Abstract
Background
Despite the high prevalence of adenomyosis in hysterectomy specimens of endometrial carcinoma (EC) patients, the relationship between adenomyosis and EC prognosis appears unclear.
Objective
To assess the prognostic value of coexistent adenomyosis in patients with EC.
Methods
A systematic review and meta‐analysis was performed by searching six electronic databases for studies reporting data on prognosis of EC patients with and without coexistent adenomyosis. Studies with patient selection based on prognostic factors were excluded. Pooled univariate hazard ratio (HR) analyses for overall survival (OS) and disease‐free survival (DRF) were performed, using EC patients without adenomyosis as a control group. For DFS, pooled multivariate HR analysis was also evaluable.
Results
Three studies of 2505 EC patients (553 with and 1952 without adenomyosis) were included. Compared with EC patients without adenomyosis, EC patients with coexistent adenomyosis showed a pooled HR of 0.533 (CI 95%, 0.329–0.864) for OS at univariate analysis; 0.536 (CI 95%, 0.334–0.859) for DFS at univariate analysis; and 0.875 (CI 95%, 0.331–2.315) for DFS at multivariate analysis.
Conclusion
In EC patients with coexistent adenomyosis, the risk of death is halved compared with EC patients without adenomyosis. However, the independence of this association needs to be verified in future studies.
Keywords: endometrium, gynecology, malignancy, myometrium, oncology, tumor
Short abstract
The risk of death is halved in EC patients with coexistent adenomyosis compared with EC patients without adenomyosis, whereas the risk of EC recurrence does not appear to be affected.
1. INTRODUCTION
Endometrial carcinoma (EC) is the most frequent gynecological tumor in high‐resource countries and the fourth in women worldwide. 1 , 2 , 3 , 4 , 5 , 6 , 7 Both incidence and number of deaths per year have increased in recent years. 1 , 2 Increase in incidence appears related to the increase in EC risk factors, such as obesity, metabolic syndrome, and causes of hyperestrogenic state, 8 while the increase in number of deaths seems to be due to poorly reproducible pathologic risk stratification of patients. 9 , 10 Hence, a more accurate and tailored risk assessment able to direct patient management is needed.
In the future, integration among all available prognostic approaches, such as The Cancer Genome Atlas program (TCGA) and Proactive Molecular Risk Classifier for Endometrial Cancer (ProMisE) molecular signatures, 2 , 7 , 11 , 12 , 13 , 14 , 15 , 16 , 17 classic histopathological factors (histotype, tumor grade, myometrial infiltration, lymph node involvement, lymphovascular space invasion), and clinical characteristics (age, body mass index, clinical stage, other diseases) 18 might lead to individualized management of patients.
In this regard, adenomyosis appears to be one of the most frequent diseases associated with EC on hysterectomy specimens. 19 , 20 Adenomyosis is defined by the migration of glands and stroma from the basal layer of the endometrium to the myometrium. 21 In a recent meta‐analysis, 22 EC patients with coexistent adenomyosis showed a significantly increased overall survival (OS). On the contrary, no difference was found in terms of disease‐free survival (DFS). However, some studies included in the meta‐analysis only included EC patients with an endometrioid histotype. 22 As the endometrioid histotype shows the best prognosis in EC patients, 17 , 23 the results from this meta‐analysis may be affected by this patient selection. The aim of the present systematic review and meta‐analysis was to assess the prognostic value of coexistent adenomyosis in patients with EC of any histotype.
2. MATERIALS AND METHODS
The study followed a protocol that a priori defined each review step and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement. 24 Each step was completed by two authors and disagreements were solved by discussion with the authors who supervised the study (PC, AM, LI, FZ, RS).
Several searches were performed in six electronic databases: MEDLINE, Google Scholar, Web of Science, Scopus, ClinicalTrials.gov, and the Cochrane Library. Databases were searched from their inception to December 2020 with several combinations of the following text words: “endometr*”; “cancer”; “carcinoma”; “neoplas*”; “malignancy”; “tumour”; “tumor”; “adenomyosis”; “myometr*”. We also screened the reference list from each eligible article.
All peer‐reviewed articles reporting data about prognosis of EC patients with and without coexistent adenomyosis were included. A priori defined exclusion criteria were literature reviews, case reports, and studies with patient selection based on prognostic factors.
The Methodological Index for Non‐Randomized Studies (MINORS) was followed for assessing the risk of bias within studies. 25 Each included study was assessed in six applicable domains related to risk of bias: (1) aim (if the study aim was clearly stated); (2) patient selection (if all consecutive patients were included); (3) data collection (if a study protocol was a priori defined and followed for data collection); (4) study endpoints (if clear endpoints were adopted); (5) endpoint assessment (if endpoints were assessed through univariate and multivariate analyses); and (6) lost to follow‐up (if patients with no prognosis data were less than 5% of the total study population).
We judged the included studies at “low risk,” “unclear risk,” or “high risk” of bias in each domain based on whether data were “reported and adequate,” “not reported,” or “reported but inadequate,” respectively.
The PICO (Population, Intervention, Comparator, Outcomes) framework was followed for data extraction. 24 “Population” in this study consists of EC patients. “Intervention” (or risk factor) consists of diagnosis of adenomyosis. “Comparator” consists of absence of adenomyosis. “Outcomes” were OS (primary outcome) and DFS (secondary outcome). OS was defined as the time interval between the date of surgery and death or the last date of follow‐up, while DFS was defined as the time interval between the date of surgery and the date of EC recurrence or the last date of follow‐up.
In the included studies, the association of adenomyosis with EC survival outcomes was assessed at univariate analysis by using the log‐rank test and the Kaplan–Meier method. Subsequently, the independent prognostic value of adenomyosis was assessed at multivariate analysis using the Cox proportional hazards model.
Hazard ratios (HR) were reported for each individual study and as a pooled estimate on forest plots, with 95% confidence interval (CI), for OS and DFS univariate analysis, and DFS multivariate analyses. EC patients without coexistent adenomyosis were considered as the reference. The random‐effects model of DerSimonian and Laird was adopted for all analyses.
The inconsistency index I 2 was adopted to quantify statistical heterogeneity among studies, as previously described. 26 , 27 Heterogeneity was judged as null for I 2 = 0%, minimal for 0% < I 2 < 25%, low for 25% < I 2 < 50%, moderate for 50% < I 2 < 75% and high for I 2 ≥ 75%.
Comprehensive Meta‐Analysis software (Biostat Inc) and Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, Cochrane Collaboration, 2014) were used as software for data analysis.
3. RESULTS
A total of 4002 papers were found through electronic database searches. Of these, 1034 papers remained after duplicate removal, 753 after title screening, and 54 after abstract screening. After full‐text assessment, three papers were included in the qualitative and quantitative analyses 28 , 29 , 30 (Figure S1).
All three included studies were designed as observational retrospective cohort studies (Table 1). The study population consisted of 2505 women with EC: 553 (22.1%) with coexistent adenomyosis and 1952 (77.9%) without adenomyosis. Mean age ranged from 52.7 to 59 years, and mean body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) ranged from 25.2 to 35.8 kg. Of the included studies with extractable data, 63.8% of patients were menopausal and 12.8% were nulliparous. EC patients had an endometrioid histotype in 88.6% of cases, FIGO Stage I in 78.7%, deep myometrial infiltration in 33.6%, and lymphovascular space invasion in 30.2% (Table 2).
TABLE 1.
Details of the included studies
| Study | Country | Setting | Type of cohort | Period of endometrial cancer diagnosis | Patient selection |
|---|---|---|---|---|---|
| 2014 Matsuo 28 | USA | Los Angeles County Medical Center, USA | Retrospective cohort | 2000–2012 | Consecutive |
| 2017 Zhang 29 | China | Hebei general Hospital, China | Retrospective cohort | 2008–2014 | Consecutive |
| 2018 Boonlak 30 | Thailandia | Srinagarind Hospital, Thailand | Retrospective cohort | 2010–2016 | Consecutive |
TABLE 2.
Details of populations included in the study
| Study |
Total patients (No). |
Adenomyosis No. (%) |
Age, mean ± SD (range) | BMI, mean ± SD |
Postmenopausal No. (%) |
Multiparity No. (%) |
Endometrioid No. (%) |
Nonendometrioid No. (%) |
Stage I No. (%) |
Stage II N (%) |
Stage III N (%) |
Stage IV N (%) |
5‐year survival No. (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
2014 Matsuo 28 |
571 | Yes | 271 (47.4) | 52.7 ± 9.6 | 35.8 ± 9.1 | — | 197 (76.7) | 229 (84.5) | 42 (15.5) | 202 (74.8) | 18 (6.7) | 38 (14.1) | 12 (4.4) | 242 (89.2) |
| No | 300 (52.5) | 52.7 ± 10.7 | 35.5 ± 10.7 | — | 187 (65.6) | 247 (82.3) | 53 (17.7) | 193 (64.3) | 32 (10.7) | 48 (16.0) | 27 (9.0) | 234 (78.2) | ||
|
2017 Zhang 29 |
1584 | Yes | 150 (9.4) | 53 | 27 | 81 (54) | — | 140 (93.3) | 10 (6.67) | 139 (92.6) | 11 (7.3) | 137 (91.3) | 13 (8.7) | 138 (92.1) |
| No | 1434 (90.5) | 55 | 26.6 | 930 (64.8) | — | 1294 (90.2) | 140 (9.76) | 1227 (85.5) | 207 (14.4) | 1212 (84.5) | 222 (15.4) | 1121 (78.2) | ||
|
2018 Boonlak 30 |
350 | Yes | 132 (37.7) | 59 (36–80) | 25.4 ± 4.8 | — | 99 (75.0) | — | — | 84 (63.7) | 14 (10.6) | 29 (21.9) | 5 (3.8) | 108 (82.1) |
| No | 218 (62.2) | 58 (31–84) | 25.2 ± 4.2 | — | 162 (74.3) | — | — | 127 (58.3) | 20 (9.2) | 52 (23.8) | 19 (8.7) | 162 (74.4) | ||
| Total | 2505 | Yes | 553 | — | — | 81 (54) | 296 (45.9) | 369 (19.3) | 52 (21.2) | 425 (21.5) | 43 (14.2) | 204 (13.4) | 30 (10) | 488 (24.3) |
| No | 1952 | — | — | 930 (64.8) | 349 (54.1) | 1541 (80.7) | 193 (78.7) | 1547 (78.4) | 259 (85.8) | 1312 (86.5) | 268 (90) | 1517 (75.5) | ||
All included studies were judged at “low risk” of bias in the aim, patient selection, data collection, and study endpoint domains. In the endpoint assessment domain, all included studies were judged at “unclear risk” of bias because multivariate analysis for OS was not performed. In the lost to follow‐up domain, all included studies were judged at “unclear risk” of bias because it was not possible to assess if patients lost to follow‐up were less than 5% of the total study population (Figure S2a).
All included studies were suitable for OS analysis, while one study was excluded from DFS analysis because it did not consider this outcome. 29
Compared with EC patients without adenomyosis, EC patients with coexistent adenomyosis showed a pooled HR of 0.533 (CI 95%, 0.329–0.864; I 2 = 26.1) for OS at univariate analysis (Figure 1); 0.536 (CI 95%, 0.334–0.859; I 2 = 41.5) for DFS at univariate analysis (Figure 2); and 0.875 (CI 95%, 0.331–2.315; I 2 = 84.5) for DFS at multivariate analysis (Figure 3).
FIGURE 1.
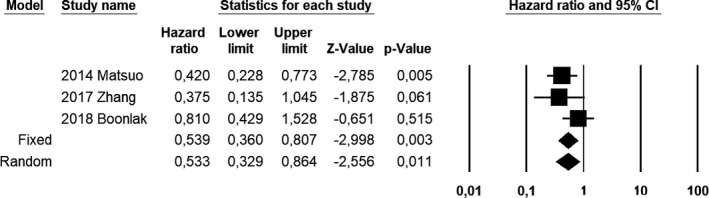
Forest plot of hazard ratios for overall survival of endometrial carcinoma patients with coexistent adenomyosis at univariate analysis; endometrial carcinoma patients without adenomyosis were used as a control group
FIGURE 2.
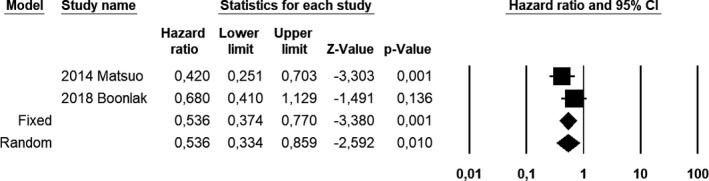
Forest plot of hazard ratios for disease‐free survival of endometrial carcinoma patients with coexistent adenomyosis at univariate analysis; endometrial carcinoma patients without adenomyosis were used as a control group
FIGURE 3.
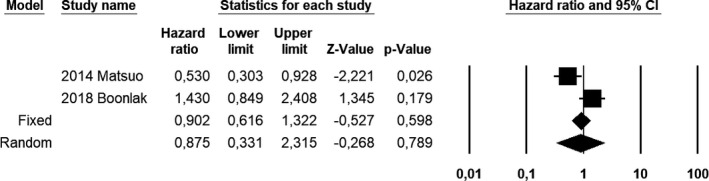
Forest plot of hazard ratios for disease‐free survival of endometrial carcinoma patients with coexistent adenomyosis at multivariate analysis; endometrial carcinoma patients without adenomyosis were used as a control group
4. DISCUSSION
The present study shows that EC patients with coexistent adenomyosis have half the risk of death and recurrence compared with EC women without adenomyosis. However, the protective effect of adenomyosis on EC recurrence was not found to be independent from other prognostic factors at multivariate analysis.
Adenomyosis is one of the most frequent findings in hysterectomy specimens from EC patients (up to 70% of cases). 19 , 20 , 28 , 31 Due to mixed results, the effect of adenomyosis on the prognosis of EC has remained unclear despite its high prevalence. On one hand, coexistent adenomyosis appears to be a risk factor for myometrial invasion, aggressive tumor behavior, and poor prognosis; however, on the other, it appears to be related to good EC prognosis.
Several hypotheses have been proposed to explain the poor prognosis in EC patients with adenomyosis. The large contact area between ectopic endometrium of adenomyosis and the muscular layer might increase the incidence and depth of myometrial infiltration of EC. 22 , 32 , 33 Furthermore, EC might follow the spread mechanism of ectopic endometrium through lymph and veins, increasing lymphovascular space invasion. 22 , 34 In addition, the poor prognosis of EC patients with adenomyosis has been associated with the malignant transformation of adenomyosis. 35 , 36 Finally, as adenomyosis reduces the contrast between the muscular layers involved in EC and adenomyosis, magnetic resonance imaging shows a decreased diagnostic accuracy in assessing depth of invasion, with potential understaging of patients. 22 , 37
In contrast, several other mechanisms might support good prognosis in patients with coexistent adenomyosis. Adenomyosis is characterized by a specific profile of cytokines with increased levels of antitumoral cytokines (such as interferon‐γ, tumor necrosis factor‐α, and interleukin 10) and decreased levels of oncogenic cytokines and growth factors (such as interleukin 1‐beta, interleukin 8, epidermal growth factor, and transforming growth factor). Such a profile of cytokines would lead to alterations in the local microenvironment, limiting tumor progression and invasiveness. 22 , 34 In addition, it has also been speculated that the thickened endometrial stroma of the adenomyotic uterus, caused by repeated inflammations with a subsequent healing process, might contribute to a mechanical block of EC invasion in the myometrium. 28 Finally, the frequent symptoms associated with adenomyosis, such as dysmenorrhea and abnormal uterine bleeding, may lead to an early EC diagnosis and then better cancer prognosis.
Our findings seem to support a positive impact of adenomyosis on the prognosis of women with EC, with the risk of death from any cause halved when compared with EC women without adenomyosis. However, the impact of adenomyosis on OS was only evaluable thorough univariate analysis. In fact, the included studies did not report multivariate analysis for OS, as shown in the risk of bias within studies assessment. Thus, the independent value of coexistent adenomyosis on the risk of death was not evaluable. Further studies with multivariate assessment of the impact of adenomyosis on OS in EC patients are necessary.
In our study, the coexistence of adenomyosis appeared to halve the risk of EC recurrence at univariate analysis; however, this finding was not significant at multivariate analysis. This suggests that this association is not independent. In fact, the association at univariate analysis may depend on other prognostic factors that might be associated with the coexistence of adenomyosis. In this regard, it would be interesting to assess the prevalence of classic prognostic histological factors (i.e. histotype, FIGO grade, deep myometrial infiltration, and lymphovascular space invasion), clinical characteristics impacting prognosis (age, body mass index), and TCGA molecular groups in EC patients with and without adenomyosis in future studies. However, further studies are also required to assess this association as only two included studies allowed data extraction for univariate and multivariate analyses regarding adenomyosis impact on DFS in EC patients.
To the best of our knowledge, this study may be the first systematic review and meta‐analysis to assess the prognostic value of coexistent adenomyosis in patients with EC, without the confounding effect of patient selection based on histological prognostic factors. Moreover, our results are supported by the overall quality of the included studies, as described in the risk of bias assessment. As for limitations, our study appears to be affected by the low number of included studies, in particular regarding DFS analyses. In addition, we were unable to perform OS multivariate analysis since the included studies did not report these. Therefore, we were unable to assess the independent value of adenomyosis on OS.
In conclusion, the risk of death is halved in EC patients with coexistent adenomyosis compared with EC patients without adenomyosis. However, this association needs to be assessed at multivariate analysis in future studies to assess the independence of the prognostic value. Furthermore, adenomyosis does not appear to affect the risk of EC recurrence. Additional data are required to confirm these findings.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
AR, AT: study conception, study design, study methods, data extraction, data analysis, manuscript preparation. DR: study conception, study design, study methods, data analysis, manuscript preparation, methods supervision. MM: study design, study methods, data analysis, manuscript preparation. PC: study conception, study design, study methods, data analysis, manuscript preparation. MA: study conception, data extraction, data analysis, manuscript preparation. ACR: data extraction, data analysis, manuscript preparation. AS: study design, data analysis, manuscript preparation, methods supervision. GZ: study design, methods supervision, whole study supervision. LI, AM, FZ, RS: study conception, study design, methods supervision, whole study supervision.
Supporting information
Fig S1
Fig S2a
Fig S2b
ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Napoli Federico II.
[Correction added on 07‐May‐2022, after first online publication: CRUI‐CARE funding statement has been added].
Raimondo D, Raffone A, Travaglino A, et al. Impact of adenomyosis on the prognosis of patients with endometrial cancer. Int J Gynecol Obstet. 2022;157:265–270. 10.1002/ijgo.13818
Diego Raimondo and Antonio Raffone equally contributed to the study.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5‐29. [DOI] [PubMed] [Google Scholar]
- 2. Raffone A, Travaglino A, Mascolo M, et al. TCGA molecular groups of endometrial cancer: pooled data about prognosis. Gynecol Oncol. 2019;155(2):374‐383. [DOI] [PubMed] [Google Scholar]
- 3. Travaglino A, Raffone A, Mascolo M, et al. TCGA molecular subgroups in endometrial undifferentiated/dedifferentiated carcinoma. Pathol Oncol Res. 2020;26(3):1411‐1416. [DOI] [PubMed] [Google Scholar]
- 4. Raffone A, Troisi J, Boccia D, et al. Metabolomics in endometrial cancer diagnosis: a systematic review. Acta Obstet Gynecol Scand. 2020;99(9):1135‐1146. [DOI] [PubMed] [Google Scholar]
- 5. Travaglino A, Raffone A, Mascolo M, et al. Clear cell endometrial carcinoma and the TCGA classification. Histopathology. 2020;76(2):336‐338. [DOI] [PubMed] [Google Scholar]
- 6. Travaglino A, Raffone A, Gencarelli A, et al. TCGA classification of endometrial cancer: the place of carcinosarcoma. Pathol Oncol Res. 2020;26(4):2067‐2073. [DOI] [PubMed] [Google Scholar]
- 7. Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular‐based classification for endometrial cancers. Br J Cancer. 2015;113:299‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med. 2020;383(21):2053‐2064. [DOI] [PubMed] [Google Scholar]
- 9. Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high‐grade endometrial carcinoma. Am J Surg Pathol. 2013;37:874‐881. [DOI] [PubMed] [Google Scholar]
- 10. Hoang LN, McConechy MK, Köbel M, et al. Histotype‐genotype correlation in 36 high‐grade endometrial carcinomas. Am J Surg Pathol. 2013;37:1421‐1432. [DOI] [PubMed] [Google Scholar]
- 11. Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: a simple, genomics‐based clinical classifier for endometrial cancer. Cancer. 2017;123:802‐813. [DOI] [PubMed] [Google Scholar]
- 12. Cancer Genome Atlas Research Network ; Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kommoss S, McConechy MK, Kommoss F, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population‐based case series. Ann Oncol. 2018;29:1180‐1188. [DOI] [PubMed] [Google Scholar]
- 14. Britton H, Huang L, Lum A, et al. Molecular classification defines outcomes and opportunities in young women with endometrial carcinoma. Gynecol Oncol. 2019;153:487‐495. [DOI] [PubMed] [Google Scholar]
- 15. Raffone A, Travaglino A, Mascolo M, et al. Histopathological characterization of ProMisE molecular groups of endometrial cancer. Gynecol Oncol. 2020;157(1):252‐259. [DOI] [PubMed] [Google Scholar]
- 16. Travaglino A, Raffone A, Mollo A, et al. TCGA molecular subgroups and FIGO grade in endometrial endometrioid carcinoma. Arch Gynecol Obstet. 2020;301(5):1117‐1125. [DOI] [PubMed] [Google Scholar]
- 17. Travaglino A, Raffone A, Stradella C, et al. Impact of endometrial carcinoma histotype on the prognostic value of the TCGA molecular subgroups. Arch Gynecol Obstet. 2020;301(6):1355‐1363. [DOI] [PubMed] [Google Scholar]
- 18. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387(10023):1094‐1108. [DOI] [PubMed] [Google Scholar]
- 19. Habiba M, Pluchino N, Petignat P, et al. Adenomyosis and endometrial cancer: literature review. Gynecol Obstet Invest. 2018;83(4):313‐328. [DOI] [PubMed] [Google Scholar]
- 20. Raffone A, Seracchioli R, Raimondo D, et al. Prevalence of adenomyosis in endometrial cancer patients: a systematic review and meta‐analysis. Arch Gynecol Obstet. 2021;303(1):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zannoni L, Ambrosio M, Raimondo D, et al. Question mark sign and transvaginal ultrasound uterine tenderness for the diagnosis of adenomyosis: a prospective validation. J Ultrasound Med. 2020;39(7):1405‐1412. [DOI] [PubMed] [Google Scholar]
- 22. An M, Duan H, Zhang Y. Prognostic significance of co‐existent adenomyosis on outcomes and tumor characteristics of endometrial cancer: a meta‐analysis. J Obstet Gynaecol Res. 2020;46(9):1851‐1863. [DOI] [PubMed] [Google Scholar]
- 23. NCCN Clinical Practice Guidelines in Oncology . Uterine Neoplasms, version 1.2021 – October 20, 2020. https://www.nccn.org/guidelines/recently‐published‐guidelines. Accessed June 24, 2021).
- 24. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slim K, Nini E, Forestier D, et al. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712‐716. [DOI] [PubMed] [Google Scholar]
- 26. Raffone A, Travaglino A, Cerbone M, et al. Diagnostic accuracy of immunochemistry for mismatch repair proteins as surrogate of microsatellite instability molecular testing in endometrial cancer. Pathol Oncol Res. 2020;26(3):1417‐1427. [DOI] [PubMed] [Google Scholar]
- 27. Raffone A, Travaglino A, Cerbone M, et al. Diagnostic accuracy of p53 immunohistochemistry as surrogate of TP53 sequencing in endometrial cancer. Pathol Res Pract. 2020;216(8):153025. [DOI] [PubMed] [Google Scholar]
- 28. Matsuo K, Cahoon SS, Gualtieri M, et al. Significance of adenomyosis on tumor progression and survival outcome of endometrial cancer. Ann Surg Oncol. 2014;21(13):4246‐4255. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Z, Yang B, Zhang W, et al. Clinicopathological characteristics and survival outcomes of patients with coexistence of adenomyosis and endometrial carcinoma. Int J Clin Exp Pathol. 2018;11(2):956‐962. [PMC free article] [PubMed] [Google Scholar]
- 30. Boonlak S, Aue‐Aungkul A, Kietpeerakool C, et al. Impact of coexisting uterine adenomyosis on the survival outcome of patients with endometrial cancer: a retrospective cohort study. Asian Pac J Cancer Prev. 2019;20(4):1185‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsuo K, Moeini A, Machida H, et al. Tumor characteristics and survival outcome of endometrial cancer arising in Adenomyosis: an exploratory analysis. Ann Surg Oncol. 2016;23:959‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ismiil N, Rasty G, Ghorab Z, et al. Adenomyosis is associated with myometrial invasion by FIGO 1 endometrial adenocancer. Int J Gynecol Pathol. 2007;26:278‐283. [DOI] [PubMed] [Google Scholar]
- 33. Ismiil N, Rasty G, Ghorab Z, et al. Adenomyosis is involved by endometrial adenocancer is a signifigant risk factor for deep myometrial invasion. Ann Dlagn Pathol. 2007;11:252‐257. [DOI] [PubMed] [Google Scholar]
- 34. Sanci M, Erkilinç S, Taylan E, et al. The effect of adenomyosis in myometrial invasion and overall survival in endometrial cancer. Int J Gynecol Cancer. 2018;28(1):145‐151. [DOI] [PubMed] [Google Scholar]
- 35. Sampson JA. Endometrial cancer of the ovary arising in endometrial tissue in that organ. Am J Obstet Gynecol. 1925;9:111‐114. [Google Scholar]
- 36. Machida H, Maeda M, Cahoon SS, et al. Endometrial cancer arising in adenomyosis versus endometrial cancer coexisting with adenomyosis: are these two different entities? Arch Gynecol Obstet. 2017;295:1459‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khalifa MA, Atri M, Klein ME, et al. Adenomyosis as a confounder to accurate endometrial cancer staging. Semin Ultrasound CT MR. 2019;40:358‐363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2a
Fig S2b


