Abstract
Background and purpose
Atrial fibrillation (AF) often remains undiagnosed in cryptogenic stroke (CS), mostly because of limited availability of cardiac long‐term rhythm monitoring. There is an unmet need for a pre‐selection of CS patients benefitting from such work‐up. A clinical risk score was therefore developed for the prediction of AF after CS and its performance was evaluated over 1 year of follow‐up.
Methods
Our proposed risk score ranges from 0 to 16 points and comprises variables known to be associated with occult AF in CS patients including age, N‐terminal pro‐brain natriuretic peptide, electrocardiographic and echocardiographic features (supraventricular premature beats, atrial runs, atrial enlargement, left ventricular ejection fraction) and brain imaging markers (multi‐territory/prior cortical infarction). All CS patients admitted to our Stroke Unit between March 2018 and August 2019 were prospectively followed for AF detection over 1 year after discharge.
Results
During the 1‐year follow‐up, 24 (16%) out of 150 CS patients with AF (detected via electrocardiogram controls, n = 18; loop recorder monitoring, n = 6) were diagnosed. Our predefined AF Risk Score (cutoff ≥4 points; highest Youden's index) had a sensitivity of 92% and a specificity of 67% for 1‐year prediction of AF. Notably, only two CS patients with <4 score points were diagnosed with AF later on (negative predictive value 98%).
Conclusions
A clinical risk score for 1‐year prediction of AF in CS with high sensitivity, reasonable specificity and excellent negative predictive value is presented. Generalizability of our score needs to be tested in external cohorts with continuous cardiac rhythm monitoring.
Keywords: atrial fibrillation, biomarker, cryptogenic stroke, NT‐proBNP, risk score
Occult atrial fibrillation (AF) often remains undiagnosed in cryptogenic stroke (CS), mostly because of limited availability of cardiac long‐term rhythm monitoring. In this prospective study, a clinical risk score for the prediction of AF in CS patients was developed. The Graz AF Risk Score was then evaluated in 150 CS patients, who were followed over 1 year for the diagnosis of AF. Our score reached a high sensitivity and reasonable specificity for predicting AF in CS patients, and had an excellent negative predictive value (98%).

INTRODUCTION
Occult atrial fibrillation (AF) is a frequent, yet undetected, cause of cryptogenic stroke (CS) and, if diagnosed, requires anticoagulation to ensure effective secondary stroke prevention [1, 2]. Randomized clinical trials failed to demonstrate the superiority of treating unselected CS patients with direct oral anticoagulants compared to standard antiplatelet treatment [3, 4]. These results support the necessity for detecting AF in CS patients, but costs and availability in daily clinical routine represent barriers in the use of long‐term cardiac rhythm monitoring [5]. Therefore, an informed pre‐selection of CS patients, who are likely to benefit from such complex diagnostic procedures, would clearly be of clinical relevance. In this context, previous studies have identified electrocardiographic, echocardiographic and neuroimaging features that were predictive for a later diagnosis of AF in CS patients [6, 7]. Moreover, laboratory biomarkers (i.e., N‐terminal pro‐brain natriuretic peptide [NT‐proBNP]) were also associated with occult AF in CS cohorts [8, 9]. Based on this available information, the aim here was to develop a clinical risk score for the prediction of AF in CS patients and to prospectively evaluate such a model in a single‐centre CS cohort over a 1‐year follow‐up period.
METHODS
Development of the Graz AF Risk Score
A literature search was conducted in MEDLINE (via PubMed) for all observational studies that have investigated the value of various markers for the prediction of AF in CS patients between 2009 and 2018. The search strategy included the following key words: ‘cryptogenic stroke’, ‘cryptogenic embolic stroke’, ‘embolic stroke’, ‘embolic stroke of undetermined source (ESUS)’ and ‘atrial fibrillation’.
The initial literature search yielded 470 abstracts. All abstracts were evaluated for studies on CS patients, regardless of study design. In a further step, residual papers were reviewed for studies reporting on predictors for a later diagnosis of AF in patients initially labelled as CS (n = 5, Table 1) [6, 7, 9, 10, 11].
TABLE 1.
Summary of selected studies on predictors for atrial fibrillation in cryptogenic stroke
| Study | Design | Cardiac rhythm monitoring | AF predictors | HR (AF Risk Score rating) |
|---|---|---|---|---|
| Favilla et al. [6] | Retrospective, single‐centre, n = 227 |
Mobile outpatient telemetry (duration 28 days) AF detection 31 patients (14%) |
Prior cortical/cerebellar infarct | 5.6 (major) |
| Miller et al. [7] | Retrospective, single‐centre, n = 156 |
Mobile outpatient telemetry (duration 21 days) AF detection 27 patients (17%) |
SPB on ECG at admission Ejection fraction 40%–50% Ejection fraction <40% Multi‐territory brain infarct |
13.7 (major) 3.6 (major) 1.8 (minor) 2.0 (minor) |
| Poli et al. [11] | Prospective, single‐centre, n = 75 |
Implantable loop recorder (duration 12 months) AF detection 25 patients (33%) |
Left atrial diameter ≥45 mm on parasternal long axis Atrial runs on 24‐h ECG (>20 beats) |
4.8 (major) 3.8 (major) |
| Thijs et al. [10] | Prospective, multi‐centre, n = 221 |
Implantable loop recorder (duration 20 months) AF detection 42 patients (19%) |
Age >75 years SPB >125 on 24‐h ECG |
5.4 (major) 3.4 (minor) |
| Kneihsl et al. yy [9] | Prospective, single‐centre, n = 143 |
Holter ECG + pulse controls (duration 15 days) AF detection 14 patients (10%) |
NT‐proBNP at admission ≥505 pg/ml |
4.2 (major) |
Abbreviations: AF, atrial fibrillation; ECG, electrocardiogram; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SPB, supraventricular premature beat.
The following predictors were identified: (i) age >60 and >75 years [10]; (ii) prior cortical or cerebellar [6] and multi‐territory [7] infarction on brain imaging; (iii) echocardiography with left atrial enlargement [11] and left ventricular ejection fraction (EF) <50% and <40% [7]; (iv) atrial runs [11] or supraventricular premature beats (SPB) >125 on 24‐h electrocardiogram (ECG) [10] or SPB on ECG at admission [7]; and (v) serum NT‐proBNP ≥505 pg/ml [9].
For the development of our AF Risk Score, these parameters were divided according to the presented hazard ratios into (i) major and (ii) minor risk criteria (Table 1). The cutoff between major and minor was set at a hazard ratio of 3.5.
In addition to the mentioned studies, the clinical condition of ‘recurrent stroke whilst on antiplatelet therapy’ was included in the minor risk category. Whilst this marker has not been analysed for AF prediction in CS patients yet, it is known to be highly indicative for AF‐related stroke in general [12, 13].
Since the influence of reduced EF on NT‐proBNP levels is pronounced, the present EF was used as a discriminator regarding NT‐proBNP as a major or minor criterion [14]. The finally proposed Graz AF Risk Score is shown in Figure 1a.
FIGURE 1.
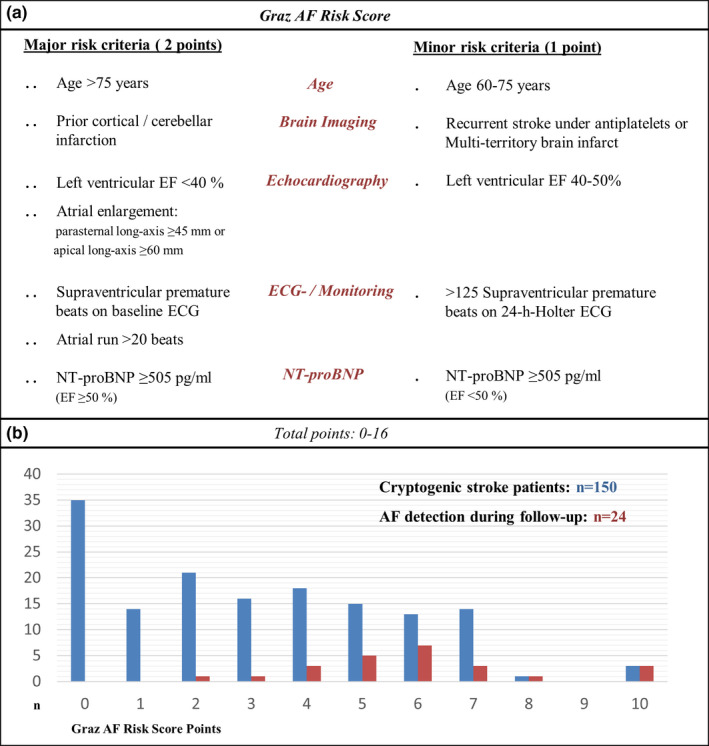
(a) The criteria of the Graz AF Risk Score. (b) The score distribution in the study cohort and the association with AF detection during the 1‐year follow‐up period
Evaluation of the Graz AF Risk Score
In this prospective observational analysis, all CS patients who were admitted to the Stroke Unit of our primary and tertiary care university hospital between March 2018 and August 2019 were included (Figure 2).
FIGURE 2.
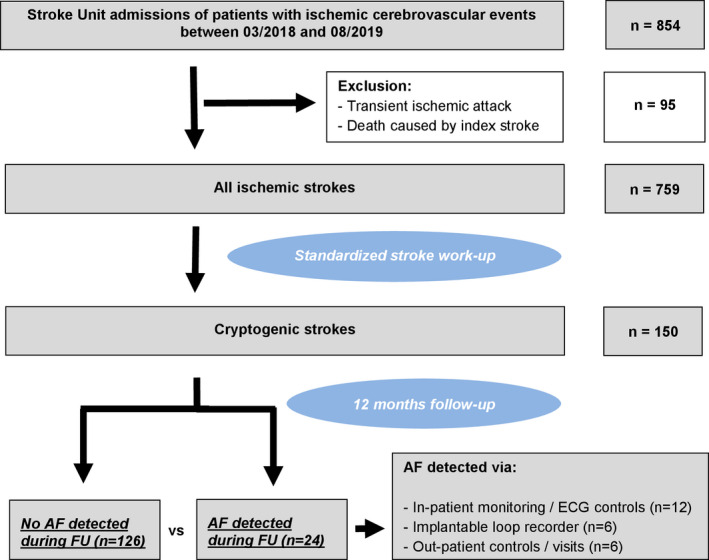
Flow diagram of selected study participants
Information on demographics, medical history (including a history of AF or other cardiac diseases), cerebrovascular risk factors, National Institutes of Health Stroke Scale score on admission and short‐term outcome, defined according to the modified Rankin Scale score at Stroke Unit discharge, were prospectively collected in all patients.
All patients underwent a thorough aetiological work‐up including medical history, brain imaging (magnetic resonance imaging [MRI] rate 90%), laboratory tests, ECG at admission, continuous ECG monitoring at the Stroke Unit, additional 24‐h Holter ECG, sonography of the extracranial and intracranial vessels, and echocardiography. Brain imaging findings were analysed by experienced neuroradiologists. Admission ECG and ECG monitoring at the Stroke Unit were first analysed by the treating stroke physicians for rhythm disorders or SPB. If any uncertainty remained, a cardiologist was consulted.
The 24‐h Holter ECG was reviewed by experienced cardiologists. If no distinct stroke aetiology was detected (according to the A‐S‐C‐O criteria), patients were classified as cryptogenic [15]. Laboratory biomarkers (NT‐proBNP) were analysed within 24 h after admission. Further details on blood sampling and NT‐proBNP analysis have been described previously [9].
Follow‐up
All included study participants were prospectively followed for a later diagnosis of AF after Stroke Unit discharge, as described below.
Patients underwent routine daily pulse controls or prolonged continuous rhythm monitoring during further hospital stay or in‐patient rehabilitation (median duration 3 weeks). ECG was performed in the case of tachycardia/arrhythmia or if clinical signs known to be associated with AF (i.e., palpitations, dyspnoea) were present. In addition, an implantable loop recorder (ILR) was offered to selected CS patients at the discretion of the treating stroke physician and further discussion with a cardiological rhythmologist.
All patients were followed for 12 months after stroke via the Elektronische Gesundheitsakte, an electronic information system documenting medical records including all prescribed medications in Austria. If the indication of a prescribed anticoagulation was unclear, a telephone follow‐up was performed with the patient or the treating general practitioner to identify the underlying diagnosis. In addition, the Medical Documentation and Communication Network of Styria (MEDOCS), which provides electronic data from all public hospitals in the province of Styria, was reviewed for relevant data [16].
Atrial fibrillation was diagnosed if episodes lasted ≥30 s or if it was classified in the electronic records [17].
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp, Armonk, NY, USA).
Pearson's chi‐squared or Fisher's exact tests were used for the comparison of dichotomous variables. Quantitative variables were tested for Gaussian distribution with the Kolmogorov–Smirnov test. If a Gaussian distribution was identified, a two‐sample independent t test was used. For non‐parametric data, the Mann–Whitney U test was applied. A p value less than 0.05 was considered statistically significant.
The Graz AF Risk Score was calculated for every selected study participant and evaluated for its predictive value for the occurrence of AF during the 1‐year follow‐up. For this purpose, Youden's index was used to define the cutoff score with the highest sensitivity and specificity for the occurrence of AF in CS patients and was compared to other proposed AF risk scores calculated from our study data (CHADS2 score, STAF score and AS5F score) [18, 19].
To test for multicollinearity between score parameters, the variance inflation factor was calculated via linear regression models. Furthermore, logistic regression models were used to test for interactions between the AF Risk Score variables.
RESULTS
Between March 2018 and August 2019, 854 patients with ischaemic cerebrovascular events were admitted to the Stroke Unit of our university hospital. Patients who had transient ischaemic attacks were excluded from the study as were patients who died because of their index stroke (n = 95), leading to a final study cohort of 759 patients (Figure 2). Of these, 150 patients (mean age 66.7 ± 15.3 years; female 43.3%) remained with the diagnosis of CS after thorough aetiological work‐up.
During a 1‐year follow‐up period, 24 of these 150 CS patients were diagnosed with AF (16%) after a mean time of 45 days after discharge (range 2–323 days). All these patients were then treated with oral anticoagulation. AF was detected on ECG monitoring/repeated ECG controls during in‐patient rehabilitation (n = 12) or on out‐patient/hospital treatment due to AF‐related symptoms/cerebrovascular events (n = 6). In an additional six patients, AF was diagnosed via ILR, which had been implanted in 24 CS patients.
Atrial fibrillation versus non‐AF in CS patients
Cryptogenic stroke patients who were diagnosed with AF during 1‐year follow‐up were older (75.0 vs. 65.1 years, p < 0.001) and more often had pre‐treatment with antiplatelets at admission (50.0% vs. 13.5%, p < 0.001) compared to non‐AF CS patients.
On brain imaging, the AF subgroup more often had old cortical or cerebellar infarcts (45.8% vs. 23.8%, p = 0.026) and multi‐territory acute brain infarcts (33.3% vs. 15.9%, p = 0.044). On echocardiography, left atrial enlargement (25% vs. 7.1%, p = 0.008) prevailed in CS patients with a later diagnosis of AF. Reduced left ventricular EF below 50% tended to be more frequent in the AF group (16.7% vs. 5.6%, p = 0.077).
Cardiac rhythm monitoring showed an increased number of SPB >125/24 h (76.5% vs. 26.2%, p < 0.001), atrial runs (17.3% vs. 0.8%, p < 0.001) and SPB on admission ECG (12.5% vs. 2.4%, p = 0.02) in AF patients. Furthermore, such patients also had higher baseline NT‐proBNP levels and exceeded the cutoff of 505 pg/ml more often compared to the non‐AF group (58.8% vs. 17.5%, p < 0.001) (Table 2).
TABLE 2.
Demographics, clinical data and laboratory parameters of cryptogenic stroke patients dichotomized by the detection of atrial fibrillation within 1 year after stroke
| Variable/clinical finding |
CS patients (n = 150) |
AF (n = 24) |
No AF (n = 126) |
p value a |
|---|---|---|---|---|
|
Demographics |
||||
| Age, years (mean, SD) | 66.7 ± 15.3 | 75.0 ± 6.3 | 65.1 ± 15.9 | <0.001 |
| Age >75 years | 50 (33.3) | 13 (54.2) | 37 (29.4) | 0.018 |
| Age >60 years | 103 (68.7) | 23 (95.8) | 80 (63.5) | 0.003 |
| Female, n, % | 65 (43.3) | 10 (41.7) | 55 (43.7) | 0.521 |
| Medical history/clinical presentation, n, % | ||||
| Arterial hypertension | 100 (66.6) | 17 (70.8) | 83 (65.9) | 0.637 |
| Dyslipidaemia | 72 (48.0) | 11 (45.8) | 61 (48.4) | 0.817 |
| Diabetes | 24 (16.0) | 2 (8.3) | 22 (17.5) | 0.264 |
| Smoking | 36 (24.0) | 4 (16.7) | 32 (25.4) | 0.359 |
| Previous stroke | 16 (10.6) | 4 (16.7) | 12 (9.5) | 0.299 |
| Pre‐existing antiplatelet therapy | 29 (19.3) | 12 (50.0) | 17 (13.5) | <0.001 |
| NIHSS at presentation | 4 (0–22) | 4 (0–22) | 3 (0–18) | 0.063 |
| neuroimaging, n, % | ||||
| Old cortical/cerebellar infarct | 41 (27.3) | 11 (45.8) | 30 (23.8) | 0.026 |
| Multi‐territory brain infarct | 28 (18.7) | 8 (33.3) | 20 (15.9) | 0.044 |
| echocardiography, n, % | ||||
| Ejection fraction <40% | 2 (1.3) | 1 (4.2) | 1 (0.8) | 0.295 |
| Ejection fraction <50% | 11 (7.3) | 4 (16.7) | 7 (5.6) | 0.077 |
| Atrial enlargement (moderate–severe) | 15 (10.0) | 6 (25.0) | 9 (7.1) | 0.008 |
| ECG/24‐h ECG, n, % | ||||
| SPB on ECG at admission | 5 (3.3) | 2 (12.5) | 3 (2.4) | 0.020 |
| SPB >125 over 24 h | 46 (30.7) | 13 (54.2) | 33 (26.2) | <0.001 |
| Atrial run ≥20 beats | 4 (2.7) | 3 (17.6) | 1 (0.8) | <0.001 |
|
Laboratory parameters at admission, n, % |
||||
| NT‐proBNP (pg/ml), mean (SD) | 605 (995) | 1088 (1203) | 497 (912) | 0.012 |
| NT‐proBNP >505 pg/ml | 36 (24.0) | 14 (58.3) | 22 (17.5) | <0.001 |
| AF Risk Score, median (min–max) | 3 (0–10) | 6 (2–10) | 2 (0–8) | <0.001 |
| outcome, n, % | ||||
| mRS 0–2 at Stroke Unit discharge | 92 (61.3) | 13 (54.2) | 79 (62.7) | 0.432 |
| Recurrent ischaemic stroke | 10 (6.7) | 2 (8.3) | 8 (6.3) | 0.721 |
Abbreviations: AF, atrial fibrillation; CS, cryptogenic stroke; ECG, electrocardiogram; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SPB, supraventricular premature beat.
Demonstrated p value was determined by comparing cryptogenic stroke patients with/without AF.
Graz AF Risk Score
In the entire cohort of CS patients, the median Graz AF Risk Score was 3 points (range 0–10 points).
The most prevalent risk factors for AF were age >60 years (103/150; 68.7%), >125 SPB on 24‐h Holter ECG (46/150; 30.7%) and old cortical or cerebellar infarction on neuroimaging (41/150; 27.3%). 35 patients (23.3%) did not have a single risk factor for the subsequent diagnosis of AF (Figure 1b).
In a further step, the Graz AF Risk Score was evaluated for its predictive value in AF detection in CS patients during a 1‐year follow‐up period. The area under the curve of the score values obtained for a later detection of AF was 0.85 (95% confidence interval 0.78–0.92), which is higher than other proposed AF risk scores that were tested in our cohort (Figure 3). According to the highest Youden’s index (0.59), the best cutoff value to predict AF in CS patients was identified as ≥4 points, achieving a sensitivity of 92% and a specificity of 67%. The diagnostic yield for AF detection was 34% (22/64) at this cutoff. Notably, only two patients with <4 score points were diagnosed with AF later on (negative predictive value 98%).
FIGURE 3.
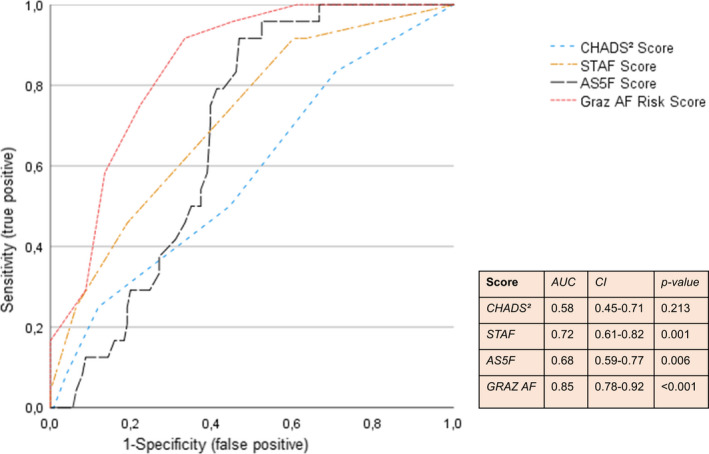
Receiver operating characteristic curves of different risk scores (CHADS2 score, STAF score, AS5F score, Graz AF Risk Score) to predict new‐onset atrial fibrillation in cryptogenic stroke patients
Except for a weak interaction between age and old cortical/cerebellar infarcts (p = 0.02), tests for multicollinearity and interactions did not reveal any significant results between the parameters included in the Graz AF Risk Score.
DISCUSSION
This prospective observational study proposes a novel clinical score to identify CS patients with an increased risk for a subsequent diagnosis of AF as their most likely underlying stroke aetiology.
Although earlier studies in this field suggested the use of long‐term cardiac rhythm monitoring with implantable event recorders in unselected CS patients, the high efforts and costs that are needed for such tools may not outweigh the relatively low diagnostic yield of about 10% within the first 12 months of observation [5, 10, 20]. This supports the necessity to identify those CS patients who are likely to benefit from such complex diagnostic procedures.
For this reason, the present Graz AF Risk Score was developed, which is the first score to predict AF in CS patients using a combination of ECG, neuro/cardiac imaging findings and blood biomarkers, which have all been strongly associated with occult AF in recent studies [6, 7, 9, 10, 11].
Although prior studies identified various risk factors for occult AF, the use of a single predictor might not be sufficient, as hazard ratios for AF detection in CS patients remained relatively low in large population‐based studies [21]. Therefore, AF risk scores based on clinical, neuroimaging and echocardiographic features have been developed [18, 19, 21, 22, 23]. Whilst these scores presented reasonable sensitivities and specificities for AF, they were not developed in a CS cohort [18, 21] or only reported on the early in‐hospital AF prediction [19, 21, 24]. It was therefore not surprising that the CHADS2 score, the STAF score and the AS5F score did not reach diagnostic accuracy in comparison to our proposed AF Risk Score.
A recently published registry‐based score (AF‐ESUS score) performed well to predict new incident AF after acute ischaemic stroke of undetermined aetiology. However, it was limited by its retrospective study design, the non‐uniform aetiological stroke work‐up and other known limitations of register‐based studies such as registration bias [23]. A further recognized AF risk score derives from a retrospective US registry study including over 9000 CS patients with a median follow‐up of 2.6 years [21]. The so‐called HAVOC score includes age, hypertension, valvular heart disease, peripheral arterial disease, obesity, congestive heart failure and coronary artery disease [21]. Whilst high HAVOC scores had a reasonable predictive value for occult AF (diagnostic yield 32% as opposed to 34% in our model), low scores were shown to be insufficient to exclude AF in a post hoc analysis of the CRYSTAL AF data. The authors therefore concluded that the HAVOC score might only partly cover the pathophysiological spectrum of AF and that other ‘non‐traditional’ features associated with AF should be included in future AF risk scores [25].
When prospectively evaluating the Graz AF Risk Score in our cohort, the best cutoff level of 4 points demonstrated high sensitivity and acceptable specificity (92% and 67%, respectively) for detecting AF in CS patients. The AF detection rate was 34% at this cutoff, which is markedly higher than in most investigations that have been published to date [1, 26] and comparable to a recent study which used clinical and echocardiographic features to pre‐select for ILR monitoring in a small CS cohort (1‐year detection rate 33%) [11].
Moreover, the first study presenting data on how many patients would have been missed if low pre‐test scores had been applied to a routine CS population is reported here. In our cohort, only two patients with AF Risk Scores <4 were diagnosed with AF during the follow‐up period, resulting in a high negative predictive value of 98%. Such patients are therefore very unlikely to have an underlying AF that caused the stroke and should be re‐evaluated for other possibly unrecognized stroke aetiologies (e.g., non‐stenosing aggressive carotid artery plaque, active cancer etc.) [27]. The inclusion of NT‐proBNP, the most widely known blood biomarker of heart failure and atrial cardiopathy, has strongly contributed to our results. Nevertheless, the sensitivity of the NT‐proBNP cutoff value of 505 pg/ml for predicting AF on long‐term follow‐up was relatively low (59%) compared to previous results from our group in a different study population on early in‐hospital AF prediction (sensitivity 86%) [9]. This might arise from a direct effect of AF episodes on NT‐proBNP. If AF occurs rarely or only in short episodes, NT‐proBNP levels might remain relatively low compared to patients with a high AF burden [28]. This could explain that baseline NT‐proBNP levels decreased with increasing time intervals between the index event and AF (data not shown). Larger multi‐centre studies are necessary to define the ideal NT‐proBNP cutoff for predicting occult AF in CS patients.
Apart from the uniform analysis of baseline NT‐proBNP levels, another strength of our study is the predefined standardized stroke work‐up at our centre with a high brain MRI rate of 90% and a thorough cardiac assessment in all included patients. Besides a classical (cardio)embolic infarct pattern on brain MRI, systolic and diastolic cardiac dysfunction were also associated with AF detection in CS patients. Increased atrial afterload due to heart failure and consecutive atrial myocyte stretching and arterial wall stress have been identified as possible mechanisms of atrial cardiopathy. Such tissue changes can lead to abnormal electric propagation and might cause SPB, atrial runs and, finally, AF [29]. This is also supported by the high SPB burden in patients who were diagnosed with AF during the follow‐up period in our study.
The main limitation of this work arises from the single‐centre design. Moreover, the moderate sample size precluded the analysis of the effect size of single parameters that were included in our score. More detailed investigations of single AF predictors could refine the cutoff values used and evaluate the strength of each marker to further improve the suggested risk score. A further limitation resides in the fact that only every sixth CS patient in our cohort had undergone continuous cardiac rhythm monitoring during the follow‐up period. Although a pragmatic predefined approach was used to follow all CS patients over 1 year, the generalizability of our score needs to be tested in external cohorts with continuous cardiac rhythm monitoring. However, a significant underestimation of AF detection is unlikely as the detection rate at 1 year (16%) after stroke was comparable to the results of the CRYSTAL AF trial (12%) which used ILR monitoring in all included patients [10].
In conclusion, this study emphasizes the utility of pre‐selectlng CS patients for further complex and expensive cardiac long‐term monitoring procedures and proposes a score that might help to identify CS patients who are at high risk for occult AF. Of note, low Graz AF Risk Scores had a high negative predictive value, which might imply a re‐evaluation of alternative stroke aetiologies in such patients.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Markus Kneihsl: Conceptualization (supporting); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); writing—original draft (lead); writing—review and editing (equal). Egbert Bisping: Conceptualization (supporting); formal analysis (supporting); methodology (supporting); supervision (supporting); writing—review and editing (supporting). Daniel Scherr: Investigation (supporting); writing—review and editing (supporting). Harald Mangge: Investigation (supporting); methodology (supporting); writing—review and editing (supporting). Simon Fandler‐Höfler: Data curation (supporting); writing—review and editing (supporting). Isabella Colonna: Data curation (supporting); investigation (supporting). Sebastian Eppinger: Data curation (supporting); investigation (supporting). Edith Hofer: Data curation (supporting); formal analysis (supporting); methodology (supporting). Franz Fazekas: Conceptualization (lead); investigation (supporting); project administration (supporting); supervision (supporting). Christian Enzinger: Conceptualization (supporting); supervision (supporting); writing—review and editing (supporting). Thomas Gattringer: Conceptualization (equal); investigation (supporting); methodology (supporting); project administration (lead); supervision (lead); writing—original draft (supporting); writing—review and editing (lead).
ETHICAL APPROVAL
Ethical approval for this study was obtained from the Medical University of Graz (REC number 29–285 ex 16/17).
ACKNOWLEDGEMENTS
The authors would like to thank Steiermärkische Krankenanstaltengesellschaft m.b.H. (KAGES) and Medtronic PLC for their organizational support.
Kneihsl M, Bisping E, Scherr D, et al. Predicting atrial fibrillation after cryptogenic stroke via a clinical risk score—a prospective observational study. Eur J Neurol. 2022;29:149–157. doi: 10.1111/ene.15102
Statistical analyses were performed by Markus Kneihsl and Egbert Bisping.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors
DATA AVAILABILITY STATEMENT
Source data from this study are available from the corresponding authors upon reasonable request.
REFERENCES
- 1. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467‐2477. [DOI] [PubMed] [Google Scholar]
- 2. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154:1121‐1201. [DOI] [PubMed] [Google Scholar]
- 3. Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378:2191‐2201. [DOI] [PubMed] [Google Scholar]
- 4. Diener HC, Sacco RL, Easton JD, et al., RE‐SPECT ESUS Steering Committee and Investigators. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380:1906‐1917. [DOI] [PubMed] [Google Scholar]
- 5. Diamantopoulos A, Sawyer LM, Lip GY, et al. Cost‐effectiveness of an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke. Int J Stroke. 2016;11:302‐312. [DOI] [PubMed] [Google Scholar]
- 6. Favilla CG, Ingala E, Jara J, et al. Predictors of finding occult atrial fibrillation after cryptogenic stroke. Stroke. 2015;46:1210‐1215. [DOI] [PubMed] [Google Scholar]
- 7. Miller DJ, Khan MA, Schultz LR, et al. Outpatient cardiac telemetry detects a high rate of atrial fibrillation in cryptogenic stroke. J Neurol Sci. 2013;324:57‐61. [DOI] [PubMed] [Google Scholar]
- 8. Fonseca AC, Brito D, Pinho e Melo T, Geraldes R, Canhão P, Caplan LR et al. N‐terminal pro‐brain natriuretic peptide shows diagnostic accuracy for detecting atrial fibrillation in cryptogenic stroke patients. Int J Stroke. 2014;9:419‐425. [DOI] [PubMed] [Google Scholar]
- 9. Kneihsl M, Gattringer T, Bisping E, et al. Blood biomarkers of heart failure and hypercoagulation to identify atrial fibrillation‐related stroke. Stroke. 2019;50:2223‐2226. [DOI] [PubMed] [Google Scholar]
- 10. Thijs VN, Brachmann J, Morillo CA, et al. Predictors for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF. Neurology. 2016;86:261‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poli S, Diedler J, Härtig F, et al. Insertable cardiac monitors after cryptogenic stroke—a risk factor based approach to enhance the detection rate for paroxysmal atrial fibrillation. Eur J Neurol. 2016;23:375‐381. [DOI] [PubMed] [Google Scholar]
- 12. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453‐1468. [DOI] [PubMed] [Google Scholar]
- 13. Diener HC, Weimar C, Weber R. Antiplatelet therapy in secondary stroke prevention—state of the art. J Cell Mol Med. 2010;14:2552‐2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rørth R, Jhund PS, Yilmaz MB, et al. Comparison of BNP and NT‐proBNP in patients with heart failure and reduced ejection fraction. Circ Heart Fail. 2020;13:e006541. [DOI] [PubMed] [Google Scholar]
- 15. Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: the ASCO (phenotypic) classification of stroke. Cerebrovasc Dis. 2009;27:502‐508. [DOI] [PubMed] [Google Scholar]
- 16. Gell G, Madjaric M, Leodolter W, Köle W, Leitner H. HIS purchase projects in public hospitals of Styria, Austria. Int J Med Inform. 2000;59:147‐155. [DOI] [PubMed] [Google Scholar]
- 17. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50:e1‐e88. [DOI] [PubMed] [Google Scholar]
- 18. Suissa L, Bertora D, Lachaud S, Mahagne MH. Score for the targeting of atrial fibrillation (STAF): a new approach to the detection of atrial fibrillation in the secondary prevention of ischemic stroke. Stroke. 2009;40:2866‐2868. [DOI] [PubMed] [Google Scholar]
- 19. Uphaus T, Weber‐Krüger M, Grond M, et al. Development and validation of a score to detect paroxysmal atrial fibrillation after stroke. Neurology. 2019;92:e115‐e124. [DOI] [PubMed] [Google Scholar]
- 20. Carrazco C, Golyan D, Kahen M, Black K, Libman RB, Katz JM. Prevalence and risk factors for paroxysmal atrial fibrillation and flutter detection after cryptogenic ischemic stroke. J Stroke Cerebrovasc Dis. 2018;27:203‐209. [DOI] [PubMed] [Google Scholar]
- 21. Kwong C, Ling AY, Crawford MH, Zhao SX, Shah NH. A clinical score for predicting atrial fibrillation in patients with cryptogenic stroke or transient ischemic attack. Cardiology. 2017;138:133‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshioka K, Watanabe K, Zeniya S, et al. A score for predicting paroxysmal atrial fibrillation in acute stroke patients: iPAB score. J Stroke Cerebrovasc Dis. 2015;24:2263‐2269. [DOI] [PubMed] [Google Scholar]
- 23. Ntaios G, Perlepe K, Lambrou D, et al. Identification of patients with embolic stroke of undetermined source and low risk of new incident atrial fibrillation: the AF‐ESUS score. Int J Stroke. 2021;16:29‐38. [DOI] [PubMed] [Google Scholar]
- 24. Haeusler KG, Kirchhof P, Kunze C, et al. Systematic monitoring for detection of atrial fibrillation in patients with acute ischaemic stroke (MonDAFIS): a randomised, open‐label, multicentre study. Lancet Neurol. 2021;20:426‐436. [DOI] [PubMed] [Google Scholar]
- 25. Zhao SX, Ziegler PD, Crawford MH, Kwong C, Koehler JL, Passman RS. Evaluation of a clinical score for predicting atrial fibrillation in cryptogenic stroke patients with insertable cardiac monitors: results from the CRYSTAL AF study. Ther Adv Neurol Disord. 2019;12:1756286419842698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ziegler PD, Rogers JD, Ferreira SW, et al. Long‐term detection of atrial fibrillation with insertable cardiac monitors in a real‐world cryptogenic stroke population. Int J Cardiol. 2017;244:175‐179. [DOI] [PubMed] [Google Scholar]
- 27. Kneihsl M, Enzinger C, Wünsch G, et al. Poor short‐term outcome in patients with ischaemic stroke and active cancer. J Neurol. 2016;263:150‐156. [DOI] [PubMed] [Google Scholar]
- 28. Winter Y, Wolfram C, Schaeg M, et al. Evaluation of costs and outcome in cardioembolic stroke or TIA. J Neurol. 2009;256:954‐963. [DOI] [PubMed] [Google Scholar]
- 29. Kamel H, Okin PM, Longstreth WT Jr, Elkind MS, Soliman EZ. Atrial cardiopathy: a broadened concept of left atrial thromboembolism beyond atrial fibrillation. Future Cardiol. 2015;11:323‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Source data from this study are available from the corresponding authors upon reasonable request.


