Abstract
Background and purpose
Aneurysm wall enhancement (AWE) of intracranial aneurysms on magnetic resonance imaging has been described in previous studies as a surrogate marker of instability. With this study, an updated literature overview and summary risk estimates of the association between AWE and different specific outcomes (i.e., rupture, growth or symptomatic presentation) for both cross‐sectional and longitudinal studies are provided.
Methods
The PRISMA guideline was followed and a search was performed of PubMed and Embase to 1 January 2021 for studies that reported on AWE and aneurysm instability. In cross‐sectional studies, AWE was compared between patients with stable and unstable aneurysms. In longitudinal studies, AWE of stable aneurysms was assessed at baseline after which patients were followed longitudinally. Risk ratios were calculated for longitudinal studies, prevalence ratios for cross‐sectional studies and then the ratios were pooled in a random‐effects meta‐analysis. Also, the performance of AWE to differentiate between stable and unstable aneurysms was evaluated.
Results
Twelve studies were included with a total of 1761 aneurysms. In cross‐sectional studies, AWE was positively associated with rupture (prevalence ratio 11.47, 95% confidence interval [CI] 4.05–32.46) and growth or symptomatic presentation (prevalence ratio 4.62, 95% CI 2.85–7.49). Longitudinal studies demonstrated a positive association between AWE and growth or rupture (risk ratio 8.00, 95% CI 2.14–29.88). Assessment of the performance of AWE showed high sensitivities, mixed specificities, low positive predictive values and high negative predictive values.
Conclusions
Although AWE is positively associated with aneurysm instability, current evidence mostly supports the use of its absence as a surrogate marker of aneurysm stability.
Keywords: aneurysm, gadolinium, inflammation, magnetic resonance angiography, risk assessment
This meta‐analysis demonstrates that, although aneurysm wall enhancement (AWE) is positively associated with aneurysm instability, current evidence mostly supports the use of its absence as a surrogate marker of aneurysm stability. Extensive prospective studies are warranted to determine the predictive potential of AWE.
INTRODUCTION
Rupture of an intracranial aneurysm causes a subarachnoid hemorrhage, which is a significant health problem due to its high associated morbidity and mortality rates [1, 2]. The increased utilization of brain imaging has resulted in a consistent increase of incidentally discovered unruptured intracranial aneurysms, warranting adequate risk assessment to counsel the risk of future rupture [3, 4]. Current guidelines advocate elective treatment of aneurysms with a high estimated risk of rupture [5]. However, current prediction models like the PHASES score (5‐year estimated risk of rupture) have significant shortcomings [6]. For example, the majority of ruptured aneurysms have low PHASES scores and would presumably not have received treatment if evaluated before the occurrence of rupture [7]. When the estimated risk of rupture is low, it is common practice to monitor for aneurysm growth using serial follow‐up imaging [8]. Aneurysm growth during follow‐up is frequently used in studies as a proxy of aneurysm rupture because it substantially increases the risk of rupture compared to stable aneurysms, with a yearly rupture rate of 3.1% versus 0.1%, respectively [9]. Hence, constant effort is needed to optimize risk assessment methods.
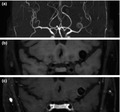
Magnetic resonance angiography (MRA) is an established, noninvasive imaging method frequently used for follow‐up imaging of unruptured intracranial aneurysms. Although a time‐of‐flight sequence, averting the need for intravenous gadolinium, is most commonly used to assess aneurysm growth, there is an increasing interest in the use of additional contrast‐enhanced magnetic resonance imaging (MRI) sequences (Figure 1) [10, 11]. In particular, multiple studies indicate that gadolinium enhancement of the aneurysm vessel wall on MRI may be a surrogate marker of aneurysm instability since histopathological studies demonstrate a correlation with inflammatory changes, a critical process behind aneurysm progression [12, 13, 14, 15, 16]. The evaluation of aneurysm wall enhancement (AWE) can thereby potentially improve the current risk assessment of incidentally found intracranial aneurysms. However, it is currently unclear how AWE performs for different types of aneurysm instability (i.e., growth, rupture or symptomatic presentation) and how the estimates differ between cross‐sectional and longitudinal studies. To answer this question, both cross‐sectional and longitudinal studies were reviewed comparing the presence of AWE between stable aneurysms and those with growth, rupture or symptomatic presentation. Additionally, the performance of AWE was assessed as a predictor of aneurysm instability during follow‐up.
FIGURE 1.
Example of aneurysm wall enhancement in a patient with a left middle cerebral artery aneurysm. All images are coronal projections and were made during the same scanning session. (a) Coronal maximum intensity projection of 3D time‐of‐flight MRA. High spatial resolution, fat‐saturated 3D T1 SPACE black blood MRI before (b) and after (c) administration of gadolinium, showing circumferential aneurysm wall enhancement
METHODS
This systematic review and meta‐analysis was conducted according to the Preferred Reporting in Systematic Reviews and Meta‐Analysis (PRISMA) statement [17].
Search strategy and eligibility criteria
A search strategy for PubMed and Embase was constructed with the assistance of a medical information specialist (Table S1). Studies published until 1 January 2021 that reported on adults with intracranial aneurysms and evaluated the association between AWE on MRI and stable versus unstable aneurysms were included. Unstable aneurysms were defined as those with growth, rupture or symptomatic presentation (e.g., cranial nerve palsy related to the aneurysm). Both cross‐sectional and longitudinal studies were included. The study types differ concerning the moment at which vessel wall imaging is performed. In the cross‐sectional studies, imaging was compared between patients with stable and unstable aneurysms (as defined previously). Vessel wall imaging was performed at the same time as the assessment of aneurysm stability. In longitudinal studies, imaging was performed at the start of follow‐up of stable aneurysms only, enabling the assessment of AWE as a predictor of instability during follow‐up. Conference abstracts were excluded as were non‐original studies (letters, editorials or reviews), studies describing non‐saccular aneurysms, written in a language other than English, including 10 or fewer patients, and those using other contrast agents than gadolinium. Reference lists of all included studies, as well as relevant reviews, were screened for potentially missed studies. When multiple studies were published (partially) based on the same patient cohort, and they described similar outcomes, the study describing the largest cohort was chosen.
Study selection
The first author (RM) screened titles and abstracts after removal of duplicates. Full texts of potentially relevant articles were independently evaluated by two reviewers (RM and MWA) for final inclusion in the systematic review and meta‐analysis. Disagreements between the two reviewers were solved by consensus or by consulting a third reviewer.
Data extraction and quality assessment
A data extraction file was constructed to systematically extract data regarding patient, aneurysm, imaging and study characteristics. Data extraction was done independently by two reviewers (RM and MWA). Study quality was assessed using the Methodological Index for Non‐randomized Studies (MINORS) for all included studies [18]. This tool encompasses 12 items relating to potential areas of bias. Each item receives a score from 0 to 2, resulting in overall scores ranging from 0 to 24. A high score indicates a low risk of bias, whereas a low score indicates a high risk of bias. The assessment was performed independently by two reviewers (RM and MWA). Disagreements were solved between the two authors by consensus, as was the case for potential exclusion of a study based on the MINORS quality assessment.
Statistical analyses
If studies described different enhancement patterns, such as circumferential or partial, this outcome was dichotomized to the presence or absence of any pattern of enhancement to facilitate data synthesis. Based on study design and outcome, studies were divided into one of three categories: (i) cross‐sectional studies with rupture as the outcome, (ii) cross‐sectional studies with growth or symptomatic presentation as the outcome and (iii) longitudinal studies with growth or rupture as the outcome. When a study described multiple outcomes, patients were divided over the categories based on the corresponding outcome of interest. The risk ratios (RRs) for longitudinal studies and prevalence ratios (PRs) for cross‐sectional studies were calculated, with corresponding 95% confidence intervals (CIs), for the association between AWE and outcomes. For each category, the ratios were then pooled in a random‐effects (DerSimonian and Laird) meta‐analysis, with a continuity correction of 0.5 for cells with a frequency of zero. Sensitivity, specificity, positive and negative predictive values were also calculated, with true positives representing unstable aneurysms with AWE and true negatives stable aneurysms without AWE.
Heterogeneity amongst studies was tested for using I 2 and χ 2 and the between‐study heterogeneity was classified as moderate (I 2 ≥ 30%), substantial (I 2 ≥ 50%) or considerable (I 2 ≥ 75%) [19, 20]. If at least substantial heterogeneity was identified or the p value of χ 2 was <0.05, the potential source of heterogeneity was explored by conducting subgroup analysis (where applicable) based on the following a priori defined variables: AWE assessment (qualitative, quantitative), aneurysm size (<7 mm, ≥7 mm), geographical region (Japan, Finland, other), outcome (growth, symptomatic, growth and symptomatic). Geographical region groups (Japan, Finland, other) are based on the groups used in the PHASES model, which is an estimator of the 5‐year rupture risk of unruptured aneurysms [6]. All statistical analysis was performed using R (version 4.0.2) with the meta and metafor packages [21]. p values <0.05 were considered statistically significant. Contour‐enhanced funnel plots were used and Egger's test was performed to evaluate the risk of publication bias if at least 10 studies were included [22] A p value threshold of <0.10 was used as an indicator of potential publication bias [23]. Data are available from the authors upon request.
Results
Study characteristics
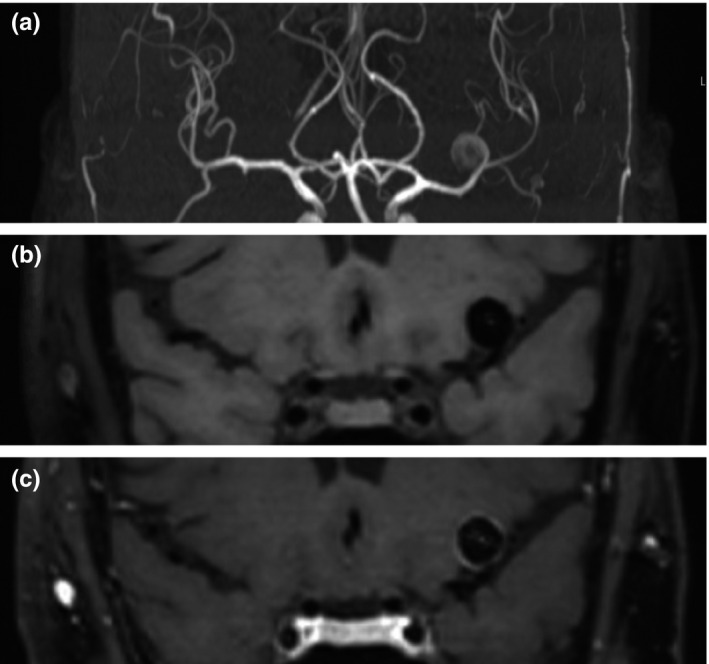
The literature search resulted in a total of 1206 unique records. Twenty‐one full‐text articles were assessed for eligibility after the screening of titles and abstracts, of which a total of 12 studies met the inclusion and exclusion criteria (Figure 2) [24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35]. Ten studies had a cross‐sectional design and performed vessel wall imaging in a total of 1551 aneurysms, of which 454 were unstable (Table 1). Two studies had a longitudinal design and performed vessel wall imaging in 210 aneurysms, of which 16 became unstable during follow‐up. Two studies primarily performed a quantitative assessment of AWE [27, 30]. For these studies, the number of stable and unstable aneurysms with and without AWE were calculated based on the sensitivity and specificity associated with the used cutoff value. The cutoff value represented the point at which the used quantitative assessment method most optimally differentiated between stable and unstable aneurysms, as determined via the receiver operating curve. The discriminative performance belonging to various AWE definitions used by the different studies is listed in Table S5. The different definitions of symptomatic and growing aneurysms are listed in Table S2. MRI field strengths ranged from 1.5 T to 7 T and some differences in scan protocols were observed (Table 2). One of the nine excluded articles did not describe the contrast agent used for vessel wall imaging [36]. It was not possible to verify the details by contacting the authors, leading to the final decision to exclude the study. The risk of bias of individual studies based on the MINORS quality assessment was considered low, and none was excluded (Table S3).
FIGURE 2.
PRISMA flow diagram
TABLE 1.
Study characteristics
| Study | Recruiting period | Country | Study type | Outcome | Follow‐up duration | Total patients | Mean age ± SD | Percent female | Total aneurysms | Stable aneurysms | Unstable aneurysms | Mean aneurysm size ± SD (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nagahata et al. [29] | September 2011–July 2013 | Japan | Cross‐sectional | Rupture | N/A | 117 | 68 ± 12 | 67 | 144 | 83 | 61 | Median 5 (IQR 3)/4 (IQR 3)b |
| Wang et al. [26] | February 2016–April 2017 | China | Cross‐sectional | Rupture | N/A | 91 | 57 ± 10 | 68 | 106 | 87 | 19 | 7.5 ± 6.3 |
| Omodaka et al. [27] | December 2013–May 2015 | Japan | Cross‐sectional | Rupture | N/A | 82 | 62 ± 10 | 77 | 104 | 76 | 28 | 5.4 ± 2.2 |
| Matsushige et al. [28] | Apr.il 2017–December 2018 | Japan | Cross‐sectional | Growth | N/A | NR | 73 ± 10 | 78 | 60 | 33 | 27 | 5.3 ± (SE 0.3) |
| Edjlali et al. [35] | November 2012–January 2016 | France | Cross‐sectional | Growth, rupture, symptomatic | N/A | 263 | 59 ± 13 | 67 | 333 | 276 | 57 | Median 5 (IQR 4) |
| Omodaka et al. [30] | March 2014–March 2017 | Japan | Cross‐sectional | Growth, symptomatic | N/A | NR | Median 70 (range 34–81)/65 (range 41–80)a | 78 | 95 | 69 | 26 | Median 5.5 (range 3.2–11.4)/4.9 (range 3.0–7.5)e |
| Zhu et al. [32] | June 2014–July 2018 | China | Cross‐sectional | Symptomatic | N/A | 139 | 58 ± 11 | 60 | 166 | 87 | 79 | 8.6 ± 5.4 |
| Fu et al. [34] | October 2014–October 2019 | China | Cross‐sectional | Symptomatic | N/A | 267 | 58 ± 11 | 59 | 341 | 248 | 93 | Median 5.4 (IQR 3.5–8.4) |
| Wang et al. [31] | February 2016 – February 2018 | China | Cross‐sectional | Symptomatic | N/A | 80 | 57 ± 10 | 56 | 89 | 58 | 31 | 7.5 ± 6.8 |
| Zhong et al. [33] | June 2017–May 2019 | China | Cross‐sectional | Symptomatic | N/A | 100 | Median 59 (range 35–82) | 68 | 113 | 80 | 33 | Median 5.98 (IQR 4.33–9.7) |
| Gariel et al. [24] | 2012–2018 | France | Longitudinal | Growth | 24 months (IQR 12–35) | 129 | 58 ± 13 | 69 | 145 | 133 | 12 | 4.1 (range 3.3–5.9) |
| Vergouwen et al. [25] | February 2014 – October 2015 | Netherlands | Longitudinal | Growth, rupture | 27 months (IQR 20–31) | 57 | 50 (47–53)/60 (55–68)/55 (52–61)d | 65 | 65 | 61 | 4 |
1–2.9: 17 3–4.9: 19 5–6.9: 20 ≥7: 15c |
Abbreviations: IQR, interquartile range; N/A, not applicable; NR, not reported; SE, standard error. Studies are arranged based on study design and outcome.
aDenoted as median age for unstable/stable aneurysms.
bDenoted as aneurysm size for ruptured/unruptured aneurysms.
cDenoted as size category: count.
dAneurysm based and denoted as median (IQR) for unstable aneurysms with enhancement/stable aneurysms with enhancement/aneurysms without enhancement.
eDenoted as aneurysm size for unstable/stable aneurysms.
TABLE 2.
Vessel wall imaging characteristics
| Study | Brand | Field strength | Contrast agent | Sequence | TR/TE (ms) | FOV (mm) | Matrix | Resolution (mm) | Scan duration per sequence (min) | Post‐contrast scan timinga |
|---|---|---|---|---|---|---|---|---|---|---|
| Matsushige (2019) [28] | GE Healthcare | 1.5 T | 0.1 mmol/kg Gd‐BT‐DO3A | 3D T1 (CUBE) FSE | 550/10 | 240 x 240 | 256 x 256 | 0.9 x 0.9 x 1.0 | 03:39 | NR |
| Edjlali (2018) [35] | GE Healthcare | 3 T | 10 ml gadoteric acid | 3D T1 (CUBE) FSE | 600/11.5 | 230 x 230 x 160 |
288 x 288 x 160 Interpolated to 512 x 512 x 320 |
0.9 x 0.9 x 1 Interpolated to 0.45 x 0.4 5 x 0.5 |
04:16 | 1 min |
| Omodaka (2016) [27] | GE Healthcare | 1.5 T or 3 T | 0.1 mmol/kg gadodiamide or gadopentetate dimeglumine | 3D T1 FSE |
1.5 T protocol: 500/minimum 3 T protocol: 600/minimum |
256 | 256 x 256 x 176 | 0.5 x 0.5 x 0.5 |
1.5 T protocol: 03:36 3 T protocol: 03:41 |
5 min |
| Wang (2018) [26] | GE Healthcare | 3 T | 20 ml Gd‐BOPTA | 2D T1 FSE | 580/11 | 160 x 160 | 384 x 224 | 0.4 x 0.7 x 1.2 | 03:08 | NR |
| Nagahata (2016) [29] | Philips | 3 T | 0.1 mmol/kg meglumine gadoterate | T1 MSDE−3D‐TSE | Protocol 1: 425/13 protocol 2: 350/6.3 | 230 x 230 |
Protocol 1: 368 x 327 protocol 2: 340 x 337 |
Protocol 1: 0.63 x 0.7 x 1.4 protocol 2: 0.68 x 0.68 x 0.7 |
Protocol 1: 03:61 protocol 2: 07:45 |
NR |
| Gariel (2020) [24] | GE Healthcare | 3 T | 10 ml gadoteric acid | 3D T1 FSE | 600/11.5 | 230 x 230 x 160 |
288 x 288 x 160 Interpolated to 512 x 512 x 320 |
0.9 x 0.9 x 1 Interpolated to 0.45 x 0.45 x 0.5 |
04:16 | NR |
| Vergouwen (2019) [25] | Philips | 3 T or 7 T | 0.1 mmol/kg gadobutrol | 3D T1 FSE |
3 T protocol: 1500/36 7 T protocol: 3952/37 |
3 T protocol: 200 x 166 x 45 7 T protocol: 250 x 250 x 190 |
NR |
3 T protocol: 0.5 x 0.5 x 0.5 7 T protocol: 0.8 x 0.8 x 0.8 |
3 T protocol: 08:03 7 T protocol: 11:00 |
NR |
| Omodaka (2019) [30] | GE Healthcare | 1.5 T or 3 T | 0.1 mmol/kg gadopentetate dimeglumine | 3D T1 FSE |
1.5 T protocol: 500/minimum 3 T protocol: 600/minimum |
256 | 256 x 256 x 176 | 0.5 x 0.5 x 0.5 |
1.5 T protocol: 03:36 3 T protocol: 03:41 |
5 min |
| Fu (2021) [34] | Siemens Healthcare | 3 T | 0.1 mmol/kg gadopentetate chelate | 2D or 3D T1 FSE |
2D protocol: 430/10 3D protocol: 800/14 |
2D protocol: 140 x 81 3D protocol: 192 x 192 |
2D protocol: 256 x 166 3D protocol: NR |
2D protocol: 0.55 x 0.53 3D protocol: 0.6 x 0.6 x 0.6 |
2D protocol: 4:55 to 8:34 3D protocol: 7:36 |
NR |
| Wang (2019) [31, 38] | GE Healthcare | 3T | 0.1 mmol/kg Gd‐BOPTA | T1 | 580/11 | 160 x 160 | 384 x 224 | 0.4 x 0.7 x 1.2 | 03:08 | NR |
| Zhu (2020) [32] | Siemens Healthcare | 3T | 0.1 mmol/kg Gd‐DTPA |
Protocol 1: 2D T1 FSE protocol 2: 3D T1 FSE (SPACE) |
Protocol 1: 581/20 protocol 2: 900/5.6 |
Protocol 1: 100 x 100 protocol 2: 160 x 160 |
NR |
Protocol 1: 0.4 x 0.4 x 1.5 protocol 2: 0.5 x 0.5 x 0.5 |
Protocol 1: 05:00 protocol 2: 08:00 |
NR |
| Zhong (2020) [33] | Siemens Healthcare | 3T | 0.1 mmol/kg Gd‐DTPA | T1 FSE | 590/15 | 130 x 30 | 256 x 256 | 0.4 x 0.1 x 2.0 | 05:00 | 1 min |
Abbreviations: AWE, aneurysm wall enhancement; FOV, field of view; FSE, fast spin echo; NR, not reported; TR/TE, repetition time/echo time.
aApproximate duration between injection of gadolinium and vessel wall imaging.
Association between AWE and rupture
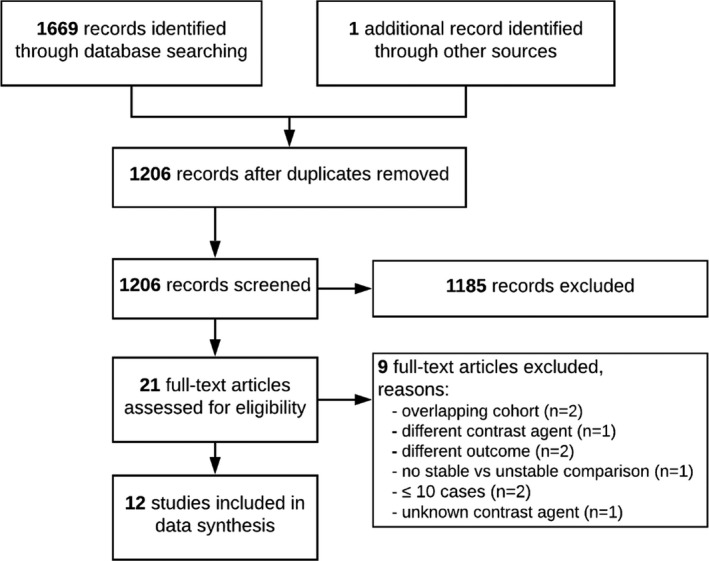
A total of four cross‐sectional studies comparing patients with ruptured and unruptured aneurysms were identified. The studies describe 656 aneurysms with 134 events. Pooling of the data resulted in a PR of 11.47 (95% CI 4.05–32.46), with substantial heterogeneity between the individual studies (I2 = 53%, p = 0.09), as depicted in Figure 3a. Subgroup analysis could not be performed adequately due to the low number of studies per subgroup. The discriminative performance is depicted in Table 3.
FIGURE 3.
Meta‐analysis of the association between AWE and aneurysm instability. (a) Cross‐sectional studies with aneurysm rupture as the outcome. (b) Cross‐sectional studies with aneurysm growth or symptomatic presentation as the outcome. (c) Longitudinal studies with aneurysm growth or rupture as the outcome. The size of the squares is proportional to the weight of each study, with the horizontal lines representing the 95% CI of the RR or PR estimate. The diamonds represent the pooled estimate with 95% CI. Studies are arranged based on effect size
TABLE 3.
Discriminative performance of aneurysm wall enhancement
| Study | Design | Outcome | AWE groups | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Reproducibility | |
|---|---|---|---|---|---|---|---|---|---|
| Inter‐rater | Intra‐rater | ||||||||
| Edjlali et al. [35] | CS | Rupture | No/focal thick/ circumferential thin/circumferential thick (>1 mm) | 88 (70–98) | 62 (56–67) | 18 (12–26) | 98 (95–100) | NR | NR |
| Omodaka et al. [27] | CS | Rupture | Quantitative: CRstalk (cutoff value 0.64) | 75 (55–89) | 83 (73–91) | 62 (44–78) | 90 (80–96) | ICC: 0.98 (0.95–0.99) | ICC: 0.98 (0.94–0.99) |
| Wang et al. [26] | CS | Rupture | No/partial/entire | 100 (82–100) | 26 (18–37) | 23 (14–33) | 100 (85–100) | NR | NR |
| Nagahata et al. [29] | CS | Rupture | No/faint/stronga | 98 (91–100) | 82 (72–90) | 80 (69–88) | 99 (92–100) | NR | NR |
| Matsushige et al. [28] | CS | Growth | Present/absent | 46 (27–67) | 85 (68–67) | 71 (44–90) | 67 (50–80) | 0.93 (0.80–1.00) | NR |
| Edjlali et al. [35] | CS | Growth + symptomatic | No/focal thick/ circumferential thin/circumferential thick (>1 mm) | 71 (52–86) | 62 (56–67) | 17 (11–25) | 95 (91–98) | 0.82 (0.67–0.99) | 0.87 (0.74–1.0) |
| Fu et al. [34] | CS | Symptomatic | No/focal/circumferential | 82 (72–89) | 69 (63–75) | 50 (41–58) | 91 (86–95) | NR | NR |
| Wang et al. [31] | CS | Symptomatic | No/partial/entire | 100 (89–100) | 43 (30–57) | 48 (36–61) | 100 (86–100) | NR | NR |
| Zhu et al. [32] | CS | Symptomatic | Grade 0/1/2b | 94 (86–98) | 67 (56–76) | 72 (62–80) | 92 (82–97) | NR | NR |
| Zhong et al. [33] | CS | Symptomatic | No/partial/circumferential | 76 (58–89) | 69 (57–79) | 50 (36–64) | 87 (77–94) | NR | NR |
| Omodaka et al. [30] | CS | Growth + symptomatic | Quantitative: CRstalk (cutoff value 0.39) | 88 (70–98) | 62 (50–74) | 47 (33–62) | 93 (82–99) | NR | NR |
| Vergouwen et al. [25] | L | Growth + rupture | Present/absent | 100 (40–100) | 75 (63–86) | 21 (6–46) | 100 (92–100) | NR | NR |
| Gariel et al. [24] | L | Growth | Present/absent | 83 (52–98) | 59 (50–67) | 15 (8–26) | 97 (91–100) | NR | NR |
The data from the different AWE groups were used in such a way that the discriminative performance parameters reflect the performance of “any enhancement”.
Reproducibility values are kappa values unless stated otherwise, and only reported if relating to “any enhancement”. Studies are arranged based on study design and outcome.
Data in parentheses are 95% confidence intervals.
Abbreviations: AWE, aneurysm wall enhancement; CS, cross‐sectional; ICC, intraclass correlation coefficient; L, longitudinal; NR, not reported.
a’Strong’ is definite enhancement equal to choroid plexus or venous plexus and ‘faint’ is increased wall signal intensity compared to precontrast scan.
bGrade 0 is no enhancement, grade 1 is enhancement more than normal vessel wall, grade 2 is enhancement greater than pituitary infundibulum.
Association between AWE and growth or symptomatic presentation
A total of seven cross‐sectional studies comparing patients with growing or symptomatic aneurysms with stable unruptured aneurysms were identified. The studies describe a total of 1171 aneurysms with 320 events. Pooling of the data resulted in a PR of 4.62 (95% CI 2.85–7.49), with substantial heterogeneity across studies (I2 = 63%, p = 0.01), as depicted in Figure 3b. Subgroup analysis showed that heterogeneity could be explained by aneurysm size, geographical region and study outcome (Table S4). The PR for growth only (two studies; PR 3.65; 95% CI 0.92–14.38; I2 = 80%) was lower compared to symptomatic only (four studies; PR 5.76; 95% CI 3.74–8.86; I2 = 20%), although the wide confidence intervals limit comparability. The discriminative performance is depicted in Table 3.
Association between AWE and rupture or growth during follow‐up
Two studies included in this meta‐analysis had a longitudinal study design with vessel wall imaging at baseline. The first study followed a total of 57 patients with 65 aneurysms during a median period of 27 months (interquartile range 20–31 months) and recorded aneurysm growth or rupture [25]. Two aneurysms enlarged and two aneurysms ruptured during follow‐up. The second study followed a total of 129 patients with 145 aneurysms during a median period of 24 months (interquartile range 12–35 months) and recorded aneurysm growth [24]. Twelve aneurysms enlarged during follow‐up. Pooling of the data resulted in an RR estimate of 8.00 (95% CI 2.14–29.88), without signs of heterogeneity (I2 = 0%, p = 0.45), as depicted in Figure 3c. The discriminative performance is depicted in Table 3.
Publication bias
With fewer than 10 identified studies per category, it was not possible to adequately assess possible publication bias. Therefore funnel plots and Egger's test were not used.
DISCUSSION
In this systematic review and meta‐analysis, the aim was to evaluate the association between AWE and MRI and aneurysm instability. Our study resulted in three main findings. First, a positive association between the presence of AWE and aneurysm instability was identified. The association was stronger for rupture than it was for growth or symptomatic presentation in cross‐sectional studies, although both were significant. A possible explanation could be that aneurysm rupture may lead to increased inflammatory changes within or even outside the aneurysm wall, leading to more frequent detection of AWE. Secondly, its discriminative performance currently favors the use of AWE absence as a marker of stability, rather than the presence of AWE as a marker of instability. AWE relatively frequently occurs in stable aneurysms leading to generally low positive predictive values. In contrast, the absence of AWE rarely occurs in growing, symptomatic or ruptured aneurysms. In particular, only two cohorts report negative predictive values below 90%, indicating that AWE absence is associated with an unlikely risk of aneurysm instability. Thirdly, preliminary results from longitudinal studies suggest that AWE may improve the current risk assessment of unruptured intracranial aneurysms. In particular, it seems that the absence of AWE is associated with a low risk of aneurysm progression in the first 2 years of follow‐up. The absence of AWE may thereby potentially be a highly clinically relevant finding, not only because it may lead to the need for less frequent follow‐up imaging and elective treatment, but it may thereby also reduce healthcare spending and enhance practitioners' ability to relieve patients' psychological burden associated with the presence of an unruptured aneurysm.
Earlier systematic reviews also identified a positive association between AWE and aneurysm instability [37, 38, 39]. However, there are important differences based on which this study adds to the current literature. Recently published data from additional cohort studies lead to the inclusion of more than three times as many aneurysms. Also, contrary to early reviews, both cross‐sectional and longitudinal studies were included, and separate analyses were performed based on outcome. Overall, a broad and contemporary overview of the current evidence for the association between AWE and aneurysm instability was thereby provided.
An important question regarding the use of AWE remains the underlying mechanism behind AWE. It is known that inflammatory changes in the aneurysm vessel wall play a significant role in the formation and progression of intracranial aneurysms [40, 41]. The hypothesis is that AWE occurs in these regions of inflammation. Studies comparing AWE with histopathological findings after elective clipping demonstrated an association between AWE and inflammatory cell infiltration, vasa vasorum and neovascularization, as well as atherosclerotic changes in the aneurysm vessel wall [12, 13, 14, 15, 16]. Rates of AWE were lower amongst patients taking the anti‐inflammatory drug acetylsalicylic acid [42]. Whether acetylsalicylic acid also truly reduces the risk of aneurysm growth or rupture is currently being investigated, together with intensive blood pressure treatment, in a randomized controlled trial (PROTECT‐U trial) [43]. Additionally, a recent study also found an association between atherosclerotic proteins (lipoprotein(a)) and AWE [44]. Here, blood samples were collected from the lumen and parent artery of 19 unruptured aneurysms. The difference in lipoprotein(a) concentration between the aneurysm sac and parent artery was significantly higher in aneurysms with AWE compared to those without AWE. However, it is currently unclear if lipoprotein(a) causally influences the risk of subarachnoid hemorrhage [45]. Hemodynamic studies have shown an association between AWE and regions of low wall shear stress, which in turn has been associated with proinflammatory changes and instability [46, 47, 48, 49]. A potential pitfall of vessel wall imaging is the possibility of misinterpreting incomplete signal suppression due to recirculating or slow flow within the aneurysm dome for true wall enhancement [10, 50]. Thus, AWE seems to reflect underlying aneurysm wall changes associated with aneurysm instability. However, this comes from studies with small sample sizes, warranting more extensive replication studies.
Limitations and future perspectives
The main limitation of this study comes from the various methodological differences between studies. For example, multiple MRI scanners and field strengths were used, in addition to some differences in scan protocols. Although the precise implications are currently not known, it is a realistic possibility that the differences in field strengths and scan protocols influence the capacity to detect AWE. For example, the frequent lack of advanced blood suppression techniques (e.g., MSDE or DANTE) may lead to more frequent false‐positive detections of AWE due to blood flow mimicking AWE [51]. However, false‐positive AWE due to an imaging artifact should be independent of aneurysm stability. Therefore, considering that positive AWE infrequently occurs amongst stable aneurysms, the extent of this particular issue may be limited. Also, only a few articles mention the time between gadolinium injection and the post‐contrast vessel wall imaging. These are pitfalls of current AWE research, which should be addressed in future studies. Another limitation of current AWE research and studies concerning aneurysm growth is the various definitions used for growth. Although it is known that inter‐rater agreement is generally high for 2D aneurysm size measurements on an individual time of flight MRA, the reliability drops significantly for the detection of growth and the smallest detectable change in size is at least 1 mm [52]. Also, the interval between follow‐up imaging for detecting growth is often neglected, assuming a stable growth rate and perhaps missing relevant information regarding the growth pattern. Now that more advanced imaging assessment methods are becoming available, the debate should be whether conventional 2D aneurysm measurements, such as maximum diameter, are still sufficient. Instead, volumetric measurements, with the support of automatic and semi‐automatic segmentation software, may provide a more reliable standardized approach, richer information and higher sensitivity [52, 53]. Concerning the field strength of 1.5 T versus 3 T, preference should go to 3 T due to its higher signal‐to‐noise ratio, which is advantageous for intracranial vessel wall imaging [10].
Several studies further classify AWE into different categories, such as circumferential AWE, partial AWE or different thicknesses of enhancement. To facilitate data synthesis for this meta‐analysis, these different categories were not incorporated. Instead, differences between absence and any enhancement was looked at, possibly underestimating the discriminative performance. Also, some studies used quantitative cutoff values for AWE. Since these cutoff values are calculated based on data of their own cohorts, this may lead to an overestimation of the relationship between AWE and instability. Selection bias may also influence the study results. For example, only aneurysms with a conservative treatment recommendation, which are generally low‐risk aneurysms, were included in longitudinal studies. In cross‐sectional studies, vessel wall imaging was performed at the time of instability, leaving it unknown whether enhancement was present before the occurrence of instability. It should also be mentioned that, whilst it is frequently accepted to describe symptomatic aneurysms as unstable, most studies did not investigate different symptoms. This may therefore represent a future direction of research. Finally, adequate assessment of publication bias was not possible due to the identification of fewer than 10 studies per category. Therefore, it is recommended that overall risk estimates are interpreted with some caution.
Regarding future studies, evaluating the predictive ability of AWE for aneurysm growth or rupture is to be encouraged. Ideally, vessel wall imaging could be used to identify aneurysms at high risk of future instability since this would enable improved selection for elective treatment. However, this would require better positive predictive values than currently demonstrated by longitudinal studies. A large prospective multicenter study is currently ongoing, which may provide more clarity regarding this topic [54]. Furthermore, the use of advanced imaging modalities, such as positron emission tomography scanning, specifically focused on visualizing aneurysm wall inflammation may lead to identifying unstable aneurysms with a higher degree of certainty. In addition, standardized quantitative evaluation of AWE may improve discriminative performance after the identification of an optimal cutoff value. Also, it may facilitate accurate detection of AWE changes during follow‐up, which may precede aneurysm growth [24]. Indeed, a reliable method should be available. A recent study assessed different approaches for quantitively evaluating AWE and showed that the pituitary‐stalk to aneurysm contrast ratio (CRstalk) provided the most reliable measurements [55]. The CRstalk provided good inter‐rater reliability, with no significant differences between different MRI scanner manufacturers and field strengths. Further analyses by Fu et al. [34] showed that addition of a quantitative method (Wall Enhancement Index) to a qualitative method (AWE patterns) may enhance the discriminative performance of AWE. Specifically, combining both methods resulted in an even better discriminative performance of AWE, compared to the two methods by themselves. They also demonstrated a higher inter‐rater agreement of the quantitative approach compared to the qualitative approach, although both were excellent.
Priority should be given to further standardization of the AWE definition and reporting standards, since current reporting differences limit data synthesis and may thereby significantly slow down future development and potential translation to clinical practice. A reporting guideline with specific recommendations for AWE studies may help facilitate this and improve the overall quality of AWE studies.
CONCLUSION
This meta‐analysis demonstrates that AWE is positively associated with aneurysm instability. However, its discriminative performance mainly advocates the use of AWE absence as a marker of aneurysm stability. This is also supported by preliminary results from longitudinal studies, suggesting that AWE absence may function as a predictor of aneurysm stability during follow‐up. Extensive prospective studies are warranted to determine the predictive potential of AWE.
CONFLICT OF INTERESTS
None.
AUTHOR CONTRIBUTIONS
Rob Molenberg: Conceptualization (equal); data curation (equal); formal analysis (equal); writing—original draft (equal). Marlien W. Aalbers: Conceptualization (equal); data curation (equal); writing—review and editing (equal). Auke P. A. Appelman: Writing—review and editing (equal). Maarten Uyttenboogaart: Conceptualization (equal); writing—review and editing (equal). J. Marc C. van Dijk: Conceptualization (equal); supervision (equal); writing—review and editing (equal).
Supporting information
Appendix S1
Molenberg R, Aalbers MW, Appelman APA, Uyttenboogaart M, van Dijk JMC. Intracranial aneurysm wall enhancement as an indicator of instability. Eur J Neurol. 2021;28:3837–3848. 10.1111/ene.15046
See commentary by C. Zhu and M. Mossa‐Basha on page 3550
DATA AVAILABILITY STATEMENT
Data are available from the authors upon request.
REFERENCES
- 1. Etminan N, Chang HS, Hackenberg K, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta‐analysis. JAMA Neurol. 2019;76:588‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rinkel GJE, Algra A. Long‐term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10:349‐356. [DOI] [PubMed] [Google Scholar]
- 3. Jalbert JJ, Isaacs AJ, Kamel H, Sedrakyan A. Clipping and coiling of unruptured intracranial aneurysms among Medicare beneficiaries, 2000 to 2010. Stroke. 2015;46:2452‐2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee WK, Oh CW, Lee H, Lee KS, Park H. Factors influencing the incidence and treatment of intracranial aneurysm and subarachnoid hemorrhage: time trends and socioeconomic disparities under a universal healthcare system. J Neurointerv Surg. 2019;11:159‐165. [DOI] [PubMed] [Google Scholar]
- 5. Thompson BG, Brown RD, Amin‐Hanjani S, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2368‐2400. [DOI] [PubMed] [Google Scholar]
- 6. Greving JP, Wermer MJ, Brown RD Jr, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13:59‐66. [DOI] [PubMed] [Google Scholar]
- 7. Rutledge C, Jonzzon S, Winkler EA, et al. Small aneurysms with low PHASES scores account for most subarachnoid hemorrhage cases. World Neurosurg. 2020;139:e580‐e584. [DOI] [PubMed] [Google Scholar]
- 8. Molenberg R, Aalbers MW, Metzemaekers JDM, et al. Clinical relevance of short‐term follow‐up of unruptured intracranial aneurysms. Neurosurg Focus. 2019;47:E7. [DOI] [PubMed] [Google Scholar]
- 9. Brinjikji W, Zhu YQ, Lanzino G, et al. Risk factors for growth of intracranial aneurysms: a systematic review and meta‐analysis. AJNR Am J Neuroradiol. 2016;37:615‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mandell DM, Mossa‐Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. Am J Neuroradiol. 2017;38:218‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santarosa C, Cord B, Koo A, et al. Vessel wall magnetic resonance imaging in intracranial aneurysms: principles and emerging clinical applications. Interv Neuroradiol. 2020;26:135‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Larsen N, Flüh C, Saalfeld S, et al. Multimodal validation of focal enhancement in intracranial aneurysms as a surrogate marker for aneurysm instability. Neuroradiology. 2020;62:1627‐1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larsen N, Von Der Brelie C, Trick D, et al. Vessel wall enhancement in unruptured intracranial aneurysms: an indicator for higher risk of rupture? High‐resolution MR imaging and correlated histologic findings. Am J Neuroradiol. 2018;39:1617‐1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimonaga K, Matsushige T, Ishii D, et al. Clinicopathological insights from vessel wall imaging of unruptured intracranial aneurysms. Stroke. 2018;49:2516‐2529. [DOI] [PubMed] [Google Scholar]
- 15. Quan K, Song J, Yang Z, et al. Validation of wall enhancement as a new imaging biomarker of unruptured cerebral aneurysm. Stroke. 2019;50:1570‐1573. [DOI] [PubMed] [Google Scholar]
- 16. Zhong W, Su W, Li T, et al. Aneurysm wall enhancement in unruptured intracranial aneurysms: a histopathological evaluation. J Am Heart Assoc. 2021;10:e018633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712‐716. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viechtbauer W. Conducting meta‐analyses in R with the metafor. J Stat Softw. 2010;36:1‐48. [Google Scholar]
- 22. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61:991‐996. [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gariel F, Ben Hassen W, Boulouis G, et al. Increased wall enhancement during follow‐up as a predictor of subsequent aneurysmal growth. Stroke. 2020;51:1868‐1872. [DOI] [PubMed] [Google Scholar]
- 25. Vergouwen MDI, Backes D, Van Der Schaaf IC, et al. Gadolinium enhancement of the aneurysm wall in unruptured intracranial aneurysms is associated with an increased risk of aneurysm instability: a follow‐up study. Am J Neuroradiol. 2019;40:1112‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang GX, Wen L, Lei S, et al. Wall enhancement ratio and partial wall enhancement on MRI associated with the rupture of intracranial aneurysms. J Neurointerv Surg. 2018;10:569‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omodaka S, Endo H, Niizuma K, et al. Quantitative assessment of circumferential enhancement along the wall of cerebral aneurysms using MR imaging. Am J Neuroradiol. 2016;37:1262‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsushige T, Shimonaga K, Ishii D, et al. Vessel wall imaging of evolving unruptured intracranial aneurysms. Stroke. 2019;50:1891‐1894. [DOI] [PubMed] [Google Scholar]
- 29. Nagahata S, Nagahata M, Obara M, et al. Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three‐dimensional turbo spin‐echo sequence with motion‐sensitized driven‐equilibrium: a sign of ruptured aneurysm? Clin Neuroradiol. 2016;26:277‐283. [DOI] [PubMed] [Google Scholar]
- 30. Omodaka S, Endo H, Niizuma K, et al. Circumferential wall enhancement in evolving intracranial aneurysms on magnetic resonance vessel wall imaging. J Neurosurg. 2019;131:1262‐1268. [DOI] [PubMed] [Google Scholar]
- 31. Wang GX, Gong MF, Zhang D, et al. Wall enhancement ratio determined by vessel wall MRI associated with symptomatic intracranial aneurysms. Eur J Radiol. 2019;112:88‐92. [DOI] [PubMed] [Google Scholar]
- 32. Zhu C, Wang X, Eisenmenger L, et al. Wall enhancement on black‐blood MRI is independently associated with symptomatic status of unruptured intracranial saccular aneurysm. Eur Radiol. 2020;30:6413‐6420. [DOI] [PubMed] [Google Scholar]
- 33. Zhong W, Du Y, Guo Q, et al. The clinical and morphologic features related to aneurysm wall enhancement and enhancement pattern in patients with anterior circulation aneurysms. World Neurosurg. 2020;134:e649‐e656. [DOI] [PubMed] [Google Scholar]
- 34. Fu Q, Wang Y, Zhang Y, et al. Qualitative and quantitative wall enhancement on magnetic resonance imaging is associated with symptoms of unruptured intracranial aneurysms. Stroke. 2021;52:213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edjlali M, Guédon A, Ben Hassen W, et al. Circumferential thick enhancement at vessel wall MRI has high specificity for intracranial aneurysm instability. Radiology. 2018;289:181‐187. [DOI] [PubMed] [Google Scholar]
- 36. Hu P, Yang Q, Wang DD, Guan SC, Zhang HQ. Wall enhancement on high‐resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm. Neuroradiology. 2016;58:979‐985. [DOI] [PubMed] [Google Scholar]
- 37. Texakalidis P, Hilditch CA, Lehman V, Lanzino G, Pereira VM, Brinjikji W. Vessel wall imaging of intracranial aneurysms: systematic review and meta‐analysis. World Neurosurg. 2018;117:453‐458.e1. [DOI] [PubMed] [Google Scholar]
- 38. Wang X, Zhu C, Leng Y, Degnan AJ, Lu J. Intracranial aneurysm wall enhancement associated with aneurysm rupture: a systematic review and meta‐analysis. Acad Radiol. 2019;26:664‐673. [DOI] [PubMed] [Google Scholar]
- 39. Larson AS, Lehman VT, Lanzino G, Brinjikji W. Lack of baseline intracranial aneurysm wall enhancement predicts future stability: a systematic review and meta‐analysis of longitudinal studies. Am J Neuroradiol. 2020;41:1606‐1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chalouhi N, Ali MS, Jabbour PM, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32:1659‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jabbarli R, Rauschenbach L, Dinger TF, et al. In the wall lies the truth: a systematic review of diagnostic markers in intracranial aneurysms. Brain Path. 2020;30:437‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roa JA, Zanaty M, Ishii D, et al. Decreased contrast enhancement on high‐resolution vessel wall imaging of unruptured intracranial aneurysms in patients taking aspirin. [Published online March 6, 2020]. J Neurosurg. 2020;134(3):902‐908. https://thejns.org/view/journals/j‐neurosurg/aop/article‐10.3171‐2019.12.JNS193023/article‐10.3171‐2019.12.JNS193023.xml. Accessed February 25, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vergouwen MDI, Rinkel GJ, Algra A, et al. Prospective randomized open‐label trial to evaluate risk factor management in patients with unruptured intracranial aneurysms: study protocol. Int J Stroke. 2018;13:992‐998. [DOI] [PubMed] [Google Scholar]
- 44. Ishii D, Zanaty M, Roa JA, et al. Concentration of Lp(a) (Lipoprotein[a]) in aneurysm sac is associated with wall enhancement of unruptured intracranial aneurysm. [published online February 10, 2021]. Stroke. 2021;52(4):1465‐1468. [DOI] [PubMed] [Google Scholar]
- 45. Larsson SC, Gill D, Mason AM, et al. Lipoprotein(a) in Alzheimer, atherosclerotic, cerebrovascular, thrombotic, and valvular disease: Mendelian randomization investigation. Circulation. 2020;141:1826‐1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiao W, Qi T, He S, et al. Low wall shear stress is associated with local aneurysm wall enhancement on high‐resolution MR vessel wall imaging. Am J Neuroradiol. 2018;39:2082‐2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khan MO, Toro Arana V, Rubbert C, et al. Association between aneurysm hemodynamics and wall enhancement on 3D vessel wall MRI. [Published online January 10, 2020]. J Neurosurg. 2020;134:1‐11. https://thejns.org/view/journals/j‐neurosurg/134/2/article‐p565.xml. Accessed February 25, 2021. [DOI] [PubMed] [Google Scholar]
- 48. Boussel L, Rayz V, McCulloch C, et al. Aneurysm growth occurs at region of low wall shear stress: patient‐specific correlation of hemodynamics and growth in a longitudinal study. Stroke. 2008;39:2997‐3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hadad S, Mut F, Chung BJ, et al. Regional aneurysm wall enhancement is affected by local hemodynamics: a 7 T MRI study. [Published online December 24, 2020]. Am J Neuroradiol. 2020;42:464–470. http://www.ajnr.org/content/early/2020/12/24/ajnr.A6927.long. Accessed February 25, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cornelissen BMW, Leemans EL, Slump CH, Marquering HA, Majoie CBLM, van den Berg R. Vessel wall enhancement of intracranial aneurysms: fact or artifact? Neurosurg Focus. 2019;47:E18. [DOI] [PubMed] [Google Scholar]
- 51. Kalsoum E, Chabernaud Negrier A, Tuilier T, et al. Blood flow mimicking aneurysmal wall enhancement: a diagnostic pitfall of vessel wall MRI using the postcontrast 3D turbo spin‐echo MR imaging sequence. Am J Neuroradiol. 2018;39:1065‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Timmins KM, Kuijf HJ, Vergouwen MDI, et al. Reliability and agreement of 2D and 3D measurements on MRAs for growth assessment of unruptured intracranial aneurysms. Am J Neuroradiol. 2021; 10.3174/ajnr.A7186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu X, Haraldsson H, Wang Y, et al. A volumetric metric for monitoring intracranial aneurysms: repeatability and growth criteria in a longitudinal MR imaging study. Am J Neuroradiol. 2021; 10.3174/ajnr.A7190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. L'Allinec V, Chatel S, Karakachoff M, et al. Prediction of unruptured intracranial aneurysm evolution: the UCAN project. Neurosurgery. 2020;87:150‐156. [DOI] [PubMed] [Google Scholar]
- 55. Roa JA, Zanaty M, Osorno‐Cruz C, et al. Objective quantification of contrast enhancement of unruptured intracranial aneurysms: a high‐resolution vessel wall imaging validation study. [Published online February 7, 2020]. J Neurosurg. 2020;134(3):862‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data are available from the authors upon request.


