durston, S. (2010). Basic impairments in regulating the speed‐accuracy tradeoff predict symptoms of attention‐deficit/hyperactivity disorder. Biological Psychiatry, 68, 1114–1119. [DOI] [PubMed] [Google Scholar]
- Pietrzak, R.H. , Mollica, C.M. , Maruff, P. , & Snyder, P.J. (2006). Cognitive effects of immediate‐release methylphenidate in children with attention‐deficit/hyperactivity disorder. Neuroscience and Biobehavioral Reviews, 30, 1225–1245. [DOI] [PubMed] [Google Scholar]
- Pievsky, M.A. , & McGrath, R.E. (2018). The neurocognitive profile of attention‐deficit/hyperactivity disorder: A review of meta‐analyses. Archives of Clinical Neuropsychology, 33, 143–157. [DOI] [PubMed] [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R‐project.org/.
- Romer, D. , Betancourt, L.M. , Brodsky, N.L. , Giannetta, J.M. , Yang, W. , & Hurt, H. (2011). Does adolescent risk taking imply weak executive function? A prospective study of relations between working memory performance, impulsivity, and risk taking in early adolescence. Developmental Science, 14, 1119–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia, K. , Halari, R. , Cubillo, A. , Smith, A.B. , Mohammad, A.M. , Brammer, M. , & Taylor, E. (2011). Methylphenidate normalizes fronto‐striatal underactivation during interference inhibition in medication‐nave boys with attention‐deficit hyperactivity disorder. Neuropsychopharmacology, 36, 1575–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia, K. , Halari, R. , Mohammad, A.M. , Taylor, E. , & Brammer, M. (2011). Methylphenidate normalizes frontocingulate underactivation during error processing in attention‐deficit/hyperactivity disorder. Biological Psychiatry, 70, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweren, L. , Hoekstra, P. , van Lieshout, M. , Oosterlaan, J. , Lambregts‐Rommelse, N. , Buitelaar, J. , … Hartman, C. (2018). Long‐term effects of stimulant treatment on ADHD symptoms, social–emotional functioning, and cognition. Psychological Medicine, 49, 217–223. [DOI] [PubMed] [Google Scholar]
- St Clair‐Thompson, H.L. , & Gathercole, S.E. (2006). Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Quarterly Journal of Experimental Psychology, 59, 745–759. [DOI] [PubMed] [Google Scholar]
- Swanson, J. , Arnold, L.E. , Kraemer, H. , Hechtman, L. , Molina, B. , Hinshaw, S. , … & Wigal, T. (2008). Evidence, interpretation, and qualification from multiple reports of long‐term outcomes in the multimodal treatment study of children with ADHD (MTA) Part I: Executive summary. Journal of Attention Disorders, 12, 4–14. [DOI] [PubMed] [Google Scholar]
- Swanson, J.M. , Arnold, L.E. , Molina, B.S.G. , Sibley, M.H. , Hechtman, L.T. , Hinshaw, S.P. , … & Kraemer, H.C. (2017). Young adult outcomes in the follow‐up of the multimodal treatment study of attention‐deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. Journal of Child Psychology and Psychiatry, 58, 663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G.‐J. , Volkow, N.D. , Wigal, T. , Kollins, S.H. , Newcorn, J.H. , Telang, F. , … & Swanson, J.M. (2013). Long‐term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS One, 8, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Detailed description of all the neuropsychological tasks used.
Figure S1. Memory search letters.
Figure S2. Shifting set task.
Figure S3. Baseline speed.
Table S1. Correlations for the outcome measures of the ANT tasks.


