Abstract
Background
Canine atopic dermatitis (cAD) is a common chronic relapsing pruritic skin disease for which management commonly relies on life‐long use of immunomodulatory drugs. A number of the medications used are associated with adverse effects and the potential for complications during long‐term use.
Hypothesis
The goal of the study was to determine if a complete and balanced diet formulated for therapeutic benefit could contribute towards management of cAD. We hypothesised that the diet would reduce pruritus while also reducing the requirement for medication during the study period.
Animals, materials and methods
Forty privately owned dogs, having undergone a comprehensive diagnosis for cAD, were randomly allocated to two groups, each group being fed one of two diets (test or control) for up to nine months. We assessed pruritus, Canine Atopic Dermatitis Extent and Severity Index‐(4th iteration) and medication score, the latter reflecting the medication required to maintain a satisfactory quality of life for the animal.
Results
Both diets were well‐accepted and ‐tolerated. There was a significant improvement in the pruritus score after three months of feeding the therapeutic diet (P = 0.0001). No such improvement was observed at any time point in the group of dogs given the control diet. There was a reduced drug requirement for dogs receiving the therapeutic diet after three months (P = 0.058), and that decrease was significant at six months (P = 0.021) and nine months (P = 0.018). No improvement was seen at any time point in the control group.
Conclusion
The results suggest that a novel therapeutic diet can assist in the management of cAD by helping to control pruritus and reducing reliance on medication.
Background – Canine atopic dermatitis (cAD) is a common chronic relapsing pruritic skin disease for which management commonly relies on life‐long use of immunomodulatory drugs. A number of the medications used are associated with adverse effects and the potential for complications during long‐term use. Hypothesis – The goal of the study was to determine if a complete and balanced diet formulated for therapeutic benefit could contribute towards management of cAD. We hypothesised that the diet would reduce pruritus while also reducing the requirement for medication during the study period. Conclusion – The results suggest that a novel therapeutic diet can assist in the management of cAD by helping to control pruritus and reducing reliance on medication.

Résumé
Contexte
La dermatite atopique canine (cAD) est une dermatose prurigineuse récidivante chronique fréquente dont la gestion nécessite souvent l’utilisation d’immunomodulateurs à vie. Un grand nombre de traitement est associé à des effets secondaires et complications potentielles dans leur utilisation au long cours.
Hypothèses
Le but de cette étude est de déterminer si un régime alimentaire complet et équilibré peut contribuer à la gestion de cAD. Nous supposons que l’alimentation pourrait réduire le prurit ainsi que le besoin de traitement au cours de la période de l’étude.
Sujets, matériels et méthodes
Quarante chiens de propriétaires, ayant eu un diagnostic détaillé de cAD, ont été réparti au hasard en deux groupes, chaque groupe ayant été nourri par une ou deux alimentations (test ou contrôle) pendant neuf mois. Nous déterminons le prurit, le CADESI‐4 et le score médicamenteux, ce dernier reflétant les traitements nécessaires pour maintenir une qualité de vie satisfaisante pour l’animal.
Résultats
Les deux alimentations sont bien tolérées et acceptées. Il y avait une amélioration significative de score de prurit après trois mois de régime thérapeutique (P = 0.0001). Cette amélioration n’a pas été observée dans le groupe de chiens recevant le régime contrôle. Il y avait une diminution des besoins en traitement pour les chiens recevant le régime thérapeutique après trois mois (P = 0.058), et cette diminution était significative à six mois (P = 0.021) et neuf mois (P = 0.018). Aucune amélioration n’a été observée à aucun moment dans le groupe contrôle.
Conclusion
Les résultats suggèrent que le régime thérapeutique peut s’associer à la gestion de cAD en aidant à contrôler le prurit et diminuer le besoin de traitement.
Resumen
Introducción
la dermatitis atópica canina (cAD) es una enfermedad cutánea pruriginosa recidivante crónica común para la que el tratamiento suele depender del uso de fármacos inmunomoduladores durante toda la vida. Varios de los medicamentos utilizados están asociados con efectos adversos y la posibilidad de complicaciones durante el uso a largo plazo.
Hipótesis
el objetivo del estudio fue determinar si una dieta completa y equilibrada formulada para obtener un beneficio terapéutico podría contribuir al manejo de la cAD. Presumimos que la dieta reduciría el prurito y al mismo tiempo reduciría la necesidad de medicación durante el período de estudio.
Animales, materiales y métodos
Cuarenta perros de propietarios particulares tras haberse sometido a un estudio diagnóstico completo de cAD, fueron asignados aleatoriamente a dos grupos, cada grupo alimentado con una de las dos dietas (prueba o control) durante un máximo de nueve meses. Se evaluó el prurito, el índice de extensión y gravedad de la dermatitis atópica canina (cuarta revisión) y la puntuación de la medicación, esta última reflejando la medicación necesaria para mantener una calidad de vida satisfactoria para el animal.
Resultados
Ambas dietas fueron bien aceptadas y toleradas. Hubo una mejora significativa en la valoración de prurito después de tres meses de alimentar la dieta terapéutica (P = 0,0001). No se observó tal mejora en ningún momento en el grupo de perros que recibió la dieta de control. Hubo un requerimiento reducido de medicamentos para los perros que recibieron la dieta terapéutica después de tres meses (P = 0,058), y esa disminución fue significativa a los seis meses (P = 0,021) y nueve meses (P = 0,018). No se observó ninguna mejora en ningún momento en el grupo de control.
Conclusión
los resultados sugieren que una dieta terapéutica novedosa puede ayudar en el manejo de la cAD al ayudar a controlar el prurito y reducir la dependencia de la medicación.
Zusammenfassung
Hintergrund
Die atopische Dermatitis des Hundes (cAD) ist eine häufige wiederkehrende juckende Erkrankung, bei der ein Management häufig auf lebenslanger Verabreichung von immunmodulatorischen Medikamenten beruht. Eine Vielzahl der verwendeten Medikamente haben Nebenwirkungen und die Möglichkeit von Komplikationen, wenn sie langfristig eingesetzt werden.
Hypothese
Das Ziel dieser Studie war es festzustellen, ob eine komplette und ausgewogene Ernährung, die zu therapeutischen Zwecken formuliert wurde, beim Management der cAD beitragen kann. Wir stellten die Hypothese auf, dass die Diät den Juckreiz reduzieren würde, während sie ebenso die Notwendigkeit einer Medikation während der Studienperiode reduzieren könnte.
Tiere, Materialien und Methoden
Vierzig Hunde im Privatbesitz, bei denen eine umfassende Diagnose von cAD gestellt wurde, wurden zufällig in zwei Gruppen eingeteilt; jeder Gruppe wurden bis zu neun Monate lang eine von zwei Diäten (Test oder Kontrolle) gefüttert. Wir erfassten den Pruritus, den Canine Atopic Dermatitis Extent and Severity Index‐(4te Auflage) und die medizinische Bewertung, wobei letztere die Medikation reflektiert, die nötig ist, um eine zufriedenstellende Lebensqualität des Tieres zu erhalten.
Ergebnisse
Beide Diäten wurden gerne gefressen und gut toleriert. Nach drei Monaten der therapeutischen Nahrung bestand eine signifikante Verbesserung bei den Prurituswerten (P = 0,0001). Eine derartige Verbesserung wurde zu keinem Zeitpunkt bei der Hundegruppe beobachtet, welche die Kontrolldiät erhielt. Nach drei Monaten bestand eine geringere Notwendigkeit (P = 0,058) bei jenen Hunden, die die therapeutische Diät erhielten, Medikamente einzusetzen. Diese Reduktion war nach sechs (P = 0,021) und neun Monaten (P = 0,018) signifikant. Zu keinem der Zeitpunkte wurde eine derartige Verbesserung in der Kontrollgruppe gesehen.
Schlussfolgerungen
Die Ergebnisse zeigen, dass eine neuartige therapeutische Diät beim Management der cAD helfen kann, den Juckreiz zu kontrollieren und den Einsatz von Medikamenten zu reduzieren.
要約
背景
犬アトピー性皮膚炎 (cAD) は、一般的な慢性再発性掻痒性皮膚疾患であり、その治療には免疫調整剤を生涯にわたって使用することが必要である。しかし、使用されている薬剤の中には、副作用や長期使用による合併症の可能性があるものも少なくない。
仮説
本研究の目的は、治療効果を目的とした完全でバランスのとれた食事が、cADの管理に貢献できるかどうかを判断することである。我々は、この食事が掻痒症を軽減すると同時に、研究期間中の投薬の必要性を軽減するという仮説を立てた。
被験動物、材料、方法
cADの総合診断を受けたオーナー所有犬40頭を2グループに無作為に割り振り、それぞれのグループに2種類の食事 (試験食または対照食) のいずれかを最長9カ月間与えた。掻痒、Canine Atopic Dermatitis Extent and Severity Index‐(4th iteration)、投薬スコアを評価した。
結果
どちらの食事もよく受け入れられ、忍容性も高かった。療法食を与えて3ヶ月後には、痒みのスコアに有意な改善が見られた (P = 0.0001) 。対照食を与えられた犬では、どの時点でもこのような改善は見られなかった。療法食を与えた犬では、3ヶ月後には薬剤の必要量が減少し (P = 0.058) 、その減少は6ヶ月後 (P = 0.021) と9ヶ月後 (P = 0.018) にも有意に見られた。対照群では、どの時点でも改善は見られなかった。
結論
今回の結果から、新しい治療食は、掻痒を抑え、薬への依存度を減らすことで、cADの管理を助けることができると考えられる。
摘要
背景
犬特应性皮炎(cAD)是一种常见的慢性复发性瘙痒性皮肤病, 其治疗通常依赖于终生使用免疫调节药物。使用的许多药物长期使用期间, 可能存在不良反应和并发症。
假设
本研究的目的是确定为达到疗效而制定的完整和平衡的日粮是否有助于cAD管理。我们假设日粮将减少瘙痒, 同时也减少研究期间药物的需求。
动物、材料和方法
将40只私家犬 (已接受cAD综合诊断) 随机分配至两组, 每组喂食两种日粮 (试验或对照) 中的一种, 持续长达9个月。我们评估了瘙痒、犬特应性皮炎程度和严重指数 (第4版) 和药物评分, 后者对应维持动物满意生活质量所需的药物。
结果
两种日粮均被广泛接受且耐受。饲喂处方粮三个月后, 瘙痒评分显著改善(P = 0.0001)。在给予对照饲料的犬组中, 在任何时间点均未观察到此类改善。3个月后, 吃处方粮的犬的药物需求降低(P = 0.058), 并且在6个月(P = 0.021)和9个月(P = 0.018)时降低显著。对照组在任何时间点均未观察到改善。
结论
结果表明, 新奇处方粮可通过帮助控制瘙痒和减少对药物的依赖来帮助cAD的管理。
Resumo
Contexto
A dermatite atópica canina (DAC) é uma dermatopatia crônica pruriginosa e recidivante comum para a qual o manejo consiste na utilização contínua de drogas imunomoduladoras. Vários dos medicamentos utilizados são associados à reações adversas e ao potencial de complicações com o uso a longo prazo.
Hipótese
O objetivo do estudo foi determinar se uma dieta completa e balanceada formulada para fins terapêuticos poderia contribuir no manejo da DAC. Nossa hipótese foi a de que a dieta poderia reduzir o prurido a longo prazo, reduzindo também o requerimento de medicações durante o período do estudo.
Animais, materiais e métodos
Quarenta cães de propriedade privada que passaram por um diagnóstico criterioso de DAC, foram aleatoriamente alocados em dois grupos, cada grupo sendo alimentado com uma das duas dietas (teste ou controle) por até nove meses. Nós avaliamos prurido, CADESI [Canine Atopic Dermatitis Extent and Severity Index (4th iteration)], e escore de medicação, o último estando relacionado à medicação necessária para manter uma qualidade de vida satisfatória para o animal.
Resultados
Ambas as dietas foram bem aceitas e toleradas. Houve uma melhora significativa no escore de prurido após três meses consumindo a dieta terapêutica (P = 0,0001). Nenhuma melhora foi observada em nenhum tempo experimental no grupo de cães tratados com a dieta controle. Observou‐se uma redução no requerimento de medicamentos nos cães quer receberam a dieta terapêutica após três meses (P = 0,058), e esta redução foi significativa após seis meses (P = 0,021) e nove meses (P = 0.018). Não foi observada nenhuma melhora em nenhum tempo experimental no grupo controle.
Conclusão
Os resultados sugerem que a nova dieta terapêutica pode auxiliar no controle da DAC pela redução do prurido e da dependência de medicação.
Introduction
Canine atopic dermatitis (cAD) is a multifactorial disease associated with immunological dysregulation and skin barrier abnormalities. 1 Treatment is based mainly on the use of anti‐inflammatory and/or immunomodulatory drugs, while the use of epidermal barrier treatments is mainly considered supportive. 2 Immunomodulatory drugs unfortunately may be associated with detrimental adverse effects. It is well‐known that dietary components or additives may positively influence the course of human atopic dermatitis (AD). 3 The influence of these phytochemicals on allergic reactions has been studied in some detail and potential efficacy is established. 4 In veterinary medicine, the role of essential fatty acids and some anti‐oxidants has been assessed. 5 , 6 , 7 Dietary supplements often are advocated for mild to moderate atopic disease as part of a multimodal approach, and some studies have shown a benefit. However, administration increases the burden of treatment, requiring dosing at minimum once per day. Therefore, incorporation of helpful ingredients into a complete meal format can add to convenience, with the potential to see concomitant improvements in compliance. What is more, it has been illustrated that food‐integrated nutrients are more effective than supplements. 8 While some diets have been developed for the management of AD in dogs, well‐controlled studies are still lacking. 9
The goal of this study was to assess the clinical efficacy of a diet specifically developed to target the pathological mechanism of cAD to assist in the long‐term management of this condition.
Methods and materials
Owner consent, Ethics and General Data Protection Regulation (GDPR) statement
The protocol was approved by the local Kanton Zurich ethical committee (ZH114/16) as well as the Royal Canin Ethical Research Committee. An informed consent form was signed by all dog owners who participated. Any personal data relating to participants in the study was the minimum required and was held only for as long as needed to execute the study, according to GDPR.
Dogs
Privately owned atopic dogs with nonseasonal pruritus were included. All dogs were treated in private practice clinics. The diagnosis of AD was made using standard criteria, after exclusion of ectoparasites and other complicating diseases such as bacterial and yeast infections. 10 Dogs with active bacterial and/or yeast infections, as well as individuals responding adequately to an eight weeks elimination diet and re‐challenge with previous diet, namely, dogs classified as food allergic were not included. Moreover, dogs with ongoing allergen‐specific immunotherapy for <12 months or dogs controlled with this intervention alone were not enrolled. Included dogs needed to be receiving ongoing ectoparasite treatment and this treatment was continued throughout the study. Concomitant medications (see Appendix 1 for list), excluding all nutritional supplements, were allowed throughout the study to comply with ethics and ensure an optimal quality of life (QoL), and were carefully recorded throughout.
Inclusion consultation and initial clinical scoring
All enrolment and clinical evaluations were performed by qualified and experienced veterinary dermatologists. Included dogs were initially evaluated for skin lesions and pruritus using Canine Allergic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) and Hill’s pruritus Visual Analog Score (pVAS), respectively. 11 Medications used during the month before inclusion were recorded. A medication score was made as published previously. 12 Briefly, as depicted in Table 1, monthly treatments were recorded, with the specific drug, dose and frequency of use being attributed a numerical score. The overall score for each month was determined by summing daily scores. The figures reported for medication score are the monthly totals divided by the number of days in the month, thereby providing a daily average.
Table 1.
Medication scoring system (as described previously 12 ) used to grade medication consumption by dogs at the start of the study and at each subsequent check point
| Medication and dose range | Score attributed |
|---|---|
| No concurrent medication | 0 |
| Shampoo therapy | 5 |
| Ear medication (topical) | 5 |
| Other topical therapy | 5 |
| Antihistamines | 10 |
| Frequent antibiotics (>21 days) | 20 |
| Less frequent antibiotics (<21 days) | 10 |
| Prednisolone | |
| ≥1 mg/kg/day | 40 |
| 0.5–1 mg/kg/day | 30 |
| 0.2–0.5 mg/kg/day | 20 |
| ≤0.2 mg/kg/day | 10 |
| Ciclosporin (5 mg/kg) | |
| Once each day | 30 |
| Every other day | 20 |
| Every three days | 10 |
| Every four days | 5 |
| Oclacitinib | |
| Twice daily | 40 |
| Once each day | 30 |
| Every other day | 20 |
| Every three days | 10 |
Follow‐up consultations and drop‐out
Follow‐up consultations were made after one month (phone call), three, six and, when possible, nine months. The one month consultation was aimed at recording any problems associated with the study, for example diet palatability, in addition to recording the medication and pruritus scores. Consultations after three, six and nine months consisted of general examination of the dogs, CADESI‐04, pVAS and medication score. At each time point the consumption of the diets was reported by the owners using a binary yes/no question with comments if needed. In addition, throughout the study owners were asked to evaluate the quality of the dogs' stools in order to assess how well the diet was being tolerated. Stool quality was assessed according to a five‐point scale, illustrated by picture (grade 5, hard; 4, well‐formed, easy to pick up; 3, well‐formed but soft; 2, very wet but not liquid; and 1, liquid diarrhoea).
Owners were permitted to withdraw their dogs from the study at any time and for any reason. They were encouraged to feed their dogs exclusively with the diet provided for at least three months. In the event of a dog being withdrawn, owners who were not satisfied with the efficacy of the diet were given the opportunity to swap to the test diet for the remainder of the study period. In this case the diet provided was renamed (code Pebble) to maintain blinding. Drop‐out reasons were recorded. Dogs maintained on any of the diets for a minimum of three months were considered for statistical analyses.
Diets
The study was double‐blinded and placebo‐controlled. Enrolled dogs were randomly allocated into one of the two study groups. One group of dogs received the test diet (code Flame) while the other group received a standard premium diet (placebo: code Ice). Both of the diets contained chicken as the protein source and were produced at a commercial manufacturing facility in France. The diets were dry kibble format (Figure 1), and were complete and balanced for dogs at maintenance energy requirement (95 kcal/kg0.75 NRC 2006). 13 A comparison of the key nutritional components of the two diets can be found in Table 2. The diets were packaged in identical neutral bags, differentiated only by their code names. Both diets were composed of standard pet food materials, the test diet was supplemented with turmeric and licorice extracts. The turmeric extract (Curcuma longa) was provided by Arjuna (Kerala, India) and contained 95% curcuminoids with enhanced bioavailability. The licorice extract (Glycyrrhiza glabra), supplied by Naturex (Avignon, France) was prepared from the root of the plant. The extract was standardised for glycyrrhizin concentration (12% w/w). The concentration of the omega‐6 fatty acid linoleic acid in the Flame diet was increased by replacing a proportion of poultry and pork fat in the Ice diet with soya oil and fish oil. The omega‐3 fatty acids were supplemented using the fish oil. As stated previously, neither the owners nor the investigators were informed as to the nature of the diet. Owners were instructed to feed only the study diets provided for the entirety of the study. When dogs were transitioning from their previous diet to the study diets, owners were advised to change gradually over six days (two days each of 75%:25%, 50%:50%, 25%:75%).
Figure 1.
Physical appearance of dry format diets.
As Pebble diet was the same as the Flame diet, it is not shown here.
Table 2.
Concentrations of key nutritional components of the two diets fed during the study. Details of the diets are given for the proximates and a group of other ingredients which differed significantly between the Test (Flame) and Control (Ice) diets
| Units | Flame | Ice | |
|---|---|---|---|
| Dry matter | % | 90.50 | 90.51 |
| Moisture | % | 9.50 | 9.49 |
| Protein | % | 22.54 | 22.51 |
| Fat | % | 13.97 | 13.99 |
| Ash | % | 6.90 | 6.89 |
| CFIB (crude fibre) | % | 2.90 | 2.90 |
| Linoleic acid | % | 3.90 | 2.20 |
| EPA+DHA | % | 0.54 | 0.01 |
| Vitamin E | mg/kg | 900.64 | 162.14 |
| Taurine | mg/kg | 4500 | 600 |
| Lutein | mg/kg | 5 | 0.74 |
| Curcuma extract | mg/kg | 300 | 0 |
| Licorice root extract | mg/kg | 200 | 0 |
EPA, eicosapentaenoic acid, DHA, docosahexaenoic acid.
Outcome measures
Drop‐out reasons and unexpected events were recorded. The percentages of dogs with >30% and >50% improvement of the clinical score were computed for both groups. Additionally, the number of dogs returning to normal (CADESI‐04 < 16, pVAS < 2.5 and medication score < 10) were recorded in both groups.
Statistical methods
Statistical analyses were performed using JMP v14 (JMP; Cary, NC, USA). Linear mixed models were used for evaluating the impact of diet, time and time‐by‐diet interactions on CADESI‐04, pVAS and medication score. Animal was modelled as random term.
Log‐transformation was used when necessary to meet the statistical assumptions of a linear mixed model (normally distributed residuals and homoscedasticity). Tukey's honestly significant difference (HSD) was used for the post hoc multiple comparisons. Level of significance was set at 5%. Medians (min–max) were provided for the studied parameters.
Results
Study population detail
Forty dogs were initially included and allocated to one of the two study groups, one to be fed the test diet Flame (n = 21) and the other to be fed the placebo diet Ice (n = 19). The male:female ratio was 21:19 and the mean age was 5.2 years (SD ±1.78). No age or sex difference was found between groups. Some breeds were over‐represented, in particular French bulldogs (n = 11), West Highland white terriers (n = 3), Labrador retrievers (n = 3) and American Staffordshire terriers (n = 3). Multiple dogs of the same breed were present in both groups.
At inclusion, the median CADESI‐04 scores in the Flame and Ice groups were 19 (11–59) and 18 (6–69), respectively. The median pruritus scores were 5.5 (2.8–6.8) and 4.6 (3–8.5) respectively. The medication scores were 20 (10–45) and 30 (5–60), respectively. There was no significant difference between the groups for any of the three parameters at inclusion.
Six dogs were not considered for subsequent analysis because they did not receive the diet for the required minimum of three months. Three were in the Flame group (one refused the diet, one developed persistent diarrhoea five weeks after diet transition, and one developed mammary tumours). Three were in the Ice group (one dog refused the diet, two were lost to follow‐up). Six owners elected to feed their dogs with the Pebble diet after three months of feeding (n = 5; four Flame, one Ice) or six months (n = 1, Ice).
One month data were available for 36 dogs (19 Flame, 17 Ice), while three month data were analysed for 32 dogs (17 Flame, 15 Ice). Six month analyses were made on 24 dogs (12 Flame, 12 Ice) and nine month analyses on 21 dogs (14 Flame, 7 Ice). There was no significant difference between the two diet groups for dropout frequency (P = 0.75).
Score comparisons
Pruritus (pVAS)
There was clear evidence of an improvement in the pruritus score for the Flame diet group after three months compared to the start of the study, the median score declining from a baseline value of 5.5 to 3.0 (ranges 3–7.6 at baseline and 0–6 at three months; P =0.0001). There was no significant difference between pVAS within the Flame group between 0 and six months (median 4.35 to 2.13), but the score reduction was significant by nine months from baseline 5.1 to 2.8 (3–6.3 and 1–3.8, respectively; P = 0.0043). Within the Ice group, no changes in pVAS were observed between any time points. Figure 2a shows the change in pVAS during the nine month study period. Variation in baseline pVAS was a consequence of cases leaving the study before each time point.
Figure 2.
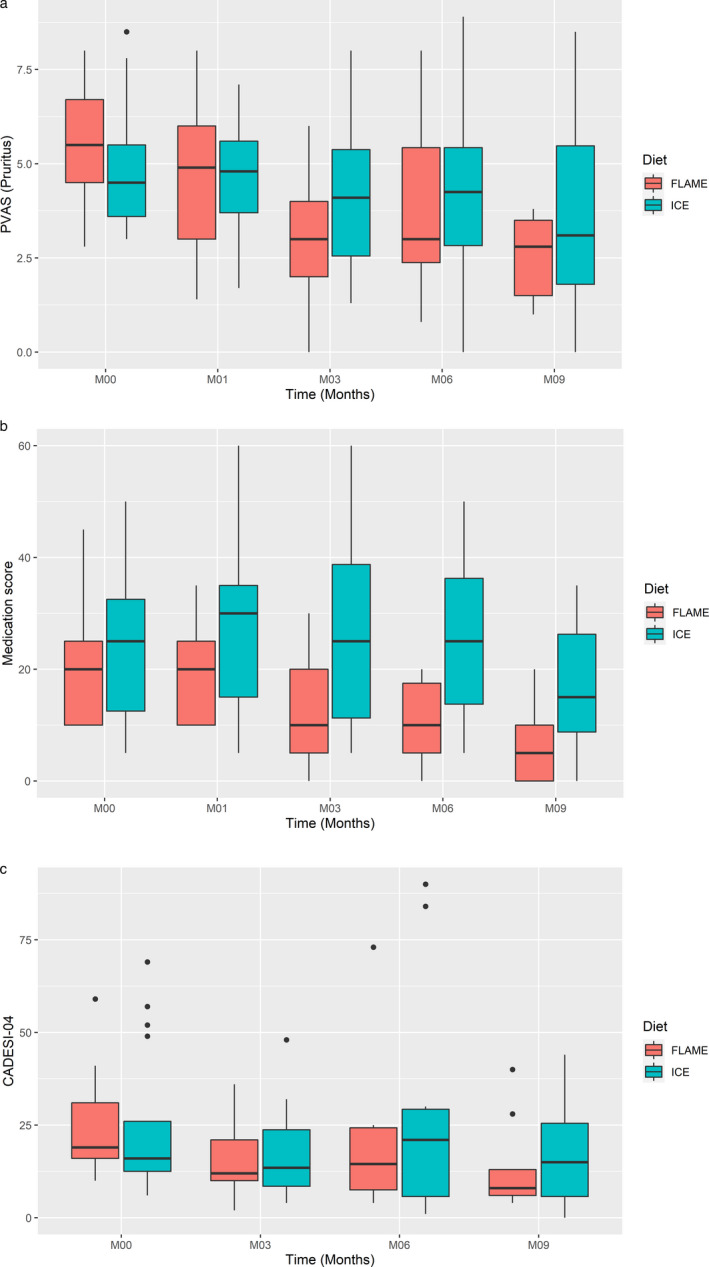
Standard boxplots (showing median with 25th to 75th percentiles) for clinical parameters assessed for two diet groups over the nine month feeding period.
Case numbers at each time point, Flame/Ice: time zero 21/19; three months 17/15; six months 12/12; nine months 14/7). Scores as a function of time and diet using: (a) Pruritus Visual Analog Score (pVAS); (b) medication; and (c) Canine Allergic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04).
Medication score
The medication score was used to determine whether and to what extent the diets were able to reduce the dependency on nondietary interventions in order to maintain an acceptable QoL. After three months, the median medication score had decreased from 20 to 10 (ranges 10–30 at baseline and 0–30 at three months; P = 0.058) for the Flame diet group. By contrast, in the Ice diet group the median medication score remained the same between baseline and three months at 25 (5–50 and 5–60, respectively; NSD). After six months the medication score showed a significant reduction in the Flame group (median 20 versus 10, ranges 10–30 and 0–20; P = 0.021), which was maintained up to nine months (20 versus 5, ranges 10–30 and 0–20; P = 0.018). In addition, the average medication scores at six and nine months were significantly lower than those at one month (P = 0.050 and P = 0.039, respectively). No significant changes in scores were observed for the Ice diet group between any of the time points. Figure 2b shows the medication score during the nine month study period.
CADESI‐04
There was a reduction in median CADESI‐04 score from 16 (10–59) at the beginning of the feeding period to 12 (2–36) after three months in the Flame diet group. The median score in the Flame group was 14.5 (4–73) at six months, and then 8 (4–40) after nine months. In the Ice group, the median CADESI‐04 after three months of feeding decreased from 18 (6–69) to 13.5 (4–48), increasing to 21 (1–84) after six months, before dropping again to 15 (0–44) by the end of the study. None of these figures showed a significant change from baseline CADESI‐04 values. Figure 2c shows CADESI‐04 scores for both diet groups during the nine month study period.
Score improvement
At each time point the number of dogs showing a > 30% and >50% improvement of the clinical scores were computed (Table 3). The analysis shows that for 11 of 11 data points (CADESI‐04, pVAS and medication score combined) there was a larger proportion of cases that improved >30% in the Flame group than in the Ice group. This also was seen for nine of 11 of the data at the >50% threshold. Table 3 also illustrates that, in the Flame diet group, there was typically a progressive increase in the proportion of dogs showing >30% and >50% improvement over time. The pattern was not repeated in the Ice diet group.
Table 3.
Improvement of the clinical score for both diet groups: the percentage of cases at each assessment point which showed either a > 30% or a > 50% improvement in pruritus (Visual Analog Score, pVAS), Canine Allergic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) or medication score
| Flame | One month | Three months | Six months | Nine months | Ice | One month | Three months | Six months | Nine months |
|---|---|---|---|---|---|---|---|---|---|
| pVAS | pVAS | ||||||||
| >30% | 47 | 82 | 46 | 64 | >30% | 17 | 36 | 31 | 36 |
| >50% | 21 | 29 | 46 | 60 | >50% | 0 | 21 | 8 | 18 |
| CADESI‐04 | CADESI‐04 | ||||||||
| >30% | 44 | 42 | 72 | >30% | 37 | 31 | 42 | ||
| >50% | 22 | 29 | 55 | >50% | 25 | 23 | 25 | ||
| Medication | Medication | ||||||||
| >30% | 10 | 53 | 64 | 82 | >30% | 6 | 7 | 8 | 18 |
| >50% | 5 | 29 | 36 | 73 | >50% | 6 | 7 | 8 | 9 |
Return to normal
Finally, it is of note that six dogs eventually achieved a normal score for the three parameters monitored in the Flame group, whereas just one dog attained this level of improvement in the Ice group.
Dogs transferred to Pebble diet
Owners elected to change the diet for six dogs. Data for these dogs were included in the analysis before but not after the change was performed. Dogs which transitioned to Pebble did not show a significant improvement: it should, however, be taken into account that four of these dogs were already on the same Flame diet and that, in the main study, it was shown that an improvement was present after three months. This suggests that stabilisation of the improvement could be expected, and was indeed the case for three of four dogs. One of the two dogs in the Ice diet group improved after switching to the Pebble diet.
Palatability and digestive tolerance
As mentioned in the Study population section above, one dog in each group refused the diet. Owners reported that the diets were well‐accepted by all other dogs. In the Flame group, one dog developed chronic diarrhoea several weeks after the diet transition. This dog was removed from the study; causality was not established. In this group, one dog had very dry faeces (grade 5), 10 had grade 4 (optimal according to the scale used) and six had grade 3 faeces. In the Ice diet group, the stools were considered grade 5 in two dogs, while 14 had grade 4 and one grade 3 faeces.
Discussion
This study demonstrates that a commercially‐prepared diet supplemented with a targeted mixture of antioxidants and with a modified fatty‐acid composition reduces pruritus and has a drug‐sparing effect on dogs with AD. There was modest itch improvement following one month of feeding the diet, although statistical significance was reached only after three months. The lesional scores showed improvement in both groups at certain times but statistical significance was not reached. This is probably because the study was somewhat underpowered for CADESI‐04, accentuated by the fact that only moderate to mild cases were included, thereby reducing the scope for improvement of CADESI‐04 score. The main purpose of the study was to achieve itch improvement with reduced requirement for medication, factors likely to positively impact the QoL of affected patients as well as their owners. 14
The clearly illustrated drug‐sparing effect also is of value because drugs used for the control of the clinical signs of cAD may be associated with adverse effects, compliance issues and, in some cases, considerable financial cost. The reduction of the medication score was evident after three months and became significant from six months onwards. It should be emphasized that AD is a life‐long disease with early onset of clinical signs. In this regard, any safe, easy‐to‐use intervention capable of decreasing the dose or dose‐duration of potentially problematic drugs is welcomed.
Both diets tested in this study were formulated to deliver high‐quality maintenance nutrition and were comparable in all aspects apart from a small group of ingredients included in the Flame test diet to target mechanistic aspects of canine atopic dermatitis. Amongst these, vitamin E and lutein provide antioxidant benefits to the skin. 6 , 15 In support of this activity, lutein has been found to concentrate in the skin following oral consumption in humans. 16 The essential omega‐6 polyunsaturated fatty acid linoleate is known to play a vital role in maintaining the structural integrity of the epidermal barrier, intrinsic as it is in the formation of certain ceramides. 17 What is more, linoleic acid intake has been shown to influence aspects of integument performance as well as skin lipid composition in dogs. 7 , 18 Taurine has been reported to contribute to keratinocyte hydration, thereby also contributing to barrier function. 19 The theoretical immunomodulatory role of the test diet is provided by three components: omega‐3 fatty acids, turmeric and licorice. Multiple studies have indicated a role for the omega‐3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the management of canine skin inflammation. 5 , 20 Turmeric is a source of phenolic compounds known as curcuminoids. These have significant anti‐inflammatory capacity, particularly in relation to cell‐mediated immunoreactivity. 21 The inclusion of licorice root extract could provide an immunomodulatory benefit. A constituent of licorice, the triterpenoid saponin glycyrrhizin, has demonstrated the ability to suppress interleukin (IL)‐4 levels and restore the immune balance of T helper (TH1/TH2) cells in a mouse allergy model. 22 In addition, it was shown that glycyrrhizin attenuates B‐cell production of allergen‐specific immunoglobulin (Ig)E and IgG1 and can act as a mast cell stabilizer. These all are activities which have potential value to influence the immune dysregulation underlying cAD. In combination, this group of functional ingredients appears to have value in the management of disease symptoms.
Previous studies have indicated the value of a dietary approach to the management of canine allergic skin disease. Success with respect to food‐derived allergies has been especially impressive. 23 , 24 In cases where the allergy is at least partly environmentally derived, results have been more variable. A recent review elegantly summarises much of the work to date. 25 A common issue highlighted is that many of the purported nutritional or nutraceutical‐based therapeutics still lack efficacy data derived from well‐designed, placebo‐controlled studies.
There was excellent acceptance and digestive tolerance of the diets used in the study. The test diet was refused by only one dog and one other developed chronic diarrhoea, although no association between the diet and the gastrointestinal signs was established. There was some improvement in all scores in the group of dogs using the control diet, although statistical significance was not reached. The moderate improvement in the control group could have been a consequence of the benefits of providing high‐quality nutrition to all participants.
It was notable that the main improvements in the test diet group were observed after three months. It is not surprising as other studies have shown that nutritional therapies can often take some time to build to maximal skin effects. 18 , 20 Classical drug administration would consequently still be important during the early phase of dietary treatment in dogs suffering from severe AD. It is possible, however, that the diet alone may be sufficient to adequately control the clinical signs of atopic dogs with mild or moderate clinical signs. Further studies are needed to confirm this potential benefit. The results from this study were encouraging over the nine month duration. The number of cases lost before each time point was not ideal, reducing the number of cases completing the full study. What is more, it may have been valuable to increase the study period to establish whether the diet could maintain the reduced medication requirement for an extended period. A further limitation of the study was inclusion of mild and moderate cases only. It would have been interesting to see if the diet also could contribute to the management of severely atopic dogs.
Conclusion
In summary, to the best of the authors' knowledge, this is the first time that a diet designed specifically to assist in the management of cAD has been shown to be efficacious in the framework of a double‐blinded, placebo‐controlled study. Importantly, it is seen that the QoL of atopic dogs could be improved using the dietary intervention and that the approach might mitigate against complications associated with drug therapy, providing significant benefit in a relatively short time frame.
Author contributions
Adrian Watson: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Validation, Writing‐original draft, Writing‐review & editing. Ana Rostaher: Data curation, Investigation, Methodology, Resources, Writing‐review & editing. Nina Fischer: Data curation, Investigation, Methodology, Resources, Writing‐review & editing. Claude Favrot: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing‐original draft, Writing‐review & editing.
Supporting information
Appendix 1: List of medications permitted for management of AD during the study
Acknowledgements
We would like to thank the following people for their expert contribution to the study:
Marie‐Christine Cadiergues, Nöelle Cochet‐Faivre, Claudia Nett‐Mettler, Didier Pin, Silvia Rüfenacht and Sébastien Viaud for clinical input, and Jeremy Laxalde for statistical guidance.
List of medications permitted for management of AD during the study
Prednisolone
Dexamethasone
Apoquel
Atopica
Cetirizine
Itraconazole
Cefalexin
Amoxicillin/Clavulanic acid
Baytril
Cortavance Spray
Malaseb Shampoo
Pyoderm Shampoo
Etiderm Shampoo
EasOtic
Ear drops containing dexamethasone and enrofloxacin
Surolan
Source of Funding: The work described was funded by Royal Canin SAS.
Conflicts of Interest: Adrian Watson is an employee of Royal Canin SAS.
References
- 1. Nuttall TJ, Marsella R, Rosenbaum MR et al. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. J Am Vet Med Assoc 2019; 254: 1,291–1,300. [DOI] [PubMed] [Google Scholar]
- 2. Olivry T, DeBoer DJ, Favrot C et al. Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet Res 2015; 11: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma S, Naura AS. Potential of phytochemicals as immune‐regulatory compounds in atopic diseases: a review. Biochem Pharmacol 2020; 173: 113,790. [DOI] [PubMed] [Google Scholar]
- 4. Magrone T, Jirillo E. Influence of polyphenols on allergic immune reactions: mechanisms of action. Proc Nutr Soc 2012; 71: 316–321. [DOI] [PubMed] [Google Scholar]
- 5. Bensignor E, Morgan DM, Nuttall T. Efficacy of an essential fatty acid‐enriched diet in managing canine atopic dermatitis: a randomized, single‐blinded, cross‐over study. Vet Dermatol 2008; 19: 156–162. [DOI] [PubMed] [Google Scholar]
- 6. Plevnik Kapun A, Salobir J, Levart A et al. Vitamin E supplementation in canine atopic dermatitis: improvement of clinical signs and effects on oxidative stress markers. Vet Rec 2014; 175: 560. [DOI] [PubMed] [Google Scholar]
- 7. Popa I, Watson AL, Solgadi A et al. Linoleate‐enriched diet increases both linoleic acid esterified to omega hydroxy very long chain fatty acids and free ceramides of canine stratum corneum without effect on protein‐bound ceramides and skin barrier function. Arch Dermatol Res 2018; 310: 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olivry T, DeBoer DJ, Favrot C et al. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet Dermatol 2010; 21: 233–248. [DOI] [PubMed] [Google Scholar]
- 9. Witzel‐Rollins A, Murphy M, Becvarova I et al. Non‐controlled, open‐label clinical trial to assess the effectiveness of a dietetic food on pruritus and dermatologic scoring in atopic dogs. BMC Vet Res 2019; 15: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Favrot C, Steffan J, Seewald W et al. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet Dermatol 2010; 21: 23–31. [DOI] [PubMed] [Google Scholar]
- 11. Hill PB, Lau P, Rybnicek J. Development of an owner‐assessed scale to measure the severity of pruritus in dogs. Vet Dermatol 2007; 18: 301–308. [DOI] [PubMed] [Google Scholar]
- 12. Fischer N, Tarpataki N, Leidi F et al. An open study on the efficacy of a recombinant Der f 2 (Dermatophagoides farinae) immunotherapy in atopic dogs in Hungary and Switzerland. Vet Dermatol 2018; 29: 337–e118. [DOI] [PubMed] [Google Scholar]
- 13. NRC (National Research Council) Energy . In: Nutrient Requirements of Dogs and Cats. The National Academies Press. Washington, D.C, USA: 2006; 22–48. [Google Scholar]
- 14. Linek M, Favrot C. Impact of canine atopic dermatitis on the health‐related quality of life of affected dogs and quality of life of their owners. Vet Dermatol 2010; 21: 456–462. [DOI] [PubMed] [Google Scholar]
- 15. Roberts RL, Green J, Lewis B. Lutein and zeaxanthin in eye and skin health. Clin Dermatol 2009; 27: 195–201. [DOI] [PubMed] [Google Scholar]
- 16. Obana A, Gohto Y, Nakazawa R et al. Effect of an antioxidant supplement containing high dose lutein and zeaxanthin on macular pigment and skin carotenoid levels. Sci Rep 2020; 10: 10,262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rabionet M, Gorgas K, Sandhoff R. Ceramide synthesis in the epidermis. Biochim Biophys Acta 2014; 1 841: 422–434. [DOI] [PubMed] [Google Scholar]
- 18. Marsh KA, Ruedisueli FL, Coe SL et al. Effects of zinc and linoleic acid supplementation on the skin and coat quality of dogs receiving a complete and balanced diet. Vet Dermatol 2000; 11: 277–284. [Google Scholar]
- 19. Janeke G, Siefken W, Carstensen S et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol 2003; 121: 354–361. [DOI] [PubMed] [Google Scholar]
- 20. Saevik BK, Bergvall K, Holm BR et al. A randomized, controlled study to evaluate the steroid sparing effect of essential fatty acid supplementation in the treatment of canine atopic dermatitis. Vet Dermatol 2004; 15: 137–145. [DOI] [PubMed] [Google Scholar]
- 21. Yadav VS, Mishra KP, Singh DP et al. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol 2005; 27: 485–497. [DOI] [PubMed] [Google Scholar]
- 22. Han S, Sun L, He F et al. Anti‐allergic activity of glycyrrhizic acid on IgE‐mediated allergic reaction by regulation of allergy‐related immune cells. Sci Rep 2017; 7: 7,222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olivry T, Bexley J, Mougeot I. Extensive protein hydrolyzation is indispensable to prevent IgE‐mediated poultry allergen recognition in dogs and cats. BMC Vet Res 2017; 13: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bizikova P, Olivry T. A randomized, double‐blinded crossover trial testing the benefit of two hydrolysed poultry‐based commercial diets for dogs with spontaneous pruritic chicken allergy. Vet Dermatol 2016; 27: 289–e70. [DOI] [PubMed] [Google Scholar]
- 25. Marchegiani A, Fruganti A, Spaterna A et al. Impact of nutritional supplementation on canine dermatological disorders. Vet Sci 2020; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: List of medications permitted for management of AD during the study


