Abstract
Background
In the Hokusai VTE Cancer study, the risk of major bleeding was 2.9% higher in the edoxaban group compared with the dalteparin group, mainly due to more gastrointestinal bleedings in patients with gastrointestinal cancer. The identification of risk factors for gastrointestinal bleeding may help to guide the use of DOACs in these patients.
Objectives
To evaluate risk factors for gastrointestinal bleeding in patients with gastrointestinal cancer receiving edoxaban.
Patients/Methods
In this nested case‐control study in patients with gastrointestinal cancer randomized to edoxaban in the Hokusai VTE Cancer study, cases (patients with clinically relevant gastrointestinal bleeding during treatment) were randomly matched to three controls (patients who had no gastrointestinal bleeding). Data for the 4‐week period prior to bleeding were retrospectively collected. Odds ratios (ORs) were calculated in a crude conditional logistic regression model and a multivariable model adjusted for age, sex, and cancer type.
Results
Twenty‐four cases and 64 matched controls were included. In the multivariable analysis, advanced cancer, defined as regionally advanced or metastatic cancer (OR 3.6, 95% CI 1.01–12.6) and low hemoglobin levels (OR 4.8, 95% CI 1.5–16.0) were significantly associated with bleeding. There was no significant difference in patients with resected tumors (OR 0.4, 95% CI 0.1–1.4), or in patients on chemotherapy (OR 1.3, 95% CI 0.5–3.5).
Conclusion
Advanced cancer and low hemoglobin levels were associated with an increased risk of gastrointestinal bleeding in patients with gastrointestinal cancer receiving edoxaban. We were unable to identify other risk factors, mainly due to limited statistical power.
Keywords: factor Xa Inhibitors, gastrointestinal neoplasms, hemorrhage, risk factors, venous thrombosis
Essentials.
Patients with gastrointestinal cancer using edoxaban are at risk of gastrointestinal bleeding
We assessed risk factors for gastrointestinal bleeding in patients with gastrointestinal cancer
Advanced cancer was significantly associated with gastrointestinal bleeding
Hemoglobin <10 g/dl was significantly associated with gastrointestinal bleeding
1. INTRODUCTION
Venous thromboembolism (VTE) is a frequent complication in patients with cancer, which requires anticoagulation for as long as the cancer is active. 1 , 2 Direct oral anticoagulants (DOACs) are currently a recommended treatment option based on the results of several trials showing non‐inferiority compared with low‐molecular‐weight heparins (LMWH) with respect to recurrence and major bleeding. 3 , 4 , 5 , 6 However, current guidelines recommend that DOACs should be used with caution in patients with gastrointestinal cancer due to an increased risk of gastrointestinal bleeding. 7 , 8 , 9
In the Hokusai VTE Cancer study, which compared the direct oral factor Xa inhibitor edoxaban with dalteparin, edoxaban was non‐inferior for the primary outcome, which was the composite of recurrent VTE and major bleeding during the 12 months of follow‐up (hazard ratio [HR], 0.97; 95% confidence interval [CI], 0.70–1.36; p = .006 for non‐inferiority). 3 Compared with dalteparin, the risk of recurrent VTE was 3.4% lower in the edoxaban group, while the risk of major bleeding was 2.9% higher. The difference in major bleeding was largely explained by a higher risk of gastrointestinal bleeding in patients with cancers of the upper gastrointestinal tract, such as esophageal and gastric cancer, but also in patients with pancreatic and hepatobiliary cancer. 3 , 10
The mechanisms responsible for the higher risk of gastrointestinal bleeding with DOACs remain unclear. The identification of risk factors for gastrointestinal bleeding in patients with gastrointestinal cancer may help to guide the use of DOACs in these patients. Therefore, the aims of this study were to evaluate risk factors for gastrointestinal bleeding in patients with gastrointestinal cancer receiving edoxaban and identify patients with gastrointestinal cancer at low risk of bleeding in whom edoxaban can be safely prescribed.
2. METHODS
2.1. Hokusai VTE cancer study
A post‐hoc nested case‐control study within the Hokusai VTE Cancer study was performed to identify risk factors for gastrointestinal bleeding in patients with gastrointestinal cancer. The rationale and main results of the Hokusai VTE cancer study have been published previously. 3 , 11 In this open‐label trial, 1050 patients with cancer‐associated VTE were randomized between July 2015 and December 2016 to either oral edoxaban (60 mg once daily) or subcutaneous dalteparin (200 IU/kg once daily for 1 month followed by 150 IU/kg once daily thereafter). The dose of edoxaban was reduced to 30 mg in patients with a creatinine clearance of 30 to 50 ml per minute or a body weight of 60 kg or less or in those receiving concomitant treatment with potent P‐glycoprotein inhibitors. Patients were followed for 12 months with a minimum treatment duration of 6 months. The primary outcome was the composite of recurrent VTE and major bleeding. Secondary outcomes were recurrent VTE, major bleeding, clinically relevant non‐major bleeding, and mortality. Major bleeding was defined according to the International Society on Thrombosis and Haemostasis (ISTH) criteria as overt bleeding that was associated with a decrease in the hemoglobin level of 2 g/dl or more, led to a transfusion of 2 or more units of blood, occurred in a critical site, or contributed to death. 12 Clinically relevant non‐major bleeding was defined as overt bleeding (i.e. symptomatic or visualized by examination) not meeting the criteria for major bleeding but requires medical attention or is associated with discomfort for the subject such as pain, or impairment of activities of daily life. Outcomes were adjudicated by an independent committee whose members were unaware of treatment allocation.
2.2. Study design
In this nested case‐control study, cases were patients with gastrointestinal cancer who developed major or clinically relevant non‐major upper or lower gastrointestinal bleeding during edoxaban treatment or up to 72 h after discontinuation. At the time of gastrointestinal bleeding, cases were matched by days since randomization to three controls, which were randomly selected by incidence density sampling from the study group still at risk at that specific time, i.e. patients receiving edoxaban without major or clinically relevant non‐major gastrointestinal bleeding at the time of matching. With this method cases are matched to controls on duration of follow‐up, allowing for control of the confounding effect of time in the analysis. 13 , 14 Additionally, in nested case‐control studies, cases occurring later in the follow‐up are eligible to be controls for earlier cases. 13 , 14 For example, if a case developed major gastrointestinal bleeding 90 days after randomization, three patients with gastrointestinal cancer who were using edoxaban and had not developed gastrointestinal bleeding at day 90 were randomly selected as controls (Figure 1). Matched control patients who developed major or clinically relevant non‐major gastrointestinal bleeding after the time of matching were transferred to the case group. For patients serving as both a control and a case, a minimum of 4 weeks between being assigned as a control and later as a case was required (Figure 1). With this method it is not possible for cases to be their own control, since controls are always matched to other cases because of this time difference. In this study three controls were chosen, since with a limited number of cases, power increases when more controls are added to the study, but usually not beyond four patients per case. 13 , 15
FIGURE 1.
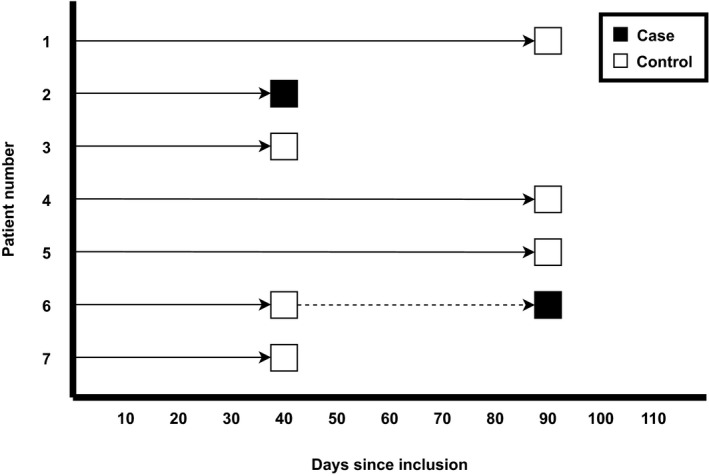
Example of 2 cases with 6 randomly matched controls
2.3. Data collection
Baseline data from included patients were routinely collected in the Hokusai VTE Cancer study. Additional clinical data were retrospectively collected by the participating centers for the 4‐week period prior to the gastrointestinal bleeding event (cases) or the 4‐week period before the day at which patients were matched (controls). The variables included were chosen based on their known association with bleeding in cancer patients as well as in the non‐cancer population or based on their potential protective properties in gastrointestinal bleeding. 16 , 17 , 18 , 19 , 20 , 21 , 22 The following data were collected: resected or non‐resected tumors (patients with resected tumors at the moment of inclusion of the Hokusai VTE Cancer study, which had a recurrence at the time of this case‐control study, were classified as non‐resected); local or advanced disease (advanced cancer is defined as locally advanced or metastatic cancer); cancer therapy (different types of chemotherapy or surgery); interventions (e.g., endoscopy); concomitant medications including proton‐pump inhibitors, corticosteroids, non‐steroidal anti‐inflammatory drugs (NSAIDs) and antiplatelet agents; edoxaban dose; weight; and laboratory results (kidney function, hemoglobin and platelets).
2.4. Statistical analysis
Baseline characteristics were presented using descriptive statistics. The association between the possible risk factors and gastrointestinal bleeding was evaluated by calculating odds ratios (ORs) with 95% confidence intervals using a crude conditional logistic regression model. In such a model, cases are compared only with their matched controls. Additionally, in a multivariable conditional logistic regression model, we adjusted for age (continuous), sex, and grouped tumor type in which the tumors we separated in three different groups (1: colorectal, 2: hepatobiliary or pancreatic, or 3: upper gastrointestinal cancer). In this model we corrected for sex, as it affects other potential risk factors assessed, such as hemoglobin levels and weight. Additionally we corrected for age and tumor type, since the difference in significantly associated risk factors for bleeding could potentially be explained in part due to different characteristics or treatment strategies in different tumor types and patients of older age (e.g. more often advanced disease at diagnosis in pancreatic cancer, or less often chemotherapy in elder patients due to poor performance status). 23 , 24 Each variable assessed was added separately to this model to calculate adjusted odds ratios.
Two sensitivity analyses were performed. In the first sensitivity analysis, an unconditional logistic regression analysis was performed in which data from all cases and controls were compared, regardless of matching, adjusting for (1) the number of days since inclusion and (2) the number of days since inclusion, age, sex, and grouped tumor type. In the second sensitivity analysis we excluded the controls who became a case later to assess whether the association would change without the possibility of a case also serving as a control. In this analysis these patients were assessed as cases only which results in the same number of cases, but fewer controls. For the association of hemoglobin levels with risk of bleeding a cut‐off value of 10 g/dl (6.2 mmol/L) was used, since a hemoglobin level below 10 g/dl has shown to be a significant predictor for clinically relevant bleeding in patients treated with edoxaban. 25 R version 3.6.1 was used for all analyses (https://www.R‐project.org).
3. RESULTS
Of the 1050 patients enrolled in the Hokusai VTE Cancer study, 522 were randomly allocated to edoxaban of whom 165 (32%) had gastrointestinal cancer. During the 12‐month study period, 29 of these 165 patients (18%) developed at least one on‐treatment major or clinically relevant non‐major gastrointestinal bleed at a median of 98.5 days (standard deviation [SD] 98.1) after randomization. All 29 cases were randomly matched to three controls from the at‐risk group by time since randomization. Of the controls, 11 were included as cases later during follow‐up. Out of these 116 cases and controls, from 42 different centers worldwide, additional data was collected from 101 patients (24 cases and 77 controls). Data could not be obtained for 5 cases and 10 controls due to various reasons. Out of the 77 controls, for 13 patients, data from the matched case could not be collected. Therefore, 24 cases and 64 matched controls were included in the conditional logistic regression model. In the unconditional logistic regression model, all 24 cases and 77 controls could be included. In the main analysis seven controls were later used as a case.
Baseline characteristics of cases and controls are shown in Table 1. Cases were older (median age 66 vs. 63 years; p = .33) and more often male (79% vs. 64%; p = .27). There was a numerical difference in upper gastrointestinal tumors (29% vs. 25%; p = .69) and lower gastrointestinal tumors (48% vs. 58%; p = .96). Of the 24 cases, 14 patients (58%) had a major bleeding event and 10 patients (42%) had a clinically relevant non‐major bleeding event. The site of bleeding was the upper gastrointestinal tract in 16 (67%) patients and the lower gastrointestinal tract in 8 (33%). Of the 16 upper gastrointestinal bleeding events, seven patients had gastric or esophageal cancer, five had hepatobiliary or pancreatic cancer, and four had colorectal cancer. Of these 16 patients, 13 (81%) had non‐resected tumors. All eight lower gastrointestinal bleeding events occurred in patients with colorectal cancer, of which, seven (88%) had non‐resected tumors. All of the clinically relevant bleeding events were before the stop of study drug administration or on the same day that the study drug was stopped. For subjects in whom the stop of the study drug and bleeding event occurred on the same day, the bleeding event was the cause of the study drug discontinuation in all instances. There were no fatal bleeding events.
TABLE 1.
Baseline characteristics of cases and matched controls
| Cases (n = 24) | Controls (n = 64) | p‐Value | |
|---|---|---|---|
| Mean days since inclusion to bleeding event/matching date (SD) | 91.8 (93.8) | 90.1 (92.7) | .94 |
| Mean age, years (SD) | 66.3 (11.5) | 63.4 (12.2) | .33 |
| Male sex, n (%) | 19 (79.2) | 41 (64.1) | .27 |
| Upper gastro‐intestinal cancer, n (%) | 7 (29.2) | 16 (25) | .69 |
| Esophagus | 4 (16.7) | 10 (15.7) | |
| Stomach | 3 (12.5) | 6 (4.4) | |
| Hepatobiliary and pancreatic cancer, n (%) | 4 (16.7) | 11 (17.2) | .95 |
| Pancreas | 2 (8.3) | 8 (12.5) | |
| Cholangiocarcinoma | 1 (4.2) | 2 (3.1) | |
| Gall bladder | 1 (4.2) | 0 | |
| Liver | 1 (1.6) | 1 (4.2) | |
| Lower gastro‐intestinal cancer, n (%) | 14 (48.3) | 37 (57.8) | .96 |
| Colon | 4 (16.7) | 22 (34.3) | |
| Rectum | 8 (33.3) | 15 (23.4) | |
| Body weight at baseline, kg (SD) | 80.3 (16.3) | 79.3 (17.9) | .82 |
| Antiplatelet therapy at baseline, n (%) | 1 (4.2) | 5 (7.8) | .90 |
| Concomitant use of P‐glycoprotein inhibitors, n (%) | 1 (4.2) | 1 (1.6) | .47 |
| Reduced‐dose edoxaban, n (%) | 6 (25) | 15 (23.4) | 1.00 |
| Mean platelet count × 109/L (SD) | 192 (115) | 213 (110) | .44 |
| Mean creatinine clearance in ml/min (SD) a | 91 (30.9) | 108 (51.1) | .13 |
Abbreviation: SD, standard deviation.
Calculated with the Cockroft and Gault formula.
In the univariable conditional logistic regression model, only a hemoglobin level below 10 g/dl (6.2 mmol/L) in the 4 weeks prior to the event was significantly associated with bleeding. 11 cases (45.8%) had a hemoglobin level <10 g/dl, compared with 10 controls (15.6%) (OR 4.0, 95% CI 1.4–11.1) (Table 2). There was a non‐statistically significant increased risk of bleeding in patients with advanced disease (defined as regionally advanced or metastatic cancer) (OR 2.7, 95% CI 0.9–8.7), platelet count <150 × 109/L (OR 1.9, 95% CI 0.6–6.3), and creatinine clearance <50 ml/min (OR 2.7, 95% 0.4–19.4). There was a non‐significant decreased risk of bleeding in patients with resected tumors (OR 0.4, 95% CI 0.1–1.4). There were no differences in bleeding in patients who were receiving chemotherapy (OR 1.3, 95% CI 0.5–3.5), antiplatelet agents (OR 1.0, 95% CI 0.1–9.6), NSAIDs (OR 0.7, 95% CI 0.1–8.4), or proton pump inhibitors (PPI) (OR 0.9, 95% CI 0.3–2.7) in the month prior to bleeding, or had a body weight <60 kg (OR 1.2, 95% CI 0.3–4.2).
TABLE 2.
Conditional logistic regression results
|
Cases (n = 24) |
Controls (n = 64) |
Univariable analyses OR (95% CI) |
Multivariable analyses a OR (95% CI) |
|
|---|---|---|---|---|
| Resected vs. non‐resected b | 4 (16.7) | 20 (31.2) | 0.4 (0.1–1.4) | 0.4 (0.1–1.4) |
| Stage | ||||
| Local disease | 1 (4.2) | 6 (9.4) | 1.3 (0.1–15.1) | 0.9 (0.1–9.6) |
| Locally advanced | 6 (25.0) | 11 (17.2) | 4.0 (0.7–24.7) | 5.7 (0.8–41.2) |
| Metastasis | 13 (54.2) | 27 (42.2) | 2.7 (0.7–10.1) | 3.1 (0.8–12.2) |
| Liver metastasis | 8 (33.3) | 21 (32.8) | 1.1 (0.4–2.9) | 1.1 (0.4–3.4) |
| Lung metastasis | 7 (29.2) | 11 (17.2) | 2.2 (0.7–7.0) | 3.2 (0.9–11.1) |
| Advanced disease | 19 (79.2) | 38 (59.4) | 2.7 (0.9–8.7) | 3.6 (1.01–12.6) |
| Surgery in 4 weeks prior to bleeding | 0 | 2 (3.1) | — | — |
| Endoscopy in 4 weeks prior to bleeding | 3 (12.5) | 3 (4.7) | 3.7 (0.6–23.0) | 3.8 (0.6–24.6) |
| Chemotherapy in 4 weeks prior to bleeding | 12 (50.0) | 29 (45.3) | 1.3 (0.5–3.5) | 1.3 (0.5–3.5) |
| Pyrimidine analogues | 5 (20.8) | 17 (26.6) | 0.8 (0.2–2.3) | 0.7 (0.2–2.5) |
| Irinotecan | 2 (8.3) | 3 (4.7) | 1.7 (0.3–10.2) | 2.4 (0.3–19.6) |
| Platinum based | 9 (37.5) | 16 (25.0) | 2.1 (0.7–6.0) | 2.4 (0.8–7.3) |
| Taxanes | 1 (4.2) | 6 (9.4) | 0.4 (0.1–3.9) | 0.2 (0.0–2.6) |
| Bevacizumab | 2 (8.3) | 3 (4.7) | 1.6 (0.3–9.4) | 1.5 (0.2–13.3) |
| Chemotherapy in week prior to bleeding | 4 (16.7) | 22 (34.4) | 0.3 (0.1–1.3) | 0.4 (0.1–1.4) |
| Corticosteroids in 2 weeks prior to bleeding | 3 (13.0) | 13 (20.3) | 0.6 (0.2–2.4) | 0.5 (0.1–2.3) |
| PPI in 2 weeks prior to bleeding | 6 (26.1) | 19 (30.2) | 0.9 (0.3–2.7) | 1.0 (0.3–3.1) |
| NSAIDs in 2 weeks prior to bleeding | 1 (4.2) | 3 (4.7) | 0.7 (0.1–7.4) | 0.7 (0.1–7.8) |
| Antiplatelet use in 2 weeks prior to bleeding | 1 (4.2) | 3 (4.7) | 1 (0.1–9.6) | 0.9 (0.1–9.6) |
| Full edoxaban dose | 16 (66.7) | 47 (74.6) | 0.6 (0.2–1.8) | 0.3 (0.1–1.4) |
| Hypertension treatment in 4 weeks prior to bleeding | 5 (20.8) | 17 (26.6) | 0.7 (0.2–2.2) | 0.8 (0.2–2.5) |
| History of overt gastrointestinal bleeding | 1 (4.7) | 3 (4.2) | 1.0 (0.1–9.6) | 1.3 (0.2–10.1) |
| Weight <60 kg in 4 weeks prior to bleeding | 4 (17.4) | 10 (16.7) | 1.2 (0.3–4.2) | 2.6 (0.4–14.6) |
| ≥1 risk factors for bleeding c | 20 (83.3) | 41 (64.1) | 3.2 (0.9–12.1) | 3.5 (0.9–13.8) |
| Hemoglobin <10 g/dl in 4 weeks prior to bleeding | 11 (45.8) | 10 (15.6) | 4.0 (1.4–11.1) | 4.8 (1.5–16.0) |
| Platelet count <150 × 109/L in 4 weeks prior to bleeding | 7 (29.2) | 12 (18.8) | 1.9 (0.6–6.3) | 1.4 (0.4–5.0) |
| Creatinine clearance <50 ml/min in 4 weeks prior to bleeding d | 2 (8.7) | 2 (3.4) | 2.7 (0.4–19.4) | 1.5 (0.2–12.6) |
Bold indicate statistical significance values.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; NSAID, non‐steroidal anti‐inflammatory drugs; OR, odds ratio; PPI, proton pump inhibitor.
Adjusted for age, sex and grouped tumor type (colorectal; hepatobiliary and pancreatic; upper gastrointestinal).
Non‐resected could be resected at baseline but reflect recurrence during the study period.
Risk factors for bleeding defined in the Hokusai VTE Cancer study as: locally advanced or metastatic cancer, brain metastasis, bevacizumab or antiplatelet use, recent surgery.
Calculated with the Cockroft and Gault formula.
In the multivariable conditional logistic regression analysis corrected for age, sex and tumor type, advanced disease in the month prior to the event was significantly associated with gastrointestinal bleeding (OR 3.6, 95% CI 1.01–12.6) as was a hemoglobin level below 10 g/dl (OR 4.8, 95% CI 1.5–16.0). The odds ratios for a low platelet count (OR 1.4, 95% CI 0.4–5.0) and a creatinine clearance <50 ml/min (OR 1.5, 95% CI 0.2–14.9) were lower compared with the crude analysis. Similar to the univariable analysis, there were no differences in bleeding risk with use of chemotherapy (OR 1.3, 95% CI 0.5–3.5), antiplatelet agents (OR 0.9, 95% CI 0.1–9.6), NSAIDs (OR 0.7, 95% CI 0.1–7.8), or PPIs (OR 1.0, 95% CI 0.3–3.1). There was a non‐significant lower risk of bleeding in patients with resected tumors during the study period (OR 0.4, 95% CI 0.1–1.4) In the multivariable analysis, there was an increased but not significant risk of bleeding in patients who had endoscopy in the month prior to the bleeding event (OR 3.8, 95% CI 0.6–24.6), lung metastasis (OR 3.2, 95% CI 0.9–11.1), platinum based chemotherapy (OR 2.4, 95% CI 0.8–7.3), or a body weight <60 kg (OR 2.6, 95% CI 0.4–14.6) (Table 2).
Since additional data could not be collected for all cases, there were several controls without a matched case. These additional control patients were not included in the primary analysis but were considered in sensitivity analysis which showed similar results as the conditional logistic regression model (Table 3). In the multivariable analysis, only a hemoglobin level below 10 g/dl was significantly associated with gastrointestinal bleeding (OR 4.1, 95% CI 1.4–12.6). Additionally, in the second sensitivity analysis, the seven controls who became a case later were excluded as controls. These patients were left in the analysis as cases only, resulting in 24 cases and 57 controls. The results were broadly similar to the main conditional logistic regression analysis. The odds ratios in the multivariable analysis for advanced disease and hemoglobin levels <10 g/L were 4.0 (95% CI 1.1–14.3) and 4.2 (95% CI 1.3–13.8) respectively.
TABLE 3.
Unconditional logistic regression with days since inclusion as a covariate
|
Cases (n = 24) |
Controls (n = 77) |
Univariable analyses + time since inclusion OR (95% CI) |
Multivariable analyses a OR (95% CI) |
|
|---|---|---|---|---|
| Resected vs. non‐resected b | 4 (16.7) | 22 (28.6) | 0.5 (0.1–1.6) | 0.5 (0.1–1.5) |
| Stage | ||||
| Local disease | 1 (4.2) | 7 (9.1) | 0.8 (0.1–6.8) | 0.7 (0.1–6.4) |
| Locally advanced | 6 (25.0) | 15 (19.5) | 2.2 (0.5–10.4) | 2.7 (0.6–13.8) |
| Metastasis | 13 (54.2) | 33 (42.9) | 2.1 (0.6–8.7) | 2.3 (0.7–9.6) |
| Liver metastasis | 8 (33.3) | 26 (33.8) | 1.0 (0.4–2.5) | 1.0 (0.3–2.7) |
| Lung metastasis | 7 (29.2) | 11 (14.3) | 2.5 (0.8–7.3) | 3.0 (0.9–9.5) |
| Advanced disease | 19 (79.2) | 48 (62.3) | 2.3 (0.8–7.7) | 2.6 (0.9–9.0) |
| Surgery in 4 weeks prior to bleeding | 0 | 2 (2.6) | — | — |
| Endoscopy in 4 weeks prior to bleeding | 3 (12.5) | 3 (3.9) | 3.5 (0.6–20.3) | 4.0 (0.7–25.1) |
| Chemotherapy in 4 weeks prior to bleeding | 12 (50.0) | 36 (46.8) | 1.1 (0.4–2.9) | 1.2 (0.5–3.3) |
| Pyrimidine analogues | 5 (20.8) | 21 (27.3) | 0.7 (0.2–2.0) | 0.7 (0.2–2.1) |
| Irinotecan | 2 (8.3) | 4 (5.2) | 1.6 (0.2–9.0) | 2.0 (0.2–11.9) |
| Platinum based | 9 (37.5) | 17 (22.1) | 2.1 (0.8–6.0) | 2.7 (0.9–8.0) |
| Taxanes | 1 (4.2) | 7 (9.1) | 0.4 (0.0–2.5) | 0.3 (0.0–2.4) |
| Bevacizumab | 2 (8.3) | 3 (3.9) | 2.2 (0.3–14.2) | 2.3 (0.3–17.3) |
| Chemotherapy in week prior to bleeding | 4 (16.7) | 27 (35.1) | 0.4 (0.1–1.1) | 0.4 (0.1–1.1) |
| Corticosteroids in 2 weeks prior to bleeding | 3 (13.0) | 14 (18.2) | 0.7 (0.1–2.3) | 0.6 (0.1–2.4) |
| PPI in 2 weeks prior to bleeding | 6 (26.1) | 23 (30.3) | 0.8 (0.3–2.2) | 0.9 (0.3–2.7) |
| NSAIDs in 2 weeks prior to bleeding | 1 (4.2) | 3 (3.9) | 1.1 (0.1–9.5) | 1.0 (0.0–9.1) |
| Antiplatelet use in 2 weeks prior to bleeding | 1 (4.2) | 3 (3.9) | 1.1 (0.1–8.7) | 1.1 (0.1–11.4) |
| Full edoxaban dose | 16 (66.7) | 59 (77.6) | 0.6 (0.2–1.7) | 0.4 (0.1–1.3) |
| Hypertension treatment in 4 weeks prior to bleeding | 5 (20.8) | 22 (28.6) | 0.7 (0.2–1.9) | 0.6 (0.2–2.0) |
| History of overt gastrointestinal bleeding | 1 (4.7) | 3 (4.2) | 1.0 (0.1–8.7) | 1.1 (0.1–11.1) |
| Weight <60 kg in 4 weeks prior to bleeding | 4 (17.4) | 10 (13.7) | 1.3 (0.3–4.5) | 2.8 (0.5–16.1) |
| ≥1 risk factors for bleeding c | 20 (83.3) | 51 (58.0) | 2.6 (0.8–9.8) | 2.8 (0.9–11.1) |
| Hemoglobin <10 g/dl in 4 weeks prior to bleeding | 11 (45.8) | 15 (19.5) | 3.5 (1.3–9.5) | 4.1 (1.4–12.6) |
| Platelet count <150 × 109/L in 4 weeks prior to bleeding | 7 (29.2) | 15 (19.5) | 1.7 (0.6–4.9) | 1.5 (0.5–4.4) |
| Creatinine clearance <50 ml/min in 4 weeks prior to bleeding d | 2 (8.7) | 3 (4.2) | 2.3 (0.3–15.4) | 1.5 (0.2–11.8) |
Bold indicate statistical significance values.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; NSAID, non‐steroidal anti‐inflammatory drugs; OR, odds ratio; PPI, proton pump inhibitor.
Adjusted for age, sex and grouped tumor type (colorectal; hepatobiliary and pancreatic; upper gastrointestinal).
Non‐resected could be resected at baseline but reflect recurrence during the study period.
Risk factors for bleeding defined in the Hokusai VTE Cancer study as: locally advanced or metastatic cancer, brain metastasis, bevacizumab or antiplatelet use, recent surgery.
Calculated with the Cockroft and Gault formula.
4. DISCUSSION
In this nested case‐control study of patients with gastrointestinal cancer enrolled in the Hokusai VTE Cancer study, those with locally advanced disease or distant metastasis had a more than 3‐fold higher risk of major or clinically relevant non‐major gastrointestinal bleeding during treatment with edoxaban, whereas those with a hemoglobin level below 10 g/dl (6.2 mmol/L) had a more than 4‐fold higher risk. These findings suggest that caution is warranted when using edoxaban for treatment of acute VTE in patients with advanced gastrointestinal cancer. We did not identify modifiable risk factors (e.g. chemotherapy) nor protective factors (e.g. PPI) for gastrointestinal bleeding.
In the Hokusai VTE Cancer and SELECT‐D trials, an increased risk of gastrointestinal bleeding was observed in patients randomized to DOACs, mostly in those with cancers of the upper gastrointestinal tract. A potential explanation is the local anticoagulant effect of DOACs in the gut lumen, since substantial amounts of active drug are excreted in the faeces, 26 which could lead to bleeding from vulnerable intestinal lesions. Patients with advanced disease are more likely to have unresectable luminal tumors in the gastrointestinal tract, which could explain the increased risk of bleeding in cases and the numerically lower bleeding risk in patients with resected tumors (OR 0.4, 95% CI 0.1–1.4). Notably, the increased risk of gastrointestinal bleeding with DOACs was not observed in the CARAVAGGIO trial, which evaluated apixaban for cancer‐associated VTE. 6 Reasons for this disparity are unclear, but may be related to intrinsic properties of apixaban, selection of fewer patients with advanced (unresected) gastrointestinal cancer, or may represent a random finding.
A hemoglobin level below 10 g/dl in the month prior to bleeding also appeared to be associated with gastrointestinal bleeding, which is in line with findings from modeling studies that identified anemia as a consistent predictor. 16 Whether a low hemoglobin is a true predictor or simply a reflection of occult blood loss is unknown. Since all major bleeding events in this study were classified as such due to overt bleeding associated with a hemoglobin drop or need for transfusion of two or more units of blood, in some cases bleeding may have already started at the time of hemoglobin measurement.
The main limitation of this study is the modest number of cases which resulted in limited statistical power and therefore the results should merely be regarded as exploratory. Consequently, we were unable to analyze major and clinically relevant non‐major bleeding separately, although it is likely that risk factors are consistently associated with both types of bleeding. Nonetheless, our results provide a detailed description of factors associated with gastrointestinal bleeding events occurring during edoxaban treatment. The present data show that 79% (19 out of 24) of bleeding events occurred in patients with advanced disease at the time of bleeding. Additionally to the analyzed variables in this study, we aimed to collect data on the presence of mucositis and alcohol intake, since these are important risk factors for bleeding, however, these data were only available for very few cases, therefore these variables were left out of the analysis. For the other variables included in the conditional logistic regression analyses, no data were missing. No multiple comparisons correction was applied, since it likely would have resulted in no outcomes being significantly associated with clinically relevant bleeding due to the small sample size. Therefore, for example, the possible true effect of advanced disease on bleeding risk would have been diminished. Nonetheless, we would like to stress that a false positive effect due to the number of variables assessed is possible.
Several previous studies have assessed risk factors for bleeding in patients with various cancer types. In a retrospective cohort study by Angelini of over 400 000 cancer patients treated with rivaroxaban or apixaban, the risk of major bleeding was higher in those with metastatic disease, chronic kidney disease, or a platelet count below 100 × 109/L. 17 In the RIETE registry, an international registry which collects data on patients with VTE, an eGFR <30 ml/min, immobility (i.e. total bed rest with bathroom privileges for ≥4 days), distant metastasis, and recent bleeding were identified as risk factors for bleeding in 3805 cancer patients receiving either vitamin K antagonists or LMWH. 18 Finally, terminal cancer and chronic kidney disease were identified as risk factors for major bleeding in the COMMAND VTE registry, a multicenter retrospective registry in Japan. 19 The present study only focused on risk factors for bleeding in patients with gastrointestinal cancer. Although we observed a higher proportion of cases than controls with a platelet count below 150 × 109/L (29.1% vs. 18.8%), this difference did not meet statistical significance (OR 1.4, 95% CI 0.4–5.0). We were unable to assess renal insufficiency as a risk factor because few such patients were included in the analysis.
Compared with non‐cancer patients, there are several additional risk factors that can increase the risk of bleeding in the cancer population. For example, cancer patients have an increased risk of gastrointestinal bleeding, because chemotherapy agents such as irinotecan, taxanes and platinum‐based regimens can cause mucositis. 20 Unfortunately, we could not retrieve information on the frequency of mucositis during the study period. Nonetheless, there was a higher but non‐significant risk of bleeding in patients receiving platinum‐based chemotherapy (OR 2.4, 95% CI 0.8–7.3). Additionally, cancer patients often undergo surgery or endoscopy procedures during the course of their disease, which is associated with an important risk of bleeding. 21 In this study, endoscopy in the month prior to bleeding did show a non‐significant increased risk of bleeding (OR 3.8, 95% CCI 0.6–24.6), however, no cases underwent surgery in the month prior to bleeding.
The high risk of gastrointestinal bleeding in cancer patients may be decreased by use of PPIs. This notion is supported by a subgroup analysis of the COMPASS trial, which compared rivaroxaban vs aspirin and pantoprazole vs. placebo in a 2 × 2 factorial design. 22 Pantoprazole significantly reduced the risk of bleeding in patients with evidence of gastrointestinal lesions detected by gastroscopy compared with placebo (HR 0.45, 95% CI 0.27–0.74), with a number needed to treat of 982. It is unknown whether pantoprazole has similar protective effects in patients with tumor lesions of the intestinal tract. Although 28% of all patients in our study were using PPIs, there was no trend towards a protective effect in these patients (OR 1.0, 95% CI 0.3–3.1).
To our knowledge this is the first study that assessed risk factors for gastro‐intestinal bleeding in patients with gastrointestinal cancer. Since the majority of major bleeding events in the Hokusai VTE cancer trial were gastrointestinal bleeding events in patients with gastrointestinal cancer, this study was needed to assess important risk factors which were associated with an increased risk of gastrointestinal bleeding in these patients. Besides tumor stage and hemoglobin levels, other potential risk factors for bleeding such as the use of concomitant medication and cancer therapy were assessed, which could possibly help to guide safe use of DOACs in patients with gastrointestinal bleeding. Most guidelines suggest against the use of DOACs in patients with gastrointestinal tumors due to the increased risk of bleeding, usually without consideration of cancer stage or whether the tumor was resected. 8 , 9 Given that the group of patients with gastrointestinal tumors is heterogeneous, DOACs could potentially be safe in some patient subgroups. The present data suggest that edoxaban is associated with a lower risk of bleeding in patients with local or resected gastrointestinal tumors, although the limited sample size precludes definite conclusions.
In conclusion, in this case‐control study, regionally advanced or metastatic gastrointestinal cancer was associated with an increased risk of gastrointestinal bleeding in patients using edoxaban for treatment of VTE. These findings call for additional studies on the safety of edoxaban specifically in patients with local, resected luminal gastrointestinal cancers in whom the risk‐benefit balance may be favorable.
CONFLICT OF INTEREST
Floris T.M. Bosch reports no conflicts of interest. Frits I. Mulder reports no conflicts of interest. Menno V. Huisman reports Grants ZonMw Dutch Healthcare Fund. Grants and consultation fees from Boehringer‐Ingelheim, Pfizer‐BMS, Bayer Health Care, Aspen, and Daiichi‐Sankyo, to the hospital. Jeffrey I. Zwicker reports research funding from Incyte and Quercegen; consultancy services to Sanofi, CSL, and Parexel; and honoraria from advisory board participation with Pfizer/Bristol Myers Squibb (BMS), and Portola. Marcello Di Nisio reports personal fees from Bayer, Daiichi Sankyo, Sanofi, Pfizer, Leo Pharma, and Aspen outside the present work. Marc Carrier reports Research Funding: BMS, Pfizer and Leo Pharma. Honoraria: Bayer, BMS, Pfizer, Leo Pharma, Sanofi and Servier. Annelise Segers reports grants from Daiichi Sankyo Pharma, during the conduct of the study; grants from Anthos Therapeutics, grants from Janssen Pharmaceuticals, grants from Tetherex, outside the submitted work. Peter Verhamme reports Honoraria for lectures/consultancy: Bayer, Boehringer, BMS, Pfizer, Daiichi‐Sankyo, Leo Pharma, Anthos Pharmaceuticals. Saskia Middeldorp reports grants and fees paid to her institution from GlaxoSmithKline, BMS/Pfizer, Aspen, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Sanofi, and Portola. Jeffrey I. Weitz was a consultant for and received honoraria from Anthos, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi‐Sankyo, Ionis, Janssen, Novartis, and Pfizer. Michael A. Grosso is employed by Daiichi Sankyo. Anil Duggal is employed by Daiichi Sankyo. Harry R. Büller reports research support from Sanofi‐aventis, Bayer HealthCare, Bristol‐Myers Squibb, Daiichi‐Sankyo, GlaxoSmithKline, Pfizer, Roche, IONIS, Boehringer Ingelheim, Eli Lilly, Novartis. Consultant from Sanofi‐aventis, Bayer HealthCare, Bristol‐Myers Squibb, Daiichi‐Sankyo, GlaxoSmithKline, Pfizer, Roche, IONIS, Boehringer Ingelheim, Eli Lilly, Novartis. Scientific advisory board from Sanofi‐aventis, Bayer HealthCare, Bristol‐Myers Squibb, Daiichi‐Sankyo, GlaxoSmithKline, Pfizer, Roche, IONIS, Boehringer Ingelheim, Eli Lilly, Novartis. Tzu‐Fei Wang reports advisory board honoraria from Servier and grants from Leo Pharma. Frits I. Mulder and David Garcia report no conflicts of interest. Pieter Willem Kamphuisen reports research funding from Daiichi Sankyo. Gary E. Raskob reports payment for consultant services from the following companies: AMAG Pharma, Anthos Therapeutics, Bayer Healthcare, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Itreas, Janssen, Novartis, Pfizer, Portola, Tetherex, XaTek. Nick van Es reports advisory board fees from Bayer, Daiichi Sankyo, and LEO Pharma, which were transferred to his institution.
AUTHOR CONTRIBUTIONS
F.T. Bosch and N. van Es designed the study protocol. F.T. Bosch, M.V. Huisman, J.I. Zwicker, M. di Nisio, M. Carrier, P. Verhamme, T‐F. Wang and D. Garcia collected additional data for this study. F.T. Bosch performed the analyses and wrote the first draft of the manuscript. F.I. Mulder, N. van Es and H.R. Büller critically reviewed the first draft of the manuscript. The second draft was critically reviewed by all included authors. All authors have read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
This study received financial support from Daiichi Sankyo Global. We would like to acknowledge Sandro Aquilanti, Lisa Baumann Kreuziger, Aart Beeker, Jan Beyer‐Westendorf, Zoltan Boda, Marianne Brodmann, Patrick Carroll, Ad Dees, Antoine Elias, Anna Falanga, Carme Font Puig, Enruiqe Gallardo, Fernando Garcia‐Bragado, Angelo Ghirarduzzi, Mihály Gurzó, Chirstiam Rojas Hernandez, Thomas Horacek, Davide Imberti, Agnes Lee, Corrado Lodigiani, Rien van Marwijk‐Kooy, Ara Metjian, Guy Meyer, Patrick Mismetti, Ingrid Pabinger, Ettore Porreca, Amparo Santamaria, Piet Vercauter, Adriana Visonà, Peter Westerweel and Erik Yeo for providing additional data.
Bosch FTM, Mulder FI, Huisman MV, et al. Risk factors for gastrointestinal bleeding in patients with gastrointestinal cancer using edoxaban. J Thromb Haemost. 2021;19:3008–3017. 10.1111/jth.15516
Manuscript handled by: Sabine Eichinger
Final decision: Sabine Eichinger, 26 August 2021
REFERENCES
- 1. Mulder FI, Horváth‐Puhó E, van Es N, et al. Venous thromboembolism in cancer patients: a population‐based cohort study. Blood. 2021;137:1959‐1969. [DOI] [PubMed] [Google Scholar]
- 2. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38:496‐520. [DOI] [PubMed] [Google Scholar]
- 3. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med. 2018;378(7):615‐624. [DOI] [PubMed] [Google Scholar]
- 4. Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT‐D). J Clin Oncol. 2018;36:2017‐2023. [DOI] [PubMed] [Google Scholar]
- 5. McBane RD, Wysokinski WE, Le‐Rademacher JG, et al. Apixaban and dalteparin in active malignancy‐associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18:411‐421. [DOI] [PubMed] [Google Scholar]
- 6. Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382:1599‐1607. [DOI] [PubMed] [Google Scholar]
- 7. Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566‐e581. [DOI] [PubMed] [Google Scholar]
- 8. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2019;38:496‐520. [DOI] [PubMed] [Google Scholar]
- 9. Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kraaijpoel N, Di Nisio M, Mulder F, et al. Clinical impact of bleeding in cancer‐associated venous thromboembolism: results from the Hokusai VTE cancer study. Thromb Haemost. 2018;118:1439‐1449. [DOI] [PubMed] [Google Scholar]
- 11. van Es N, Di Nisio M, Bleker SM, et al. Edoxaban for treatment of venous thromboembolism in patients with cancer. Thromb Haemost. 2015;114:1268‐1276. [DOI] [PubMed] [Google Scholar]
- 12. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. Scientific and Standardization Committee Communication. J Thromb Haemost. 2005;3:692‐694. [DOI] [PubMed] [Google Scholar]
- 13. Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case‐control studies: III. Design options. Am J Epidemiol. 1992;135:1042‐1050. [DOI] [PubMed] [Google Scholar]
- 14. Szklo M, Nieto FJ. Epidemiology: Beyond the Basics, 4th edn. Jones & Bartlett Learning; 2019. [Google Scholar]
- 15. Cologne J, Langholz B. Selecting controls for assessing interaction in nested case‐control studies. J Epidemiol. 2003;13:193‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Winter MA, van Es N, Büller HR, Visseren FLJ, Nijkeuter M. Prediction models for recurrence and bleeding in patients with venous thromboembolism: a systematic review and critical appraisal. Thromb Res. 2021;199:85‐96. [DOI] [PubMed] [Google Scholar]
- 17. Angelini DE, Radivoyevitch T, McCrae KR, Khorana AA. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am J Hematol. 2019;94:780‐785. [DOI] [PubMed] [Google Scholar]
- 18. Trujillo‐Santos J, Nieto J, Tiberio G, et al. Predicting recurrences or major bleeding in cancer patients with venous thromboembolism. Thromb Haemost. 2008;100:435‐439. [PubMed] [Google Scholar]
- 19. Nishimoto Y, Yamashita Y, Kim K, et al. Risk factors for major bleeding during anticoagulation therapy in cancer‐associated venous thromboembolism ‐ from the COMMAND VTE Registry. Circ J. 2020;84:2006‐2014. [DOI] [PubMed] [Google Scholar]
- 20. Thomsen M, Vitetta L. Adjunctive treatments for the prevention of chemotherapy‐ and radiotherapy‐induced mucositis. Integr Cancer Ther. 2018;17:1027‐1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen D, Afzal N, Sohn S, et al. Postoperative bleeding risk prediction for patients undergoing colorectal surgery. Surgery. 2018;164:1209‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moayyedi P, Eikelboom JW, Bosch J, et al. Pantoprazole to prevent gastroduodenal events in patients receiving rivaroxaban and/or aspirin in a randomized, double‐blind, placebo‐controlled trial. Gastroenterology. 2019;157:403‐412.e5. [DOI] [PubMed] [Google Scholar]
- 23. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10:10‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim JH. Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol. 2015;21:5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di Nisio M, Raskob G, Büller H, et al. Prediction of major and clinically relevant bleeding in patients with VTE treated with edoxaban or vitamin K antagonists. Thromb Haemost. 2017;117:784‐793. [DOI] [PubMed] [Google Scholar]
- 26. Desai J, Kolb J, Weitz J, Aisenberg J. Gastrointestinal bleeding with the new oral anticoagulants – defining the issues and the management strategies. Thromb Haemost. 2013;110:205‐212. [DOI] [PubMed] [Google Scholar]


