Abstract
Aim
Impaired awareness of hypoglycaemia (IAH) affects about 25% of patients with type 1 diabetes (T1DM). IAH can be reversed by strict avoidance of hypoglycaemia for at least 3 weeks. Adjunctive treatment with sodium glucose cotransporter 2 inhibitors may reduce the risk of hypoglycaemia through reduction of glucose variability. We tested the hypothesis that short‐term use of dapagliflozin may improve awareness of hypoglycaemia in people with T1DM and IAH.
Materials and Methods
Fifteen patients with T1DM and IAH were included in this randomized double‐blind, placebo‐controlled cross‐over trial (age 49.7 ± 14.6 years, 40% men, disease duration 24.1 ± 14.2 years, glycated haemoglobin 7.5 ± 0.8% (58.6 ± 8.4 mmol/mol). They were treated with dapagliflozin 10 mg once daily or matching placebo, with a washout period of 2 weeks. At the end of each treatment period, participants underwent a modified hyperinsulinaemic normoglycaemic‐hypoglycaemic glucose clamp (glucose nadir 2.5 mmol/L). Blinded continuous glucose monitors were used in the final treatment weeks.
Results
Treatment with dapagliflozin significantly improved glycated haemoglobin [−0.32 ± 0.10 vs. 0.22 ± 0.13% (−4.1 ± 0.9 vs. 2.3 ± 1.4 mmol/mol), dapagliflozin vs. placebo, p = .007] and glucose variability (standard deviation, 2.6 ± 0.2 vs. 3.1 ± 0.3 mmol/L, p = .029), but did not affect the frequency of hypoglycaemia. During the hypoglycaemic clamp, dapagliflozin did not affect symptom responses (8.0 ± 3.4 vs. 5.2 ± 1.6, p = .31), but significantly reduced the need for exogenous glucose to maintain hypoglycaemia (3.2 ± 0.3 vs. 4.1 ± 0.4 mg/kg/min, p = .022).
Conclusions
Eight weeks of treatment with dapagliflozin did not restore hypoglycaemic awareness in people with T1DM and impaired awareness of hypoglycaemia, but ameliorated some clinical aspects.
Keywords: dapagliflozin, hypoglycaemia, randomized trial, SGLT2 inhibitor, type 1 diabetes
1. INTRODUCTION
Iatrogenic hypoglycaemia is the most frequent, acute complication of insulin therapy in people with type 1 diabetes. On average, people with type 1 diabetes experience two to three hypoglycaemic events per week 1 , 2 and each year one severe hypoglycaemic event, 3 defined by appearance of cognitive impairment of such a degree that it requires external assistance for recovery. 4 Timely recognition of (the typical symptoms of) hypoglycaemia is critical to prevent severe hypoglycaemia. Recurrent hypoglycaemia can induce a process of habituation leading to the syndrome of impaired awareness of hypoglycaemia (IAH), which affects about 25% of patients with type 1 diabetes. 1 , 5 These people have lost the capacity to detect hypoglycaemia in a timely manner, thus increasing the risk to develop severe hypoglycaemia up to six‐fold. 6
Risk factors for IAH include a recent history of hypoglycaemia, low C‐peptide levels and longer diabetes duration. 7 , 8 , 9 Marked glucose variability may contribute to both the development and persistence of IAH, possibly mediated by increased incidence of hypoglycaemia, following or not following (too aggressive) corrections of recurrent hyperglycaemia. 1 , 10 Meticulous avoidance of hypoglycaemia for at least 3 weeks has been shown to reverse IAH. 11 , 12 However, the often associated deterioration of glycaemic control [i.e. rise of glycated haemoglobin (HbA1c)] is an important limitation of this strategy and in daily clinical practice many patients revert back upon retightening of glucose control.
Sodium‐glucose co transporter 2 (SGLT‐2) inhibitors selectively inhibit SGLT‐2 in the proximal tubules of the kidney, leading to decreased reabsorption of filtered glucose and an increase in urinary glucose excursion. 13 , 14 SGLT‐2 inhibitors have been shown to improve glucose control without increasing the incidence of hypoglycaemia in people with type 1 diabetes. 15 , 16 Furthermore, time in range (TIR) has been reported to increase during treatment with an SGLT‐2 inhibitor, 17 reflecting reduced glucose variability. More stability in day‐to‐day glucose control may ameliorate awareness of hypoglycaemia in patients with IAH because of reduced exposure to hypoglycaemia. We therefore hypothesized that SGLT‐2 inhibition would be helpful in restoring hypoglycaemic awareness in people with IAH. To test this hypothesis, we investigated the effect of short‐term treatment with the SGLT‐2 inhibitor dapagliflozin on counter‐regulatory responses to insulin‐induced hypoglycaemia in patients with type 1 diabetes and IAH.
2. MATERIALS AND METHODS
2.1. Study design
This was a randomized, double‐blind, placebo‐controlled cross‐over intervention performed at the Radboud University Medical Center (Nijmegen, the Netherlands). The study was approved by the local institutional review board and performed according to the principles of the Declaration of Helsinki. All participants provided written informed consent.
2.2. Study population
Patients with type 1 diabetes were recruited from the outpatient diabetes clinic of the Radboud University Medical Center. Patients were included between November 2018 and August 2019. Criteria for inclusion were: age 18‐75 years, type 1 diabetes duration ≥1 year, body mass index 19‐40 kg/m2, insulin treatment according to basal‐bolus insulin regimen, HbA1c <9% (75 mmol/mol), and the presence of IAH as assessed by a score of ≥3 on the Dutch modified version of the Clarke questionnaire. 18 Key exclusion criteria were current treatment with or known intolerance to SGLT‐2 inhibitors, treatment with glucose‐ or immune‐modifying agents other than insulin, history of cardiovascular disease and/or severe kidney failure, diabetes‐related complications (except for background retinopathy and asymptomatic peripheral neuropathy) and history of diabetic ketoacidosis requiring medical intervention within 1 month before screening.
2.3. Study procedure
Participants first came for a screening visit, which included medical history and standard physical examination (including body weight, height, blood pressure, pulse rate and screening for peripheral neuropathy). Blood was sampled for determination of HbA1c and serum creatinine.
After inclusion, patients were randomly assigned to treatment with dapagliflozin or matching placebo for 8 weeks in a cross‐over fashion, with a 2‐week washout period between treatment periods. Participants were enrolled by the investigator and were assigned to dapagliflozin or placebo treatment according to a randomization list that was managed by the pharmacy department of our hospital, to ensure the double‐blinded study design. Randomization was done by a computer program with the use of blocks of two subjects, to ensure that equal numbers of participants would start treatment with either dapagliflozin or placebo. Before start of the study medication, patients received ketone meters and were advised about how to identify potential symptoms of (normoglycaemic) diabetic ketoacidosis (e.g. nausea, vomiting). Patients were instructed to contact the study site in case of (suspected) symptoms of ketoacidosis, and if self‐measured blood ketone readings were ≥1.5 mmol/L, irrespective of blood glucose levels. Dapagliflozin and placebo capsules were dosed 10 mg once daily. After start of the study medication, patients were instructed to reduce prandial insulin levels by 10% to decrease the risk of hypoglycaemia. Participants were asked to perform four‐point daily blood glucose profiles and to keep a glucose diary for the duration of the study. Insulin doses were adjusted according to the glucose profiles, aiming for fasting and pre‐meal blood glucose levels of 4‐7 mmol/L without the occurrence of hypoglycaemia. In weeks 1, 2, 4 and 6, insulin dose adaptations and potential side effects were documented by telephone consultation. Patients recorded any hypoglycaemic event in their glucose diary, and whether they needed help from someone. During the final week of each treatment period, subjects completed seven‐point glucose profiles, and wore a blinded continuous glucose monitor (CGM) (Dexcom G6; Dexcom Inc., San Diego, CA, USA) for at least 5 days.
At the end of each treatment period of 8 weeks, subjects underwent a hyperinsulinaemic euglycaemic‐hypoglycaemic glucose clamp (nadir, 2.5 mmol/L). Participants were asked to come to the clinical research facility after an overnight fast, having abstained from alcohol, caffeine and smoking for 24 h and from strenuous exercise for 48 h. Participants were asked to reduce the basal insulin dose to avoid hypoglycaemia the day and night before the clamp. The clamps were rescheduled in case of hypoglycaemia (glucose ≤3.0 mmol/L). After arrival at the research facility, one intravenous cannula was inserted into the antecubital vein for infusion of insulin and glucose, and the other cannula was inserted in a retrograde way in a forearm vein. This forearm was placed in a heated box (55°C) so that arterialized blood could be obtained. Glucose levels were determined every 5 min using Biosen C‐Line (EKF Diagnostics, Cardiff, UK). 19 Glucose 20% (Baxter BV, Deerfield, IL, USA) and insulin (insulin aspart; Novo Nordisk, Bagsvaerd, Denmark) were infused in the contralateral arm. Baseline hyperglycaemia was corrected as needed with a small bolus of insulin. Subsequently, a hyperinsulinaemic (60 mU/m2/min) euglycaemic‐hypoglycaemic glucose clamp was initiated. The duration of the euglycaemic phase (target glucose, 5.0 mmol/L) was 30 min, after which glucose levels were allowed to fall to 2.5 mmol/L over ~35 min and maintained there for another 45 min. Blood samples were collected at several timepoints (i.e. at baseline, after 30 min of euglycaemia, after 20 and 45 min of hypoglycaemia, and after recovery from hypoglycaemia or 90 min after hypoglycaemia if not fully recovered) for the measurement of catecholamines, insulin, glucagon, cortisol and growth hormone. Participants were also asked to rate hypoglycaemic symptom scores by a validated questionnaire at those timepoints. 18 Symptoms were scored from 0 (none) to 6 (severe) and divided into autonomic symptoms (e.g. sweating, trembling and palpitations), neuroglycopenic symptoms (e.g. confusion, blurred vision and difficulty speaking), general symptoms (e.g. nausea and headache) and dummy symptoms (pain in the legs and yellow vision). 18 , 19
At the end of the hypoglycaemic phase, participants were asked to estimate the current glucose level, and to eat as much as they thought would be necessary to recover from hypoglycaemia. Insulin infusion was stopped at that moment and glucose infusion was tapered until stop over 35 min. Glucose levels were measured until 90 min after hypoglycaemia or until glucose levels reached ≥8.0 mmol/L. A questionnaire on appetite was administered at the abovementioned timepoints during the clamp and after recovery. This questionnaire consisted of a visual analogue scale (0‐100 mm) on which patients rated hunger, fullness, prospective consumption, desire to eat and thirst (maximal score 500 mm). 20
2.4. Study outcomes
The primary study endpoint was the symptom score in response to insulin‐induced hypoglycaemia during the hyperinsulinaemic clamp. A power calculation aimed at finding an increase of at least 40% in hypoglycaemia‐induced autonomic symptom score response with a power of 80% yielded a total number of participants of 15, where drop‐outs would be replaced. Differences in symptom scores were calculated between euglycaemia and the second hypoglycaemic timepoint (after 45 min of hypoglycaemia). Secondary outcome measures included plasma levels of counter‐regulatory hormones in response to clamped hypoglycaemia, maximal glucose excursion after hypoglycaemia, time until recovery from hypoglycaemia (defined as a glucose level above 4.0 mmol/L), glucose infusion rates during euglycaemia and hypoglycaemia, self‐reported appetite scores during and after hypoglycaemia, and amount of calories and carbohydrates consumed after hypoglycaemia. Other secondary outcomes were the change in total daily insulin dose, body weight and HbA1c, as well as mean 24‐h glucose levels, glucose variability and percentages of time spent above, in and below range, as derived from CGM downloads. TIR was defined as glucose levels between 3.9 and 10.0 mmol/L, according to predefined Dexcom G6‐settings and endorsed by a recent consensus statement. 21 Glucose variability was defined by both the average standard deviation and the coefficient of variation of 24‐h glucose levels.
2.5. Measurements
HbA1c was measured by the TOSOH G8 HPLC‐analyser, distributed by Sysmex. Plasma adrenaline and noradrenaline were analysed by high‐performance liquid chromatography combined with fluorometric detection. 19 Plasma insulin was assessed by an in‐house radioimmunoassay. 22 Plasma glucagon was measured by radioimmunoassay (Eurodiagnostica, Malmö, Sweden). Plasma growth hormone and cortisol were determined using a routine analysis method with an Electrochemiluminescent Immunoassay on a Modular Analytics E170 (Roche Diagnostics, GmbH, Mannheim, Germany).
2.6. Statistical analysis
Data were analysed using IBM SPSS statistics version 25 (IBM Corp., Armonk, NY, USA). All data are expressed as the mean ± SEM, unless otherwise specified. p < .05 was considered statistically significant. We tested for normality using the Shapiro‐Wilk test and QQ plots. Differences in means within groups were analysed using paired Student t‐tests (when normally distributed) and Wilcoxon signed rank tests (when not normally distributed). Serial data were analysed by two‐way repeated‐measures ANOVA.
3. RESULTS
In total, 15 patients with type 1 diabetes and IAH were included; two participants withdrew consent (one because of a wish to become pregnant, one because of fear for possible side effects) before start of the study, both of whom were replaced. Because of this, 15 patients completed the study, baseline characteristics are shown in Table 1. Results related to the two hyperinsulinaemic‐hypoglycaemic clamps were based on 14 patients because one patient only completed one of the two clamps. The patients were generally well‐controlled and the majority was on an insulin pump. Seven patients were on real‐time CGM and three patients used flash glucose monitoring.
TABLE 1.
Baseline characteristics
| n = 15 | |
|---|---|
| Age, years | 49.7 ± 14.6 |
| Male gender | 6 (40) |
| Weight, kg | 77.5 ± 13.1 |
| BMI, kg/m2 | 25.1 ± 3.0 |
| Score on modified Clarke questionnaire | 3.0 [3.0, 4.0] |
| Complications | |
| Retinopathy | 0 (0) |
| Neuropathy | 1 (6.7) |
| Nephropathy | 0 (0) |
| Duration of diabetes, years | 24.1 ± 14.2 |
| Insulin therapy | |
| CSII | 9 (60) |
| MDI | 6 (40) |
| Insulin dose, IU/day | 42.4 ± 19.4 |
| Insulin dose, IU/kg | 0.55 ± 0.2 |
| HbA1c, % (mmol/mol) | 7.5 ± 0.8 (58.6 ± 8.4) |
| Creatinine, μmol/L | 71.9 ± 16.9 |
Note: Data are presented as number (%), mean ± SD or median [IQR].
Abbreviations: BMI, body mass index; CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections.
3.1. Hypoglycaemic glucose clamps
Before start of the clamp, glucose levels were lower after dapagliflozin than after placebo treatment (7.1 ± 0.6 vs. 10.1 ± 0.8 mmol/L, p = .002). During the clamp, mean glucose levels for the euglycaemic phase (5.0 ± 0.1 vs. 4.8 ± 0.1 mmol/L, p = .07) and the hypoglycaemic phase (2.8 ± 0.0 vs. 2.8 ± 0.0 mmol/L, p = .84) were similar for dapagliflozin and placebo, respectively (Figure 1), although nadir plasma glucose levels slightly differed (2.5 ± 0.0 vs. 2.4 ± 0.0 mmol/L, p = .031). Plasma insulin levels were comparable during both clamps. At baseline, beta‐hydroxybutyrate (BHB) levels were higher after dapagliflozin than after placebo treatment [0.65 (0.22, 0.92) mmol/L vs. 0.06 (0.05, 0.37) mmol/L, p = .003], but suppressed to a similar extent during hypoglycaemia [0.03 (0.03, 0.07) mmol/L vs. 0.03 (0.03, 0.03), p = .18]. Symptom scores in response to hypoglycaemia did not differ between treatment with dapagliflozin and placebo (mean difference from euglycaemia 8.0 ± 3.4 vs. 5.2 ± 1.6, p = .31) (Figure 2).Treatment with dapagliflozin also did not alter counter‐regulatory hormone responses to hypoglycaemia, when compared with placebo treatment (Figure 3).
FIGURE 1.

A, Glucose levels and B, glucose infusion rate (GIR) during the hyperinsulinaemic clamps after treatment with dapagliflozin (closed circles) or placebo (open circles). eu, 30‐min euglycaemic phase; hypo, 45‐min hypoglycaemic phase; recovery, recovery phase after hypoglycaemia. *p < .05
FIGURE 2.
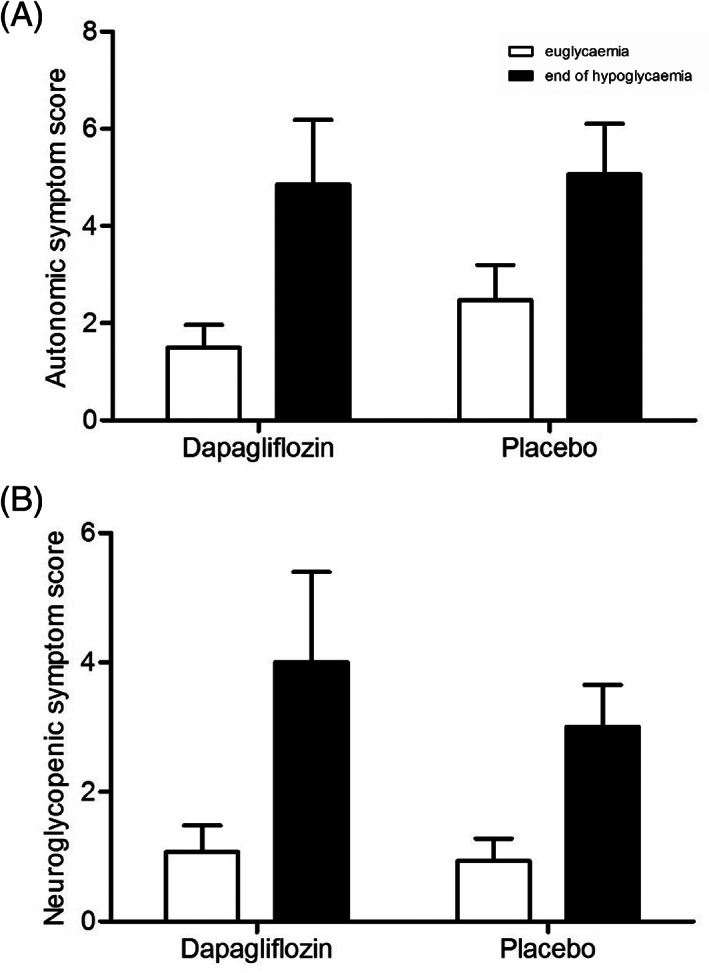
A, Autonomic and B, neuroglycopenic symptom scores during clamped euglycaemia (white bars) and hypoglycaemia (black bars), after dapagliflozin and placebo treatment
FIGURE 3.

(A‐E) Levels of counter‐regulatory hormones during hyperinsulinaemic clamps after treatment with dapagliflozin (closed circles) or placebo (open circles). A, adrenaline; B, noradrenaline; C, cortisol; D, growth hormone; E, glucagon. hypo, 45 min hypoglycaemic phase; rec, recovery phase after hypoglycaemia
Mean glucose infusion rates did not differ between treatments during euglycaemia (3.0 ± 0.4 vs. 3.6 ± 0.4 mg/kg/min, p = .14), but were significantly lower with dapagliflozin than with placebo during hypoglycaemia (3.2 ± 0.3 vs. 4.1 ± 0.4 mg/kg/min, p = .022) (Figure 1). Mean time until glycaemic recovery after hypoglycaemia was 17.5 ± 2.1 min after dapagliflozin treatment and 21.8 ± 2.1 min after placebo treatment (p = .17). Maximal glucose excursion during recovery after 45 min of hypoglycaemia was 8.0 ± 0.2 mmol/L after dapagliflozin treatment and 8.0 ± 0.2 mmol/L after placebo treatment (p = .96). There were no differences in appetite scores at either timepoint during and amount of carbohydrates and calories consumed after the clamp between the two treatments.
3.2. Treatment periods
Compared with placebo, 8 weeks dapagliflozin treatment significantly decreased HbA1c [−0.32 ± 0.10 vs. 0.22 ± 0.13% (−4.1 ± 0.9 vs. 2.3 ± 1.4 mmol/mol), p = .007]. Total daily insulin dose did not change and was not different at the end of the two 8‐week treatment periods between dapagliflozin and placebo (35.9 ± 3.2 vs. 37.1 ± 3.5 IU, p = .28). Compared with placebo, dapagliflozin also reduced body weight (−2.3 ± 0.6 vs. –0.1 ± 0.5 kg, p = .033). The median number of self‐reported hypoglycaemic events per person per week was 0.9 (0.4, 2.4) with dapagliflozin and 1.0 (0.3, 1.4) with placebo treatment (p = .70). Two episodes of severe hypoglycaemia were recorded, one in each of the treatment periods, both in the same participant.
Mean 24‐h glucose levels during the final dapagliflozin treatment weeks were 7.6 ± 0.3 mmol/L and 8.2 ± 0.4 mmol/L with placebo treatment (difference, −0.6 ± 0.3 mmol/L, p = .075). Glucose variability reflected by the standard deviation of glucose levels was significantly lower during treatment with dapagliflozin as compared with placebo (2.6 ± 0.2 vs. 3.1 ± 0.3 mmol/L, p = .029). The coefficient of variation showed a trend towards lower glucose variability during treatment with dapagliflozin as compared with placebo (33.6 ± 0.0 vs. 36.9 ± 0.0%, p = .07). The percentage of time spent in range was not significantly different after both treatment periods (72.9 ± 3.3 vs. 68.0 ± 4.2%, p = .19). Although TIR was higher in participants already using real‐time CGM (or flash glucose monitoring) than in participants who did not, dapagliflozin did not further improve TIR in either subgroup when compared with placebo. Median percentage of time spent below range (glucose <3.0 mmol/L) and mean percentage of time spent above range (glucose >10.0 mmol/L) did not differ between treatment periods [0.4 (0.0, 3.7) vs. 1.5 (0.3, 2.1)%, p = .76 and 20.7 ± 3.3 vs. 26.0 ± 4.1%, p = .15, respectively].
3.3. Adverse effects
One patient had a genital infection during dapagliflozin treatment, treated with antimycotic therapy, another had a urinary tract infection during placebo treatment, treated with antibiotics. One participant suffered from flu‐like symptoms for 3 days during dapagliflozin treatment, which were self‐limiting. Other adverse events included food poisoning, shoulder bursitis and an ankle fracture. Diabetic ketoacidosis did not occur during either treatment period.
4. DISCUSSION
The main finding of this study is that 8 weeks of treatment with dapagliflozin did not restore hypoglycaemic awareness in people with type 1 diabetes and IAH, but ameliorated one aspect of IAH. Indeed, treatment with dapagliflozin reduced the need for exogenous glucose to maintain hypoglycaemia during the clamp, reflecting greater endogenous glucose appearance. Dapagliflozin also reduced HbA1c and improved glucose variability without increasing the frequency of hypoglycaemia or the time spent below the range of normoglycaemia.
To our knowledge, this is the first study specifically examining the effect of treatment with an SGLT‐2 inhibitor in people with type 1 diabetes and IAH. Our data of improved glucose control and reduced glucose variability are in line with previous studies that investigated SGLT‐2 inhibitor treatment in people with type 1 diabetes. 16 , 17 , 23 , 24 , 25 , 26 , 27 , 28 Remarkably, these improvements were achieved against a background of already reasonably‐well glucose control and, similar to previous studies, without increasing the risk of (severe) hypoglycaemic events or the time spent in hypoglycaemia. 15 , 16 , 17 , 23 , 24 , 25 In other words, treatment with dapagliflozin shifted the inverse relationship between HbA1c and incidence of hypoglycaemia to the left, but contrary to our aims, this effect was entirely because of a change in the first rather than the second component.
Symptom scores in response to clamped hypoglycaemia were numerically higher after dapagliflozin treatment when compared with placebo, but this difference was not statistically significant. Dapagliflozin treatment was associated with a reduced glucose infusion rate during the hypoglycaemic condition of the clamp, reflecting lower external glucose requirements and consequently a greater endogenous glucose rising capacity to maintain the same blood glucose level. As no single counter‐regulatory hormone response was enhanced by dapagliflozin, this effect may be because of the composite glucose‐increasing effect of all counter‐regulatory hormones combined or to increased beta‐adrenergic sensitivity, a reduction of which has been demonstrated in people with IAH. 29 , 30 Given the mechanism of action of SGLT2 inhibitors, we would have expected higher glucagon levels in the dapagliflozin arm. We have not been able to show such increases in glucagon in patients with type 1 diabetes treated with SGLT‐2 inhibitors. This may perhaps be because of glucagon levels being already somewhat elevated because of loss of the inhibitory effect of endogenous insulin secretion. Beta‐hydroxybutyrate levels before start of the clamps were higher after dapagliflozin than after placebo treatment. However, although ketones may be used as an alternative for glucose, these levels were almost completely suppressed during hypoglycaemia, thus unlikely to explain the differences in glucose requirements.
Apart from reduced need for exogenous glucose to maintain hypoglycaemia, treatment with dapagliflozin was not better than placebo in enhancing counter‐regulatory hormone responses to or symptomatic awareness of hypoglycaemia during the clamp. The most obvious explanation for these results is that dapagliflozin did not affect hypoglycaemia event rates, presumably because the participants were more concerned about (reducing) hyper‐ than hypoglycaemic excursions. 31 Although this study cannot claim clear beneficial effects of dapagliflozin on IAH, there are several points to consider. First, the frequency of hypoglycaemia in our study was around one episode per week, which is already low for people with type 1 diabetes in general, let alone for a population with IAH. The proportion of time spent below range was similarly low, averaging 0.4% and 1.5% for dapagliflozin and placebo treatment, respectively. Second, the TIR of about 70% in both study arms was much higher than observed in other studies on SGLT‐2 inhibitor treatment 17 , 23 , 24 , 26 , 27 , 28 and close to or above the target recommended by current guidelines. 21 Both points suggest that optimal treatment was already achieved in the placebo arm in terms of avoiding hypoglycaemia without deteriorating overall glucose control, perhaps because of study instructions to intensify glucose monitoring, with or without CGM. Indeed, about a third of the participants had retained sufficient awareness after both treatments to identify correctly the second part of the clamp as being hypoglycaemic. Although speculative, we would posit that this effect may have reduced our participants' interests in further avoiding hypoglycaemia to improve awareness, but rather use dapagliflozin to limit the occurrence of hyperglycaemic events, thus improving overall glucose control without increasing this risk.
Impaired awareness of hypoglycaemia is a complex clinical syndrome that is difficult to reverse. Although various agents and interventions have been shown to enhance counter‐regulatory responses to hypoglycaemia, 32 , 33 , 34 , 35 few have been tried in longer‐term studies and none was effective in restoring hypoglycaemic awareness. This includes CGM, although its use reduces the risk of severe hypoglycaemia as the main consequence of IAH. 36 One explanation for the failure to resolve IAH may relate to many people with IAH being more concerned about hyperglycaemia and associated complications and prone to underestimating the risks of hypoglycaemia. 31 , 37 Use of SGLT‐2 inhibitors may play a role in improving IAH, although its use should be carefully balanced against the risk of diabetic ketoacidosis. 38 We did not observe any episode of ketoacidosis in our trial, but the risk of ketoacidosis remains a controversial issue when it comes to approving treatment with SGLT‐2 inhibitors for patients with type 1 diabetes. 39 , 40
Strengths of our study include the study design (randomized, double‐blind and placebo‐controlled), the use of glucose clamps to measure awareness of hypoglycaemia and the use of (blinded) CGMs. Our study also has limitations. First, we did not perform glucose clamps at baseline to measure hypoglycaemic symptom scores. This would have increased the study burden to participants. The duration of the study was relatively short when compared with other studies. However, 3 weeks of hypoglycaemia avoidance is reportedly sufficient to improve hypoglycaemic awareness 11 , 12 and we showed meaningful results with respect to glucose variability and HbA1c, so that a longer study duration is unlikely to have produced different results. The cross‐over design may have influenced the results by carry‐over effects, despite the 2‐week washout period between the treatment periods. This study design was chosen because the high statistical power allowing substantially fewer study participants than parallel designed studies, particularly given the intensity of the interventions scheduled (particularly hypoglycaemic clamps). Cross‐over studies generally result in more homogeneous study populations. Moreover, we used block randomization to minimize the impact of carry‐over effects, which was not found by formal testing.
In conclusion, 8 weeks of treatment with dapagliflozin did not restore hypoglycaemic awareness in people with type 1 diabetes and IAH, but ameliorated one aspect of IAH. Treatment with dapagliflozin lowered HbA1c without increasing the risk of hypoglycaemia, and decreased glucose variability in people with type 1 diabetes and IAH. In theory this should result in less time in hypoglycaemia when aiming at a stable HbA1c, but larger studies, including more subjects with and without CSII and CGM, are needed to determine the exact value of adding SGLT‐2 inhibitors on hypoglycaemia awareness.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
CJT, BEdG and LAvM designed the study. LAvM performed the experiments and collected the data. LAvM analysed the data and wrote the first version of the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages. The guarantor accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14505.
ACKNOWLEDGMENTS
The authors thank Karin Saini, Eveline Otters‐van Bodegom, Sanne Houba, Anja Rasing and Jordi van Unnik (Radboudumc Technology Center Clinical Studies) for assistance during the glucose clamps. We also thank Clementine Verhulst, Julia van Heck and Anneke Hijmans for assistance during the clamps and for performing laboratory experiments. The authors also thank all the volunteers for their participation.
van Meijel LA, Tack CJ, de Galan BE. Effect of short‐term use of dapagliflozin on impaired awareness of hypoglycaemia in people with type 1 diabetes. Diabetes Obes Metab. 2021;23(11):2582-2589. 10.1111/dom.14505
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169‐3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostenson CG, Geelhoed‐Duijvestijn P, Lahtela J, Weitgasser R, Markert Jensen M, Pedersen‐Bjergaard U. Self‐reported non‐severe hypoglycaemic events in Europe. Diabet Med. 2014;31(1):92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pedersen‐Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev. 2004;20(6):479‐486. [DOI] [PubMed] [Google Scholar]
- 4. International Hypoglycaemia Study G . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetes Care. 2017;40(1):155‐157. [DOI] [PubMed] [Google Scholar]
- 5. de Galan BE, Schouwenberg BJ, Tack CJ, Smits P. Pathophysiology and management of recurrent hypoglycaemia and hypoglycaemia unawareness in diabetes. Neth J Med. 2006;64(8):269‐279. [PubMed] [Google Scholar]
- 6. Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med. 2008;25(4):501‐504. [DOI] [PubMed] [Google Scholar]
- 7. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902‐1912. [DOI] [PubMed] [Google Scholar]
- 8. Group UKHS . Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140‐1147. [DOI] [PubMed] [Google Scholar]
- 9. Schouwenberg BJ, Coenen MJ, Paterson AD, et al. Genetic determinants of impaired awareness of hypoglycemia in type 1 diabetes. Pharmacogenet Genomics. 2017;27(9):323‐328. [DOI] [PubMed] [Google Scholar]
- 10. Kovatchev BP, Cox DJ, Farhy LS, Straume M, Gonder‐Frederick L, Clarke WL. Episodes of severe hypoglycemia in type 1 diabetes are preceded and followed within 48 hours by measurable disturbances in blood glucose. J Clin Endocrinol Metab. 2000;85(11):4287‐4292. [DOI] [PubMed] [Google Scholar]
- 11. Fanelli CG, Epifano L, Rambotti AM, et al. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short‐term IDDM. Diabetes. 1993;42(11):1683‐1689. [DOI] [PubMed] [Google Scholar]
- 12. Dagogo‐Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43(12):1426‐1434. [DOI] [PubMed] [Google Scholar]
- 13. Biester T, Aschemeier B, Fath M, et al. Effects of dapagliflozin on insulin‐requirement, glucose excretion and ss‐hydroxybutyrate levels are not related to baseline HbA1c in youth with type 1 diabetes. Diabetes Obes Metab. 2017;19(11):1635‐1639. [DOI] [PubMed] [Google Scholar]
- 14. Hediger MA, Rhoads DB. Molecular physiology of sodium‐glucose cotransporters. Physiol Rev. 1994;74(4):993‐1026. [DOI] [PubMed] [Google Scholar]
- 15. Pieber TR, Famulla S, Eilbracht J, et al. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4‐week, randomized, placebo‐controlled trial (EASE‐1). Diabetes Obes Metab. 2015;17(10):928‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perkins BA, Cherney DZ, Partridge H, et al. Sodium‐glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8‐week open‐label proof‐of‐concept trial. Diabetes Care. 2014;37(5):1480‐1483. [DOI] [PubMed] [Google Scholar]
- 17. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT‐1): 24 week results from a multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):864‐876. [DOI] [PubMed] [Google Scholar]
- 18. Janssen MM, Snoek FJ, Heine RJ. Assessing impaired hypoglycemia awareness in type 1 diabetes: agreement of self‐report but not of field study data with the autonomic symptom threshold during experimental hypoglycemia. Diabetes Care. 2000;23(4):529‐532. [DOI] [PubMed] [Google Scholar]
- 19. Rooijackers HM, Wiegers EC, van der Graaf M, et al. A single bout of high‐intensity interval training reduces awareness of subsequent hypoglycemia in patients with type 1 diabetes. Diabetes. 2017;66(7):1990‐1998. [DOI] [PubMed] [Google Scholar]
- 20. Zoon HF, de Graaf C, Boesveldt S. Food Odours direct specific appetite. Foods. 2016;5(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abbink EJ, Walker AJ, van der Sluijs HA, Tack CJ, Smits P. No role of calcium‐ and ATP‐dependent potassium channels in insulin‐induced vasodilation in humans in vivo. Diabetes Metab Res Rev. 2002;18(2):143‐148. [DOI] [PubMed] [Google Scholar]
- 23. Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of Dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT‐1 52‐week study. Diabetes Care. 2018;41(12):2552‐2559. [DOI] [PubMed] [Google Scholar]
- 24. Mathieu C, Dandona P, Phillip M, et al. Glucose variables in type 1 diabetes studies with Dapagliflozin: pooled analysis of continuous glucose monitoring data from DEPICT‐1 and ‐2. Diabetes Care. 2019;42(6):1081‐1087. [DOI] [PubMed] [Google Scholar]
- 25. Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of Canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38(12):2258‐2265. [DOI] [PubMed] [Google Scholar]
- 26. Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of Dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT‐2 study): 24‐week results from a randomized controlled trial. Diabetes Care. 2018;41(9):1938‐1946. [DOI] [PubMed] [Google Scholar]
- 27. Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. 2018;41(12):2560‐2569. [DOI] [PubMed] [Google Scholar]
- 28. Famulla S, Pieber TR, Eilbracht J, et al. Glucose exposure and variability with Empagliflozin as adjunct to insulin in patients with type 1 diabetes: continuous glucose monitoring data from a 4‐week, randomized, placebo‐controlled trial (EASE‐1). Diabetes Technol Ther. 2017;19(1):49‐60. [DOI] [PubMed] [Google Scholar]
- 29. Korytkowski MT, Mokan M, Veneman TF, Mitrakou A, Cryer PE, Gerich JE. Reduced beta‐adrenergic sensitivity in patients with type 1 diabetes and hypoglycemia unawareness. Diabetes Care. 1998;21(11):1939‐1943. [DOI] [PubMed] [Google Scholar]
- 30. Fritsche A, Stefan N, Haring H, Gerich J, Stumvoll M. Avoidance of hypoglycemia restores hypoglycemia awareness by increasing beta‐adrenergic sensitivity in type 1 diabetes. Ann Intern Med. 2001;134(9 Pt 1):729‐736. [DOI] [PubMed] [Google Scholar]
- 31. Rogers HA, de Zoysa N, Amiel SA. Patient experience of hypoglycaemia unawareness in type 1 diabetes: are patients appropriately concerned? Diabet Med. 2012;29(3):321‐327. [DOI] [PubMed] [Google Scholar]
- 32. de Galan BE, Tack CJ, Lenders JW, et al. Theophylline improves hypoglycemia unawareness in type 1 diabetes. Diabetes. 2002;51(3):790‐796. [DOI] [PubMed] [Google Scholar]
- 33. Farrell CM, McNeilly AD, Fournier P, et al. A randomised controlled study of high intensity exercise as a dishabituating stimulus to improve hypoglycaemia awareness in people with type 1 diabetes: a proof‐of‐concept study. Diabetologia. 2020;63(4):853‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vele S, Milman S, Shamoon H, Gabriely I. Opioid receptor blockade improves hypoglycemia‐associated autonomic failure in type 1 diabetes mellitus. J Clin Endocrinol Metab. 2011;96(11):3424‐3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Debrah K, Sherwin RS, Murphy J, Kerr D. Effect of caffeine on recognition of and physiological responses to hypoglycaemia in insulin‐dependent diabetes. Lancet. 1996;347(8993):19‐24. [DOI] [PubMed] [Google Scholar]
- 36. van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open‐label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893‐902. [DOI] [PubMed] [Google Scholar]
- 37. Speight J, Barendse SM, Singh H, et al. Cognitive, behavioural and psychological barriers to the prevention of severe hypoglycaemia: a qualitative study of adults with type 1 diabetes. SAGE Open Med. 2014;2:2050312114527443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Danne T, Garg S, Peters AL, et al. International consensus on risk Management of Diabetic Ketoacidosis in patients with type 1 diabetes treated with sodium‐glucose cotransporter (SGLT) inhibitors. Diabetes Care. 2019;42(6):1147‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. European Medicines Agency. First Oral Add‐on Treatment to Insuline for Treatment of Certain Patients with Type 1 Diabetes, 2019. https://www.ema.europa.eu/en/news/first‐oral‐add‐treatment‐insulin‐treatment‐certain‐patients‐type‐1‐diabetes.
- 40. Siegmund T, Ampudia‐Blasco FJ, Schnell O. Two clinical cases of adjunctive use of a SGLT‐2 inhibitor in type 1 diabetes. Diabetes Res Clin Pract. 2020;162:108131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


